Wear and Corrosion Resistance of Thermally Formed Decorative Oxide Layers on Austenitic Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Surface Topography and Roughness Tests
2.3. X-Ray Diffraction Measurements
2.4. Microhardness and Friction Wear Resistance Tests
2.5. Corrosion Resistance Tests
2.6. Wettability Tests
3. Results and Discussion
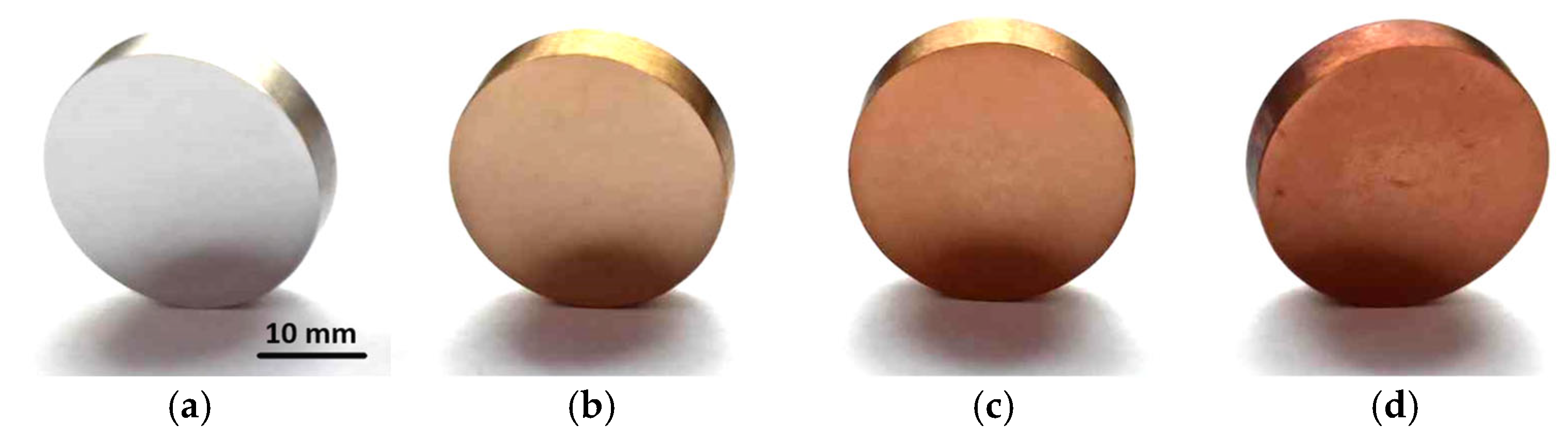
3.1. Surface Appearance
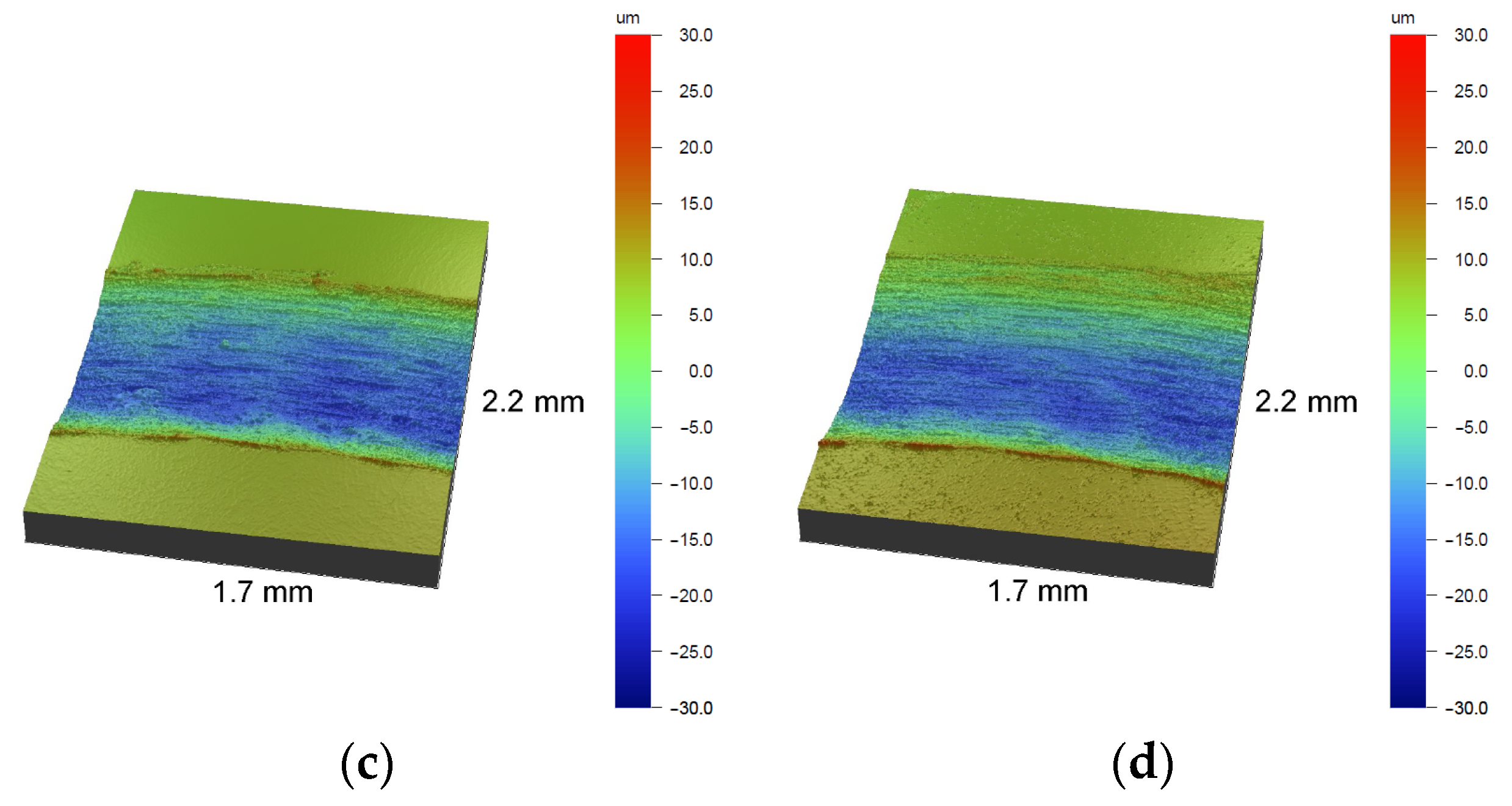
3.2. Surface Topography and Roughness
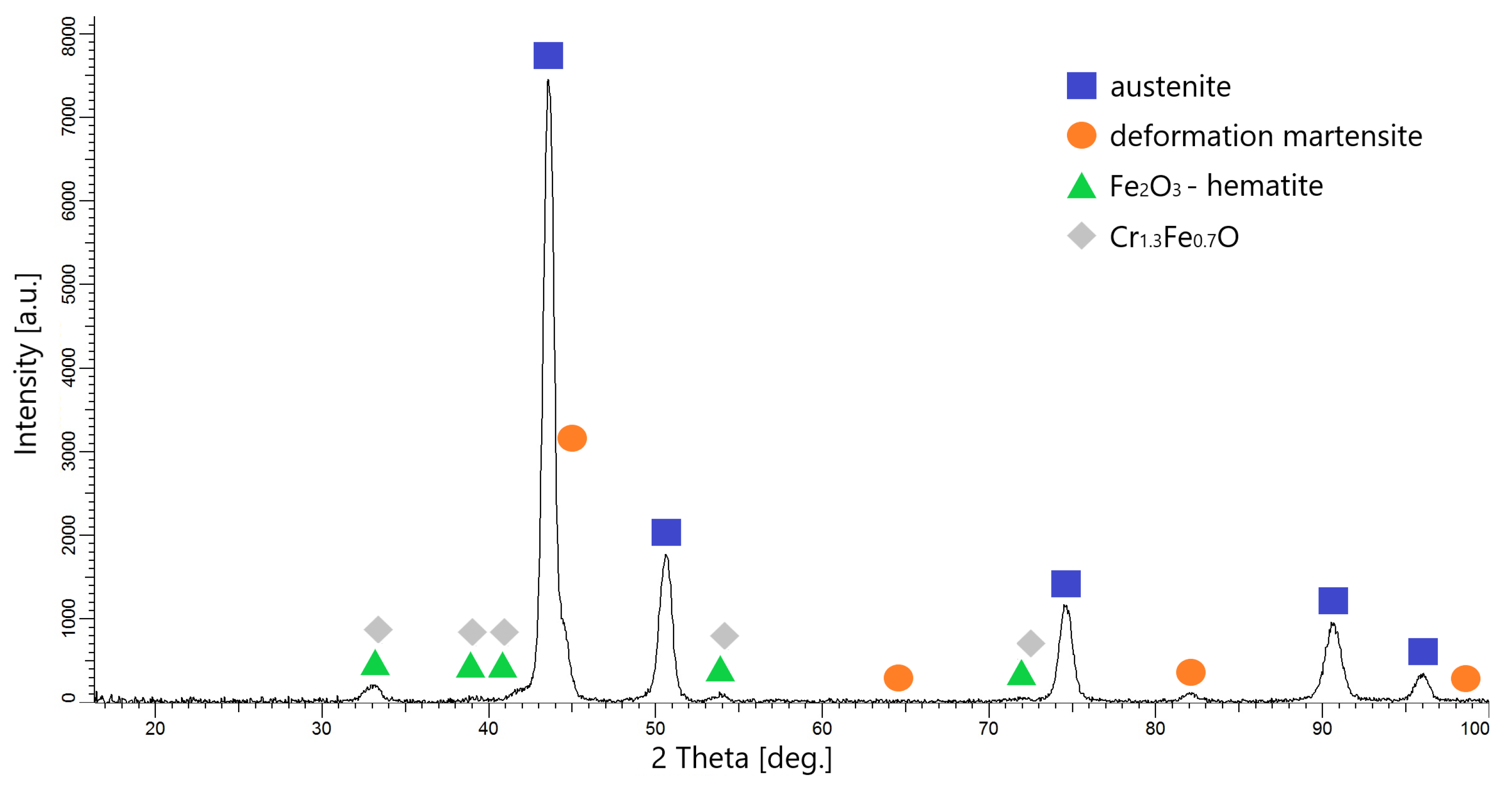
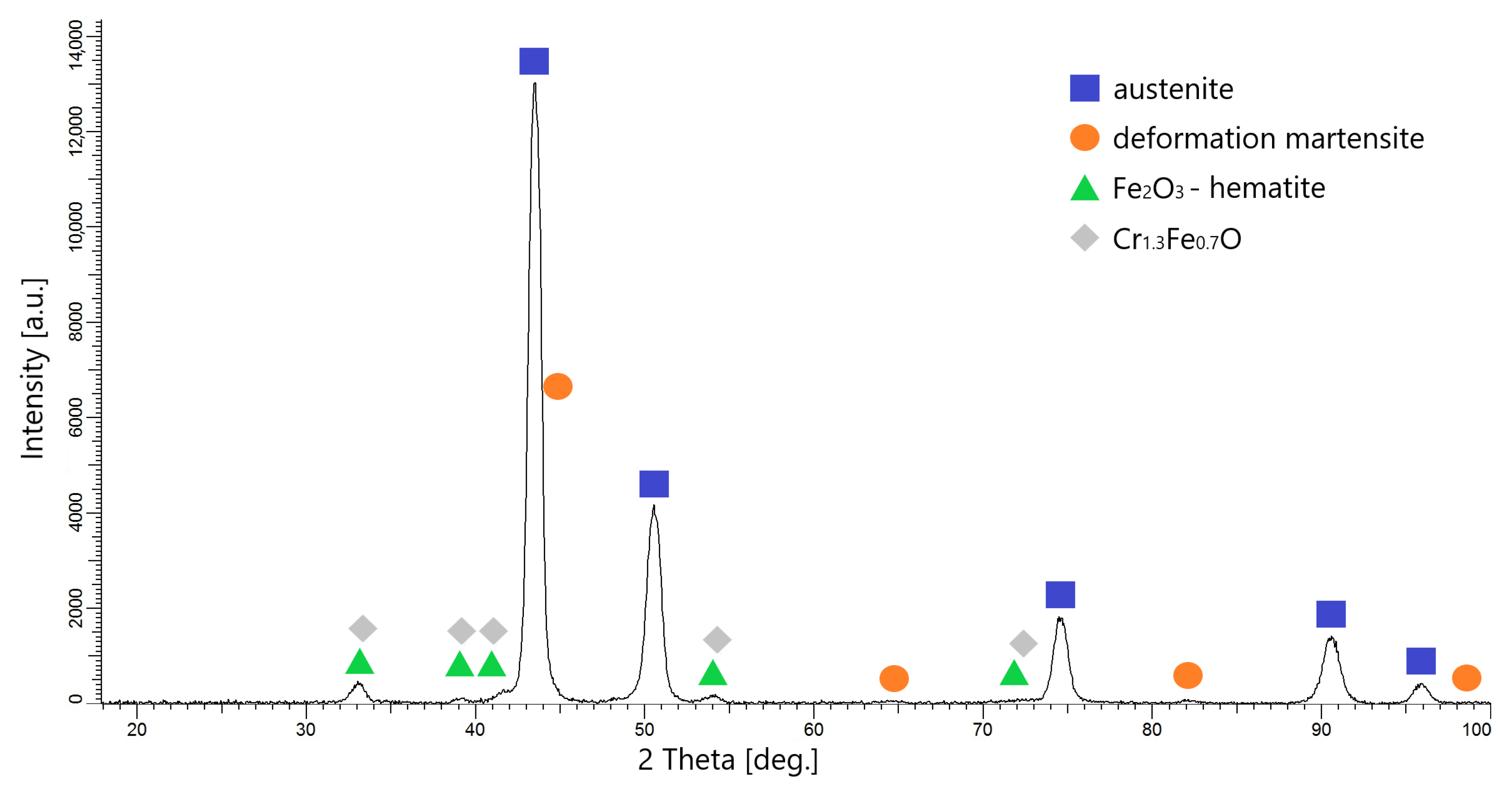
3.3. Microstructure Analysis
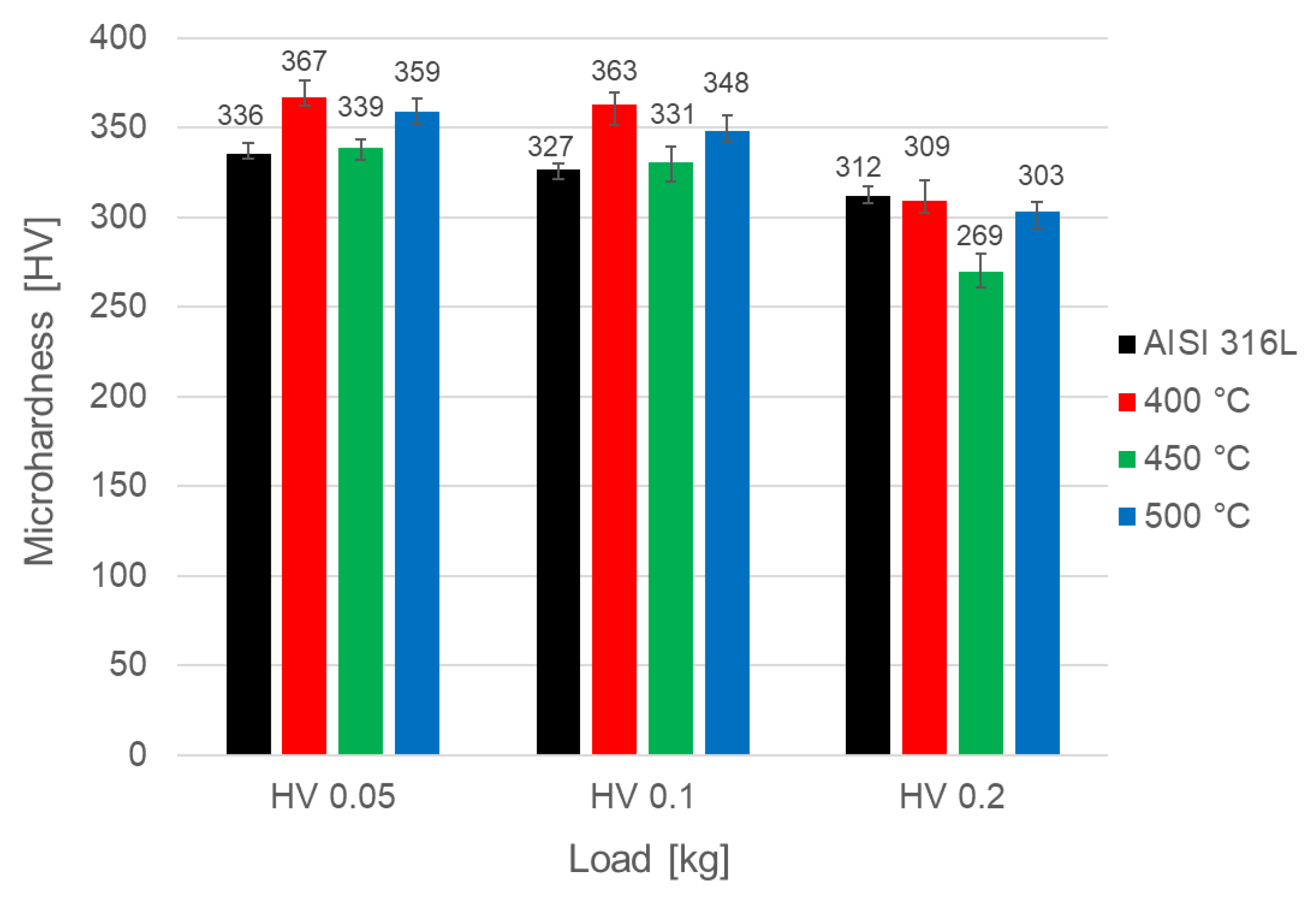
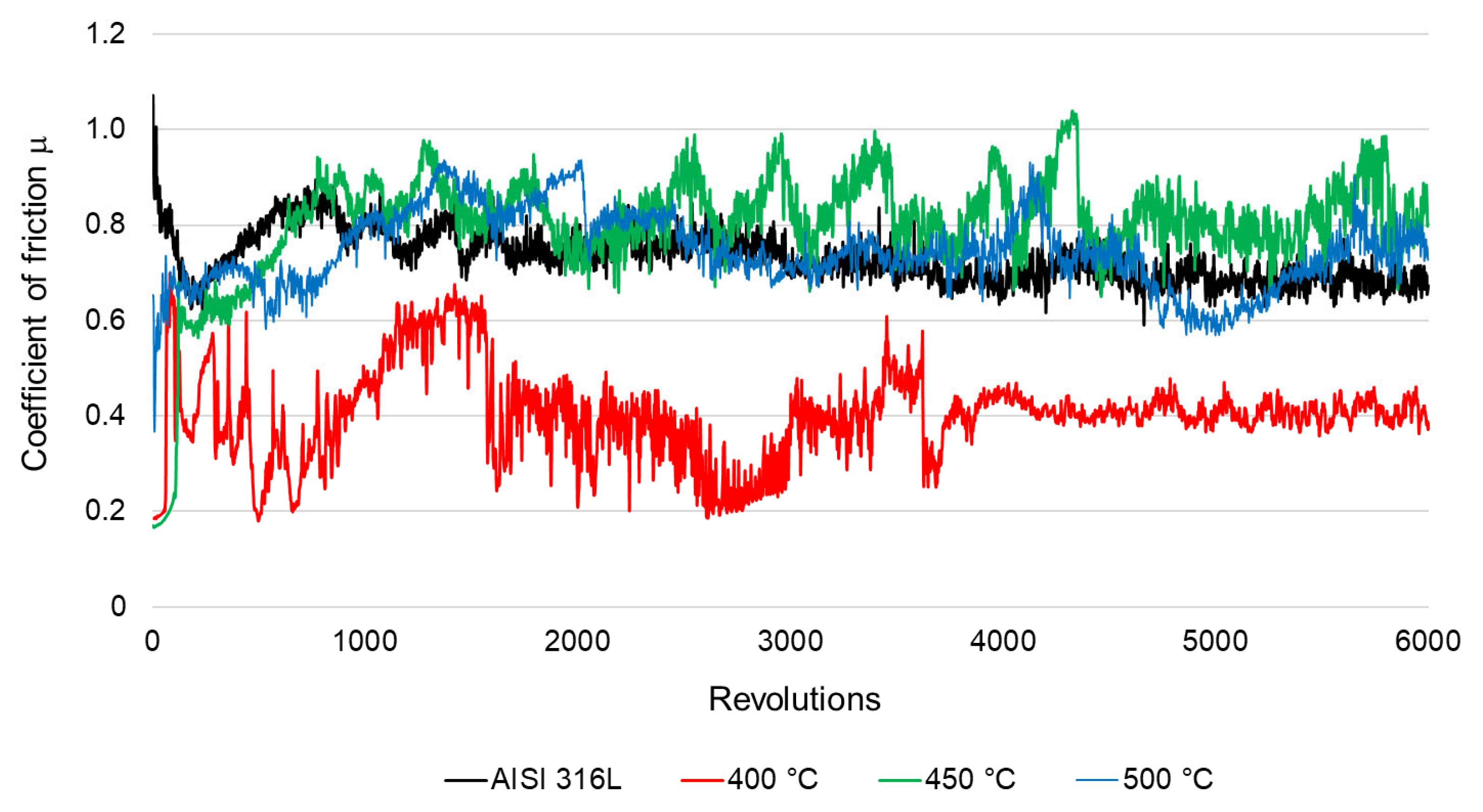
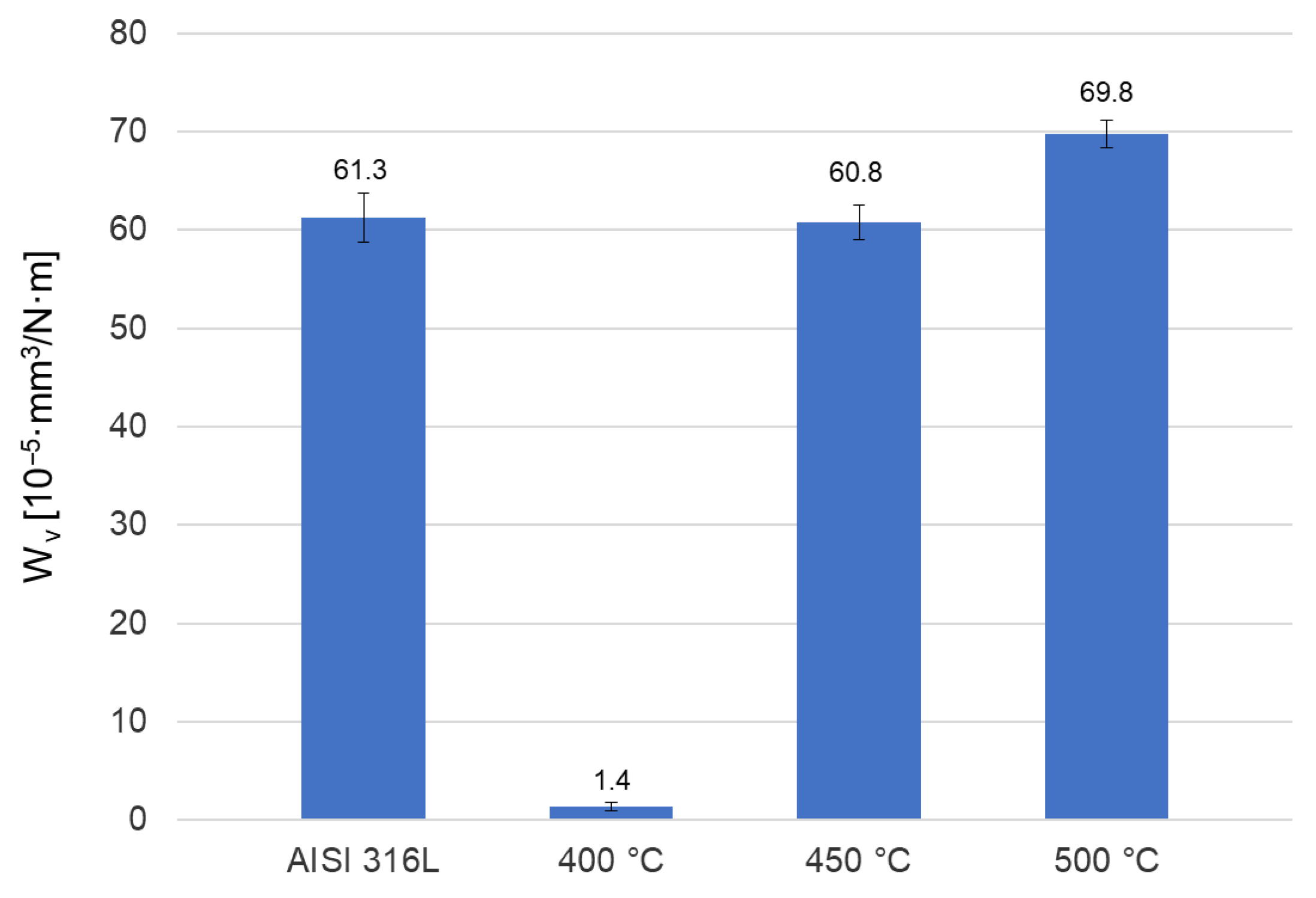
3.4. Microhardness and Friction Wear Resistance Analysis
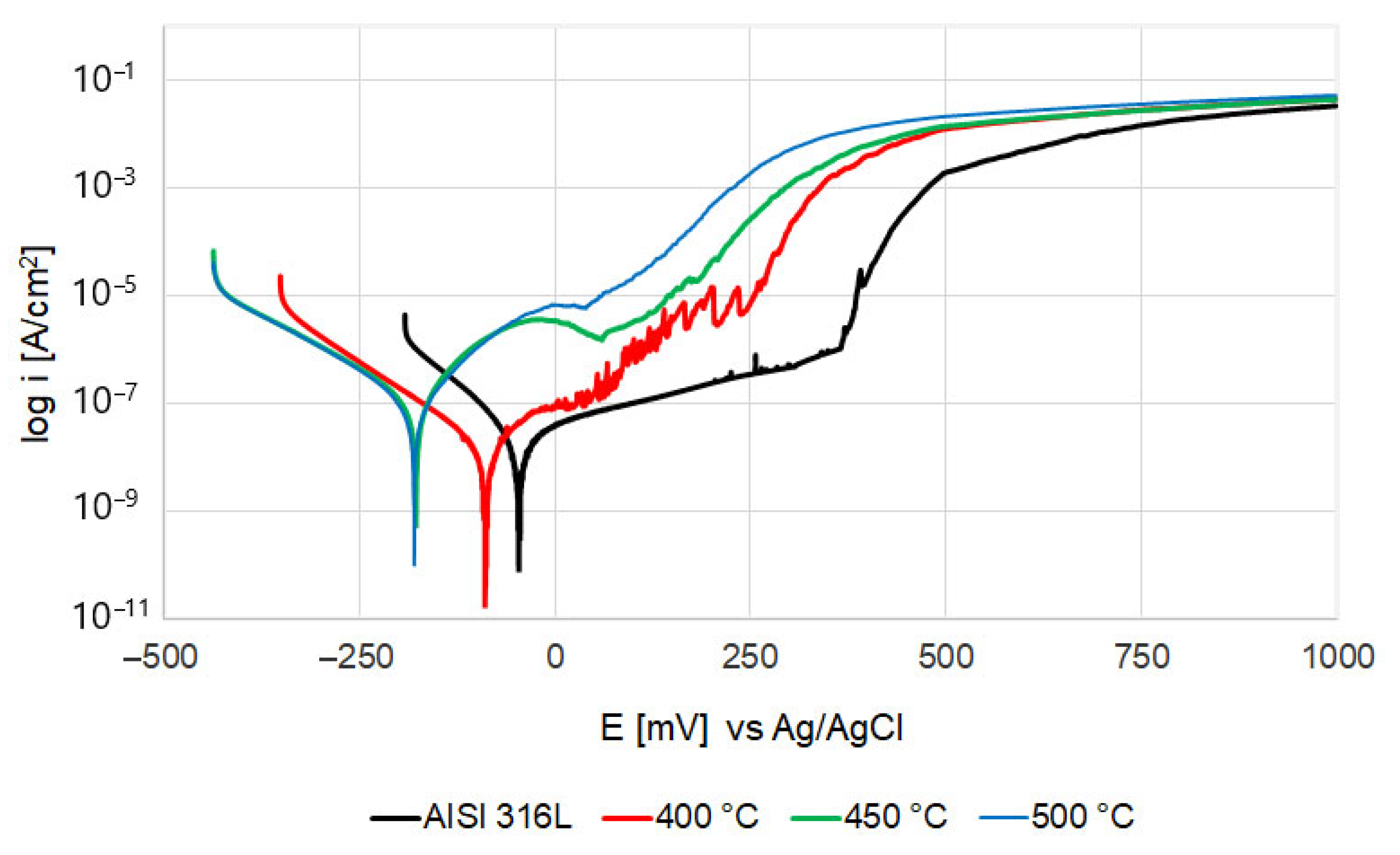
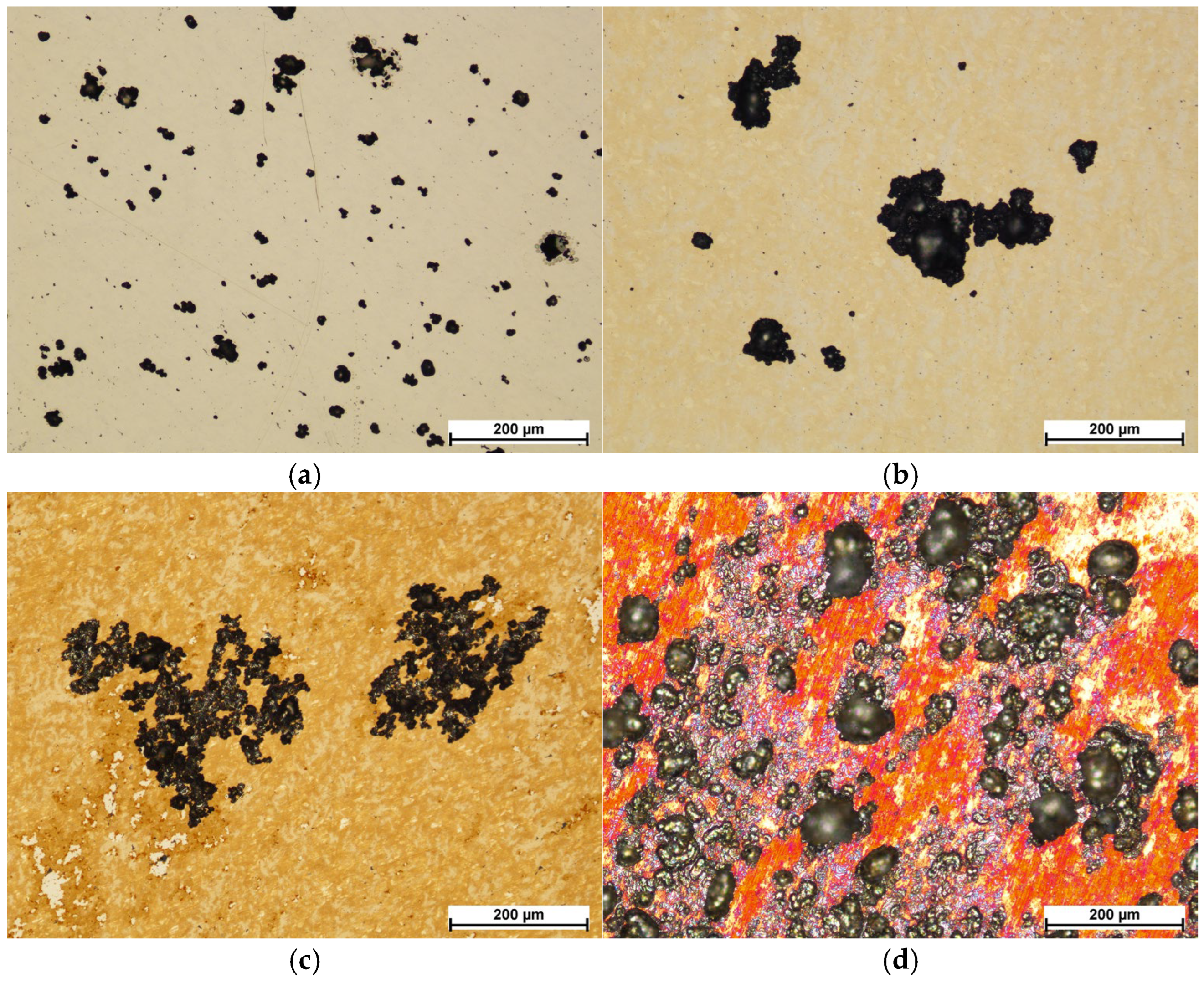
3.5. Corrosion Resistance Analysis
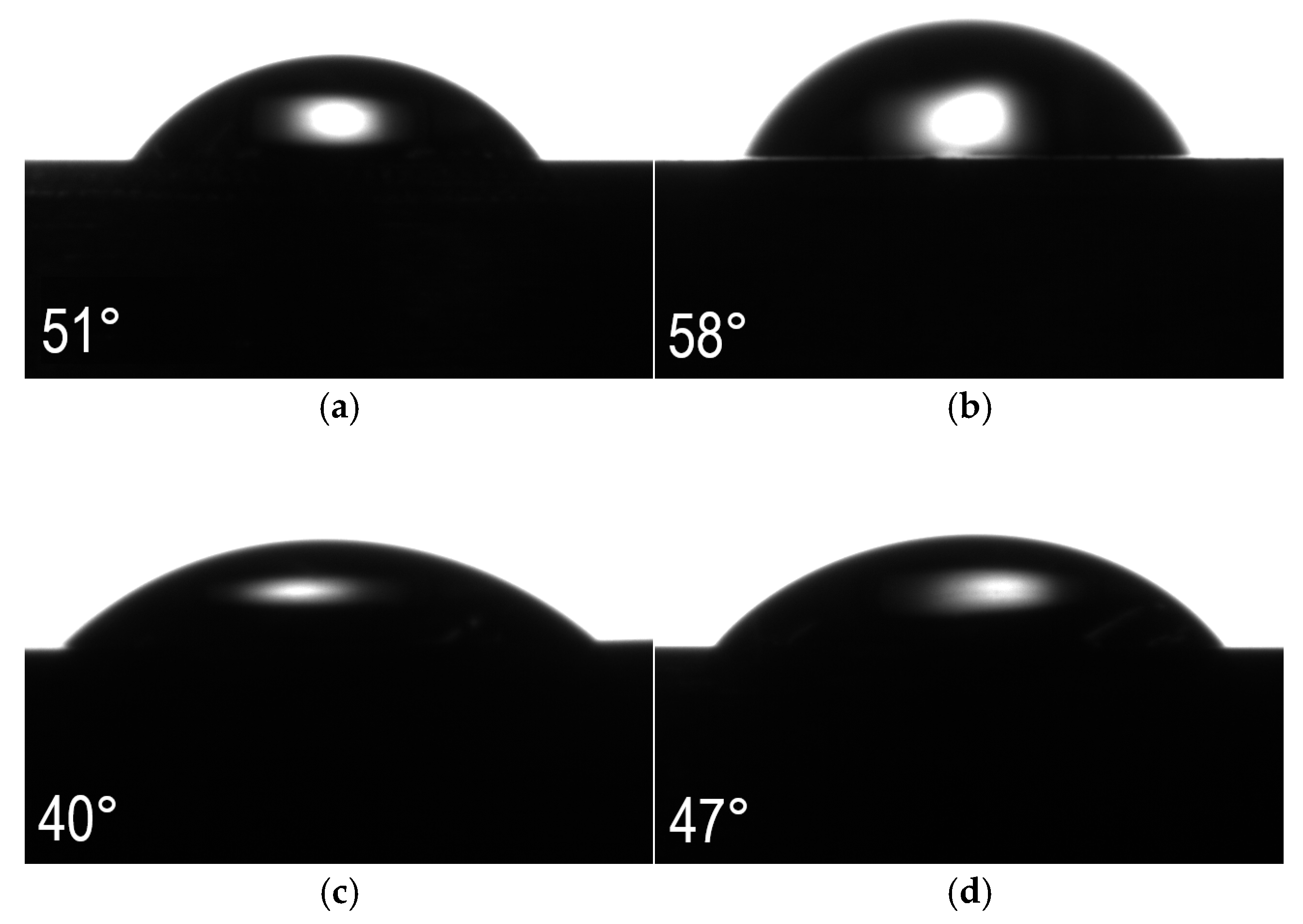
3.6. Wettability Analysis
4. Conclusions
- The phases formed on the surfaces after oxidation were Fe2O3 (hemetite) and Cr1.3Fe0.7O. The layers obtained are very thin and were not observable on their cross-sections during microscopic examination.
- Roughness tests using an optical profilometer showed that roughness tends to increase with the oxidation process temperature.
- Due to their small thickness, the layers obtained did not significantly increase the microhardness of the tested steel. The hardness measured at the lowest loads (0.05, 0.1 kg) increased the most after oxidation at 400 °C, likely due to the presence of deformation-induced martensite in the surface layer.
- The “ball-on-disc” tribological tests showed that the layer formed during oxidation at 400 °C, which had the lowest roughness and highest hardness, had a significant impact on reducing the friction coefficient and the wear rate. Explaining the phenomenon of very good wear resistance obtained after this process requires in-depth analyses, which will be the subject of further research.
- Corrosion tests showed a clear deterioration of corrosion resistance after oxidation at the two highest temperatures (450 °C and 500 °C). The steel oxidized at 400 °C presented a corrosion potential and a breakdown potential slightly lower than the polished steel in its initial state, but it obtained significantly better corrosion current density and polarization resistance values. After the corrosion tests, larger pits were present than on the steel in its initial state, but there were definitively fewer of them.
- All tested sample surfaces showed a hydrophilic character. The steel after oxidation at 400 °C had the lowest surface wettability, which may also contributed to the good corrosion resistance of this layer.
- The steel changed color to gold after oxidation, which may be a positive feature in applications where decorative qualities are important.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nouri, A.; Wen, C. Stainless Steels in Orthopedics. In Structural Biomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 67–101. ISBN 978-0-12-818831-6. [Google Scholar]
- Laybidi, F.H.; Bahrami, A. Antibacterial Properties of ZnO-Containing Bioactive Glass Coatings for Biomedical Applications. Mater. Lett. 2024, 365, 136433. [Google Scholar] [CrossRef]
- Beni, B.H.; Bahrami, A.; Rajabinezhad, M.; Abbasi, M.S.; Laybidi, F.H. Electrophoretic Deposition of Bioactive Glass 58S- xSi3N4 (0 < x < 20 Wt.%) Nanocomposite Coating on AISI 316L Stainless Steel Substrate for Biomedical Applications. Surf. Interfaces 2024, 55, 105380. [Google Scholar] [CrossRef]
- Rossi, C.; De Mongeot, F.B.; Ferrando, G.; Manzato, G.; Meyer, M.; Parodi, L.; Sgobba, S.; Sortino, M.; Vaglio, E. Study on Properties of AISI 316L Produced by Laser Powder Bed Fusion for High Energy Physics Applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2023, 1055, 168459. [Google Scholar] [CrossRef]
- Lacerda, F.G.B.; Tavares, S.S.M.; Perez, G.; Garcia, P.S.P.; Pimenta, A.R. Failure Investigation of an AISI 316L Pipe of the Flare System in an Off-Shore Oil Platform. Eng. Fail. Anal. 2025, 167, 108939. [Google Scholar] [CrossRef]
- Borowski, T. Enhancing the Corrosion Resistance of Austenitic Steel Using Active Screen Plasma Nitriding and Nitrocarburising. Materials 2021, 14, 3320. [Google Scholar] [CrossRef]
- Li, C.X.; Georges, J.; Li, X.Y. Active Screen Plasma Nitriding of Austenitic Stainless Steel. Surf. Eng. 2002, 18, 453–457. [Google Scholar] [CrossRef]
- Yazıcı, M.; Çomaklı, O.; Yetim, T.; Yetim, A.F.; Çelik, A. Investigation of Mechanical, Tribological and Magnetic Properties after Plasma Nitriding of AISI 316L Stainless Steel Produced with Different Orientations Angles by Selective Laser Melting. Surf. Coat. Technol. 2023, 467, 129676. [Google Scholar] [CrossRef]
- Singh, A.; Thirumurugesan, R.; Krishnakumar, S.; Rani, R.; Chandramouli, S.; Parameswaran, P.; Mythili, R. Performance Evaluation of Plasma Nitrided 316L Stainless Steel during Long Term High Temperature Sodium Exposure. Nucl. Eng. Technol. 2023, 55, 1468–1475. [Google Scholar] [CrossRef]
- Lu, Y.; Li, D.; Ma, H.; Liu, X.; Wu, M.; Hu, J. Enhanced Plasma Nitriding Efficiency and Properties by Severe Plastic Deformation Pretreatment for 316L Austenitic Stainless Steel. J. Mater. Res. Technol. 2021, 15, 1742–1746. [Google Scholar] [CrossRef]
- Hirano, M.; Miura, K.; Ohtsu, N. Hydrogen-Free Plasma Nitriding Process for Fabrication of Expanded Austenite Layer on AISI 316 Stainless Steel Surface. Materials 2025, 18, 140. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Ma, C.; Hai, X.; Song, K. 316L Austenitic Stainless Steel Deformation Organization and Nitriding-Strengthened Layer Relationships. Appl. Sci. 2025, 15, 2352. [Google Scholar] [CrossRef]
- Zatkalíková, V.; Drímalová, P.; Balin, K.; Slezák, M.; Markovičová, L. Electrochemical Behavior of Plasma-Nitrided Austenitic Stainless Steel in Chloride Solutions. Materials 2024, 17, 4189. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, B.; Kamiński, J.; Kulikowski, K.; Borowski, T.; Sobiecki, J.R.; Wierzchoń, T. The Effect of Nitriding Temperature of AISI 316L Steel on Sub-Zero Corrosion Resistance in C2H5OH. Materials 2024, 17, 3056. [Google Scholar] [CrossRef] [PubMed]
- Andriūnas, P.; Čerapaitė-Trušinskienė, R.; Galdikas, A. Modeling of Plasma Nitriding of Austenitic Stainless Steel through a Mask. Coatings 2024, 14, 1014. [Google Scholar] [CrossRef]
- Guardian-Tapia, R.; Rosales-Cadena, I.; Roman-Zubillaga, J.L.; Gonzaga-Segura, S.R. Mechanical and Microstructural Characterization of AISI 316L Stainless Steel Superficially Modified by Solid Nitriding Technique. Coatings 2024, 14, 1167. [Google Scholar] [CrossRef]
- Corujeira Gallo, S.; Dong, H. Study of Active Screen Plasma Processing Conditions for Carburising and Nitriding Austenitic Stainless Steel. Surf. Coat. Technol. 2009, 203, 3669–3675. [Google Scholar] [CrossRef]
- Souza, R.M.; Ignat, M.; Pinedo, C.E.; Tschiptschin, A.P. Structure and Properties of Low Temperature Plasma Carburized Austenitic Stainless Steels. Surf. Coat. Technol. 2009, 204, 1102–1105. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Wang, S.; Peng, Y.; Gong, J.; Somers, M.A.J. On the Fatigue Behavior of Low-Temperature Gaseous Carburized 316L Austenitic Stainless Steel: Experimental Analysis and Predictive Approach. Mater. Sci. Eng. A 2020, 793, 139651. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Feng, Y.; Wang, X.; Peng, Y.; Gong, J. Exploration on the Fatigue Behavior of Low-Temperature Carburized 316L Austenitic Stainless Steel at Elevated Temperature. Mater. Sci. Eng. A 2022, 850, 143562. [Google Scholar] [CrossRef]
- Tsujikawa, M.; Yoshida, D.; Yamauchi, N.; Ueda, N.; Sone, T.; Tanaka, S. Surface Material Design of 316 Stainless Steel by Combination of Low Temperature Carburizing and Nitriding. Surf. Coat. Technol. 2005, 200, 507–511. [Google Scholar] [CrossRef]
- Borgioli, F. The Expanded Phases Formed in Stainless Steels by Means of Low-Temperature Thermochemical Treatments: A Corrosion Perspective. Metals 2024, 14, 1371. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, C.X.; Dong, H.; Bell, T. Low Temperature Plasma Nitrocarburising of AISI 316 Austenitic Stainless Steel. Surf. Coat. Technol. 2005, 191, 195–200. [Google Scholar] [CrossRef]
- Borowski, T.; Kulikowski, K.; Spychalski, M.; Rożniatowski, K.; Rajchel, B.; Adamczyk-Cieślak, B.; Wierzchoń, T. Mechanical Behavior of Nitrocarburised Austenitic Steel Coated with N-DLC by Means of DC and Pulsed Glow Discharge. Arch. Metall. Mater. 2022, 67, 317–324. [Google Scholar] [CrossRef]
- Kajzer, A.; Ceglarska, M.; Sura, N.; Kajzer, W.; Borowski, T.; Tarnowski, M.; Pilecki, Z. Effect of Nitrided and Nitrocarburised Austenite on Pitting and Crevice Corrosion Resistance of 316 LVM Steel Implants. Materials 2020, 13, 5484. [Google Scholar] [CrossRef]
- Zhang, M.; Zhai, L.; Xue, Y.; Xu, Y.; Wu, W.; Jiang, Y.; Gong, J. Unusual Intermediate Layer Precipitation in Low-Temperature Salt Bath Nitrocarburized 316L Austenitic Stainless Steel. Surf. Coat. Technol. 2024, 494, 131521. [Google Scholar] [CrossRef]
- Jafarpour, S.M.; Dalke, A.; Biermann, H. Different Approaches for Plasma Nitrocarburizing of Austenitic Stainless Steel Using a Plasma-Activated Solid Carbon Precursor in a Hot-Wall Reactor. J. Mater. Res. Technol. 2025, 34, 1791–1802. [Google Scholar] [CrossRef]
- Xue, Y.; Zhai, L.; Xu, Y.; Wu, W.; Jiang, Y.; Gong, j. Low-Temperature Nitrocarburized 316L Stainless Steel: Residual Stress and Mechanical Properties. Surf. Interfaces 2025, 62, 106247. [Google Scholar] [CrossRef]
- Lee, I.; Barua, A. Behavior of the S-Phase of Plasma Nitrocarburized 316L Austenitic Stainless Steel on Changing Pulse Frequency and Discharge Voltage at Fixed Pulse-off Time. Surf. Coat. Technol. 2016, 307, 1045–1052. [Google Scholar] [CrossRef]
- Borowski, T.; Kulikowski, K.; Adamczyk-Cieślak, B.; Rożniatowski, K.; Spychalski, M.; Tarnowski, M. Influence of Nitrided and Nitrocarburised Layers on the Functional Properties of Nitrogen-Doped Soft Carbon-Based Coatings Deposited on 316L Steel under DC Glow-Discharge Conditions. Surf. Coat. Technol. 2020, 392, 125705. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, Z.; Su, Z.; Li, Y.; Yi, A.; Zheng, J.; Zhu, J.; Li, K.; Chen, K.; Liao, Z.; et al. Mechanical Property and Biocompatibility of Multilayer Ti-DLC Films with Different Ti Target Power by Hybrid PVD/PECVD on 316L Stainless Steel. Surf. Coat. Technol. 2025, 496, 131672. [Google Scholar] [CrossRef]
- Wongpanya, P.; Wongpinij, T.; Photongkam, P.; Siritapetawee, J. Improvement in Corrosion Resistance of 316L Stainless Steel in Simulated Body Fluid Mixed with Antiplatelet Drugs by Coating with Ti-Doped DLC Films for Application in Biomaterials. Corros. Sci. 2022, 208, 110611. [Google Scholar] [CrossRef]
- Yazdanpanah, A.; Pietri, A.D.; Ben Hjal, A.; Khodabakhshi, M.; Biasiolo, L.; Dabalà, M. Electrochemical and Localized Corrosion Characteristics of Kolsterised and DLC-Coated 316LVM Stainless Steel for Biomedical Applications. Appl. Surf. Sci. 2025, 693, 162808. [Google Scholar] [CrossRef]
- Vicente, F.A.; Ramos, B.B.; Pereira, D.G.; Bendo, T.; Hammes, G.; De Mello, J.D.B.; Binder, C. Effect of Thermochemical Treatment as Buffer Layers on the Tribological Performance of Stainless Steel with DLC Films. Wear 2025, 570, 205998. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ma, Z.; Chen, S.; Ma, M.; Yang, D.; An, X.; Zhao, Y.; Chen, L.; Liu, L.; et al. High-Loading 316L Stainless Steel by Integrated Low-Temperature Surface Nitriding and DLC Deposition. Surf. Coat. Technol. 2025, 496, 131716. [Google Scholar] [CrossRef]
- Jellesen, M.S.; Christiansen, T.L.; Hilbert, L.R.; Møller, P. Erosion–Corrosion and Corrosion Properties of DLC Coated Low Temperature Gas-Nitrided Austenitic Stainless Steel. Wear 2009, 267, 1709–1714. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Q.; Liu, Y.; Wang, S.; Abel, E.W. Reduction of Bacterial Adhesion on Modified DLC Coatings. Colloids Surf. B Biointerfaces 2008, 61, 182–187. [Google Scholar] [CrossRef]
- Li, C.X.; Bell, T. Corrosion Properties of Active Screen Plasma Nitrided 316 Austenitic Stainless Steel. Corros. Sci. 2004, 46, 1527–1547. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, K.; Wen, W.; Lu, D.; Long, J.; Meng, Y.; Peng, M.; Wei, F. Coupled Effects of TRIP and TWIP in Metastable Austenitic Stainless Steel via Optimization of Stacking Fault Energy. Mater. Charact. 2025, 220, 114656. [Google Scholar] [CrossRef]
- Das, A.; Chakraborti, P.C.; Tarafder, S.; Bhadeshia, H.K.D.H. Analysis of Deformation Induced Martensitic Transformation in Stainless Steels. Mater. Sci. Technol. 2011, 27, 366–370. [Google Scholar] [CrossRef]
- Tian, Y.; Borgenstam, A.; Hedström, P. Comparing the Deformation-Induced Martensitic Transformation with the Athermal Martensitic Transformation in Fe-Cr-Ni Alloys. J. Alloys Compd. 2018, 766, 131–139. [Google Scholar] [CrossRef]
- Borowski, T.; Jeleńkowski, J.; Psoda, M.; Wierzchoń, T. Modifying the Structure of Glow Discharge Nitrided Layers Produced on High-Nickel Chromium-Less Steel with the Participation of an Athermal Martensitic Transformation. Surf. Coat. Technol. 2010, 204, 1375–1379. [Google Scholar] [CrossRef]





| C | Cr | Mn | Mo | N | Ni | P | S | Si |
|---|---|---|---|---|---|---|---|---|
| ≤0.03 | 16–18 | ≤2 | 2–3 | ≤0.1 | 10–14 | ≤0.045 | ≤0.03 | ≤1 |
| Material | Ra [nm] | Rz [nm] |
|---|---|---|
| AISI 316L | 33.1 ± 2.9 | 989.6 ± 76.6 |
| 400 °C | 51.1 ± 1.1 | 1933.0 ± 274.8 |
| 450 °C | 84.2 ± 2.0 | 5266.7 ± 222.7 |
| 500 °C | 190.7 ± 8.6 | 11,231.6 ± 71.7 |
| Material | icorr [nA/cm2] | Ecorr [mV] | Rpol [kΩ∙cm2] | Epit [mV] |
|---|---|---|---|---|
| AISI 316L | 227 ± 138 | −28 ± 14 | 468 ± 153 | 420 ± 8 |
| 400 °C | 41 ± 14 | −62 ± 19 | 909 ± 217 | 398 ± 26 |
| 450 °C | 79 ± 18 | −177 ± 8 | 275 ± 51 | 267 ± 14 |
| 500 °C | 109 ± 14 | −196 ± 15 | 190 ± 17 | 160 ± 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borowski, T.; Wójcik, H.; Spychalski, M.; Adamczyk-Cieślak, B. Wear and Corrosion Resistance of Thermally Formed Decorative Oxide Layers on Austenitic Steel. Metals 2025, 15, 707. https://doi.org/10.3390/met15070707
Borowski T, Wójcik H, Spychalski M, Adamczyk-Cieślak B. Wear and Corrosion Resistance of Thermally Formed Decorative Oxide Layers on Austenitic Steel. Metals. 2025; 15(7):707. https://doi.org/10.3390/met15070707
Chicago/Turabian StyleBorowski, Tomasz, Hubert Wójcik, Maciej Spychalski, and Bogusława Adamczyk-Cieślak. 2025. "Wear and Corrosion Resistance of Thermally Formed Decorative Oxide Layers on Austenitic Steel" Metals 15, no. 7: 707. https://doi.org/10.3390/met15070707
APA StyleBorowski, T., Wójcik, H., Spychalski, M., & Adamczyk-Cieślak, B. (2025). Wear and Corrosion Resistance of Thermally Formed Decorative Oxide Layers on Austenitic Steel. Metals, 15(7), 707. https://doi.org/10.3390/met15070707








