Evolution of Solid Products Formed During the Cathodic Decomposition of Chalcopyrite at Different Energetic Conditions in Acetic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Tests
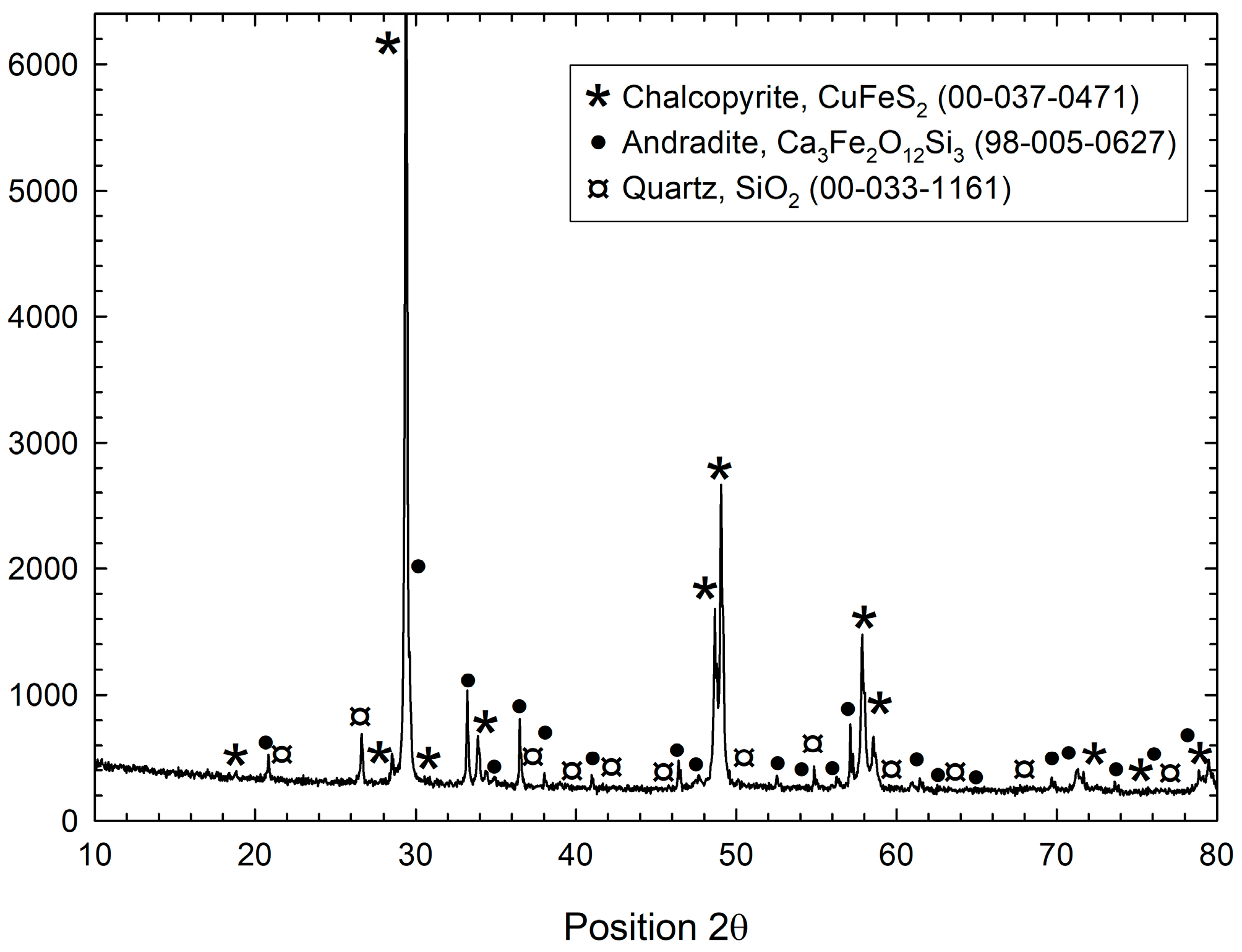
2.3. Characterization of the Solid Products Formed
3. Results and Discussion
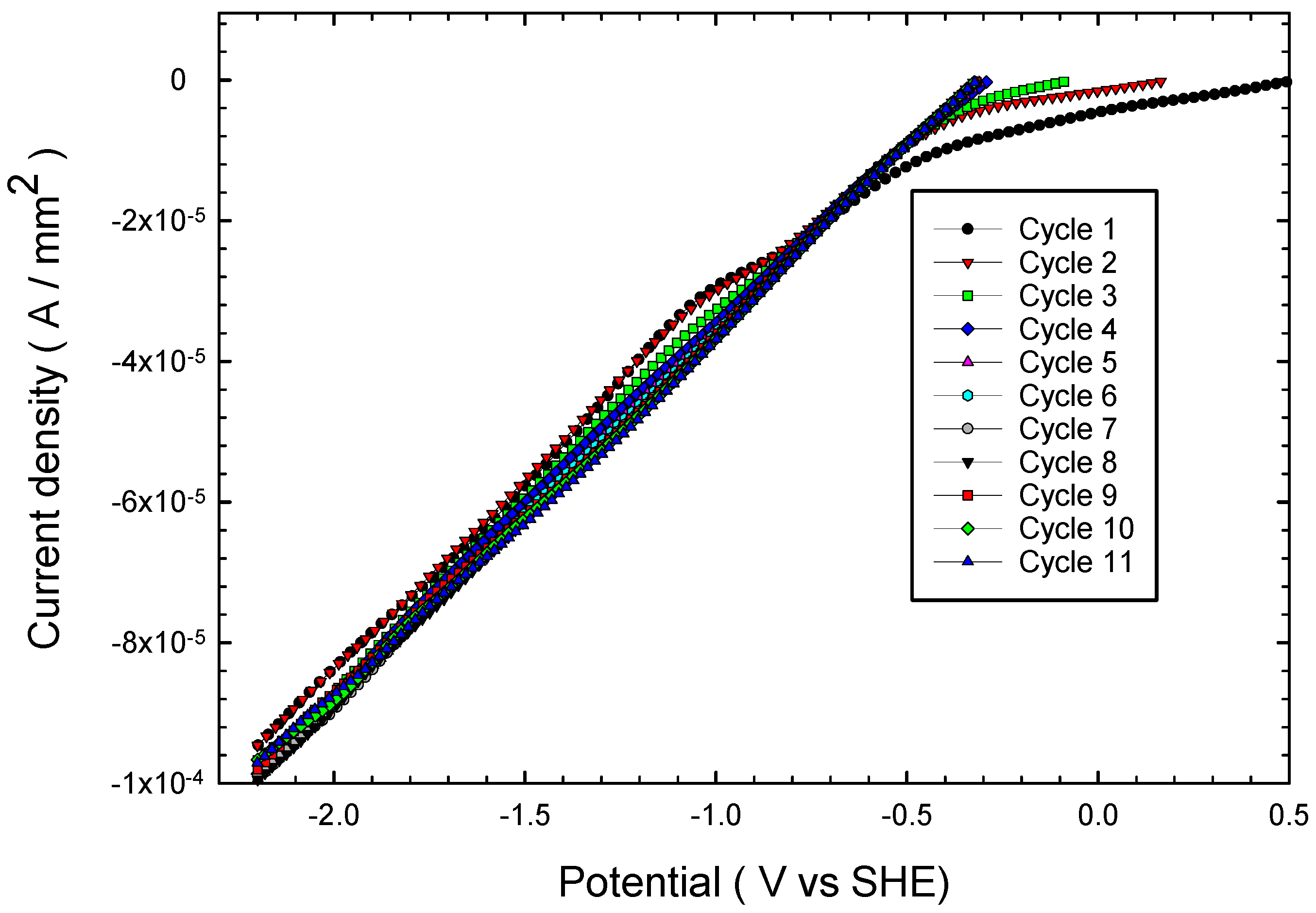
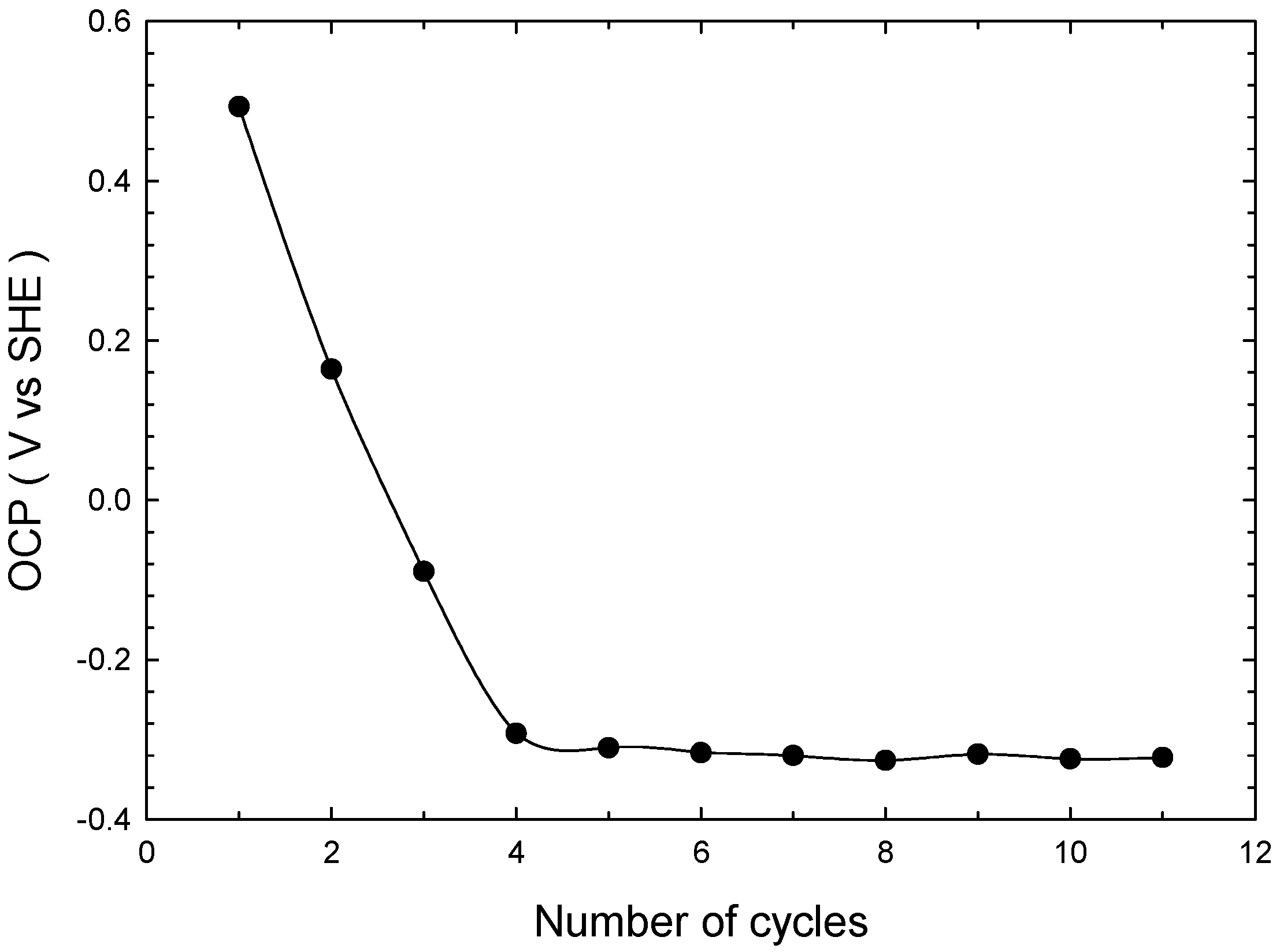
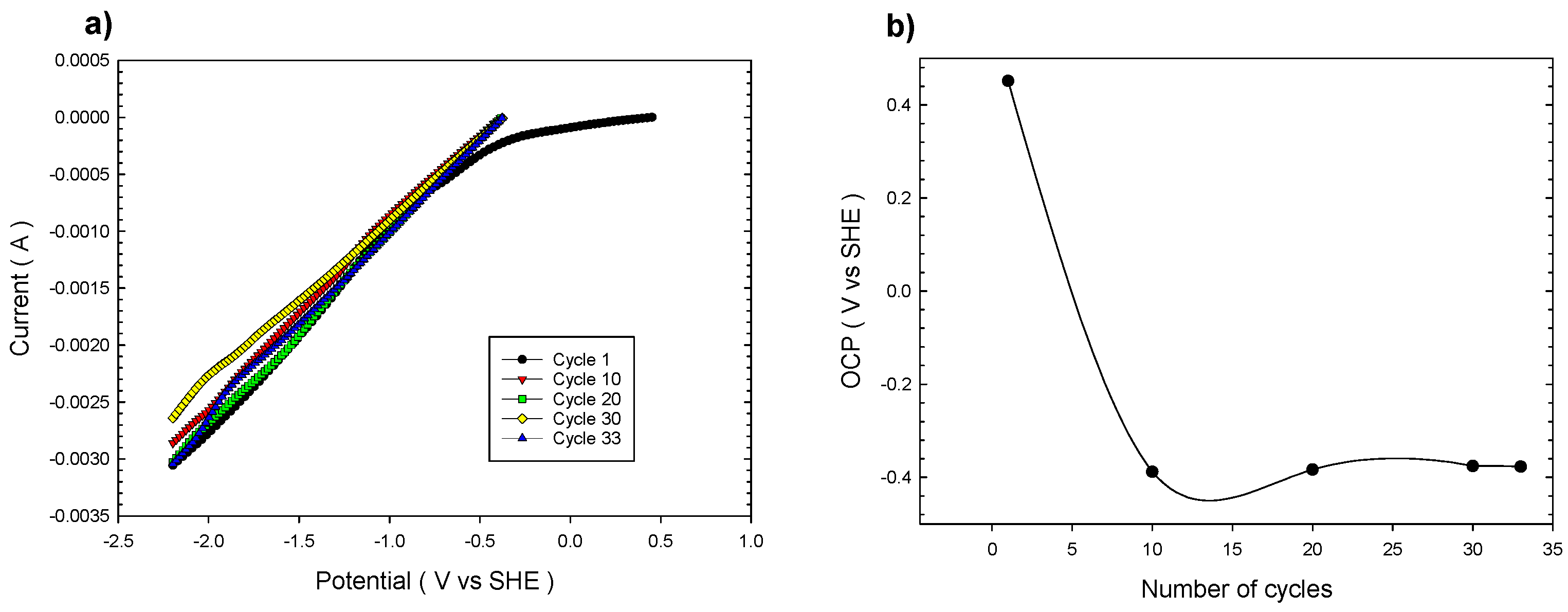
3.1. Effect of Multiple Cathodic Polarizations on Chalcopyrite Reduction
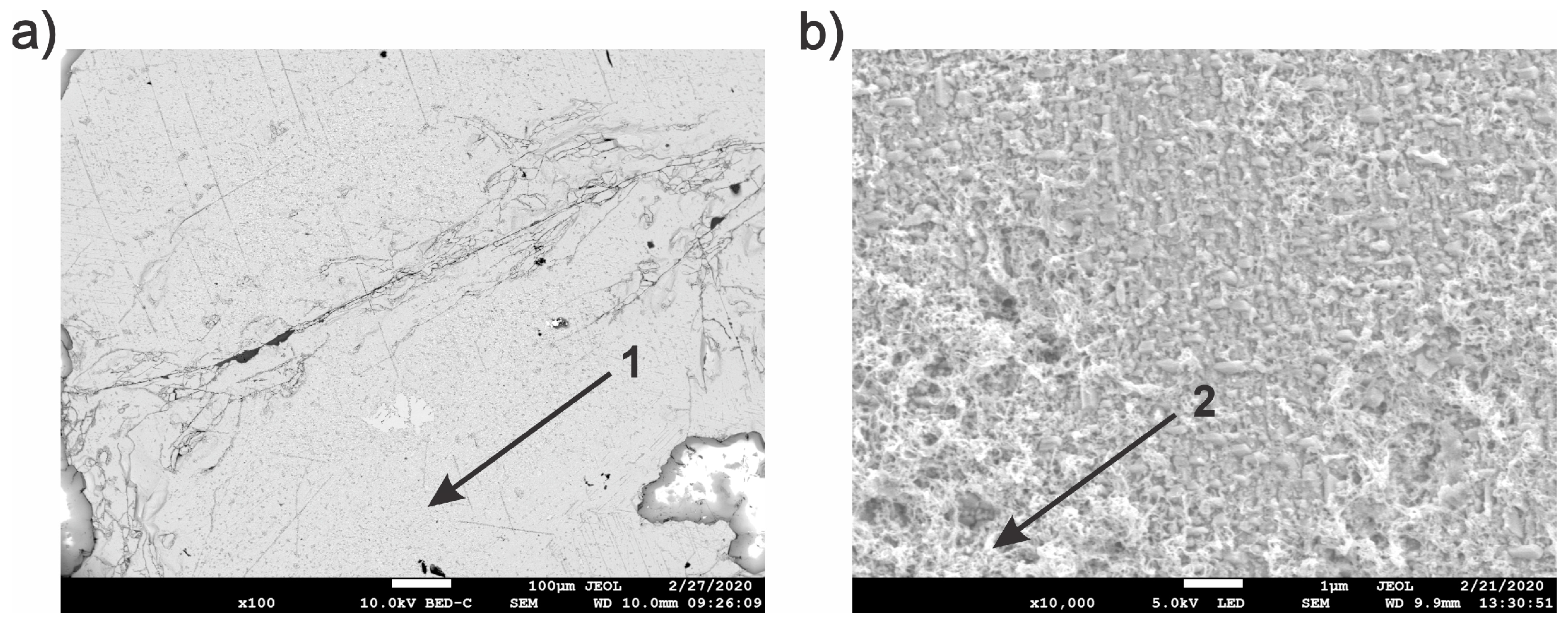
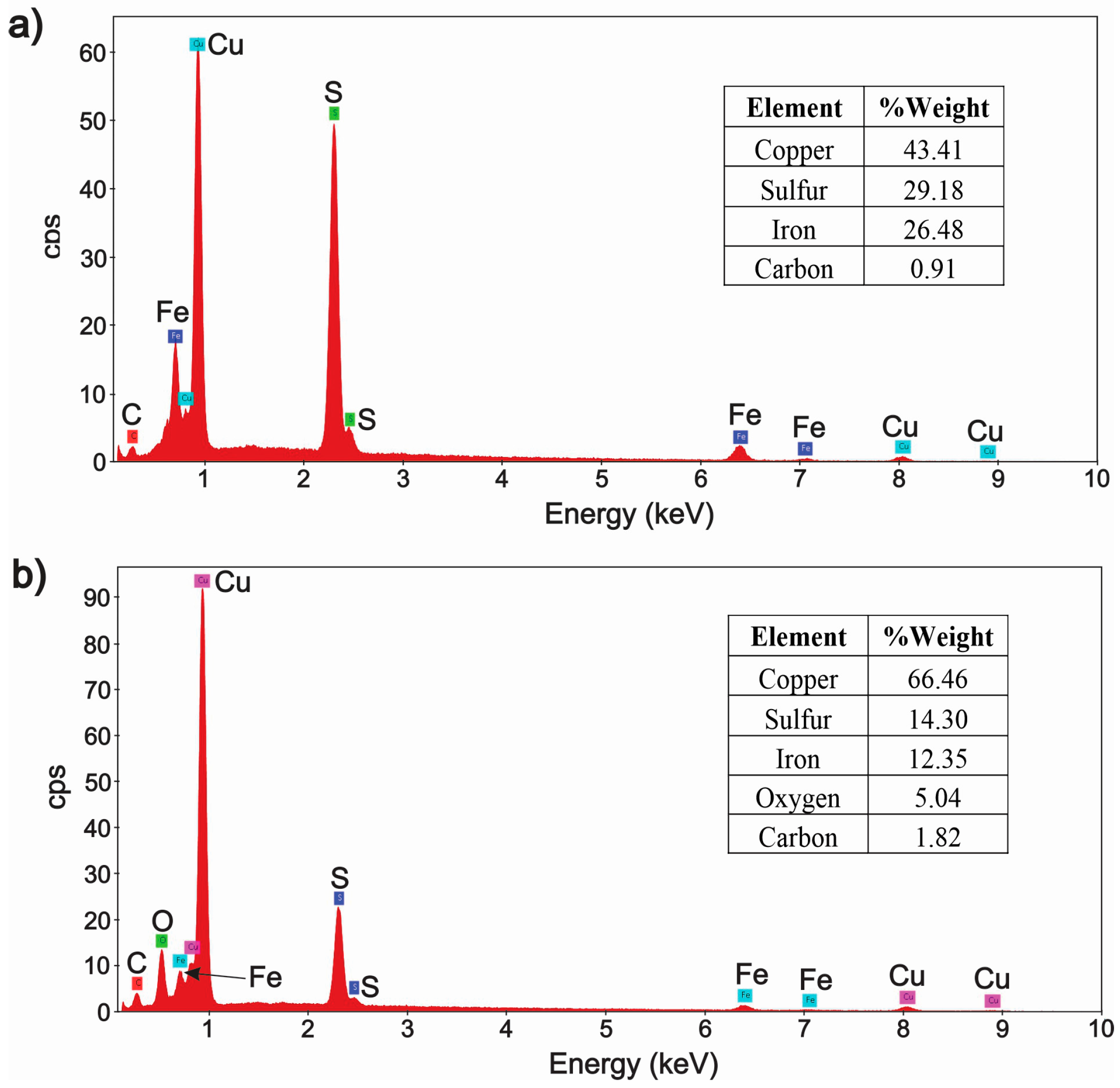
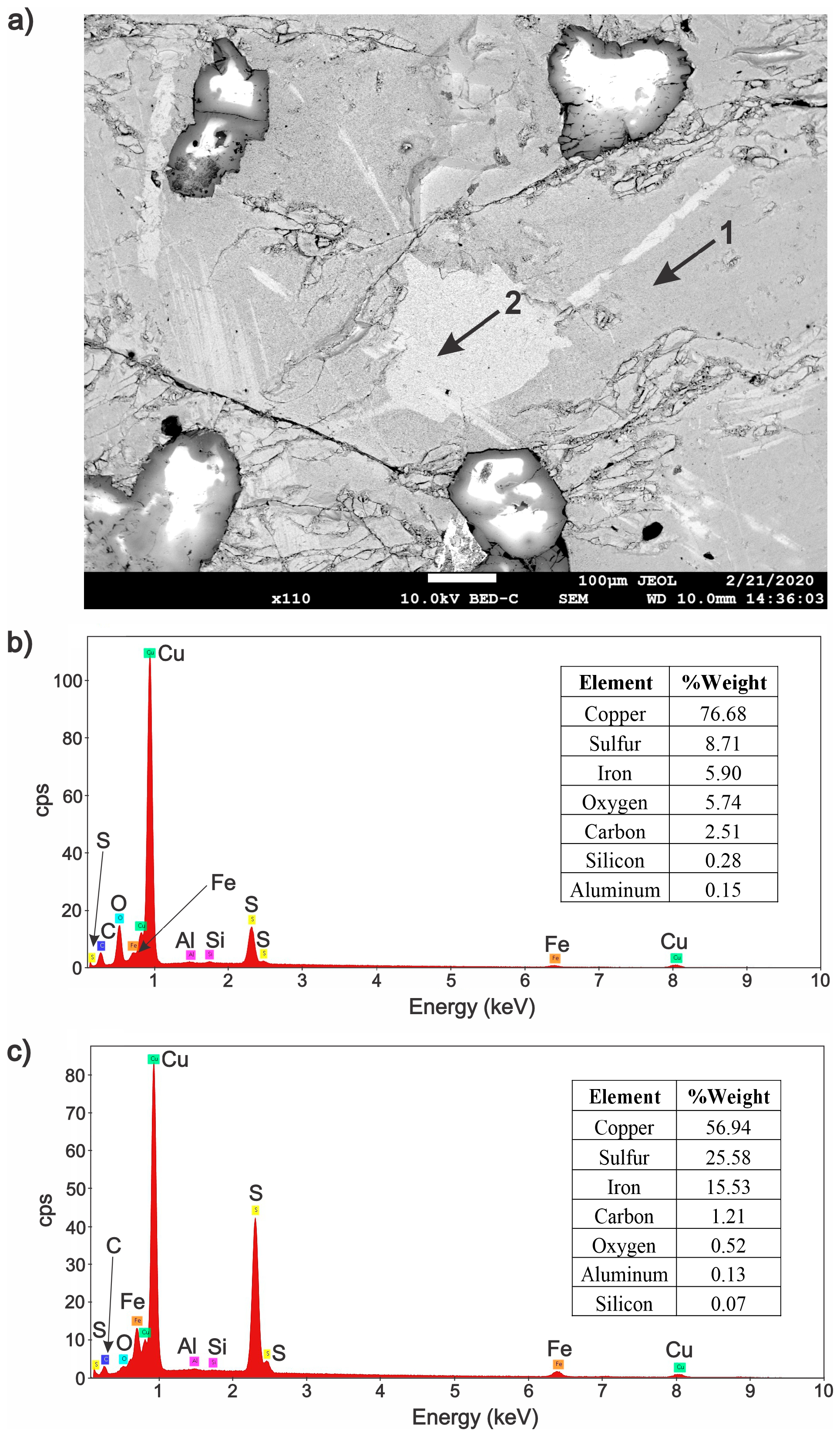
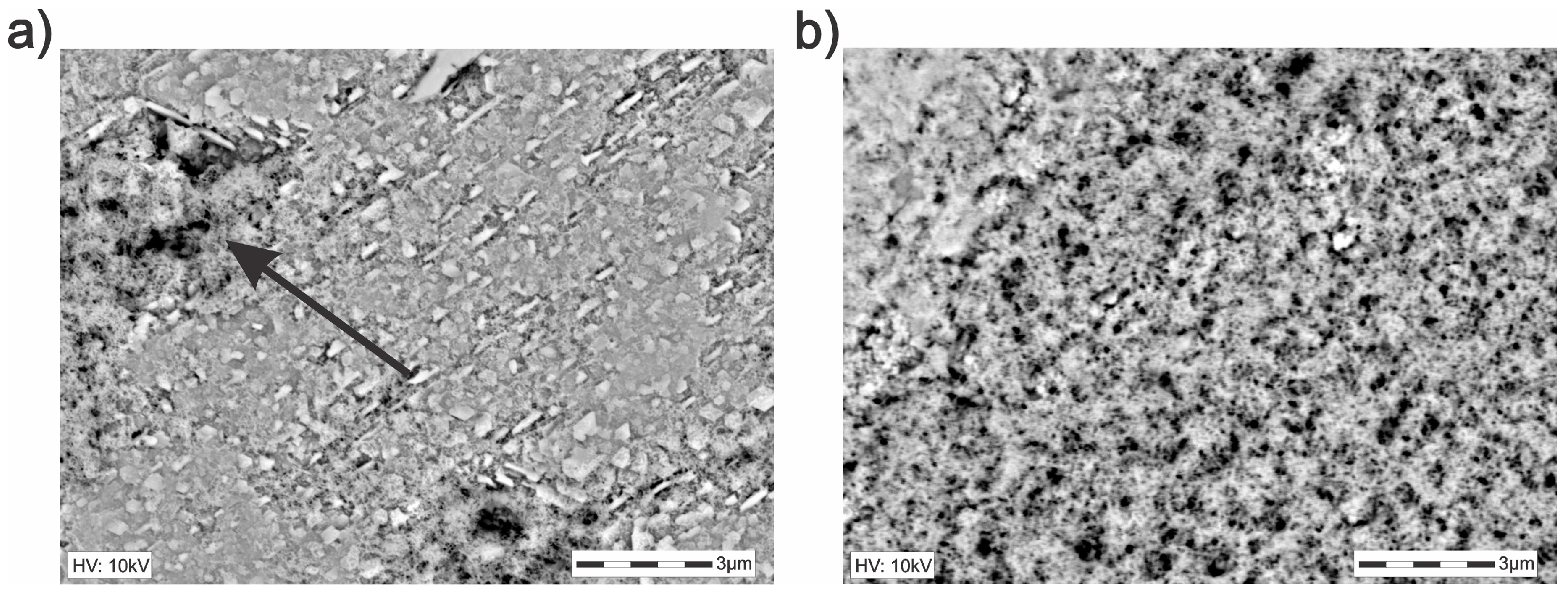
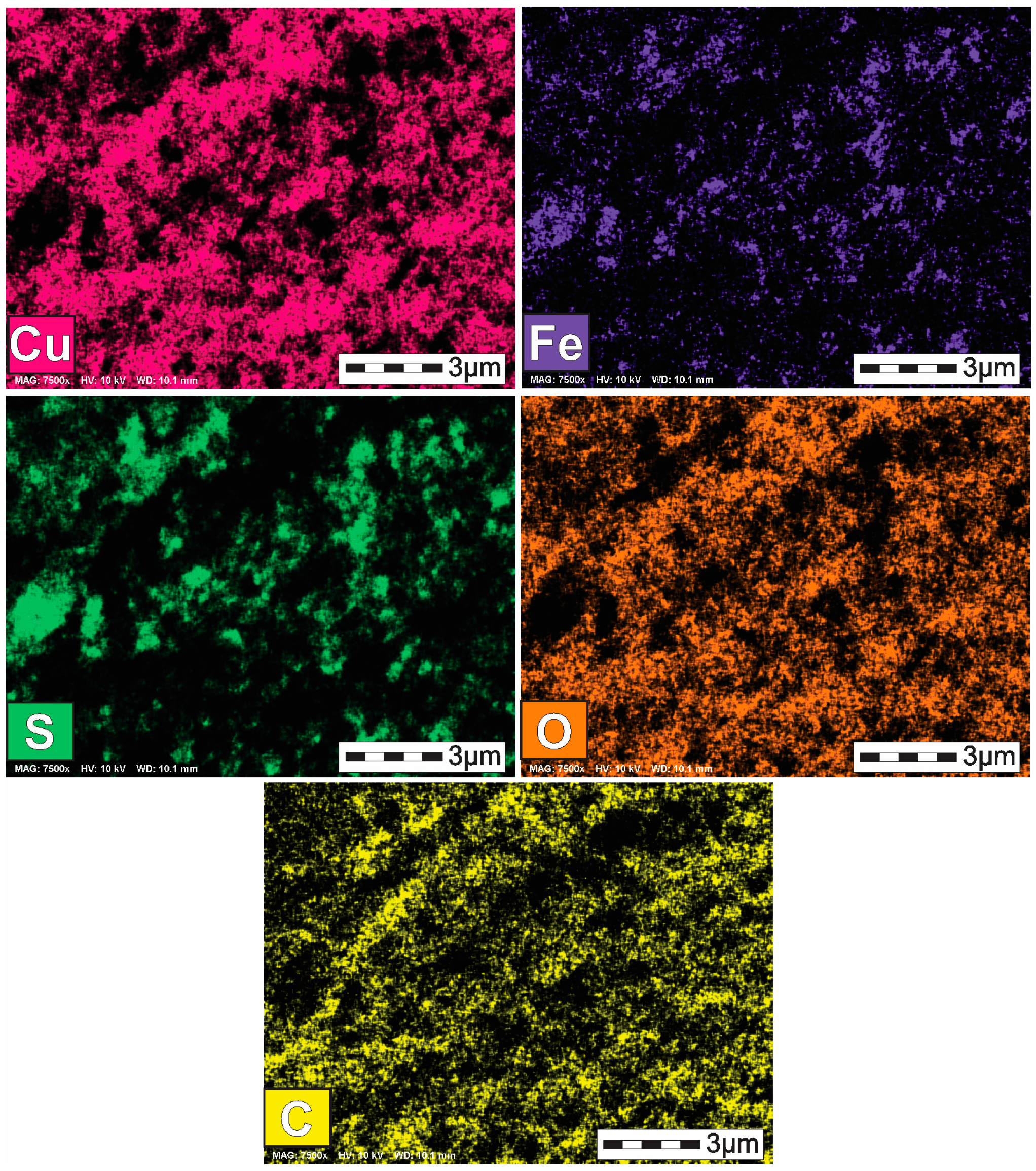
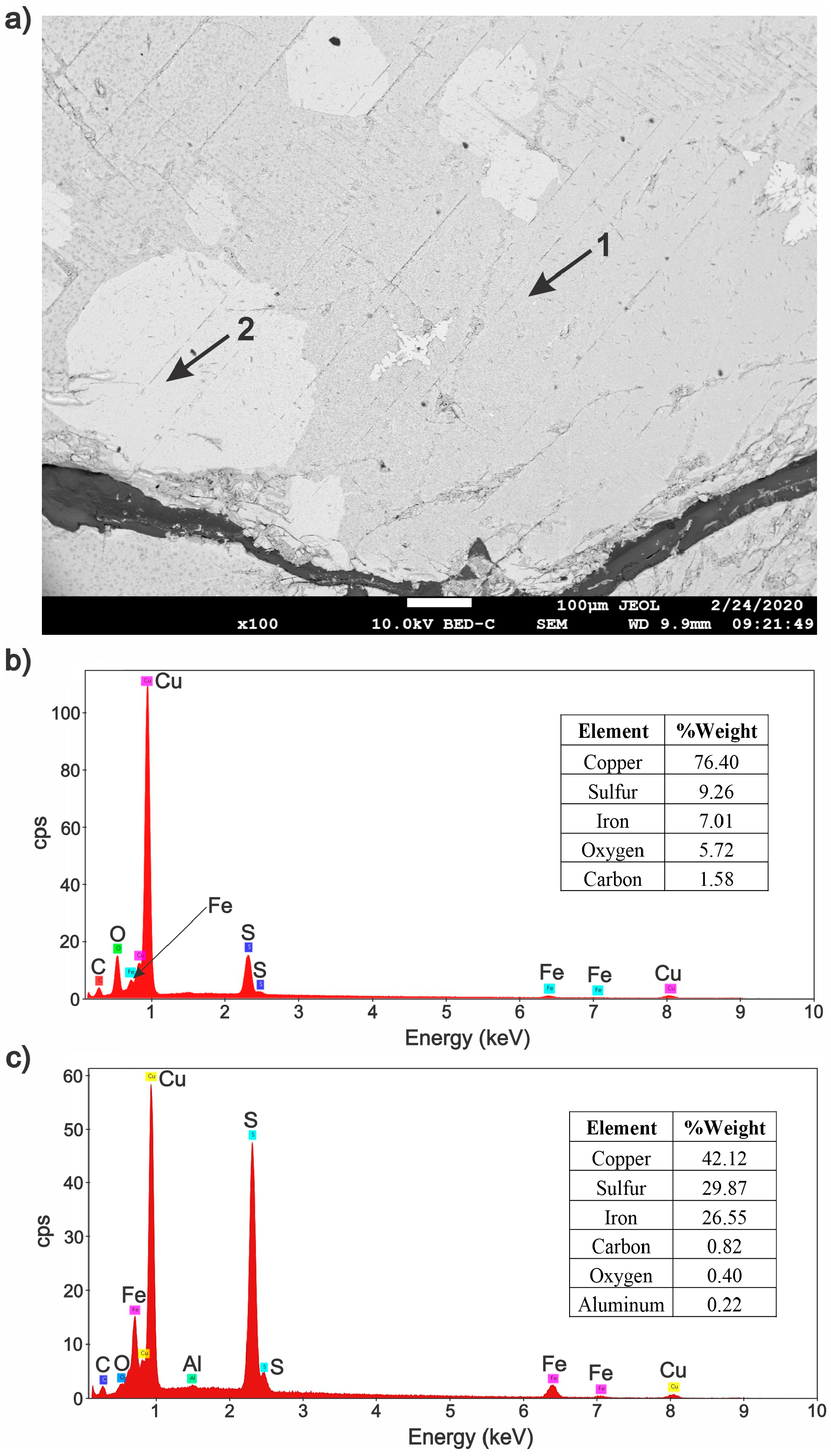
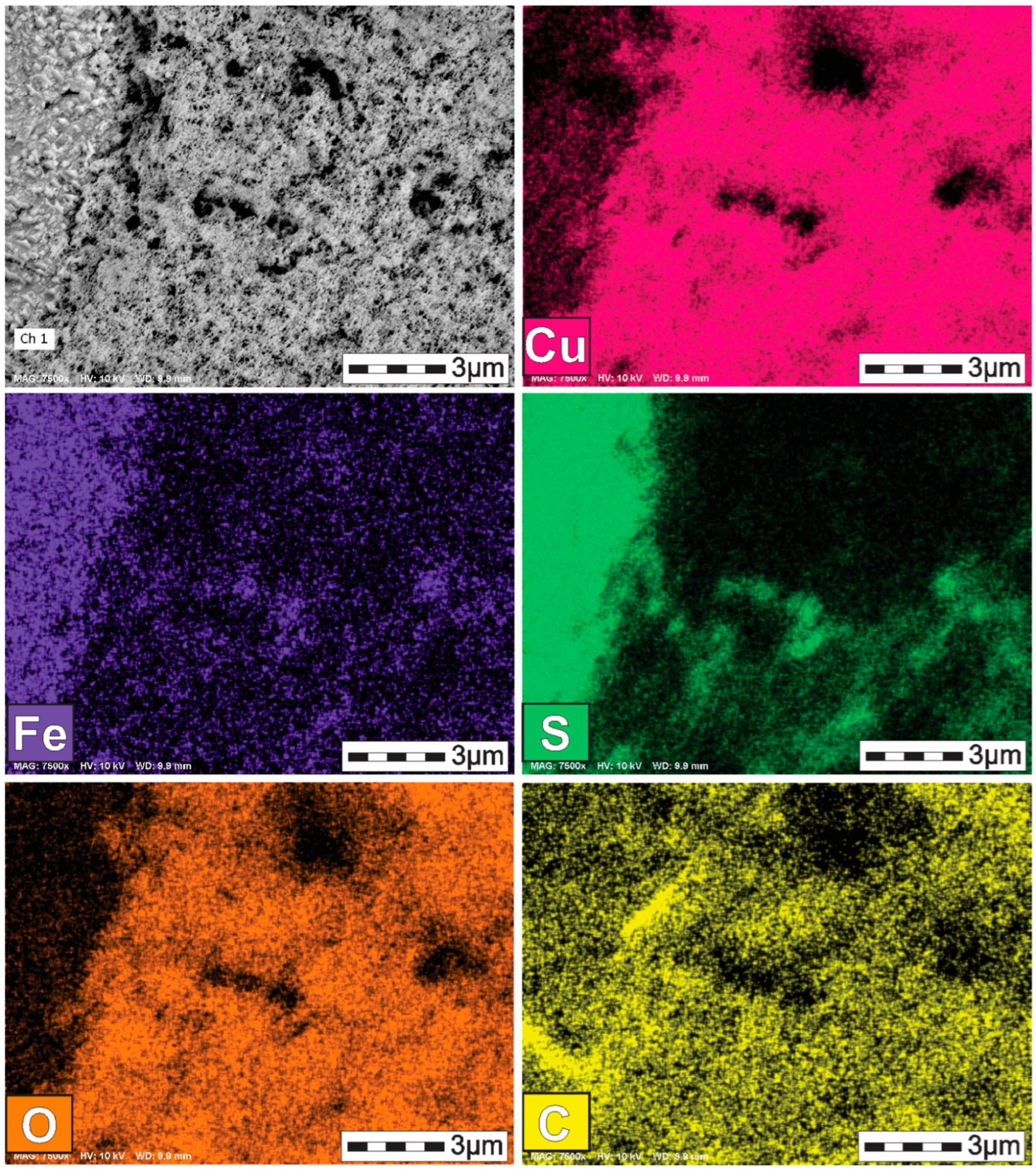
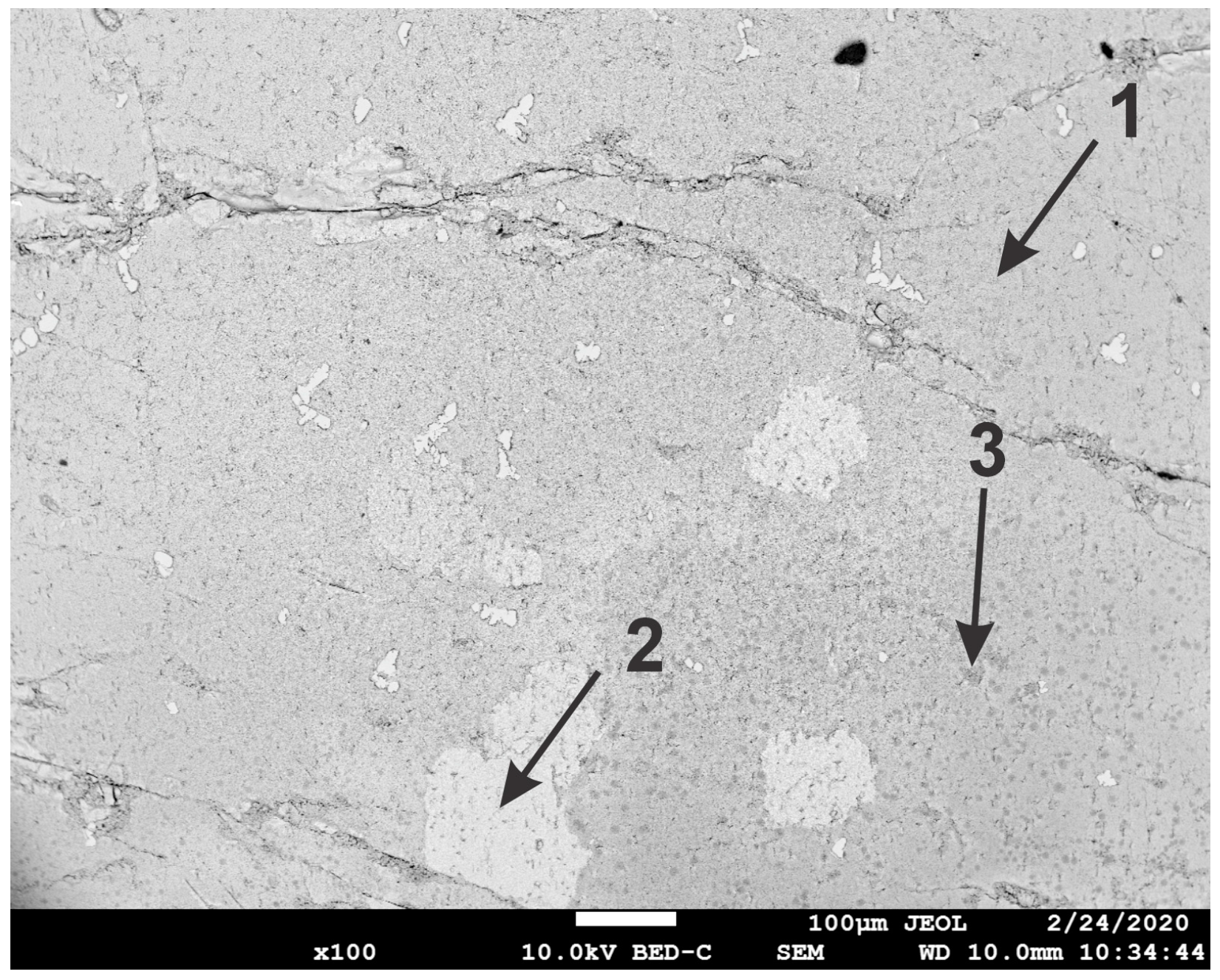
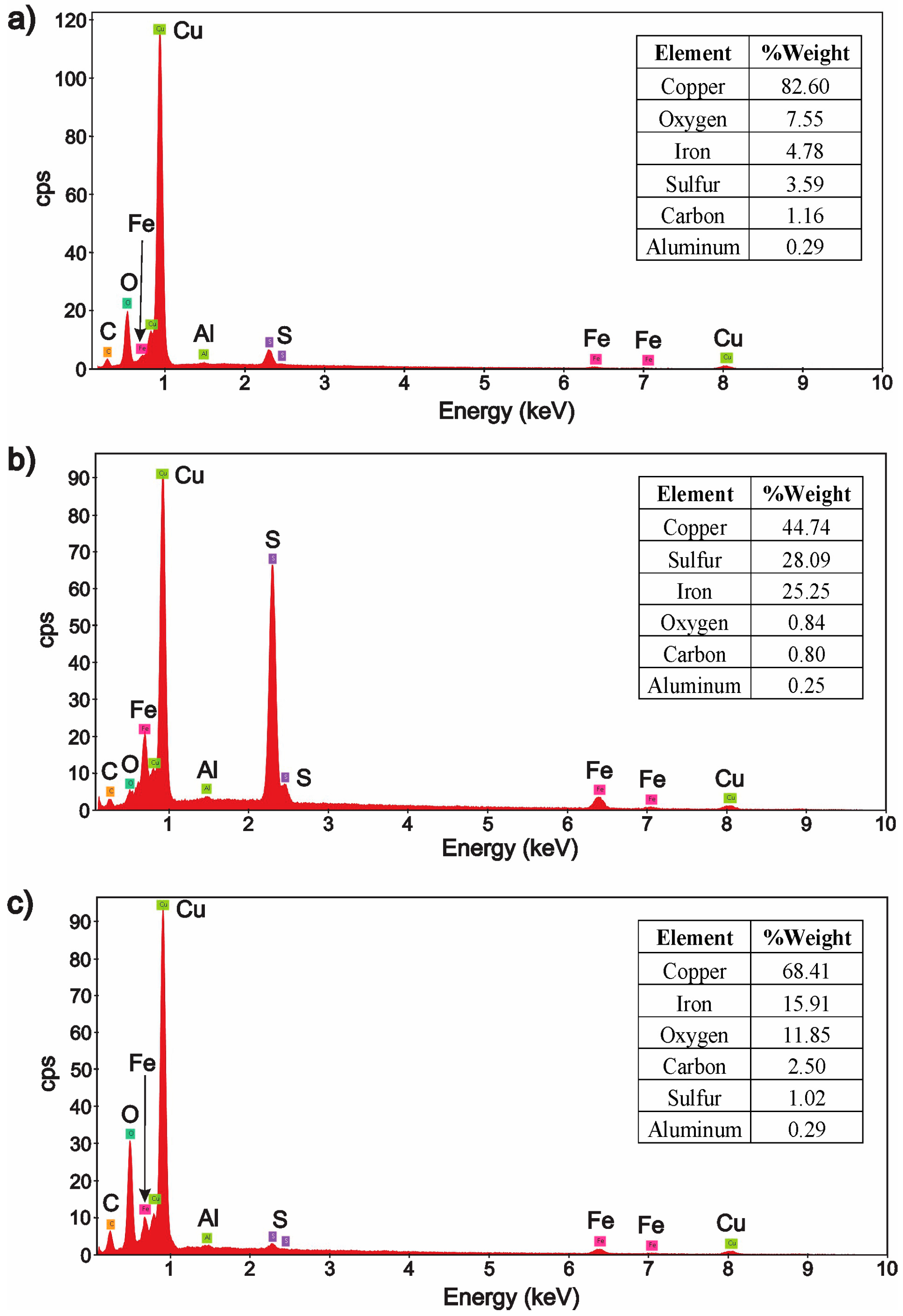
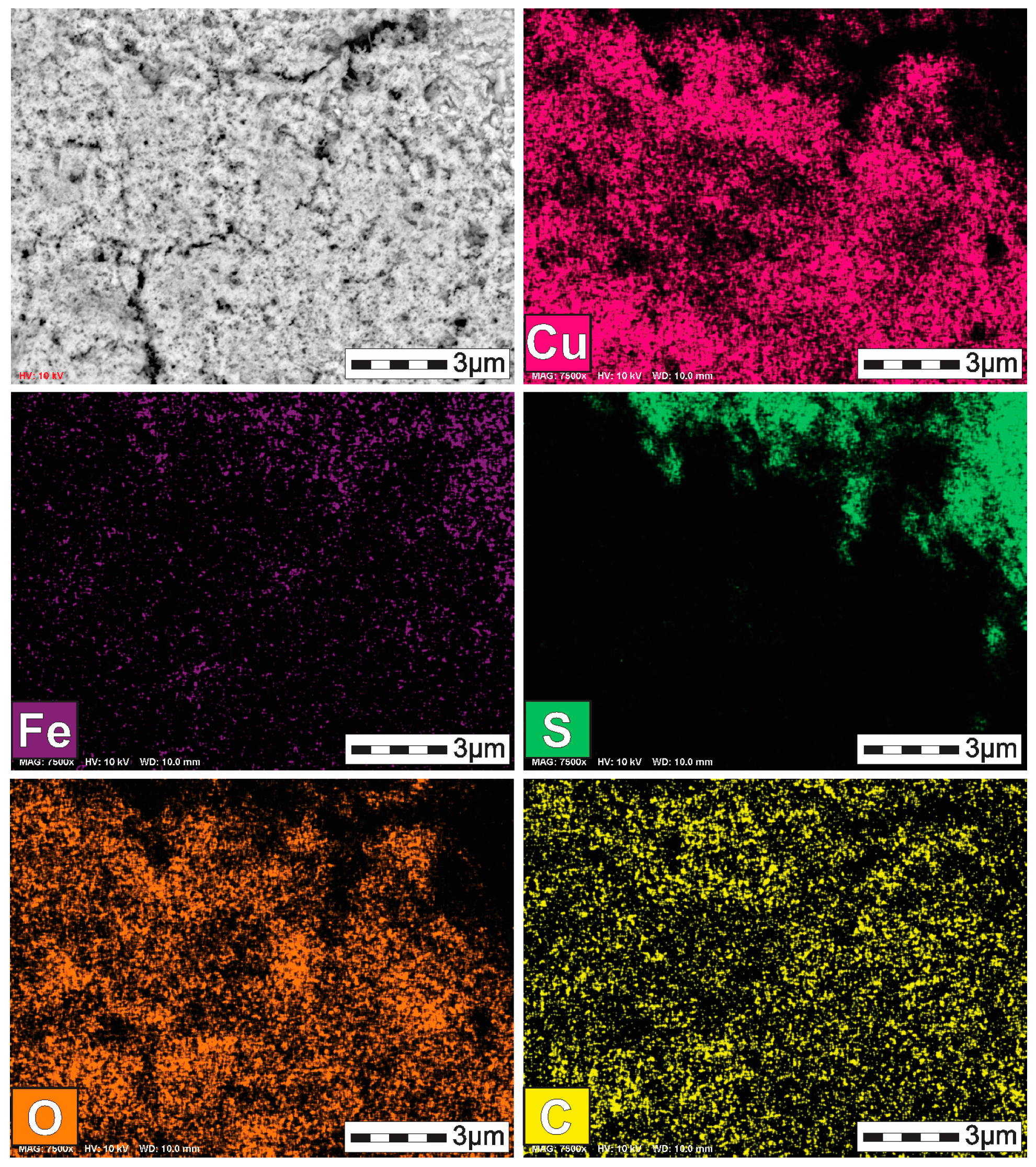
3.2. Characterization of the Solid Products Formed During Different Polarization Cycles Using Field Emission Scanning Electron Microscopy
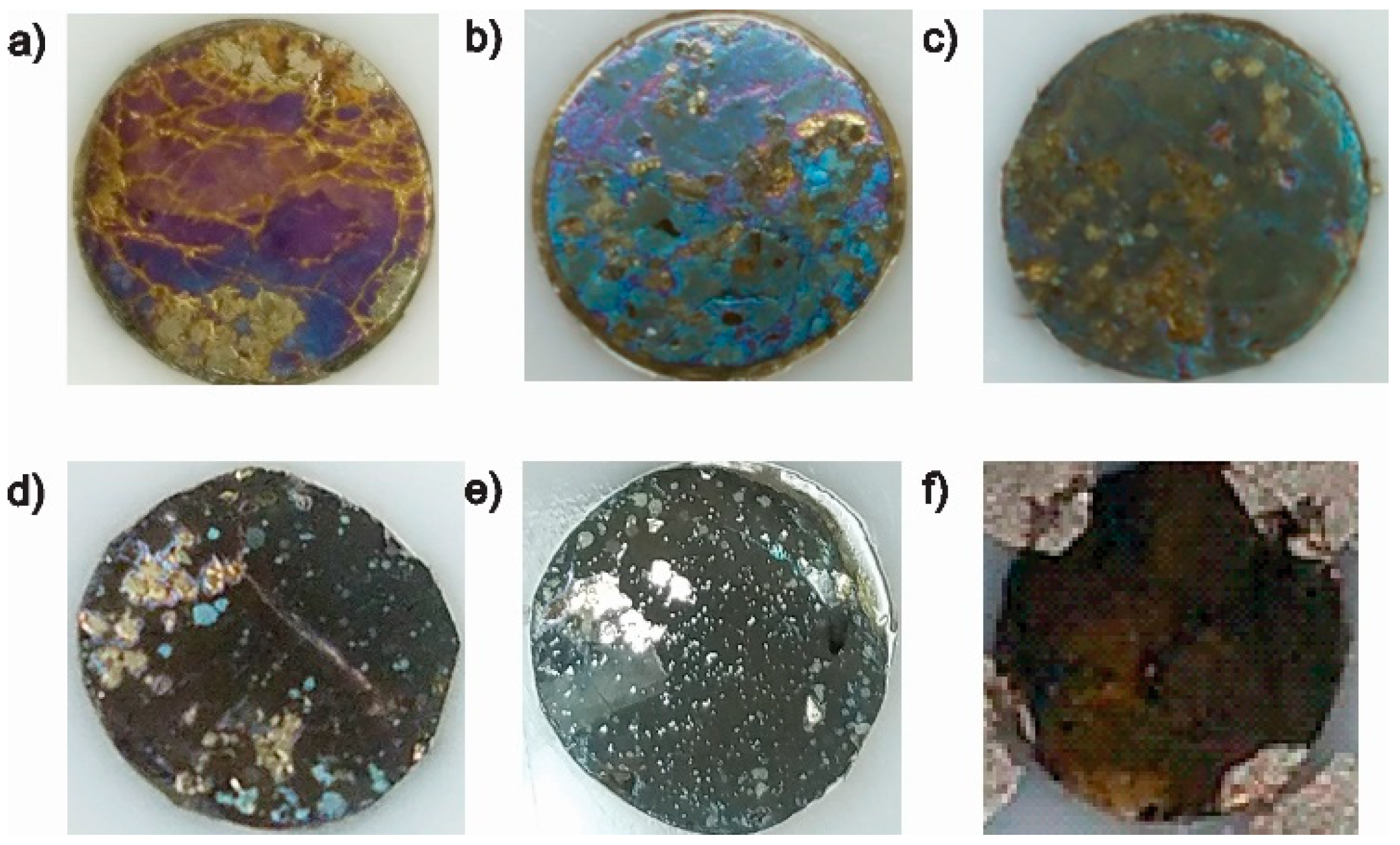
3.2.1. Solid Products Formed During 1, 3, and 5 Polarization Cycles
3.2.2. Solid Products Formed During Seven Polarization Cycles
3.2.3. Solid Products Formed During Nine Polarization Cycles
3.2.4. Solid Products Formed During 11 and 33 Polarization Cycles
4. Conclusions
- Chalcopyrite can be transformed to less refractory copper phases (copper sulfide, copper oxides, metallic copper) using acetic acid as a leaching medium during electro-assisted reduction.
- When chalcopyrite is subjected to multiple cathodic polarization cycles in 3 M acetic acid, the conventional passivation phenomenon caused by the formation of sulfur layers does not occur; the current density is maintained during 11 polarization cycles.
- Chalcopyritic iron is continuously released when chalcopyrite polarization is increased from 1 to 11 cycles.
- OCP continuously decreased from 0.5 to −0.3 V vs. SHE at 1 and 11 polarization cycles, respectively, revealing the formation of less refractory copper phases.
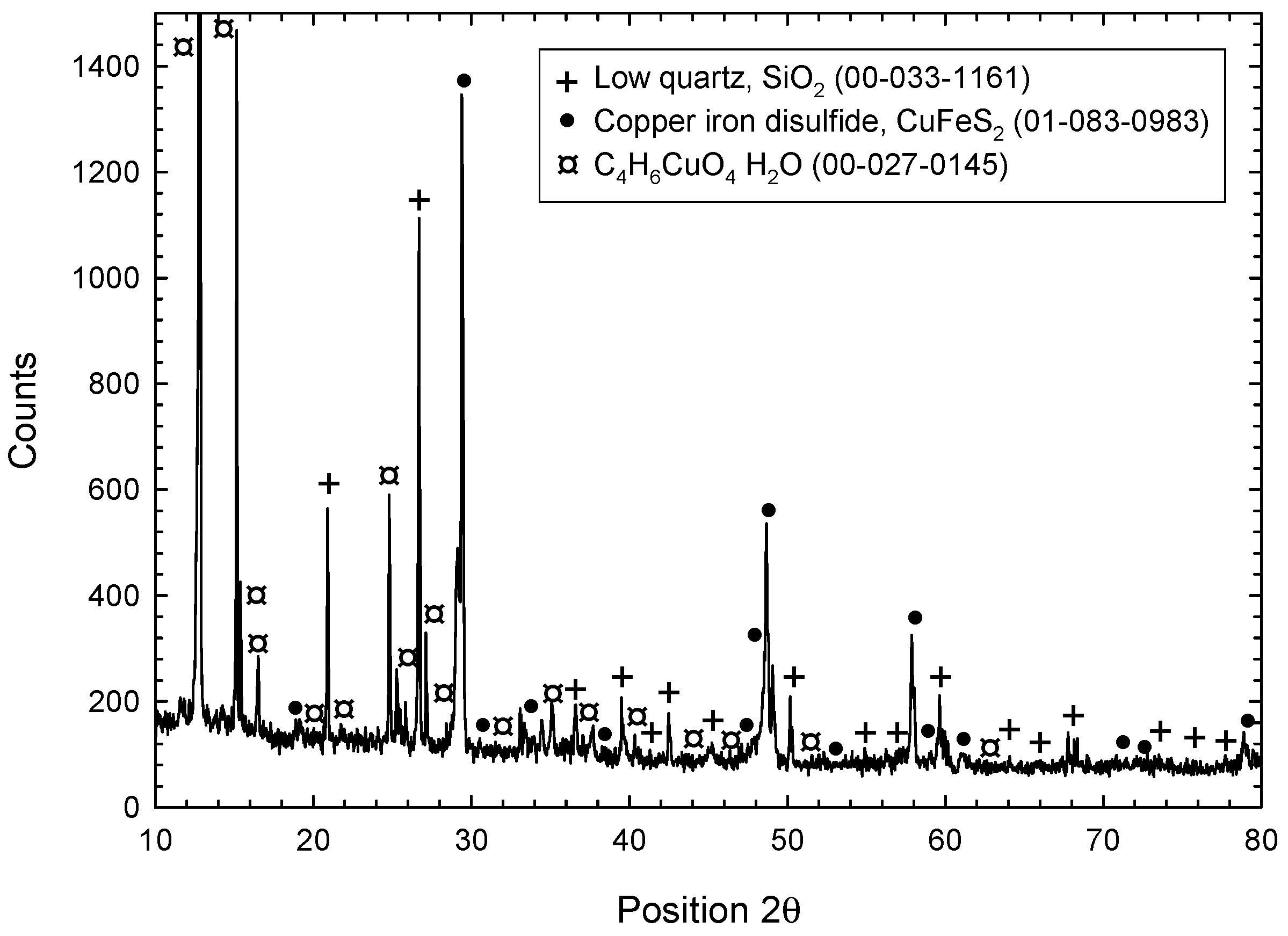
- Characterization of solid products by FESEM-EDS techniques revealed that bornite is mainly formed at 1 cathodic polarization cycle, while copper sulfides and copper can be formed when the polarization is increased up to 7, 9, and 11 cycles.
- Metallic copper can be oxidized when it is withdrawn from the electrochemical cell producing copper oxides species.
- Cathodic polarization of chalcopyrite performed during 33 cycles from OCP to −2.2 V vs. SHE, showed the possibility of increasing the chalcopyrite transformation to less refractory phases, i.e., metallic copper. The latter can react with oxygen and electro-adsorbed acetate, producing copper acetate-type species (C4H6CuO4H2O).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daehn, K.; Allanore, A. Electrolytic production of copper from chalcopyrite. Curr. Opin. Electrochem. 2020, 22, 110–119. [Google Scholar]
- Martínez-Gómez, V.J.; Fuentes-Aceituno, J.C.; Garibay, R.P.; Ordaz-Hernández, K.; Siller, D.M.P. Effect of galena during the electro-assisted reductive leaching of a chalcopyrite concentrate in HCl solutions. Miner. Eng. 2023, 203, 108355. [Google Scholar] [CrossRef]
- Burkin, A.R. Solid-State Transformations during Leaching. Min. Sci. Eng. 1969, 1, 4–14. [Google Scholar]
- Ammou-Chokroum, M.; Cambazoglu, M.; Steinmetz, D. Oxydation ménagée de la chalcopyrite en solution acide: Analyse cinétique des réactions. I. Modèles chimiques. Bull. Société Française Minéralogie Cristallogr. 1977, 100, 149–161. [Google Scholar]
- Munoz, P.B.; Miller, J.D.; Wadsworth, M.E. Reaction mechanism for the acid ferric sulfate leaching of chalcopyrite. Metall. Trans. B 1979, 10, 149–158. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar]
- Yang, Y.; Harmer-Bassell, S.; Chen, M. Synchrotron-based XPS and NEXAFS study of surface chemical species during electrochemical oxidation of chalcopyrite. Hydrometallurgy 2015, 156, 89–98. [Google Scholar]
- Castillo-Magallanes, N.; Cruz, R.; Lázaro, I. Effect of organic agentes on the oxidation process of chalcopyrite in a sulfuric acid solution. Electrochim. Acta 2020, 355, 136789. [Google Scholar]
- Crundwell, F.K.; Van Aswegen, A.; Bryson, L.J.; Biley, C.; Craig, D.; Marsicano, V.D.; Keartland, J.M. The effect of visible light on the dissolution of natural chalcopyrite (CuFeS2) in sulphuric acid solutions. Hydrometallurgy 2015, 158, 119–131. [Google Scholar]
- Khoshkhoo, M.; Dopson, M.; Shchukarev, A.; Sandström, Å. Chalcopyrite leaching and bioleaching: An X-ray photoelectron spectroscopic (XPS) investigation on the nature of hindered dissolution. Hydrometallurgy 2014, 149, 220–227. [Google Scholar]
- Yoo, K.; Kim, S.K.; Lee, J.C.; Ito, M.; Tsunekawa, M.; Hiroyoshi, N. Effect of chloride ions and leaching rate of chalcopyrite. Miner. Eng. 2010, 23, 471–477. [Google Scholar] [CrossRef]
- Allanore, A.; Lavelaine, H.; Birat, J.P.; Valentin, G.; Lapicque, F. Experimental investigation of cell design for the electrolysis of iron oxide suspensions in alkaline electrolyte. J. Appl. Electrochem. 2010, 40, 1957–1966. [Google Scholar] [CrossRef]
- Tian, Z.; Li, H.; Wei, Q.; Qin, W.; Yang, C. Effects of redox potential on chalcopyrite leaching: An overview. Miner. Eng. 2021, 172, 107135. [Google Scholar] [CrossRef]
- Nicol, M.; Miki, H.; Zhang, S. The anodic behaviour of chalcopyrite in chloride solutions: Voltammetry. Hydrometallurgy 2017, 171, 198–205. [Google Scholar] [CrossRef]
- O’Connor, G.M.; Eksteen, J.J. A critical review of the passivation and semiconductor mechanisms of chalcopyrite leaching. Miner. Eng. 2020, 154, 106401. [Google Scholar] [CrossRef]
- Liu, X.J.; Liao, Y.; Ma, H.; Liu, Q. Electrochemical characterizations and galvanic effect of chalcopyrite leaching and passivation-A review. Miner. Eng. 2024, 210, 108673. [Google Scholar] [CrossRef]
- Baba, A.A.; Ayinla, K.I.; Adekola, F.A.; Ghosh, M.K.; Ayanda, O.S.; Bale, R.B.; Sheik, A.R.; Pradhan, S.R. A Review on Novel Techniques for Chalcopyrite Ore Processing. Int. J. Min. Eng. Miner. Process. 2012, 1, 1–16. [Google Scholar]
- Prasad, S.; Pandey, B.D. Alternative processes for treatment of chalcopyrite—A review. Miner. Eng. 1998, 11, 763–781. [Google Scholar] [CrossRef]
- Liu, X.; Du, Z.; Sun, C.; Zhang, N. A review on the electrochemical analysis of sulfide minerals—Pyrite, chalcopyrite, and galena. Green Smart Min. Eng. 2025, 2, 18–31. [Google Scholar] [CrossRef]
- Allanore, A. Electrochemical Engineering for Commodity Metals Extraction. Electrochem. Soc. Interface 2017, 26, 63. [Google Scholar] [CrossRef]
- Dreisinger, D.; Abed, N. A fundamental study of the reductive leaching of chalcopyrite using metallic iron part I: Kinetic analysis. Hydrometallurgy 2002, 66, 37–57. [Google Scholar] [CrossRef]
- Biegler, T.; Swift, D.A. The Electrolytic Reduction of Chalcopyrite in Acid Solution. J. Appl. Electrochem. 1976, 6, 229–235. [Google Scholar]
- Blech, I.A.; Kruger, J. Further Evidence for Monoatomic Hydrogen as a Darkening Agent in Chemography. J. Electrochem. Soc. 1984, 131, 2455. [Google Scholar]
- Ye, J.; Zang, Y.; Wang, Q.; Zhang, Y.; Sun, D.; Zhang, L.; Wang, G.; Zheng, X.; Zhu, J. Nitrogen doped FeS2 nanoparticles for efficient and stable hydrogen evolution reaction. J. Energy Chem. 2021, 56, 283–289. [Google Scholar] [CrossRef]
- Li, Z.; Jalil, S.A.; Singh, S.C.; Li, W.; Wei, X.; Guo, C. Significantly enhanced electrocatalytic activity of copper for hydrogen evolution reaction through femtosecond laser blackening. Int. J. Hydrogen Energy 2021, 46, 10783–10788. [Google Scholar]
- Fujimura, T.; Kunimoto, M.; Fukunaka, Y.; Homma, T. Analysis of the hydrogen Evolution reaction at Ni micro-patterned electrodes. Electrochim. Acta 2021, 368, 137678. [Google Scholar]
- Marinović, V.; Despić, A.R. Cathodic Hydrogen Evolution from Aqueous Solutions of Acetic Acid. Russ. J. Electrochem. 2004, 40, 995–999. [Google Scholar] [CrossRef]
- Martínez-Gómez, V.J.; Fuentes-Aceituno, J.C.; Pérez-Garibay, R.; Lee, J.-C. A phenomenological study of the electro-assisted reductive leaching of chalcopyrite. Hydrometallurgy 2016, 164, 54–63. [Google Scholar]
- Biegler, T.; Constable, D.C. Continuous electrolytic reduction of a chalcopyrite slurry. J. Appl. Electrochem. 1977, 7, 175–179. [Google Scholar]
- Jasso-Recio, L.D.; Fuentes-Aceituno, J.C.; Pérez-Garibay, R. Electro-assisted reduction of chalcopyrite using acetic acid. The feasibility to use weak and less aggressive acids. Miner. Eng. 2024, 205, 108482. [Google Scholar]
- Martínez-Gómez, V.J.; Fuentes-Aceituno, J.C.; Pérez-Garibay, R.; Lee, J.-C. A study of the electro-assisted reductive leaching of a chalcopyrite concentrate in HCl solutions. Part I: Kinetic behavior and nature of the chalcopyrite reduction. Hydrometallurgy 2018, 181, 195–205. [Google Scholar] [CrossRef]
| Sample | Number of Cycles | Fe Released (mg/L) |
|---|---|---|
| M1 | 1 | 0.085 |
| M2 | 3 | 0.428 |
| M3 | 5 | 0.820 |
| M4 | 7 | 1.47 |
| M5 | 9 | 1.75 |
| M6 | 11 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasso-Recio, L.D.; Fuentes-Aceituno, J.C.; Pérez-Garibay, R.; Enríquez-Farías, A.V.; Flores-Valdés, A.; Torres-Torres, J. Evolution of Solid Products Formed During the Cathodic Decomposition of Chalcopyrite at Different Energetic Conditions in Acetic Acid. Metals 2025, 15, 672. https://doi.org/10.3390/met15060672
Jasso-Recio LD, Fuentes-Aceituno JC, Pérez-Garibay R, Enríquez-Farías AV, Flores-Valdés A, Torres-Torres J. Evolution of Solid Products Formed During the Cathodic Decomposition of Chalcopyrite at Different Energetic Conditions in Acetic Acid. Metals. 2025; 15(6):672. https://doi.org/10.3390/met15060672
Chicago/Turabian StyleJasso-Recio, Laura Denisse, Juan Carlos Fuentes-Aceituno, Roberto Pérez-Garibay, Aldo Valentín Enríquez-Farías, Alfredo Flores-Valdés, and Jesús Torres-Torres. 2025. "Evolution of Solid Products Formed During the Cathodic Decomposition of Chalcopyrite at Different Energetic Conditions in Acetic Acid" Metals 15, no. 6: 672. https://doi.org/10.3390/met15060672
APA StyleJasso-Recio, L. D., Fuentes-Aceituno, J. C., Pérez-Garibay, R., Enríquez-Farías, A. V., Flores-Valdés, A., & Torres-Torres, J. (2025). Evolution of Solid Products Formed During the Cathodic Decomposition of Chalcopyrite at Different Energetic Conditions in Acetic Acid. Metals, 15(6), 672. https://doi.org/10.3390/met15060672








