Phosphorus Removal in Metallurgical-Grade Silicon via a Combined Approach of Si-Fe Solvent Refining and SiO2-TiO2-CaO-CaF2 Slag Refining
Abstract
1. Introduction
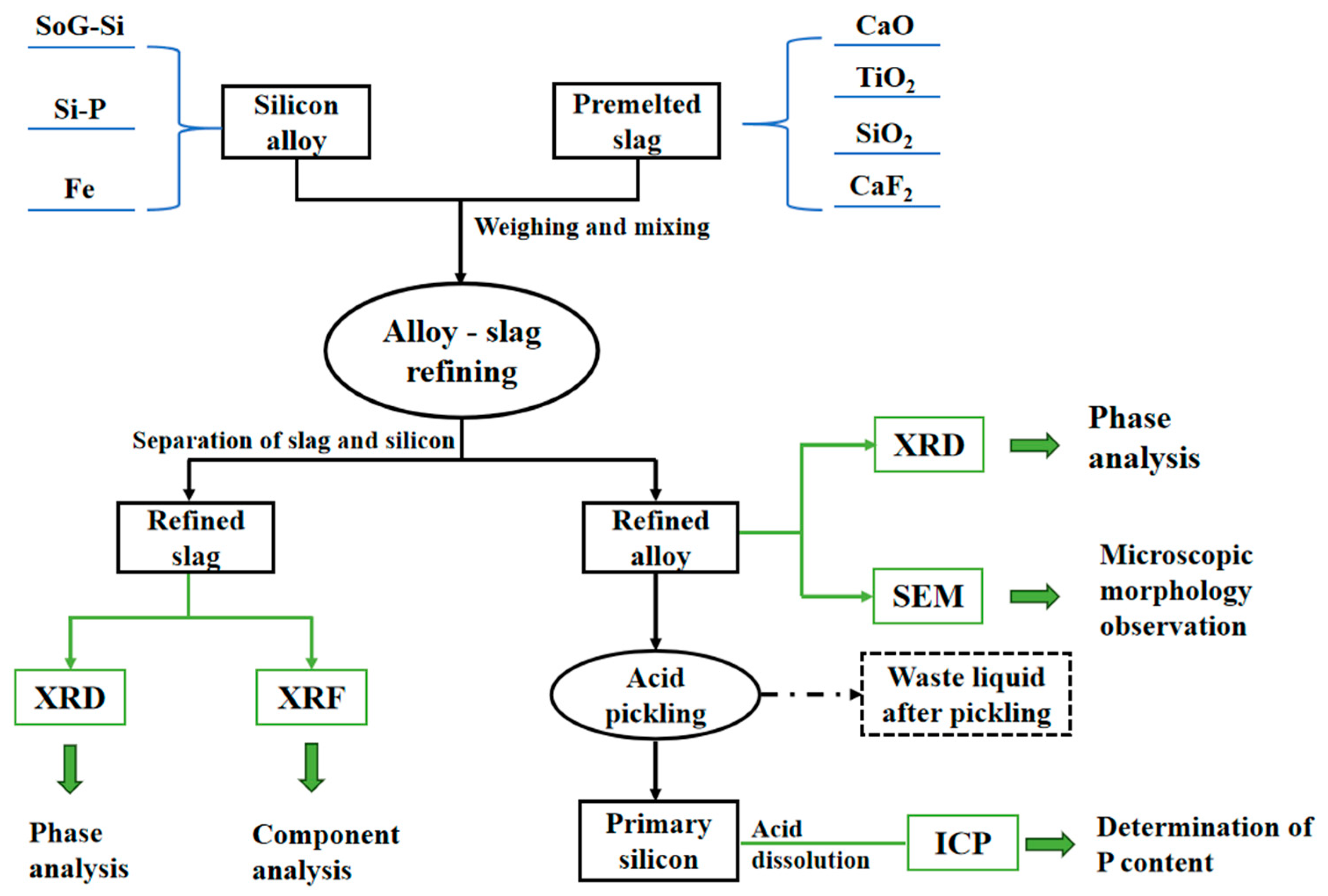
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Procedure
3. Results and Discussion
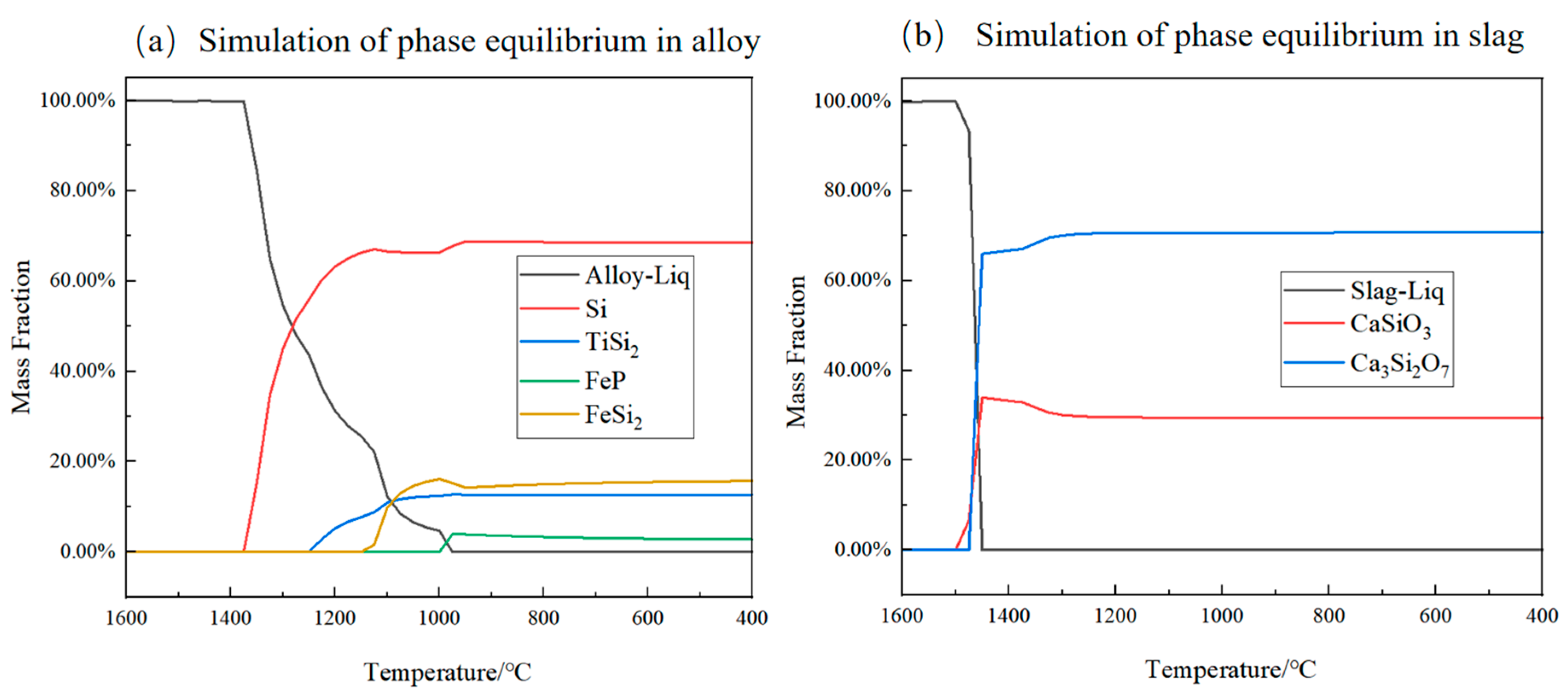
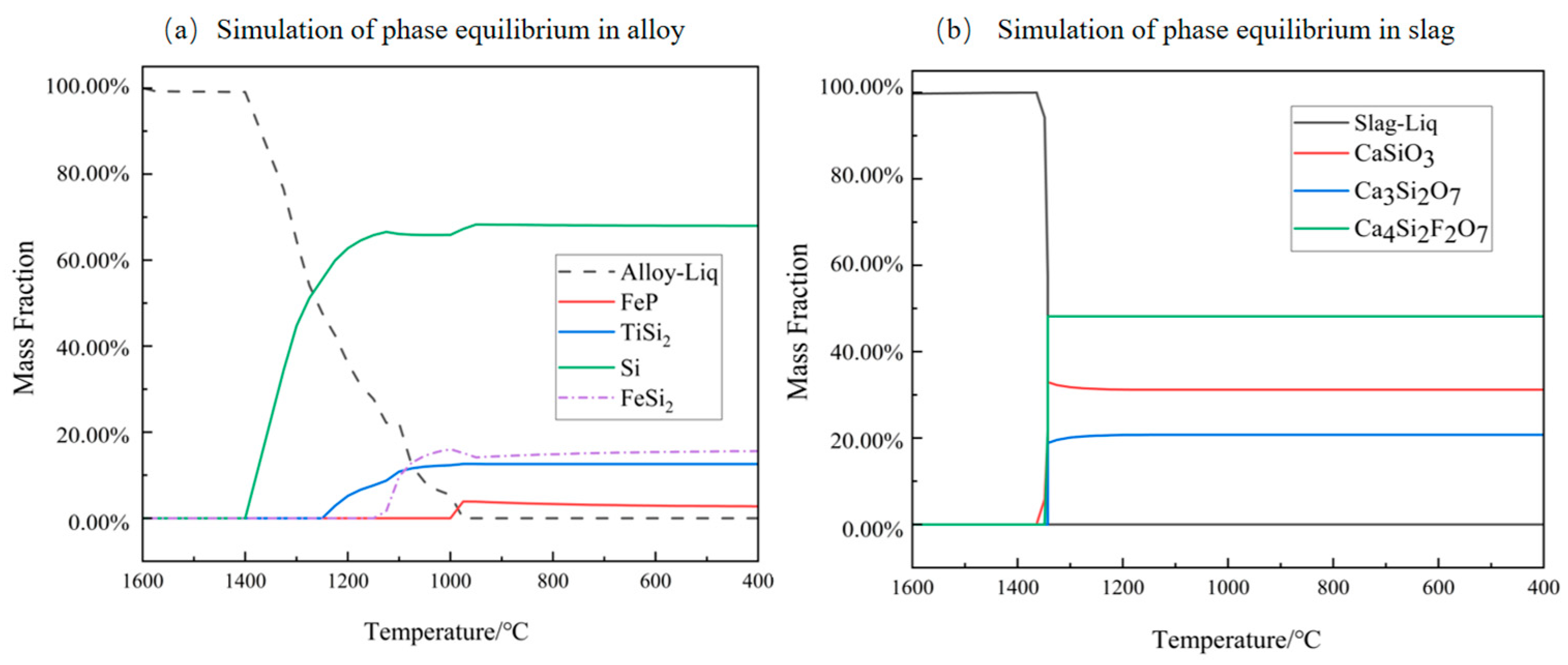
3.1. Thermodynamic Analysis of P Removal in Alloy-Slagging Refining
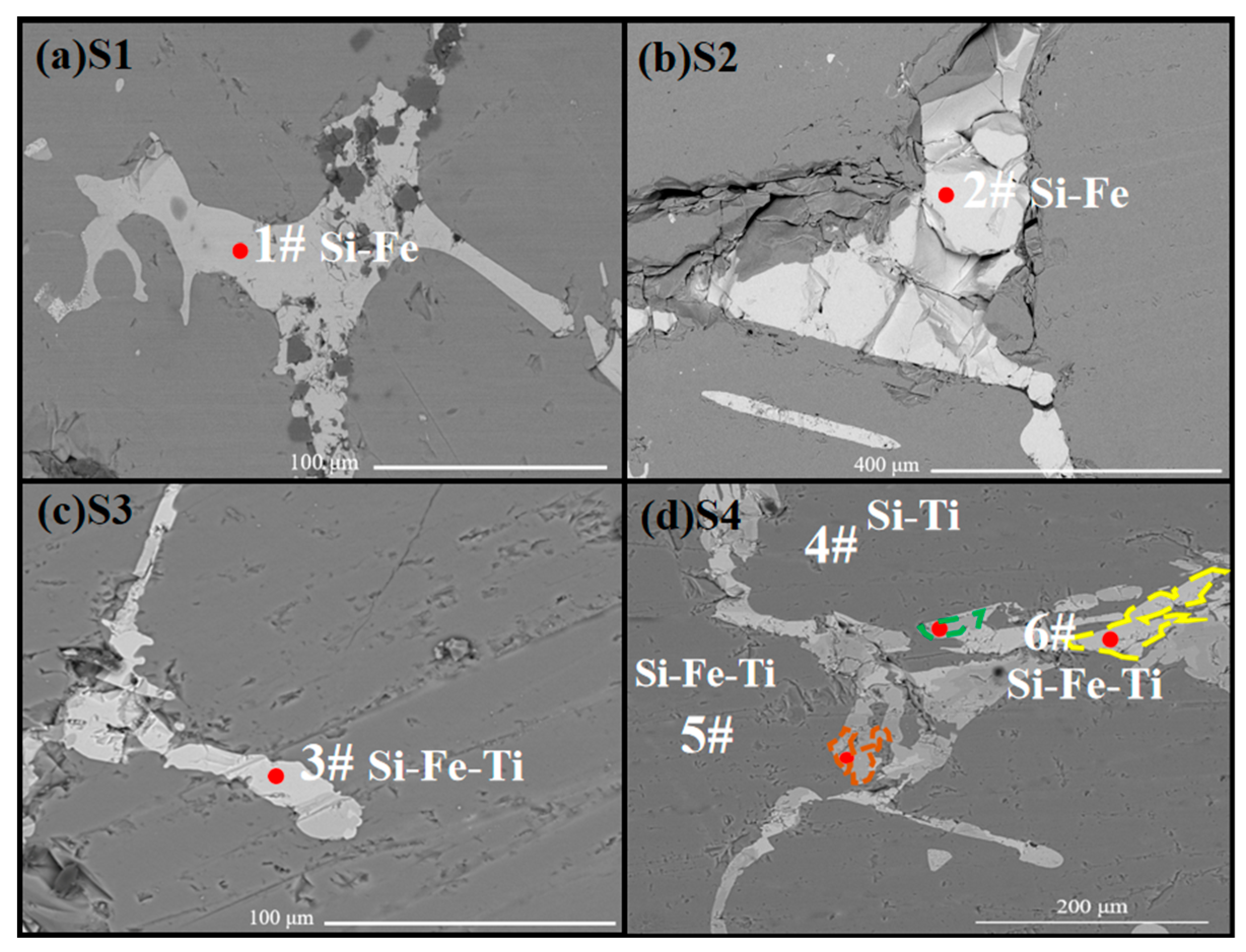
3.2. Microstructural Morphological Analysis
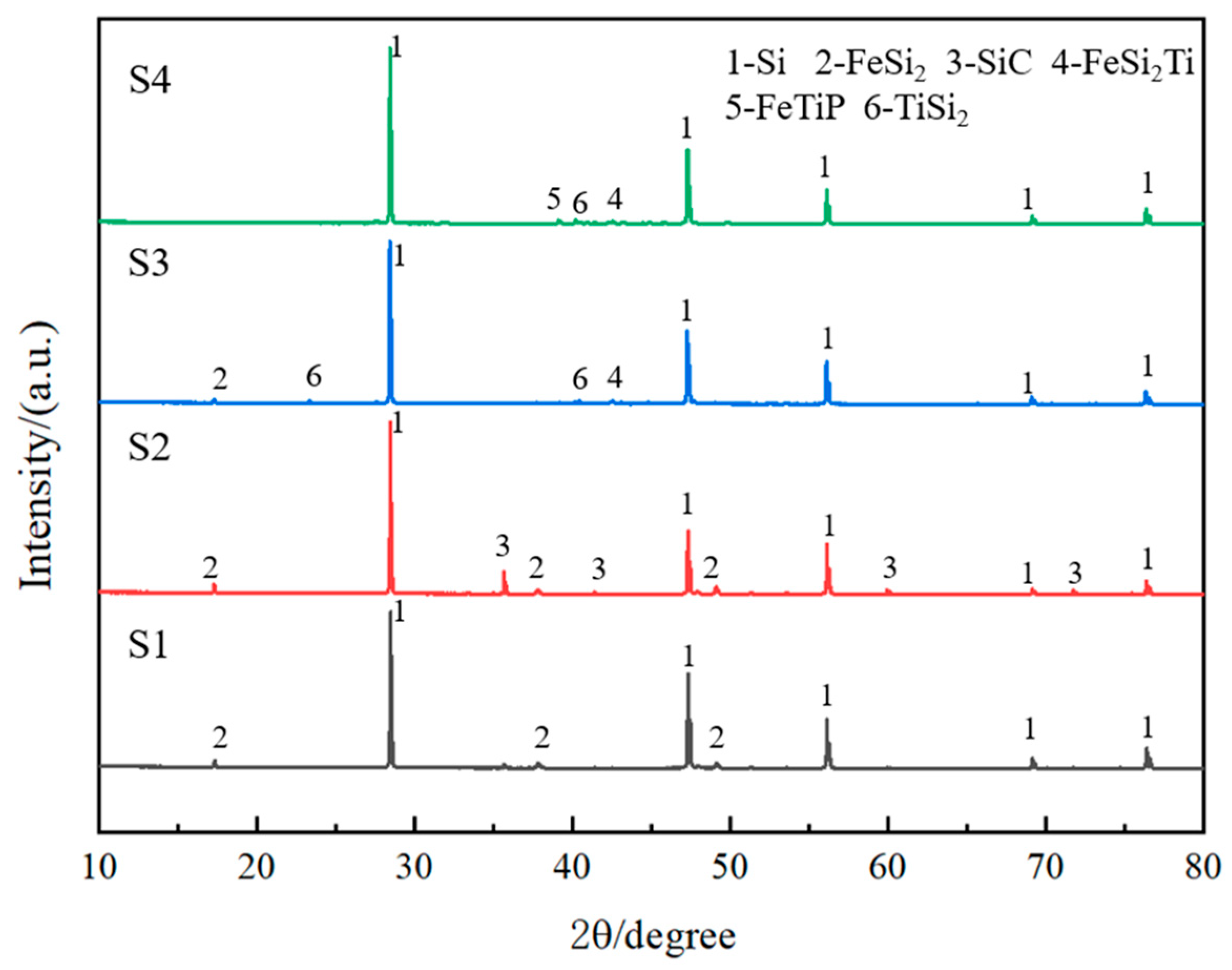
3.2.1. The Effect of Slag Component Variation on Phosphorus Removal Efficiency
3.2.2. The Effect of TiO2 Content Variation on Phosphorus Removal Efficiency
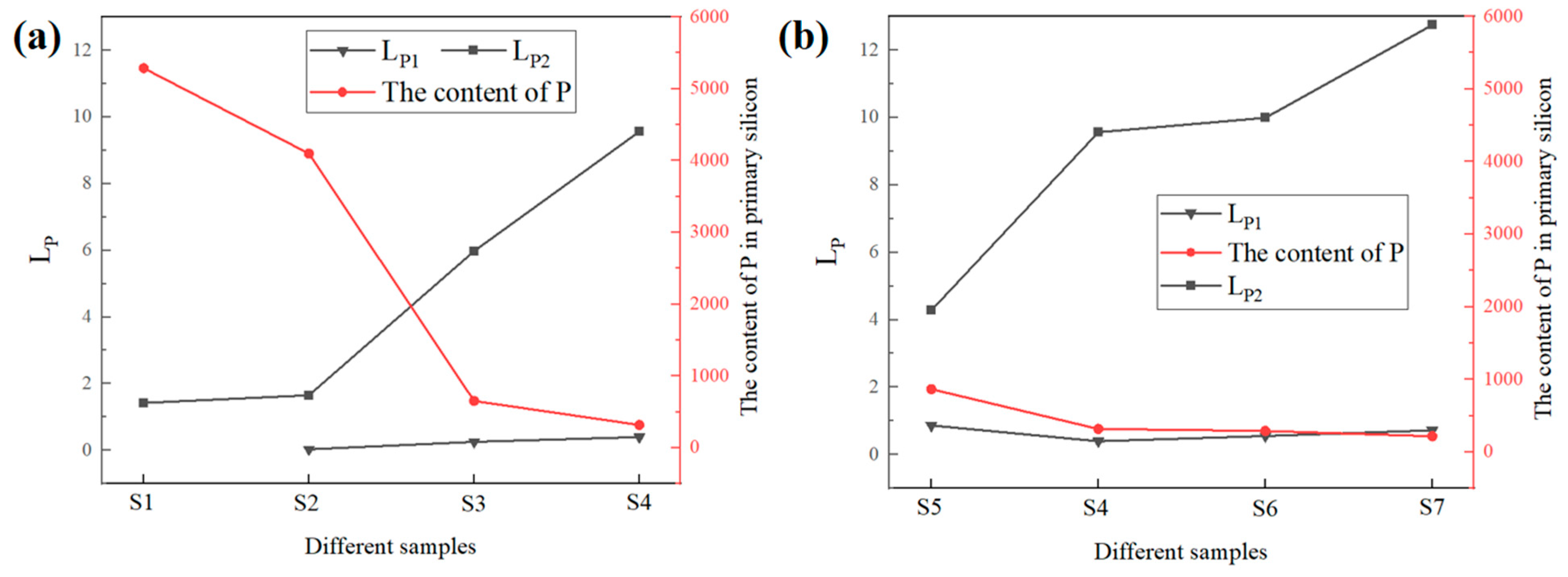
3.3. Compositional Analysis
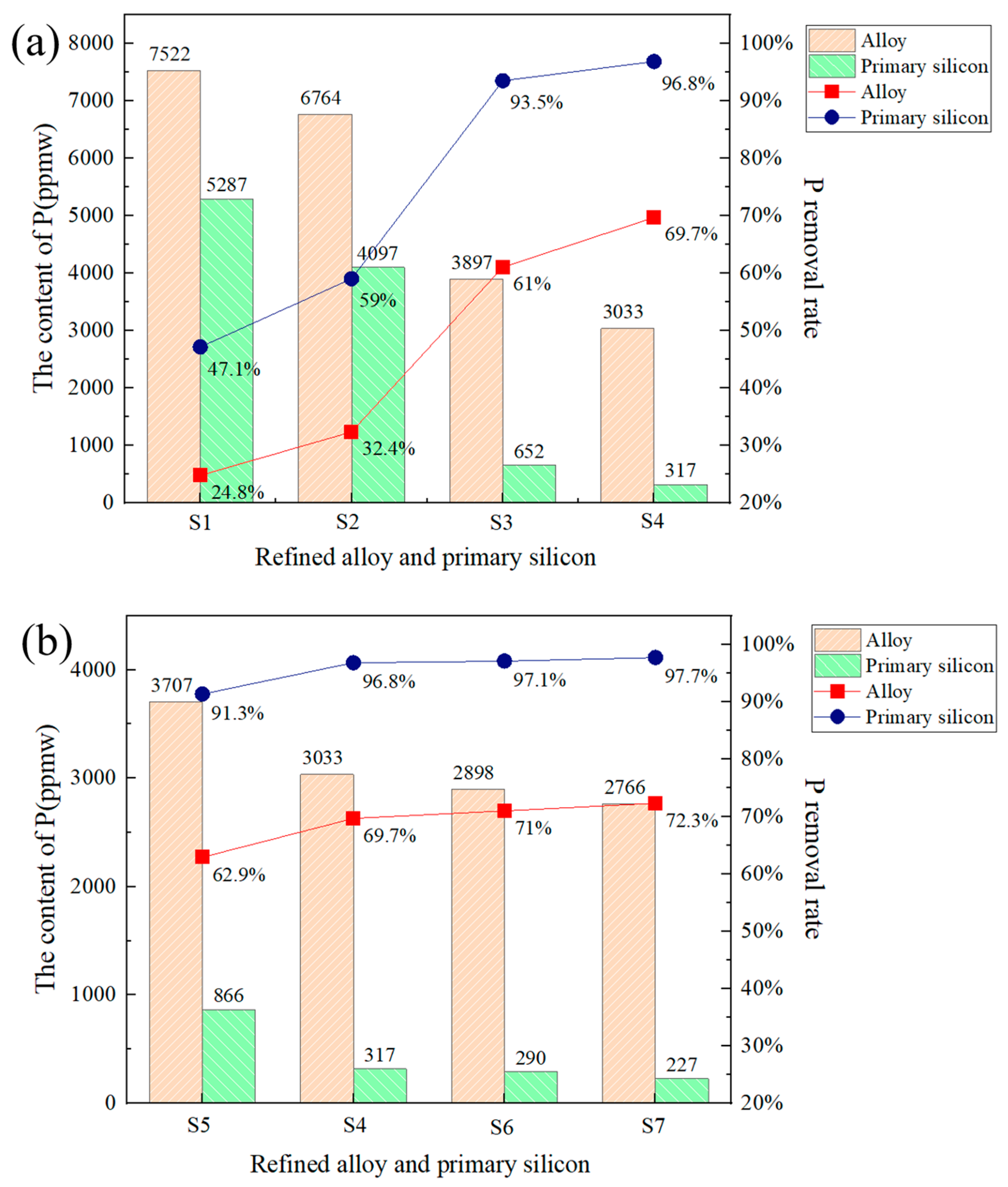
3.4. Phosphorus Removal Efficiency
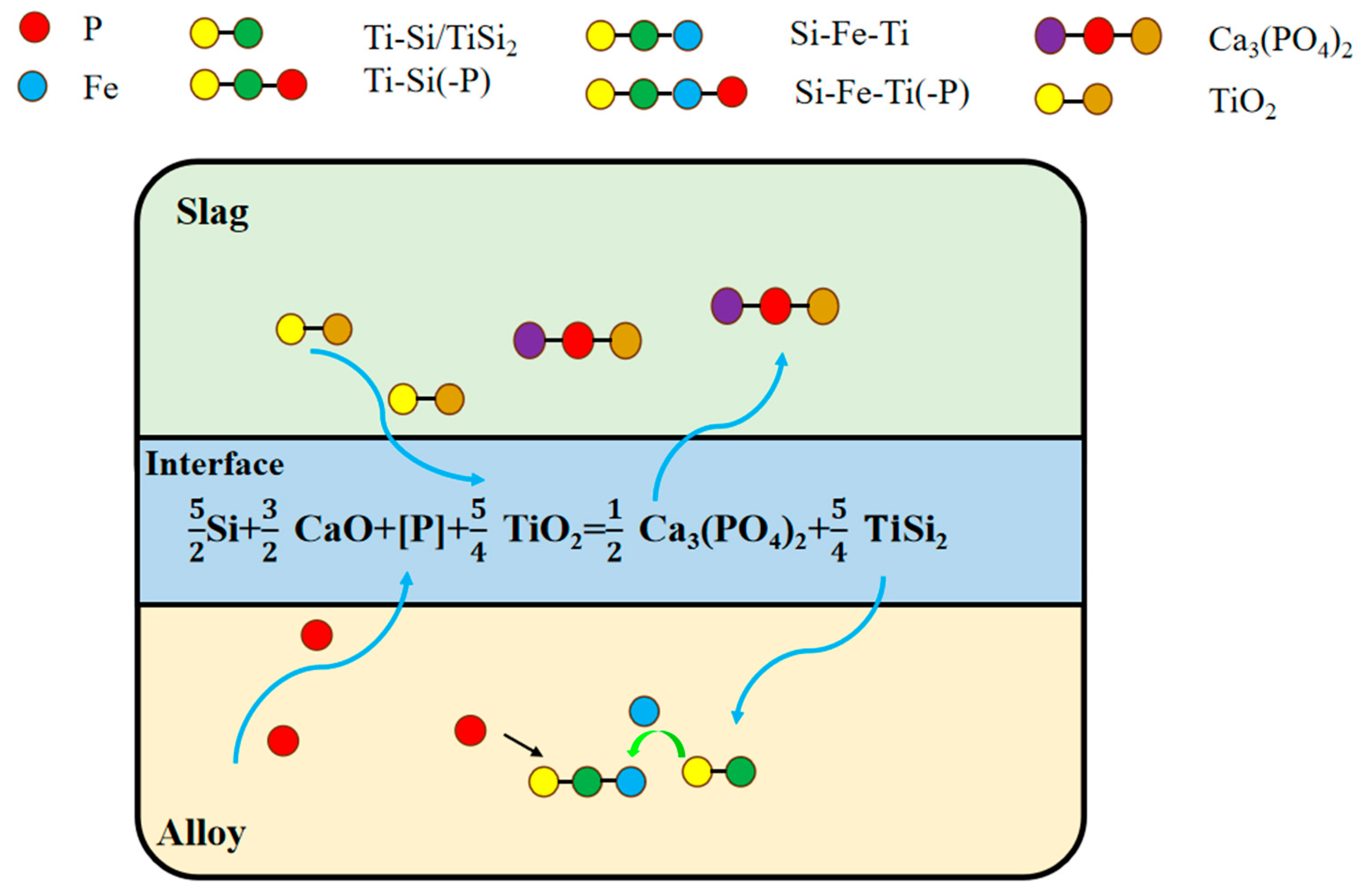
3.5. Dephosphorization Mechanism Analysis
- (1)
- Oxidation of slagging agent: A portion of P in molten silicon migrated to the silicon–slag interface, where it was oxidized by the highly oxidizing slagging agent. This oxidation primarily resulted in the formation of P-containing compounds, predominantly calcium phosphate (Ca3(PO4)2). During this process, SiO2 functioned as an acidic matrix and supplied an abundant oxygen source, thereby facilitating the oxidation of P impurities into the slag phase. CaO created a strongly alkaline environment, enabling the effective formation of stable calcium phosphate compounds that subsequently entered the slag phase. The partial reduction in TiO2 produces Ti, which reacted with Fe and Si in the alloy to form the Si-Fe-Ti ternary phase and the TiSi2 phase, effectively capturing P impurities. Additionally, CaF2 lowered the melting point and enhanced the fluidity of the slag agent, thereby intensifying the reaction at the slag–silicon interface.
- (2)
- P-rich phase formation: Simultaneously, at elevated temperatures, P diffused toward the slag phase while TiO2 in the slag underwent chemical reduction. The equilibrium phase calculations and experimental findings collectively indicated that nearly all the Ti in the slag was reduced and migrated into the silicon matrix. This process resulted in phase reconfiguration within the alloy and the formation of a Si-Fe-Ti ternary phase. Excess Ti further reacted with silicon to form the TiSi2 phase. Both the Si-Fe-Ti ternary phase and the TiSi2 phase exhibited a strong affinity for P. According to SEM-EDS analysis, the majority of P in the refined alloy phases was concentrated in these two aforementioned phases. When the TiO2 content in the slag increased, the proportion of P-rich phases in the alloy also increased correspondingly, thereby enhancing the efficiency of P removal. This demonstrated that most of the P in the alloy phases was enriched in the Si-Fe-Ti and TiSi2 phases, which can subsequently be separated from the silicon matrix through acid pickling.
4. Conclusions
- (1)
- Thermodynamic theoretical calculation: Thermodynamic simulation calculations were conducted using FactSage software to predict the changes in the composition of thermodynamic equilibrium phases and liquid phases during the smelting process. These simulations allowed for the inference of various precipitated phases and their precipitation sequence (primary Si → TiSi2 → FeSi2 → FeP) in the alloy phase upon reaching thermodynamic equilibrium, thereby providing preliminary confirmation of the feasibility of this method.
- (2)
- Influence of slag composition: In this study, the initial exploration focused on the influence of refining a Si-10 wt. % Fe alloy using slags with varying compositions during the dephosphorization process (no slag addition, binary slag SiO2-TiO2, ternary slag SiO2-CaO-TiO2, and quaternary slag SiO2-TiO2-CaO-CaF2). The results indicated that when the quaternary slag system SiO2-TiO2-CaO-CaF2 was employed, the incorporation of CaF2 enhanced the slag’s fluidity, lowered its melting point, facilitated interfacial reactions, and ultimately led to the most efficient P removal.
- (3)
- TiO2 content: Based on the SiO2-TiO2-CaO-CaF2 system, the TiO2 content in the slag was varied within the range of 5–20 wt. %. As the TiO2 content increased, the P removal rate also showed an upward trend. However, due to the substantial decrease in primary silicon yield at higher TiO2 levels, the optimal TiO2 content was determined to be 10 wt. %. Under these conditions, utilizing a Si-10wt. %Fe alloy and a slag composition of 48 wt. %CaO-32 wt. %SiO2-10 wt. %TiO2-10 wt. %CaF2, a P removal rate of 96.8% was achieved.
- (4)
- Analysis of the dual impurity removal mechanism: Based on the theoretical calculations and experimental results, the removal of P in this study was primarily achieved through two mechanisms. A portion of P was removed via slag oxidation. Due to the high alkalinity of CaO and the oxygen potential of SiO2, P was oxidized into stable Ca3(PO4)2 at the slag–silicon interface. Secondly, the remaining phosphorus within the alloy was removed through reduction with TiO2. This process facilitated the migration of P to the Fe-Si-Ti ternary phase, where it was subsequently captured by TiSi2 formed during the reaction.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ranjan, S.; Balaji, S.; Panella, R.A. Silicon solar cell production. Comput. Chem. Eng. 2011, 35, 1439–1453. [Google Scholar] [CrossRef]
- He, Y.F.; Ma, W.H.; Xing, A.M.; Hu, M.; Liu, S.; Yang, X.; Li, J.; Du, S.; Zhou, W. A review of the process on the purification of metallurgical grade silicon by solvent refining. Mater. Sci. Semicond. Process. 2022, 141, 106438. [Google Scholar] [CrossRef]
- Chen, H.; Morita, K.; Ma, X.; Chen, Z.; Wang, Y. Boron removal for solar-grade silicon production by metallurgical route: A review. Sol. Energy Mater. Sol. Cells 2019, 203, 110169. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, K.; Zheng, D.; Ma, W.; Dai, Y. Phosphorus removal from upgraded metallurgical-grade silicon by vacuum directional solidification. Vacuum 2017, 146, 159–163. [Google Scholar] [CrossRef]
- Sekar, S.; Manickam, S.; Perumalsamy, R. Enhancement of the quality of mc-Si ingot grown by vacuum directional solidification furnace with growth rate increase reducing the cost of the wafer for PV application: Carbon and oxygen analysis. Vacuum 2025, 231, 113816. [Google Scholar] [CrossRef]
- Meteleva-Fischer, Y.V.; Yang, Y. Slag treatment followed by acid leaching as a route to Solar-Grade Silicon. Jom 2012, 64, 957–967. [Google Scholar] [CrossRef]
- Zhu, M.; Azarov, A.; Monakhov, E.; Tang, K.; Safarian, J. Phosphorus separation from metallurgical-grade silicon by magnesium alloying and acid leaching. Sep. Purif. Technol. 2020, 240, 116614. [Google Scholar] [CrossRef]
- Arman, H.; Stefan, A.; Michael, M. Boron removal from silicon melt by gas blowing technique. High Temp. Mater. Process. 2022, 41, 69–91. [Google Scholar]
- Lai, H.; Sheng, Z.; Li, J.; Xing, P.; Luo, X. Enhanced separation of phosphorus from metallurgical grade silicon by CaAl2Si2 phase reconstruction. Sep. Purif. Technol. 2018, 191, 257–265. [Google Scholar] [CrossRef]
- Huang, F.; Lu, Q.; Wu, M.; Zhao, L. Purification of metallurgical-grade silicon by Sn-Si solvent refining with different tin content. Silicon 2022, 14, 103–113. [Google Scholar] [CrossRef]
- Mei, J.; Yu, W.; Hou, P.; Xue, Y.; Xin, Y.; Yan, K. Purification of metallurgical grade silicon via the Mg-Si solvent refining and acid leaching process. Silicon 2020, 13, 341–350. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Ma, W.; Yang, B. Boron removal from metallurgical grade silicon using a refining technique of calcium silicate molten slag containing potassium carbonate. Silicon 2014, 7, 247–252. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, G.; Azarov, A.; Monakhov, E.; Tang, K.; Müller, M.; Safarian, J. Effects of La2O3 addition into CaO-SiO2 slag: Structural evolution and impurity separation from Si-Sn alloy. Metall. Mater. Trans. B 2021, 52, 3045–3063. [Google Scholar] [CrossRef]
- He, Q.; He, N.; Yang, D.; Wu, J.; Wei, K.; Ma, W. Oxidation kinetics and mechanism of boron in metallurgical-grade silicon melt by CaO-SiO2 slag refining. Metall. Mater. Trans. B 2022, 53, 1841–1850. [Google Scholar] [CrossRef]
- Zhang, C.; Lai, H.; Zhang, Y.; Sheng, Z.; Li, J.; Xing, P.; Luo, X. Extraction of phosphorus from metallurgical grade silicon using a combined process of Si-Al-Ca solvent refining and CaO-CaF2 slag treatment. Sep. Purif. Technol. 2020, 232, 115954. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhao, Q.; Tao, M.; Du, J.; Huang, X.; Liu, C. Preparation of FeCoNi medium entropy alloy from Fe3+-Co2+-Ni2+ solution system. Int. J. Miner. Metall. Mater. 2025, 32, 92–101. [Google Scholar] [CrossRef]
- Yan, Z.; Zhao, Q.; Han, C.; Mei, X.; Liu, C.; Jiang, M. Effects of iron oxide on crystallization behavior and spatial distribution of spinel in stainless steel slag. Int. J. Miner. Metall. Mater. 2024, 31, 292–300. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, Z.; Liu, J.; Qian, G.; Du, B. The mechanism of boron removal from silicon alloy by electric field using slag treatment. Sep. Purif. Technol. 2018, 199, 134–139. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Zuo, Q.; Ban, B.; Chen, J. Simultaneously removal of P and B from Si by Sr and Zr co-addition during Al-Si low-temperature solvent refining. Int. J. Miner. Metall. Mater. 2023, 30, 365–377. [Google Scholar] [CrossRef]
- Meteleva-Fischer, Y.V.; Yang, Y.; Boom, R.; Kraaijveld, B.; Kuntzel, H. Microstructure of metallurgical grade silicon during alloying refining with calcium. Intermetallics 2012, 25, 9–17. [Google Scholar] [CrossRef]
- Zhu, M.; Yue, S.; Wu, G.; Tang, K.; Xu, Y.; Safarian, J. P removal from Si by Si-Ca-Al alloying-leaching refining: Effect of Al and the CaAl2Si2 phase. Sep. Purif. Technol. 2021, 271, 118675. [Google Scholar] [CrossRef]
- Huang, L.; Danaei, A.; Thomas, S.; Xing, P.; Li, J.; Luo, X.; Barati, M. Solvent extraction of phosphorus from Si-Cu refining system with calcium addition. Sep. Purif. Technol. 2018, 204, 205–212. [Google Scholar] [CrossRef]
- Ren, Y.; Morita, K. Low-temperature process for the fabrication of low-boron content bulk Si from Si–Cu solution with Zr addition. ACS Sustain. Chem. Eng. 2020, 8, 6853–6860. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.; Wang, X.; Kong, J.; Xing, P.; Zhuang, Y.; Luo, X. Synthesis and separation of Si-Fe alloy to produce high-purity silicon. J. Mater. Res. Technol. 2019, 8, 4470–4476. [Google Scholar] [CrossRef]
- Khajavi, L.T.; Morita, K.; Yoshikawa, T.; Barati, M. Removal of boron from silicon by solvent refining using ferrosilicon alloys. Metall. Mater. Trans. 2015, 46, 615–620. [Google Scholar] [CrossRef]
- Taposhe, G.I.A.; Khajavi, L.T. Removal of phosphorus from Si-Fe alloy by CaO-Al2O3-SiO2-Na2O slag refining. JOM 2021, 73, 729–735. [Google Scholar] [CrossRef]
- Esfahani, S.; Barati, M. Purification of metallurgical silicon using iron as impurity getter, part II: Extent of silicon purification. Met. Mater. Int. 2011, 17, 1009–1015. [Google Scholar] [CrossRef]
- Esfahani, S.; Barati, M. Purification of metallurgical silicon using iron as an impurity getter part I: Growth and separation of Si. Met. Mater. Int. 2011, 17, 823–829. [Google Scholar] [CrossRef]
- Deng, X.; Li, S.; Wen, J.; Wei, K.; Zhang, M.; Yang, X.; Ma, W. Mechanism of enhancing phosphorus removal from metallurgical grade silicon by Si-Fe-Ti phase reconstruction. Metall. Mater. Trans. B 2021, 52, 625–632. [Google Scholar] [CrossRef]
- Taposhe, G.I.A.; Khajavi, L.T. Thermodynamics of impurities removal from Si–Fe alloy by CaO–Al2O3–SiO2–Na2O slag refining. Metall. Mater. Trans. B 2022, 53, 4019–4028. [Google Scholar] [CrossRef]
- Li, M.; Utigard, T.; Barati, M. Removal of boron and phosphorus from silicon using CaO-SiO2-Na2O-Al2O3 flux. Metall. Mater. Trans. B 2014, 45, 221–228. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Tafaghodi Khajavi, L. Phosphorus removal from Si-Fe alloy using SiO2-Al2O3-CaO Slag. Metall. Mater. Trans. B 2019, 50, 1773–1781. [Google Scholar] [CrossRef]
- Sakiani, H.; Tabaian, S.H.; Chen, J.; Li, J.; Ban, B. Investigating boron and phosphorus removal from silicon by Si-Ti and Si-Ti-Fe alloying systems. Sep. Purif. Technol. 2020, 250, 117227. [Google Scholar] [CrossRef]
- Gu, H.Z.; Cao, J.; Wu, J.J.; Wei, K.; Ma, W. A novel method for preparing Si–Ti alloy by co-reduction of silicon slag and Ti-bearing blast furnace slag. Metall. Mater. Trans. B 2023, 54, 2815–2823. [Google Scholar] [CrossRef]
- Park, H.J.; Min, J.D.; Song, S.H. The effect of CaF2 on the viscosities and structures of CaO-SiO2(-MgO)-CaF2 slags. Metall. Mater. Trans. B 2002, 33, 723–729. [Google Scholar] [CrossRef]
- Ju, J.; Yang, K.; Zhu, Z.; Gu, Y.; Chang, L.Z. Effect of CaF2 and CaO/Al2O3 on viscosity and structure of TiO2-bearing slag for electroslag remelting. Iron Steel Res. Int. 2021, 28, 1541–1550. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. Reprint of: FactSage thermochemical software and databases. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Shahbazian, F.; Sichen, D.; Mills, K.C.; Seetharaman, S. Experimental studies of viscosities of some CaO-CaF2-SiO2 slags. Ironmak. Steelmak. 2013, 26, 193–199. [Google Scholar] [CrossRef]
- Shahbazian, F. Experimental studies of the viscosities in the CaO-FeO-SiO2-CaF2 slag. Scand. J. Metall. 2001, 30, 302–308. [Google Scholar] [CrossRef]
- Thomas, S.; Huang, L.; Barati, M. A review of slag refining of crude Silicon. JOM 2020, 73, 260–281. [Google Scholar] [CrossRef]
- Putera, P.D.A.; Avarmaa, L.K.; Petrus, H.T.; Chen, J.; Brooks, G.A.; Rhamdhani, M.A. Multistage refining of silicon using CaO–SiO2–Al2O3 and CaO–SiO2–MgO slags. J. Sustain. Metall. 2025, 11, 895–907. [Google Scholar] [CrossRef]



| No. | Slag Composition (wt. %) | |||
|---|---|---|---|---|
| SiO2 | TiO2 | CaO | CaF2 | |
| S1 | 0 | 0 | 0 | 0 |
| S2 | 90 | 10 | 0 | 0 |
| S3 | 36 | 10 | 54 | 0 |
| S4 | 32 | 10 | 48 | 10 |
| S5 | 34 | 5 | 51 | 10 |
| S6 | 30 | 15 | 45 | 10 |
| S7 | 28 | 20 | 42 | 10 |
| Point | Si | Fe | Ti | P | Ca | Possible Phases | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | at. % | wt. % | at. % | wt. % | at. % | wt. % | at. % | wt. % | at. % | ||
| 1# | 54.52 | 69.46 | 42.52 | 27.13 | -- | 2.96 | 3.40 | -- | FeSi2 | ||
| 2# | 53.40 | 68.96 | 44.84 | 29.17 | 0.47 | 0.36 | 1.29 | 1.51 | -- | FeSi2 | |
| 3# | 41.92 | 56.76 | 29.58 | 20.06 | 27.03 | 21.39 | 1.41 | 1.72 | 0.06 | 0.06 | FeSi2Ti |
| 4# | 58.48 | 70.67 | 0.63 | 0.38 | 40.69 | 28.74 | 0.17 | 0.19 | 0.03 | 0.03 | TiSi2 |
| 5# | 55.56 | 68.54 | 9.80 | 6.06 | 33.55 | 24.19 | 1.02 | 1.14 | 0.08 | 0.07 | Si64.3Fe7.4Ti28.3 |
| 6# | 41.76 | 56.39 | 29.20 | 19.75 | 26.86 | 21.20 | 2.17 | 2.65 | 0.01 | 0.009 | FeSi2Ti |
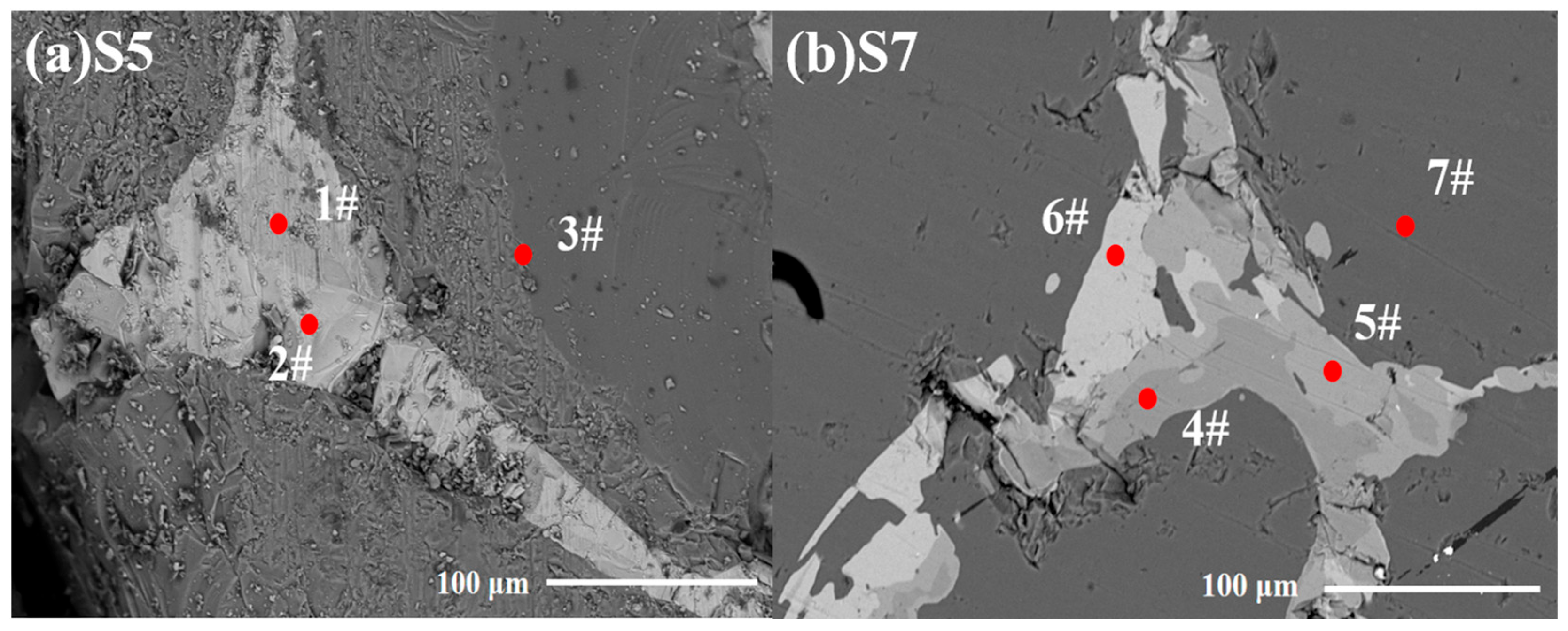
| Point | Si | Fe | Ti | P | Possible Phases | ||||
|---|---|---|---|---|---|---|---|---|---|
| wt. % | at. % | wt. % | at. % | wt. % | at. % | wt. % | at. % | ||
| 1# | 46.51 | 63.08 | 52.12 | 35.41 | 0.37 | 0.29 | 1.00 | 1.22 | FeSi2 |
| 2# | 31.97 | 46.38 | 36.69 | 26.19 | 29.08 | 25.50 | 2.25 | 2.93 | FeSi2Ti |
| 3# | 99.38 | 99.67 | 0.34 | 0.17 | 0.28 | 0.16 | -- | -- | Si |
| 4# | 49.94 | 63.14 | 5.13 | 3.25 | 43.90 | 35.45 | 1.02 | 1.16 | TiSi2 |
| 5# | 48.29 | 61.62 | 11.75 | 7.51 | 37.34 | 27.85 | 2.62 | 3.02 | Si64.3Fe7.4Ti28.3 |
| 6# | 32.85 | 47.16 | 35.54 | 25.10 | 28.58 | 23.83 | 3.03 | 3.90 | FeSi2Ti |
| 7# | 100 | 100 | -- | -- | -- | -- | -- | -- | Si |
| No. | Element Content (wt. %) | ||||||
|---|---|---|---|---|---|---|---|
| Si | Ca | Fe | Ti | P | O | F | |
| S2 | 46.13 | -- | 0.32 | 0.17 | 0.01 | 53.37 | -- |
| S3 | 23.06 | 34.94 | 0.08 | 0.22 | 0.05 | 41.65 | -- |
| S4 | 21.00 | 31.99 | 0.51 | 0.84 | 0.06 | 38.97 | 6.63 |
| S5 | 19.80 | 35.24 | 0.83 | 0.25 | 0.16 | 39.04 | 4.68 |
| S6 | 19.76 | 35.30 | 0.69 | 0.42 | 0.08 | 38.32 | 5.43 |
| S7 | 19.93 | 35.08 | 0.27 | 0.58 | 0.10 | 38.29 | 5.75 |
| No. | Reactions | △Gθ (J/mol) | Temp. (°C) |
|---|---|---|---|
| (1) | [P] + 5/2Si + 3/2CaO + 5/4TiO2 = 1/2Ca3(PO4)2 + 5/4TiSi2 | 78.89T − 433,055.4 | 800–1600 |
| (2) | [P] + 5/2Si + 3/2CaO + 5/4TiO2 + 5/4Fe = 1/2Ca3(PO4)2 + 5/4FeSi2 + 5/4Ti | 98.45T − 122,218.6 | 800–1600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Zhao, Q.; Li, J. Phosphorus Removal in Metallurgical-Grade Silicon via a Combined Approach of Si-Fe Solvent Refining and SiO2-TiO2-CaO-CaF2 Slag Refining. Metals 2025, 15, 668. https://doi.org/10.3390/met15060668
Zhong Y, Zhao Q, Li J. Phosphorus Removal in Metallurgical-Grade Silicon via a Combined Approach of Si-Fe Solvent Refining and SiO2-TiO2-CaO-CaF2 Slag Refining. Metals. 2025; 15(6):668. https://doi.org/10.3390/met15060668
Chicago/Turabian StyleZhong, Yi, Qing Zhao, and Juncheng Li. 2025. "Phosphorus Removal in Metallurgical-Grade Silicon via a Combined Approach of Si-Fe Solvent Refining and SiO2-TiO2-CaO-CaF2 Slag Refining" Metals 15, no. 6: 668. https://doi.org/10.3390/met15060668
APA StyleZhong, Y., Zhao, Q., & Li, J. (2025). Phosphorus Removal in Metallurgical-Grade Silicon via a Combined Approach of Si-Fe Solvent Refining and SiO2-TiO2-CaO-CaF2 Slag Refining. Metals, 15(6), 668. https://doi.org/10.3390/met15060668






