Effect of Scanning Speed on Wear and Corrosion Behaviors of High-Speed Laser-Cladded Cu-TiC Coating
Abstract
1. Introduction
2. Experimental Methods
2.1. Material Preparation
2.2. Microstructure Characterization
2.3. Wear Test
2.4. Electrochemical Measurement
3. Results and Discussion
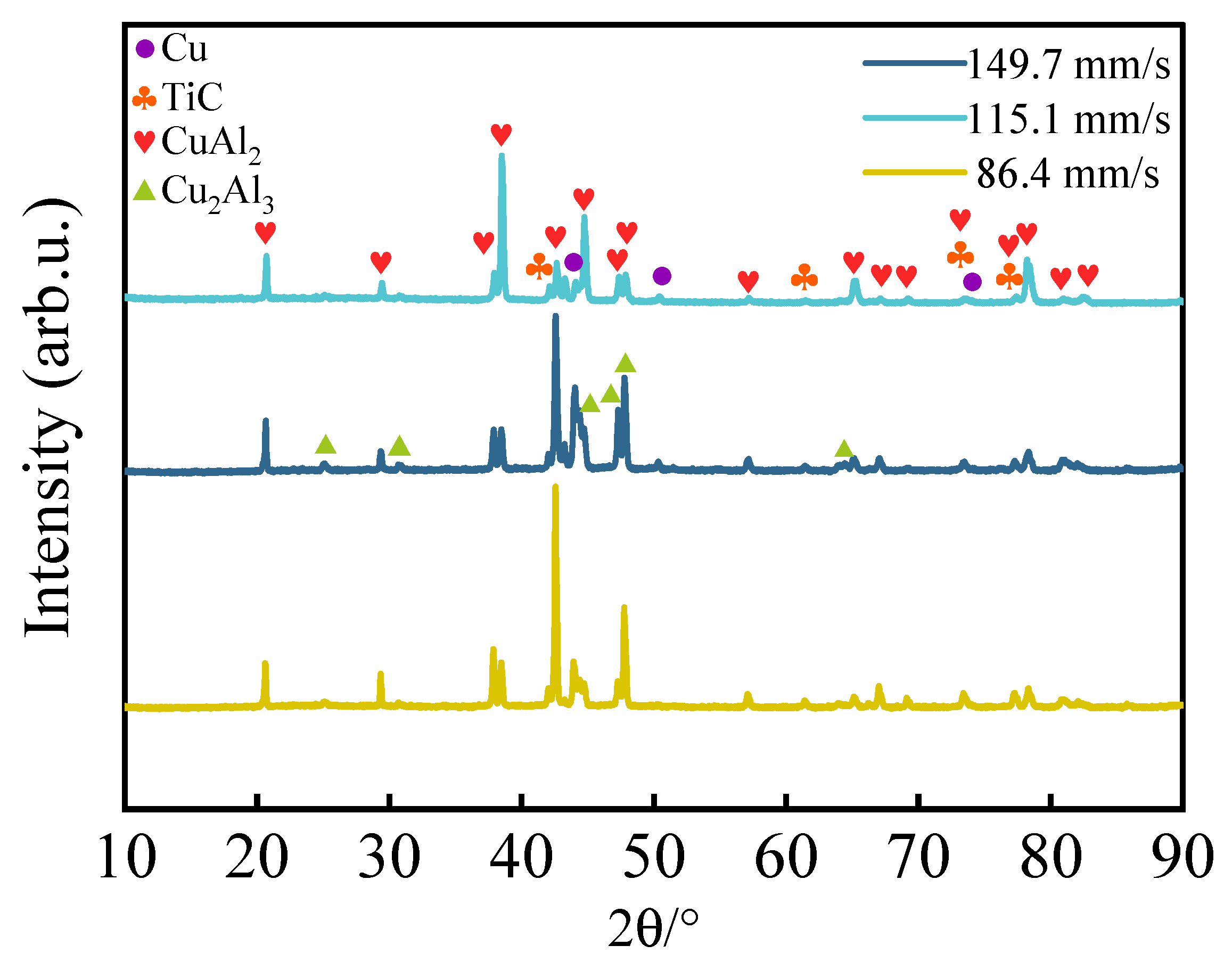
3.1. Phase and Microstructure
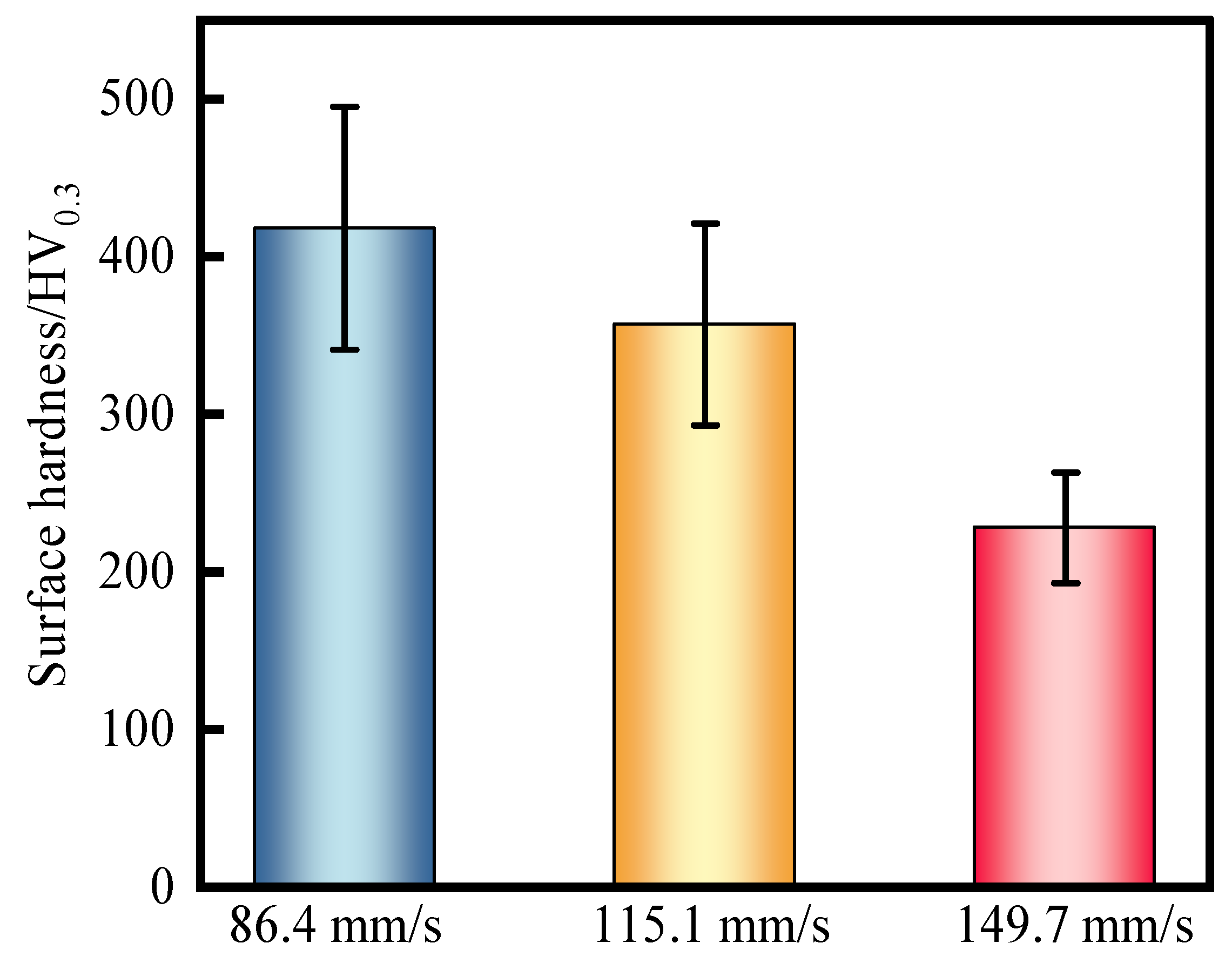
3.2. Hardness
3.3. Wear Performance
3.3.1. COF and Wear Rate
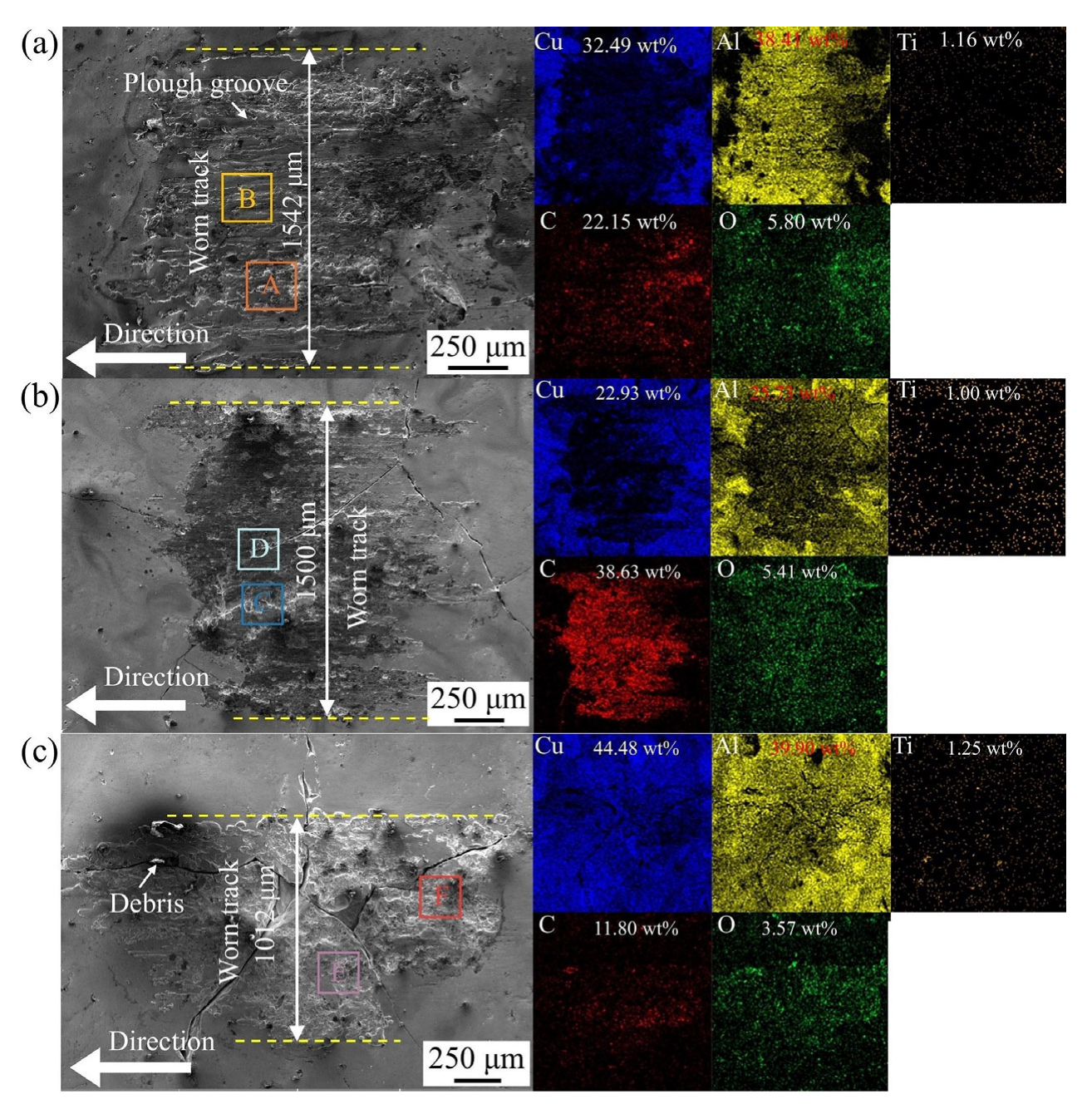
3.3.2. Mapping Analysis of Worn Track on Coatings
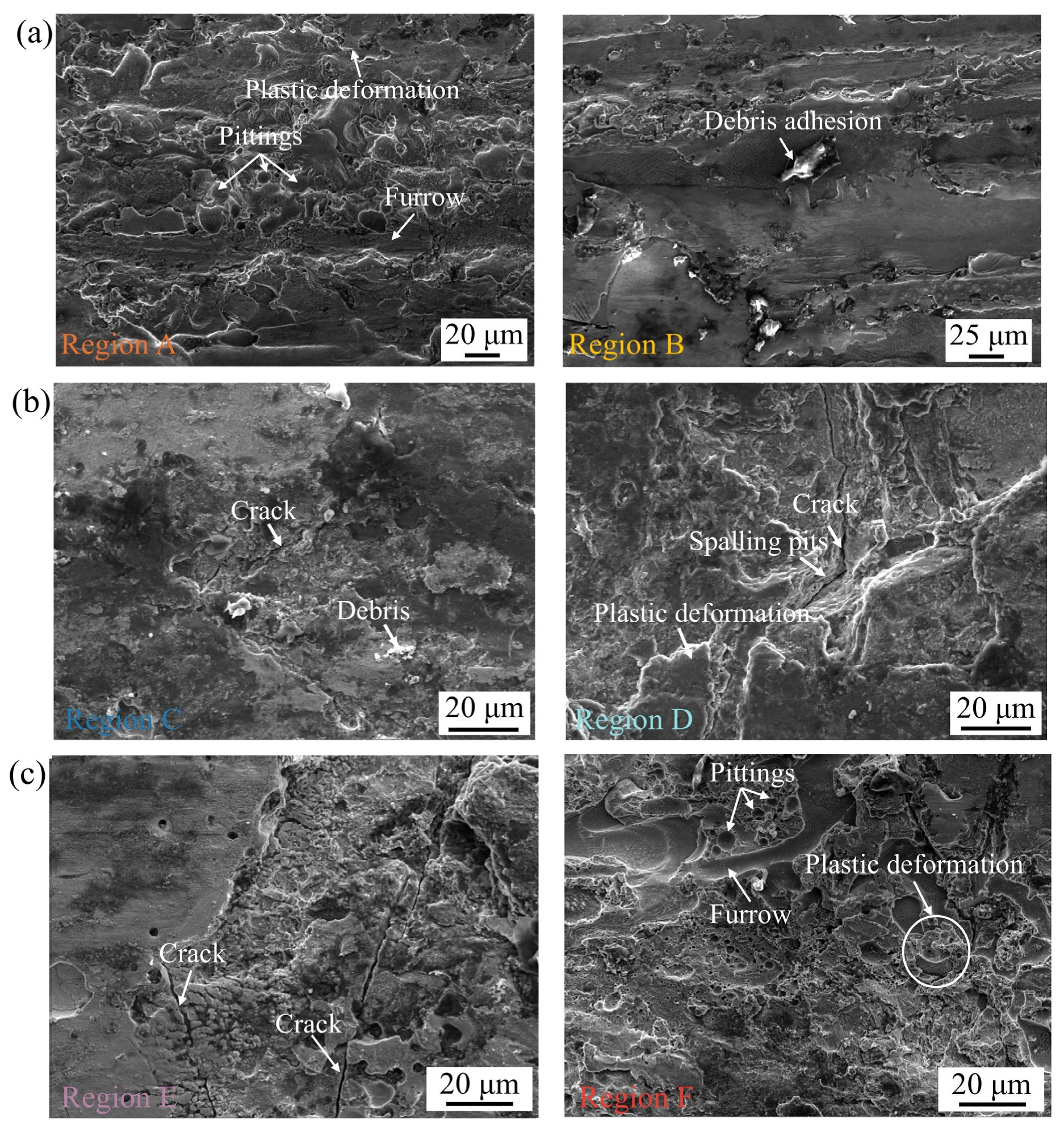
3.3.3. Morphologies of Worn Track on Coatings
3.3.4. Wear Mechanism
3.4. Electrochemical Corrosion Behavior
3.4.1. PPC
3.4.2. Electrochemical Impedance Spectroscopy Tests
4. Conclusions
- The scanning speed significantly affected the phase composition and grain morphology of the coating. At a low scanning speed (86.4 mm/s), the higher heat input promoted the formation of the CuAl2 phase, and the grains were mainly columnar crystals. With the increase in the scanning speed (149.7 mm/s), the cooling rate accelerated, the proportion of Cu2Al3 phase increased, the grain size refined, and it transformed into a coexisting structure of equiaxed crystals and cellular crystals. The TiC particles were uniformly distributed, with good interface bonding and inhibition of elemental segregation.
- The coating with a high scanning speed (149.7 mm/s) exhibited the best wear resistance, and its wear rate was 17.9% lower than that of the substrate. The wear mechanism was mainly abrasive wear and adhesive wear, accompanied by slight oxidative wear. High-speed cladding inhibited crack propagation and hard particle spalling by refining grains and strengthening the TiC interface, thereby enhancing the load-bearing capacity of the coating.
- The increase in scanning speed significantly improved the electrochemical performance of the coating. The corrosion current density of the coating with a high scanning speed (149.7 mm/s) was reduced to 7.36 × 10−7 A·cm−2, and the polarization resistance reached 23,813 Ω·cm2. The improvement in corrosion resistance was attributed to the formation of a dense passivation film and the blocking of the Cl− diffusion path.
- The scanning speed of 149.7 mm/s was the optimal parameter, and its coating had the characteristics of a low wear rate, high corrosion resistance, and stable tribological behavior. It applies to extreme marine environments with high salt spray and high mechanical load, such as conductive rings on ships.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Gao, G.; Fu, R.; Chen, J.; Qian, P.; Wang, H.; Yang, Z.; Wu, G. Influence of rainwater acid concentration on the current-carrying friction for C/Cu contact pairs. Wear 2025, 572–573, 205970. [Google Scholar] [CrossRef]
- Gao, Q.; Li, Q.; Chen, W.; Wang, W.; Wu, Z.; Zhang, Z.; Wan, Y.; Feng, Y.; Wang, D. Enhanced current-carrying tribological properties of copper-based microporous friction pairs containing slow-release polyaniline conductive grease. Tribol. Int. 2025, 201, 110240. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Ma, G.; Li, G.; Zhao, H.; Li, Y.; Tan, N.; Wang, H. Laser cladding Mo-based coatings on copper alloys to improve the current-carrying tribological properties of Cu/Al friction pairs. Surf. Coat. Technol. 2024, 487, 130989. [Google Scholar] [CrossRef]
- Luo, X.; Xiong, Y.; Liu, X.; Feng, X.; Zhang, Z.; Yin, F.; Zheng, Z. Atomistic study of the friction and wear behaviors of Cu/SiC nanocomposite: The interaction among reinforcement particle, matrix and counterpart. Appl. Surf. Sci. 2025, 696, 163010. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Qin, L.-X.; Qiu, W.-T.; Li, S.-F.; Jiang, Y.-B.; Jia, Y.-L.; Li, Z. Electrical sliding friction wear behaviors and mechanisms of Cu–Sn matrix composites containing MoS2/graphite. Wear 2024, 548–549, 205388. [Google Scholar] [CrossRef]
- Wang, X.; Song, K.; Feng, J.; Xing, J.; Hang, T.; Zhang, Y. Ablation resistance and current-carrying friction performance of WMoCu alloy with different Mo contents. Tribol. Int. 2025, 203, 110413. [Google Scholar] [CrossRef]
- Cheng, X.; Wei, K.; Li, H.; Teng, N.; Xu, S.; Chen, Q.; Gong, X.; He, Y.; Yan, S. A Ni-Cu/CuPP composite coating with good wear resistance and long-term corrosion resistance for seawater applications. Tribol. Int. 2025, 202, 110393. [Google Scholar] [CrossRef]
- Liang, Y.; Liao, Z.Y.; Zhang, L.L.; Cai, M.W.; Wei, X.S.; Shen, J. A review on coatings deposited by extreme high–speed laser cladding: Processes, materials, and properties. Opt. Laser Technol. 2023, 164, 109472. [Google Scholar] [CrossRef]
- Yuan, W.; Zhu, L.; Luo, C.; Liu, H.; Chen, Z.; He, Y.; Han, E. Enhanced CO2 separation properties by incorporating acid-functionalized graphene oxide into polyimide membrane. High Perform. Polym. 2021, 33, 405–416. [Google Scholar] [CrossRef]
- Jian, Y.; Liu, Y.; Qi, H.; He, P.; Huang, G.; Huang, Z. Effects of scanning speed on the microstructure, hardness and corrosion properties of high-speed laser cladding Fe-based stainless coatings. J. Mater. Res. Technol. 2024, 29, 3380–3392. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Y.; Wan, Y.; Jeyaprakash, N.; Wang, Y.; Ma, K.; Yang, J. Influence of scanning speed on microstructure and corrosion resistance of Fe-based amorphous coatings by high-speed laser cladding. Surf. Coat. Technol. 2024, 479, 130449. [Google Scholar] [CrossRef]
- Gan, R.; Liu, Z.; Kong, Y.; Chang, Y.; Shen, Y.; Li, J.; Ning, H. Preparation and properties of Cu/Cu-Sn alloy cladding layers on titanium alloy by laser cladding. J. Alloys Compd. 2025, 1020, 179547. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, H.; He, H.; Cao, H. Content effects of in-situ synthesis TiC for grain refinement, porosity suppression and performance enhancement in wire arc additive manufactured Al-Cu alloy. J. Mater. Process. Technol. 2025, 340, 118875. [Google Scholar] [CrossRef]
- Chen, R.; Deng, J.; Luo, H.; Fu, C.; Wang, R.; Chen, H.; Wang, Q.; Xiao, X.; Yang, B. Laminate structure improves the ductility of Cu-Al2O3/Cu composites fabricated by accumulative roll bonding. Mater. Today Commun. 2025, 46, 112454. [Google Scholar] [CrossRef]
- Hao, J.; Chen, C.; Zhao, Y.; Tong, M.; Zhao, D.; Ke, Y.; Liu, G.; Li, R.; Wang, B. Microstructure evolution and aging strengthening behavior of in-situ TiC nano-reinforced Al-Si-Cu-Mg alloy. Mater. Charact. 2025, 222, 114808. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Tan, N.; Mou, H.; Xing, Z.; Liu, J.; Du, X.; Li, J.; Cai, Z.; Wang, H. Microstructure and tribological properties of ultrasonic vibration assisted high-speed laser cladding (CoCrNi) 88Al6Ti6-cBN coatings. Intermetallics 2025, 178, 108644. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, X.; Zhao, H.; Duan, B.; Zhou, L.; Mou, H.; Ma, G.; Wang, H. Microstructure and tribological properties of FeCrNi-WC medium entropy alloy composite coatings prepared by high-speed laser cladding with different preheating temperatures. Surf. Coat. Technol. 2025, 504, 132047. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Cheng, Y.-H.; He, B.; Wan, Y.-X.; Chen, H.; Wang, Y.-F.; Yang, J.-Y. Enhancement of high-entropy alloy coatings with multi-scale TiC ceramic particles via high-speed laser cladding: Microstructure, wear and corrosion. Appl. Surf. Sci. 2025, 685, 162061. [Google Scholar] [CrossRef]
- Liu, J.; Shao, Q.; Cui, X.; Jin, G.; Wen, X.; Shi, T.; Tian, H. Research on in situ generation mechanism and tribological properties of superhard high entropy ceramic coatings by high speed laser cladding. Ceram. Int. 2025; in press. [Google Scholar] [CrossRef]
- Shuke, T.; Zhao, F.; Wu, G.; Liu, M.; Godfrey, A.; Xie, J.; Liu, X. Dowel-like Morphology of Cu2Al3 Enhances Shear Strength of Interfacial Layers in Cu-Al Composites. Acta Mater. 2025, 284, 120589. [Google Scholar]
- Zhou, J.-L.; Cheng, Y.-H.; Wan, Y.-X.; Wang, Y.-F.; Chen, Y.-X.; Liang, X.-B. Solidification characteristics and microstructure of TaNbZrTi refractory high entropy coating by extreme high-speed laser cladding. Int. J. Refract. Met. Hard Mater. 2023, 115, 106257. [Google Scholar] [CrossRef]
- Cui, N.; Zhao, T.; Wang, Z.; Zhao, Y.; Chao, Y.; Lin, H.; Li, D. Strengthening Effect on Microstructures and Properties with CuAl2 and Mg2Si in Forged 2A50 Alloy Under Different T6 Heat Treatment. J. Alloys Compd. 2025, 1010, 178310. [Google Scholar] [CrossRef]
- Wang, D.; Ding, Z.; Bao, J.; Zhao, B.; Qi, G.; Zhang, G.; Zheng, G. Friction and wear behavior of TiB2-TaC-TiC ceramic materials under high-temperature stress and oxidation conditions. Ceram. Int. 2025, 51, 290–300. [Google Scholar] [CrossRef]
- Jiang, G.-Y.; Zhang, J.-W. The influence of laser power on the microstructure and friction performance of laser-prepared TiC-NbC composite coatings on stainless steel surfaces. Mater. Today Commun. 2024, 41, 110812. [Google Scholar] [CrossRef]
- Mo, K.; Cao, L.; Zhao, D.; Long, Y.; Zhong, J.; Zhong, J.; Bi, G. Study on the microstructure and wear resistance of laser clad martensitic stainless steel 420 with different content of TiC. Mater. Today Commun. 2025, 46, 112615. [Google Scholar] [CrossRef]
- Rojacz, H.; Pichelbauer, K.; Mayrhofer, P.H. Hardmetal scrap and TiC-NiMo reinforced Fe3Al claddings: A sustainable solution with enhanced wear resistance and thermal stability. Surf. Coat. Technol. 2025, 500, 131904. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, Z.; Feng, Y.; Zhang, X.; Yang, J.; Huang, T. Comparative study on microstructure evolution, mechanical properties, and wear behavior of TiC and B4C single-reinforced and hybrid-reinforced Al–Mg–Si alloys by vacuum hot-press sintering. J. Mater. Res. Technol. 2024, 31, 2063–2076. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Zhao, Z.; Liu, C.; Lu, H.; Liu, X.; Song, X. Microstructural characteristics and wear-resistant mechanisms of WC-TiC-co coatings with varying TiC contents. Int. J. Refract. Met. Hard Mater. 2024, 122, 106730. [Google Scholar] [CrossRef]
- Cao, F.; Cui, H.; Song, X.; Gao, L.; Liu, M.; Qiao, Q.; Kong, H. Fabrication of multi-scale TiC and stainless steel composite coatings via circular oscillating laser towards superior wear and corrosion resistance of aluminum alloy. J. Mater. Sci. Technol. 2024, 177, 191–204. [Google Scholar] [CrossRef]
- Wang, R.; Ye, S.; Cheng, P.; Xie, Z.; Wang, Y.; Zhang, Y.; Li, G.; Wu, W.; Lu, X. Microstructure and wear resistance of in-situ TiC reinforced Stellite 6 coating using PTA cladding. J. Mater. Res. Technol. 2023, 27, 2656–2669. [Google Scholar] [CrossRef]
- Liu, Z.; Kong, D. Effects of TiC mass fraction on microstructure, corrosive–wear and electrochemical properties of laser cladded CoCrFeNiMo high–entropy alloy coatings. Tribol. Int. 2023, 186, 108640. [Google Scholar]
- Zhang, Y.; Liu, Y.; Wang, J.; Hu, D.; Li, J. Microstructure and wear resistance of direct laser-deposited TiC-enhanced aluminum-based composite coating for brake discs. Surf. Coat. Technol. 2023, 455, 129193. [Google Scholar] [CrossRef]
- Li, Y.; Fu, H.; Ma, T.; Wang, K.; Yang, X.; Lin, J. Microstructure and wear resistance of AlCoCrFeNi-WC/TiC composite coating by laser cladding. Mater. Charact. 2022, 194, 112479. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Meng, F.; Yu, T.; Ma, Z.; Qu, S.; Sun, Z. Effect of TiC content on the microstructure and wear performance of in situ synthesized Ni-based composite coatings by laser direct energy deposition. Surf. Coat. Technol. 2022, 444, 128678. [Google Scholar] [CrossRef]
- Gao, Z.-T.; Wang, R.-Q.; Ke, L.-C.; Liu, Y.; Gao, Z.-M.; Zhang, C.-W.; Yu, Y. Morphology distribution and corrosion resistance of in-situ TiC/CoCrFeNi high-entropy alloy coating. Mater. Charact. 2025, 224, 115078. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Zhang, J.; Yang, G.; Zhang, Y.; Wang, T.; Lei, J. Enhancement of the electrochemical corrosion resistance of Ti6Al4V alloy reinforced by nano- and micro-TiC particles through directed energy deposition. Corros. Sci. 2023, 221, 111343. [Google Scholar] [CrossRef]
- Song, H.; Shao, H.; Zhou, Y.; Zhao, C.; Chi, J.; Dong, G.; Bi, J.; Zheng, Q.; Jia, X.; Starostenkov, M.D.; et al. Effect of TiC ceramic particles and circular oscillating laser beam on corrosion resistance in fusing zone of 2195 Al-Li alloy. Corros. Sci. 2025, 252, 112986. [Google Scholar] [CrossRef]
- Li, X.; Ni, J.; Wang, Z.; Li, J.; Xu, Y.; Zhou, S.; Xu, K.; Hao, X.; Hu, C.; Na, X.; et al. Electrochemical corrosion behavior of hot-deformed NdFeB magnet with different content of nano-TiC. J. Alloys Compd. 2022, 917, 165518. [Google Scholar] [CrossRef]
- Han, B.; Zhu, S.; Dong, W.; Bai, Y.; Ding, H.; Luo, Y.; Di, P. Improved mechanical performance and electrochemical corrosion of WC-Al2O3 composite in NaCl solution by adding the TiC additives. Int. J. Refract. Met. Hard Mater. 2021, 99, 105566. [Google Scholar] [CrossRef]
- Wu, W.; Ma, L.; Huang, S.; He, W.; Song, L.; Lv, T.; Xu, Q. Comparison of electrochemical characteristics and passive film properties of selective laser melted and wrought TA15 alloys in sulfuric acid solution. Corros. Sci. 2024, 236, 112254. [Google Scholar] [CrossRef]
- Su, L.; Liu, W.; Liu, Y.; Jiang, Y.; Wang, Y.; Li, Z.; You, K.; Wang, C.; Liu, G. Dual-stage corrosion mechanisms and antibacterial enhancement of Cu-Sn-P alloys with Si and Al microalloying in simulated body fluid. Mater. Des. 2025, 251, 113635. [Google Scholar] [CrossRef]






| Element | Al | Zn | Cr | Mg | Mn | Cu | Fe | Si |
|---|---|---|---|---|---|---|---|---|
| Content | Bal. | 5.10–6.10 | 0.18–0.28 | 2.10–2.90 | 0.30 | 1.20–2.00 | 0.50 | 0.40 |
| Laser Power (W) | Spot Diameter (mm) | Scanning Speed (mm/s) | Angular Velocity (r/min) | Powder Feed (g/min) | Overlap Rate (%) |
|---|---|---|---|---|---|
| 1600 | 3 | 86.4 | 15 | 15 | 70 |
| 115.1 | 20 | ||||
| 149.7 | 26 |
| Sample | Ecorr (V) | icorr (A·cm−2) | βa (mV·dec−1) | βc (mV·dec−1) | Rp (Ω·cm2) |
|---|---|---|---|---|---|
| 86.4 mm/s | −1.0 | 1.2 × 10−4 | 196 | 118 | 280 |
| 115.1 mm/s | −0.6 | 2.8 × 10−5 | 378 | 311 | 2642 |
| 149.7 mm/s | −0.5 | 7.4 × 10−7 | 46 | 336 | 23,813 |
| Parameters | 86.4 mm/s | 115.1 mm/s | 149.7 mm/s |
|---|---|---|---|
| Rs (Ω·cm2) | 10.6 ± 0.06 | 10.7 ± 0.07 | 10.2 ± 0.07 |
| Qf (×10−3 Ω−1·cm−2·Sn) | 4.0 ± 0.45 | 2.6 ± 0.08 | 1.6 ± 0.05 |
| nf | 0.31 ± 0.01 | 0.91 ± 0.01 | 0.88 ± 0.01 |
| Rf (Ω·cm2) | 22.1 ± 1.30 | 230.5 ± 28.09 | 453.2 ± 30.12 |
| Qdl (×10−3 Ω−1·cm−2·Sn) | 3.11 ± 0.25 | 4.44 ± 0.50 | 5.10 ± 0.04 |
| ndl | 0.71 ± 0.05 | 0.54 ± 0.07 | 0.92 ± 0.04 |
| Rct (Ω·cm2) | 538 ± 86.78 | 1388 ± 106.89 | 3388 ± 179.09 |
| χ2 (10−3) | 4.22 | 1.70 | 1.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Zhou, Y.; Zuo, X. Effect of Scanning Speed on Wear and Corrosion Behaviors of High-Speed Laser-Cladded Cu-TiC Coating. Metals 2025, 15, 641. https://doi.org/10.3390/met15060641
Cheng S, Zhou Y, Zuo X. Effect of Scanning Speed on Wear and Corrosion Behaviors of High-Speed Laser-Cladded Cu-TiC Coating. Metals. 2025; 15(6):641. https://doi.org/10.3390/met15060641
Chicago/Turabian StyleCheng, Shiya, Yuankai Zhou, and Xue Zuo. 2025. "Effect of Scanning Speed on Wear and Corrosion Behaviors of High-Speed Laser-Cladded Cu-TiC Coating" Metals 15, no. 6: 641. https://doi.org/10.3390/met15060641
APA StyleCheng, S., Zhou, Y., & Zuo, X. (2025). Effect of Scanning Speed on Wear and Corrosion Behaviors of High-Speed Laser-Cladded Cu-TiC Coating. Metals, 15(6), 641. https://doi.org/10.3390/met15060641





