Comparative Analysis of Lead Ions and Ammonium Salts in Malachite Sulfurization-Assisted Flotation Based on Surface Layer Durability
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
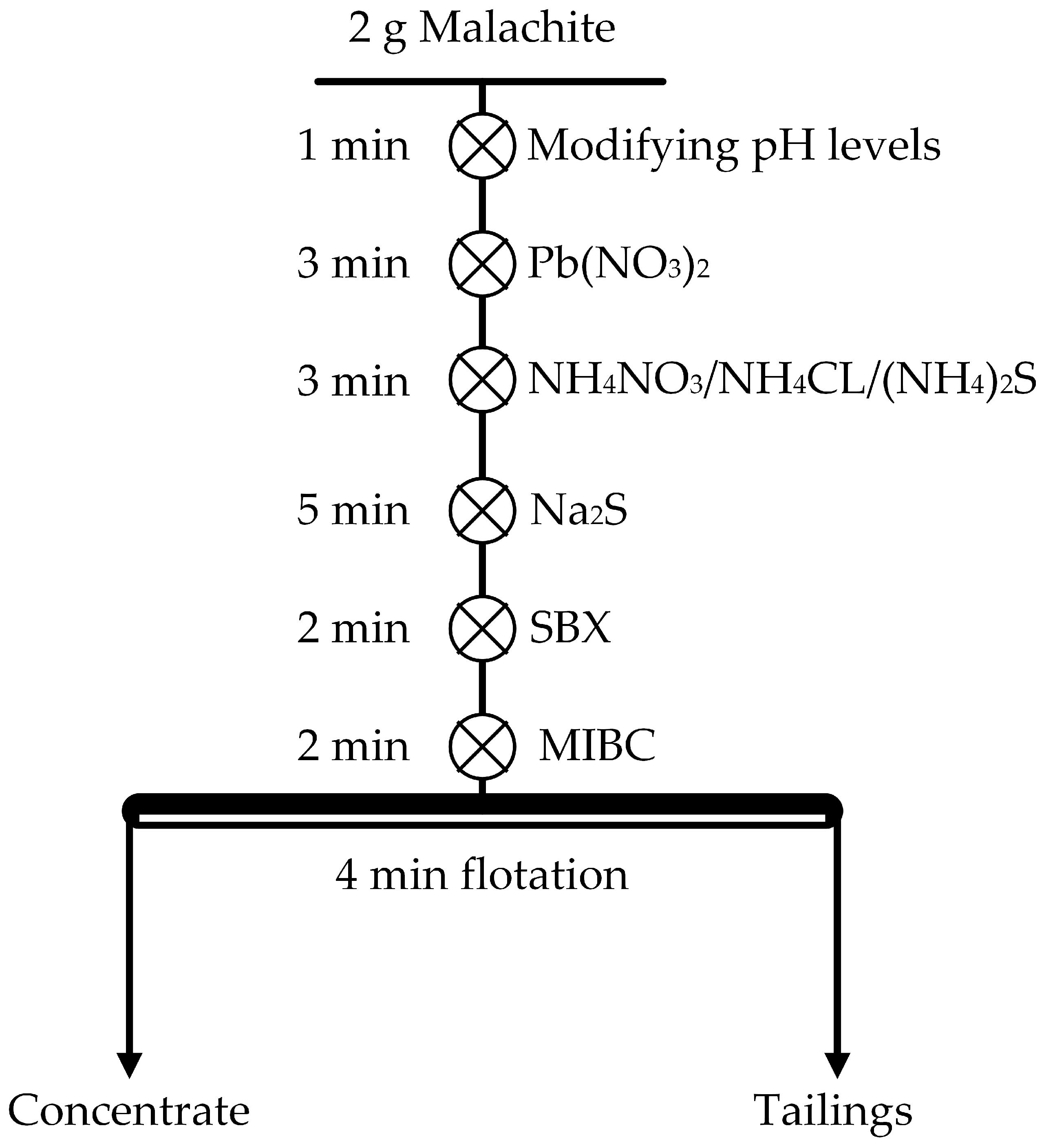
2.2. Flotation Experiments
2.3. AFM Measurements
2.4. ToF-SIMS Measurements
2.5. FESEM-EDS Measurements
2.6. FTIR Measurements
2.7. Contact Angle Measurements
3. Results and Discussion
3.1. Effect of Xanthate and Lead Ions on Single-Flotation Recovery
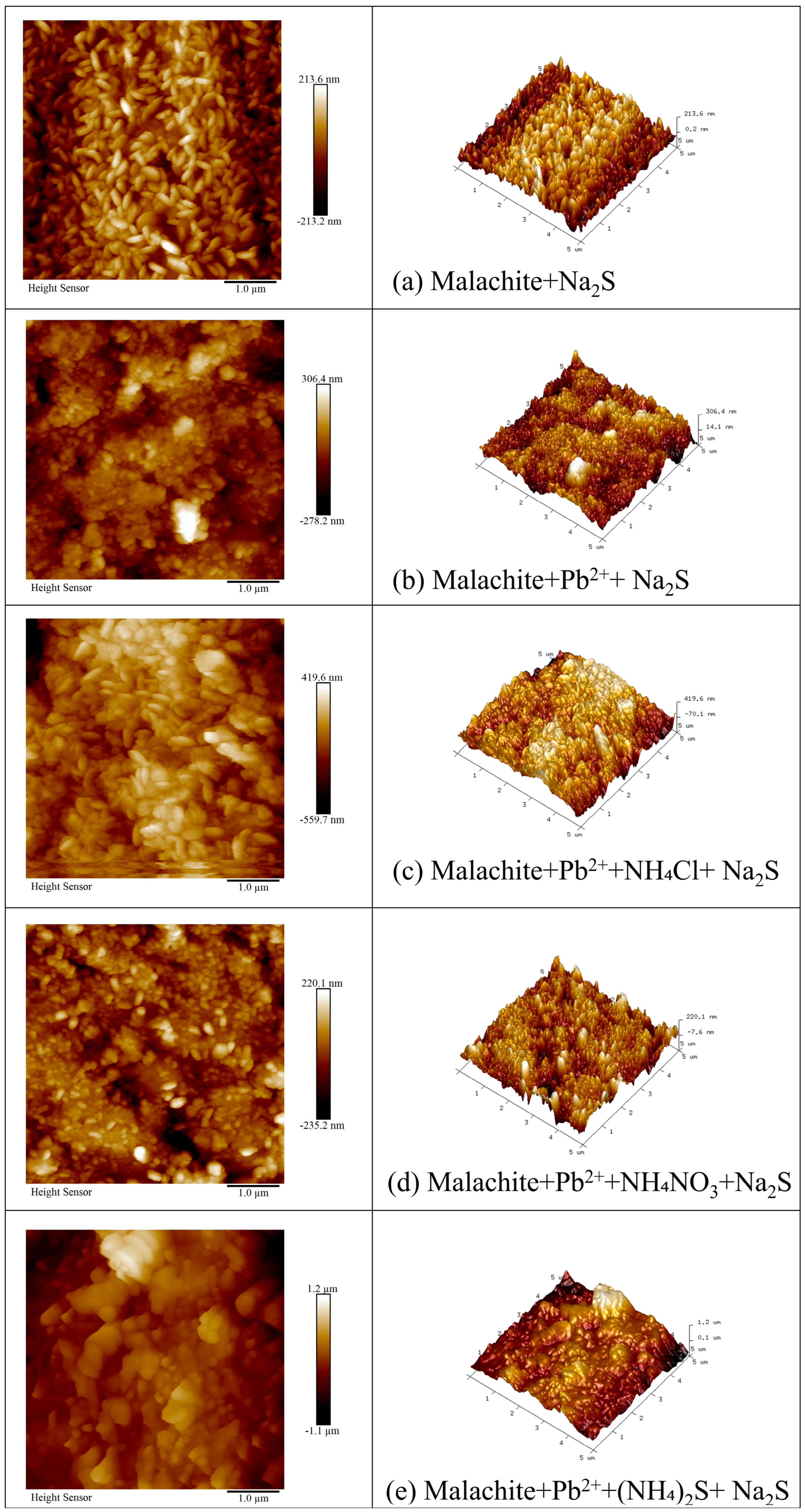
3.2. AFM Analysis
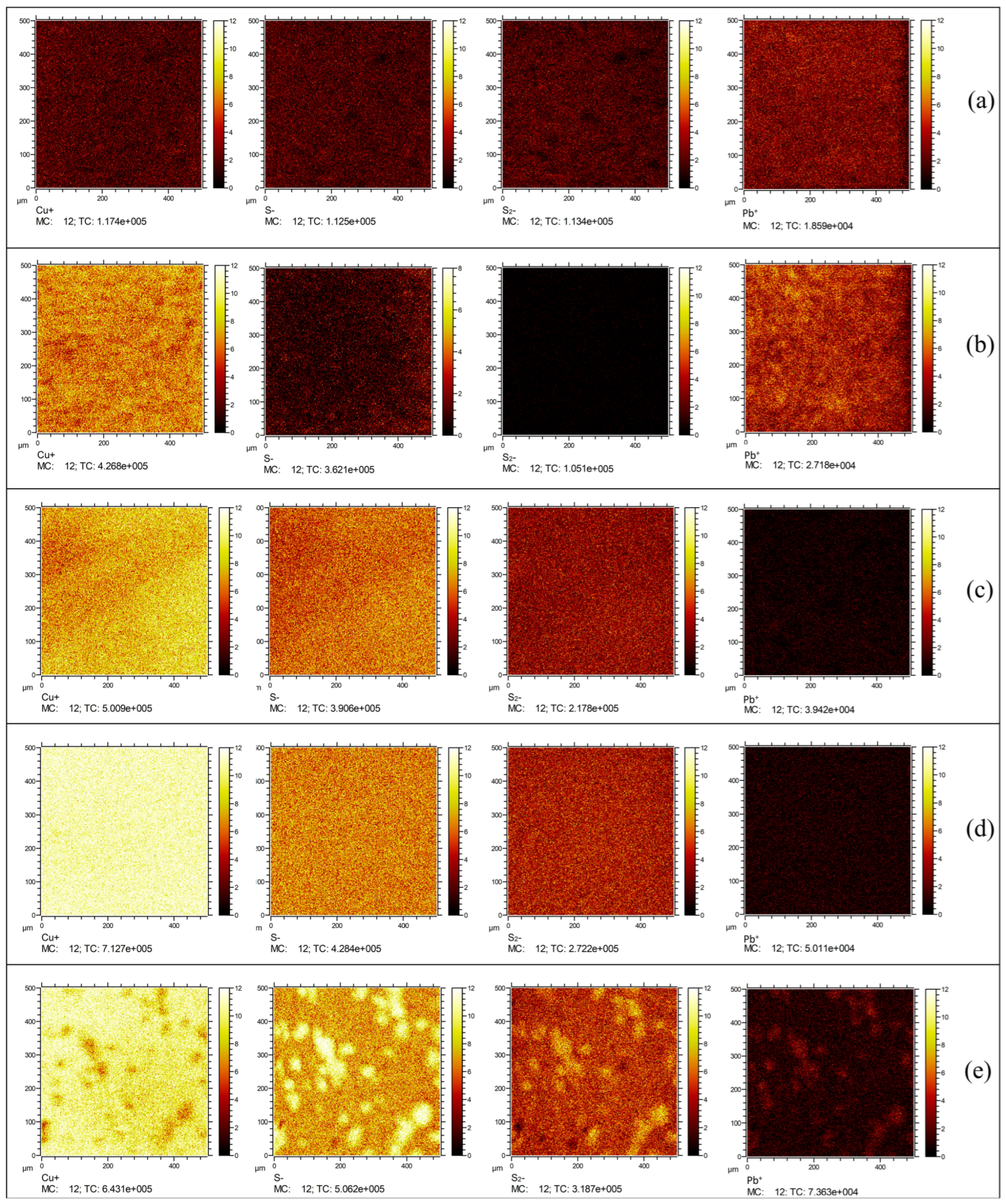
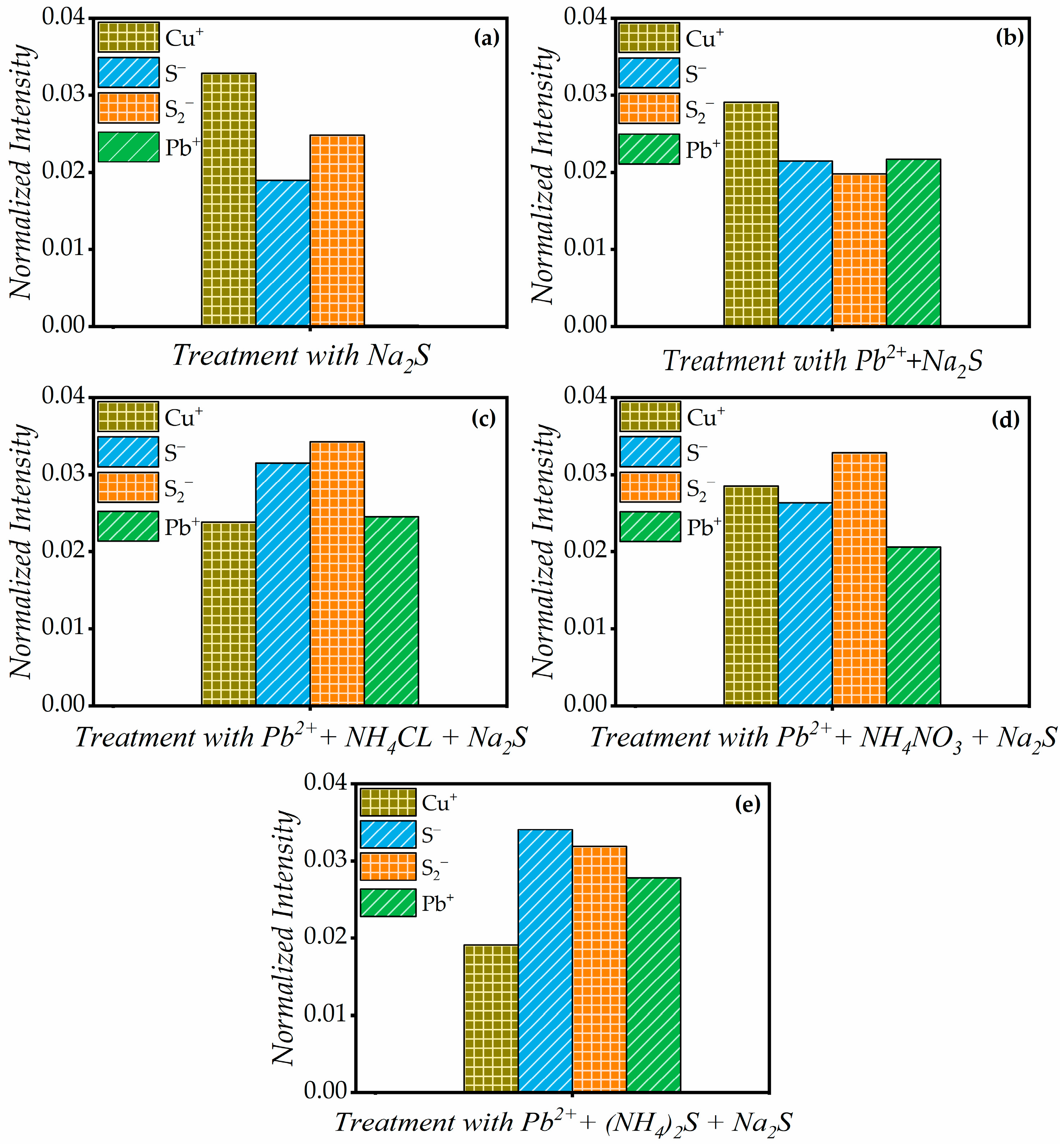
3.3. ToF-SIMS Analysis
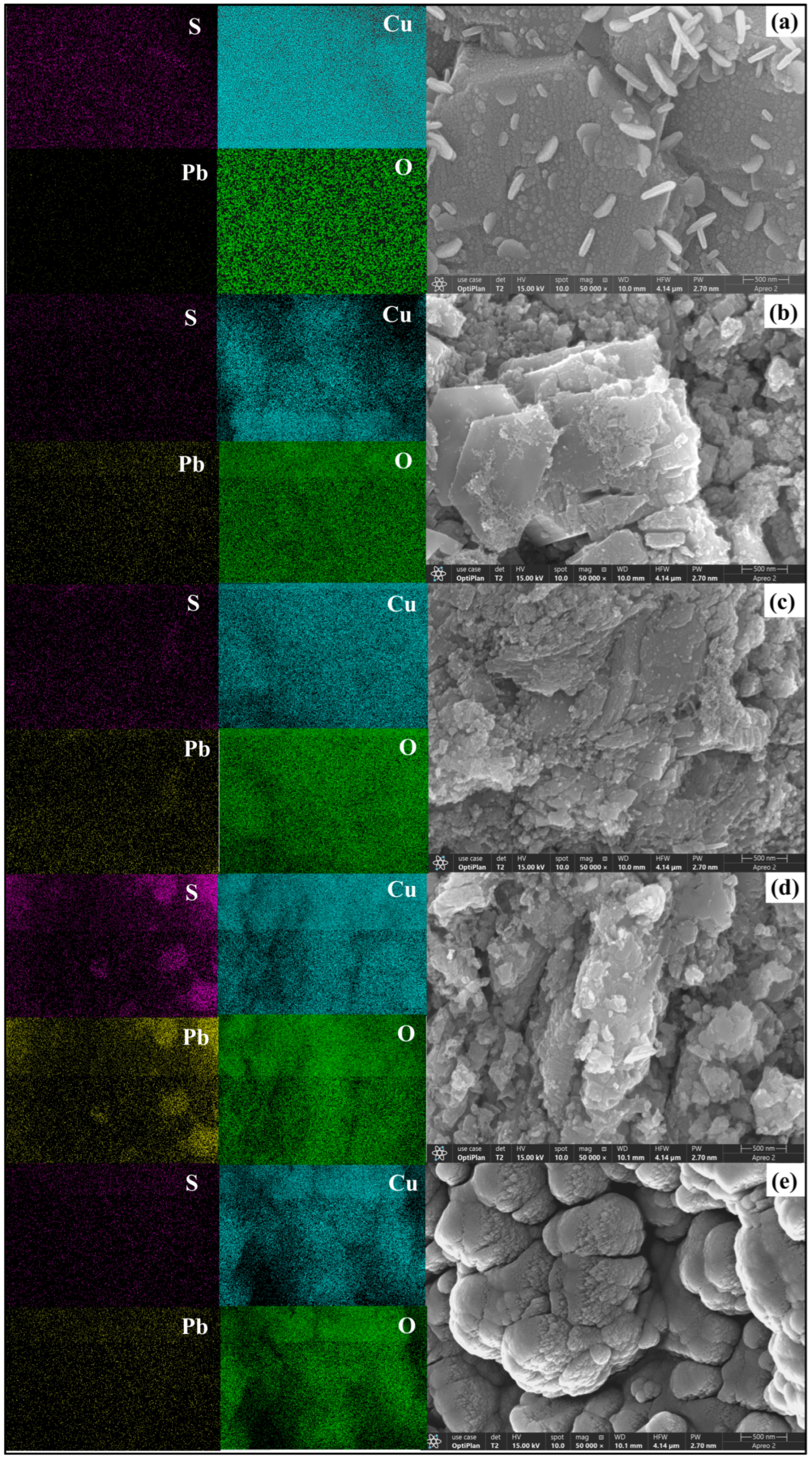
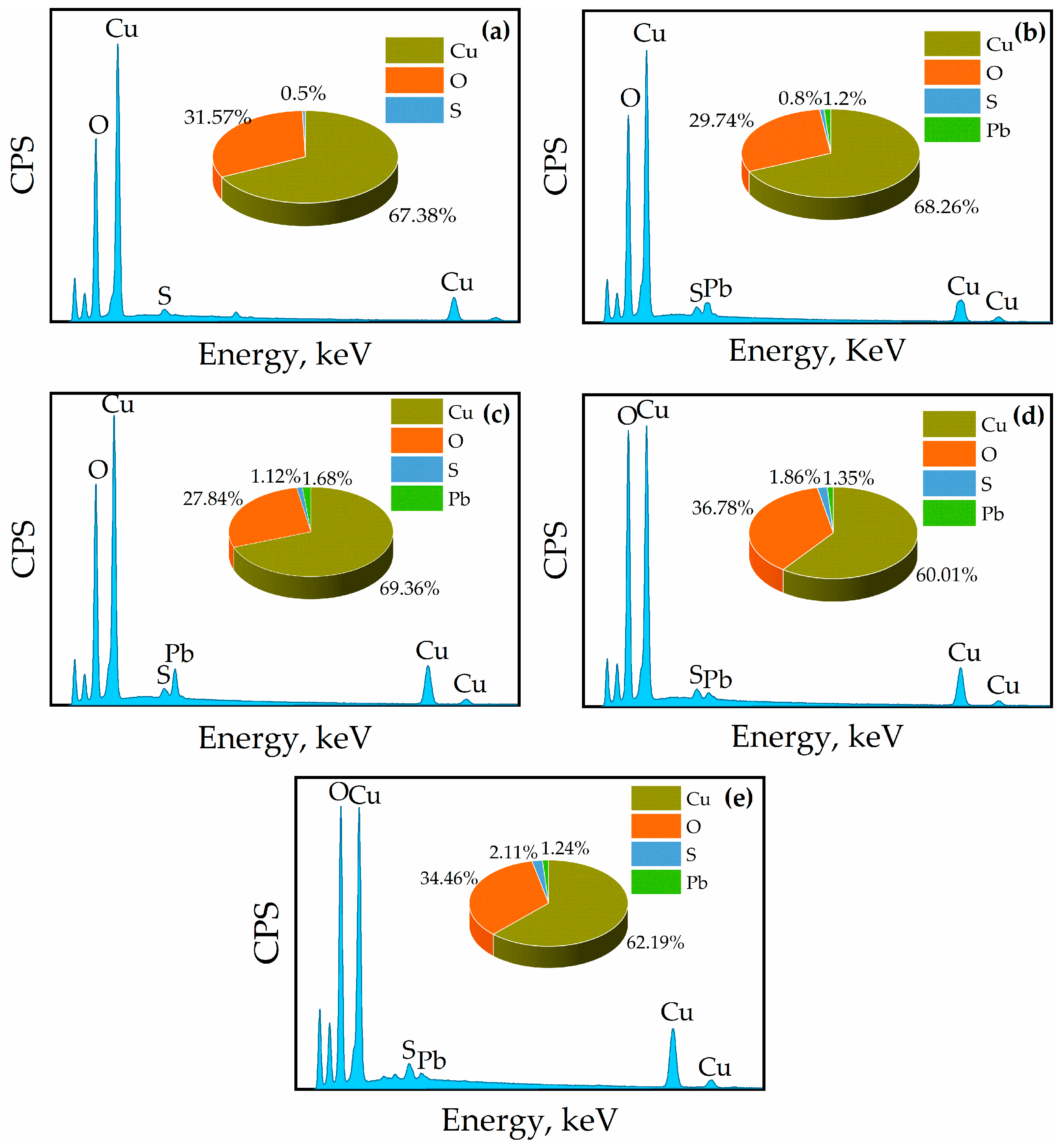
3.4. FESEM-EDS Analysis
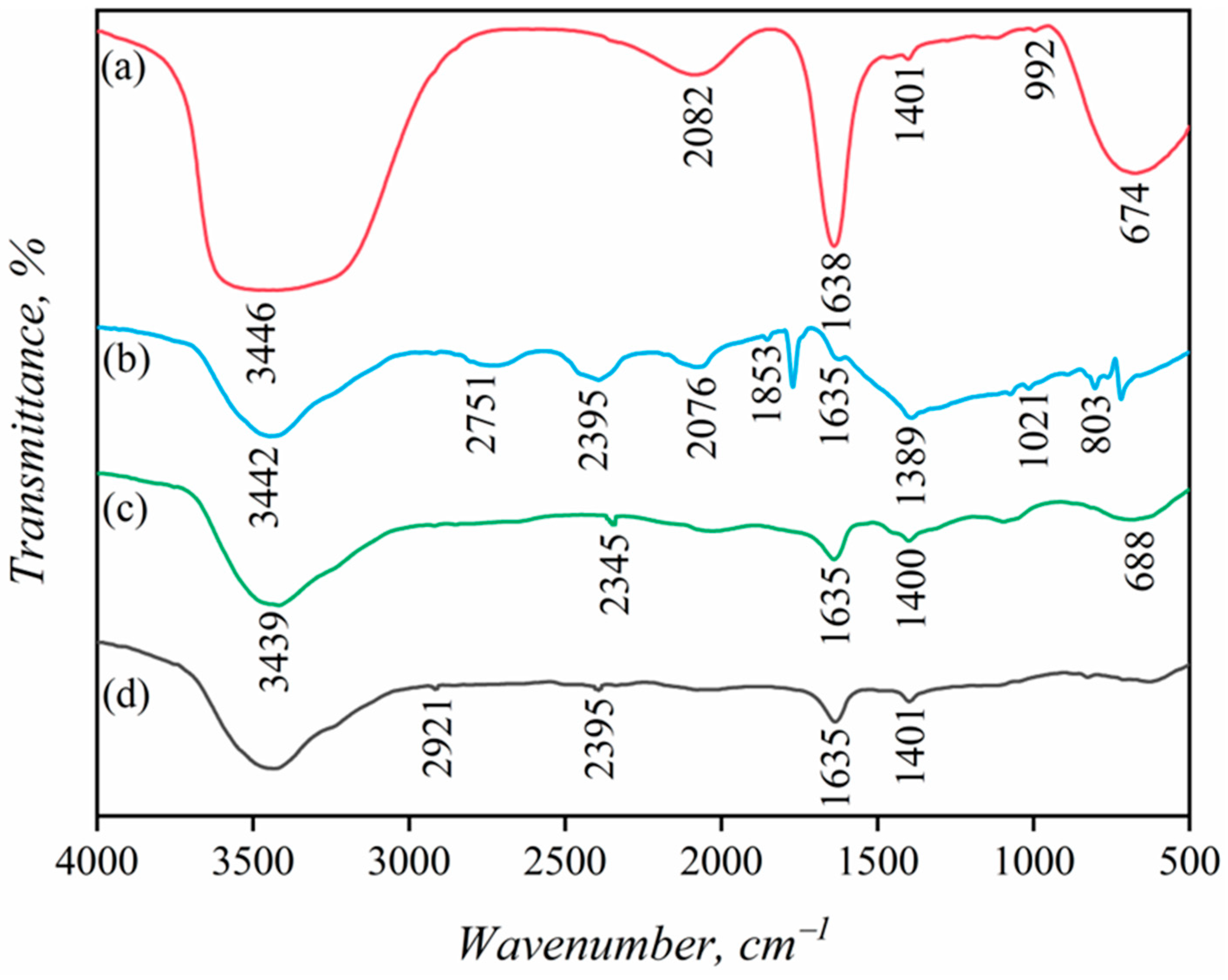
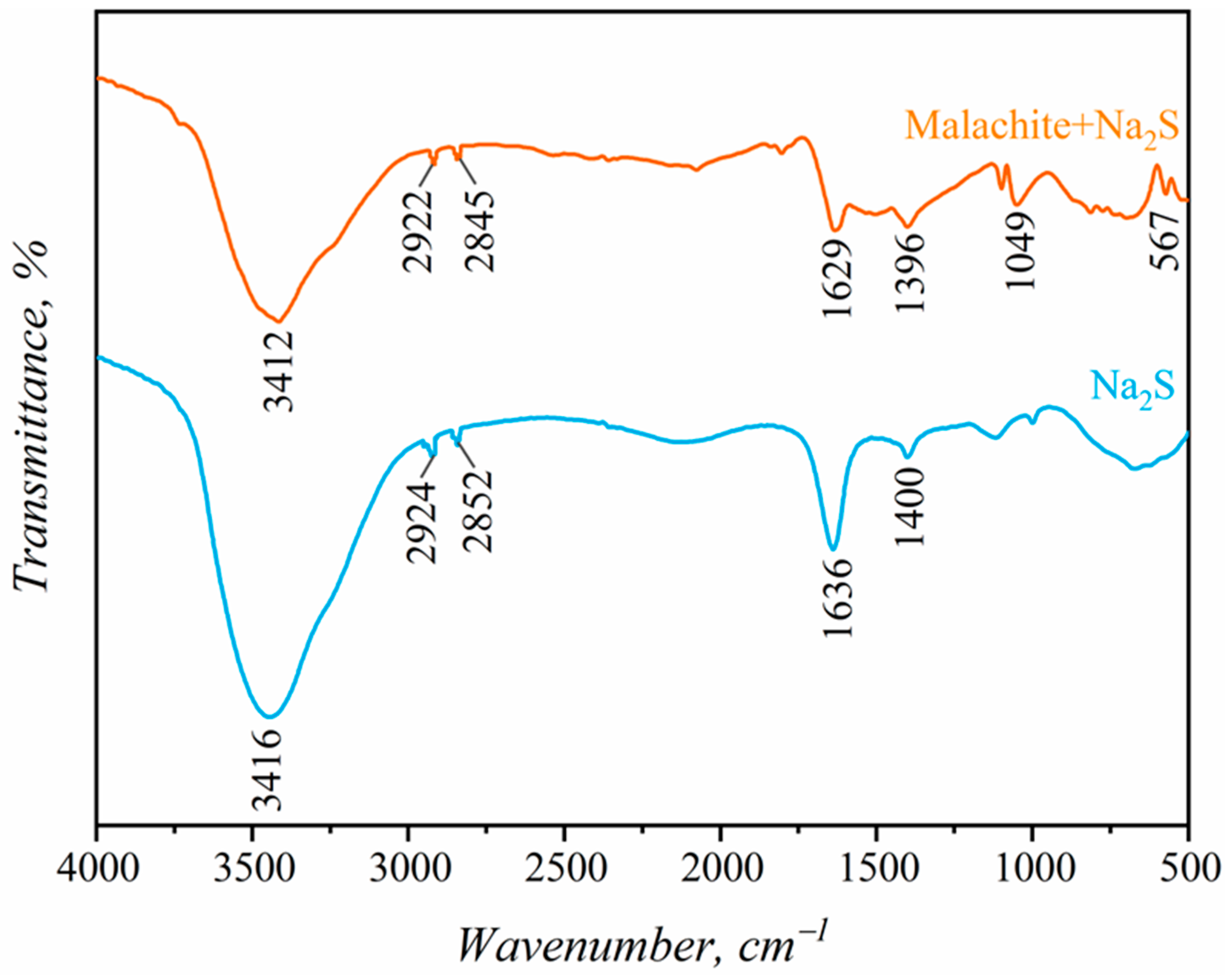
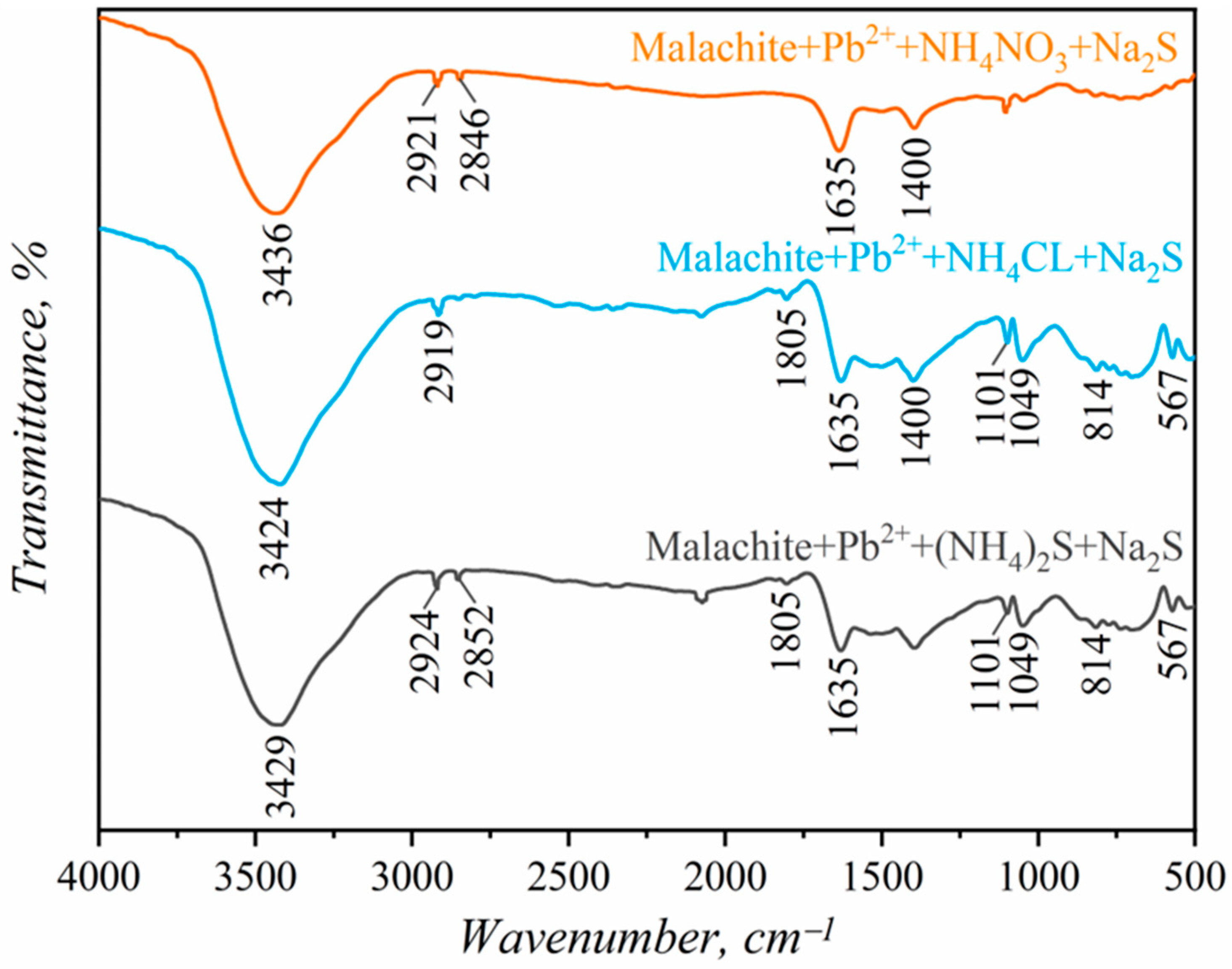
3.5. FT-IR Analysis
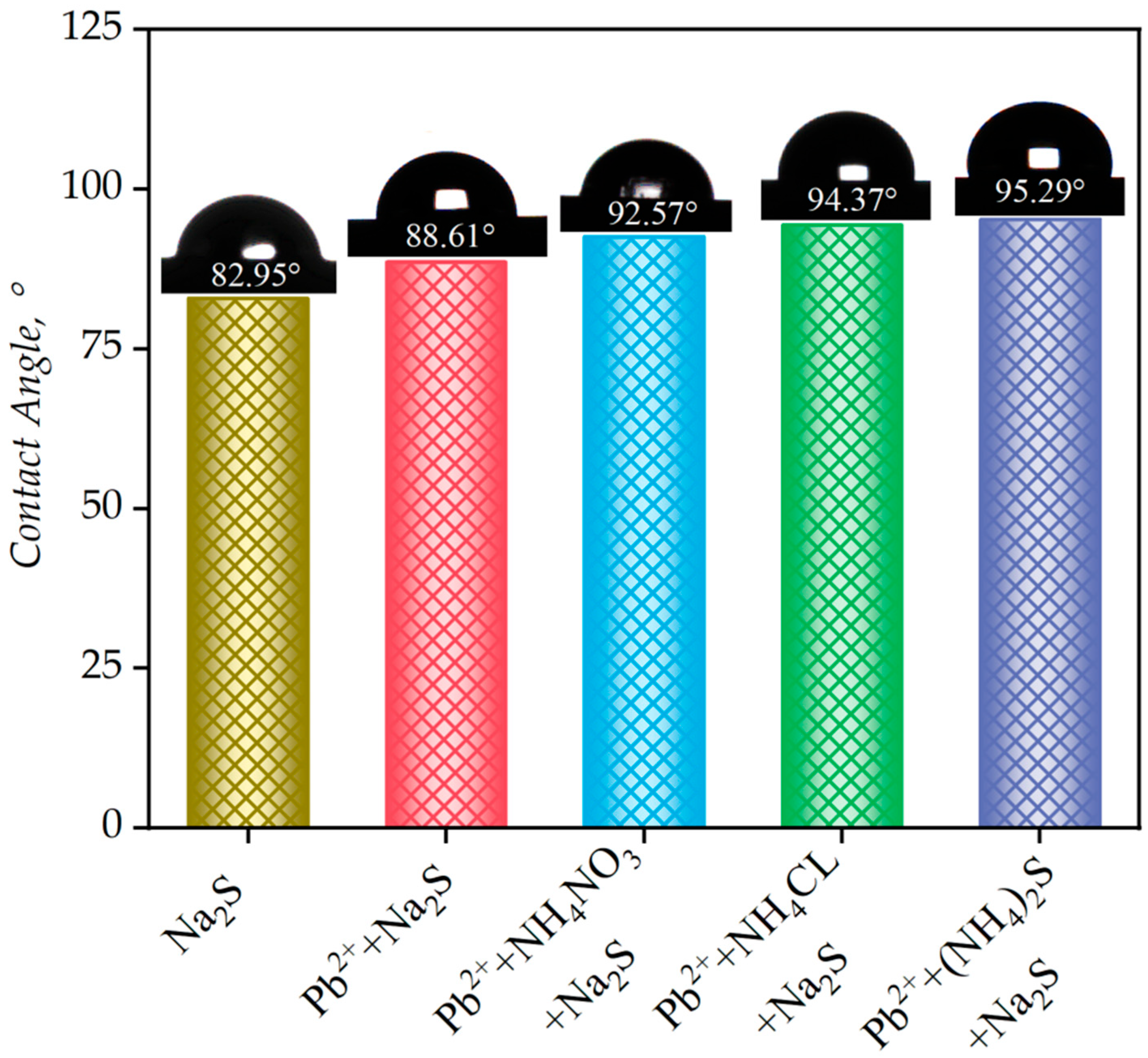
3.6. Contact Angle Analysis
4. Conclusions
- 1.
- Micro-flotation results showed a 94.6% recovery at optimal SBX concentrations, which was further improved by the Pb2+ + (NH4)2S + Na2S system, indicating superior flotation performance. Excessive Pb2+ caused over-sulfurization, reducing reactivity and efficiency. Controlling Pb2+ activation and SBX concentration is key for optimal recovery.
- 2.
- The ToF-SIMS analysis showed higher signal intensities for Cu+ and sulfur anions, suggesting more effective sulfide ion adsorption on the surface compared to traditional sulfurization. The Pb2+ + (NH4)2S + Na2S system produced significant Pb-S and Cu-S species, generated more active sites for xanthate-assisted adsorption, and promoted the growth of sulfurized species.
- 3.
- FESEM-EDS analysis revealed significant changes in the malachite surface morphology after sulfurization. The Pb2+ + NH4+ system formed dense Pb-S and Cu-S structures with increased sulfur content, enhancing sulfurization and flotation recovery. In contrast, the Pb and Cu contents decreased due to the formation of lead–sulfur and copper-sulfide compounds.
- 4.
- The contact angle measurements showed that the activation systems significantly enhanced surface hydrophobicity, with the highest contact angle resulting from the excellent durability and hydrophobicity of the copper-sulfide film, compared to traditional sulfurization.
- 5.
- FTIR analysis confirmed the formation of stable Cu-S species on the malachite surface after activation with Pb2+ and NH4+ ions, which enhances SBX adsorption and improves hydrophobicity and floatability. Key FTIR peaks for Cu-S and N=O further indicate enhanced sulfurization-assisted flotation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schipper, B.W.; Lin, H.C.; Meloni, M.A.; Wansleeben, K.; Heijungs, R.; van der Voet, E. Estimating global copper demand until 2100 with regression and stock dynamics. Resour. Conserv. Recycl. 2018, 132, 28–36. [Google Scholar] [CrossRef]
- Hu, Q.; Gu, Y. Copper economic dynamics: Navigating resource scarcity, price volatility, and green growth. Resour. Policy 2024, 89, 104462. [Google Scholar] [CrossRef]
- Ciacci, L.; Fishman, T.; Elshkaki, A.; Graedel, T.E.; Vassura, I.; Passarini, F. Exploring future copper demand, recycling and associated greenhouse gas emissions in the EU-28. Glob. Environ. Chang. 2020, 63, 102093. [Google Scholar] [CrossRef]
- Castillo, E.; Eggert, R. Reconciling Diverging Views on Mineral Depletion: A Modified Cumulative Availability Curve Applied to Copper Resources. Resour. Conserv. Recycl. 2020, 161, 104896. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, G.; Song, S.; Li, H. Interaction of gangue minerals with malachite and implications for the sulfidization flotation of malachite. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 679–684. [Google Scholar] [CrossRef]
- Jena, S.S.; Tripathy, S.K.; Mandre, N.R.; Venugopal, R.; Farrokhpay, S. Sustainable Use of Copper Resources: Beneficiation of Low-Grade Copper Ores. Minerals 2022, 12, 545. [Google Scholar] [CrossRef]
- Evans, A.M. Ore Geology and Industrial Minerals: An Introduction, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Mao, Y.B.; Deng, J.S.; Wen, S.M.; Fang, J.J. Reaction kinetics of malachite in ammonium carbamate solution. Chem. Pap. 2015, 69, 1187–1192. [Google Scholar] [CrossRef]
- Deng, J.; Lai, H.; Wen, S.; Li, S. Confirmation of interlayer sulfidization of malachite by TOF-SIMS and principal component analysis. Minerals 2019, 9, 204. [Google Scholar] [CrossRef]
- Zhou, R.; Chander, S. Kinetics of sulfidization of malachite in hydrosulfide and tetrasulfide solutions. Int. J. Miner. Process. 1993, 37, 257–272. [Google Scholar] [CrossRef]
- Zuo, Q.; Wu, D.; Cao, J.; Wang, Z.; Wen, S.; Huang, L.; Chen, H. Corrosion activation by ammonium fluoride enhances the separation of chrysocolla and quartz by sulfidation flotation. Miner. Eng. 2022, 189, 107864. [Google Scholar] [CrossRef]
- Wang, H.; Wen, S.; Liu, D.; Han, G.; Feng, Q. Surface characteristic and sulfidization-xanthate flotation behaviours of malachite as influenced by ferric ions. Colloids Surf. A Physicochem. Eng. Asp. 2023, 668, 131469. [Google Scholar] [CrossRef]
- Deepa, H.K.; Reddy, A.J.; Sharma, K. Temperature-dependent Growth Mechanism of PVP-based Copper Sulfide (CuS) Nanocomposite. J. Mines Met. Fuels 2023, 71, 58–65. [Google Scholar] [CrossRef]
- Liu, R.; Liu, D.; Li, J.; Li, J.; Liu, Z.; Jia, X.; Yang, S.; Li, J.; Ning, S. Sulfidization mechanism in malachite flotation: A heterogeneous solid-liquid reaction that yields CuxSy phases grown on malachite. Miner. Eng. 2020, 154, 106420. [Google Scholar] [CrossRef]
- Lu, W.; Wen, S.; Liu, D.; Wang, H.; Feng, Q. A novel method for improving sulfidization xanthate flotation of malachite: Copper–ammonium synergistic activation. Appl. Surf. Sci. 2023, 618, 156660. [Google Scholar] [CrossRef]
- Liu, D.; Fang, J.; Zhang, X.; Wen, S.; Wei, Z.; Bai, S.; Xu, B.; Zhang, W. Sulfidisation promotion effect of ammonium sulfate on flotation of copper oxide ore. Adv. Mater. Res. 2012, 524–527, 1109–1114. [Google Scholar] [CrossRef]
- Liu, M.; Chen, D.; Hu, B.; He, P.; Chen, Y.; Zeng, H.; Zhang, C.; Zhu, J. New insights into the activation mechanism of ammonium ions on the malachite sulfidization flotation. Miner. Eng. 2024, 205, 108452. [Google Scholar] [CrossRef]
- Wang, H.; Wen, S.; Han, G.; Feng, Q. Adsorption characteristics of Pb(II) species on the sulfidized malachite surface and its response to flotation. Sep. Purif. Technol. 2021, 264, 118440. [Google Scholar] [CrossRef]
- Muanda, M.M.; Omalanga, P.P.D. Influence of lead nitrate on sulfurizing flotation of a copper-cobalt oxide ore. Walailak J. Sci. Technol. 2021, 18, 9319. [Google Scholar] [CrossRef]
- Bai, L.; Liu, J.; Han, Y.; Jiang, K.; Zhao, W. Effects of xanthate on flotation kinetics of chalcopyrite and talc. Minerals 2018, 8, 369. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. Flotation behavior and mechanism of α-bromododecanoic acid as collector on the flotation separation of spodumene from feldspar and quartz. J. Mol. Liq. 2021, 336, 116303. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. The flotation behavior and adsorption mechanism of a new cationic collector on the separation of spodumene from feldspar and quartz. Sep. Purif. Technol. 2021, 264, 118445. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, M.; Gui, X.; Cao, Y.; Babel, B.; Rudolph, M.; Weber, S.; Kappl, M.; Butt, H.J. The application of atomic force microscopy in mineral flotation. Adv. Colloid Interface Sci. 2018, 256, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Jiao, F.; Zhu, H.; Xu, L.; Qin, W. Mitigating the negative effects of feldspar slime on spodumene flotation using mixed anionic/cationic collector. Miner. Eng. 2021, 168, 106813. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Hart, B. TOF-SIMS studies of surface chemistry of minerals subjected to flotation separation—A review. Miner. Eng. 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Yao, J.; Sun, Y.; Yin, W.; Sun, H.; Yang, S. Study on the effects of a novel imidazoline siliconophilic collector in the desilication process of magnesite: Separation experiments, adsorption mechanisms, and separation model. Sep. Purif. Technol. 2025, 353, 128461. [Google Scholar] [CrossRef]
- Yazid, H.; Bouzid, T.; Regti, A.; El Himri, M.; El Haddad, M. Experimental and DFT insights into the adsorption competition of two cationic dyes on activated carbon derived from walnut shells in aqueous solutions. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100940. [Google Scholar] [CrossRef]
- Naboulsi, A.; Haydari, I.; Bouzid, T.; Grich, A.; Aziz, F.; Regti, A.; El Himri, M.; El Haddad, M. Fixed-bed adsorption of pesticide agricultural waste using cross-linked adsorptive hydrogel composite beads. Environ. Sci. Pollut. Res. 2024, 31, 32320–32338. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, S.; Feng, Q.; Liu, Y. Activation mechanism of lead ions in the flotation of sulfidized azurite with xanthate as collector. Miner. Eng. 2021, 163, 106809. [Google Scholar] [CrossRef]
- Grich, A.; Bouzid, T.; Naboulsi, A.; Regti, A.; El Himri, M.; El Haddad, M. Synthesis and optimization of activated carbon from Doum (Chamaerops humilis) fiber via pyrolysis-assisted H3PO4 activation for removal of bisphenol A and α-Naphthol. Diam. Relat. Mater. 2024, 145, 111061. [Google Scholar] [CrossRef]
- Cram, A.G.; Davies, M.B. A Raman and IR spectrophotomeric study of some basic lead nitrate compounds and their thermal decomposition products. J. Inorg. Nucl. Chem. 1976, 38, 1111–1117. [Google Scholar] [CrossRef]
- Das, K.C.; Dhar, S.S. Rapid catalytic degradation of malachite green by MgFe2O4 nanoparticles in presence of H2O2. J. Alloys Compd. 2020, 828, 154462. [Google Scholar] [CrossRef]
- Saha, B.; Das, G. Malachite nanoparticle: A new basic hydrophilic surface for pH-controlled adsorption of bovine serum albumin with a high loading capacity. J. Phys. Chem. C 2009, 113, 15667–15675. [Google Scholar] [CrossRef]
- Krainer, S.; Hirn, U. Contact angle measurement on porous substrates: Effect of liquid absorption and drop size. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126503. [Google Scholar] [CrossRef]
- Wang, Z.; Han, G.; Feng, Q. Selective flotation of galena and sphalerite using N-(phosphonomethyl) iminodiacetic acid as an eco-friendly depressant. Green Smart Min. Eng. 2024, 1, 96–103. [Google Scholar] [CrossRef]


| Elements | Cu | Fe | Mn | Al2O3 | SiO2 | CaO | MgO | Others |
|---|---|---|---|---|---|---|---|---|
| Wt. (%) | 55.78 | 0.12 | 0.40 | 0.53 | 2.18 | 0.69 | 0.34 | 39.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.M.; Wang, H.; Shen, P.; Liu, D. Comparative Analysis of Lead Ions and Ammonium Salts in Malachite Sulfurization-Assisted Flotation Based on Surface Layer Durability. Metals 2025, 15, 601. https://doi.org/10.3390/met15060601
Ibrahim AM, Wang H, Shen P, Liu D. Comparative Analysis of Lead Ions and Ammonium Salts in Malachite Sulfurization-Assisted Flotation Based on Surface Layer Durability. Metals. 2025; 15(6):601. https://doi.org/10.3390/met15060601
Chicago/Turabian StyleIbrahim, Ayman M., Han Wang, Peilun Shen, and Dianwen Liu. 2025. "Comparative Analysis of Lead Ions and Ammonium Salts in Malachite Sulfurization-Assisted Flotation Based on Surface Layer Durability" Metals 15, no. 6: 601. https://doi.org/10.3390/met15060601
APA StyleIbrahim, A. M., Wang, H., Shen, P., & Liu, D. (2025). Comparative Analysis of Lead Ions and Ammonium Salts in Malachite Sulfurization-Assisted Flotation Based on Surface Layer Durability. Metals, 15(6), 601. https://doi.org/10.3390/met15060601








