Tunable Microwave Absorption Performance of Ni-TiN@CN Nanocomposites with Synergistic Effects from the Addition of Ni Metal Elements
Abstract
1. Introduction
2. Experimental Section
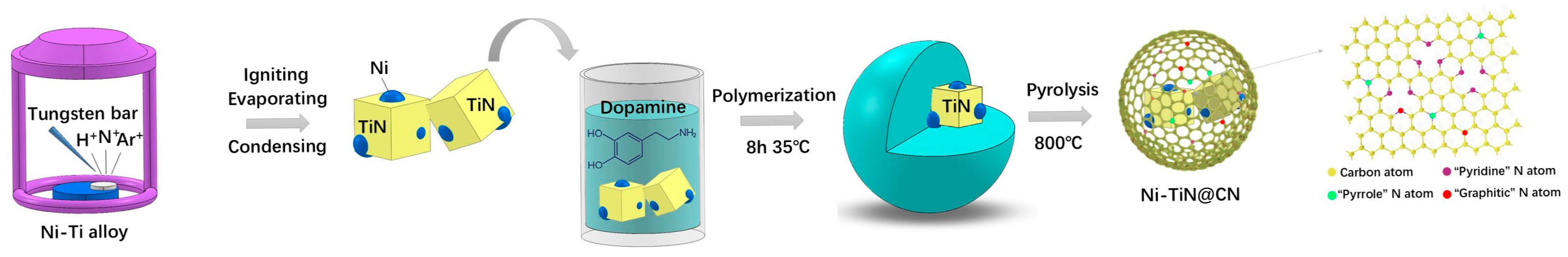
2.1. Synthesis of the Ni-TiN Nanocubes
2.2. Synthesis of Ni-TiN@CN Nanocomposites
2.3. Characterization of Ni-TiN@CN Nanocomposites
3. Results and Discussion
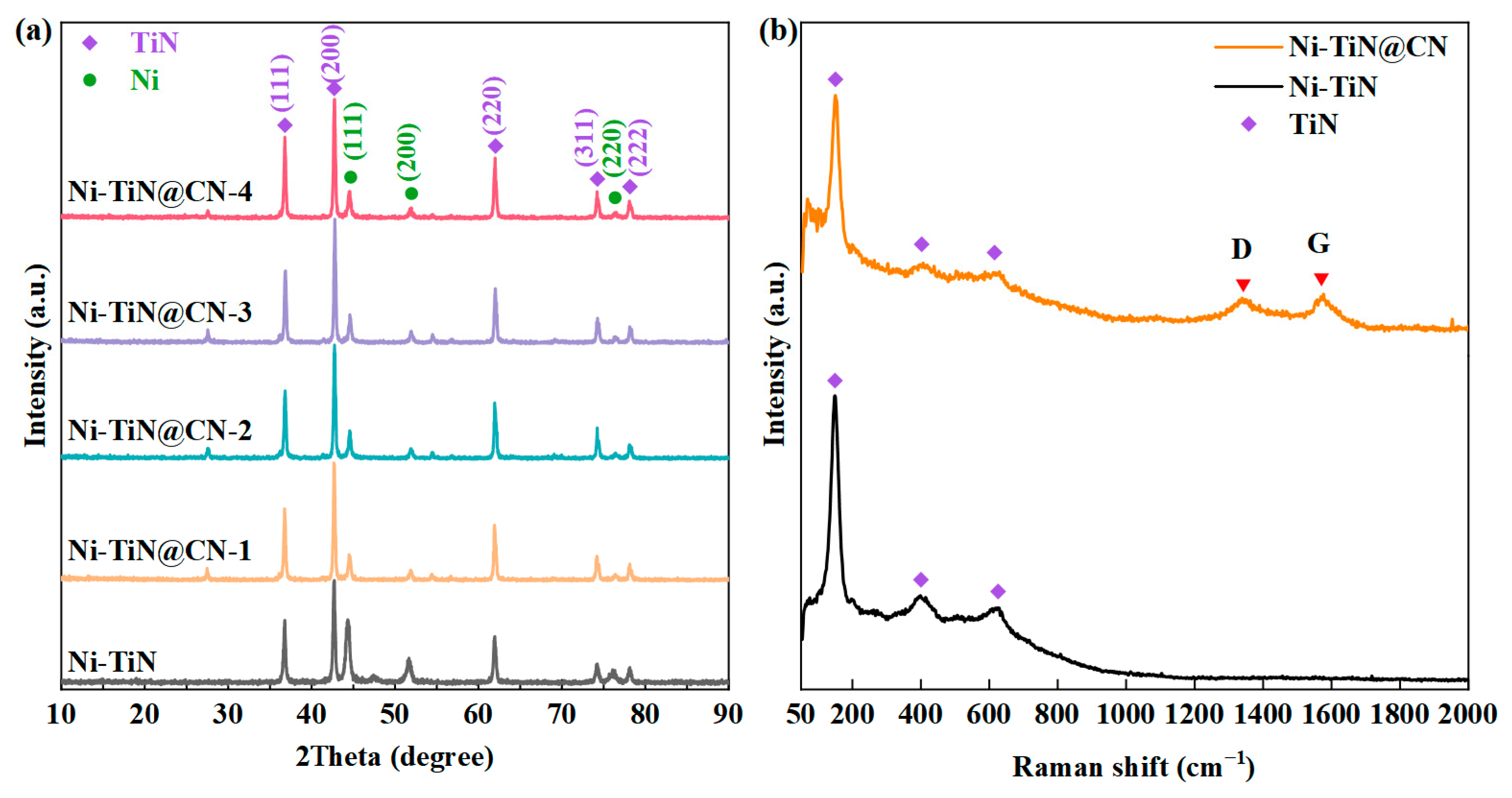
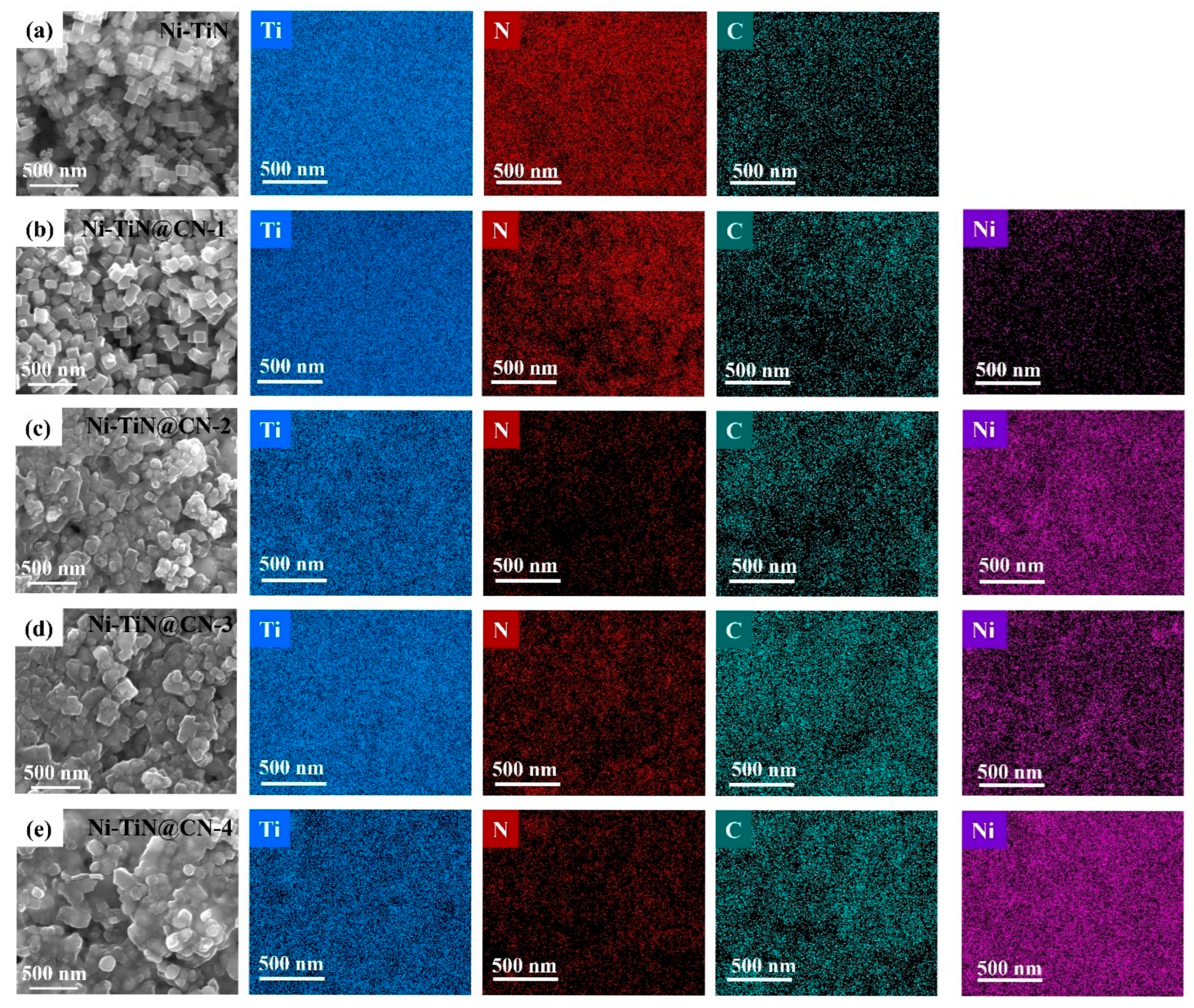
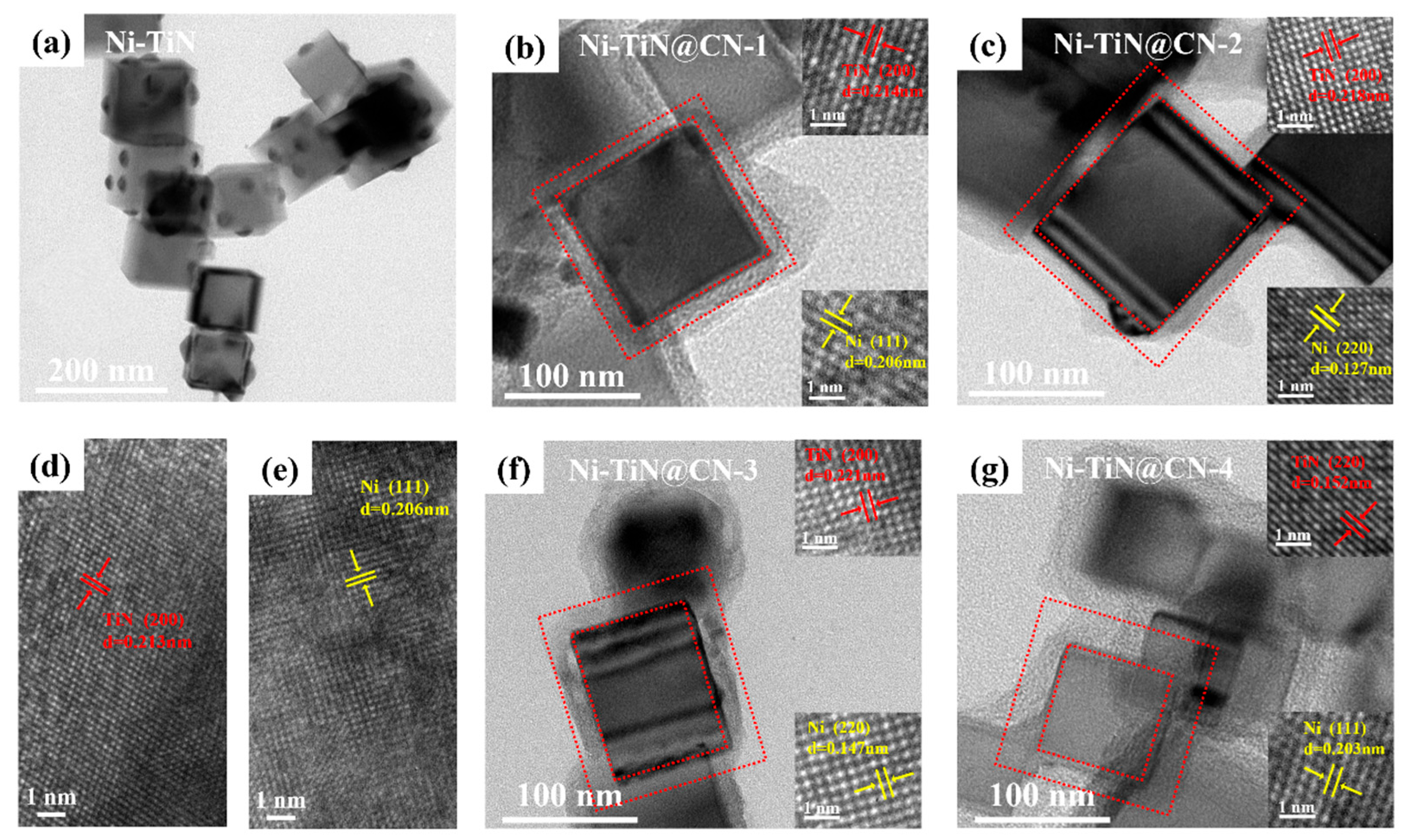
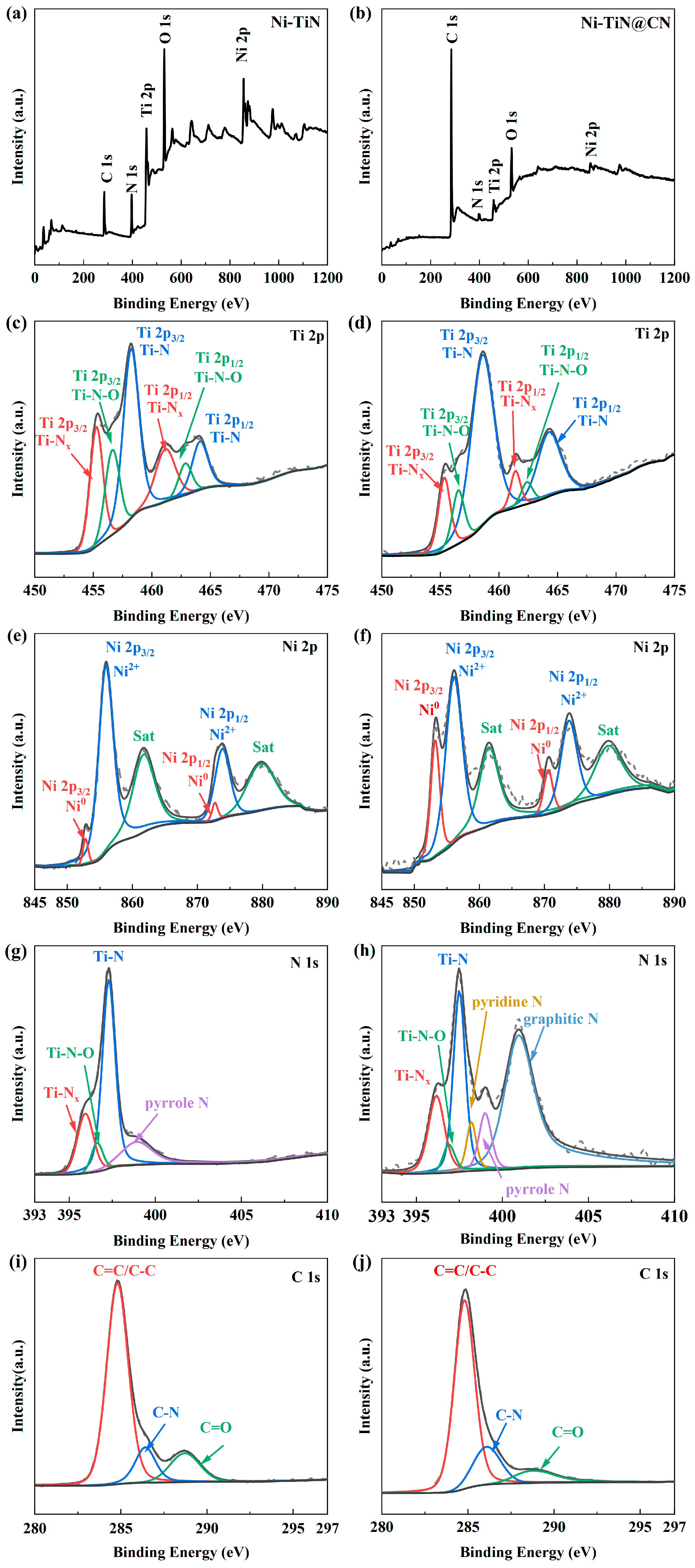
3.1. Investigation of Structure Properties
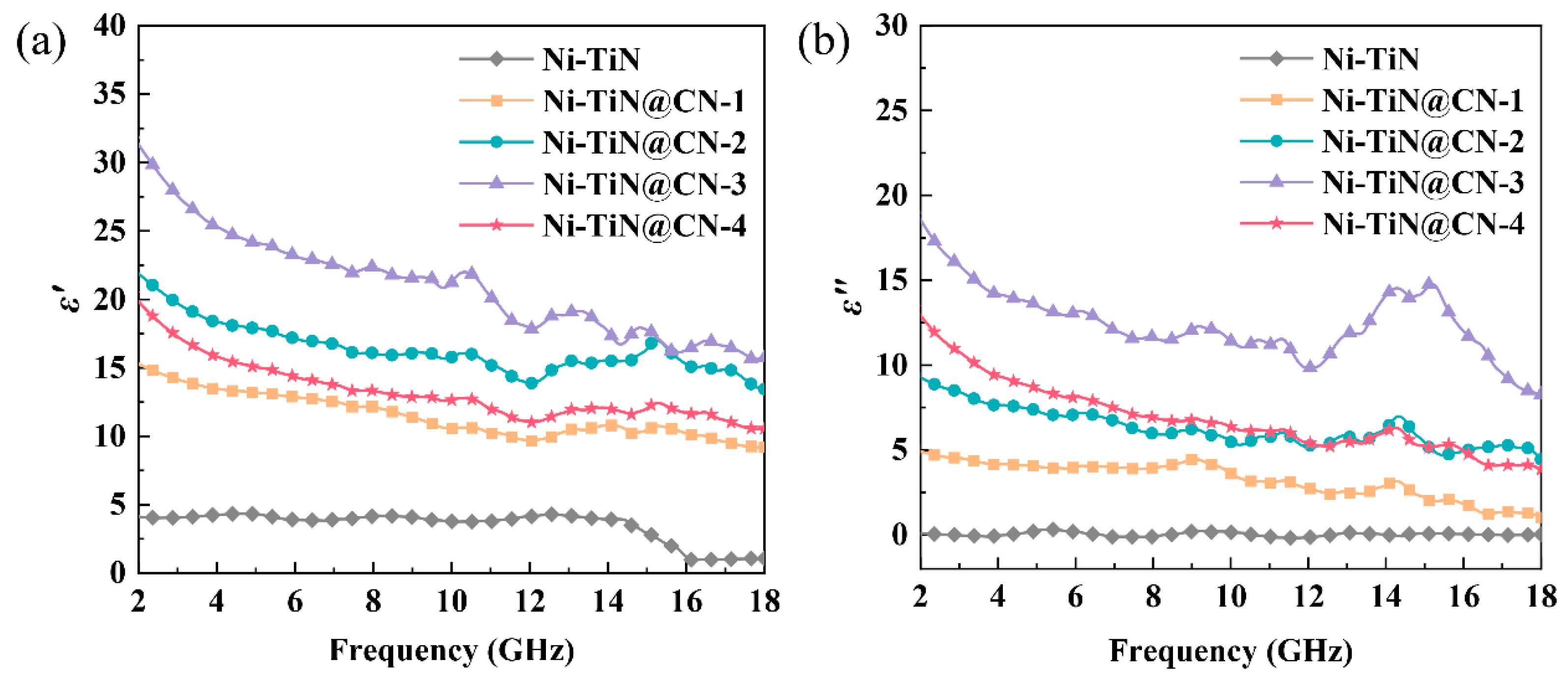
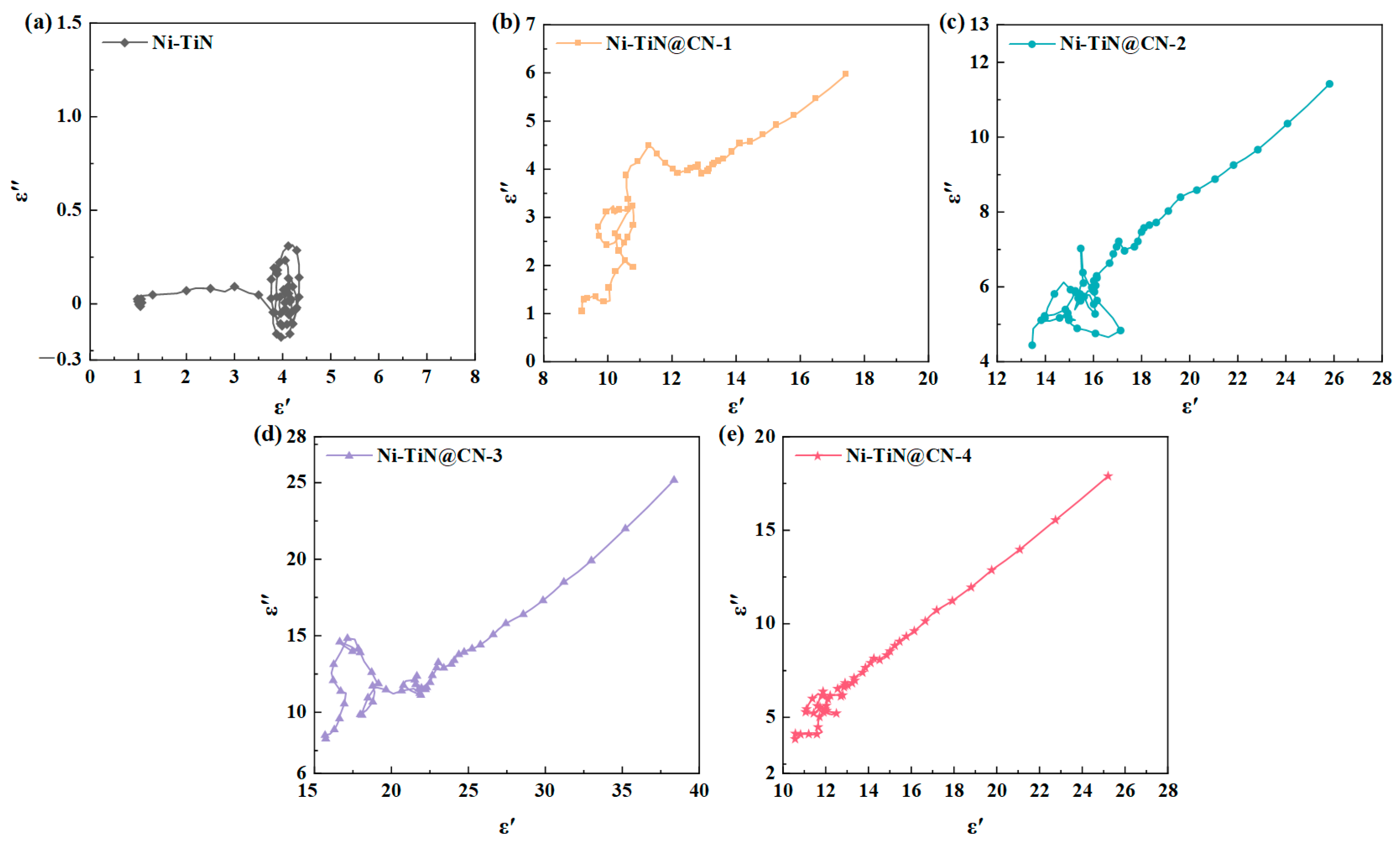
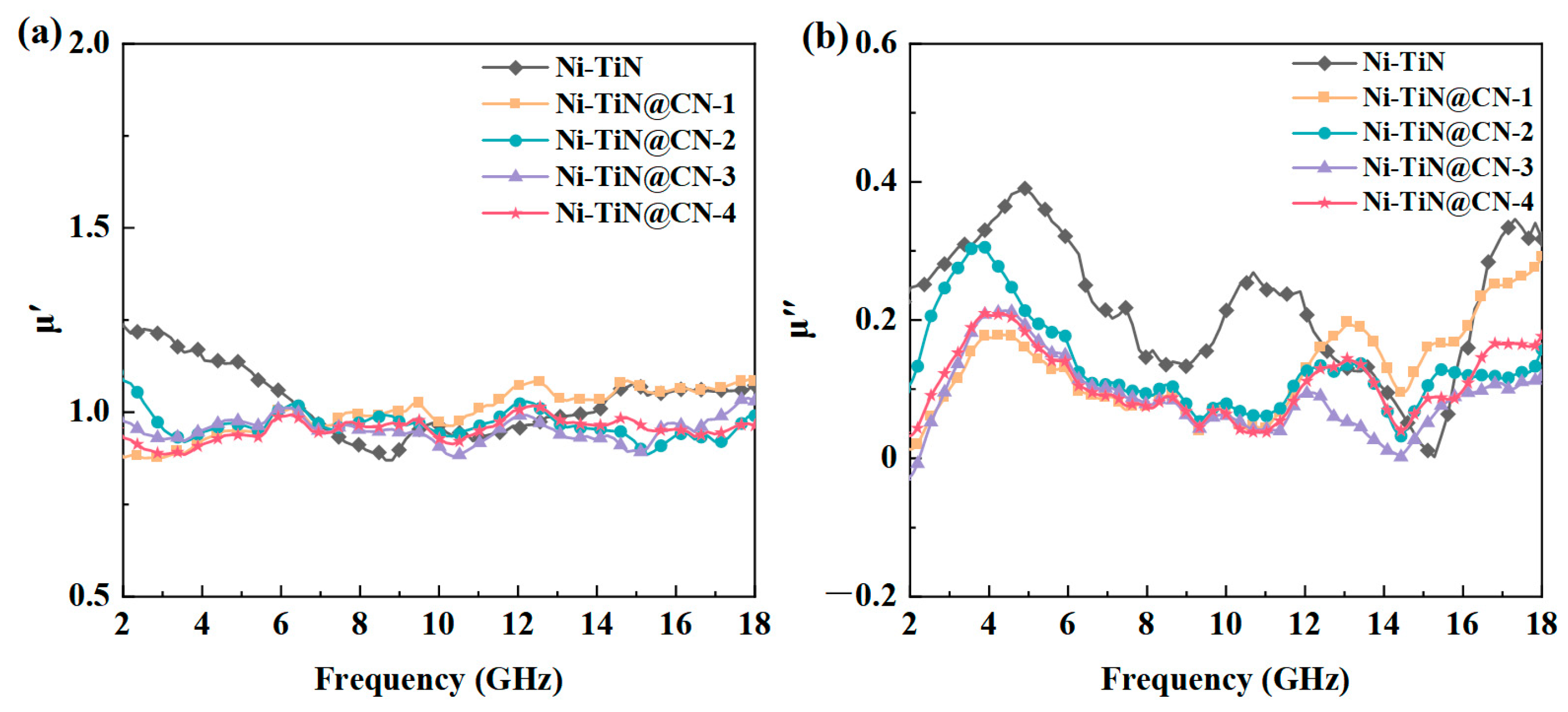
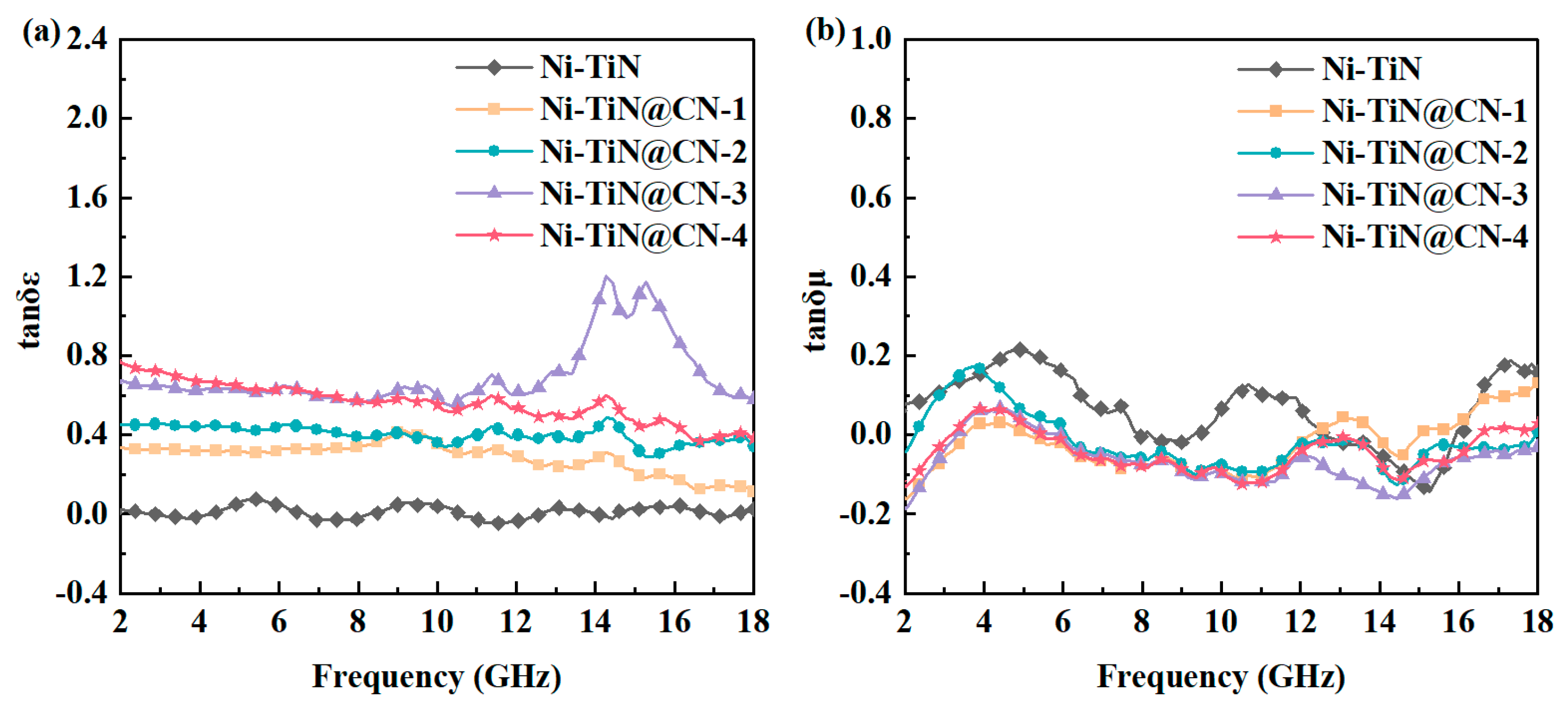
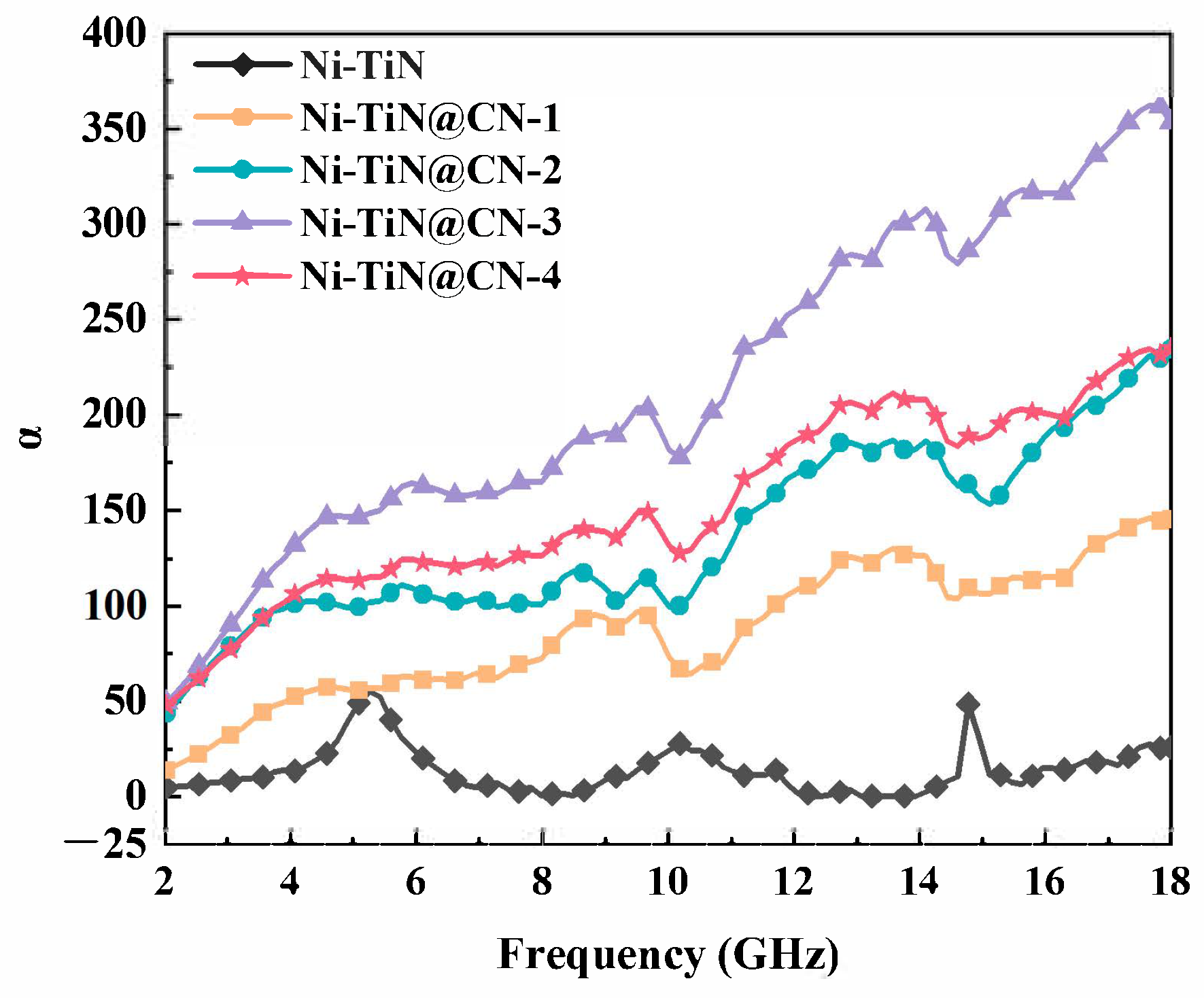
3.2. Electromagnetic Parameters and Microwave Absorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wen, X.; Qin, Y.; Hao, Y.; Chang, G.; Huang, K.; Lei, M.; Shen, X.J.; He, Y.; Pan, X.C.; Wu, Y.F. Fe-Loaded Two-Dimensional Nitrogen-Doped Carbon for Electromagnetic Wave Absorption. Chin. Phys. Lett. 2024, 41, 128401. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Bain, M.K. Electrically conductive nanocomposites of polyaniline with poly(vinyl alcohol) and methylcellulose. J. Appl. Polym. Sci. 2008, 110, 2849–2853. [Google Scholar] [CrossRef]
- Ganguly, S.; Bhawal, P.; Ravindren, R.; Das, N.C. Polymer Nanocomposites for Electromagnetic Interference Shielding: A Review. J. Nanosci. Nanotechnol. 2018, 11, 7641–7669. [Google Scholar] [CrossRef]
- Hong, X.Y.; Wang, Q.; Tang, Z.H.; Khan, W.Q.; Zhou, D.W.; Feng, T.F. Synthesis and Electromagnetic Absorbing Properties of Titanium Carbonitride with Quantificational Carbon Doping. J. Phys. Chem. C 2016, 120, 148–156. [Google Scholar] [CrossRef]
- Shafieizadegan Esfahani, A.R.; Katbab, A.A.; Dehkhoda, P.; Karami, H.R.; Barikani, M.; Sadeghi, S.H.H.; Ghorbani, A. Preparation and characterization of foamed polyurethane/silicone rubber/graphite nanocomposite as radio frequency wave absorbing material: The role of interfacial compatibilization. Environ. Sci. Technol. 2012, 72, 382–389. [Google Scholar] [CrossRef]
- Yang, S.; Sun, X.X.; Ning, Y.H.; Yuan, Y.; Luo, B.L.; Yin, W.L.; Li, Y.B. Effectively tuning electromagnetic absorption of carbon-based nanocomposites by phase transition. Carbon 2022, 190, 47–56. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Gong, C.H.; Liu, S.C.; Zhang, M.M.; Shi, Y.P.; Zhang, J.W. Fabrication of TiN/Carbon nanofibers by electrospinning and their electromagnetic wave absorption properties. J. Alloys Compd. 2018, 735, 1488–1493. [Google Scholar] [CrossRef]
- Chen, W.M.; Jin, H.C.; Xie, S.; Xie, H.Y.; Zhu, J.F.; Ji, H.X.; Wan, L.J. TiN nanocrystal anchored on N-doped graphene as effective sulfur hosts for high-performance lithium-sulfur batteries. J. Energy Chem. 2021, 54, 16–22. [Google Scholar] [CrossRef]
- Shi, H.M.; Zhang, H.; Chen, Z.; Wang, T.; Wang, L.; Zeng, W.; Zhang, G.H.; Duan, H.G. Synthesis of TiN nanostructures by Mg-assisted nitriding TiO2 in N2 for lithium ion storage. Chem. Eng. J. 2018, 336, 12–19. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, Y.; Liu, H.M.; Li, D.; Wang, Y.B.; Xu, C.C.; Tian, Y.P.; Meng, H.J. TiN/Fe2N/C composite with stable and broadband high-temperature microwave absorption. Int. J. Miner. Metall. Mater. 2024, 31, 2508–2517. [Google Scholar] [CrossRef]
- Shi, Y.P.; Li, D.; Si, H.X.; Jiang, Z.Y.; Li, M.Y.; Gong, C.H. TiN/BN composite with excellent thermal stability for efficiency microwave absorption in wide temperature spectrum. J. Mater. Sci. Technol. 2022, 130, 249–255. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhang, L.; Si, H.X.; Zhang, Y.; Li, C.P.; Zhang, L.; Zhang, J.W.; Gong, C.H. TiN nanofiber metacomposites for efficient electromagnetic wave absorption: Insights on multiple reflections and scattering effects. J. Mater. Sci. Technol. 2025, 233, 69–79. [Google Scholar] [CrossRef]
- Li, C.P.; Li, D.; Zhang, S.; Ma, L.; Zhang, L.; Zhang, J.W.; Gong, C.H. Interface Engineering of Titanium Nitride Nanotube Composites for Excellent Microwave Absorption at Elevated Temperature. Nano-Micro Lett. 2024, 16, 168. [Google Scholar] [CrossRef]
- Mohapatra, S.R.; Sahu, B.; Chandrasekhar, M.; Kumar, P.; Kaushik, S.D.; Rath, S.; Singh, A.K. Effect of cobalt substitution on structural, impedance, ferroelectric and magnetic properties of multiferroic Bi2Fe4O9 ceramics. Ceram. Int. 2016, 42, 12352–12360. [Google Scholar] [CrossRef]
- Wang, S.J.; Zhang, X.; Hao, S.Y.; Qiao, J.; Wang, Z.; Wu, L.L.; Liu, J.R.; Wang, F.L. Nitrogen-Doped Magnetic-Dielectric-Carbon Aerogel for High-Efficiency Electromagnetic Wave Absorption. Nano-Micro Lett. 2024, 16, 16. [Google Scholar] [CrossRef]
- Yu, D.; Shi, G.M.; Shi, F.N.; Bao, X.K.; Li, S.T.; Li, Q. N-doped carbon nanofiber embedded with TiN nanoparticles: A type of efficient microwave absorbers with lightweight and wide-bandwidth. J. Alloys Compd. 2022, 920, 165791. [Google Scholar] [CrossRef]
- Zhu, B.; Cui, Y.; Lv, D.F.; Xu, K.Z.; Chen, Y.J.; Wei, Y.N.; Wei, H.Y.; Bu, J.L. Synthesis of setaria viridis-like TiN fibers for efficient broadband electromagnetic wave absorption in the whole X and Ku bands. Appl. Surf. Sci. 2020, 533, 147439. [Google Scholar] [CrossRef]
- Hassel, A.W.; Neelakantan, L.; Zelenkevych, A.; Ruh, A.; Spiegel, M. Selective de-alloying of NiTi by oxochloridation. Corros. Sci. 2008, 50, 1368–1375. [Google Scholar] [CrossRef]
- Marchenko, E.S.; Shishelova, A.A.; Baigonakova, G.A.; Polyakov, N.A.; Botryakova, I.G. Mechanical properties and corrosion resistance of porous nickel titanium alloys synthesized in different reactive atmospheres. Phys. Scr. 2024, 99, 015939. [Google Scholar] [CrossRef]
- Shi, G.M.; Yin, J.C.; Li, Q.; Ji, L.; Li, S.T.; Shi, F.N. Facile preparation and properties of cubic TiN@CN nanocapsules as electrode materials for supercapacitors and as microwave absorbers. J. Mater. Sci.-Mater. Electron. 2020, 31, 10574–10584. [Google Scholar] [CrossRef]
- Xiang, M.Q.; Song, M.; Zhu, Q.S.; Yang, Y.F.; Hu, C.Q.; Liu, Z.W.; Zhao, H.D.; Ge, Y. Inducing two-dimensional single crystal TiN arrays with exposed {1 1 1} facets by a novel chemical vapor deposition with excellent electrocatalytic activity for hydrogen evolution reaction. Chem. Eng. J. 2021, 404, 126451. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, X.Q.; Zhang, Y.; Yin, D.Z.; Gong, C.H.; Yu, L.G.; Zhang, J.W.; Zhang, Z.J. Ferromagnetism and microwave electromagnetism of iron-doped titanium nitride nanocrystals. J. Phys. Chem. Lett. 2012, 116, 26006–26012. [Google Scholar] [CrossRef]
- Jaeger, D.; Patscheider, J. A complete and self-consistent evaluation of XPS spectra of TiN. J. Electron. Spectrosc. Relat. Phenom. 2012, 185, 523–534. [Google Scholar] [CrossRef]
- Chu, Y.Q.; Huang, Z.K.; Wang, X.H.; Zhou, M.L.; Zhao, F.M. Highly dispersed silver imbedded into TiN submicrospheres for electrochemical detecting of hydrogen peroxide. Sci. Rep. 2020, 10, 22126. [Google Scholar] [CrossRef]
- Haunold, T.; Rupprechter, G. LiOx-modification of Ni and Co3O4 surfaces: An XPS, LEIS and LEED study. Surf. Sci. 2021, 713, 121915. [Google Scholar] [CrossRef]
- Xu, Y.; Tu, W.G.; Zhang, B.W.; Yin, S.M.; Huang, Y.Z.; Kraft, M.; Xu, R. Nickel nanoparticles encapsulated in few-layer nitrogen-doped graphene derived from metal-organic frameworks as efficient bifunctional electrocatalysts for overall water splitting. Adv. Mater. 2017, 29, 1605957. [Google Scholar] [CrossRef]
- Milošv, I.; Strehblow, H.H.; Navinšek, B.; Metikoš-Huković, M. Electrochemical and thermal oxidation of TiN coatings studied by XPS. Surf. Interface Anal. 1995, 23, 529–539. [Google Scholar] [CrossRef]
- Li, S.T.; Shi, G.M.; Li, Q.; Shi, F.N.; Wang, X.L.; Yang, L.M. One-step synthesis and performances of Ni@CN nanocapsules with superior dual-function as electrocatalyst and microwave absorbent. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126162. [Google Scholar] [CrossRef]
- Wei, D.C.; Liu, Y.Q.; Wang, Y.; Zhang, H.L.; Huang, L.P.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Wu, G.L.; Cheng, Y.H.; Yang, Z.H.; Jia, Z.R.; Wu, H.J.; Yang, L.J.; Li, H.L.; Guo, P.Z.; Lv, H.L. Design of carbon sphere/magnetic quantum dots with tunable phase compositions and boost dielectric loss behavior. Chem. Eng. J. 2018, 333, 519–528. [Google Scholar] [CrossRef]
- Jia, Z.R.; Gao, Z.G.; Lan, D.; Lan, D.; Cheng, Y.H.; Wu, G.L.; Wu, H.J. Effects of filler loading and surface modification on electrical and thermal properties of epoxy/montmorillonite composite. Chin. Phys. B 2018, 27, 117806. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, Y.; Zhao, H.Q.; Lv, H.L.; Qi, X.D.; Cao, J.M.; Ji, G.B.; Du, Y.W. Engineering morphology configurations of hierarchical flower-like MoSe2 spheres enable excellent low-frequency and selective microwave response properties. Chem. Eng. J. 2019, 372, 390–398. [Google Scholar] [CrossRef]
- Yang, L.J.; Huang, K.M.; Yang, X.Q. Dielectric properties of N,N-Dimethylformamide aqueous solutions in external electromagnetic fields by molecular dynamics simulation. J. Mater. Chem. A 2009, 114, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.F.; Zhang, S.Z.; Chu, W.S.; Li, H.; Chen, Y.J.; Chen, B.Q.; Chen, B.B.; Liu, H.Z. Tailoring conductive network nanostructures of ZIF-derived cobalt-decorated N-doped graphene/carbon nanotubes for microwave absorption applications. J. Colloid. Interface Sci. 2021, 591, 463–473. [Google Scholar] [CrossRef]
- Murugaraj, P.; Mainwaring, D.E.; Tonkin, D.C.; Kobaisi, M.A. Probing the dynamics of water in chitosan polymer films by dielectric spectroscopy. J. Appl. Polym. Sci. 2010, 120, 1307–1315. [Google Scholar] [CrossRef]
- Quan, B.; Liang, X.H.; Ji, G.B.; Cheng, Y.; Liu, W.; Ma, J.N.; Zhang, Y.N.; Li, D.R.; Xu, G.Y. Dielectric polarization in electromagnetic wave absorption: Review and perspective. J. Alloys Compd. 2017, 728, 1065–1075. [Google Scholar] [CrossRef]
- Liu, C.Y.; Xu, Q.K.; Tang, Y.; Wang, Z.R.; Ma, R.Y.; Ma, N.; Du, P.Y. Zr4+ doping-controlled permittivity and permeability of BaFe12-xZrxO19 and the extraordinary EM absorption power in the millimeter wavelength frequency range. J. Mater. Chem. C 2016, 40, 9532–9543. [Google Scholar] [CrossRef]
- Xu, P.; Han, X.J.; Wang, C.; Zhou, D.H.; Lv, Z.S.; Wen, A.H.; Wang, X.H.; Zhang, B. Synthesis of electromagnetic functionalized Nickel/Polypyrrole Core/Shell composites. J. Phys. Chem. B 2008, 112, 10443–10448. [Google Scholar] [CrossRef]
- Jiang, W.C.; Sun, M.X.; Zhang, K.; Wu, F.; Xie, A.M.; Wang, M.Y. Three-dimensional (3D) α-Fe2O3/polypyrrole (PPy) nanocomposite for effective electromagnetic absorption. Aip Adv. 2016, 6, 065021. [Google Scholar] [CrossRef]
- Kong, L.; Yin, X.W.; Yuan, X.Y.; Zhang, Y.J.; Liu, X.M.; Cheng, L.F.; Zhang, L.T. Electromagnetic wave absorption properties of graphene modified with carbon nanotube/poly(dimethyl siloxane) composites. Carbon 2014, 73, 185–193. [Google Scholar] [CrossRef]
- Li, D.R.; Liang, X.H.; Quan, B.; Cheng, Y.; Ji, G.B.; Du, Y.W. Investigating the synergistic impedance match and attenuation effect of Co@C composite through adjusting the permittivity and permeability. Mater. Res. Express 2017, 4, 035604. [Google Scholar] [CrossRef]
- Yan, L.L.; Zhang, M.; Zhao, S.C.; Sun, T.J.; Zhang, B.; Cao, M.S.; Qin, Y. Wire-in-tube ZnO@carbon by molecular layer deposition: Accurately tunable electromagnetic parameters and remarkable microwave absorption. Chem. Eng. J. 2020, 382, 122860. [Google Scholar] [CrossRef]
- Bai, Y.W.; Shi, G.M.; Gao, J.; Shi, F.N. Synthesis, crystal structure of a iron-manganese bimetal MOF and its graphene composites with enhanced microwave absorption properties. J. Phys. Chem. Solids 2021, 148, 109657. [Google Scholar] [CrossRef]
- Sun, M.X.; Xiong, Z.M.; Zhang, Z.W.; Chen, C.C.; Qin, L.; Wang, D.R.; Wu, F.; Liu, P.B. One-dimensional Ag@NC-Co@NC composites with multiphase core-shell hetero-interfaces for boosting microwave absorption. Compos. Sci. Technol. 2022, 228, 109663. [Google Scholar] [CrossRef]
- Wang, H.C.; Yan, Z.R.; An, J.; He, J.; Hou, Y.L.; Yu, H.Y.; Ma, N.; Yu, G.H.; Sun, D.B. Iron cobalt/polypyrrole nanoplates with tunable broadband electromagnetic wave absorption. RSC Adv. 2016, 6, 92152–92158. [Google Scholar] [CrossRef]
- Liao, X.F.; Peng, J.H.; Zhang, L.B.; Hu, T.; Li, J. Enhanced carbothermic reduction of ilmenite placer by additional ferrosilicon. J. Alloys Compd. 2017, 708, 1110–1116. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Q.; Fu, Y.; Liu, T. A review on carbon/magnetic metal composites for microwave absorption. J. Mater. Sci. Technol. 2021, 86, 91–109. [Google Scholar] [CrossRef]
- Gao, S.; Yang, H.Y.; Wang, G.; Yin, P. Excellent electromagnetic wave absorbing properties of two-dimensional carbon-based nanocomposite supported by transition metal carbides Fe3C. Carbon 2020, 162, 438–444. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, G.; Liu, L.; Guo, L.; Yu, S. Binary synergistic enhancement of dielectric and microwave absorption properties: A composite of arm symmetrical PbS dendrites and polyvinylidene fluoride. Nano Res. 2016, 10, 284–294. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.F.; Ren, Y.J.; Zheng, Q.; Wang, L.J.; Jiang, W. Anionic MOF derived Bimetallic NixCoy@Nano-porous carbon composites toward strong and efficient electromagnetic wave absorption. J. Mater. 2022, 8, 852–862. [Google Scholar] [CrossRef]
- Shi, G.M.; Lv, S.H.; Cheng, X.B.; Wang, X.L.; Li, S.T. Enhanced microwave absorption properties of modified Ni@C nanocapsules with accreted N doped C shell on surface. J. Mater. Sci.-Mater. Electron. 2018, 29, 17483–17492. [Google Scholar] [CrossRef]
- He, J.; Shan, D.Y.; Yan, S.Q.; Luo, H.; Cao, C.; Peng, Y.H. Magnetic FeCo nanoparticles-decorated Ti3C2 MXene with enhanced microwave absorption performance. J. Magn. Magn. Mater. 2019, 492, 165639. [Google Scholar] [CrossRef]
- Kuchi, R.; Nguyen, H.M.; Dongquoc, P.C.; Van, H.A.; Viet, D.D.; Kim, D.D.; Kim, J.J. Optimization of FeNi/SWCNT composites by a simple co-arc discharge process to improve microwave absorption performance. J. Alloys Compd. 2021, 852, 156712. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Meng, H.J.; Shi, Y.P.; Zhang, X.F.; Liu, C.X.; Wang, Y.; Gong, C.H.; Zhang, J.W. TiN/Ni/C ternary composites with expanded heterogeneous interfaces for efficient microwave absorption. Compos. Part B 2020, 193, 108028. [Google Scholar] [CrossRef]
- Dong, S.X.; Li, J.; Li, N.; Zhang, S.; Li, B.; Zhang, Q.L.; Ge, L.Q. Enhanced broadband microwave absorption of Fe/C core-shell nanofibers in X and Ku bands. Ceram. Int. 2023, 49, 8181–8189. [Google Scholar] [CrossRef]
- Gou, G.J.; Hua, W.L.; Liu, K.Y.; Cheng, F.; Xie, X.L. Bimetallic MOF@wood-derived hierarchical porous carbon composites for efficient microwave absorption. Diam. Relat. Mater. 2024, 141, 110688. [Google Scholar] [CrossRef]
- Sun, G.H.; Jia, Z.R.; Xu, S.; Hu, D.Q.; Zhang, C.H.; Wu, G.L. Synergistic regulation of dielectric-magnetic dual-loss and triple heterointerface polarization via magnetic MXene for high-performance electromagnetic wave absorption. J. Mater. Sci. Technol. 2022, 113, 128–137. [Google Scholar] [CrossRef]
- Li, W.X.; Qi, H.X.; Guo, F.; Du, Y.; Song, N.J.; Liu, Y.Y.; Chen, Y.Q. Co nanoparticles supported on cotton-based carbon fibers: A novel broadband microwave absorbent. J. Alloys Compd. 2019, 772, 760–769. [Google Scholar] [CrossRef]
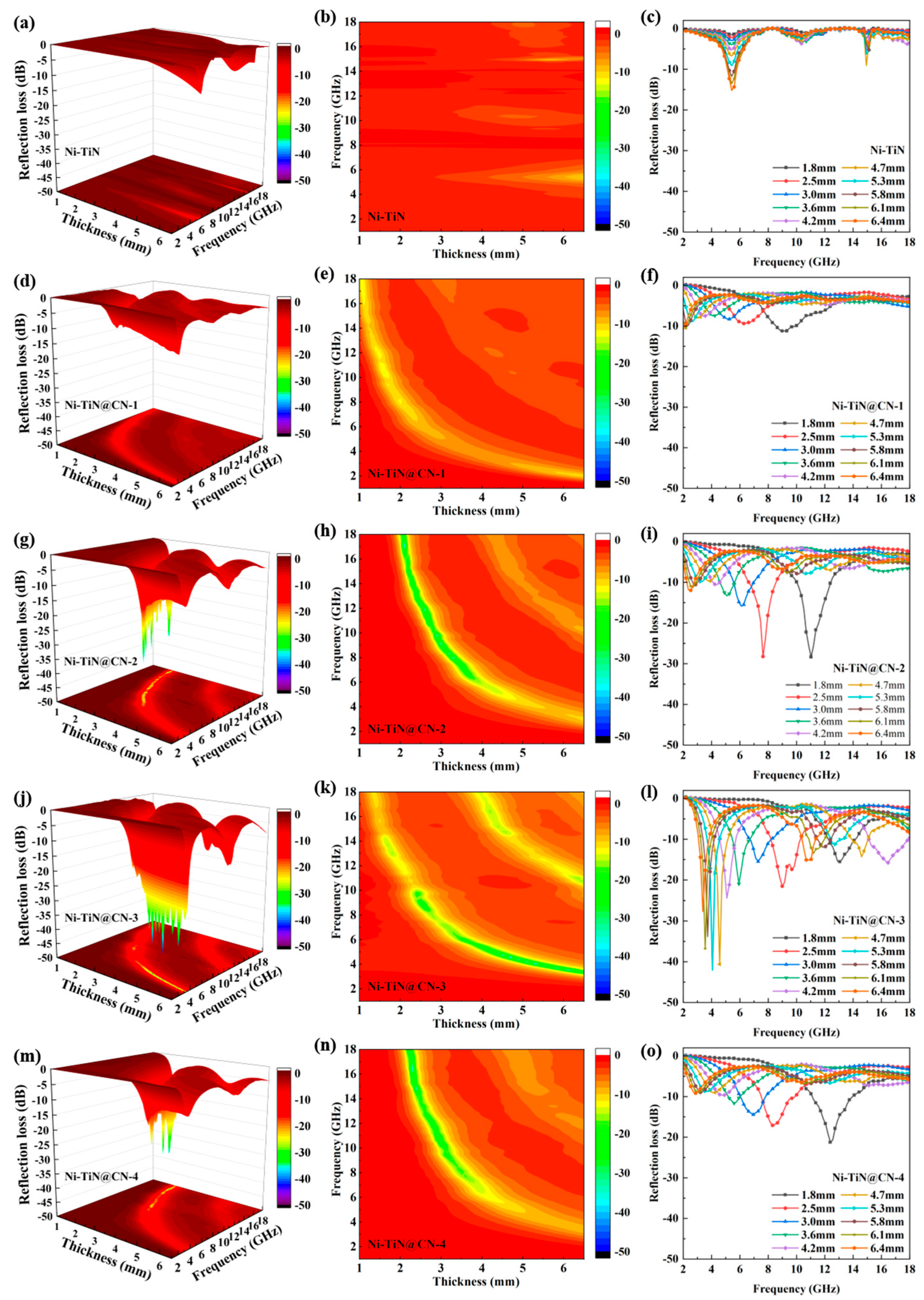
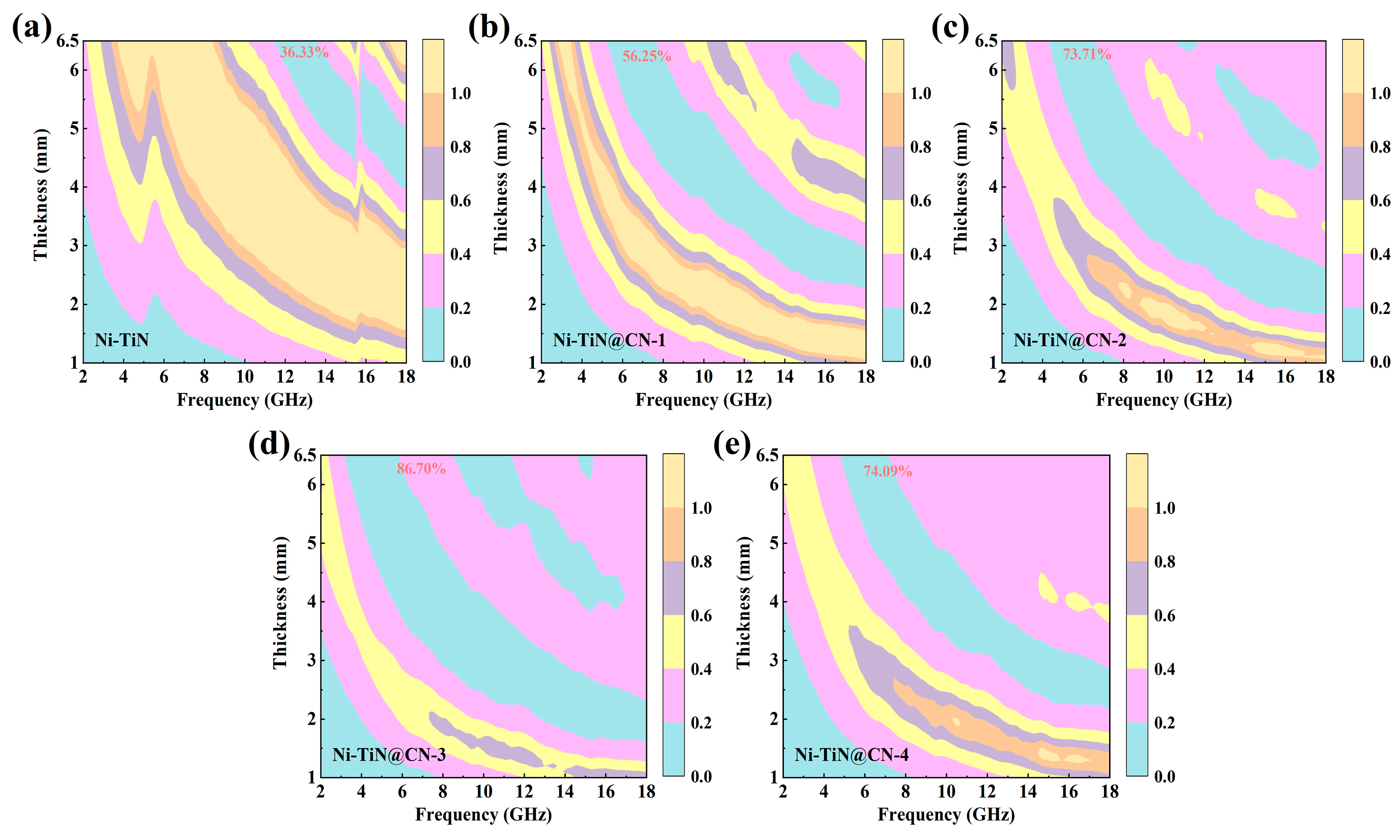
| Samples | Minimum RL Value (dB) | Thickness (mm) | Frequency (GHz) | EAB ≤ −10 dB (GHz) | EAB ≤ −20 dB (GHz) |
|---|---|---|---|---|---|
| Ni-TiN | −15.21 | 6.4 | 5.42 | 0.77 | 0 |
| Ni-TiN@CN-1 | −13.34 | 1.6 | 10.35 | 1.41 | 0 |
| Ni-TiN@CN-2 | −37.25 | 3.3 | 7.97 | 8.7 | 1.4 |
| Ni-TiN@CN-3 | −42.05 | 5.4 | 4.06 | 14.90 | 2.2 |
| Ni-TiN@CN-4 | −35.71 | 2.4 | 16.3 | 7.61 | 0.32 |
| Samples | Minimum RL Value (dB) | Thickness (mm) | Frequency (GHz) | EAB ≤−10 dB (GHz) | Ref. |
|---|---|---|---|---|---|
| Ni-C NCs | −38.3 | 2.4 | 11.7 | 3.1 | [51] |
| FeCo/Ti3C2Tx MXene | −28.72 | 1.6 | 17.2 | 8.8 | [52] |
| FeNi/SWCNT | −42.5 | 2.7 | 7.4 | 4.9 | [53] |
| TiN/Ni/C | −35.1 | 1.7 | 12.4 | 3.6 | [54] |
| Fe/C NFs | −20.6 | 2.5 | 11.52 | 6.24 | [55] |
| Ni-TiN@CN-3 | −42.05 | 5.4 | 4.06 | 14.90 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Shi, G. Tunable Microwave Absorption Performance of Ni-TiN@CN Nanocomposites with Synergistic Effects from the Addition of Ni Metal Elements. Metals 2025, 15, 597. https://doi.org/10.3390/met15060597
Li Q, Shi G. Tunable Microwave Absorption Performance of Ni-TiN@CN Nanocomposites with Synergistic Effects from the Addition of Ni Metal Elements. Metals. 2025; 15(6):597. https://doi.org/10.3390/met15060597
Chicago/Turabian StyleLi, Qian, and Guimei Shi. 2025. "Tunable Microwave Absorption Performance of Ni-TiN@CN Nanocomposites with Synergistic Effects from the Addition of Ni Metal Elements" Metals 15, no. 6: 597. https://doi.org/10.3390/met15060597
APA StyleLi, Q., & Shi, G. (2025). Tunable Microwave Absorption Performance of Ni-TiN@CN Nanocomposites with Synergistic Effects from the Addition of Ni Metal Elements. Metals, 15(6), 597. https://doi.org/10.3390/met15060597





