CALPHAD-Assisted Analysis of Fe-Rich Intermetallics and Their Effect on the Mechanical Properties of Al-Fe-Si Sheets via Continuous Casting and Direct Rolling
Abstract
1. Introduction
2. Materials and Methods
2.1. CALPHAD Calculations
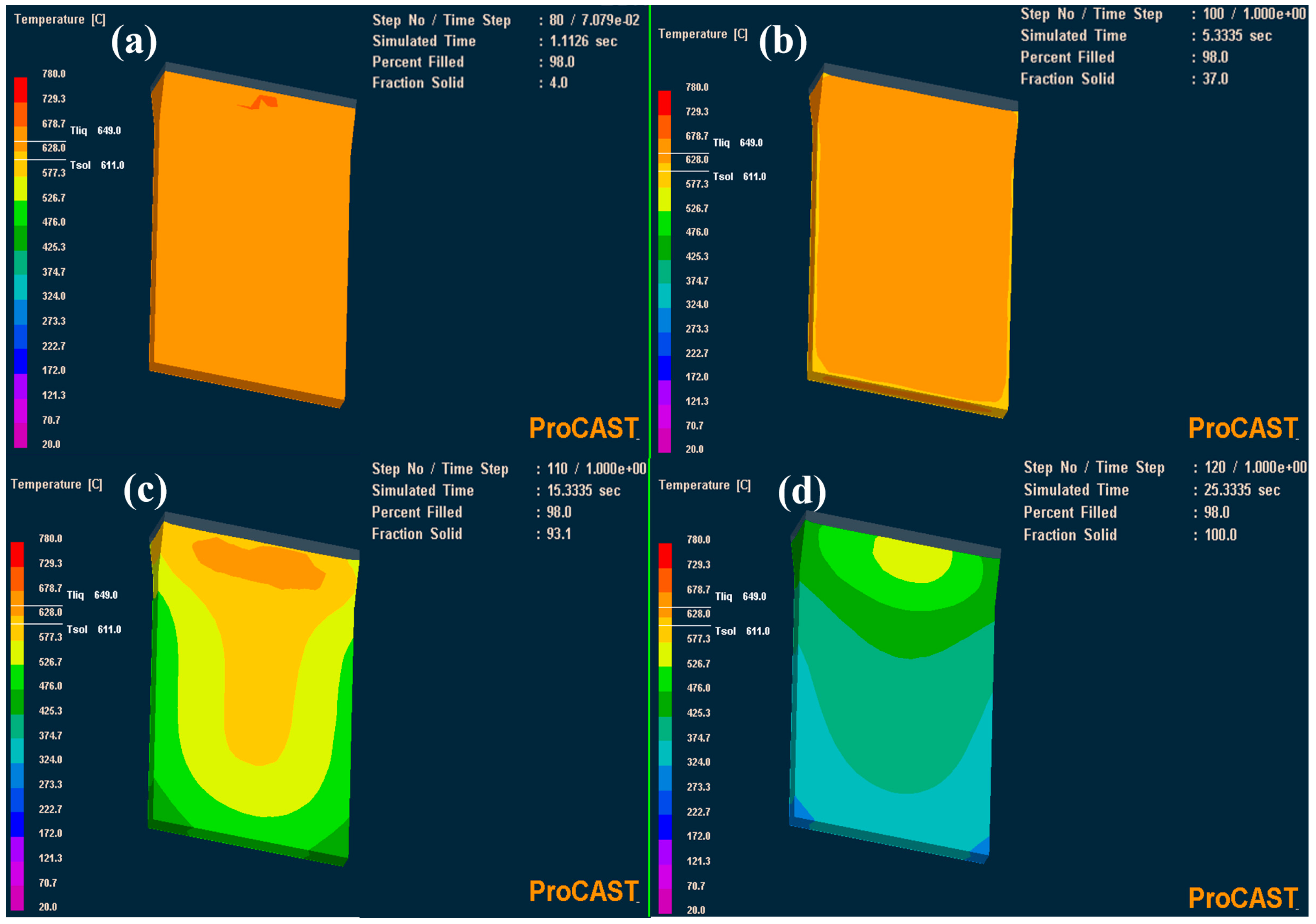
2.2. Casting and Direct Rolling Processing
2.3. Microstructural Observation
2.4. Hardness, Tensile, and Electrochemical Tests
3. Results and Discussion
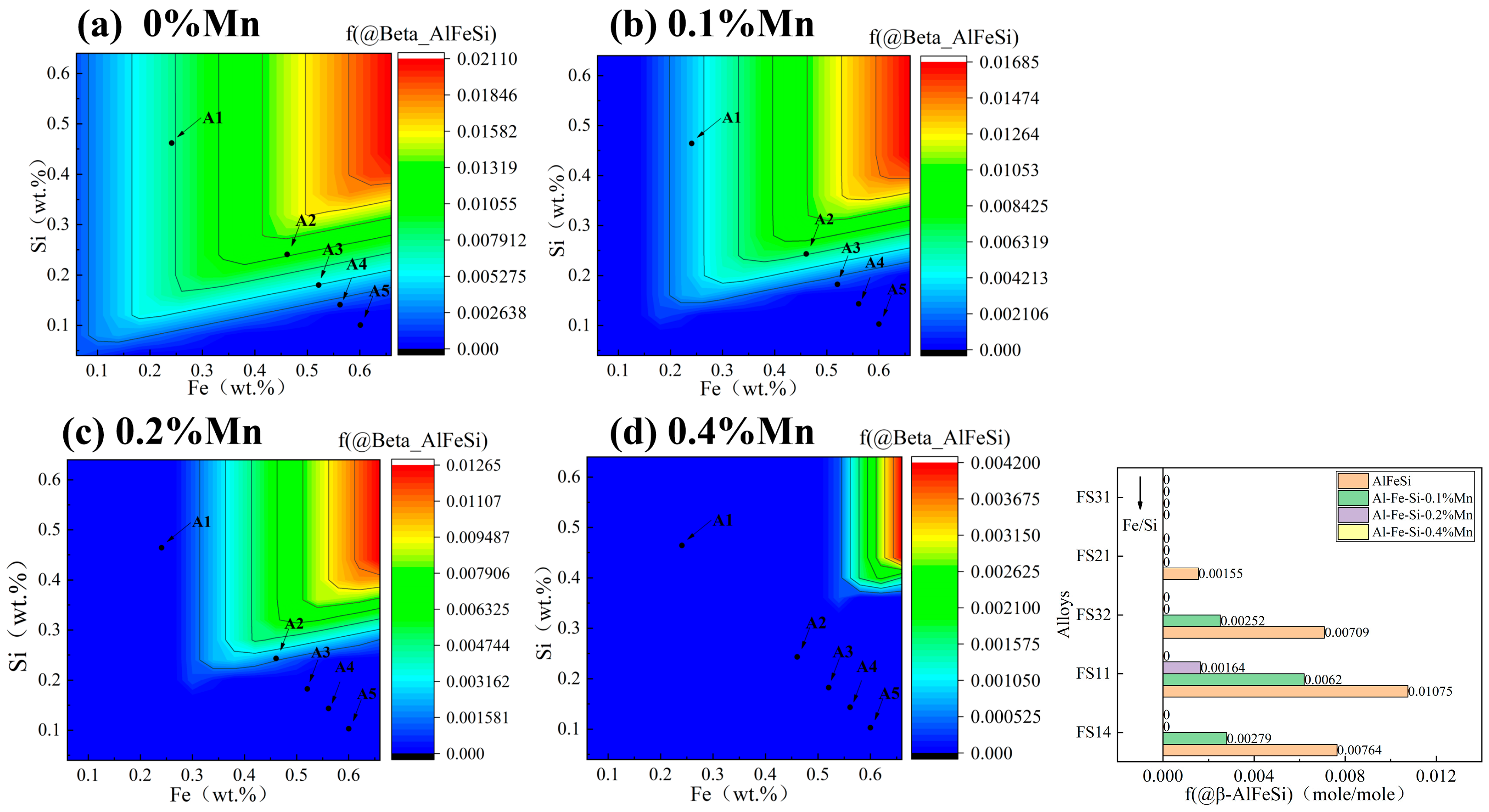
3.1. Contour Maps of Fe and Si Levels in Al-Fe-Si Alloys
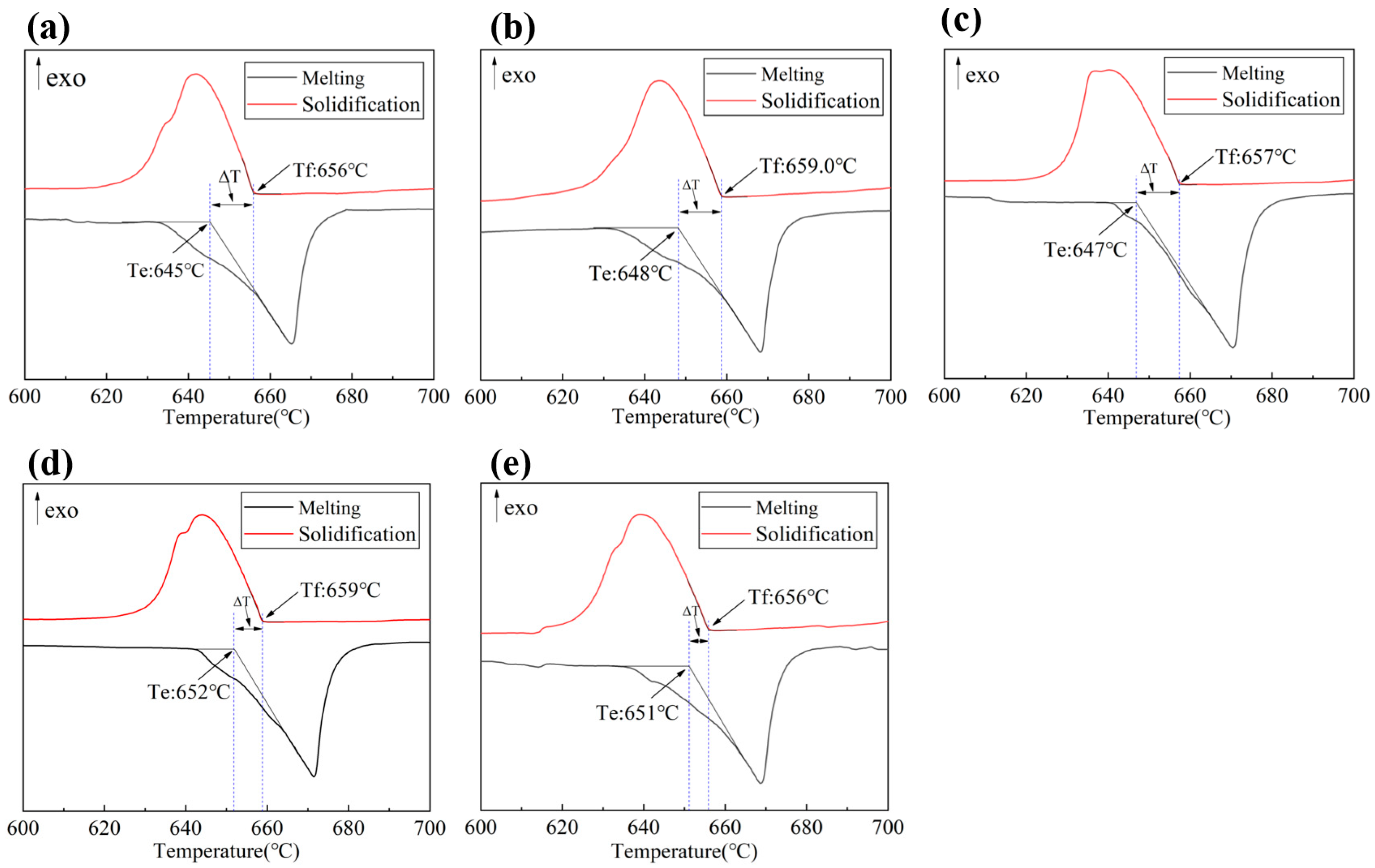
3.2. The DSC Curves of the Five Experimental Alloys
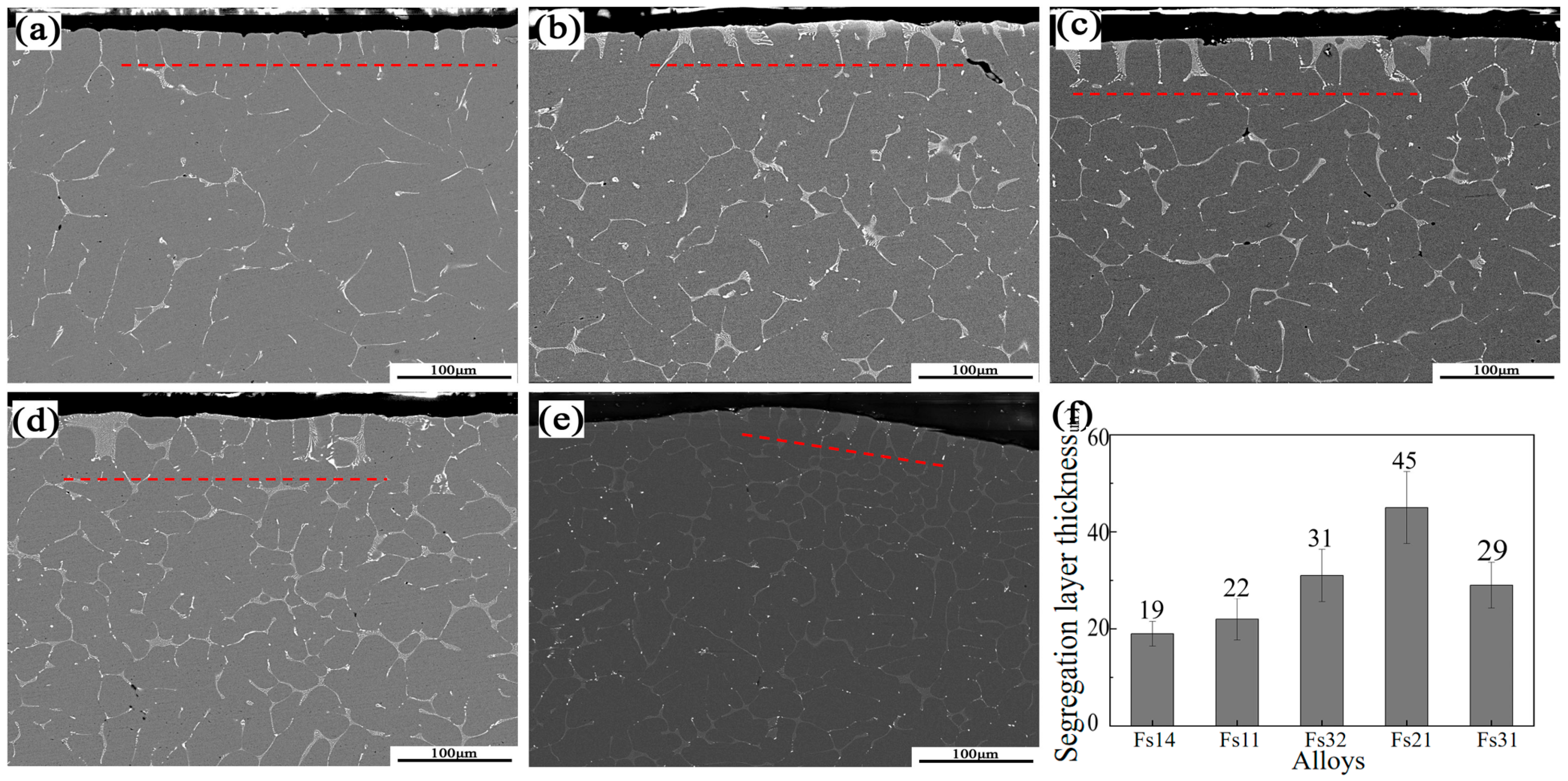
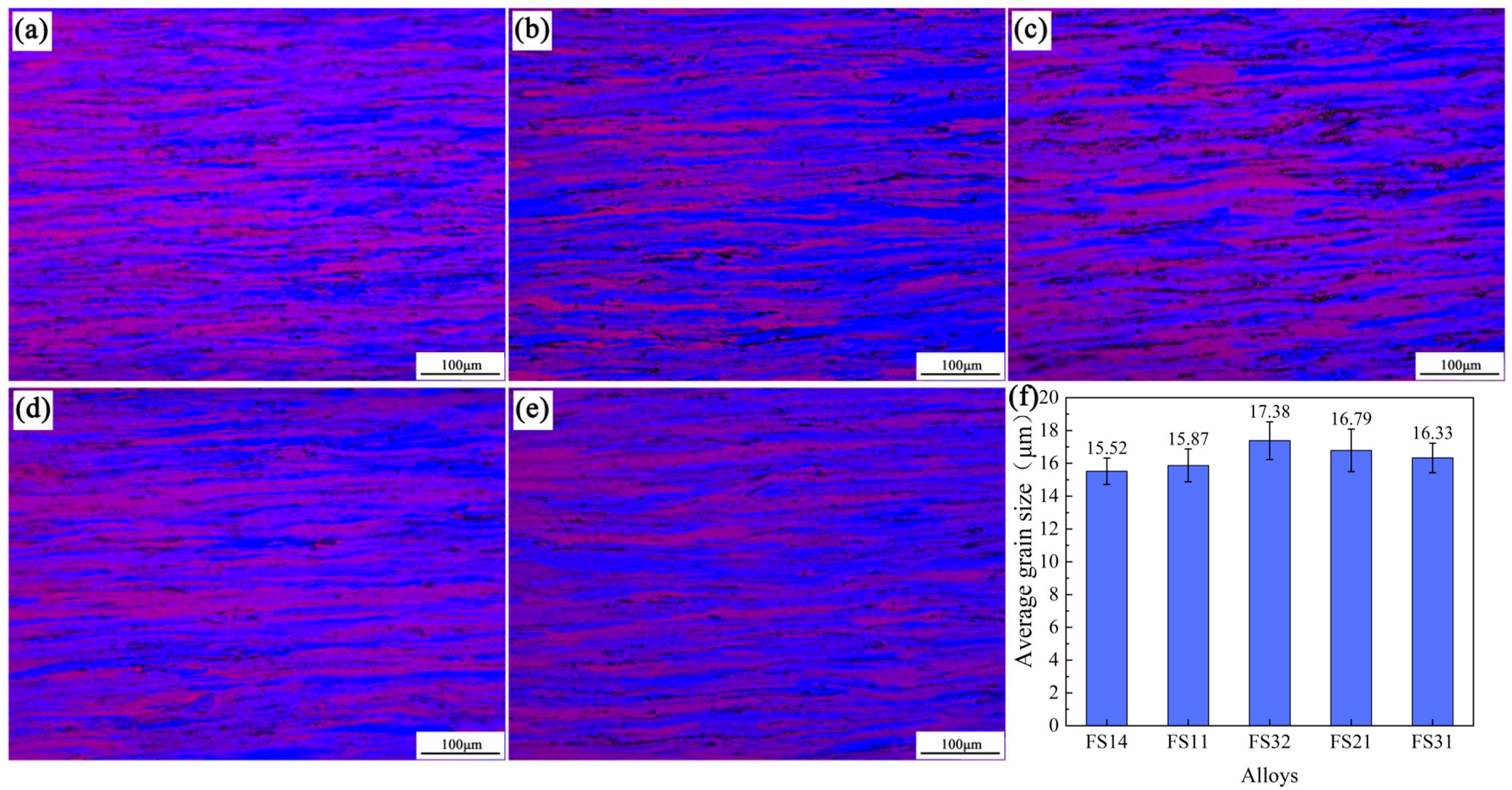
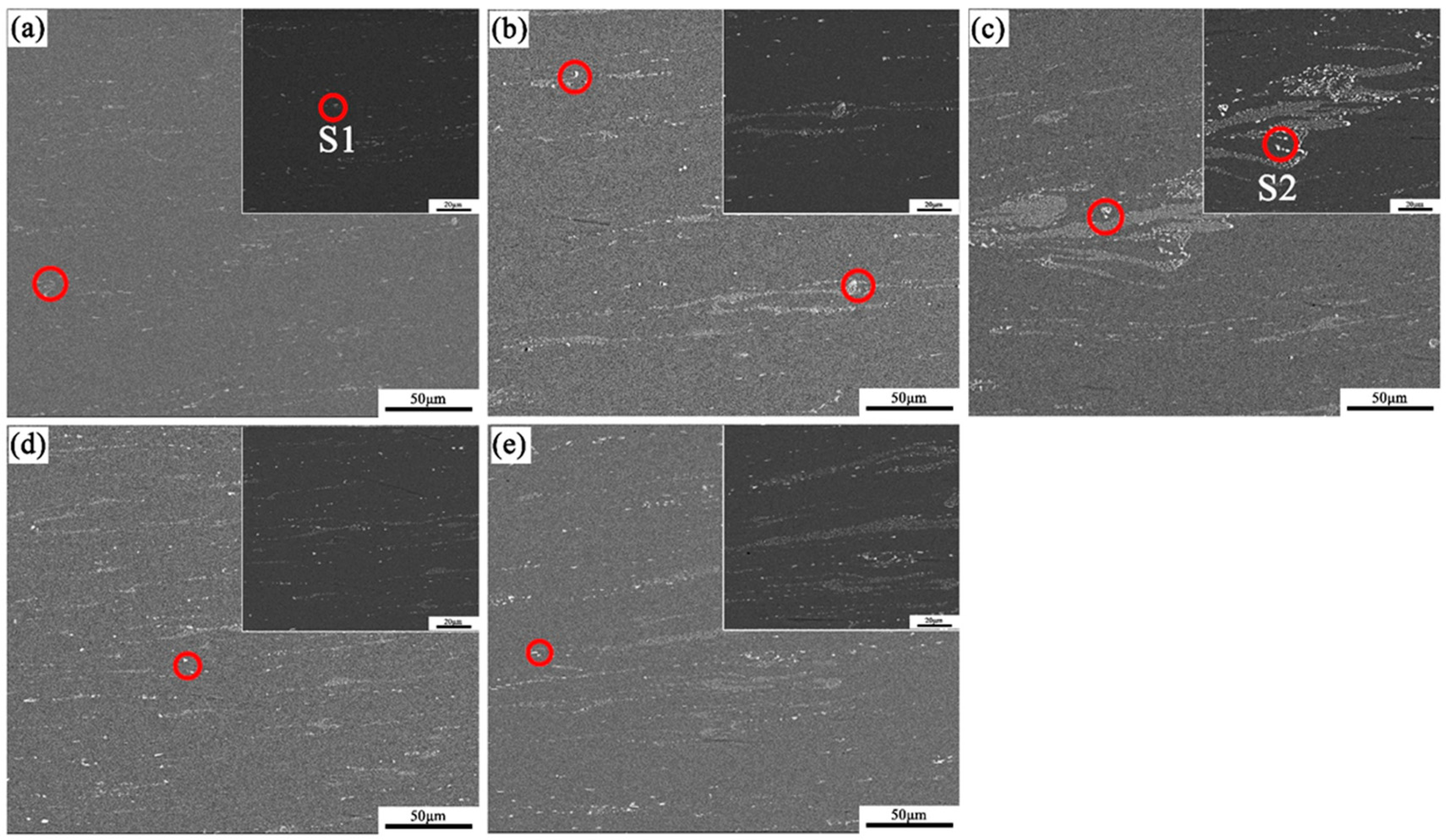
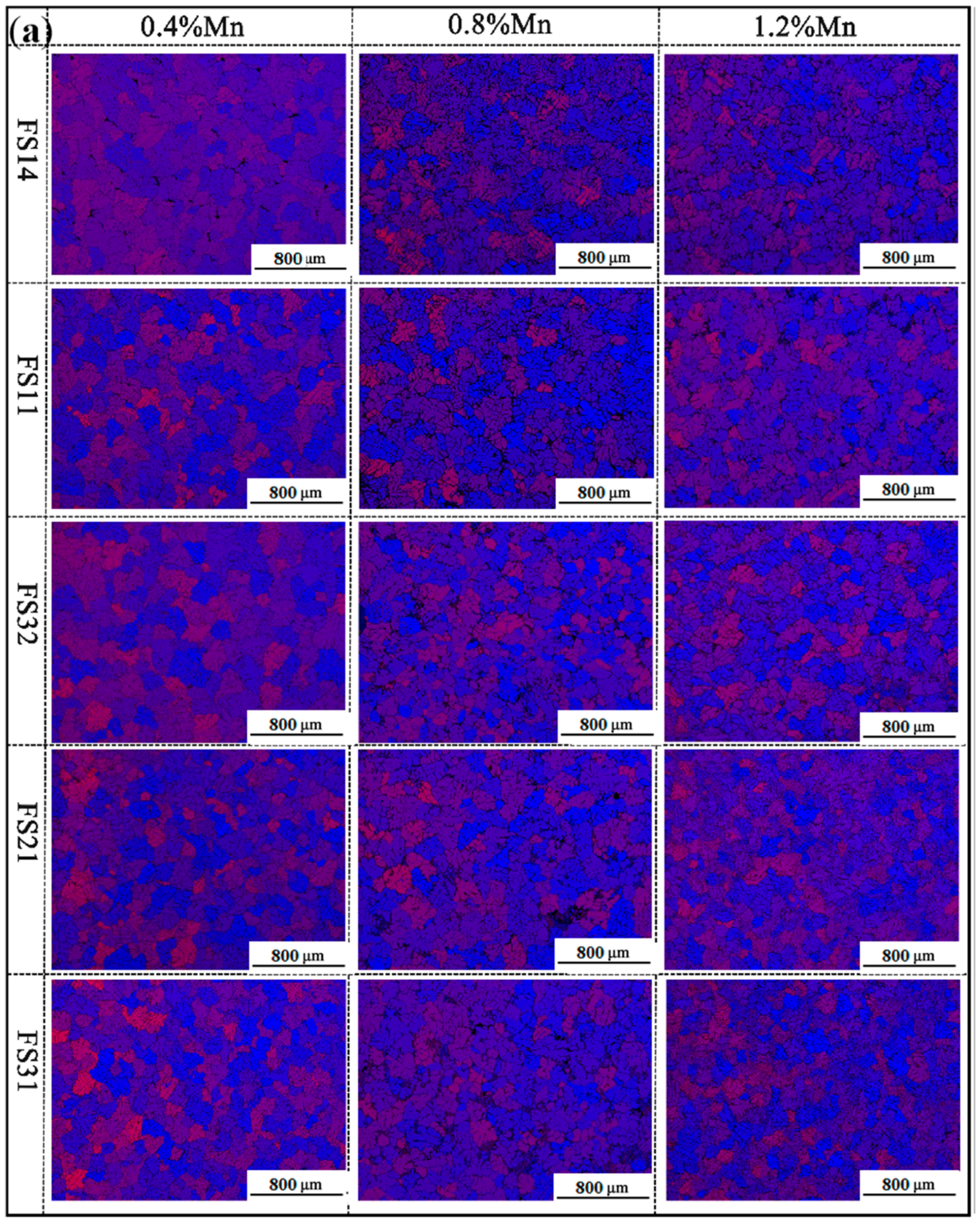
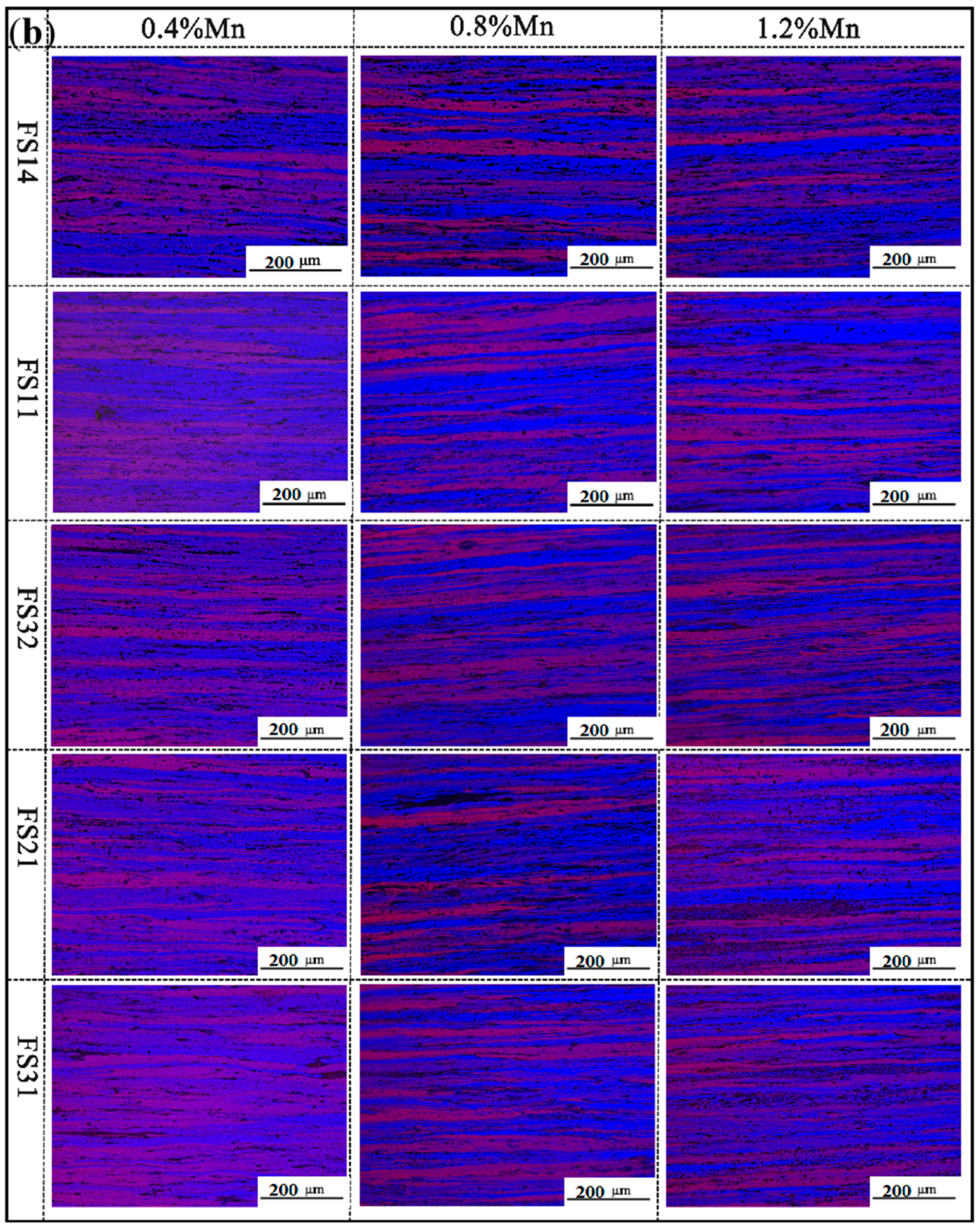
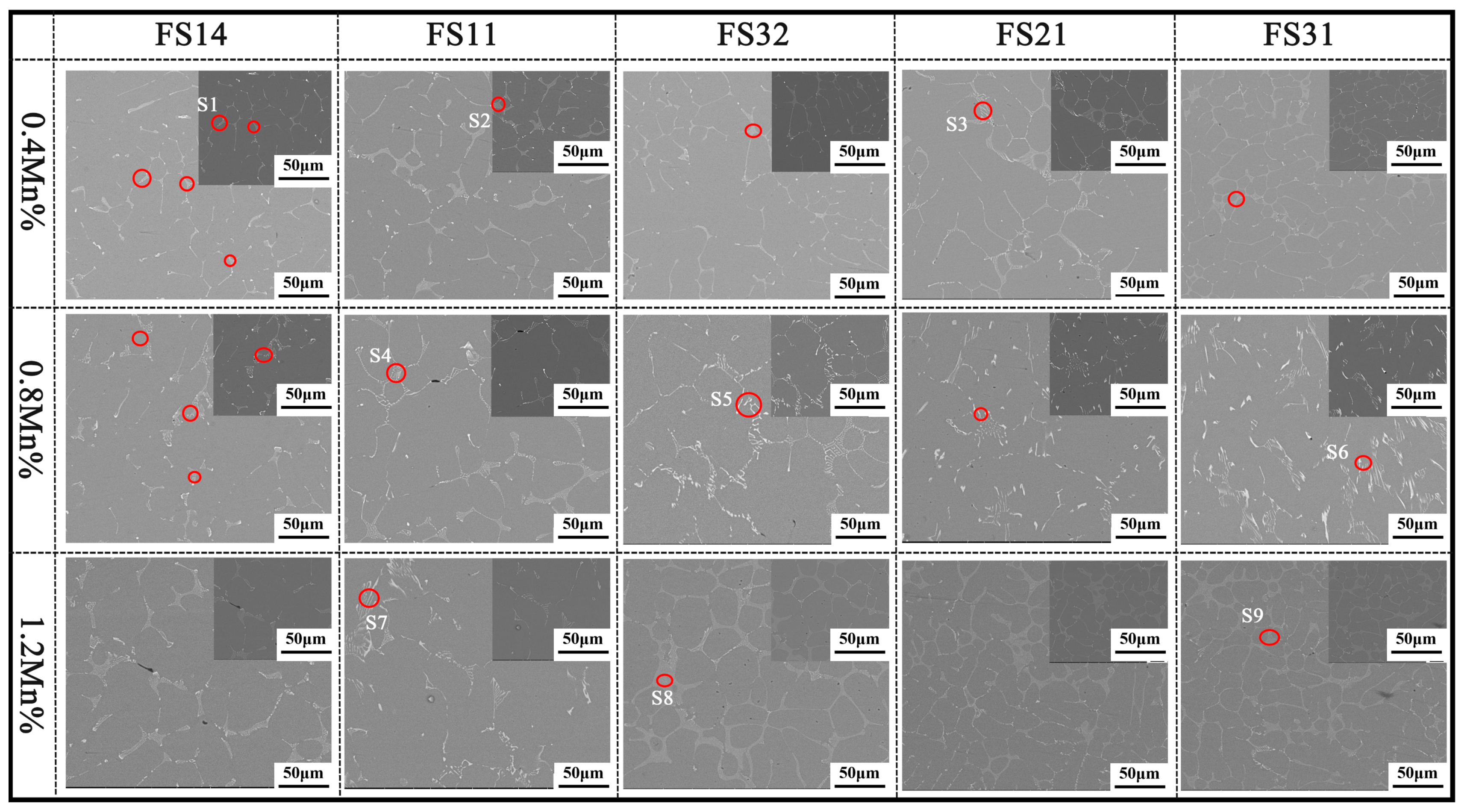
3.3. The Microstructure
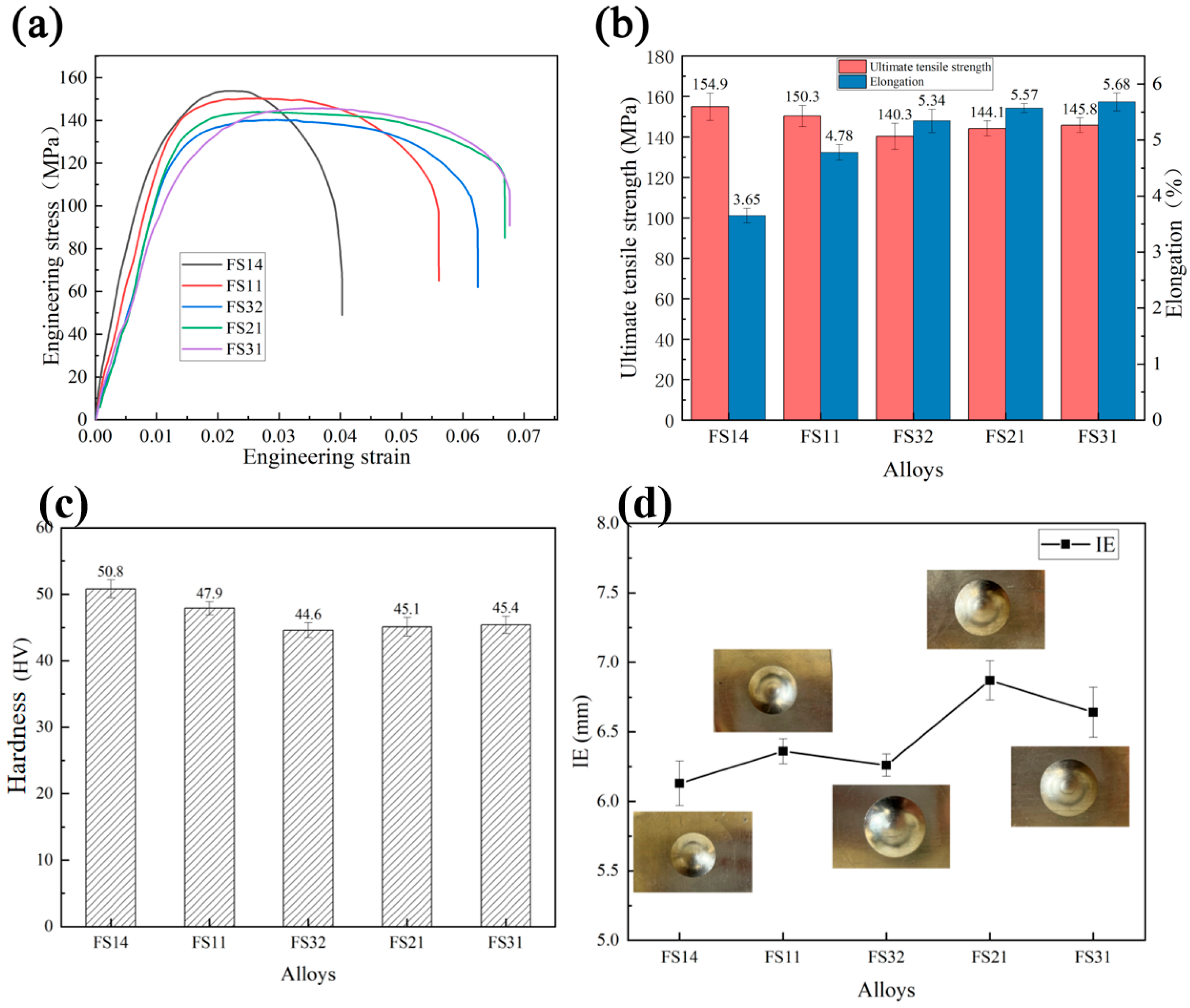
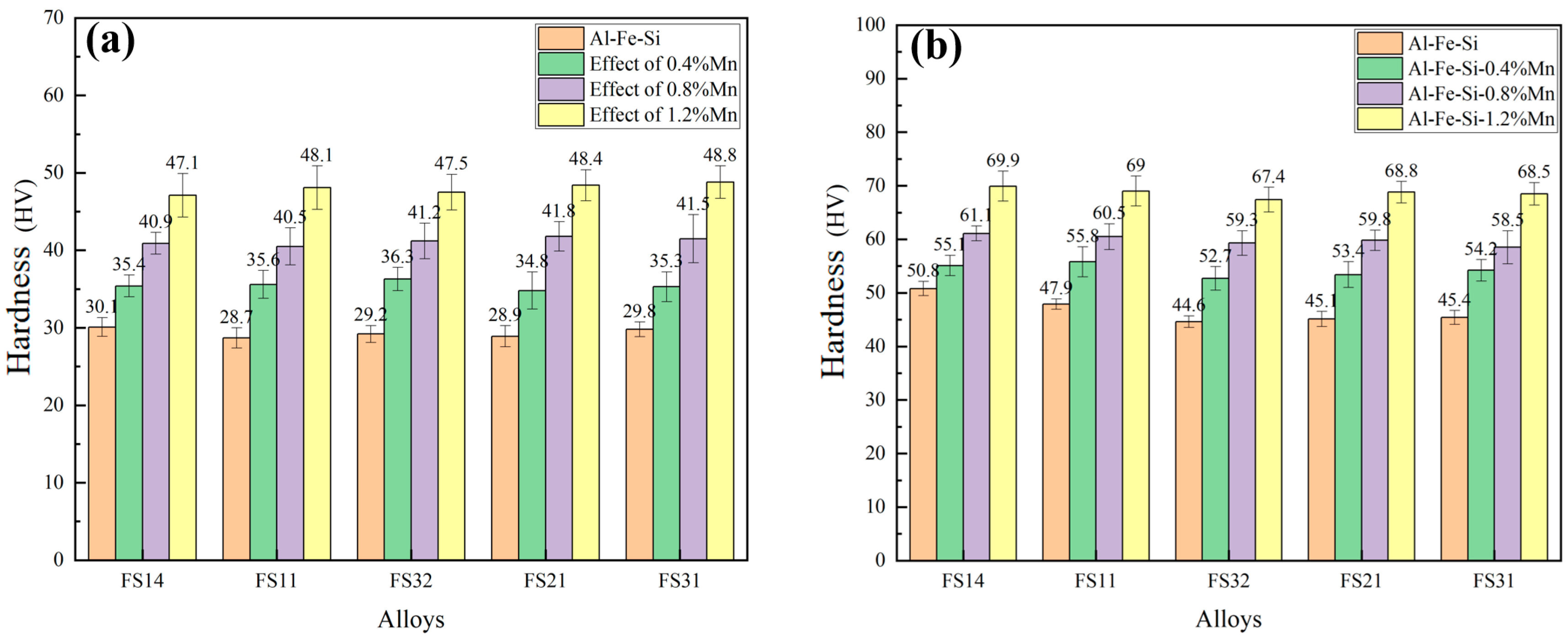
3.4. Evolution of Microstructure and Mechanical Properties of Al-Fe-Si Alloy Sheets
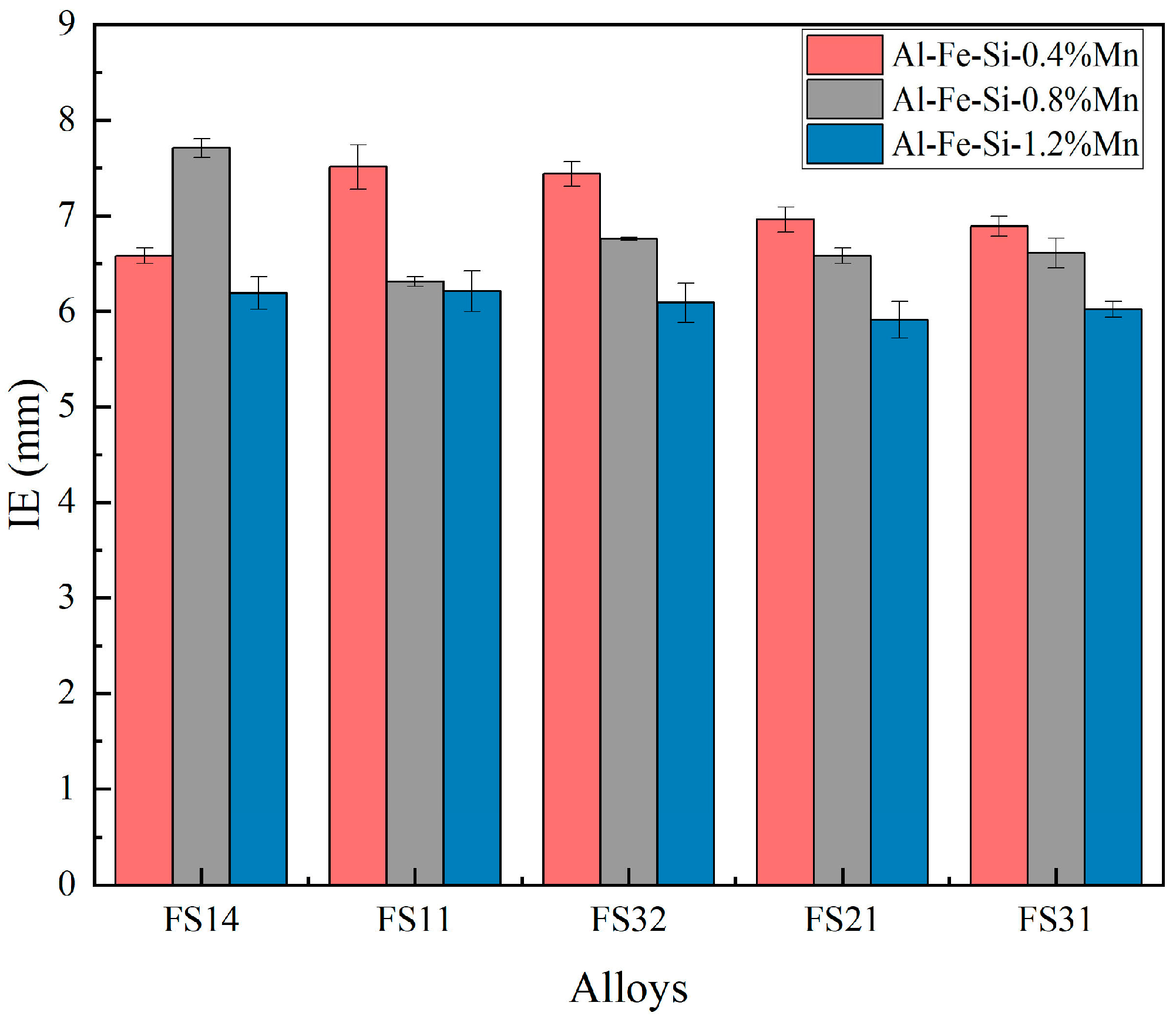
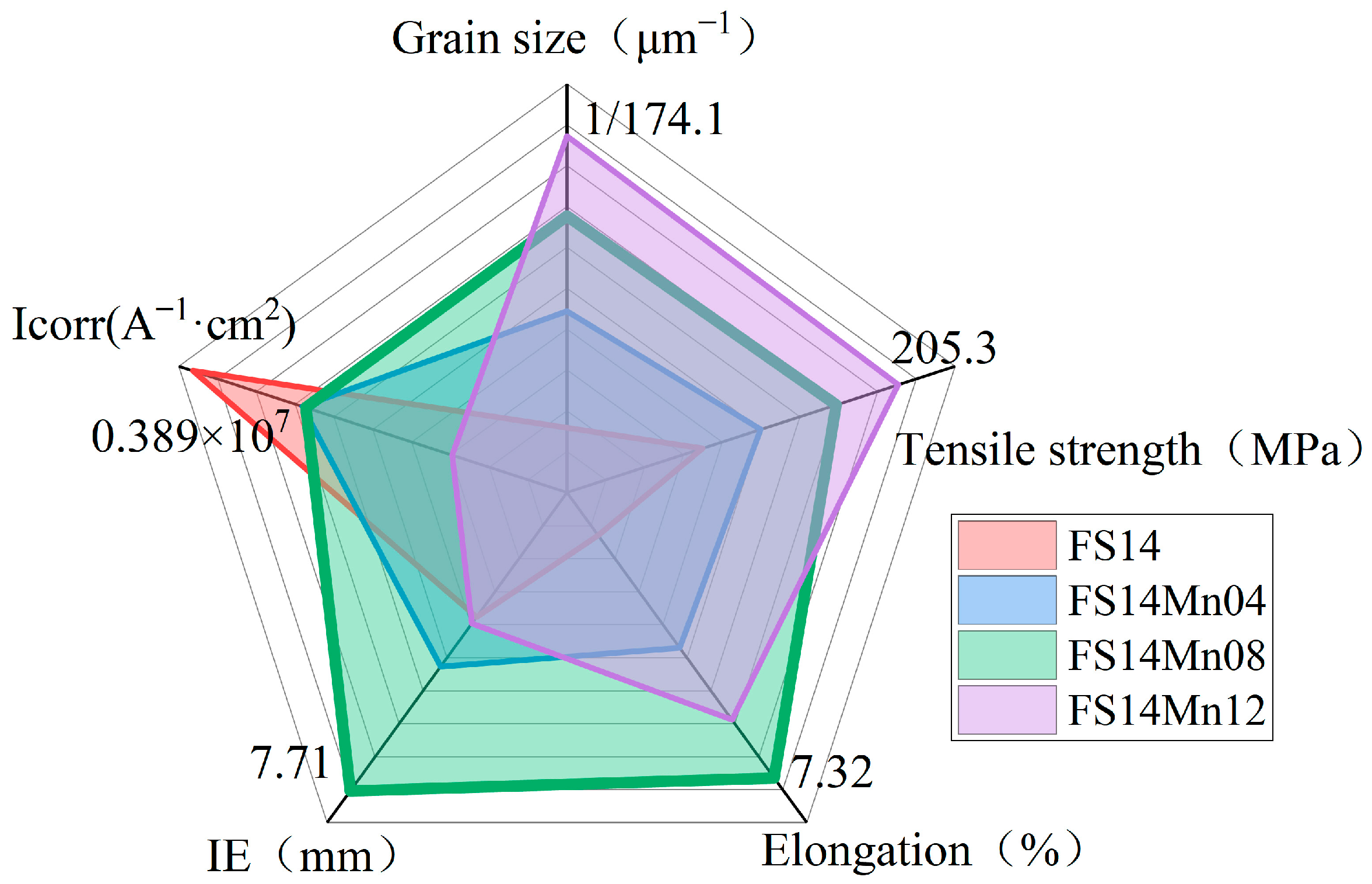
3.5. The Effect of Mn Content on the Comprehensive Properties of Al-Fe-Si Alloys for TBCCR
4. Conclusions
- (1)
- Phase evolution in Al-Fe-Si alloys was first analyzed via CALPHAD to establish composition–microstructure relationships. Five representative alloys with Fe/Si atomic ratios spanning from 1:4 to 3:1 were selected for experimental validation (designated FS14-FS31). Among the alloys with different Fe/Si ratios, the FS31 alloy demonstrated the narrowest solidification interval of 5.5 °C. In the casting ingots, the α-phase exhibited surface segregation tendencies, while the β-phase showed no such inclination, a phenomenon linked to the chemical potential and activity of elements during solidification.
- (2)
- Microstructural and surface segregation analyses revealed that the FS31 alloy (Fe/Si = 3:1, Fe + Si = 0.7%) achieved optimal mechanical performance: ultimate tensile strength of 145.8 MPa, elongation to failure of 5.7%, accompanied by a cupping value of 6.64 mm. This composition demonstrates the critical role of maintaining 0.7% total Fe + Si content with a 3:1 atomic ratio for balanced castability and product performance.
- (3)
- Based on the findings of Al-Fe-Si alloys, the Mn-modified microstructure was quantitatively assessed through CALPHAD-guided composition design (0.4–1.2 wt % Mn). The addition of Mn was found to refine the grain structure and enhance the strength. The FS14 alloy displayed minimal surface segregation after Mn addition. Ductility remained stable at ≤0.8 wt.% Mn but degraded at 1.2 wt.% Mn, indicating a threshold for Mn content optimization.
- (4)
- The TBCCR-optimized Al-Fe-Si-Mn alloy (Fe + Si = 0.7%, Fe/Si = 1:4, 0.8 wt.% Mn) demonstrated superior comprehensive properties: enhanced ultimate tensile strength = 189.4 MPa, improved elongation to failure = 7.32%, and elevated cupping value = 7.71 mm. This composition achieves an optimal balance between grain refinement, mechanical performance (strength–ductility synergy), formability, and corrosion resistance. Property optimization originates from the coordinated control of phase formation through Mn microalloying, providing a compositional design framework for high-performance TBCCR alloys.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, R.; Zhao, W.; Zhang, L.; Li, X.; Guan, S. Microstructure and corrosion resistance of commercial purity aluminum sheet manufactured by continuous casting direct rolling after ultrasonic melt pre-treatment. J. Mater. Res. Technol. 2023, 22, 1522–1532. [Google Scholar] [CrossRef]
- Zhao, J.R.; Hung, F.Y.; Chen, B.J. Effects of heat treatment on a novel continuous casting direct rolling 6056 aluminum alloy: Cold rolling characteristics and tensile fracture properties. J. Mater. Res. Technol. 2021, 11, 535–547. [Google Scholar] [CrossRef]
- Haga, T.; Tkahashi, K.; Ikawaand, M.; Watari, H. Twin roll casting of aluminum alloy strips. J. Mater. Process. Technol. 2004, 153–154, 42–47. [Google Scholar] [CrossRef]
- Gras, C.; Meredith, M.; Hunt, J.D. Microdefects formation during the twin-roll casting of Al–Mg–Mn aluminium alloys. J. Mater. Process. Technol. 2005, 167, 62–72. [Google Scholar] [CrossRef]
- Slagsvold, M. Effect of Fe and Si Content in Aluminium Alloys as a Result of Increased Recycling: Testing of High Purity Aluminium Alloys in Uniaxial Tension. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2011. [Google Scholar]
- Lan, X.; Li, K.; Wang, J.; Yang, M.; Lu, Q.; Du, Y. Developing Al–Fe–Si alloys with high thermal stability through tuning Fe, Si contents and cooling rates. Intermetallics 2022, 144, 107505. [Google Scholar] [CrossRef]
- Li, Z.; Qin, L.; Guo, B.; Yuan, J.; Zhang, Z.; Li, W.; Mi, J. Characterization of the Convoluted 3D Intermetallic Phases in a Recycled Al Alloy by Synchrotron X-ray Tomography and Machine Learning. Acta Metall. Sin. (Engl. Lett.) 2022, 35, 115–123. [Google Scholar] [CrossRef]
- Karlík, M.; Mánik, T.; Lauschmann, H. Influence of Si and Fe on the distribution of intermetallic compounds in twin-roll cast Al–Mn–Zr alloys. J. Alloys Compd. 2012, 515, 108–113. [Google Scholar] [CrossRef]
- Birol, Y. Thermomechanical processing of a twin-roll cast Al–1Fe–0.2Si alloy. J. Mater. Process. Technol. 2008, 202, 564–568. [Google Scholar] [CrossRef]
- Barekar, N.S.; Dhindaw, B.K. Twin-Roll Casting of Aluminum Alloys—An Overview. Mater. Manuf. Process. 2014, 29, 651–661. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Damoah, L.N.W.; Robertson, D.G. Removal of Iron from Aluminum: A Review. Miner. Process. Extr. Metall. Rev. 2012, 33, 99–157. [Google Scholar] [CrossRef]
- Luo, K.; Wang, Z.; Meng, L.; Guo, Z. Removal of iron for aluminum recovery from scrap aluminum alloy by supergravity separation with manganese addition. Chem. Eng. Process.-Process Intensif. 2022, 173, 108841. [Google Scholar] [CrossRef]
- Song, X.; Gao, M.; Yang, B.; Guan, R. Modification and refinement of Fe-containing phases, mechanical properties and strengthening mechanisms in Al–Fe alloys via Cr alloying and continuous rheo-extrusion. Mater. Sci. Eng. A 2022, 850, 143557. [Google Scholar] [CrossRef]
- Bo, L.; Xiangxiang, H.; Rui, X.; Yuliang, Z.; Yemao, L.; Huaqiang, X. Evolution of iron-rich intermetallics and its effect on the mechanical properties of Al–Cu–Mn–Fe–Si alloys after thermal exposure and high-temperature tensile testing. J. Mater. Res. Technol. 2023, 23, 2527–2541. [Google Scholar] [CrossRef]
- Green, J.A.S. Aluminum Recycling and Processing for Energy Conservation and Sustainability; ASM International: Almere, The Netherlands, 2007. [Google Scholar]
- Zhang, Y.; Liu, Y.; Han, Y.; Wei, C.; Gao, Z. The role of cooling rate in the microstructure of Al–Fe–Si alloy with high Fe and Si contents. J. Alloys Compd. 2009, 473, 442–445. [Google Scholar] [CrossRef]
- Sun, J.; Takahashi, A.; Higashi, K.; Yamamoto, T.; Komarov, S. Fabrication of CrSi2-Containing Master Alloys for Modification of Fe-Containing Intermetallic Compounds in Aluminum Alloys. Materials 2022, 15, 7836. [Google Scholar] [CrossRef]
- Que, Z.; Zhou, Y.; Wang, Y.; Mendis, C.L.; Fan, Z. Effects of Mg addition on the Al6(Fe,Mn) intermetallic compounds and the grain refinement of α-Al in Al-Fe-Mn alloys. Mater. Charact. 2021, 171, 110758. [Google Scholar] [CrossRef]
- Ren, J.; Fang, X.; Chen, D.; Cao, C.; He, Y.; Liu, J. The Effect of Heat Treatments on the Microstructural Evolution of Twin-Roll-Cast Al-Fe-Si Alloys. J. Mater. Eng. Perform. 2021, 30, 4401–4410. [Google Scholar] [CrossRef]
- Bacaicoa, I.; Luetje, M.; Zeismann, F.; Geisert, A.; Fehlbier, M.; Brueckner-Foit, A. On the role of Fe-content on the damage behavior of an Al-Si-Cu alloy. Procedia Struct. Integr. 2019, 23, 33–38. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Zhou, Y.; Chong, X.; Li, X.; Zhang, H.; Nagaumi, H. Exploring crystal structures, stability and mechanical properties of Fe, Mn-containing intermetallics in Al-Si Alloy by experiments and first-principles calculations. J. Alloys Compd. 2021, 876, 160022. [Google Scholar] [CrossRef]
- Liu, C.L.; Du, Q.; Parson, N.C.; Poole, W.J. The interaction between Mn and Fe on the precipitation of Mn/Fe dispersoids in Al-Mg-Si-Mn-Fe alloys. Scr. Mater. 2018, 152, 59–63. [Google Scholar] [CrossRef]
- Kuijpers, N.C.W.; Vermolen, F.J.; Vuik, C.; Koenis, P.T.G.; Nilsen, K.E.; Zwaag, S.V.D. The dependence of the β-AlFeSi to α-Al(FeMn)Si transformation kinetics in Al–Mg–Si alloys on the alloying elements. Mater. Sci. Eng. A 2005, 394, 9–19. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, X.; Qin, J.; Zhou, W.; Ling, J.; Dong, Q.; Yu, J.; Nagaumi, H.; Zhang, B. Effect of Mn/Cr ratio on precipitation behaviors of α-Al(FeMnCr)Si dispersoids and mechanical properties of Al–Mg–Si–Cu alloys. Mater. Sci. Eng. A 2022, 860, 144269. [Google Scholar] [CrossRef]
- Wang, Y.; Zang, A.; Wells, M.; Poole, W.; Li, M.; Parson, N. Strain localization at longitudinal weld seams during plastic deformation of Al–Mg–Si–Mn–Cr extrusions: The role of microstructure. Mater. Sci. Eng. A 2022, 849, 143454. [Google Scholar] [CrossRef]
- Basak, C.B.; Meduri, A.; Babu, N.H. Influence of Ni in high Fe containing recyclable Al-Si cast alloys. Mater. Des. 2019, 182, 108017. [Google Scholar] [CrossRef]
- Xu, C.; Xiao, W.; Hanada, S.; Yamagata, H.; Ma, C. The effect of scandium addition on microstructure and mechanical properties of Al–Si–Mg alloy: A multi-refinement modifier. Mater. Charact. 2015, 110, 160–169. [Google Scholar] [CrossRef]
- Liu, X.; Jia, H.-L.; Wang, C.; Wu, X.; Zha, M.; Wang, H.-Y. Enhancing mechanical properties of twin-roll cast Al–Mg–Si–Fe alloys by regulating Fe-bearing phases and macro-segregation. Mater. Sci. Eng. A 2022, 831, 142256. [Google Scholar] [CrossRef]
- Birol, Y. Interannealing twin-roll cast Al–Fe–Si strips without homogenization. Scr. Mater. 2009, 61, 185–188. [Google Scholar] [CrossRef]
- Birol, Y. Response to annealing treatments of twin-roll cast thin Al–Fe–Si strips. J. Alloys Compd. 2008, 458, 265–270. [Google Scholar] [CrossRef]
- Penumakala, P.K.; Nallathambi, A.K.; Specht, E.; Urlau, U.; Hamilton, D.; Dykes, C. Influence of process parameters on solidification length of twin-belt continuous casting. Appl. Therm. Eng. 2018, 134, 275–286. [Google Scholar] [CrossRef]
- Lu, G.; Liang, Y.; Xu, C.; Rao, W.; Xue, Y.; Li, L.; Zhang, L.; Guan, S. Influence of Partial Er Substitution for Sc on the Microstructure, Mechanical Properties and Corrosion Resistance of Short-Processed Al-4.7Mg-0.6Mn-0.3Zr-0.3Sc Sheets. Metals 2024, 14, 1013. [Google Scholar] [CrossRef]
- Chen, S.L.; Daniel, S.; Zhang, F. The PANDAT software package and its applications. Calphad 2002, 26, 175–188. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Zhao, X.; Liu, X.; Cheng, X.; Jiang, S.; Zhang, Q. Effect of Al/Mg Ratio on the Microstructure and Phase Distribution of Zn-Al-Mg Coatings. Metals 2023, 13, 1963. [Google Scholar] [CrossRef]
- Anyalebechi, P. Effects of alloying elements and solidification conditions on secondary dendrite arm spacing in aluminum alloys. TMS Annu. Meet. 2004, 2004, 217–233. [Google Scholar]
- Easton, M.; Davidson, C.; John, D.S. Effect of Alloy Composition on the Dendrite Arm Spacing of Multicomponent Aluminum Alloys. Metall. Mater. Trans. A 2010, 41, 1528–1538. [Google Scholar] [CrossRef]
- Khan, M.H.; Das, A.; Li, Z.; Kotadia, H.R. Effects of Fe, Mn, chemical grain refinement and cooling rate on the evolution of Fe intermetallics in a model 6082 Al-alloy. Intermetallics 2021, 132, 107132. [Google Scholar] [CrossRef]

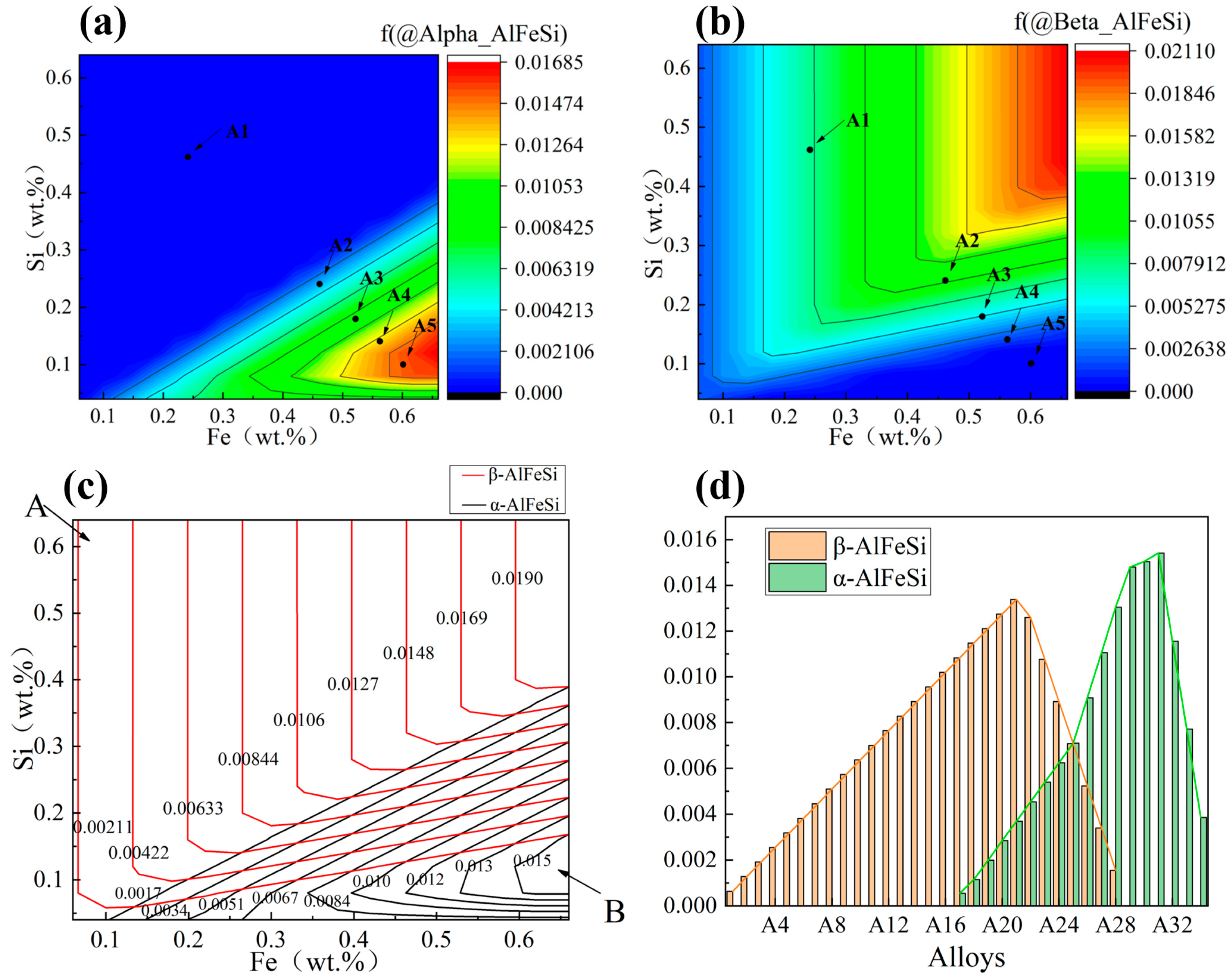




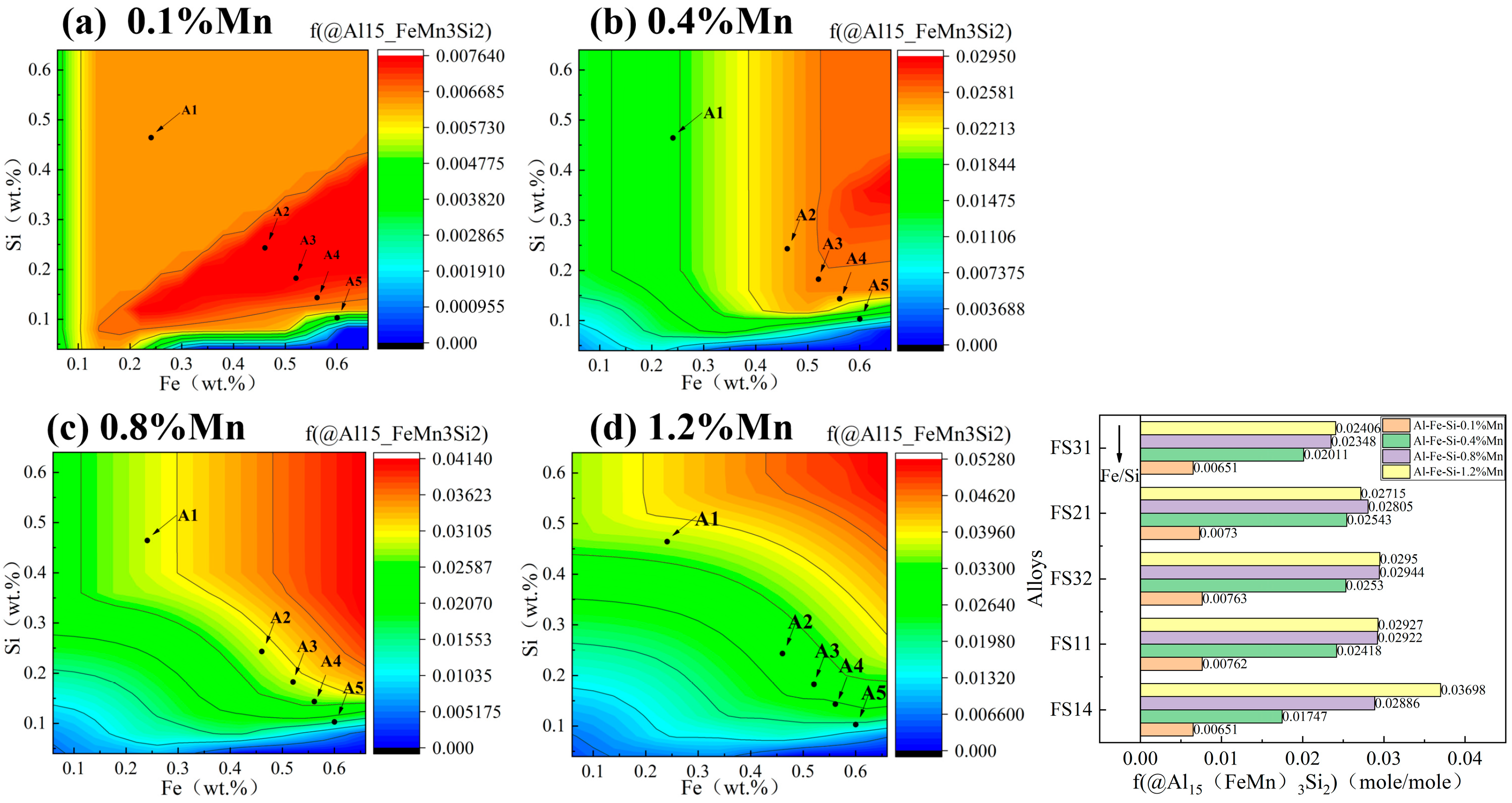



| Alloys | Fe/Si (Atom Ratio) | Nominal Composition (wt.%) | Actual Composition (wt.%) | Al (wt.%) | Intermetallics | ||
|---|---|---|---|---|---|---|---|
| Fe | Si | Fe | Si | ||||
| FS14(A1) | 1:4 | 0.24 | 0.46 | 0.23 | 0.48 | Bal. | β-AlFeSi |
| FS11(A2) | 1:1 | 0.46 | 0.24 | 0.48 | 0.25 | Bal. | β-AlFeSi (Main) and α-AlFeSi |
| FS32(A3) | 3:2 | 0.52 | 0.18 | 0.50 | 0.17 | Bal. | β-AlFeSi and α-AlFeSi |
| FS21(A4) | 2:1 | 0.56 | 0.14 | 0.59 | 0.15 | Bal. | β-AlFeSi and α-AlFeSi (Main) |
| FS31(A5) | 3:1 | 0.60 | 0.10 | 0.56 | 0.09 | Bal. | α-AlFeSi |
| Alloy | FS14 | FS11 | FS32 | FS21 | FS31 |
|---|---|---|---|---|---|
| Al-Fe-Si | 211.8/15.5 | 208.7/15.9 | 213.1/17.4 | 207.4/16.8 | 203.2/16.3 |
| Al-Fe-Si-0.4Mn | 204.6/17.1 | 196.7/16.6 | 203.9/15.8 | 193.3/14.9 | 195.6/16.7 |
| Al-Fe-Si-0.8Mn | 186.8/16.1 | 183.1/15.8 | 176.6/13.8 | 193.1/14.4 | 174.2/16.2 |
| Al-Fe-Si-1.2Mn | 174.1/15.9 | 179.5/16.4 | 174.1/15.5 | 169.6/16.2 | 172.5/15.8 |
| Element/Location | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 |
|---|---|---|---|---|---|---|---|---|---|
| Fe | 6.51 | 9.56 | 10.11 | 5.96 | 7.38 | 15.18 | 6.17 | 6.76 | 10.72 |
| Si | 2.28 | 6.08 | 1.80 | 3.12 | 4.64 | 0.58 | 4.47 | 3.46 | 2.91 |
| Mn | 1.23 | 1.01 | 1.95 | 2.66 | 3.88 | 6.50 | 4.12 | 3.33 | 9.94 |
| Al | 89.98 | 83.35 | 86.14 | 88.26 | 84.10 | 77.74 | 85.24 | 86.45 | 76.43 |
| FS14 | FS11 | FS32 | FS21 | FS31 | |
|---|---|---|---|---|---|
| 0.4 Mn | 169.9 5.35% | 169.2 8.08% | 156.4 7.02% | 159.2 5.34% | 154.5 5.92% |
| 0.8 Mn | 189.4 7.32% | 182.6 3.52% | 172.6 4.34% | 182.1 4.61% | 183.3 5.28% |
| 1.2 Mn | 205.3 6.44% | 209.7 5.52% | 202.9 3.18% | 200.1 3.37% | 210.9 4.43% |
| Alloys | Corrosion Potential Ecorr(V) | Corrosion Current Density Icorr(A·cm−2) |
|---|---|---|
| FS14 | −0.99 | 2.576 × 10−7 |
| FS14Mn04 | −0.97 | 3.283 × 10−7 |
| FS14Mn08 | −0.89 | 3.314 × 10−7 |
| FS14Mn12 | −0.83 | 5.299 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, X.; Shi, L.; Huang, S.; Xu, C.; Lu, G.; Guan, S. CALPHAD-Assisted Analysis of Fe-Rich Intermetallics and Their Effect on the Mechanical Properties of Al-Fe-Si Sheets via Continuous Casting and Direct Rolling. Metals 2025, 15, 578. https://doi.org/10.3390/met15060578
Li L, Li X, Shi L, Huang S, Xu C, Lu G, Guan S. CALPHAD-Assisted Analysis of Fe-Rich Intermetallics and Their Effect on the Mechanical Properties of Al-Fe-Si Sheets via Continuous Casting and Direct Rolling. Metals. 2025; 15(6):578. https://doi.org/10.3390/met15060578
Chicago/Turabian StyleLi, Longfei, Xiaolong Li, Lei Shi, Shouzhi Huang, Cong Xu, Guangxi Lu, and Shaokang Guan. 2025. "CALPHAD-Assisted Analysis of Fe-Rich Intermetallics and Their Effect on the Mechanical Properties of Al-Fe-Si Sheets via Continuous Casting and Direct Rolling" Metals 15, no. 6: 578. https://doi.org/10.3390/met15060578
APA StyleLi, L., Li, X., Shi, L., Huang, S., Xu, C., Lu, G., & Guan, S. (2025). CALPHAD-Assisted Analysis of Fe-Rich Intermetallics and Their Effect on the Mechanical Properties of Al-Fe-Si Sheets via Continuous Casting and Direct Rolling. Metals, 15(6), 578. https://doi.org/10.3390/met15060578







