Stainable Utilization Strategies for Basic Oxygen Furnace Slag: Properties, Processing, and Future Directions
Abstract
1. Introduction
2. Fundamental Properties of BOF Slag
2.1. Chemical Composition and Mineralogical Phases
2.2. Physical and Mechanical Properties
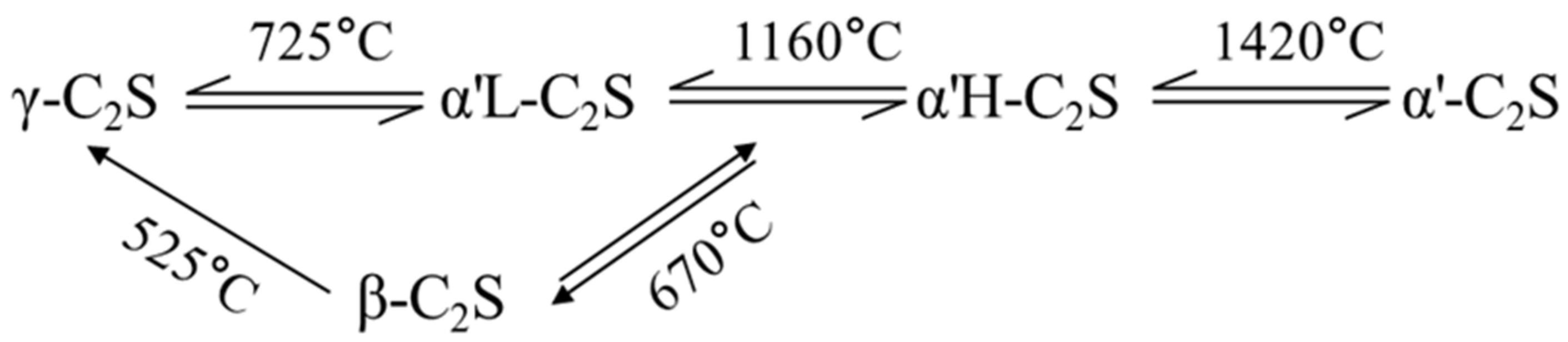
2.3. Reactivity and Volume Stability
2.4. Utilization Potential and Limitations
3. Utilization Pathways of BOF Slag
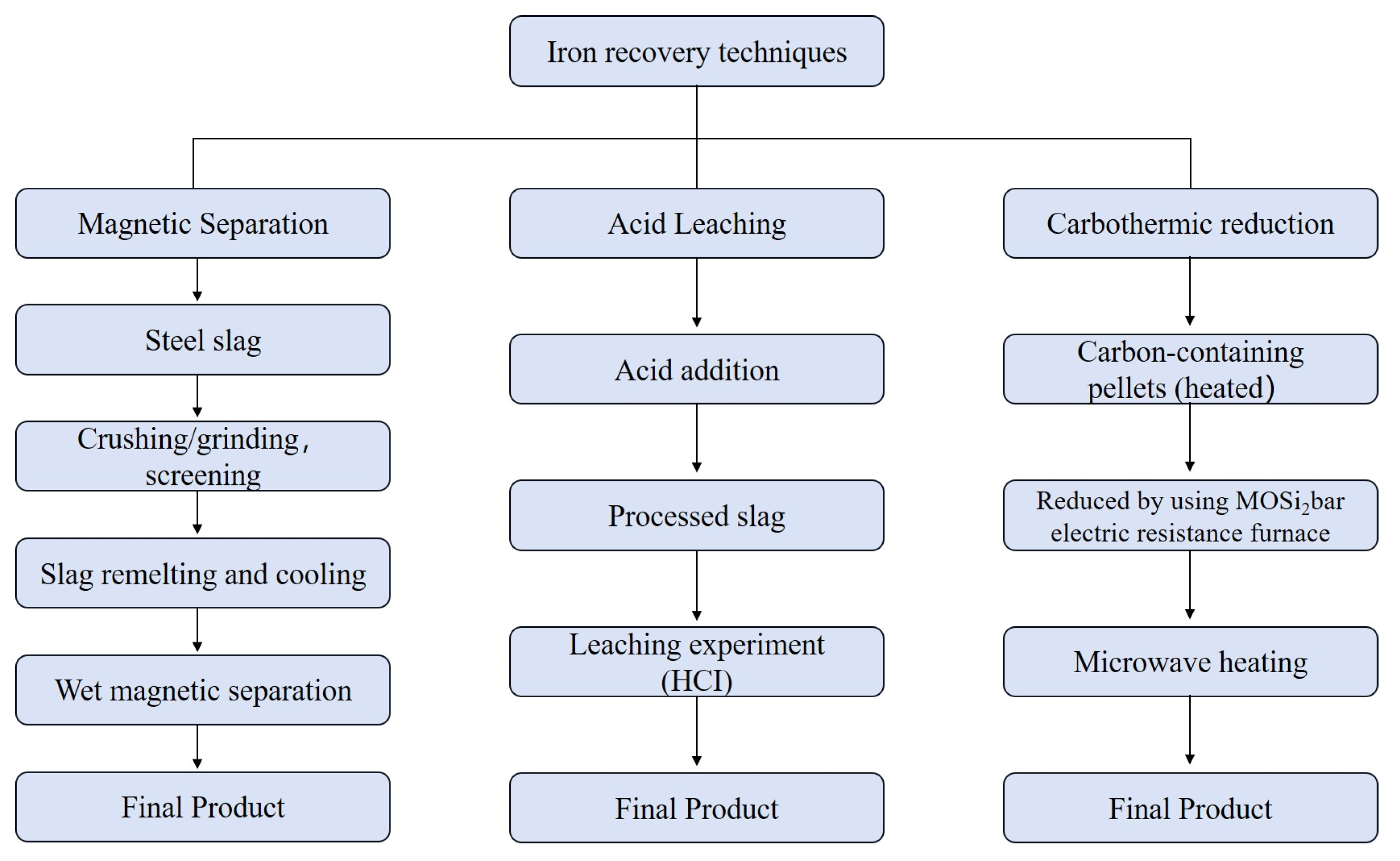
3.1. Pretreatment Technologies
3.2. Utilization of Valuable Components of BOF Slag
3.2.1. Recovery of Iron
3.2.2. Recovery of Vanadium
3.2.3. Recycling of Chromium
3.2.4. Recovery of Phosphorus
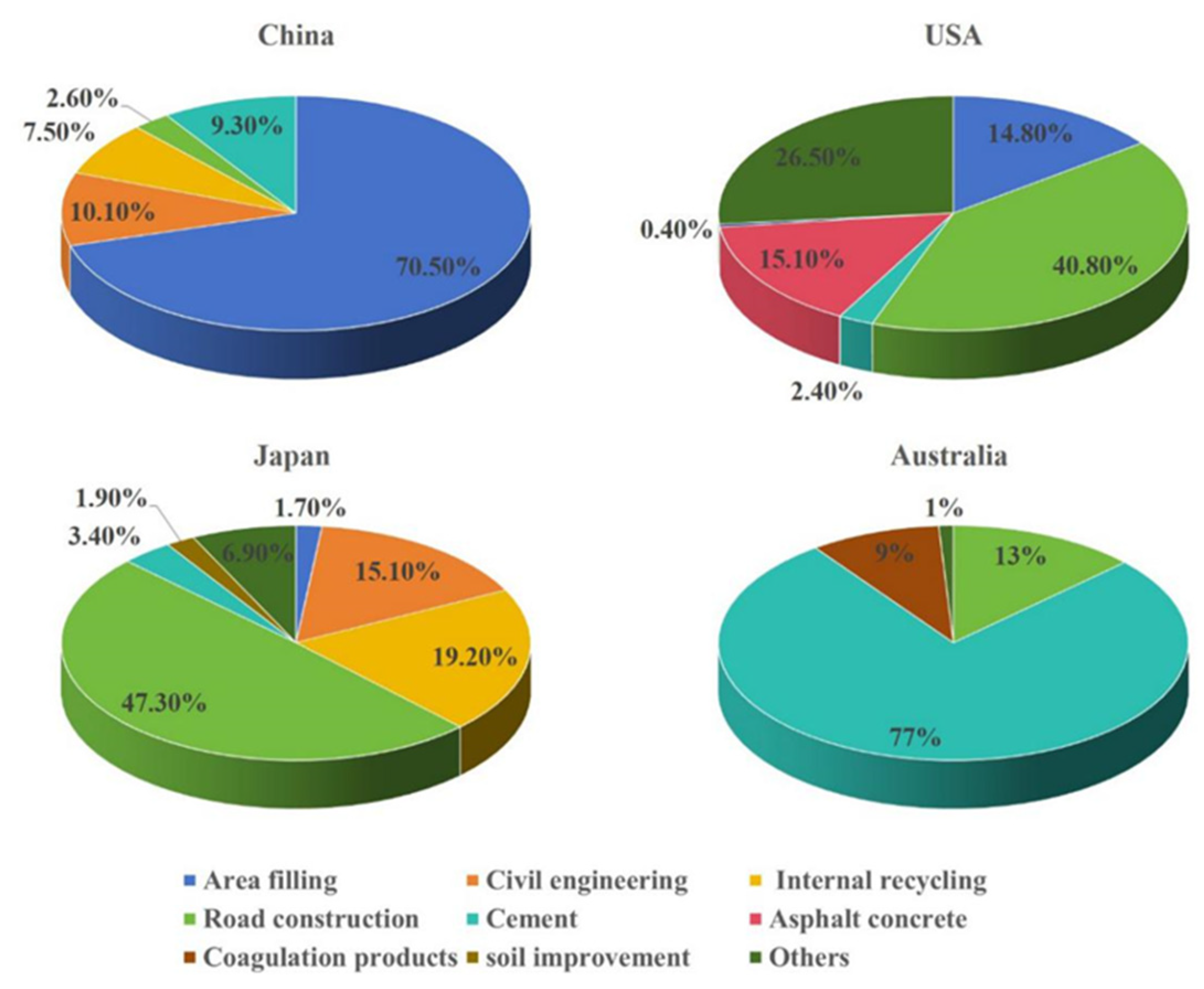
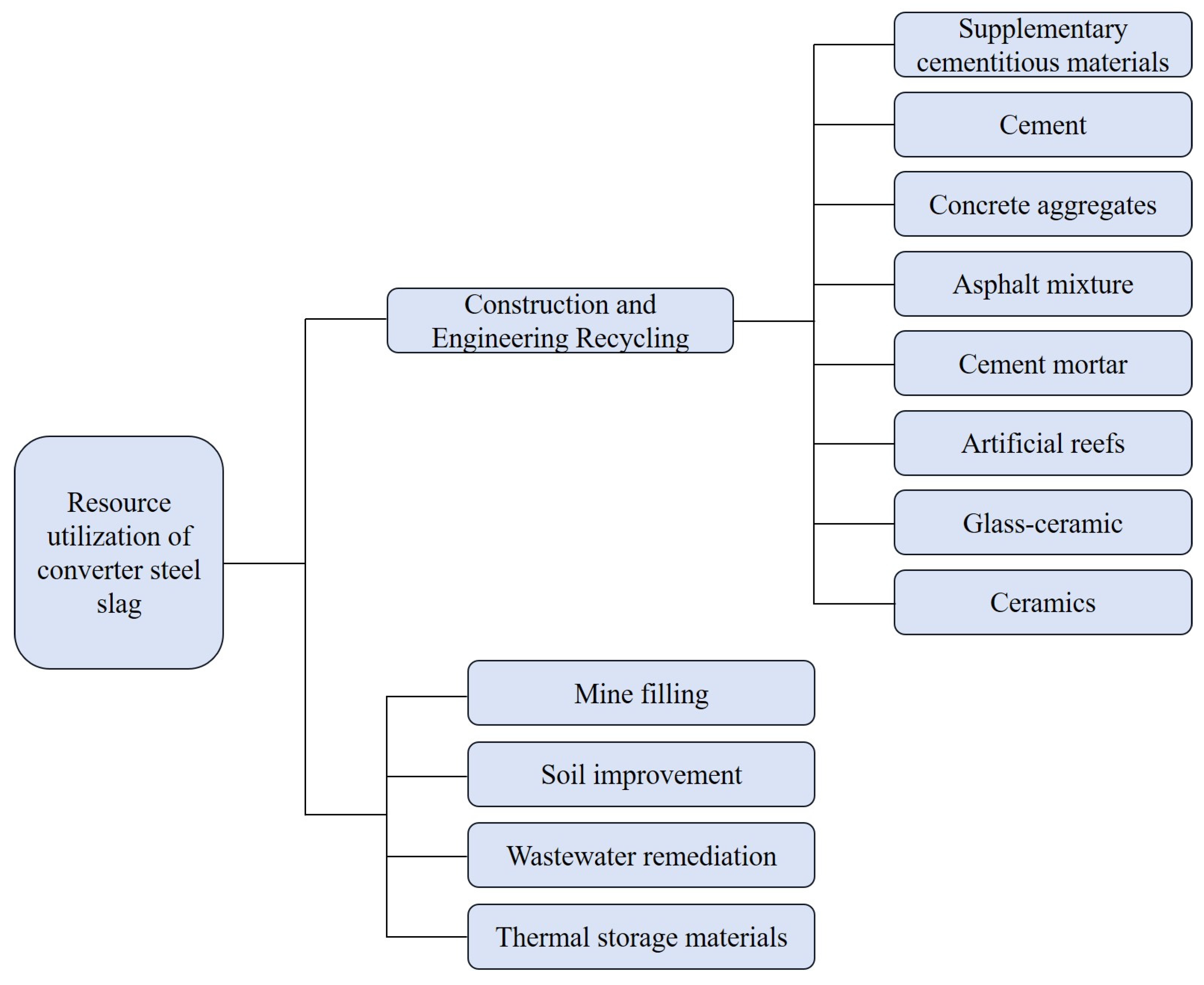
3.3. Resource Utilization of Converter BOF Slag
3.3.1. Construction and Engineering Recycling
Supplemental Cementitious Materials
Cement
Cement Mortar
Asphalt Mixture
Concrete Aggregates
Artificial Reefs
Glass-Ceramic
Ceramic Materials
3.3.2. Mine-Filling Materials
3.3.3. Soil Amendment
3.3.4. Wastewater Remediation
Adsorbents
Catalysts
Photocatalysts
3.3.5. Thermal Storage Material
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BOF | Basic oxygen furnace |
| SS | Steel slag |
References
- Nunes, V.A.; Borges, P.H.R. Recent advances in the reuse of steel slags and future perspectives as binder and aggregate for alkali-activated materials. Constr. Build. Mater. 2021, 281, 122605. [Google Scholar] [CrossRef]
- Analysis of Steel Slag Market Prospect in 2024: China’s Steel Slag Output Will Increase to 160 Million Tons. 2024. (In Chinese). Available online: https://m.chinabgao.com/freereport/97810.html (accessed on 22 March 2025).
- Yang, H.; Ma, L.; Li, Z. A Method for Analyzing Energy-Related Carbon Emissions and the Structural Changes; World Steel Statistical Yearbook 2020; World Steel Association: Brussels, Belgium, 2020. [Google Scholar]
- O’Connor, J.; Nguyen, T.B.T.; Honeyands, T.; Monaghan, B.; O’Dea, D.; Rinklebe, J.; Vinu, A.; Hoang, S.A.; Singh, G.; Kirkham, M.B.; et al. Production, characterisation, utilisation, and beneficial soil application of steel slag: A review. J. Hazard. Mater. 2021, 419, 126478. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- National Committee of the Chinese People’s Political Consultative Conference. Suggestion to Promote Comprehensive Utilization of Steel Slag Resources. 2022. Available online: http://www.chinadaily.com.cn/ (accessed on 16 June 2022).
- Chinese People’s Political Consultative Conference. Iron and Steel Slag in 2017. 2020. Available online: https://d9-wret.s3.us-west-2.amazonaws.com/assets/palladium/production/s3fs-public/atoms/files/myb1-2017-fesla.pdf (accessed on 22 February 2024).
- Zhang, Y.; Ying, Y.; Xing, L.; Zhan, G.; Deng, Y.; Chen, Z.; Li, J. Carbon dioxide reduction through mineral carbonation by steel slag. J. Environ. Sci. 2025, 152, 664–684. [Google Scholar] [CrossRef]
- Nippon Slag Association. Production and Uses of Steel Slag in Japan; Nippon Slag Association: Sapporo City, Japan, 2023. [Google Scholar]
- Liu, K.; Zhao, H.; Yuan, Z.; Zhao, F.; Chen, D.; Shi, C.; Li, X.; Wang, S.; Wang, X.; Zhang, X. Preparation and characterization of steel slag-based low, medium, and high-temperature composite phase change energy storage materials. J. Energy Storage 2023, 57, 106309. [Google Scholar] [CrossRef]
- Fu, S.; Kwon, E.E.; Lee, J. Upcycling steel slag into construction materials. Constr. Build. Mater. 2024, 444, 137882. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, P.; Wang, D. Research on mineral characteristics of converter steel slag and its comprehensive utilization of internal and external recycle. J. Clean. Prod. 2017, 156, 50–61. [Google Scholar] [CrossRef]
- Wang, G.C. (Ed.) 2—Ferrous metal production and ferrous slags. In The Utilization of Slag in Civil Infrastructure Construction; Woodhead Publishing: Cambridge, UK, 2016; pp. 9–33. [Google Scholar]
- Shu, K.; Sasaki, K. Occurrence of steel converter slag and its high value-added conversion for environmental restoration in China: A review. J. Clean. Prod. 2022, 373, 133876. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Ye, T.; Alwasiyah, S.K.; de Lasa, H.I.; Li, C.Z.; Lin, C.S.; Lu, J.Y.; Tang, G.L.; Wang, Y.Z.; Zhang, Q. Integrated and innovative steel slag utilization for iron reclamation, green material production and CO2 fixation via accelerated carbonation. J. Clean. Prod. 2016, 137, 617–631. [Google Scholar] [CrossRef]
- BS EN 1097-7; Tests for Mechanical and Physical Properties of Aggregates—Part 7: Determination of the Particle Density of Filler—Pycnometer Method. British Standards Institution (BSI): London, UK, 2022.
- ASTM D6473; Standard Test Method for Specific Gravity and Absorption of Rock for Erosion Control. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM C535; Standard Test Method for Resistance to Degradation of Large-Size Coarse Aggregate by Abrasion and Impact in the Los Angeles Machine. ASTM International: West Conshohocken, PA, USA, 2024.
- EN 1097-1; Tests for Mechanical and Physical Properties of Aggregates—Part 1: Determination of the Resistance to Wear (Micro-Deval). European Committee for Standardization (CEN): Brussels, Belgium, 2021.
- Satish Kumar, D.; Reddy, B.V.; Zhao, C.; Kim, H.J.; Kim, H.; Lee, J.K.; Kim, D.S.; Shin, H.S.; Park, S.J. Measurement of metallic iron in steel making slags. Measurement 2019, 131, 156–161. [Google Scholar] [CrossRef]
- Chang, Y.; Zhao, Z.; Zhao, D.; Zhang, D.; Xue, L. Co-treatment of steel slag and oil shale waste in cemented paste backfill: Evaluation of fresh properties, microstructure, and heavy metals immobilization. J. Environ. Manag. 2024, 349, 119406. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, P.; Qiu, J.; Guo, Z.; Sun, X.; Gu, X. Recycling hazardous steel slag after thermal treatment to produce a binder for cemented paste backfill. Powder Technol. 2022, 395, 652–662. [Google Scholar] [CrossRef]
- Li, W.; Cao, M.; Wang, D.; Chang, J. Increase in volume stability of RO phases in steel slag by combined treatment of alkali and dry carbonation. Constr. Build. Mater. 2023, 396, 132345. [Google Scholar] [CrossRef]
- Chen, Z.; Li, W.; Cao, M. Coupling effect of γ-dicalcium silicate and slag on carbonation resistance of low carbon materials. J. Clean. Prod. 2020, 262, 121385. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, J.; Chen, Z.; Liu, J. Hydration activity and expansibility of different divalent metal oxide solution compositions in steel slag. Adv. Cem. Res. 2023, 35, 408–418. [Google Scholar] [CrossRef]
- Prateek, G.; Zheng, K.; Gong, C.; Wei, S.; Zhou, X.; Yuan, Q. Preparing high strength cementitious materials with high proportion of steel slag through reverse filling approach. Constr. Build. Mater. 2023, 368, 130474. [Google Scholar] [CrossRef]
- Kim, J.-M.; Choi, S.-M.; Han, D. Improving the mechanical properties of rapid air cooled ladle furnace slag powder by gypsum. Constr. Build. Mater. 2016, 127, 93–101. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, Q. Inhibition mechanisms of steel slag on the early-age hydration of cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]
- Atia, B.M.; Radwan, H.A.; Kassab, W.A.; Sarhan, H.K.A.; Gado, M.A.; Goda, A.E. Highly efficient recovery of vanadium from Abu Zeneima ferruginous siltstone, Southwestern Sinai, Egypt, by a novel polyimine-based chelating ligand. J. Chin. Chem. Soc. 2024, 71, 507–522. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, W.; Lyu, X.; Liu, X.; Su, H.; Li, C.; Wang, H. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Qian, Q. Analysis of rock facies structure of converter slag under different processing methods. Sichuan Metall. 2019, 41, 48–51. (In Chinese) [Google Scholar]
- Diao, J.; Zhou, W.; Ke, Z.; Qiao, Y.; Zhang, T.; Liu, X.; Xie, B. System assessment of recycling of steel slag in converter steelmaking. J. Clean. Prod. 2016, 125, 159–167. [Google Scholar] [CrossRef]
- Lan, Y.-P.; Liu, Q.-C.; Meng, F.; Niu, D.-L.; Zhao, H. Optimization of magnetic separation process for iron recovery from steel slag. J. Iron Steel Res. Int. 2017, 24, 165–170. [Google Scholar] [CrossRef]
- Fisher, L.V.; Barron, A.R. The recycling and reuse of steelmaking slags—A review. Resour. Conserv. Recycl. 2019, 146, 244–255. [Google Scholar] [CrossRef]
- Li, P.; Guo, H.; Gao, J.; Min, J.; Yan, B.; Chen, D.; Seetharaman, S. Novel concept of steam modification towards energy and iron recovery from steel slag: Oxidation mechanism and process evaluation. J. Clean. Prod. 2020, 254, 119952. [Google Scholar] [CrossRef]
- Li, Y.; Dai, W.-B. Modifying hot slag and converting it into value-added materials: A review. J. Clean. Prod. 2018, 175, 176–189. [Google Scholar] [CrossRef]
- Xue, P.; He, D.; Xu, A.; Gu, Z.; Yang, Q.; Engström, F.; Björkman, B. Modification of industrial BOF slag: Formation of MgFe2O4 and recycling of iron. J. Alloys Compd. 2017, 712, 640–648. [Google Scholar] [CrossRef]
- Liu, Z.; Bi, X.; Gao, Z.; Liu, W. Carbothermal Reduction of Iron Ore in Its Concentrate-Agricultural Waste Pellets. Adv. Mater. Sci. Eng. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Agrawal, S.; Dhawan, N. Microwave Carbothermic Reduction of Low-Grade Iron Ore. Metall. Mater. Trans. B 2020, 51, 1576–1586. [Google Scholar] [CrossRef]
- Shekhar Samanta, N.; Das, P.P.; Dhara, S.; Purkait, M.K. An Overview of Precious Metal Recovery from Steel Industry Slag: Recovery Strategy and Utilization. Ind. Eng. Chem. Res. 2023, 62, 9006–9031. [Google Scholar] [CrossRef]
- Das, P.; Upadhyay, S.; Dubey, S.; Singh, K.K. Waste to wealth: Recovery of value-added products from steel slag. J. Environ. Chem. Eng. 2021, 9, 105640. [Google Scholar] [CrossRef]
- Mohamed Noor, N.; Ismail, A.K.; Jaafar, J.; Zakaria, N.A.; Abdullah, R. Heavy metal recovery from electric arc furnace steel slag by using hydrochloric acid leaching. In Proceedings of the E3S Web of Conferences, Penang, Malaysia, 28–29 November 2017; EDP Sciences: Les Ulis, France, 2018; Volume 34. [Google Scholar]
- Liu, X.; Wang, D.; Li, Z.; Ouyang, W.; Bao, Y.; Gu, C. Efficient separation of iron elements from steel slag based on magnetic separation process. J. Mater. Res. Technol. 2023, 23, 2362–2370. [Google Scholar] [CrossRef]
- Gomes, H.I.; Jones, A.; Rogerson, M.; Greenway, G.M.; Lisbona, D.F.; Burke, I.T.; Mayes, W.M. Removal and recovery of vanadium from alkaline steel slag leachates with anion exchange resins. J. Environ. Manag. 2017, 187, 384–392. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, L.; Li, W.; Liu, Y.; Wang, S. Separation and recovery of vanadium and chromium from acidic leach solution of V-Cr-bearing reducing slag. J. Environ. Chem. Eng. 2017, 5, 4702–4706. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, L.; Liu, Y.; Wang, S.; Chen, B. Extraction of vanadium from converter slag by two-step sulfuric acid leaching process. J. Clean. Prod. 2018, 170, 1089–1101. [Google Scholar] [CrossRef]
- Ju, J.; Feng, Y.; Li, H.; Xu, C.; Yang, Y. Efficient Separation and Recovery of Vanadium, Titanium, Iron, Magnesium, and Synthesizing Anhydrite from Steel Slag. Min. Metall. Explor. 2022, 39, 733–748. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, E.Y.; Chung, K.W.; Kim, R.; Jeon, H.S. A review on the metallurgical recycling of vanadium from slags: Towards a sustainable vanadium production. J. Mater. Res. Technol. 2021, 12, 343–364. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Yang, J.-Y. Vanadium extraction from steel slag: Generation, recycling and management. Environ. Pollut. 2023, 343, 123126. [Google Scholar] [CrossRef]
- An, Y.; Ma, B.; Li, X.; Chen, Y.; Wang, C.; Gao, M.; Feng, G. A review on the roasting-assisted leaching and recovery of V from vanadium slag. Process Saf. Environ. Prot. 2023, 173, 263–276. [Google Scholar] [CrossRef]
- Gao, F.; Zhou, J. Semiclassical states for critical choquard equations with critical frequency. Topol. Methods Nonlinear Anal. 2021, 57, 107–133. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y. Phase transformations of vanadium recovery from refractory stone coal by novel NaOH molten roasting and water leaching technology. RSC Adv. 2017, 7, 36917–36922. [Google Scholar] [CrossRef]
- Ji, Y.; Shen, S.; Liu, J.; Xue, Y. Cleaner and effective process for extracting vanadium from vanadium slag by using an innovative three-phase roasting reaction. J. Clean. Prod. 2017, 149, 1068–1078. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Zhou, M.; Gao, H.; Liu, J.; Xue, X. Roasting and leaching behaviors of vanadium and chromium in calcification roasting–acid leaching of high-chromium vanadium slag. Int. J. Miner. Metall. Mater. 2018, 25, 515–526. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xue, Z. Oxidation Kinetics of Vanadium Slag Roasting in the Presence of Calcium Oxide. Miner. Process. Extr. Metall. Rev. 2017, 38, 265–273. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Q.; Lv, X.; Bai, C. Effect of Mechanical Activation Treatment on the Recovery of Vanadium from Converter Slag. Metall. Mater. Trans. B: Process Metall. Mater. Process. Sci. 2017, 48, 2759–2767. [Google Scholar] [CrossRef]
- Tian, L.; Xu, Z.; Chen, L.; Liu, Y.; Zhang, T. Effect of microwave heating on the pressure leaching of vanadium from converter slag. Hydrometallurgy 2019, 184, 45–54. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, T.; Gao, H.; Liu, Y.; Zheng, X.; Xue, X. Comparison of Ultrasound-Assisted and Regular Leaching of Vanadium and Chromium from Roasted High Chromium Vanadium Slag. JOM. 2018, 70, 155–160. [Google Scholar] [CrossRef]
- Kim, E.; Spooren, J.; Broos, K.; Nielsen, P.; Horckmans, L.; Vrancken, K.C.; Quaghebeur, M. New method for selective Cr recovery from stainless steel slag by NaOCl assisted alkaline leaching and consecutive BaCrO4 precipitation. Chem. Eng. J. 2016, 295, 542–551. [Google Scholar] [CrossRef]
- Kim, E.; Spooren, J.; Broos, K.; Horckmans, L.; Quaghebeur, M. Selective recovery of Cr from stainless steel slag by alkaline roasting followed by water leaching. Hydrometallurgy 2015, 158, 139–148. [Google Scholar] [CrossRef]
- Duan, S.; Liao, H.; Cheng, F.; Song, H.; Yang, H. Investigation into the synergistic effects in hydrated gelling systems containing fly ash, desulfurization gypsum and steel slag. Constr. Build. Mater. 2018, 187, 1113–1120. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Yang, M.; Meng, Y.; Fu, Z.; Wang, M. A novel technology for the production of crystal Cr2O3 with V-Cr-bearing reducing slag. J. Environ. Chem. Eng. 2020, 8, 103799. [Google Scholar] [CrossRef]
- Du, C.; Gao, X.; Ueda, S.; Kitamura, S. Selective Leaching of P from Steelmaking Slag in Sulfuric Acid Solution. J. Sustain. Metall. 2019, 5, 594–605. [Google Scholar] [CrossRef]
- Benhelal, E.; Shamsaei, E.; Rashid, M.I. Challenges against CO2 abatement strategies in cement industry: A review. J. Environ. Sci. 2021, 104, 84–101. [Google Scholar] [CrossRef]
- Li, W.; Gao, S. Prospective on energy related carbon emissions peak integrating optimized intelligent algorithm with dry process technique application for China’s cement industry. Energy 2018, 165, 33–54. [Google Scholar] [CrossRef]
- Zhang, L.; Mabee, W.E. Comparative study on the life-cycle greenhouse gas emissions of the utilization of potential low carbon fuels for the cement industry. J. Clean. Prod. 2016, 122, 102–112. [Google Scholar] [CrossRef]
- Nair, N.; Mohammed Haneefa, K.; Santhanam, M.; Gettu, R. A study on fresh properties of limestone calcined clay blended cementitious systems. Constr. Build. Mater. 2020, 254, 119326. [Google Scholar] [CrossRef]
- Sui, S.; Shan, Y.; Li, S.; Geng, Y.; Wang, F.; Liu, Z.; Jiang, J.; Wang, L.; Yang, Z. Investigation on chloride migration behavior of metakaolin-quartz-limestone blended cementitious materials with electrochemical impedance spectroscopy method. Case Stud. Constr. Mater. 2024, 20, e03064. [Google Scholar] [CrossRef]
- Srivastava, S.; Snellings, R.; Cool, P. Clinker-free carbonate-bonded (CFCB) products prepared by accelerated carbonation of steel furnace slags: A parametric overview of the process development. Constr. Build. Mater. 2021, 303, 124556. [Google Scholar] [CrossRef]
- Liu, J.; Yu, B.; Wang, Q. Application of steel slag in cement treated aggregate base course. J. Clean. Prod. 2020, 269, 121733. [Google Scholar] [CrossRef]
- Saxena, S.; Tembhurkar, A.R. Impact of use of steel slag as coarse aggregate and wastewater on fresh and hardened properties of concrete. Constr. Build. Mater. 2018, 165, 126–137. [Google Scholar] [CrossRef]
- Wang, J.; Deng, X.; Tan, H.; Guo, H.; Zhang, J.; Li, M.; Chen, P.; He, X.; Yang, J.; Jian, S.; et al. Effect of direct electric curing on the mechanical properties, hydration process, and environmental benefits of cement-steel slag composite. Constr. Build. Mater. 2023, 406, 133382. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, B. Modification of phases evolution and heavy metal immobilization in alkali-activated MSWI FA by the incorporation of converter steel slag. J. Build. Eng. 2023, 78, 107573. [Google Scholar] [CrossRef]
- Yang, J.; Lu, J.; Wu, Q.; Xia, M.F.; Li, X. Influence of steel slag powders on the properties of MKPC paste. Constr. Build. Mater. 2018, 159, 137–146. [Google Scholar] [CrossRef]
- Li, L.; Ye, T.; Li, Y.; Wang, Z.; Feng, Z.; Liu, Z. Improvement of microporous structure and impermeability of cement mortars using fly ash and blast furnace slag under low curing pressures. Constr. Build. Mater. 2023, 401, 132890. [Google Scholar] [CrossRef]
- Mardmomen, S.; Chen, H.-L. Modeling the thermal and mechanical properties of early age concrete containing ground granulated blast furnace slag. Constr. Build. Mater. 2023, 401, 132902. [Google Scholar] [CrossRef]
- Wang, J.; Deng, X.; Tan, H.; Guo, H.; Zhang, J.; Li, M.; Chen, P.; He, X.; Yang, J.; Jian, S.; et al. The mechanical properties and sustainability of phosphogypsum-slag binder activated by nano-ettringite. Sci. Total Environ. 2023, 903, 166015. [Google Scholar] [CrossRef]
- Ma, X.; Yao, B.; Zou, J.; Ho, J.C.M.; Zhuang, X.; Wang, Q. Sodium gluconate as a retarder modified sewage sludge ash-based geopolymers: Mechanism and environmental assessment. J. Clean. Prod. 2023, 419, 138317. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, M.; He, X.; Zheng, Z.; Su, Y.; Yang, J.; Zhao, H. Effect of wet-grinding steel slag on the properties of Portland cement: An activated method and rheology analysis. Constr. Build. Mater. 2021, 286, 122823. [Google Scholar] [CrossRef]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Huo, W.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Efficient use of steel slag in alkali-activated fly ash-steel slag-ground granulated blast furnace slag ternary blends. Constr. Build. Mater. 2020, 259, 119814. [Google Scholar] [CrossRef]
- Amran, M.; Murali, G.; Khalid, N.H.A.; Fediuk, R.; Ozbakkaloglu, T.; Lee, Y.H.; Haruna, S.; Lee, Y.Y. Slag uses in making an ecofriendly and sustainable concrete: A review. Constr. Build. Mater. 2021, 272, 121942. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Yin, H.; Chen, J.; Zhang, N. Intermediate-calcium based cementitious materials prepared by MSWI fly ash and other solid wastes: Hydration characteristics and heavy metals solidification behavior. J. Hazard. Mater. 2018, 349, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Liu, Z.; Lyu, X.; Wang, X.; Liu, J.; Zhang, T. In-depth insight into the cementitious synergistic effect of steel slag and red mud on the properties of composite cementitious materials. J. Build. Eng. 2022, 52, 104449. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, W.; Li, Y.; Ba, H.; Li, N.; Ju, Y.; Zhao, B.; Jia, G.; Hu, W. The mechanism of hydration reaction of granulated blast furnace slag-steel slag-refining slag-desulfurization gypsum-based clinker-free cementitious materials. J. Build. Eng. 2021, 44, 103289. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, Z.; Huang, Y.; Gu, F.; Zhang, J.; Tang, H.; Yang, L.; Tian, W.; Rao, M.; Li, G.; et al. Mechanical properties and environmental characteristics of the synergistic preparation of cementitious materials using electrolytic manganese residue, steel slag, and blast furnace slag. Constr. Build. Mater. 2024, 411, 134480. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, S.; Yang, H.; Ni, W.; Li, J.; Zhang, G.; Teng, G. Influence on fine lead–zinc tailings solidified/stabilised by clinker-free slag-based binder. J. Environ. Chem. Eng. 2022, 10, 108692. [Google Scholar] [CrossRef]
- Du, H.; Xu, D.; Li, X.; Li, J.; Ni, W.; Li, Y.; Fu, P. Application of molten iron desulfurization slag to replace steel slag as an alkaline component in solid waste-based cementitious materials. J. Clean. Prod. 2022, 377, 134353. [Google Scholar] [CrossRef]
- Gao, T.; Dai, T.; Shen, L.; Jiang, L. Benefits of using steel slag in cement clinker production for environmental conservation and economic revenue generation. J. Clean. Prod. 2021, 282, 124538. [Google Scholar] [CrossRef]
- Zvironaite, J.; Pranckeviciene, J.; Kligys, M.; Balciunas, G.; Macanovskis, A. Alkali-silica reactivity of portland-composite cements and gravel aggregates. Ceramics 2019, 63, 76–85. [Google Scholar] [CrossRef]
- Shehata, N.; Sayed, E.T.; Abdelkareem, M.A. Recent progress in environmentally friendly geopolymers: A review. Sci. Total Environ. 2021, 762, 143166. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Noguchi, N.; Matumoto, K.; Morinaga, Y.; Chabayashi, T.; Kato, H.; Nawa, T. Characteristics of ferrite-rich portland cement: Comparison with ordinary portland cement. Front. Mater. 2019, 6, 97. [Google Scholar] [CrossRef]
- Taimasov, B.T.; Sarsenbayev, B.K.; Khudyakova, T.M.; Kolesnikov, A.S.; Zhanikulov, N.N. Development and testing of low-energy-intensive technology of receiving sulphate-resistant and road portlandcement. Eurasian Chem.-Technol. J. 2017, 19, 347–355. [Google Scholar] [CrossRef]
- Verma, Y.K.; Mazumdar, B.; Ghosh, P. CO2 emission reduction using blast furnace slag for the clinker manufacturing in cement industry. J. Indian Chem. Soc. 2020, 97, 1083–1087. [Google Scholar]
- Dai, S.; Zhu, H.; Zhang, D.; Liu, Z.; Cheng, S.; Zhao, J. Insights to compressive strength, impermeability and microstructure of micro-expansion steel slag cement under constraint conditions. Constr. Build. Mater. 2022, 326, 126540. [Google Scholar] [CrossRef]
- Jiang, Y.; Ahmad, M.R.; Chen, B. Properties of magnesium phosphate cement containing steel slag powder. Constr. Build. Mater. 2019, 195, 140–147. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, Y.; Ma, H.; Zhou, X.; Luo, Z. Upcycling steel slag in producing eco-efficient iron–calcium phosphate cement. J. Clean. Prod. 2022, 371, 133688. [Google Scholar] [CrossRef]
- Yu, X.; Chu, J.; Wu, S.; Wang, K. Production of biocement using steel slag. Constr. Build. Mater. 2023, 383, 131365. [Google Scholar] [CrossRef]
- Agrawal, U.S.; Wanjari, S.P.; Naresh, D.N. Characteristic study of geopolymer fly ash sand as a replacement to natural river sand. Constr. Build. Mater. 2017, 150, 681–688. [Google Scholar] [CrossRef]
- Xiao, J.; Qiang, C.; Nanni, A.; Zhang, K. Use of sea-sand and seawater in concrete construction: Current status and future opportunities. Constr. Build. Mater. 2017, 155, 1101–1111. [Google Scholar] [CrossRef]
- Yu, H.; Xu, P.; Zhu, Y.; Wan, X.; Zhu, H. Effect of Carbonated Steel Slag as Sand Replacement on Strength and Durability of Cement Mortar. J. Qingdao Technol. Univ. 2023, 44, 20–28. (In Chinese) [Google Scholar]
- Gencel, O.; Karadag, O.; Oren, O.H.; Bilir, T. Steel slag and its applications in cement and concrete technology: A review. Constr. Build. Mater. 2021, 283, 122783. [Google Scholar] [CrossRef]
- Pan, Z.; Zhou, J.; Jiang, X.; Xu, Y.; Jin, R.; Ma, J.; Zhuang, Y.; Diao, Z.; Zhang, S.; Si, Q.; et al. Investigating the effects of steel slag powder on the properties of self-compacting concrete with recycled aggregates. Constr. Build. Mater. 2019, 200, 570–577. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, H.; Wang, J.; Feng, Q. Preliminary investigation on the pozzolanic activity of superfine steel slag. Constr. Build. Mater. 2015, 82, 227–234. [Google Scholar] [CrossRef]
- Liu, G.; Schollbach, K.; van der Laan, S.; Tang, P.; Florea, M.V.A.; Brouwers, H.J.H. Recycling and utilization of high volume converter steel slag into CO2 activated mortars—The role of slag particle size. Resour. Conserv. Recycl. 2020, 160, 104883. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P.; Liu, J.; Wang, X.; Bai, Z. Effect of Fe and Mn on the hydration activity of f-CaO in steel slag. Constr. Build. Mater. 2024, 421, 135719. [Google Scholar] [CrossRef]
- Li, W.; Cao, M.; Wang, D.; Zhao, J.; Chang, J. Improving the hydration activity and volume stability of the RO phases in steel slag by combining alkali and wet carbonation treatments. Cem. Concr. Res. 2023, 172, 107236. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, P.; Liu, S. Microwave pre-curing of Portland cement-steel slag powder composite for its hydration properties. Constr. Build. Mater. 2018, 189, 1093–1104. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, D. Hydration and mechanical properties of cement-steel slag system incorporating different activators. Constr. Build. Mater. 2023, 363, 129981. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, D.; Luo, D. Enhanced hydration and mechanical properties of cement-based materials with steel slag modified by water glass. J. Mater. Res. Technol. 2022, 21, 1830–1842. [Google Scholar] [CrossRef]
- Tan, H.; Nie, K.; He, X.; Guo, Y.; Zhang, X.; Deng, X.; Su, Y.; Yang, J. Effect of organic alkali on compressive strength and hydration of wet-grinded granulated blast-furnace slag containing Portland cement. Constr. Build. Mater. 2019, 206, 10–18. [Google Scholar] [CrossRef]
- Polaczyk, P.; Ma, Y.; Xiao, R.; Hu, W.; Jiang, X.; Huang, B. Characterization of aggregate interlocking in hot mix asphalt by mechanistic performance tests. Road Mater. Pavement Des. 2021, 22, S498–S513. [Google Scholar] [CrossRef]
- Polaczyk, P.; Huang, B.; Shu, X.; Gong, H. Investigation into Locking Point of Asphalt Mixtures Utilizing Superpave and Marshall Compactors. J. Mater. Civ. Eng. 2019, 31, 04019188. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, I.; Lastra-González, P.; Indacoechea-Vega, I.; Castro-Fresno, D. Recyclability potential of asphalt mixes containing reclaimed asphalt pavement and industrial by-products. Constr. Build. Mater. 2019, 195, 148–155. [Google Scholar] [CrossRef]
- Motevalizadeh, S.M.; Sedghi, R.; Rooholamini, H. Fracture properties of asphalt mixtures containing electric arc furnace slag at low and intermediate temperatures. Constr. Build. Mater. 2020, 240, 117965. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, B.; Wei, H.; Chai, C.; Chen, Y. Laboratory evaluation on the performance of porous asphalt mixture with steel slag for seasonal frozen regions. Sustainability 2019, 11, 6924. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, J.; Hong, R.; Ye, S.; Jin, A. Research on low-temperature performance of steel slag/polyester fiber permeable asphalt mixture. Constr. Build. Mater. 2022, 334, 127214. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Li, M.; Wang, Z.; Zhang, T. Microwave heating uniformity, road performance and internal void characteristics of steel slag asphalt mixtures. Constr. Build. Mater. 2022, 353, 129155. [Google Scholar] [CrossRef]
- Su, J.F.; Wang, L.Q.; Xie, X.M.; Gao, X. Understanding the final surface state of self-healing microcapsules containing rejuvenator in bituminous binder of asphalt. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126287. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Zhang, Y.; Feng, S.; Lu, G.; Cao, L. Laboratory and Numerical Investigation of Microwave Heating Properties of Asphalt Mixture. Materials 2019, 12, 146. [Google Scholar] [CrossRef]
- Liu, Q.; Li, B.; Schlangen, E.; Sun, Y. Research on the mechanical, thermal, induction heating and healing properties of steel slag/steel fibers composite asphalt mixture. Appl. Sci. 2017, 7, 1088. [Google Scholar] [CrossRef]
- Xu, H.; Wu, S.; Li, H.; Zhao, Y.; Lv, Y. Study on recycling of steel slags used as coarse and fine aggregates in induction healing asphalt concretes. Materials 2020, 13, 889. [Google Scholar] [CrossRef]
- Lou, B.; Sha, A.; Barbieri, D.M.; Liu, Z.; Zhang, F. Microwave heating properties of steel slag asphalt mixture using a coupled electromagnetic and heat transfer model. Constr. Build. Mater. 2021, 291, 123248. [Google Scholar] [CrossRef]
- Lou, B.; Liu, Z.; Sha, A.; Jia, M.; Li, Y. Microwave absorption ability of steel slag and road performance of asphalt mixtures incorporating steel slag. Materials 2020, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, D.; Cannone Falchetto, A.; Cao, Y. Microwave deicing properties and carbon emissions assessment of asphalt mixtures containing steel slag towards resource conservation and waste reuse. Sci. Total Environ. 2024, 912, 169189. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, T.; Guo, H.; Wang, Z.; Wang, X. Evaluation of self-healing properties of asphalt mixture containing steel slag under microwave heating: Mechanical, thermal transfer and voids microstructural characteristics. J. Clean. Prod. 2022, 342, 130932. [Google Scholar] [CrossRef]
- Wan, J.; Wu, S.; Xiao, Y.; Chen, Z.; Zhang, D. Study on the effective composition of steel slag for asphalt mixture induction heating purpose. Constr. Build. Mater. 2018, 178, 542–550. [Google Scholar] [CrossRef]
- Yang, C.; Wu, S.; Xie, J.; Amirkhanian, S.; Liu, Q.; Zhang, J.; Xiao, Y.; Zhao, Z.; Xu, H.; Li, N.; et al. Enhanced induction heating and self-healing performance of recycled asphalt mixtures by incorporating steel slag. J. Clean. Prod. 2022, 366, 132999. [Google Scholar] [CrossRef]
- Fakhri, M.; Ahmadi, A. Evaluation of fracture resistance of asphalt mixes involving steel slag and RAP: Susceptibility to aging level and freeze and thaw cycles. Constr. Build. Mater. 2017, 157, 748–756. [Google Scholar] [CrossRef]
- Pasetto, M.; Baldo, N. Dissipated energy analysis of four-point bending test on asphalt concretes made with steel slag and RAP. Int. J. Pavement Res. Technol. 2017, 10, 446–453. [Google Scholar] [CrossRef]
- Faleschini, F.; Fernández-Ruíz, M.A.; Zanini, M.A.; Brunelli, K.; Pellegrino, C.; Hernández-Montes, E. High performance concrete with electric arc furnace slag as aggregate: Mechanical and durability properties. Constr. Build. Mater. 2015, 101, 113–121. [Google Scholar] [CrossRef]
- Lai, M.H.; Zou, J.; Yao, B.; Ho, J.C.M.; Zhuang, X.; Wang, Q. Improving mechanical behavior and microstructure of concrete by using BOF steel slag aggregate. Constr. Build. Mater. 2021, 277, 122269. [Google Scholar] [CrossRef]
- Song, Q.; Guo, M.; Wang, L.; Ling, T. Use of steel slag as sustainable construction materials: A review of accelerated carbonation treatment. Resour. Conserv. Recycl. 2021, 173, 105740. [Google Scholar] [CrossRef]
- Jung, S.; Chau, T.V.; Kim, M.; Na, W.-B. Artificial Seaweed Reefs That Support the Establishment of Submerged Aquatic Vegetation Beds and Facilitate Ocean Macroalgal Afforestation: A Review. J. Mar. Sci. Eng. 2022, 10, 1184. [Google Scholar] [CrossRef]
- Tsiamis, K.; Salomidi, M.; Gerakaris, V.; Mogg, A.O.M.; Porter, E.S.; Sayer, M.D.J.; Küpper, F.C. Macroalgal vegetation on a north European artificial reef (Loch Linnhe, Scotland): Biodiversity, community types and role of abiotic factors. J. Appl. Phycol. 2020, 32, 1353–1363. [Google Scholar] [CrossRef]
- Baalamurugan, J.; Ganesh Kumar, V.; Naveen Prasad, B.S.N.; Padmapriya, R.; Karthick, V.; Govindaraju, K. Recycling of induction furnace steel slag in concrete for marine environmental applications towards ocean acidification studies. Int. J. Environ. Sci. Technol. 2021, 19, 5039–5048. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Liu, Y.; Hu, W.; Ni, W. On the use of blast furnace slag and steel slag in the preparation of green artificial reef concrete. Constr. Build. Mater. 2016, 112, 241–246. [Google Scholar] [CrossRef]
- Layman, C.A.; Allgeier, J.E.; Lemasson, A. An ecosystem ecology perspective on artificial reef production. J. Appl. Ecol. 2020, 57, 2139–2148. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.R.; Kim, Y.R.; Yoon, S.; Kim, K. Assessment of Heavy Metals Eluted from Materials Utilized in Artificial Reefs Implemented in South Korea. J. Mar. Sci. Eng. 2022, 10, 1720. [Google Scholar] [CrossRef]
- Roslan, N.H.; Ismail, M.; Abdul-Majid, Z.; Ghoreishiamiri, S.; Muhammad, B. Performance of steel slag and steel sludge in concrete. Constr. Build. Mater. 2016, 104, 16–24. [Google Scholar] [CrossRef]
- Bodor, M.; Santos, R.M.; Cristea, G.; Salman, M.; Cizer, Ö.; Iacobescu, R.I.; Chiang, Y.W.; van Balen, K.; Vlad, M.; van Gerven, T. Laboratory investigation of carbonated BOF slag used as partial replacement of natural aggregate in cement mortars. Cem. Concr. Compos. 2016, 65, 55–66. [Google Scholar] [CrossRef]
- Xue, G.; Fu, Q.; Xu, S.; Li, J. Macroscopic mechanical properties and microstructure characteristics of steel slag fine aggregate concrete. J. Build. Eng. 2022, 56, 104742. [Google Scholar] [CrossRef]
- Chang, X.; Zhang, R.; Xiao, Y.; Chen, X.; Zhang, X.; Liu, G. Mapping of publications on asphalt pavement and bitumen materials: A bibliometric review. Constr. Build. Mater. 2020, 234, 117370. [Google Scholar] [CrossRef]
- Deng, L.; Yun, F.; Jia, R.; Li, H.; Jia, X.; Shi, Y.; Zhang, X. Effect of SiO2/MgO ratio on the crystallization behavior, structure, and properties of wollastonite-augite glass-ceramics derived from stainless steel slag. Mater. Chem. Phys. 2020, 239, 122039. [Google Scholar] [CrossRef]
- Shi, Y.; Li, B.W.; Zhao, M.; Zhang, M.X. Growth of diopside crystals in CMAS glass-ceramics using Cr2O3 as a nucleating agent. J. Am. Ceram. Soc. 2018, 101, 3968–3978. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Wu, T. Effect of Cr2O3 on the crystallization behavior of synthetic diopside and characterization of Cr-doped diopside glass ceramics. Ceram. Int. 2018, 44, 10119–10129. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, L.; Wang, Y.; Cang, D. Effects of Fe2O3 on the properties of ceramics from steel slag. Int. J. Miner. Metall. Mater. 2018, 25, 413–419. [Google Scholar] [CrossRef]
- Luo, Z.; He, F.; Zhang, W.; Xiao, Y.; Xie, J.; Sun, R.; Xie, M. Effects of fluoride content on structure and properties of steel slag glass-ceramics. Mater. Chem. Phys. 2020, 242, 122531. [Google Scholar] [CrossRef]
- OuYang, S.; Zhang, Y.; Chen, Y.; Zhao, Z.; Wen, M.; Li, B.; Shi, Y.; Zhang, M.; Liu, S. Preparation of Glass-ceramics Using Chromium-containing Stainless Steel Slag: Crystal Structure and Solidification of Heavy Metal Chromium. Sci. Rep. 2019, 9, 1964. [Google Scholar] [CrossRef]
- Shang, W.; Peng, Z.; Huang, Y.; Gu, F.; Zhang, J.; Tang, H.; Yang, L.; Tian, W.; Rao, M.; Li, G.; et al. Production of glass-ceramics from metallurgical slags. J. Clean. Prod. 2021, 317, 128220. [Google Scholar] [CrossRef]
- Bayer Ozturk, Z.; Eren Gultekin, E. Preparation of ceramic wall tiling derived from blast furnace slag. Ceram. Int. 2015, 41, 12020–12026. [Google Scholar] [CrossRef]
- Zong, Y.; Zhang, X.; Mukiza, E.; Xu, X.; Li, F. Effect of fly ash on the properties of ceramics prepared from steel slag. Appl. Sci. 2018, 8, 1187. [Google Scholar] [CrossRef]
- Pal, M.; Das, S.; Gupta, S.; Das, S.K. Thermal analysis and vitrification behavior of slag containing porcelain stoneware body. J. Therm. Anal. Calorim. 2016, 124, 1169–1177. [Google Scholar] [CrossRef]
- Tabit, K.; Waqif, M.; Saâdi, L. Anorthite-cordierite based binary ceramics from coal fly ash and steel slag for thermal and dielectric applications. Mater. Chem. Phys. 2020, 254, 123472. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Wu, J.; Liu, S.; Ma, S.; Cheng, T. Preparation and thermal shock resistance of solar thermal storage ceramics from high calcium and high iron steel slag. Ceram. Int. 2023, 50, 8099–8108. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Ouyang, S.; Li, H.; Li, X.; Li, B. Microstructural transformation of stainless steel slag-based CAMS glass ceramics prepared by SPS. Ceram. Int. 2021, 47, 1284–1293. [Google Scholar] [CrossRef]
- Zong, Y.; Wan, Q.; Cang, D. Preparation of anorthite-based porous ceramics using high-alumina fly ash microbeads and steel slag. Ceram. Int. 2019, 45, 22445–22451. [Google Scholar] [CrossRef]
- Yingliang, Z.; Zhengyu, M.; Jingping, Q.; Xiaogang, S.; Xiaowei, G. Experimental study on the utilization of steel slag for cemented ultra-fine tailings backfill. Powder Technol. 2020, 375, 284–291. [Google Scholar] [CrossRef]
- Zhang, M.; Li, K.; Ni, W.; Zhang, S.; Liu, Z.; Wang, K.; Wei, X.; Yu, Y. Preparation of mine backfilling from steel slag-based non-clinker combined with ultra-fine tailing. Constr. Build. Mater. 2022, 320, 126248. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, B.; Ren, Y.; Wu, Z.; Li, Q.; Li, K.; Zhang, M.; Yu, J.; Liu, J.; Ni, W. The Preparation Process and Hydration Mechanism of Steel Slag-Based Ultra-Fine Tailing Cementitious Filler. Gels 2023, 9, 82. [Google Scholar] [CrossRef]
- Li, J.; Yilmaz, E.; Cao, S. Influence of industrial solid waste as filling material on mechanical and microstructural characteristics of cementitious backfills. Constr. Build. Mater. 2021, 299, 124288. [Google Scholar] [CrossRef]
- Hao, J.; Zhou, Z.; Chen, Z.; Zhou, Y.; Wang, J. Damage characterization and microscopic mechanism of steel slag-cemented paste backfill under uniaxial compression. Constr. Build. Mater. 2023, 409, 134175. [Google Scholar] [CrossRef]
- Li, J.; Cao, S.; Yilmaz, E. Characterization of Macro Mechanical Properties and Microstructures of Cement-Based Composites Prepared from Fly Ash, Gypsum and Steel Slag. Minerals 2021, 12, 6. [Google Scholar] [CrossRef]
- Das, S.; Kim, G.W.; Hwang, H.Y.; Verma, P.P.; Kim, P.J. Cropping With Slag to Address Soil, Environment, and Food Security. Front. Microbiol. 2019, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Deus, A.C.F.; Büll, L.T.; Guppy, C.N.; Santos, S.d.M.C.; Moreira, L.L.Q. Effects of lime and steel slag application on soil fertility and soybean yield under a no till-system. Soil Tillage Res. 2020, 196, 104422. [Google Scholar] [CrossRef]
- Wen, T.; Yang, L.; Dang, C.; Miki, T.; Bai, H.; Nagasaka, T. Effect of basic oxygen furnace slag on succession of the bacterial community and immobilization of various metal ions in acidic contaminated mine soil. J. Hazard. Mater. 2020, 388, 121784. [Google Scholar] [CrossRef]
- Yang, L.; Wei, T.; Li, S.; Lv, Y.; Miki, T.; Yang, L.; Nagasaka, T. Immobilization persistence of Cu, Cr, Pb, Zn ions by the addition of steel slag in acidic contaminated mine soil. J. Hazard. Mater. 2021, 412, 125176. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Liu, Y.; Zhang, Y.; Ren, J.; Zeng, Y.; Huang, Z. Synthesis of biochar modified steel slag composites for passivation of multiple heavy metals in soil. J. Environ. Chem. Eng. 2024, 12, 114026. [Google Scholar] [CrossRef]
- Guerrini, I.A.; Croce, C.G.G.; Bueno, O.d.C.; Jacon, C.P.R.P.; Nogueira, T.A.R.; Fernandes, D.M.; Ganga, A.; Capra, G.F. Composted sewage sludge and steel mill slag as potential amendments for urban soils involved in afforestation programs. Urban For. Urban Green. 2017, 22, 93–104. [Google Scholar] [CrossRef]
- Das, S.; Gwon, H.S.; Khan, M.I.; Jeong, S.T.; Kim, P.J. Steel slag amendment impacts on soil microbial communities and activities of rice (Oryza sativa L.). Sci. Rep. 2020, 10, 6746. [Google Scholar] [CrossRef]
- Wang, W.; Sardans, J.; Lai, D.Y.F.; Wang, C.; Zeng, C.; Tong, C.; Liang, Y.; Peñuelas, J. Effects of steel slag application on greenhouse gas emissions and crop yield over multiple growing seasons in a subtropical paddy field in China. Field Crops Res. 2015, 171, 146–156. [Google Scholar] [CrossRef]
- Wang, M.; Lan, X.; Xu, X.; Fang, Y.; Singh, B.P.; Sardans, J.; Romero, E.; Peñuelas, J.; Wang, W. Steel slag and biochar amendments decreased CO2 emissions by altering soil chemical properties and bacterial community structure over two-year in a subtropical paddy field. Sci. Total Environ. 2020, 740, 140403. [Google Scholar] [CrossRef]
- Ning, D.; Liang, Y.; Song, A.; Duan, A.; Liu, Z. In situ stabilization of heavy metals in multiple-metal contaminated paddy soil using different steel slag-based silicon fertilizer. Environ. Sci. Pollut. Res. 2016, 23, 23638–23647. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, X.; Zhao, C.; Mou, S.; Achal, V.; Al-Misned, F.A.; Mortuza, M.G.; Gadd, G.M. Bioimmobilization of Heavy Metals in Acidic Copper Mine Tailings Soil. Geomicrobiol. J. 2016, 33, 261–266. [Google Scholar] [CrossRef]
- He, H.; Tam, N.F.Y.; Yao, A.; Qiu, R.; Li, W.C.; Ye, Z. Growth and Cd uptake by rice (Oryza sativa) in acidic and Cd-contaminated paddy soils amended with steel slag. Chemosphere 2017, 189, 247–254. [Google Scholar] [CrossRef]
- Bashir, S.; Salam, A.; Rehman, M.; Khan, S.; Gulshan, A.B.; Iqbal, J.; Shaaban, M.; Mehmood, S.; Zahra, A.; Hu, H. Effective Role of Biochar, Zeolite and Steel Slag on Leaching Behavior of Cd and Its Fractionations in Soil Column Study. Bull. Environ. Contam. Toxicol. 2019, 102, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef]
- He, H.; Xiao, Q.; Yuan, M.; Huang, R.; Sun, X.; Wang, X.; Zhao, H. Effects of steel slag amendments on accumulation of cadmium and arsenic by rice (Oryza sativa) in a historically contaminated paddy field. Environ. Sci. Pollut. Res. 2020, 27, 40001–40008. [Google Scholar] [CrossRef]
- Sahu, J.N.; Kapelyushin, Y.; Mishra, D.P.; Ghosh, P.; Sahoo, B.K.; Trofimov, E.; Meikap, B.C. Utilization of ferrous slags as coagulants, filters, adsorbents, neutralizers/stabilizers, catalysts, additives, and bed materials for water and wastewater treatment: A review. Chemosphere 2023, 325, 138201. [Google Scholar] [CrossRef]
- Han, C.; Wang, Z.; Wu, Q.; Yang, W.; Yang, H.; Xue, X. Evaluation of the role of inherent Ca2 in phosphorus removal from wastewater system. Water Sci. Technol. 2016, 73, 1644–1651. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Zhu, Y.; Bai, Z.; Luque, R.; Xuan, J. Functionalized chitosan biosorbents with ultra-high performance, mechanical strength and tunable selectivity for heavy metals in wastewater treatment. Chem. Eng. J. 2017, 325, 350–359. [Google Scholar] [CrossRef]
- Mahmoud, S.A.; Atia, B.M.; Gado, M.A. Efficient removal of toxic metals (Hg(II), Cr(III), Pb(II), Cd(II)) using high-performance polyvinyl alcohol-L-2-Amino-3-mercaptopropionic acid composite from wastewater. Int. J. Environ. Sci. Technol. 2025, 1–26. [Google Scholar] [CrossRef]
- Chen, G.; Yang, L.; Chen, J.; Miki, T.; Li, S.; Bai, H.; Nagasaka, T. Competitive mechanism and influencing factors for the simultaneous removal of Cr(III) and Zn(II) in acidic aqueous solutions using steel slag: Batch and column experiments. J. Clean. Prod. 2019, 230, 69–79. [Google Scholar] [CrossRef]
- Sarkar, C.; Basu, J.K.; Samanta, A.N. Synthesis of mesoporous geopolymeric powder from LD slag as superior adsorbent for Zinc (II) removal. Adv. Powder Technol. 2018, 29, 1142–1152. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Tian, S.; Li, K.; Yan, F.; Liu, N.; Yang, M.; Chen, X. BOF steel slag as a low-cost sorbent for vanadium (V) removal from soil washing effluent. Sci. Rep. 2017, 7, 11177. [Google Scholar] [CrossRef]
- Samanta, N.S.; Banerjee, S.; Mondal, P.; Anweshan; Bora, U.; Purkait, M.K. Preparation and characterization of zeolite from waste Linz-Donawitz (LD) process slag of steel industry for removal of Fe3+ from drinking water. Adv. Powder Technol. 2021, 32, 3372–3387. [Google Scholar] [CrossRef]
- Li, Y.; Qi, X.; Li, G.; Wang, H. Efficient removal of arsenic from copper smelting wastewater via a synergy of steel-making slag and KMnO4. J. Clean. Prod. 2021, 287, 125578. [Google Scholar] [CrossRef]
- Wang, M.; Wang, X.; Zhang, M.; Han, W.; Yuan, Z.; Zhong, X.; Yu, L.; Ji, H. Treatment of Cd(Ⅱ) and As(Ⅴ) co-contamination in aqueous environment by steel slag-biochar composites and its mechanism. J. Hazard. Mater. 2023, 447, 130784. [Google Scholar] [CrossRef]
- Liu, J.; Feng, J.; Wang, Z.; Lyu, X.; Liu, C.; Dai, H.; Bai, Z. Steel slag base geopolymer for efficient removal of Zn and Ni ions from aqueous solutions: Preparation and characterization. Mater. Lett. 2023, 340, 134173. [Google Scholar] [CrossRef]
- Huy, D.H.; Seelen, E.; Liem-Nguyen, V. Removal mechanisms of cadmium and lead ions in contaminated water by stainless steel slag obtained from scrap metal recycling. J. Water Process Eng. 2020, 36, 101369. [Google Scholar] [CrossRef]
- Sang, M.; Zhao, H.; Li, Y.; Zhu, L. The adsorption properties of steel slag-based porous geopolymer for Cu2+ removal. Miner. Eng. 2023, 201, 108225. [Google Scholar]
- Sarkar, C.; Basu, J.K.; Samanta, A.N. Microwave activated LD slag for phenolic wastewater treatment: Multi-parameter optimization, isotherms, kinetics and thermodynamics. Chem. Eng. Trans. 2017, 57, 277–282. [Google Scholar]
- Yang, L.; Qiang, X.; Wang, Z.; Li, Y.; Bai, H.; Li, H. Steel slag as low-cost adsorbent for the removal of phenanthrene and naphthalene. Adsorpt. Sci. Technol. 2018, 36, 1160–1177. [Google Scholar] [CrossRef]
- Yoon, S.; Bae, S. Development of magnetically separable Cu catalyst supported by pre-treated steel slag. Korean J. Chem. Eng. 2019, 36, 1814–1825. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Ahmad, A.; Ghangrekar, M.M. Efficient upcycling of iron scrap and waste polyethylene terephthalate plastic into Fe3O4@ C incorporated MIL-53 (Fe) as a novel electro-Fenton catalyst for the degradation of salicylic acid. Environ. Pollut. 2023, 322, 121242. [Google Scholar] [CrossRef]
- dos Santos, A.J.; da Costa Cunha, G.; Cruz, D.R.S.; Romão, L.P.C.; Martínez-Huitle, C.A. Iron mining wastes collected from Mariana disaster: Reuse and application as catalyst in a heterogeneous electro-Fenton process. J. Electroanal. Chem. 2019, 848, 113330. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.; Peng, P.; Xie, H.; Li, X.; Xu, J.; Li, W. A pilot-scale three-dimensional electrochemical reactor combined with anaerobic-anoxic-oxic system for advanced treatment of coking wastewater. J. Environ. Manag. 2020, 258, 110021. [Google Scholar] [CrossRef]
- Song, B.; Wang, Z.; Li, J.; Luo, M.; Cao, P.; Zhang, C. Sulfur-zinc modified kaolin/steel slag: A particle electrode that efficiently degrades norfloxacin in a neutral/alkaline environment. Chemosphere 2021, 284, 131328. [Google Scholar] [CrossRef]
- Wang, Z.; Song, B.; Li, J.; Teng, X. Degradation of norfloxacin wastewater using kaolin/steel slag particle electrodes: Performance, mechanism and pathway. Chemosphere 2021, 270, 128652. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Wang, J.; Yang, Y.; Xu, M.; Liu, J.; Yang, C.; Cai, Y.; He, H.; Du, Y.; et al. In-situ constructing nanostructured magnesium ferrite on steel slag for Cr(VI) photoreduction. J. Hazard. Mater. 2022, 422, 126951. [Google Scholar] [CrossRef]
- Shu, Y.; Ji, B.; Li, Y.; Zhang, W.; Zhang, H.; Zhang, J. Natural pyrite improved steel slag towards environmentally sustainable chromium reclamation from hexavalent chromium-containing wastewater. Chemosphere 2021, 282, 130974. [Google Scholar] [CrossRef]
- Sarkar, C.; Basu, J.K.; Samanta, A.N. Synthesis of MIL-53(Fe)/SiO2 composite from LD slag as a novel photo-catalyst for methylene blue degradation. Chem. Eng. J. 2019, 377, 119621. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Ab Rahim, M.H.; Alqahtani, T.M.; Witoon, T.; Lim, J.-W.; Cheng, C.K. A review on advances in green treatment of glycerol waste with a focus on electro-oxidation pathway. Chemosphere 2021, 276, 130128. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, Y.; Tang, M.; Liu, G.; Li, L. Insight into the steel converter slag composite supported three-dimensional electro-Fenton remediation of landfill leachate. J. Water Process Eng. 2023, 53, 103603. [Google Scholar] [CrossRef]
- Fang, C.; Nie, L.; Chen, H.; Yang, Y. Magnetic recyclable Co-MOF derivate-modified steel slag for tetracycline removal by integrated adsorption and Fenton-like catalysis. J. Water Process Eng. 2024, 63, 105434. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, L.; Zhang, K.; Kang, L. Synthesis of eco-friendly CaWO4/CSH nanocomposite and photocatalytic degradation of dyeing pollutant. Integr. Ferroelectr. 2017, 181, 113–122. [Google Scholar] [CrossRef]
- Inoue, T.; Chuaicham, C.; Saito, N.; Ohtani, B.; Sasaki, K. Z-scheme heterojunction of graphitic carbon nitride and calcium ferrite in converter slag for the photocatalytic imidacloprid degradation and hydrogen evolution. J. Photochem. Photobiol. A Chem. 2023, 440, 114644. [Google Scholar] [CrossRef]
- Shao, N.; Li, S.; Yan, F.; Su, Y.; Liu, F.; Zhang, Z. An all-in-one strategy for the adsorption of heavy metal ions and photodegradation of organic pollutants using steel slag-derived calcium silicate hydrate. J. Hazard. Mater. 2020, 382, 121120. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, C.; Lu, P.; Zhang, X.; Zhang, L.; Shi, J. Overcoming poisoning effects of heavy metal ions against photocatalysis for synergetic photo-hydrogen generation from wastewater. Nano Energy 2017, 38, 494–503. [Google Scholar] [CrossRef]
- Ortega-Fernández, I.; Calvet, N.; Gil, A.; Rodríguez-Aseguinolaza, J.; Faik, A.; D’Aguanno, B. Thermophysical characterization of a by-product from the steel industry to be used as a sustainable and low-cost thermal energy storage material. Energy 2015, 89, 601–609. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, H.; Zhou, J.; Cen, K. Thermal properties and friction behaviors of slag as energy storage material in concentrate solar power plants. Sol. Energy Mater. Sol. Cells 2018, 182, 21–29. [Google Scholar] [CrossRef]
- Kocak, B.; Fernandez, A.I.; Paksoy, H. Benchmarking study of demolition wastes with different waste materials as sensible thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 219, 110777. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ali, H.M.; Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: A review. Int. J. Heat Mass Transf. 2018, 127, 838–856. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y. Exploration of steel slag for thermal energy storage and enhancement by Na2CO3 modification. J. Clean. Prod. 2023, 395, 136289. [Google Scholar] [CrossRef]
- Xu, X.; Li, M.; Wang, Y.; Wu, J.; Zhou, Y.; Shen, Y. Preparation and characterization of solar absorption and thermal storage integrated ceramics from calcium and iron-rich steel slag. Ceram. Int. 2023, 49, 8381–8389. [Google Scholar] [CrossRef]
- Boquera, L.; Castro, J.R.; Fernandez, A.G.; Navarro, A.; Pisello, A.L.; Cabeza, L.F. Thermo-mechanical stability of concrete containing steel slag as aggregate after high temperature thermal cycles. Sol. Energy 2022, 239, 59–73. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Zhu, Y.; Zhang, H.; Yan, M.; Liu, D.; Hao, J. Preparation and characterization of novel low-cost sensible heat storage materials with steel slag. J. Energy Storage 2024, 76, 109643. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, Y.; Zhang, A.; Ren, J.; Xu, Q.; Wu, Y.; Zhao, Y.; Ding, Y. Investigation on low-carbon shape-stable phase change composite by steel slag and carbide slag for solar thermal energy storage. J. Energy Storage 2024, 76, 109736. [Google Scholar] [CrossRef]
- Merlin, K.; Soto, J.; Delaunay, D.; Traonvouez, L. Industrial waste heat recovery using an enhanced conductivity latent heat thermal energy storage. Appl. Energy 2016, 183, 491–503. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Huang, Y. Synthesis and characterization of form-stable carbonate/steel slag composite materials for thermal energy storage. J. Energy Storage 2022, 52, 104708. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Z.; Zhang, X.; Wu, B.; Shen, Y.; Wu, L.; Zhang, X. Steel slag-KNO3 phase change composites for thermal storage at medium-high temperature and solid waste recycling. Mater. Chem. Phys. 2023, 301, 127655. [Google Scholar] [CrossRef]
- Fukahori, R.; Nomura, T.; Zhu, C.; Sheng, N.; Okinaka, N.; Akiyama, T. Macro-encapsulation of metallic phase change material using cylindrical-type ceramic containers for high-temperature thermal energy storage. Appl. Energy 2016, 170, 324–328. [Google Scholar] [CrossRef]
- Sakellariou, K.G.; Karagiannakis, G.; Criado, Y.A.; Konstandopoulos, A.G. Calcium oxide based materials for thermochemical heat storage in concentrated solar power plants. Sol. Energy 2015, 122, 215–230. [Google Scholar] [CrossRef]
- Perejón, A.; Valverde, J.M.; Miranda-Pizarro, J.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Large-Scale Storage of Concentrated Solar Power from Industrial Waste. ACS Sustain. Chem. Eng. 2017, 5, 2265–2272. [Google Scholar] [CrossRef]
- Valverde, J.M.; Miranda-Pizarro, J.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Calcium-Looping performance of steel and blast furnace slags for thermochemical energy storage in concentrated solar power plants. J. CO2 Util. 2017, 22, 143–154. [Google Scholar] [CrossRef]
| Component | SiO2 | Al2O3 | CaO | MgO | FeO | S | P2O5 | f-CaO | Basicity (CaO/(SiO2 + P2O5)) |
|---|---|---|---|---|---|---|---|---|---|
| Range | 15–25 | 3–7 | 45–60 | 5–20 | 12–25 | 0.1–0.4 | 0–1 | 1.6–7 | 2.1–3.5 |
| Basicity | Dominant Phases (by Abundance) |
|---|---|
| 1.36 | Olivine (CaO·MgO·SiO2), rhodonite (3CaO·MgO·2SiO2), RO phase |
| 1.80 | Rhodonite, dicalcium silicate (C2S), RO phase |
| 2.51 | Tricalcium silicate (C3S), C2S, RO phase |
| 2.99 | C3S, C2S, RO phase, calcium ferrite (Ca2Fe2O5) |
| Parameter | Range/Value | Test Standard | Engineering Implication |
|---|---|---|---|
| Density | 3.3–3.6 g/cm3 | BS EN 1097-7 [16] | Aggregate grading design |
| Water absorption | <3% | ASTM D6473 [17] | Weathering resistance |
| Crushing value | 20.4–30.8% | ASTM C535 [18] | Compressive strength indicator |
| Bond work index | 0.7 (Std sand = 1.0) | Bond grindability | Grinding energy consumption |
| Abrasion coefficient (K) | 0.8 | EN 1097-1 [19] | Pavement rutting resistance |
| Brittleness index (n) | 0.2 | Dynamic impact | Crack propagation resistance |
| Component | Reaction | Expansion | Critical Temperature |
|---|---|---|---|
| f-CaO | CaO + H2O → Ca(OH)2 | 100–300% | Ambient–100 °C |
| β-C2S | β-C2S → γ-C2S | 10% | 675 °C |
| MgO | MgO + H2O → Mg(OH)2 | 77% | <120 °C |
| C3S decomposition | 3CaO·SiO2→2CaO·SiO2 + CaO | Localized | 1100–1250 °C |
| Application | Key Properties | TRL | Critical Barriers |
|---|---|---|---|
| Cement additive | C2S/C3S reactivity | 6–7 | f-CaO content < 1.5% required |
| Road aggregate | High abrasion resistance | 8 | High density (+15% transport cost) |
| Wastewater treatment | Porous structure | 5 | Heavy metal leaching risks |
| CO2 mineralization | Carbonation reactivity | 4 | Slow reaction kinetics |
| Limitation Factor | Mechanism | Mitigation Strategy |
|---|---|---|
| Volume expansion (f-CaO/MgO) | Hydration-induced damage | Steam aging (150 °C, 6 h) |
| Low pozzolanic activity | Glassy phase < 10% | Mechanical activation (<45 μm) |
| Heavy metal leaching (Cr, V) | Alkaline ion mobilization | Phosphate stabilization |
| Abrasive particles | Metallic iron residues (2–5%) | Magnetic separation (>95% recovery) |
| Method | Key Process | Advantages | Limitations |
|---|---|---|---|
| Thermal Splashing | Water spraying on molten slag for 3–4 days, followed by magnetic separation | High capacity (200–400 t/h) Mature technology | High water consumption (3–5 m3/t) Poor stability (f-CaO > 4%) |
| Rotary Drum | Integrated cooling–crushing–magnetic separation in rotating drum | Efficient Fe recovery (η > 92%) Superior stability (f-CaO < 2%) | Requires high fluidity (η > 1 Pa·s) |
| Steam Aging | Intermittent water spraying on 300–800 °C slag induces self-pulverization | High fines yield (85% < 20 mm) Low dust emission (<10 mg/m3) | Long cycle (8–12 h) High energy demand (50–80 kWh/t) |
| Air Quenching | Molten slag atomization by compressed air with heat recovery | Uniform granules (D50 = 0.5–2 mm) 65% heat recovery | Noise pollution (>85 dB) High maintenance cost |
| Pressurized Steam | Accelerated f-CaO hydration under 1.5–2.0 MPa steam | Short cycle (2–4 h) Excellent stability (f-CaO < 1%) | Capital intensive ($1.2–1.8 M/unit) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Zhang, S.; Li, K.; Zhao, T.; Meng, Q.; Guan, D.; Zhang, A. Stainable Utilization Strategies for Basic Oxygen Furnace Slag: Properties, Processing, and Future Directions. Metals 2025, 15, 537. https://doi.org/10.3390/met15050537
Ma C, Zhang S, Li K, Zhao T, Meng Q, Guan D, Zhang A. Stainable Utilization Strategies for Basic Oxygen Furnace Slag: Properties, Processing, and Future Directions. Metals. 2025; 15(5):537. https://doi.org/10.3390/met15050537
Chicago/Turabian StyleMa, Chunting, Siqi Zhang, Keqing Li, Tong Zhao, Qingxin Meng, Dongshang Guan, and Ao Zhang. 2025. "Stainable Utilization Strategies for Basic Oxygen Furnace Slag: Properties, Processing, and Future Directions" Metals 15, no. 5: 537. https://doi.org/10.3390/met15050537
APA StyleMa, C., Zhang, S., Li, K., Zhao, T., Meng, Q., Guan, D., & Zhang, A. (2025). Stainable Utilization Strategies for Basic Oxygen Furnace Slag: Properties, Processing, and Future Directions. Metals, 15(5), 537. https://doi.org/10.3390/met15050537