Use of Hydrogen Peroxide as Oxidizing Agent in Chalcopyrite Leaching: A Review
Abstract
1. Introduction
2. Use of Hydrogen Peroxide in Chalcopyrite Leaching
2.1. Physical Aspects
2.2. Chemical Aspects
2.3. Thermodynamic Aspects
2.4. Kinetic Aspects
2.5. Safety Aspects for Hydrogen Peroxide
2.6. General Aspects of the Effect of Hydrogen Peroxide on Chalcopyrite Leaching
3. Use of Hydrogen Peroxide with Novel Leachants
3.1. Chalcopyrite Leaching in the H2O2 System-Organic
3.1.1. Effect of Organic Liquids-H2O2 on Chalcopyrite Leaching
3.1.2. Kinetic Aspects in Systems H2O2-Organic
3.1.3. Case Studies H2O2 System-Organic
3.2. Chalcopyrite Leaching in H2O2 System-Inorganic Salts
Case Studies H2O2 System-Inorganic Salts
3.3. Chalcopyrite Leaching in H2O2 System-Alkaline-Amino Acids
Case Studies H2O2 System-Alkaline-Amino Acid
3.4. Leaching Kinetics in Systems with H2O2 and Novel Leachants
4. Prospects and Challenges in the Use of Hydrogen Peroxide
4.1. H2O2 Decomposition Delay
4.2. Microwave Leaching in the Presence of H2O2
4.3. Photoleaching in the Presence of H2O2
4.4. Mechanically Assisted Leaching
4.5. Non-Oxidative/Reductive and Oxidative Leaching with H2O2
5. Conclusions
6. Recommendations
- Detailed ore characterization: Thoroughly analyze the ore samples, considering their mineralogical composition, copper content, and presence of trace elements or impurities that may act as catalysts or inhibitors. This characterization will allow for a better understanding of the chalcopyrite dissolution mechanisms and a more efficient optimization of the leaching process parameters.
- Exploration of chemical combinations: Investigate combinations of H2O2 with stabilizing agents and other leaching agents to improve copper extraction efficiency and reduce operating costs. In particular, it is suggested to evaluate the interaction of H2O2 with organic solvents, amino acids, and complexing agents that can improve leaching rates, especially under low-grade copper conditions.
- Pilot-scale trials: Conduct pilot-scale trials to determine the technical, economic, and environmental feasibility of using H2O2 in large-scale chalcopyrite leaching. These trials should consider process efficiency and performance, costs associated with the safe use and handling of H2O2, and environmental risks, such as by-product or waste generation.
- Development of advanced kinetic models: Models capable of integrating the chemical complexity of the leaching system, including the reactions of H2O2 with the components of the mineral and the medium. These models must be able to accurately predict the leaching rates under different operating conditions and be useful on an industrial scale.
- Evaluation of emerging technologies: Consider innovative technologies that can complement the leaching process with H2O2, such as microwave-assisted leaching to accelerate the breakdown of the crystalline structure of chalcopyrite; photoleaching, using light to enhance the reactivity of H2O2; and mechanical leaching, increasing the surface exposure of the mineral to the leaching agents. These technologies should be evaluated in combination with H2O2 to determine their potential, increase efficiency, and reduce leaching times.
- Sustainability and H2O2 production: Investigate sustainable methods for the production of H2O2, using renewable energy sources such as solar or wind. This would help reduce the carbon footprint of the process and could make the use of H2O2 more cost-effective and environmentally friendly in the long term.
- Environmental impact and waste management: Develop strategies to minimize the environmental impact of the process, including efficient management of by-products generated during H2O2 leaching. This should include recovery and reuse of chemical components whenever possible.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marsden, J.O. Energy efficiency and copper hydrometallurgy. Hydrometallurgy 2008, 29–42. [Google Scholar]
- Herrington, R. Geological features and genetic models of mineral deposits. In SME Mining Engineering Handbook; SME: Englewood, CO, USA, 2011; Part IV. [Google Scholar]
- Barton, I.F.; Hiskey, J.B. Chalcopyrite leaching in novel lixiviants. Hydrometallurgy 2022, 207, 105775. [Google Scholar] [CrossRef]
- Ji, G.; Liao, Y.; Wu, Y.; Xi, J.; Liu, Q. A review on the research of hydrometallurgical leaching of low-grade complex chalcopyrite. J. Sustain. Metall. 2022, 8, 964–977. [Google Scholar] [CrossRef]
- Baba, A.A.; Ayinla, K.I.; Adekola, F.A.; Ghosh, M.K.; Ayanda, O.S.; Bale, R.B.; Sheik, A.R.; Pradhan, S.R. A review on novel techniques for chalcopyrite ore processing. Int. J. Min. Eng. Miner. Process. 2012, 1, 1–16. [Google Scholar]
- Brierley, C.L.; Briggs, A.P. Selection and sizing of biooxidation equipment and circuits. In Mineral Processing Plant Design, Practice and Controli; Society of Mining Engineers: Littleton, CO, USA, 2002; pp. 1540–1568. [Google Scholar]
- Hazen, N.; Robertson, J. Research and development. In SME Mineral Processing and Extractive Metallurgy Handbook; Society of Mining, Metallurgy & Exploration: Englewood, CO, USA, 2019; pp. 325–332. [Google Scholar]
- Watling, H. Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acidic sulfate, sulfate–chloride and sulfate–nitrate process options. Hydrometallurgy 2013, 140, 163–180. [Google Scholar] [CrossRef]
- de Melo Silva Cheloni, L.M.; Martins, F.L.; Pinto, L.M.; Rodrigues, M.L.M.; Leão, V.A. Chemical and biological leaching of chalcopyrite-elemental sulfur reaction products. Miner. Process. Extr. Metall. Rev. 2024, 45, 453–464. [Google Scholar] [CrossRef]
- Nyembwe, K.J.; Waanders, F.; Mkandawire, M.; Mamba, B.; Fosso-Kankeu, E. Complexity of Chalcopyrite Mineral Affecting Copper Recovery During Leaching. In Recovery of Values from Low—Grade and Complex Minerals: Development of Sustainable Processes; Wiley: Hoboken, NJ, USA, 2024; pp. 145–177. [Google Scholar]
- Hiskey, J.B.; Mansanti, J.G.; Mcnulty, T. Solution Mining-In Situ Leaching. In SME Mineral Processing & Extractive Metallurgy Handbook; Society for Mining, Metallurgy & Exploration (SME): Englewood, CO, USA, 2019; Volume 2, pp. 1191–1206. [Google Scholar]
- Kuhn, M.C.; Alley, R.D. Copper Hydrometallurgy. In SME Mineral Processing and Extractive Metallurgy Handbook; Society for Mining, Metallurgy & Exploration (SME): Englewood, CO, USA, 2019. [Google Scholar]
- Kritskii, A.; Fuentes, G.; Deveci, H. A critical review of hydrothermal treatment of sulfide minerals with Cu (II) solution in H2SO4 media. Hydrometallurgy 2024, 231, 106413. [Google Scholar] [CrossRef]
- Bao, S.; Chen, B.; Zhang, Y.; Ren, L.; Xin, C.; Ding, W.; Yang, S.; Zhang, W. A comprehensive review on the ultrasound-enhanced leaching recovery of valuable metals: Applications, mechanisms and prospects. Ultrason. Sonochem. 2023, 98, 106525. [Google Scholar] [CrossRef]
- Martins, F.L.; Leão, V.A. Chalcopyrite bioleaching in chloride media: A mini-review. Hydrometallurgy 2023, 216, 105995. [Google Scholar] [CrossRef]
- Liddicoat, J.; Dreisinger, D. Chloride leaching of chalcopyrite. Hydrometallurgy 2007, 89, 323–331. [Google Scholar] [CrossRef]
- Lundström, M.; Aromaa, J.; Forsén, O.; Hyvärinen, O.; Barker, M.H. Leaching of chalcopyrite in cupric chloride solution. Hydrometallurgy 2005, 77, 89–95. [Google Scholar] [CrossRef]
- Yévenes, L.V.; Miki, H.; Nicol, M. The dissolution of chalcopyrite in chloride solutions: Part 2: Effect of various parameters on the rate. Hydrometallurgy 2010, 103, 80–85. [Google Scholar] [CrossRef]
- Akcil, A.; Ciftci, H. A study of the selective leaching of complex sulphides from the Eastern Black Sea Region, Turkey. Miner. Eng. 2002, 15, 457–459. [Google Scholar] [CrossRef]
- Córdoba, E.; Muñoz, J.; Blázquez, M.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar]
- Dutrizac, J. Elemental sulphur formation during the ferric chloride leaching of chalcopyrite. Hydrometallurgy 1990, 23, 153–176. [Google Scholar] [CrossRef]
- Yoo, K.; Kim, S.-K.; Lee, J.-C.; Ito, M.; Tsunekawa, M.; Hiroyoshi, N. Effect of chloride ions on leaching rate of chalcopyrite. Miner. Eng. 2010, 23, 471–477. [Google Scholar] [CrossRef]
- Aydogan, S.; Ucar, G.; Canbazoglu, M. Dissolution kinetics of chalcopyrite in acidic potassium dichromate solution. Hydrometallurgy 2006, 81, 45–51. [Google Scholar] [CrossRef]
- Altundogan, H.; Boyrazli, M.; Tumen, F. A study on the sulphuric acid leaching of copper converter slag in the presence of dichromate. Miner. Eng. 2004, 17, 465–467. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Janković, Z.; Dimitrijević, M. Investigation of the kinetics of chalcopyrite oxidation by potassium dichromate. Hydrometallurgy 1994, 35, 187–201. [Google Scholar] [CrossRef]
- Sokić, M.D.; Marković, B.; Živković, D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid. Hydrometallurgy 2009, 95, 273–279. [Google Scholar] [CrossRef]
- Ikkiz, D.; Gülfen, M.; Aydın, A. Dissolution kinetics of primary chalcopyrite ore in hypochlorite solution. Miner. Eng. 2006, 19, 972–974. [Google Scholar] [CrossRef]
- Padilla, R.; Vega, D.; Ruiz, M. Pressure leaching of sulfidized chalcopyrite in sulfuric acid–oxygen media. Hydrometallurgy 2007, 86, 80–88. [Google Scholar] [CrossRef]
- Puvvada, G.; Murthy, D. Selective precious metals leaching from a chalcopyrite concentrate using chloride/hypochlorite media. Hydrometallurgy 2000, 58, 185–191. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.; Al-Harahsheh, A. Ferric chloride leaching of chalcopyrite: Synergetic effect of CuCl2. Hydrometallurgy 2008, 91, 89–97. [Google Scholar] [CrossRef]
- Skrobian, M.; Havlik, T.; Ukasik, M. Effect of NaCl concentration and particle size on chalcopyrite leaching in cupric chloride solution. Hydrometallurgy 2005, 77, 109–114. [Google Scholar] [CrossRef]
- Tchoumou, M.; Roynette, M. Leaching of complex sulphide concentrate in acidic cupric chloride solutions. Trans. Nonferrous Met. Soc. China 2007, 17, 423–428. [Google Scholar] [CrossRef]
- Ahn, J.; Wu, J.; Lee, J. A comparative kinetic study of chalcopyrite leaching using alternative oxidants in methanesulfonic acid system. Miner. Process. Extr. Metall. Rev. 2022, 43, 390–401. [Google Scholar] [CrossRef]
- Vardner, J.T.; Inaba, Y.; Jung, H.; Farinato, R.S.; Nagaraj, D.R.; Banta, S.; West, A.C. The Reductive leaching of chalcopyrite by Chromium (II) chloride for the rapid and complete extraction of copper. ChemistryOpen 2023, 12, e202200196. [Google Scholar] [CrossRef]
- Ji, G.; Liao, Y.; Xi, J.; Liu, Q.; Wu, Y.; Ma, H.; Li, J. Behavior and kinetics of copper during oxygen pressure leaching of complex chalcopyrite without acid. J. Sustain. Metall. 2023, 9, 350–362. [Google Scholar] [CrossRef]
- Miao, J.; Leng, H.; Han, B. Leaching and kinetic study of chalcopyrite without acid in an O2–H2O system. J. Sustain. Metall. 2023, 9, 1279–1288. [Google Scholar] [CrossRef]
- Karimov, K.; Tretiak, M.; Rogozhnikov, D.; Dizer, O. The Dissolution Behavior of Pyrite and Chalcopyrite During Low-Temperature Pressure Oxidation: Chalcopyrite Influence on Pyrite Oxidation. Materials 2024, 17, 5132. [Google Scholar] [CrossRef] [PubMed]
- Antonijević, M.; Janković, Z.; Dimitrijević, M. Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulphuric acid. Hydrometallurgy 2004, 71, 329–334. [Google Scholar] [CrossRef]
- Mahajan, V.; Misra, M.; Zhong, K.; Fuerstenau, M. Enhanced leaching of copper from chalcopyrite in hydrogen peroxide–glycol system. Miner. Eng. 2007, 20, 670–674. [Google Scholar] [CrossRef]
- Turan, M.D.; Altundoğan, H.S. Leaching of chalcopyrite concentrate with hydrogen peroxide and sulfuric acid in an autoclave system. Metall. Mater. Trans. B 2013, 44, 809–819. [Google Scholar] [CrossRef]
- Zandevakili, S.; Akhondi, M.R. Microwave-assisted leaching for copper recovery from the chalcopyrite concentrate of Sarcheshmeh copper complex. Int. J. Min. Geo Eng. 2022, 56, 277–284. [Google Scholar]
- Olubambi, P.A.; Potgieter, J.H. Investigations on the mechanisms of sulfuric acid leaching of chalcopyrite in the presence of hydrogen peroxide. Miner. Process. Extr. Metall. Rev. 2009, 30, 327–345. [Google Scholar] [CrossRef]
- Hu, J.; Tian, G.; Zi, F.; Hu, X. Leaching of chalcopyrite with hydrogen peroxide in 1-hexyl-3-methyl-imidazolium hydrogen sulfate ionic liquid aqueous solution. Hydrometallurgy 2017, 169, 1–8. [Google Scholar] [CrossRef]
- Ahn, J.; Wu, J.; Lee, J. Investigation on chalcopyrite leaching with methanesulfonic acid (MSA) and hydrogen peroxide. Hydrometallurgy 2019, 187, 54–62. [Google Scholar] [CrossRef]
- Nicol, M.J. The role and use of hydrogen peroxide as an oxidant in the leaching of minerals. II. alkaline solutions. Hydrometallurgy 2020, 194, 105365. [Google Scholar] [CrossRef]
- Petrović, S.J.; Bogdanović, G.D.; Antonijević, M.M.; Vukčević, M.; Kovačević, R. The extraction of copper from chalcopyrite concentrate with hydrogen peroxide in sulfuric acid solution. Metals 2023, 13, 1818. [Google Scholar] [CrossRef]
- Karppinen, A.; Seisko, S.; Lundström, M. Atmospheric leaching of Ni, Co, Cu, and Zn from sulfide tailings using various oxidants. Miner. Eng. 2024, 207, 108576. [Google Scholar] [CrossRef]
- Goor, G.; Glenneberg, J. Hydrogen Peroxide. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Yepsen, O.; Cornejo-Ponce, L.; Yepsen, R. Perspectives for Photochemical Leaching Processes of Chalcopyrite: A Solar Radical-Leaching Process. Mining 2024, 4, 352–366. [Google Scholar] [CrossRef]
- Klauber, C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution. Int. J. Miner. Process. 2008, 86, 1–17. [Google Scholar] [CrossRef]
- Moazzami, Y.; Shafaei Tonkaboni, S.Z.; Gharabaghi, M. Optimizing Leaching Parameters for Copper Extraction from Chalcopyrite Using [Bmim][HSO4] Ionic Liquid. Iran. J. Chem. Chem. Eng. 2024, 43, 2635–2648. [Google Scholar]
- Agacayak, T.; Aras, A.; Aydogan, S.; Erdemoglu, M. Leaching of chalcopyrite concentrate in hydrogen peroxide solution. Physicochem. Probl. Miner. Process. 2014, 50, 657–666. [Google Scholar]
- Ruiz-Sánchez, Á.; Lapidus, G.T. Improved process for leaching refractory copper sulfides with hydrogen peroxide in aqueous ethylene glycol solutions. In Extraction 2018: Proceedings of the First Global Conference on Extractive Metallurgy; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ruiz-Sánchez, A.; Lázaro, I.; Lapidus, G. Improvement effect of organic ligands on chalcopyrite leaching in the aqueous medium of sulfuric acid-hydrogen peroxide-ethylene glycol. Hydrometallurgy 2020, 193, 105293. [Google Scholar] [CrossRef]
- Rasouli, A. Copper Extraction from Chalcopyrite Through a Two-Step Nonoxidative/Oxidative Leaching Process. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2023. [Google Scholar]
- Schlesinger, M.E.; King, M.; Sole, K.; Davenport, W. Hydrometallurgical copper extraction: Introduction and leaching. In Extractive Metallurgy of Copper; Elsevier: Amsterdam, The Netherlands, 2011; pp. 281–322. [Google Scholar]
- Taboada, M.E.; Jamett, N.E.; Moraga, G.A.; Hernández, P.C.; Graber, T.A. Obtention of Suitable Pregnant Leach Solution (PLS) for Copper Solvent Extraction Plants from Copper Concentrate Using Hydrogen Peroxide and Iodine in a Sulfuric Acid–Chloride Medium. Metals 2024, 14, 817. [Google Scholar] [CrossRef]
- Velásquez-Yévenes, L.; Nicol, M.; Miki, H. The dissolution of chalcopyrite in chloride solutions: Part 1. The effect of solution potential. Hydrometallurgy 2010, 103, 108–113. [Google Scholar]
- Dong, Y.B.; Lin, H.; Zhou, S.; Xu, X.; Zhang, Y. Effects of quartz addition on chalcopyrite bioleaching in shaking flasks. Miner. Eng. 2013, 46, 177–179. [Google Scholar] [CrossRef]
- Dakkoune, A.; Bourgeois, F.; Po, A.; Joulian, C.; Hubau, A.; Touzé, S.; Julcour, C.; Guezennec, A.G.; Cassayre, L. Hydrometallurgical Processing of Chalcopyrite by Attrition-Aided Leaching. ACS Eng. Au 2023, 3, 195–209. [Google Scholar] [CrossRef]
- Khoshkhoo, M.; Dopson, M.; Engström, F.; Sandström, Å. New insights into the influence of redox potential on chalcopyrite leaching behaviour. Miner. Eng. 2017, 100, 9–16. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Oldfield, L.F. The oxidation-reduction reactions of hydrogen peroxide at inert metal electrodes and mercury cathodes. Trans. Faraday Soc. 1955, 51, 249–259. [Google Scholar] [CrossRef]
- Solvay. H2O2 Safety and Handling of Hydrogen Peroxide. Available online: https://www.solvay.com/sites/g/files/srpend616/files/2019-10/H2O2%20Safety%20and%20Handling%20of%20Hydrogen%20Peroxide%20-%20Mexico%20SP.pdf (accessed on 20 December 2024).
- Dutrizac, J. The dissolution of chalcopyrite in ferric sulfate and ferric chloride media. Metall. Trans. B 1981, 12, 371–378. [Google Scholar] [CrossRef]
- Adebayo, A.O.; Ipinmoroti, K.O.; Ajayi, O.O. Dissolution kinetics of chalcopyrite with hydrogen peroxide in sulphuric acid medium. Chem. Biochem. Eng. Q. 2003, 17, 213–218. [Google Scholar]
- Sokić, M.; Marković, B.; Stanković, S.; Kamberović, Ž.; Štrbac, N.; Manojlović, V.; Petronijević, N. Kinetics of chalcopyrite leaching by hydrogen peroxide in sulfuric acid. Metals 2019, 9, 1173. [Google Scholar] [CrossRef]
- Wu, J.; Ahn, J.; Lee, J. Kinetic and mechanism studies using shrinking core model for copper leaching from chalcopyrite in methanesulfonic acid with hydrogen peroxide. Miner. Process. Extr. Metall. Rev. 2021, 42, 38–45. [Google Scholar] [CrossRef]
- Dimitrijevic, M.; Urosevic, D.; Milic, S.; Sokic, M.; Markovic, R. Dissolution of copper from smelting slag by leaching in chloride media. J. Min. Metall. Sect. B Metall. 2017, 53, 407. [Google Scholar] [CrossRef]
- Arslanoğlu, H.; Yaraş, A. Chalcopyrite leaching with hydrogen peroxide in formic acid medium. Trans. Indian Inst. Met. 2020, 73, 785–792. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, Á.; Lapidus, G.T. Study of chalcopyrite leaching from a copper concentrate with hydrogen peroxide in aqueous ethylene glycol media. Hydrometallurgy 2017, 169, 192–200. [Google Scholar] [CrossRef]
- Marcial, O.J.S.; Bastida, A.N.; Bañuelos, J.E.; Martínez, O.U.V.; Luevano, L.A.; Rosales, B.S. Chalcopyrite leaching kinetics in the presence of methanol. Int. J. Chem. React. Eng. 2019, 17, 20190081. [Google Scholar]
- Liu, X.J.; Liao, Y.; Ma, H.; Liu, Q. Electrochemical characterizations and galvanic effect of chalcopyrite leaching and passivation-A review. Miner. Eng. 2024, 210, 108673. [Google Scholar] [CrossRef]
- Michałek, T.; Pacławski, K.; Fitzner, K. Chalcopyrite Leaching in the Presence of Isopropanol—The Kinetic and Mechanistic Studies. Materials 2024, 17, 824. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, A.; Lapidus, G. Decomposition of organic additives in the oxidative chalcopyrite leaching with hydrogen peroxide. Miner. Eng. 2022, 187, 107783. [Google Scholar] [CrossRef]
- Price, E.E. Copper Leaching from Chalcopyrite with an Alternative Lixiviant/Oxidant System. Master’s Thesis, The University of Arizona, Tucson, AZ, USA, 2022. [Google Scholar]
- Dinga, J.T.; Petersen, J.; Moyo, T. Effect of Addition of Various Alcohols on the Leaching of Chalcopyrite in Ferric Sulfate Media. In Proceedings of the 10th Edition of the Africa Base Metal Conference, Livingstone, Zambia, 13–15 June 2023; Available online: https://www.researchgate.net/publication/377417073 (accessed on 13 January 2025).
- Granata, G.; Miura, A.; Liu, W.; Pagnanelli, F.; Tokoro, C. Iodide-assisted leaching of chalcopyrite in acidic ferric sulfate media. Hydrometallurgy 2019, 186, 244–251. [Google Scholar] [CrossRef]
- Moraga, G.A.; Jamett, N.E.; Hernández, P.C.; Graber, T.A.; Taboada, M.E. Chalcopyrite Leaching with Hydrogen Peroxide and Iodine Species in Acidic Chloride Media at Room Temperature: Technical and Economic Evaluation. Metals 2021, 11, 1567. [Google Scholar] [CrossRef]
- Nurtazina, N.; Uvarov, N.; Azhigulova, R.; Tyapkin, P. Chalcopyrite leaching by amino acid solutions in the presence of hydrogen peroxide. Physicochem. Probl. Miner. Process. 2022, 58, 157067. [Google Scholar] [CrossRef]
- Petrović, S.J.; Bogdanović, G.D.; Antonijević, M.M. Leaching of chalcopyrite with hydrogen peroxide in hydrochloric acid solution. Trans. Nonferrous Met. Soc. China 2018, 28, 1444–1455. [Google Scholar] [CrossRef]
- Ghomi, M.A.; Mozammel, M.; Moghanni, H.; Shahkar, L. Atmospheric leaching of chalcopyrite in the presence of some polar organic reagents: A comparative study and optimization. Hydrometallurgy 2019, 189, 105120. [Google Scholar] [CrossRef]
- Solis-Marcíal, O.; Lapidus, G. Improvement of chalcopyrite dissolution in acid media using polar organic solvents. Hydrometallurgy 2013, 131, 120–126. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Yang, H.-Y.; Huang, S.-T.; Lü, Y.; Xiong, L. Ultra fast microwave-assisted leaching for the recovery of copper and tellurium from copper anode slime. Int. J. Miner. Metall. Mater. 2015, 22, 582–588. [Google Scholar] [CrossRef]
- Behera, S.; Panda, S.K.; Das, D.; Mohapatra, R.; Kim, H.; Lee, J.; Jyothi, R.; Parhi, P. Microwave assisted leaching investigation for the extraction of copper (II) and chromium (III) from spent catalyst. Sep. Purif. Technol. 2020, 244, 116842. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.W. Microwave-assisted leaching—A review. Hydrometallurgy 2004, 73, 189–203. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.; Bradshaw, S. The reality of non-thermal effects in microwave assisted leaching systems? Hydrometallurgy 2006, 84, 1–13. [Google Scholar] [CrossRef]
- Ju, Y.; Yang, S.; Ding, Y.; Sun, C.; Gu, C.; He, Z.; Qin, C.; He, H.; Xu, B. Microwave-enhanced H2O2-based process for treating aqueous malachite green solutions: Intermediates and degradation mechanism. J. Hazard. Mater. 2009, 171, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jia, K.; Lu, S.; Cao, Y.; Boczkaj, G.; Wang, C. Thermally activated natural chalcopyrite for Fenton-like degradation of Rhodamine B: Catalyst characterization, performance evaluation, and catalytic mechanism. J. Environ. Chem. Eng. 2024, 12, 111469. [Google Scholar] [CrossRef]
- Lei, J.; Ding, L.; Li, X.; Li, Y.; Wang, M.; Zhang, Y.; Zhang, Z.; Wu, D.; Jiang, K. Efficient periodate activation by chalcopyrite for levofloxacin hydrochloride degradation: Effects of sulfur species and dominant pathways of reactive oxygen species. Sep. Purif. Technol. 2024, 340, 126816. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Yu, W.; Ma, T. Insights into structural and functional regulation of chalcopyrite and enhanced mechanism of reactive oxygen species (ROS) generation in advanced oxidation process (AOP): A review. Sci. Total Environ. 2024, 919, 170530. [Google Scholar] [CrossRef]
- Laskar, C.; Dakkoune, A.; Julcour, C.; Bourgeois, F.; Biscans, B.; Cassayre, L. Case-based analysis of mechanically-assisted leaching for hydrometallurgical extraction of critical metals from ores and wastes: Application in chalcopyrite, ferronickel slag, and Ni-MH black mass. C. R. Chim. 2024, 27 (Suppl. 4), 1–16. [Google Scholar] [CrossRef]
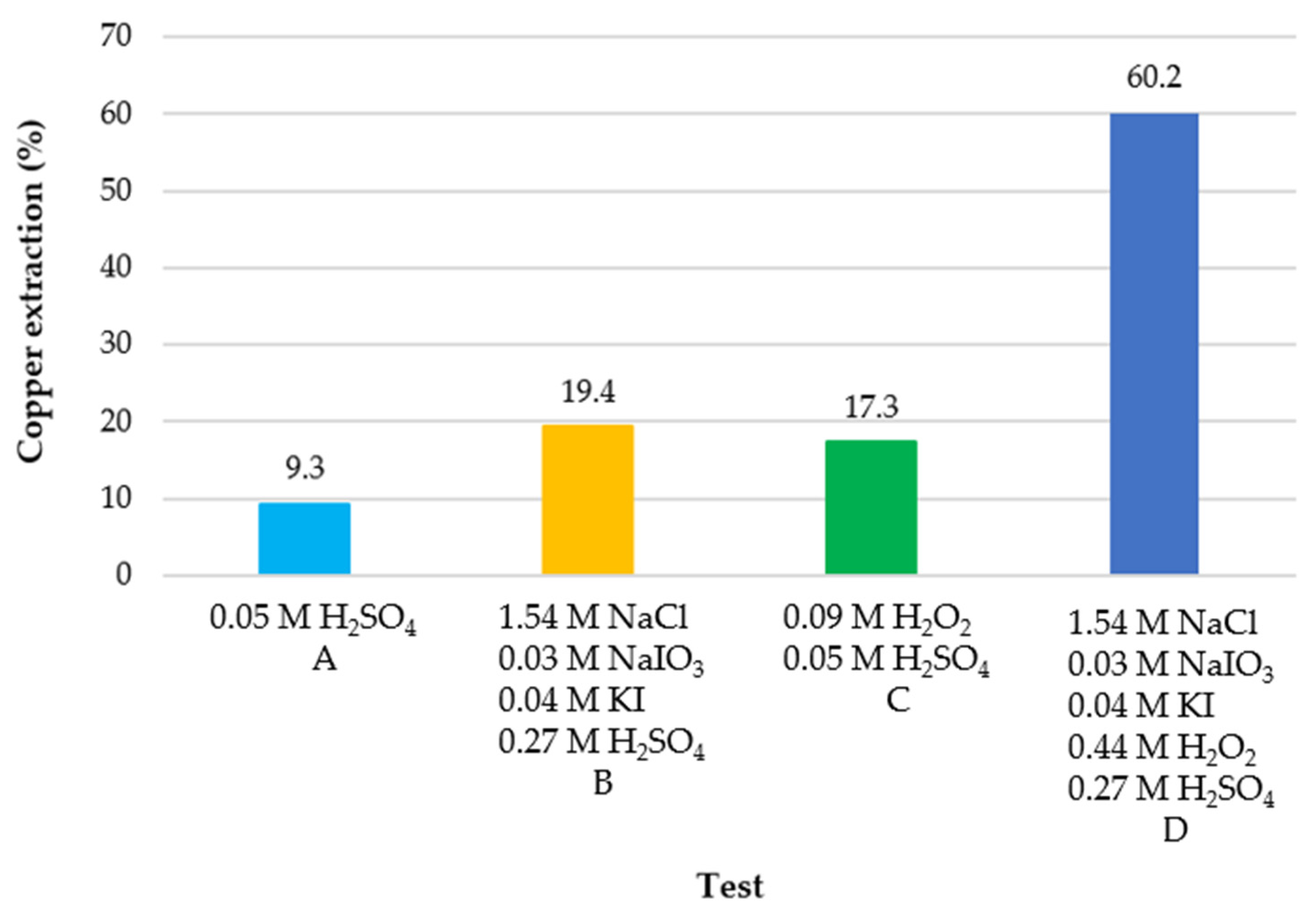
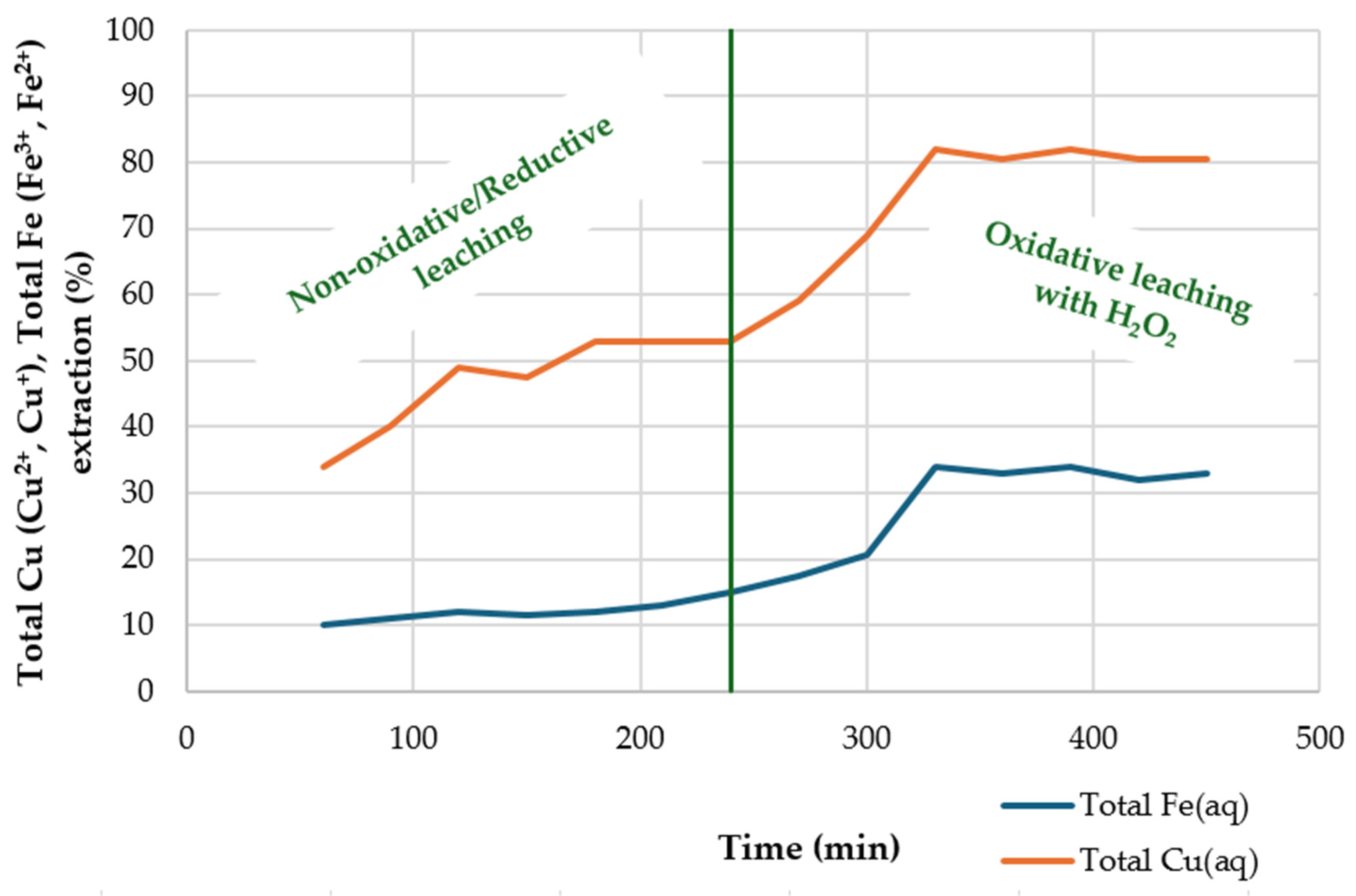
| Sample Type | Temperature (°C) | System H2O2-H2SO4 | Leaching Time (h) | Ea (kJ/mol) | Limiting Stage | References |
|---|---|---|---|---|---|---|
| Concentrate 32% Cu, 100–300 µm | 30−50 | 5.9 M [H2O2] + 0.1 M [H2SO4]. | 2 | 39 | Chemical reaction | [65] |
| Low grade ore 0.58% Cu 0–5 mm | 25−50 | 2 M [H2O2] + 2 M [H2SO4]. | 3 | 60 | Chemical reaction | [38] |
| Concentrate 27% Cu +75–37 µm | 25−45 | 1 M [H2O2] + 1.5 M [H2SO4]. | 4 | 80 | Diffusion | [66] |
| Concentrate 25% Cu +0–75 µm | 30−60 | 3 M [H2O2] + 3 M [H2SO4]. | 2 | 38.9 | Diffusion | [46] |
| Variables | Effect on H2O2 | Alternatives to Counteract the Negative Effect | Reference |
|---|---|---|---|
| Solid/ liquid ratio | Sufficient/insufficient hydrogen peroxide concentration. | Better extractions are obtained at diluted solid/liquid concentrations of 1:100. Unfavorable effects are seen at ratios of 1:50 or 1:10. | [46,52,67,69] |
| Stirring | Accelerates hydrogen peroxide degradation. Then, the concentration of hydrogen peroxide decreases due to its decomposition. | Work in the range of 0–600 rpm. | [46,65,69] |
| Temperature | It affects the stability of H2O2 which is consumed in the reaction and decomposes and influences the oxidation rate of chalcopyrite mainly in the initial leaching stage. | Higher temperatures result in higher copper extraction; however, this is a very relative concept. | [44,46] |
| pH | Higher concentrations of H2SO4 promotes the intensive decomposition of H2O2 in acidic pH ranges. | Higher concentrations of H2SO4, allow for higher copper extractions. | [46] |
| Iron or copper in solution and solid mineral particles | Catalytic decomposition | Lower kinetics caused by decomposition; could be counteracted by the addition of organic solvents. | [46] |
| Temperature (°C) | System H2O2-Leachant | Leaching Time (h) | Ea (kJ/mol) | Limiting Stage | References |
|---|---|---|---|---|---|
| 15−40 | 3 M [H2O2] + 0.6 M [H2SO4] + 5.7 M [CH3OH] | 5 | 24.27 | Chemical reaction | [71] |
| 20−50 | 1 M [H2O2] + 2 M [H2SO4] + 0.5 M [C3H8O] + 0.5 M [C3H8O] | 3 | 60.68 | Diffusion | [73] |
| 30−40 | 30% (v/v) [H2O2] + 40% (w/v) [Bmim][HSO4]*. | 3 | 49.61 | Chemical reaction | [51] |
| N° | Feeding Conditions | Optimal Leaching Conditions | Copper Extraction | Reference |
|---|---|---|---|---|
| 1 | Chalcopyrite: 65%. Cu: 25%. Fe: 32% Fe: 32% P80: 37–49 μm Concentration: 3.75 g/L | [H2O2]: 1 M [H2SO4]: 0.7 M [Ethylene glycol]: 3.5 M T: 20 °C Stirring: 600 rpm time: 24 h | Cu: 90% | [70] |
| 2 | Chalcopyrite: 89.5%. Cu: 28.8% Cu: 28.8% Fe: 26.4% Fe: 26.4% Fe: P80: 40 μm Concentration: 10 g/L | [H2O2]: 0.3 M [Metasulfonic acid]:30 g/L T: 75 °C time: 96 h | Cu: 99% | [44] |
| 3 | Chalcopyrite: 60.1%. Cu: 22.4%. Fe: 28.2% P80: 63 μm Concentration: 50 g/L | First Stage: [H2O2] = 1 M [H2SO4] = 0.007 M [Ethylene glycol] = 0.1 M [Oxalic acid] = 0.4 M Second Stage: [H2O2] = 2 M [H2SO4] = 0.007 M [Ethylene glycol] = 0.1 M [EDTA] = 0.4 M T: 26 °C Stirring: 400 rpm time 24 h. | Cu: 90% | [54] |
| 4 | Chalcopyrite: 84.6%. Cu: 28.8% Fe: 26.4% P80: 40 μm Concentration: 10 g/L | [H2O2]: 0.9 M [Metasulfonic acid]: 75 g/L T: 65 °C time: 96 h | Cu: 94% | [75] |
| 5 | Chalcopyrite: 94.5%. Cu: 31.35% Cu: 31.35% Fe: 30.01% P80: 3.55 μm Concentration: 6 g/L | [H2O2]: 1 M [H2SO4]: 1.75 M [2-propanol]: 30% v/v T: 40 °C Stirring: 100 rpm time: 5 h | Cu: 75% | [76] |
| 6 | Chalcopyrite: 94.5%. Cu: 31.35% Fe: 30.01% P80: 3.55 μm Concentration: 6 g/L | [H2O2]: 1 M [H2SO4]: 1.75 M [Ethanol]: 30% v/v T: 40 °C Stirring: 100 rpm time: 5 h | Cu: 53% | [76] |
| 7 | Cu: 31.67% Fe: 34.55%. P80: 5–100 μm Concentration: 10 g/L | [H2O2]: 1 M [H2SO4]: 0.5 M [Isopropanol]: 2 M T: 50 °C Stirring: 400 rpm time: 3 h | Cu: 70% | [73] |
| 8 | Chalcopyrite Cu: 29% Fe: 28% P80: 37–100 μm Concentration: 10 g/L | [H2O2]: 30% (v/v) [Bmim][HSO4]: 40% (w/v) T: 40 °C Stirring: 300 rpm time: 3 h | Cu: 90.32% | [51] |
| Low Level (M) | Low Level (g/L) | High Level (M) | High Level (g/L) | |
|---|---|---|---|---|
| NaCl | 0.00 | 0.00 | 1.54 | 90.00 |
| NaIO3 | 0.00 | 0.00 | 0.01 | 2.00 |
| KI | 0.00 | 0.00 | 0.01 | 2.30 |
| H2O2 | 0.09 | 3.00 | 0.44 | 15.00 |
| H2SO4 | 0.05 | 5.30 | 0.27 | 26.30 |
| Feeding Material Conditions | Leaching | Extraction | Reference |
|---|---|---|---|
| Chalcopyrite: 63.7%. Pyrite: 16.4%. Cu: 29.79% P80: 61 μm Concentration: 100 g/L | [H2O2]: 0.44 M [H2SO4]: 0.268 M [NaCl]: 1.54 M [KI]: 3.13 mM T: 20 °C Stirring: 600 rpm time: 45 min | Cu: 27% | [57] |
| Chalcopyrite: 60% Cu: 20.93% Fe: 31.22% P80: 75 μm Concentration: 12.5 g/L | [H2O2]: 0.25 M [H2SO4]: 0.5 M [NaCl]: 2 M T: 90 °C Stirring: 300 rpm time: 3 h | Cu: 39.7% | [41] |
| Chalcopyrite: 60% Cu: 20.93% Fe: 31.22% P80: 75 μm Concentration: 12.5 g/L | [H2O2]: 0.25 M [H2SO4]: 0.5 M [NaCl]: 2 M T: 90 °C Stirring: 300 rpm time: 3 h Applies microwave-assisted leaching. | Cu: 75.3% | [41] |
| Feeding Material | Leaching | Extraction |
|---|---|---|
| Chalcopyrite: 90% Cu: 35.57% Fe: 31.68% P80: 58 μm Concentration: 2 g/L | [H2O2]: 0.1 M [NaOH]: 0.1 M [Glycine]: 0.1 M T: 65 °C pH: 10 Stirring: 160 rpm time: 30 min | Cu: 7.76% |
| Chalcopyrite: 90% Cu: 35.57% Fe: 31.68 P80: 58 μm Concentration: 2 g/L | [H2O2]: 0.1 M [NaOH]: 0.1 M [Betaine]: 0.1 M T: 65 °C pH: 10 Stirring: 160 rpm time: 30 min | Cu: 6.26% |
| Chalcopyrite: 90% Cu: 35.57% Fe: 31.68 P80: 58 μm Concentration: 2 g/L | [H2O2]: 0.1 M [NaOH]: 0.1 M [Lysine]: 0.1 M T: 65 °C pH: 10 Stirring: 160 rpm time: 30 min | Cu: 2.40% |
| Temperature (°C) | System H2O2-X | Leaching Time (h) | Ea (kJ/mol) | Limiting Stage | References |
|---|---|---|---|---|---|
| 30–50 | 5.9 M [H2O2] + 0.1 M [H2SO4]. | 2 | 39.0 | Chemical reaction | [65] |
| 25–50 | 2 M [H2O2] + 2 M [H2SO4] | 3 | 60.0 | Chemical reaction | [38] |
| 30–60 | 2 M [H2O2] + 0.5 M [HCl] | 3 | 19.6 | Diffusion | [80] |
| 25–45 | 1 M [H2O2] + 1.5 M [H2SO4]. | 4 | 80.0 | Diffusion | [66] |
| 15–40 | 3 M [H2O2] + 0.6 M [H2SO4] + 5.7 M [CH3OH] | 5 | 24.3 | Chemical reaction | [71] |
| 30–60 | 3 M [H2O2] + 3 M [H2SO4] | 2 | 38.9 | Diffusion | [46] |
| 20–50 | 1 M [H2O2]+ 2 M [H2SO4] + 0.5 M [C3H8O] | 3 | 60.8 | Diffusion | [73] |
| 30–40 | 30% (v/v) [H2O2] + 40% (w/v) [Bmim][HSO4] | 3 | 49.6 | Chemical reaction | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, D.J.; Graber, T.A.; Angel-Castillo, A.H.; Hernández, P.C.; Taboada, M.E. Use of Hydrogen Peroxide as Oxidizing Agent in Chalcopyrite Leaching: A Review. Metals 2025, 15, 531. https://doi.org/10.3390/met15050531
Flores DJ, Graber TA, Angel-Castillo AH, Hernández PC, Taboada ME. Use of Hydrogen Peroxide as Oxidizing Agent in Chalcopyrite Leaching: A Review. Metals. 2025; 15(5):531. https://doi.org/10.3390/met15050531
Chicago/Turabian StyleFlores, Danny J., Teófilo A. Graber, Alejandro H. Angel-Castillo, Pía C. Hernández, and María E. Taboada. 2025. "Use of Hydrogen Peroxide as Oxidizing Agent in Chalcopyrite Leaching: A Review" Metals 15, no. 5: 531. https://doi.org/10.3390/met15050531
APA StyleFlores, D. J., Graber, T. A., Angel-Castillo, A. H., Hernández, P. C., & Taboada, M. E. (2025). Use of Hydrogen Peroxide as Oxidizing Agent in Chalcopyrite Leaching: A Review. Metals, 15(5), 531. https://doi.org/10.3390/met15050531








