Study of the Behavior and Mechanism of Sponge Iron Oxidation
Abstract
1. Introduction
2. Materials and Methods
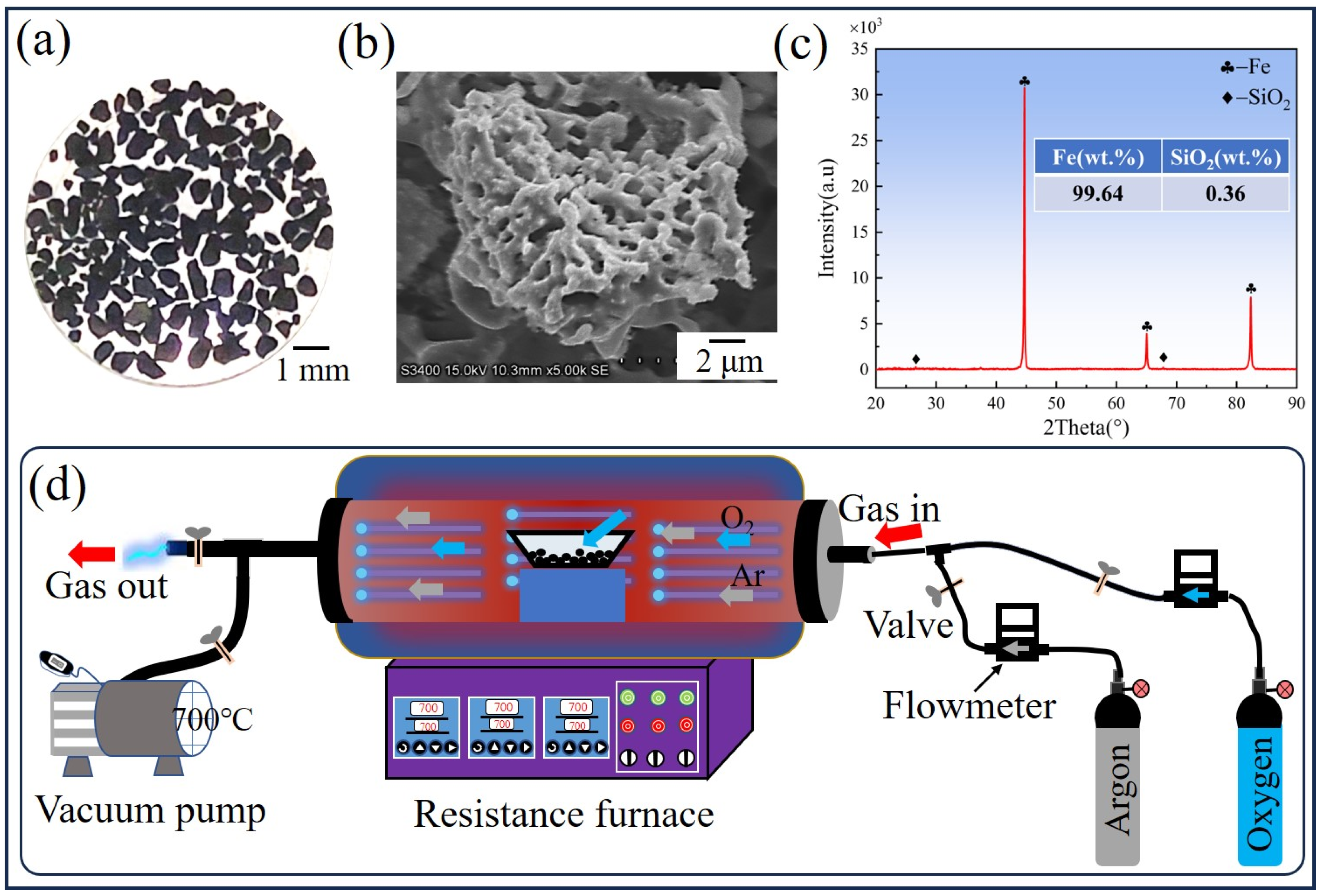
2.1. Experimental Materials and Methods
2.2. Characterization Methods
3. Experimental Results and Discussion
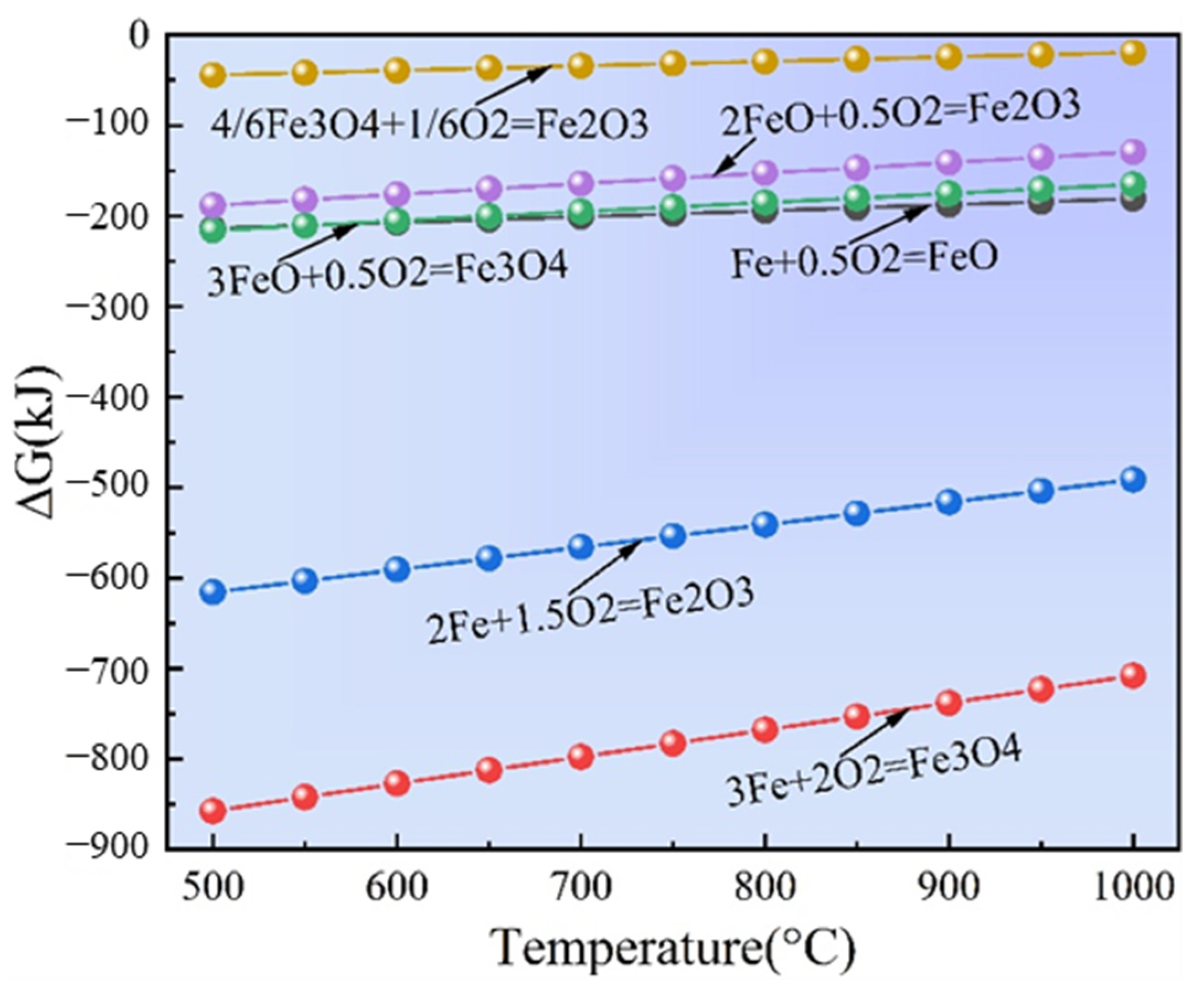
3.1. Thermodynamic Analysis of Oxidation
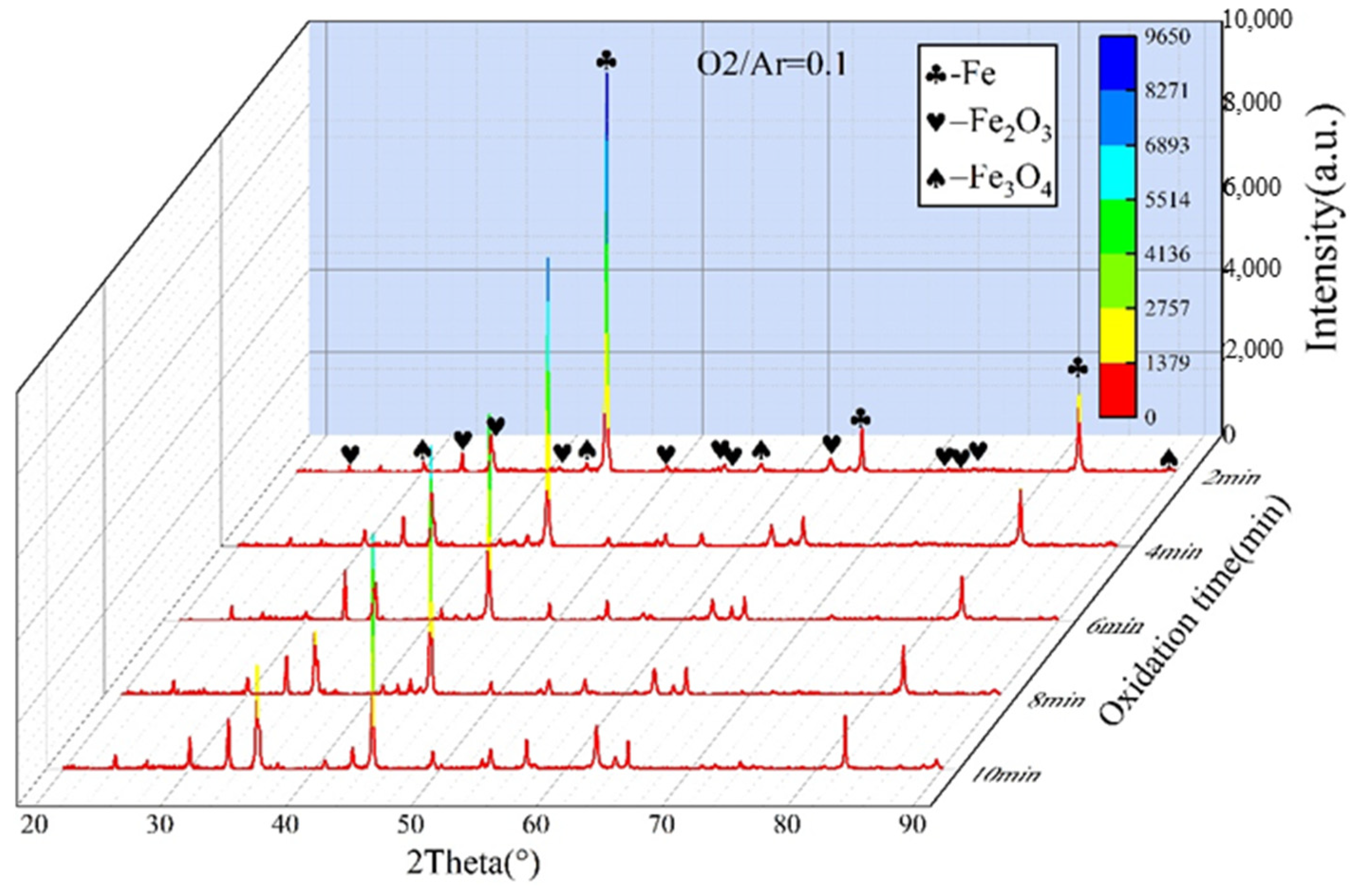
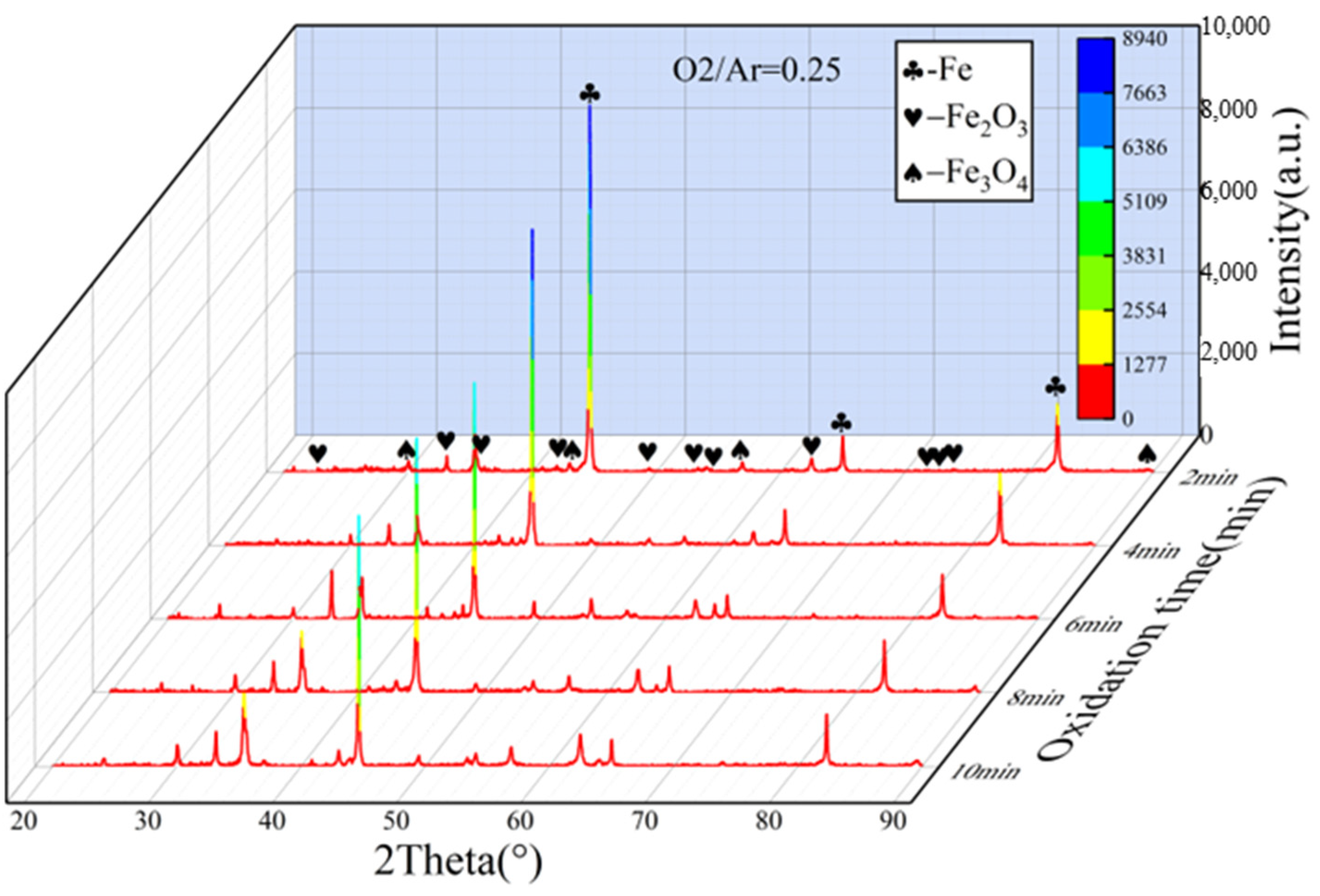
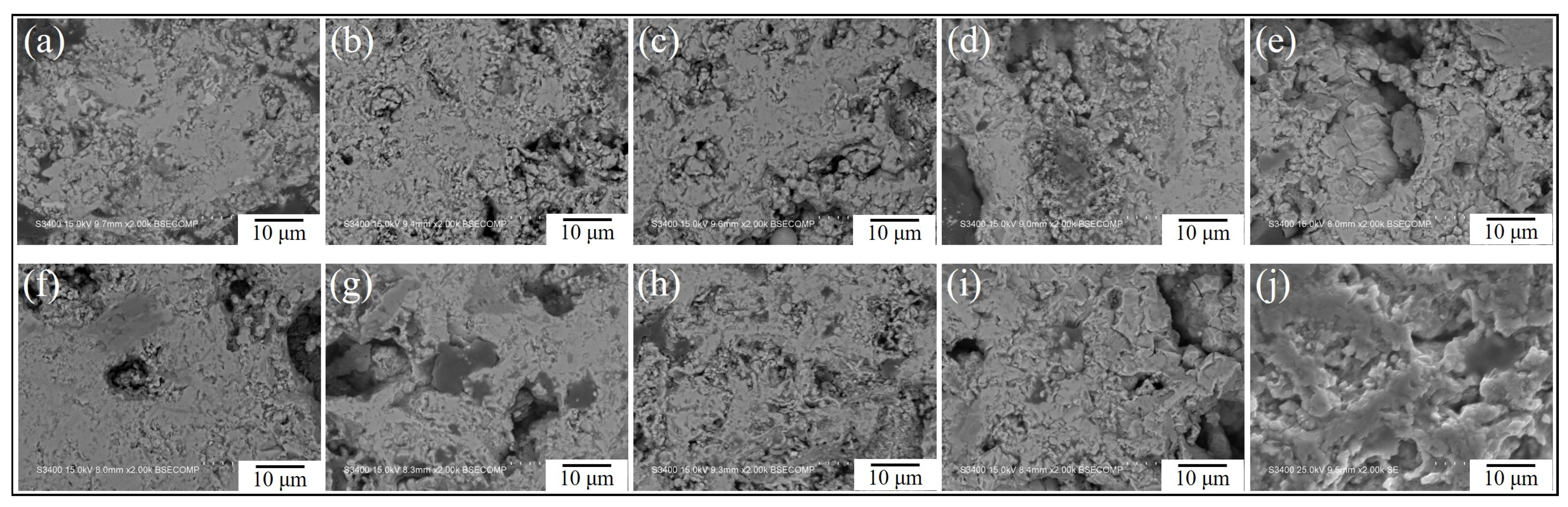
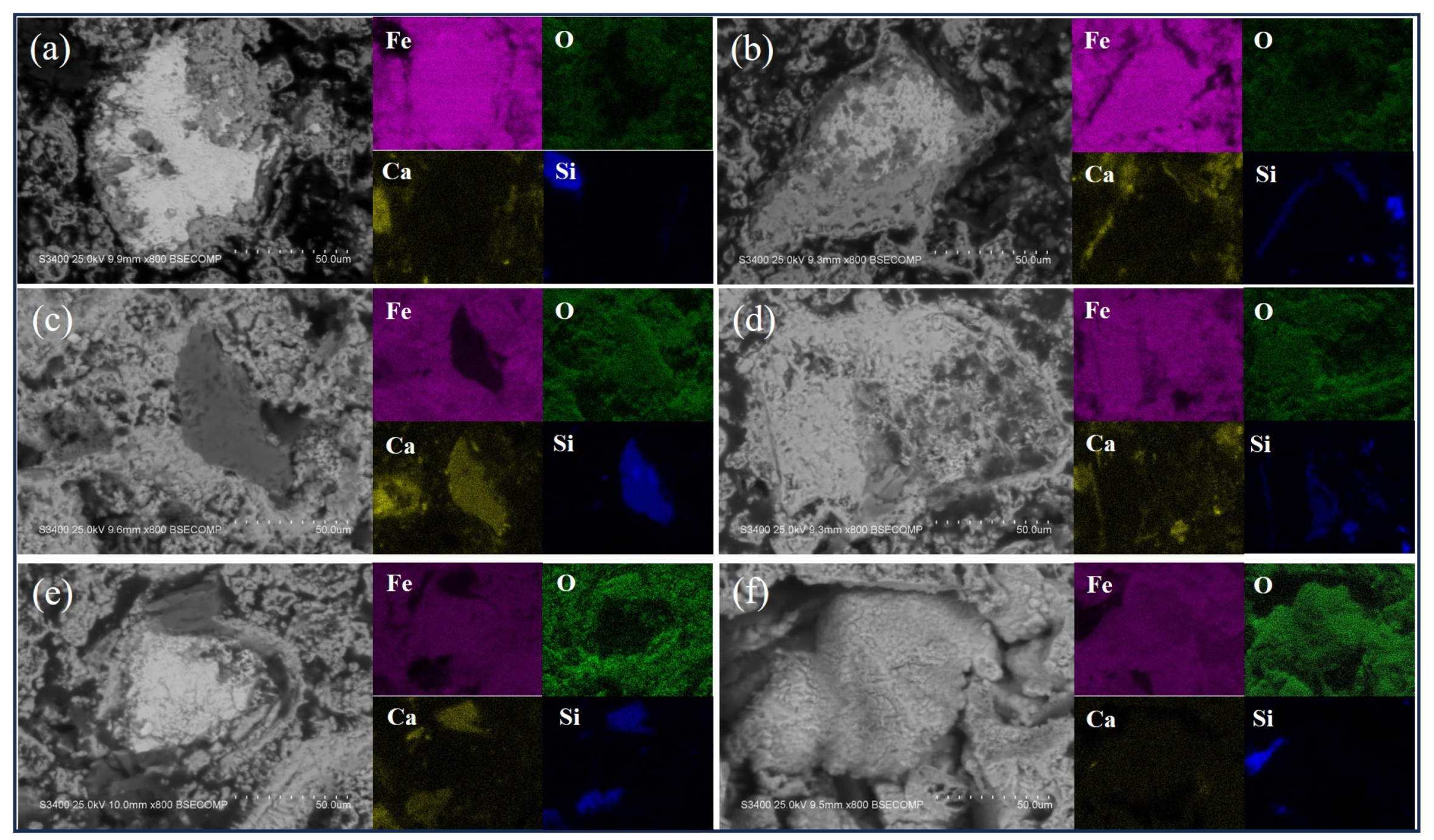
3.2. Analysis of Oxidation Behavior
3.3. Oxidation Kinetics Analysis
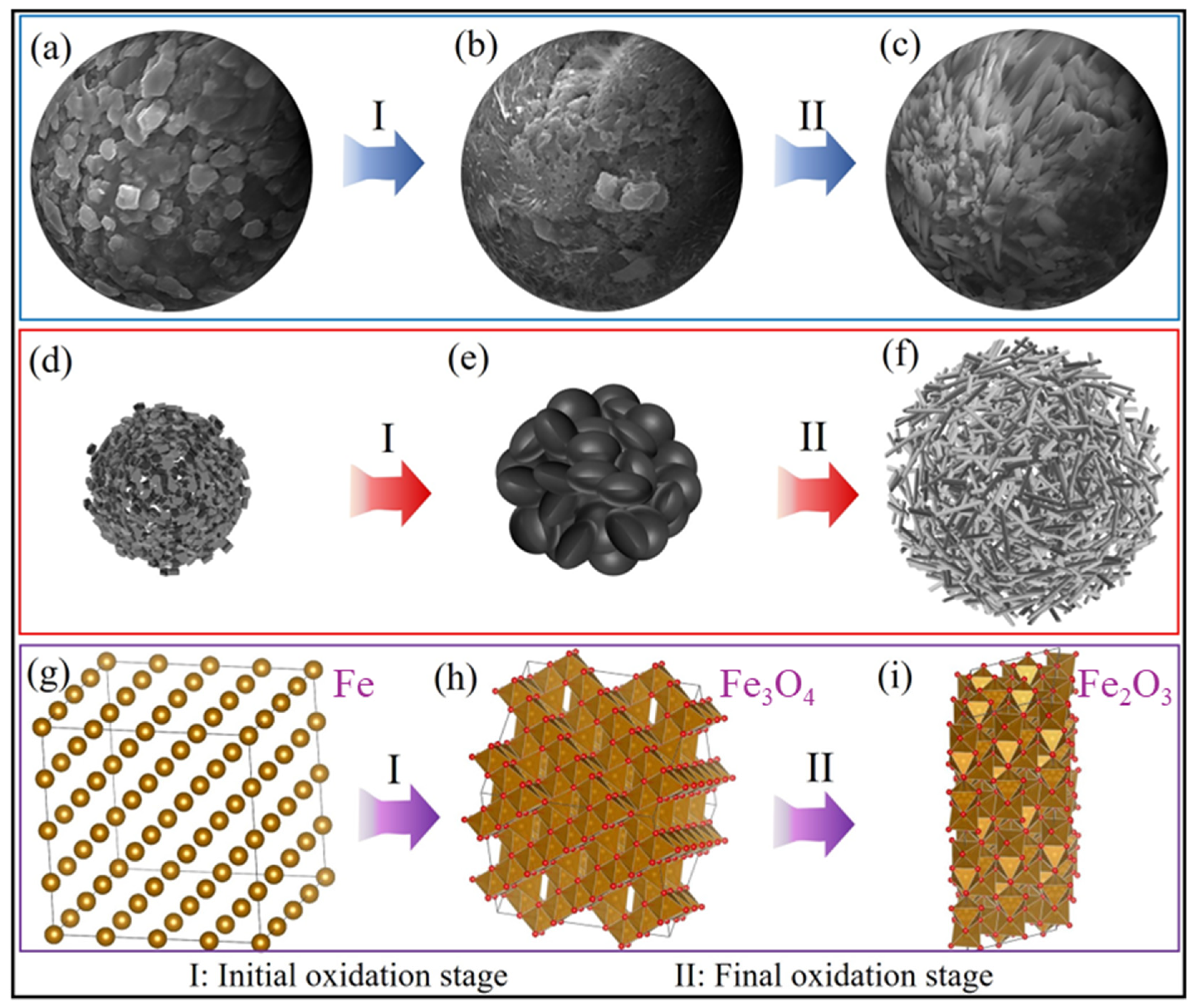
3.4. Analysis of Oxidation Mechanism
4. Conclusions
- (1)
- The oxidation products of sponge iron predominantly comprise Fe2O3 and Fe3O4, and compared with Fe3O4, Fe2O3 is the most difficult to form.
- (2)
- With the extension of oxidation time, the surface of sponge iron changed from a granular porous structure (Fe) to a dense bulk structure (Fe3O4) and was finalized as a rod structure (Fe2O3).
- (3)
- In an O2/Ar = 21/79 atmosphere, the oxide content was higher than that in an O2/Ar = 11/89 atmosphere, and the content of oxidation products gradually increased with the extension of oxidation time.
- (4)
- In an O2/Ar = 11/89 atmosphere, the n value for the entire stage was 0.68, indicating that the oxidation rate of sponge iron was relatively fast in this process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghadi, A.Z.; Radfar, N.; Valipour, M.S.; Sohn, H.Y. A Review on the Modeling of Direct Reduction of Iron Oxides in Gas-Based Shaft Furnaces. Steel Res. Int. 2023, 94, 2200742. [Google Scholar] [CrossRef]
- Cavaliere, P.; Perrone, A.; Marsano, D.; Primavera, V. Hydrogen-Based Direct Reduction of Iron Oxides Pellets Modeling. Steel Res. Int. 2023, 94, 2200791. [Google Scholar] [CrossRef]
- Cavaliere, P.; Perrone, A.; Marsano, D. Effect of reducing atmosphere on the direct reduction of iron oxides pellets. Powder Technol. 2023, 426, 118650. [Google Scholar] [CrossRef]
- Kaushik, P.; Fruehan, R.J.; Kaushik, P.; Fruehan, R.J. Behavior of direct reduced iron and hot briquetted iron in the upper blast furnace shaft: Part I. Fundamentals of kinetics and mechanism of oxidation. Metall. Mater. Trans. B 2006, 37, 715–725. [Google Scholar] [CrossRef]
- Kaushik, P.; Fruehan, R.J. Behavior of direct reduced iron and hot briquetted iron in the upper blast furnace shaft: Part II. A model of oxidation. Metall. Mater. Trans. B 2006, 37, 727–732. [Google Scholar] [CrossRef]
- Dutta, S.K.; Chokshi, Y.B. Basic Concepts of Iron and Steel Making; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Hajidavalloo, E.; Alagheband, A. Thermal analysis of sponge iron preheating using waste energy of EAF. J. Mater. Process. Tech. 2008, 208, 336–341. [Google Scholar] [CrossRef]
- Karami, M.; Koohestani, H.; Gholami, H. Investigation of the effect of geometrical shape of sponge iron on the operating parameters of the induction furnace. Can. Metall. Quart. 2023, 62, 356–361. [Google Scholar] [CrossRef]
- Fradet, Q.; Ali, M.L.; Riedel, U. Development of a porous solid model for the direct reduction of iron ore pellets. Steel Res. Int. 2022, 93, 2200042. [Google Scholar] [CrossRef]
- Cavaliere, P.; Dijon, L.; Laska, A.; Koszelow, D. Hydrogen direct reduction and reoxidation behaviour of high-grade pellets. Int. J. Hydrogen Energy 2024, 49, 1235–1254. [Google Scholar] [CrossRef]
- De Mello, J.D.B.; Binder, R.; Klein, A.N.; Hutchings, I.M. Effect of compaction pressure and powder grade on microstructure and hardness of steam oxidised sintered iron. Powder Metall. 2001, 44, 53–61. [Google Scholar] [CrossRef]
- Wang, W.F. Effect of powder type and compaction pressure on the density, hardness and oxidation resistance of sintered and steam-treated steels. J. Mater. Eng. Perform. 2007, 16, 533–538. [Google Scholar] [CrossRef]
- Daghagheleh, O.; Schenk, J.; Zheng, H.; Taferner, B.; Forstner, A.; Rosenfellner, G. Long-Term Reoxidation of Hot Briquetted Iron in Various Prepared Climatic Conditions. Steel Res. Int. 2023, 94, 2200535. [Google Scholar] [CrossRef]
- Al-Saraireh, F.M. Experimental Investigation of Thermofriction’s Impact on Surface Hardness of Steel Products’. Manuf. Technol. 2023, 23, 319–325. [Google Scholar] [CrossRef]
- Wei, X.Y.; Qian, Y. Experimental study on the low to medium temperature oxidation characteristics and kinetics of micro-size iron powder. CIESC J. 2023, 74, 2624. [Google Scholar]
- Bandopadhyay, A.; Ganguly, A.; Gupta, K.N.; Ra, H.S. Investigations on the anomalous oxidation behaviour of high-carbon gas-based direct reduced iron (DRI). Thermochim. Acta 1996, 276, 199–207. [Google Scholar] [CrossRef]
- Bodas, M.G.; Dash, D.R.; Sivaramakrishnan, C.S. Oxidation of iron powder in a fluidized bed reactor. Mater. Design 1996, 17, 167–172. [Google Scholar] [CrossRef]
- Van Diepen, A.M.; Vledder, H.J.; Langereis, C. The nature of the passivating oxide layer on iron powder. Appl. Phys. 1978, 15, 163–166. [Google Scholar] [CrossRef]
- Choisez, L.; Rooij, N.E.V.; Hessels, C.J.M.; Silva, A.K.D.; Souza Filho, I.R.; Ma, Y.; Goey, P.D.; Springer, H.; Raab, D. Phase transformations and microstructure evolution during combustion of iron powder. Acta Mater. 2022, 239, 118261. [Google Scholar] [CrossRef]
- AbdElmomen, S.S. Reoxidation of direct reduced iron in ambient air. Ironmak. Steelmak. 2014, 41, 107–111. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Nikolaev, E.V.; Vlasov, V.A.; Zhuravko, S.P. Investigation of oxidation process of mechanically activated ultrafine iron powders. IOP Conf. Ser. Mater. Sci. Eng. 2016, 110, 012093. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Vlasov, V.A.; Surzhikov, A.P.; Kupchishin, A.I. Kinetic Study of Lithium–Zinc Ferrite Synthesis Under Electron Beam Heating Conditions. Inorg. Mater. Appl. Res. 2022, 13, 494. [Google Scholar] [CrossRef]
- Lysenko, E.N.; Surzhikov, A.P.; Nikolaev, E.V.; Vlasov, V.A.; Zhuravkov, S.P.J. The oxidation kinetics study of ultrafine iron powders by thermogravimetric analysis. Therm. Anal. Calorim. 2014, 115, 1447–1452. [Google Scholar] [CrossRef]
- Yuan, Z.; Sohn, H.Y. Re-oxidation kinetics of flash reduced iron particles in O2–N2 gas mixtures relevant to a novel flash ironmaking process. ISIJ Int. 2014, 54, 1235–1243. [Google Scholar] [CrossRef]
- Agustianingrum, M.P.; Latief, F.H.; Park, N.; Lee, U. Thermal oxidation characteristics of Fex (CoCrMnNi) 100-x medium and high-entropy alloys. Intermetallics 2020, 120, 106757. [Google Scholar] [CrossRef]




| Atmosphere | 2 min | 4 min | 6 min | 8 min | 10 min |
|---|---|---|---|---|---|
| O2/Ar = 11/89 | ☑ | ☑ | ☑ | ☑ | ☑ |
| O2/Ar = 21/79 | ☑ | ☑ | ☑ | ☑ | ☑ |
| Oxidation Time (min) | O2/Ar = 0.1 | O2/Ar = 0.25 |
|---|---|---|
| 2–6 | n1 = 0.68 | n1 = 1.17 |
| 6–10 | n2 = 0.68 | n2 = 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, P.; Zhang, C.; Lu, X.; Peng, W. Study of the Behavior and Mechanism of Sponge Iron Oxidation. Metals 2025, 15, 508. https://doi.org/10.3390/met15050508
Jiang P, Zhang C, Lu X, Peng W. Study of the Behavior and Mechanism of Sponge Iron Oxidation. Metals. 2025; 15(5):508. https://doi.org/10.3390/met15050508
Chicago/Turabian StyleJiang, Pingguo, Chen Zhang, Xionggang Lu, and Wangjun Peng. 2025. "Study of the Behavior and Mechanism of Sponge Iron Oxidation" Metals 15, no. 5: 508. https://doi.org/10.3390/met15050508
APA StyleJiang, P., Zhang, C., Lu, X., & Peng, W. (2025). Study of the Behavior and Mechanism of Sponge Iron Oxidation. Metals, 15(5), 508. https://doi.org/10.3390/met15050508





