Lightweight Ti3VNbAl0.5Zrx (x = 0, 0.1, 0.5, and 1) Refractory High-Entropy Alloys with an Optimized Balance of Strength and Ductility
Abstract
1. Introduction
2. Materials and Methods
3. Results
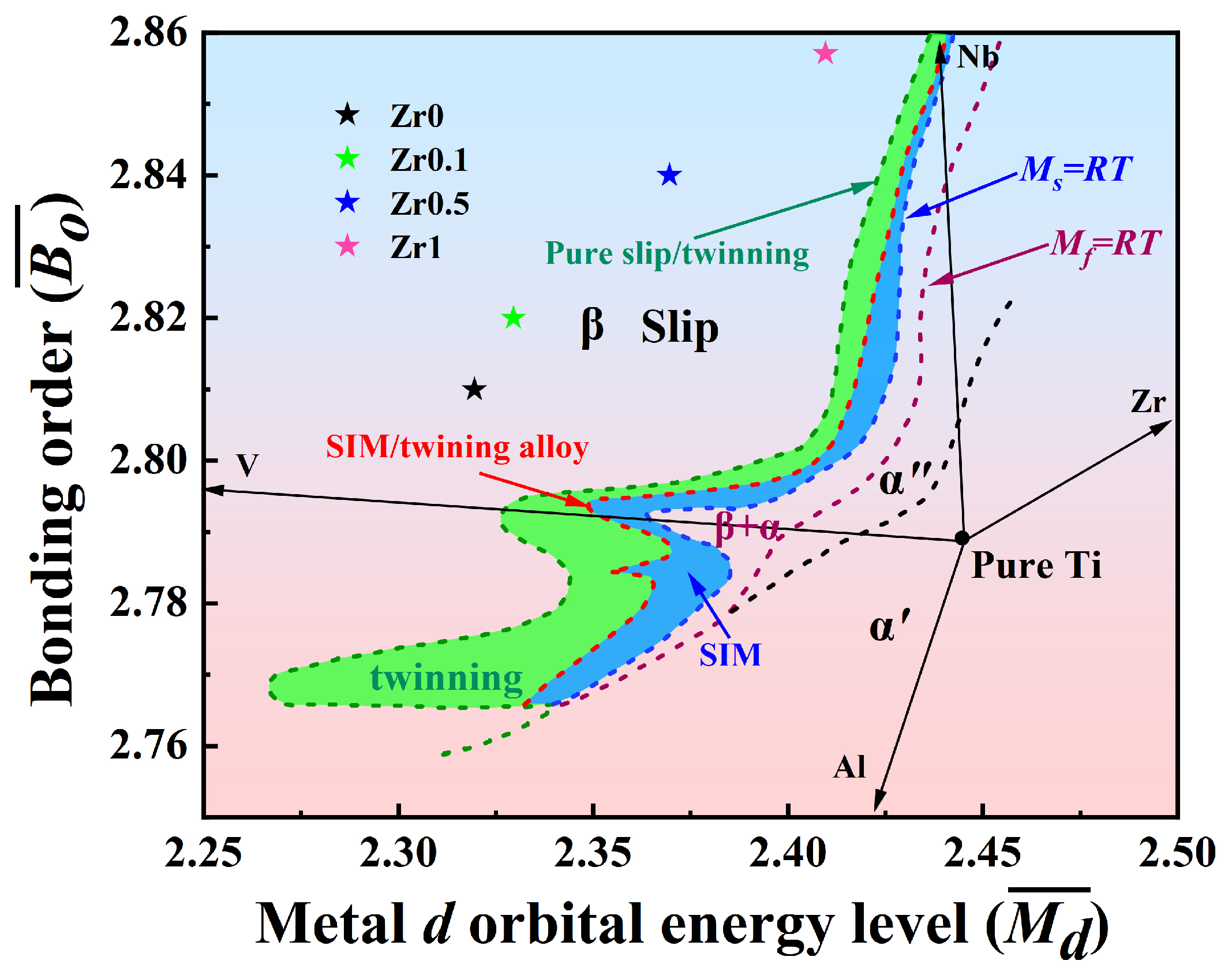
3.1. Phases and Alloys Design
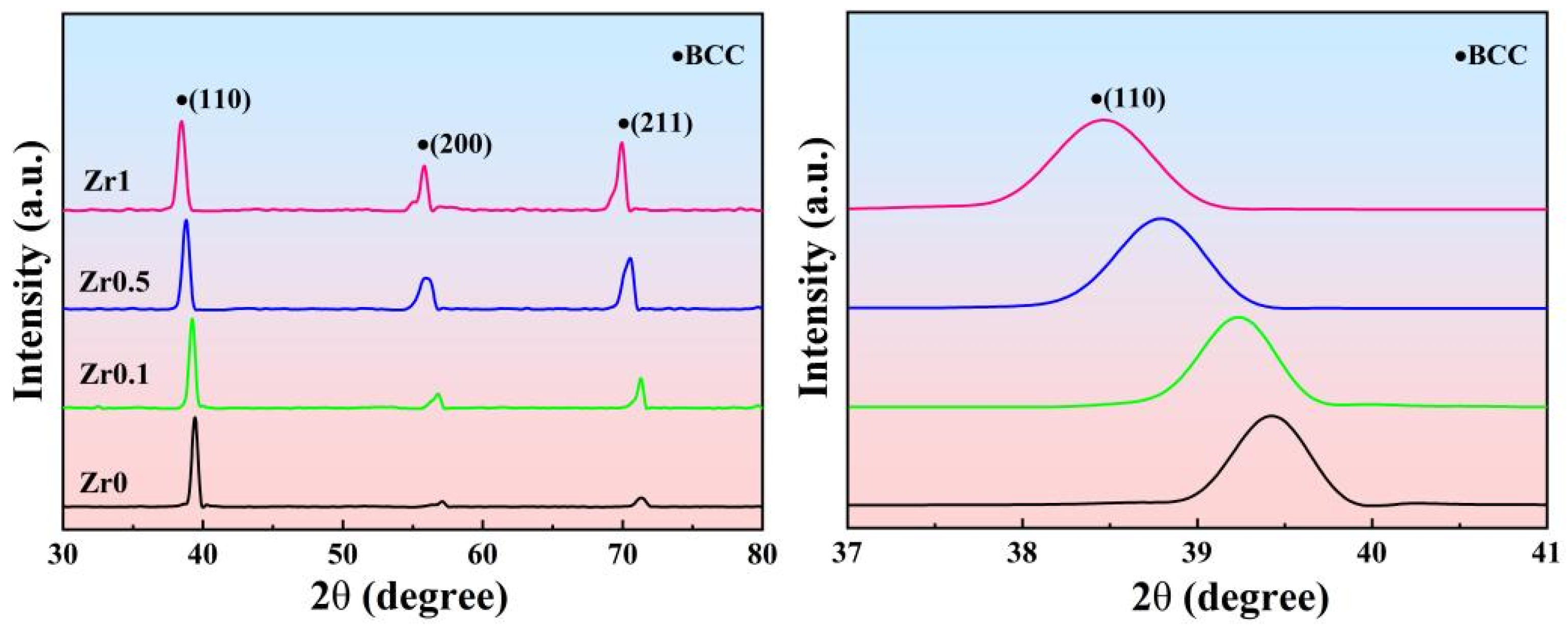
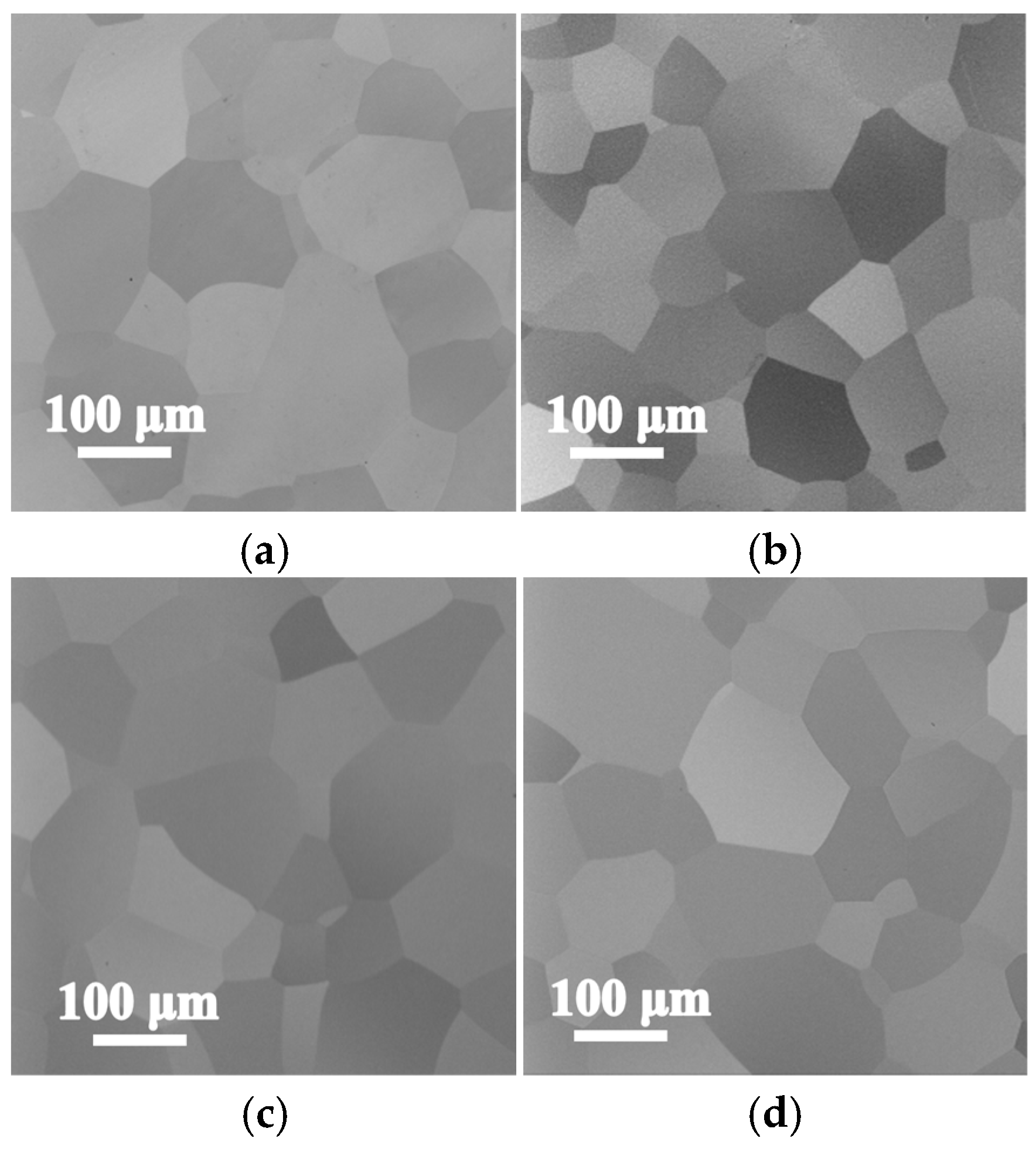
3.2. Microstructures and Phase Constitution of the Annealed Ti3VNbAl0.5Zrx Alloys
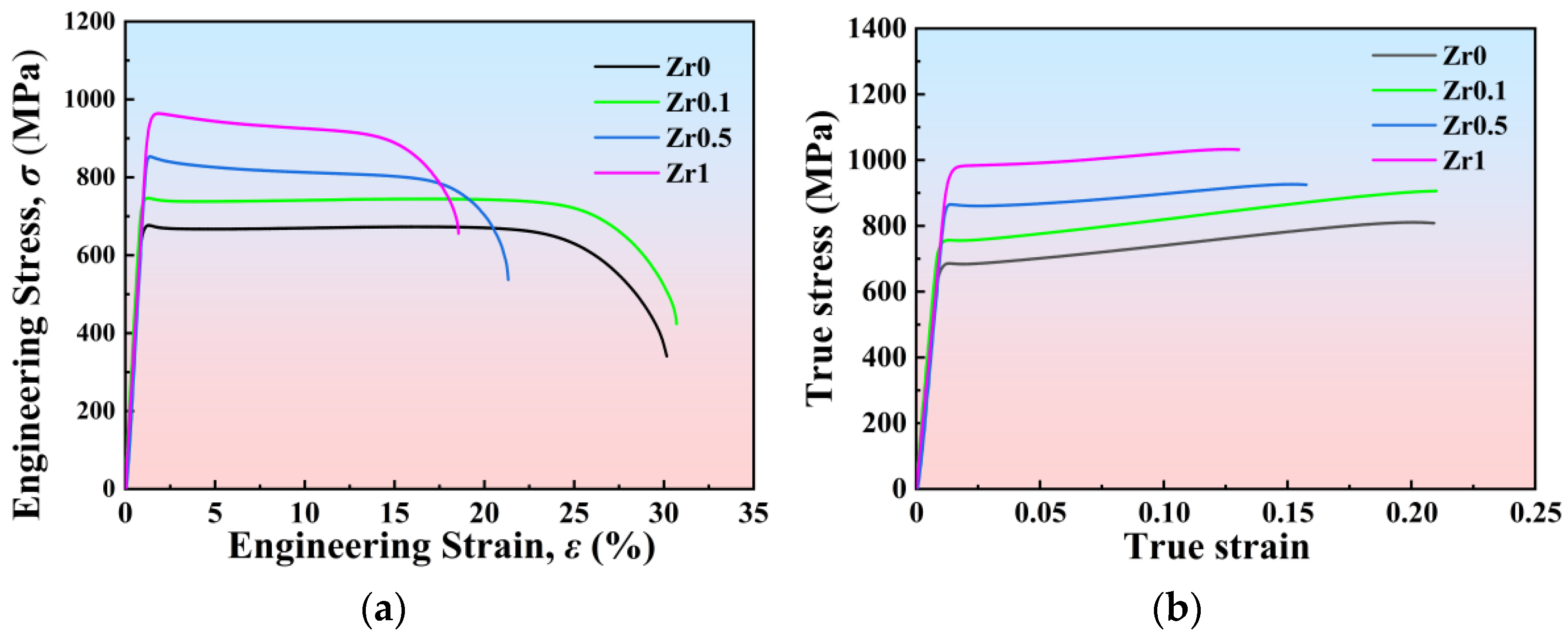
3.3. Tensile Properties of the Annealed Ti3VNbAl0.5Zrx Alloys
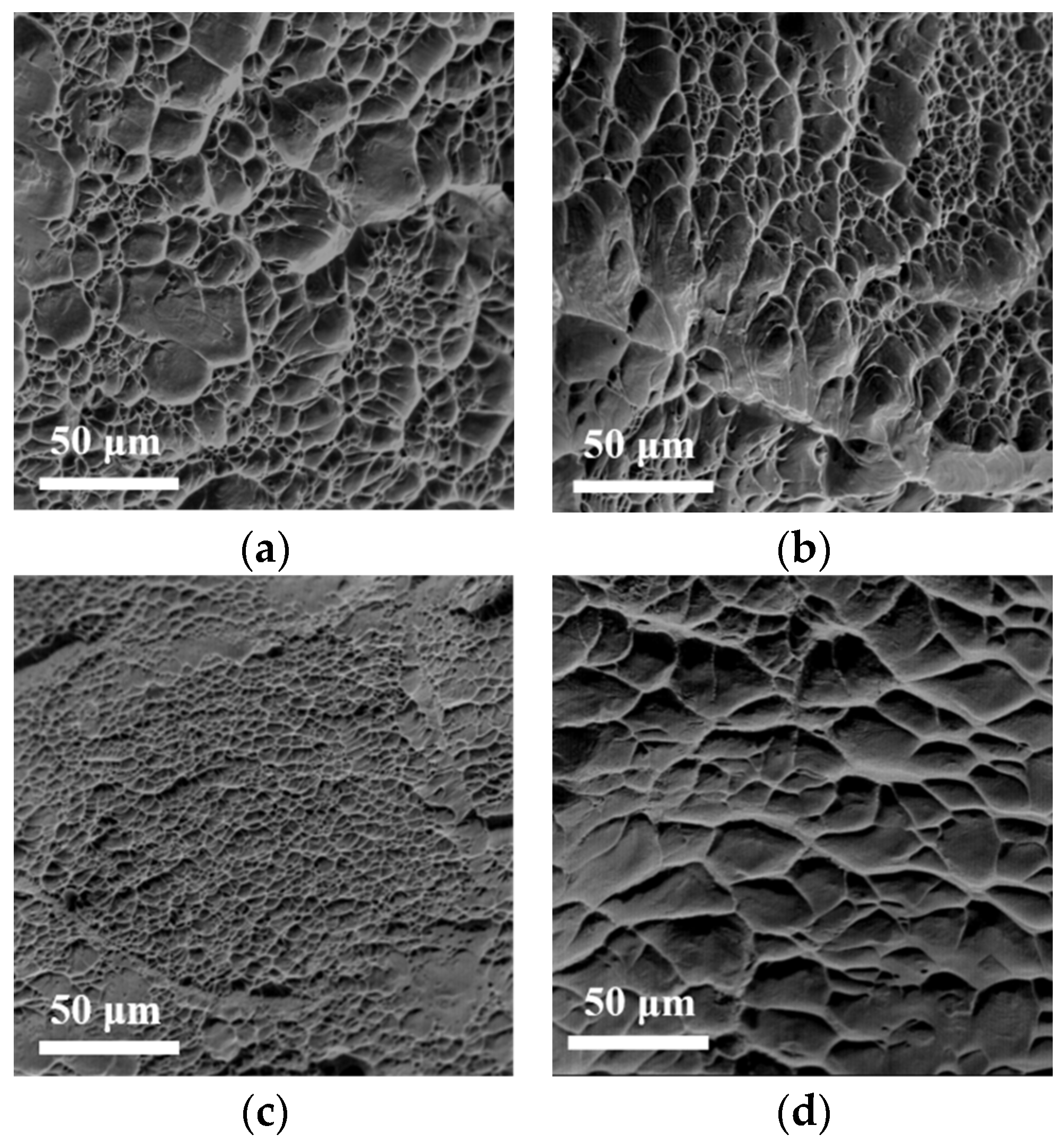
3.4. Microstructures of the Annealed Ti3VNbAl0.5Zrx Alloys After Tensile Deformation
4. Discussion
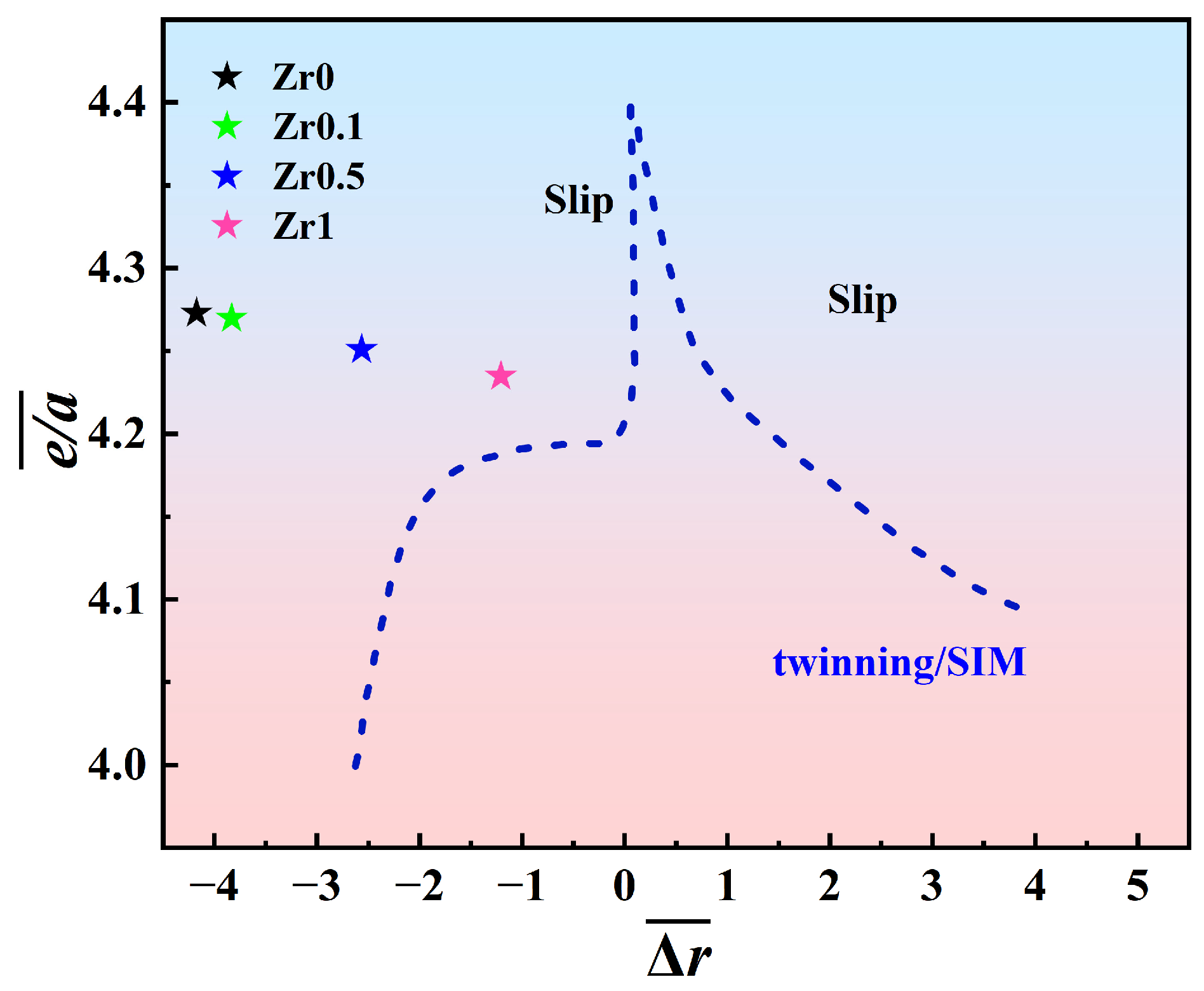
4.1. Deformation Mechanism of Annealed Ti3VNbAl0.5Zrx Alloys
4.2. Strengthening Mechanism of Annealed Ti3VNbAl0.5Zrx Alloys
5. Conclusions
- The four alloys were designed using the phase formation law and d-electron theory. By increasing the Ti content, controlling the content of Al and Zr elements, and adding the BCC structure-stabilizing element Nb, these methods effectively suppressed the formation of intermetallic compounds and B2 phases. The final results demonstrate that all the alloys successfully achieved a single-phase BCC structure;
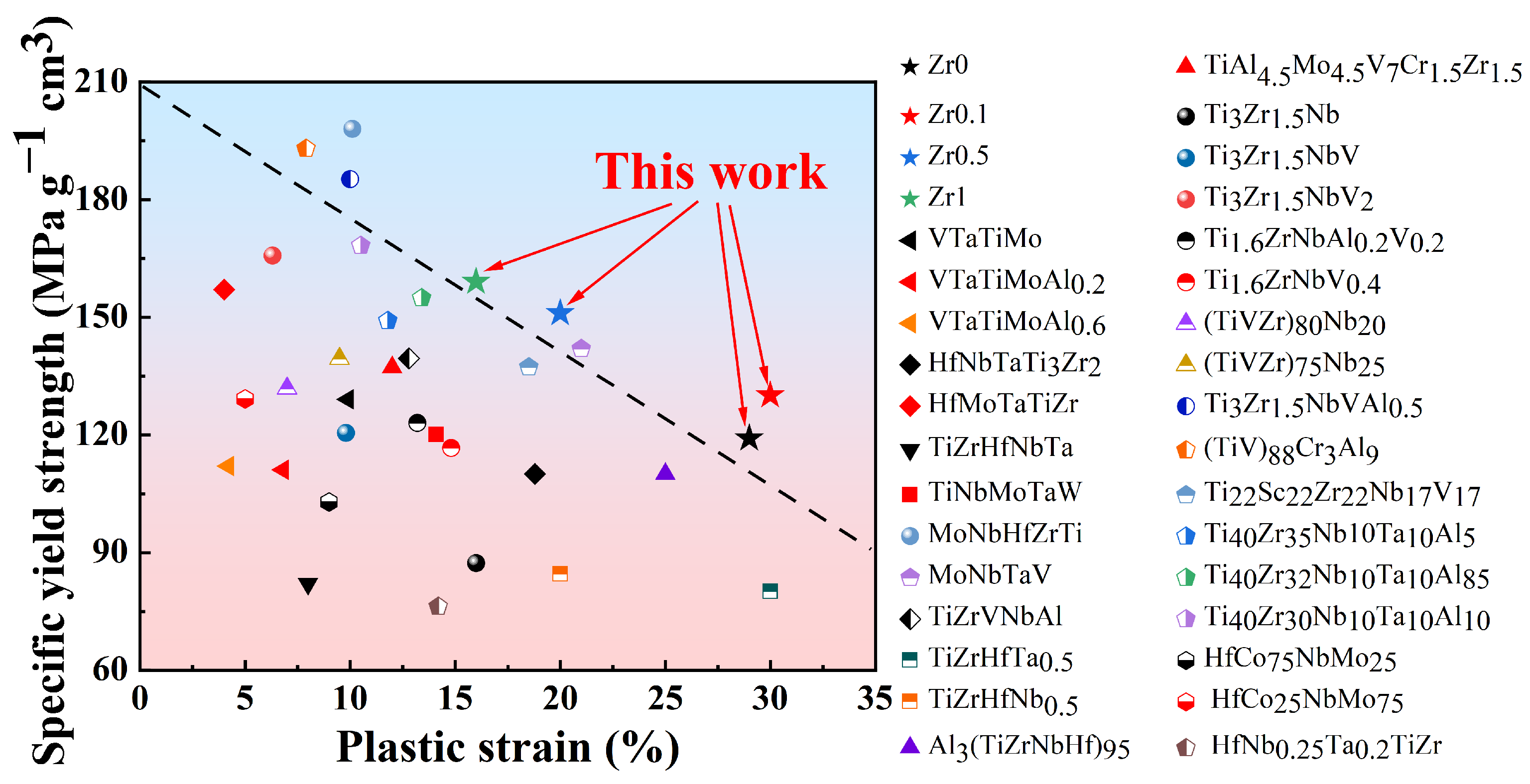
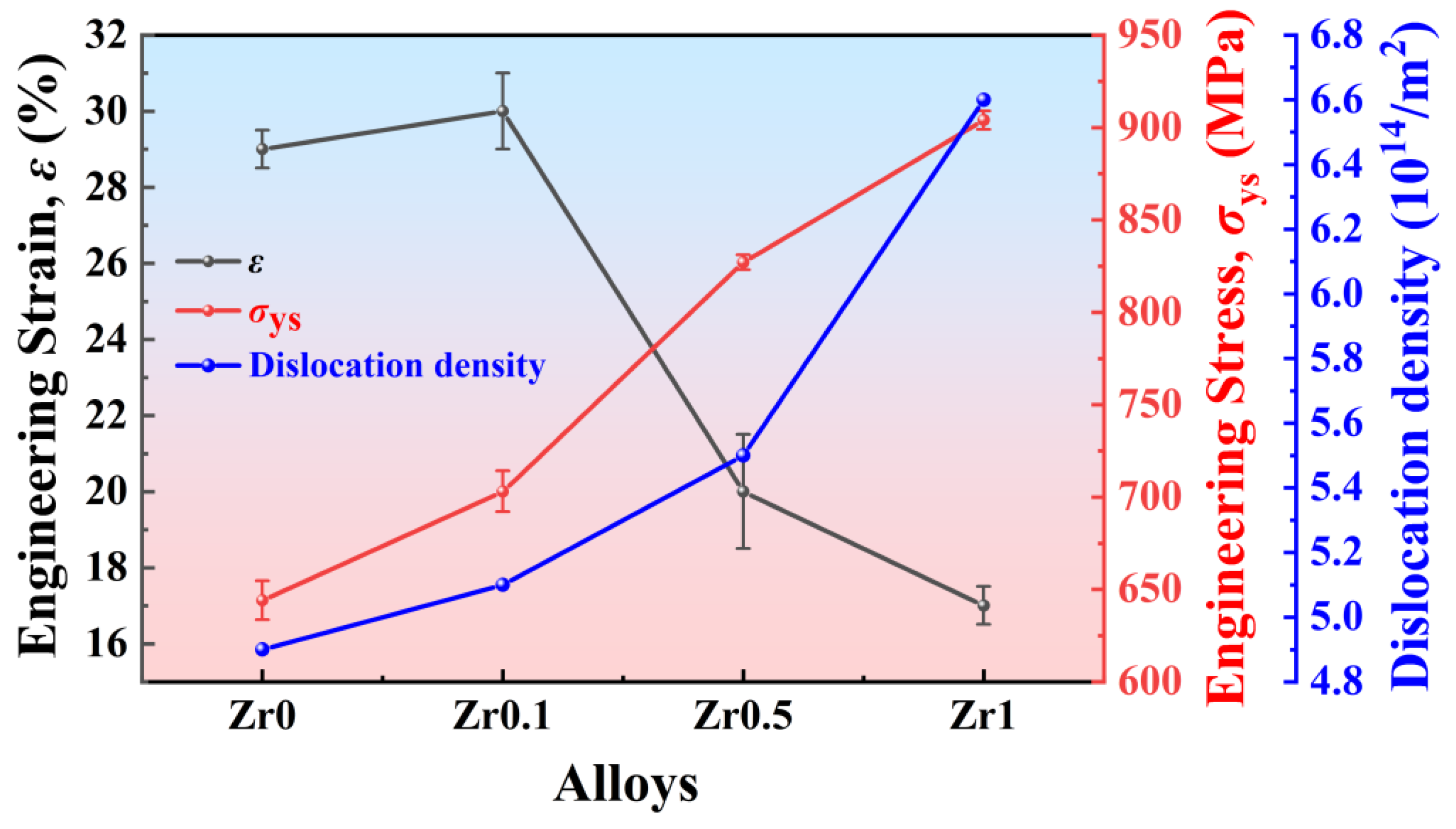
- The yield strength of Ti3VNbAl0.5Zrx (x = 0, 0.1, 0.5, and 1) LHEAs increases significantly from 644 MPa (x = 0) to 904 MPa (x = 1). This substantial enhancement in yield strength is primarily attributed to the progressive increase in Zr content. However, the increase in Zr content also leads to a notable reduction in ductility, with ductility decreasing from 29% (x = 0) to 16% (x = 1). Additionally, it is observed that these alloys exhibit weak strain-hardening ability during the deformation process. Furthermore, the specific yield strength and tensile ductility of the Ti3VNbAl0.5Zrx LHEAs are better than those of most RHEAs and LHEAs;
- The deformation mechanism of Zr1 alloys is based on dislocation slip. The observation of deformation microstructure shows that the slip bands and Taylor lattices are the main deformation products in Zr1 alloy, which makes the alloy exhibit excellent tensile plasticity at room temperature. In addition, dislocation tangles, dislocation walls, dislocation loops, and dislocation pile-ups could also be observed. These substructures increase the resistance of dislocation movement and contribute to the stability of plastic deformation, thereby contributing to the weak strain-hardening ability of the alloys.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCC | Body-centered cubic |
| LHEAs | Lightweight high-entropy alloys |
| RHEAs | Refractory high-entropy alloys |
| HEAs | High-entropy alloys |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive spectroscopy |
| BSE | Backscattered electron |
| TEM | Transmission electron microscopy |
| SAED | Selected area diffraction |
| HDDWs | High-density dislocation walls |
References
- Bridges, D.; Fieser, D.; Santiago, J.J.; Hu, A. Novel Frontiers in High-Entropy Alloys. Metals 2023, 13, 1193. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, A.X.; Zhan, S.; Liu, C.-T.; Cao, S.C. Refractory High-Entropy Alloys: A Focused Review of Preparation Methods and Properties. J. Mater. Sci. Technol. 2023, 142, 196–215. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Li, W.; Xie, D.; Li, D.; Zhang, Y.; Gao, Y.; Liaw, P.K. Mechanical Behavior of High-Entropy Alloys. Prog. Mater. Sci. 2021, 118, 100777. [Google Scholar] [CrossRef]
- Pandey, V.; Seetharam, R.; Chelladurai, H. A Comprehensive Review: Discussed the Effect of High-Entropy Alloys as Reinforcement on Metal Matrix Composite Properties, Fabrication Techniques, and Applications. J. Alloys Compd. 2024, 1002, 175095. [Google Scholar] [CrossRef]
- Yang, F.; Wang, J.; Zhang, Y.; Wu, Z.; Zhang, Z.; Zhao, F.; Huot, J.; Grobivć Novaković, J.; Novaković, N. Recent Progress on the Development of High Entropy Alloys (HEAs) for Solid Hydrogen Storage: A Review. Int. J. Hydrogen Energy 2022, 47, 11236–11249. [Google Scholar] [CrossRef]
- Arun, S.; Radhika, N.; Saleh, B. Advances in Vacuum Arc Melting for High Entropy Alloys: A Review. Vacuum 2024, 226, 113314. [Google Scholar] [CrossRef]
- Zhuo, L.; Xie, Y.; Chen, B. A Review on Recent Progress of Refractory High Entropy Alloys: From Fundamental Research to Engineering Applications. J. Mater. Res. Technol. 2024, 33, 1097–1129. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical Properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 Refractory High Entropy Alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Yu, B.; Ren, Y.; Zeng, Y.; Ma, W.; Morita, K.; Zhan, S.; Lei, Y.; Lv, G.; Li, S.; Wu, J. Recent Progress in High-Entropy Alloys: A Focused Review of Preparation Processes and Properties. J. Mater. Res. Technol. 2024, 29, 2689–2719. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, S.; Yang, S.; Luo, Q.; Jin, Y.; Xie, W.; Zhang, L.; Li, Q. Light-Weight Refractory High-Entropy Alloys: A Comprehensive Review. J. Mater. Sci. Technol. 2023, 151, 41–65. [Google Scholar] [CrossRef]
- Dou, B.; Pan, Y.; Liu, S.; Wang, B.; Cheng, B.; Wang, L.; Sun, S.; Xue, Y. Achieving Outstanding Strength-Ductility Matching in BCC Light-Weight High Entropy Alloys via High Content Ordered Nanoprecipitates. Mater. Sci. Eng. A 2024, 889, 145861. [Google Scholar] [CrossRef]
- Ren, K.; Liu, H.; Chen, R.; Tang, Y.; Guo, B.; Li, S.; Wang, J.; Wang, R.; Lu, F. Compression Properties and Impact Energy Release Characteristics of TiZrNbV High-Entropy Alloy. Mater. Sci. Eng. A 2021, 827, 142074. [Google Scholar] [CrossRef]
- Zhu, M.; Yao, L.; Liu, Y.; Zhang, M.; Li, K.; Jian, Z. Microstructure Evolution and Mechanical Properties of a Novel CrNbTiZrAlx (0.25 ≤ x ≤ 1.25) Eutectic Refractory High-Entropy Alloy. Mater. Lett. 2020, 272, 127869. [Google Scholar] [CrossRef]
- Yurchenko, N.; Panina, E.; Salishchev, G.; Stepanov, N. Structure and Mechanical Properties of Near-Eutectic Refractory Al-Cr-Nb-Ti-Zr High Entropy Alloys. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1014, 012058. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Chu, C.; Fu, Z.; Jiang, Z.; Yang, X.; Lavernia, E.J. Microstructural Evolution and Mechanical Behavior of Novel Ti1.6ZrNbAl Lightweight Refractory High-Entropy Alloys Containing BCC/B2 Phases. Mater. Sci. Eng. A 2023, 885, 145661. [Google Scholar] [CrossRef]
- Gao, F.; Deng, C.; Sun, Y.; Hu, L.; Ba, M. Lightweight Refractory High Entropy Alloys with Excellent Specific Strength and Enhanced Malleability by In-Situ Heterogeneous Structure. Mater. Charact. 2023, 203, 113127. [Google Scholar] [CrossRef]
- Qiao, D.; Liang, H.; Wu, S.; He, J.; Cao, Z.; Lu, Y.; Li, T. The Mechanical and Oxidation Properties of Novel B2-Ordered Ti2ZrHf0.5VNb0.5Alx Refractory High-Entropy Alloys. Mater. Charact. 2021, 178, 111287. [Google Scholar] [CrossRef]
- Soni, V.; Gwalani, B.; Alam, T.; Dasari, S.; Zheng, Y.; Senkov, O.N.; Miracle, D.; Banerjee, R. Phase Inversion in a Two-Phase, BCC+B2, Refractory High Entropy Alloy. Acta Mater. 2020, 185, 89–97. [Google Scholar] [CrossRef]
- Yurchenko, N.Y.; Stepanov, N.D.; Shaysultanov, D.G.; Tikhonovsky, M.A.; Salishchev, G.A. Effect of Al Content on Structure and Mechanical Properties of the AlxCrNbTiVZr (x = 0; 0.25; 0.5; 1) High-Entropy Alloys. Mater. Charact. 2016, 121, 125–134. [Google Scholar] [CrossRef]
- Heydari, H.; Tajally, M.; Habibolahzadeh, A. Calculations to Introduce Some Light High Entropy Alloys Based on Phase Formation Rules. J. Alloys Compd. 2022, 912, 165222. [Google Scholar] [CrossRef]
- Huang, R.; Tan, J.; Li, W.; Dong, Q.; Li, C.J.; Qin, X.M.; Guo, S.F.; Lu, Y.P. Effects of V Content on the Microstructure and Mechanical Properties of Nb31Ti37-Zr26Al6V Refractory Medium-Entropy Alloys. Intermetallics 2022, 143, 107472. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Yao, Z.; Zhao, W.; Fu, Y.; Jin, Z.; Dong, J.; Jin, L. Effect of Zr on Microstructure and Mechanical Properties of Novel Al0.5Mo0.5NbTi1.25V0.5Zrx Lightweight Refractory High-Entropy Alloys. J. Alloys Compd. 2025, 1024, 180085. [Google Scholar] [CrossRef]
- Feng, R.; Zhang, C.; Gao, M.C.; Pei, Z.; Zhang, F.; Chen, Y.; Ma, D.; An, K.; Poplawsky, J.D.; Ouyang, L.; et al. High-Throughput Design of High-Performance Lightweight High-Entropy Alloys. Nat. Commun. 2021, 12, 4329. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.; Jiang, L.; Wang, T.; Li, T. Effects of Electro-Negativity on the Stability of Topologically Close-Packed Phase in High Entropy Alloys. Intermetallics 2014, 52, 105–109. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Zhang, Z.; Liu, L.; Sun, L. Two Novel Zr-Rich Refractory High-Entropy Alloys with Excellent Tensile Mechanical Properties. Intermetallics 2023, 157, 107872. [Google Scholar] [CrossRef]
- Cai, Z.; Guo, Y.; Liu, J.; Liu, J.; Guo, J.; Du, X.; Huang, S. Progress in Light-Weight High Entropy Alloys. J. Wuhan Univ. Technol.-Mat. Sci. Ed. 2021, 36, 737–753. [Google Scholar] [CrossRef]
- Heydari, H.; Tajally, M.; Habibolahzadeh, A. Computational Analysis of Novel AlLiMgTiX Light High Entropy Alloys. Mater. Chem. Phys. 2022, 280, 125834. [Google Scholar] [CrossRef]
- Abdel-Hady, M.; Hinoshita, K.; Morinaga, M. General Approach to Phase Stability and Elastic Properties of β-Type Ti-Alloys Using Electronic Parameters. Scr. Mater. 2006, 55, 477–480. [Google Scholar] [CrossRef]
- Ng, C.H.; Bermingham, M.J.; Yuan, L.; Dargusch, M.S. Towards β-Fleck Defect Free Additively Manufactured Titanium Alloys by Promoting the Columnar to Equiaxed Transition and Grain Refinement. Acta Mater. 2022, 224, 117511. [Google Scholar] [CrossRef]
- Cui, S.; Liu, S.; Nie, J.; Chen, D.; Wu, X.; Qin, G.; Zhang, E. Design and Preparation of a Biomedical Titanium Alloy with Low Elastic Modulus and High Antibacterial Property Based on Ti-Mo-Ag System. J. Alloys Compd. 2022, 908, 164639. [Google Scholar] [CrossRef]
- Yang, J.K.; Zhang, C.L.; Zhang, H.; Li, J.; Zhang, J.Y.; Kuang, J.; Liu, G.; Sun, J. Spinodal Decomposition-Mediated Multi-Architectured α Precipitates Making a Metastable β-Ti Alloy Ultra-Strong and Ductile. J. Mater. Sci. Technol. 2024, 191, 106–121. [Google Scholar] [CrossRef]
- Liu, F.; Chen, S.; Wang, B.; Wang, L.; Xiao, Y.; Wang, L.; Sun, S.; Xue, Y. High Specific Yield Strength TiZrAlNbV High-Entropy Alloys via Coherent Nanoprecipitation Strengthening. Mater. Sci. Eng. A 2022, 861, 144346. [Google Scholar] [CrossRef]
- Zhao, Q.; Bolzoni, L.; Chen, Y.; Xu, Y.; Torrens, R.; Yang, F. Processing of Metastable Beta Titanium Alloy: Comprehensive Study on Deformation Behaviour and Exceptional Microstructure Variation Mechanisms. J. Mater. Sci. Technol. 2022, 126, 22–43. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Ma, Z.L.; Wang, M.; Chen, Y.W.; Tan, Y.D.; Cheng, X.W. Design of Novel Low-Density Refractory High Entropy Alloys for High-Temperature Applications. Mater. Sci. Eng. A 2019, 755, 318–322. [Google Scholar] [CrossRef]
- Li, G.; Wu, Z.; Dong, Y.; Hu, Z.; Jia, Y.; Wu, S.; Mu, Y.; Sun, K.; Jia, Y.; Wang, G. Microstructures, Properties and Preparations of Lightweight Refractory High-Entropy Alloys: A Review. J. Mater. Res. Technol. 2025, 35, 3183–3204. [Google Scholar] [CrossRef]
- Zheng, W.; Lü, S.; Wu, S.; Chen, X.; Guo, W. Development of MoNbVTax Refractory High Entropy Alloy with High Strength at Elevated Temperature. Mater. Sci. Eng. A 2022, 850, 143554. [Google Scholar] [CrossRef]
- Wang, S.; Shu, D.; Shi, P.; Zhang, X.; Mao, B.; Wang, D.; Liaw, P.K.; Sun, B. TiZrHfNb Refractory High-Entropy Alloys with Twinning-Induced Plasticity. J. Mater. Sci. Technol. 2024, 187, 72–85. [Google Scholar] [CrossRef]
- Juan, C.-C.; Tsai, M.-H.; Tsai, C.-W.; Lin, C.-M.; Wang, W.-R.; Yang, C.-C.; Chen, S.-K.; Lin, S.-J.; Yeh, J.-W. Enhanced Mechanical Properties of HfMoTaTiZr and HfMoNbTaTiZr Refractory High-Entropy Alloys. Intermetallics 2015, 62, 76–83. [Google Scholar] [CrossRef]
- Wang, S.; Wu, M.; Shu, D.; Zhu, G.; Wang, D.; Sun, B. Mechanical Instability and Tensile Properties of TiZrHfNbTa High Entropy Alloy at Cryogenic Temperatures. Acta Mater. 2020, 201, 517–527. [Google Scholar] [CrossRef]
- Han, Z.D.; Luan, H.W.; Liu, X.; Chen, N.; Li, X.Y.; Shao, Y.; Yao, K.F. Microstructures and Mechanical Properties of Ti NbMoTaW Refractory High-Entropy Alloys. Mater. Sci. Eng. A 2018, 712, 380–385. [Google Scholar] [CrossRef]
- Guo, W.; Liu, B.; Liu, Y.; Li, T.; Fu, A.; Fang, Q.; Nie, Y. Microstructures and Mechanical Properties of Ductile NbTaTiV Refractory High Entropy Alloy Prepared by Powder Metallurgy. J. Alloys Compd. 2019, 776, 428–436. [Google Scholar] [CrossRef]
- Wu, Y.; Si, J.; Lin, D.; Wang, T.; Wang, W.Y.; Wang, Y.; Liu, Z.; Hui, X. Phase Stability and Mechanical Properties of AlHfNbTiZr High-Entropy Alloys. Mater. Sci. Eng. A 2018, 724, 249–259. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, L.; Amar, A.; Liaw, P.K.; Wang, T.; Li, T.; Lu, Y. Achieving Excellent Uniform Tensile Ductility and Strength in Dislocation-Cell-Structured High-Entropy Alloys. Int. J. Plast. 2024, 181, 104079. [Google Scholar] [CrossRef]
- Huang, W.; Hou, J.; Wang, X.; Qiao, J.; Wu, Y. Excellent Room-Temperature Tensile Ductility in as-Cast Ti37V15Nb22Hf23W3 Refractory High Entropy Alloys. Intermetallics 2022, 151, 107735. [Google Scholar] [CrossRef]
- Dou, B.; Zhang, Y.; Chen, A.; Cui, J.; Li, B.; Chen, S.; Li, Y.; Liu, S.; Wang, X.; Wang, B.; et al. Grain Refinement Induced Dynamic Plasticity Improvement in Lightweight High Entropy Alloys. J. Alloys Compd. 2024, 997, 174968. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhu, Y.; Zhang, S.; Cheng, F.; Yang, J.; Su, B.; Yang, C. High Specific Yield Strength and Superior Ductility of a Lightweight Refractory High-Entropy Alloy Prepared by Laser Additive Manufacturing. Addit. Manuf. 2023, 77, 103813. [Google Scholar] [CrossRef]
- Wang, C.H.; Russell, A.M.; Cao, G.H. A Semi-Empirical Approach to the Prediction of Deformation Behaviors of β-Ti Alloys. Scr. Mater. 2019, 158, 62–65. [Google Scholar] [CrossRef]
- Liu, D.; Jin, X.; Guo, N.; Liaw, P.K.; Qiao, J.W. Non-Equiatomic FeMnCrNiAl High-Entropy Alloys with Heterogeneous Structures for Strength and Ductility Combination. Mater. Sci. Eng. A 2021, 818, 141386. [Google Scholar] [CrossRef]
- Zeng, S.; Zhou, Y.; Li, H.; Zhang, H.; Zhang, H.; Zhu, Z. Microstructure and Mechanical Properties of Lightweight Ti3Zr1.5NbVAlx (x = 0, 0.25, 0.5 and 0.75) Refractory Complex Concentrated Alloys. J. Mater. Sci. Technol. 2022, 130, 64–74. [Google Scholar] [CrossRef]
- Hong, C.; Lu, Y.H.; Zheng, H.B.; Li, Z.H.; Guo, G.J. Uniaxial Tensile Behaviors and Hall-Petch Relationship of Polycrystalline 316LN Stainless Steel via Molecular Dynamics Simulation. Comput. Mater. Sci. 2024, 244, 113195. [Google Scholar] [CrossRef]
- Labusch, R. A Statistical Theory of Solid Solution Hardening. Phys. Status Solidi B 1970, 41, 659–669. [Google Scholar] [CrossRef]
- Fleischer, R.L. Solution hardening by tetragonal dist ortions: Application to irradiation hardening in FCC crystals. Acta Metall. 1962, 10, 835–842. [Google Scholar] [CrossRef]
- Yang, L.; Xiong, K.; Jin, C.; Dai, H.; Wu, H.; Zhang, S.; He, J.; Wang, Y.; Mao, Y. Effects of Ti Content on the Tensile Ductility, Lattice Distortion and Mechanical Properties of VNbTaTi Refractory High-Entropy Alloys. J. Mater. Res. Technol. 2024, 30, 3886–3899. [Google Scholar] [CrossRef]



| Element | Melting Point (°C) | Atomic Radius (Å) | VEC | Density (g/cm3) | Pauling Electronegativity |
|---|---|---|---|---|---|
| Al | 660 | 1.43 | 3 | 2.7 | 1.61 |
| Ti | 1660 | 1.46 | 4 | 4.51 | 1.54 |
| V | 1890 | 1.32 | 5 | 6.11 | 1.63 |
| Zr | 1852 | 1.60 | 4 | 6.49 | 1.33 |
| Nb | 2468 | 1.43 | 5 | 8.57 | 1.60 |
| ∆Hmix (kJ/mol) | Al | Ti | V | Nb | Zr |
|---|---|---|---|---|---|
| Al | … | −30 | −16 | −18 | −44 |
| Ti | … | −2 | 2 | 0 | |
| V | … | −1 | 4 | ||
| Nb | … | 4 | |||
| Zr | … |
| Alloys | ΔHmix (kJ/mol) | ΔSmix (J/Kmol) | VEC | Δδ | ΔΩ | Δχ |
|---|---|---|---|---|---|---|
| Zr0 | −8.336 | 1.16 R | 4.273 | 3.78% | 2.03 | 3.37 |
| Zr0.1 | −8.312 | 1.239 R | 4.27 | 4.09% | 2.39 | 4.98 |
| Zr0.5 | −8.189 | 1.237 R | 4.251 | 4.92% | 2.72 | 7.65 |
| Zr1 | −8.063 | 1.418 R | 4.235 | 5.7% | 3 | 9.47 |
| Alloys | Experimental Density (g/cm3) | Theoretical Density (g/cm3) |
|---|---|---|
| Zr0 | 5.39 ± 0.03 | 5.37 |
| Zr0.1 | 5.41 ± 0.04 | 5.40 |
| Zr0.5 | 5.47 ± 0.02 | 5.49 |
| Zr1 | 5.68 ± 0.04 | 5.70 |
| Alloy | ||
|---|---|---|
| Zr0 | 2.70 | 2.38 |
| Zr0.1 | 2.71 | 2.39 |
| Zr0.5 | 2.74 | 2.43 |
| Zr1 | 2.76 | 2.46 |
| Zr0 | Zr0.1 | Zr0.5 | Zr1 | |
|---|---|---|---|---|
| α (Å) | 3.219 ± 0.0005 | 3.237 ± 0.0063 | 3.279 ± 0.0068 | 3.303 ± 0.0091 |
| D (μm) | 124 ± 12 | 101 ± 8 | 120 ± 13 | 110 ± 13 |
| Alloy | (%) | |
|---|---|---|
| Zr0 | 4.273 | −4.36 |
| Zr0.1 | 4.270 | −4.05 |
| Zr0.5 | 4.251 | −2.91 |
| Zr1 | 4.235 | −1.69 |
| Properties | Ti | Zr | |||
|---|---|---|---|---|---|
| R (Å) | 1.462 | 1.603 | 1.429 | 1.312 | 1.432 |
| G (GPa) | 45 | 35 | 38 | 47 | 25 |
| σy (MPa) | 140 | 207 | 105 | 150 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, H.; Wang, X.; Lan, A.; Jin, X.; Qiao, J. Lightweight Ti3VNbAl0.5Zrx (x = 0, 0.1, 0.5, and 1) Refractory High-Entropy Alloys with an Optimized Balance of Strength and Ductility. Metals 2025, 15, 503. https://doi.org/10.3390/met15050503
Fang H, Wang X, Lan A, Jin X, Qiao J. Lightweight Ti3VNbAl0.5Zrx (x = 0, 0.1, 0.5, and 1) Refractory High-Entropy Alloys with an Optimized Balance of Strength and Ductility. Metals. 2025; 15(5):503. https://doi.org/10.3390/met15050503
Chicago/Turabian StyleFang, Haoyu, Xuejiao Wang, Aidong Lan, Xi Jin, and Junwei Qiao. 2025. "Lightweight Ti3VNbAl0.5Zrx (x = 0, 0.1, 0.5, and 1) Refractory High-Entropy Alloys with an Optimized Balance of Strength and Ductility" Metals 15, no. 5: 503. https://doi.org/10.3390/met15050503
APA StyleFang, H., Wang, X., Lan, A., Jin, X., & Qiao, J. (2025). Lightweight Ti3VNbAl0.5Zrx (x = 0, 0.1, 0.5, and 1) Refractory High-Entropy Alloys with an Optimized Balance of Strength and Ductility. Metals, 15(5), 503. https://doi.org/10.3390/met15050503







