Composites Cu–Ti3SiC2 Obtained via Extrusion-Based Additive Manufacturing: Structure and Tribological Properties
Abstract
1. Introduction
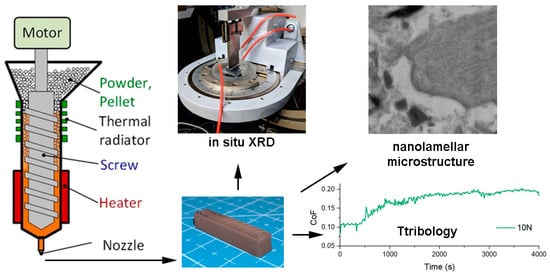
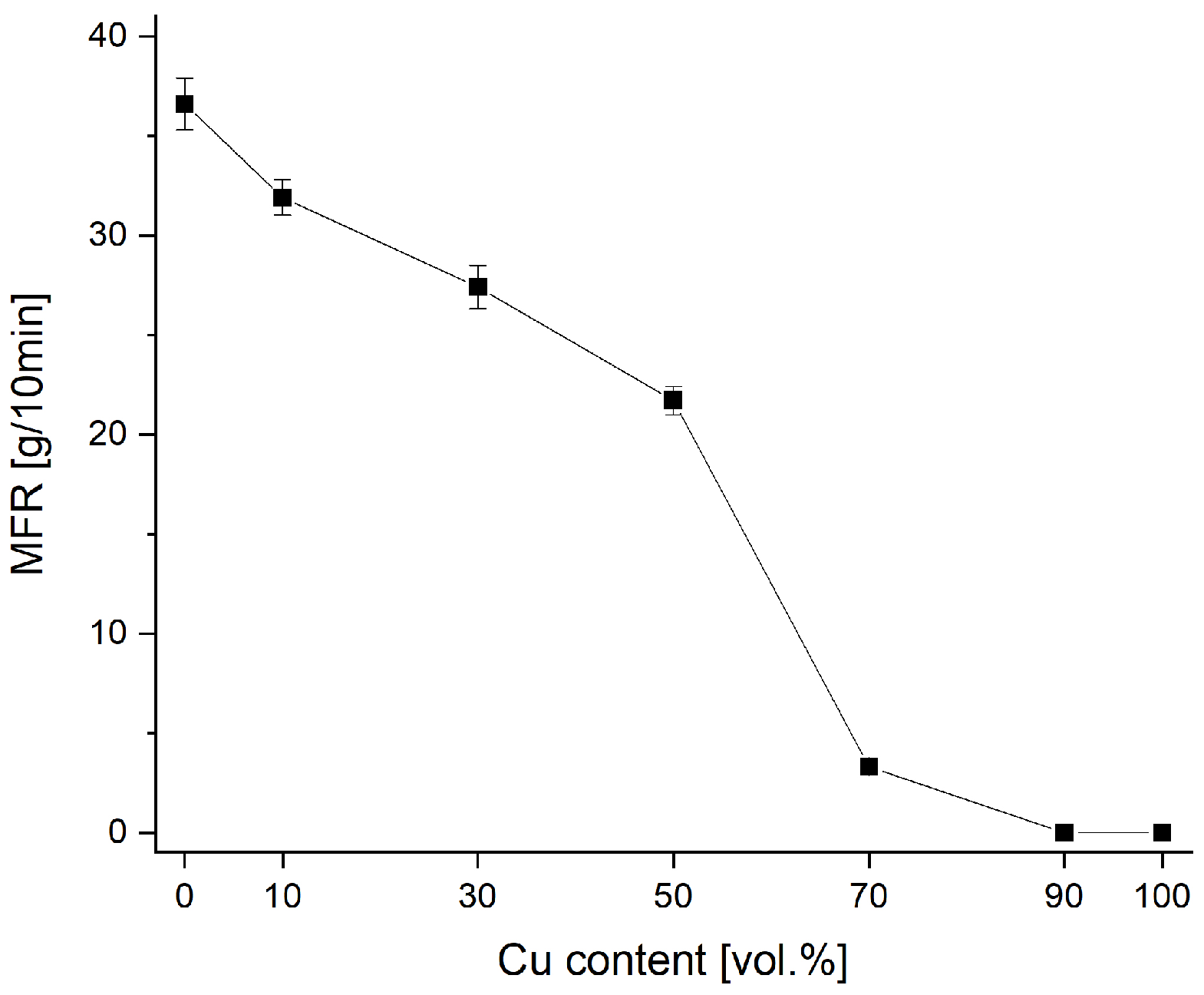
2. Materials and Methods
3. Results and Discussion
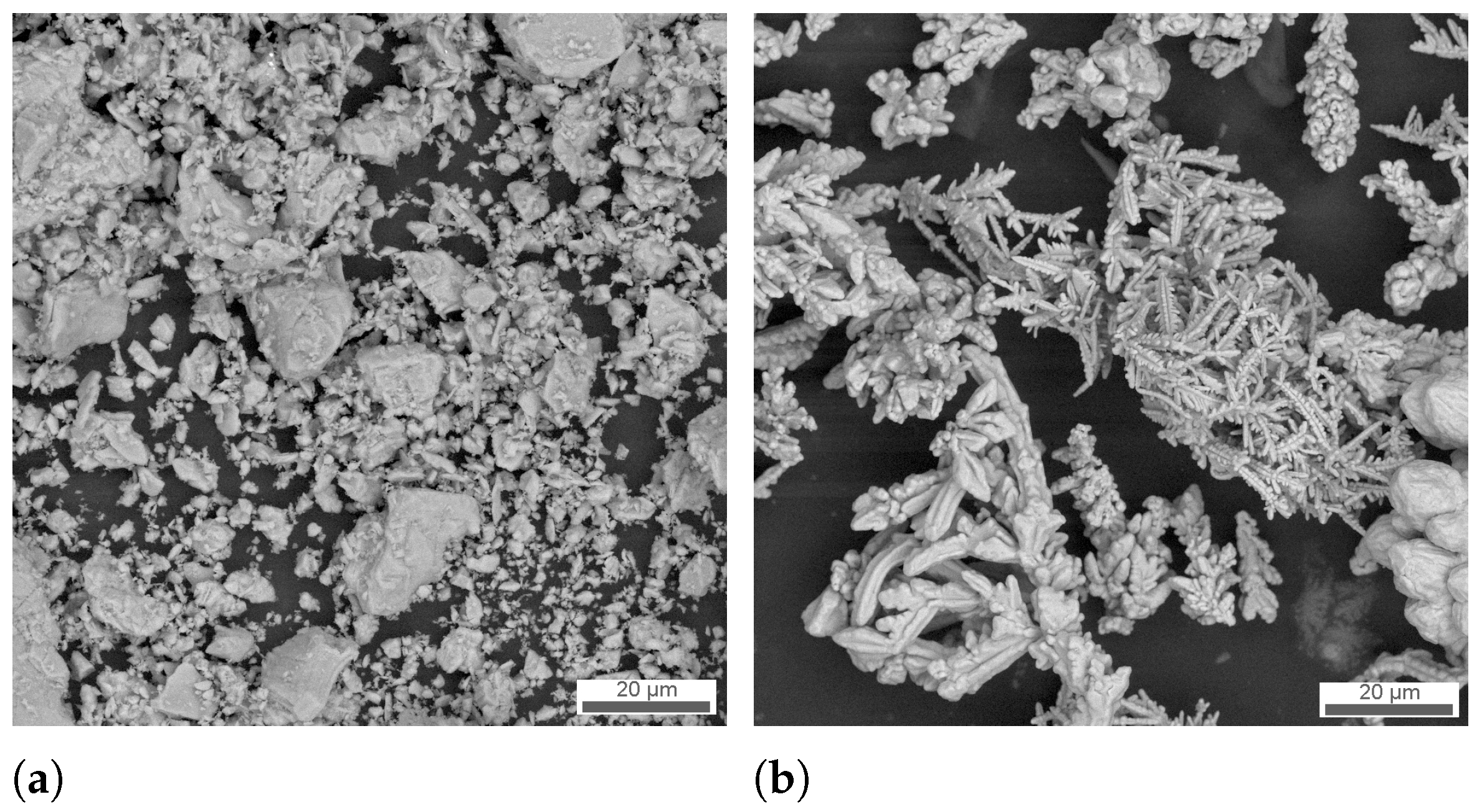
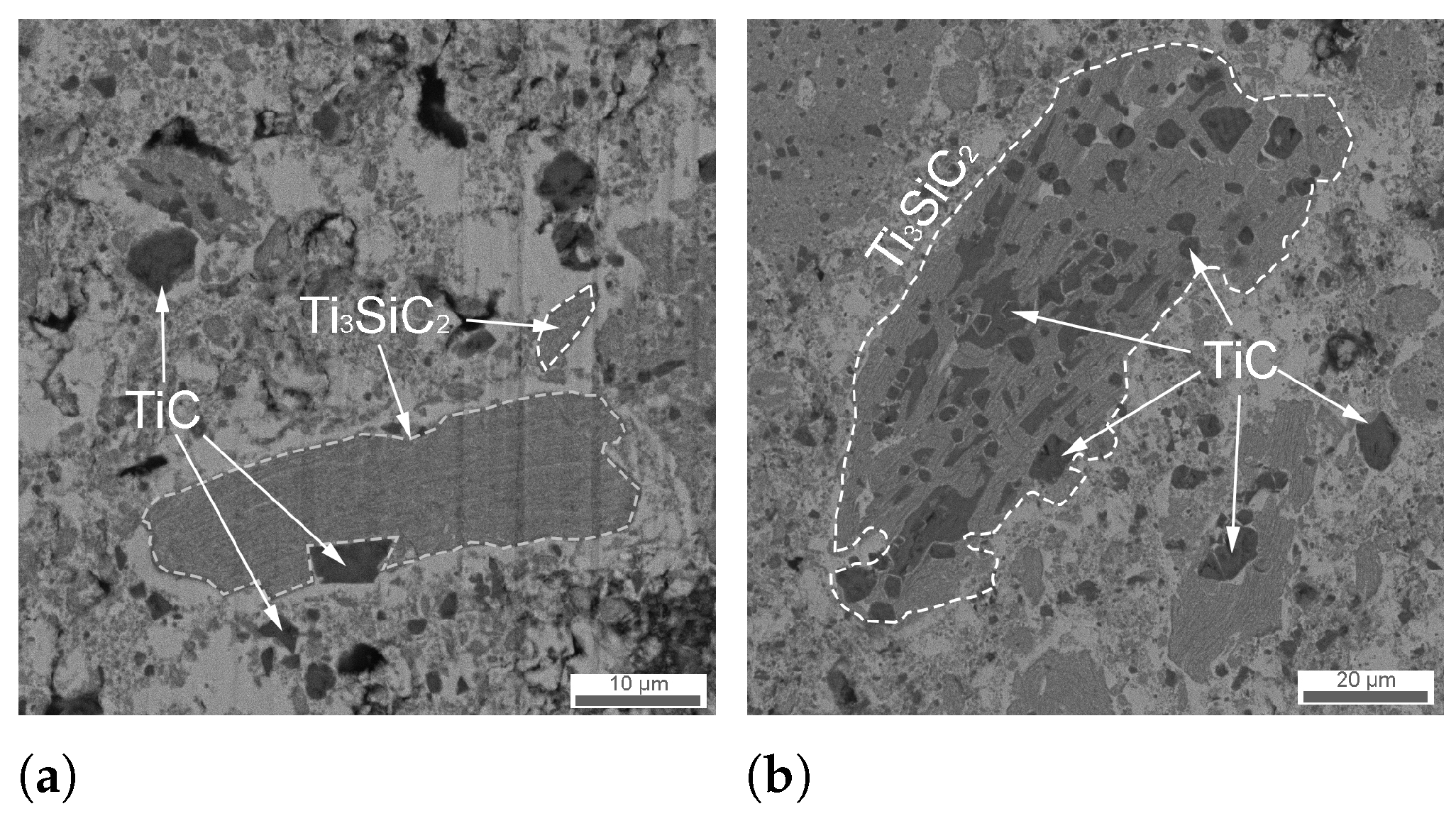
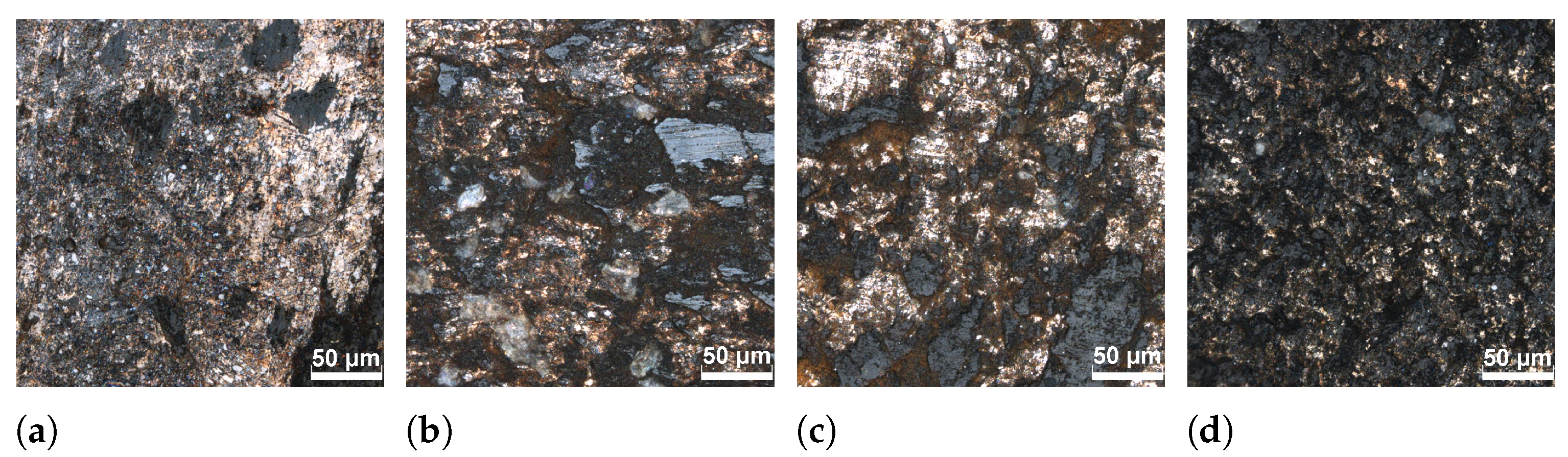
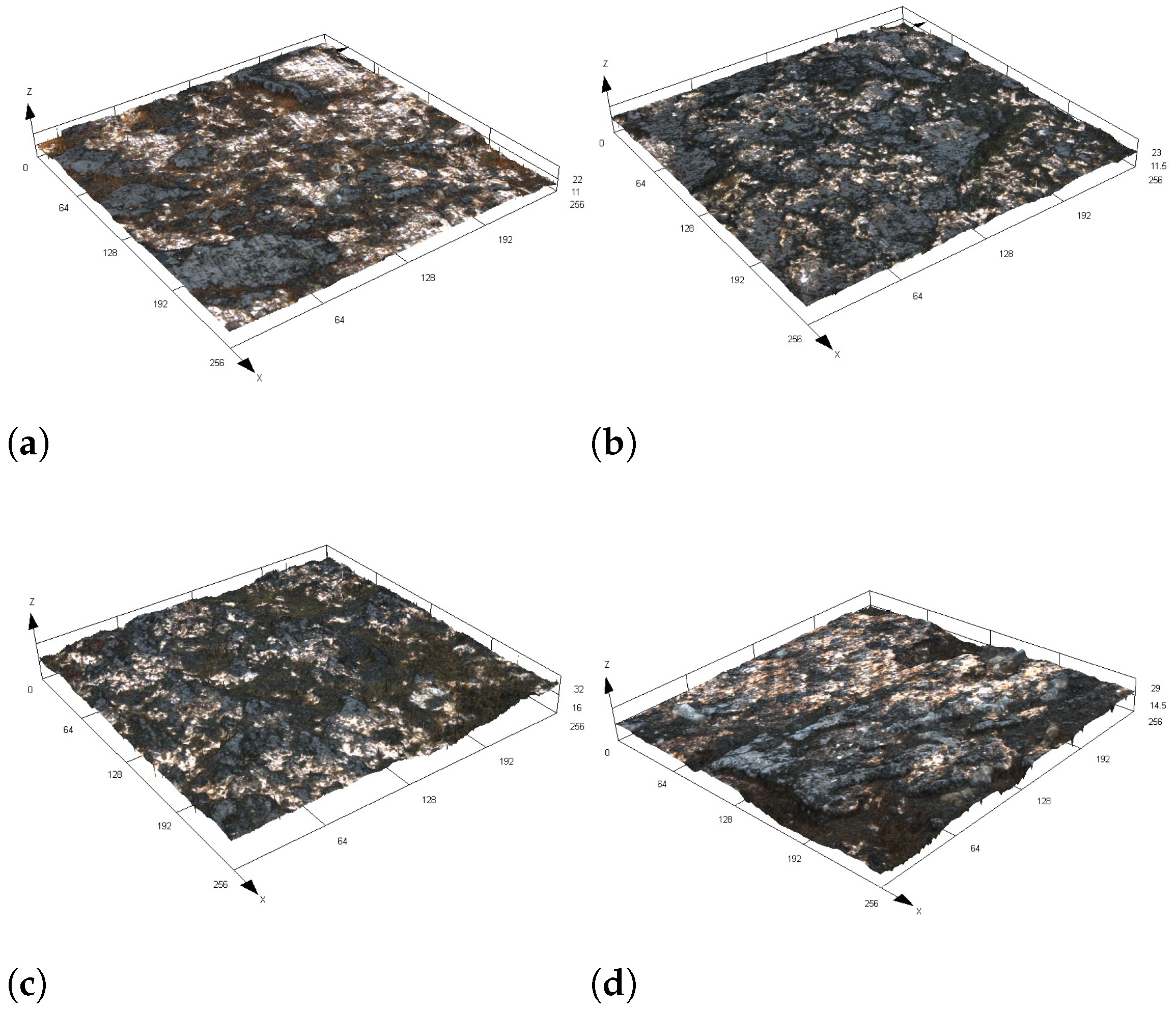
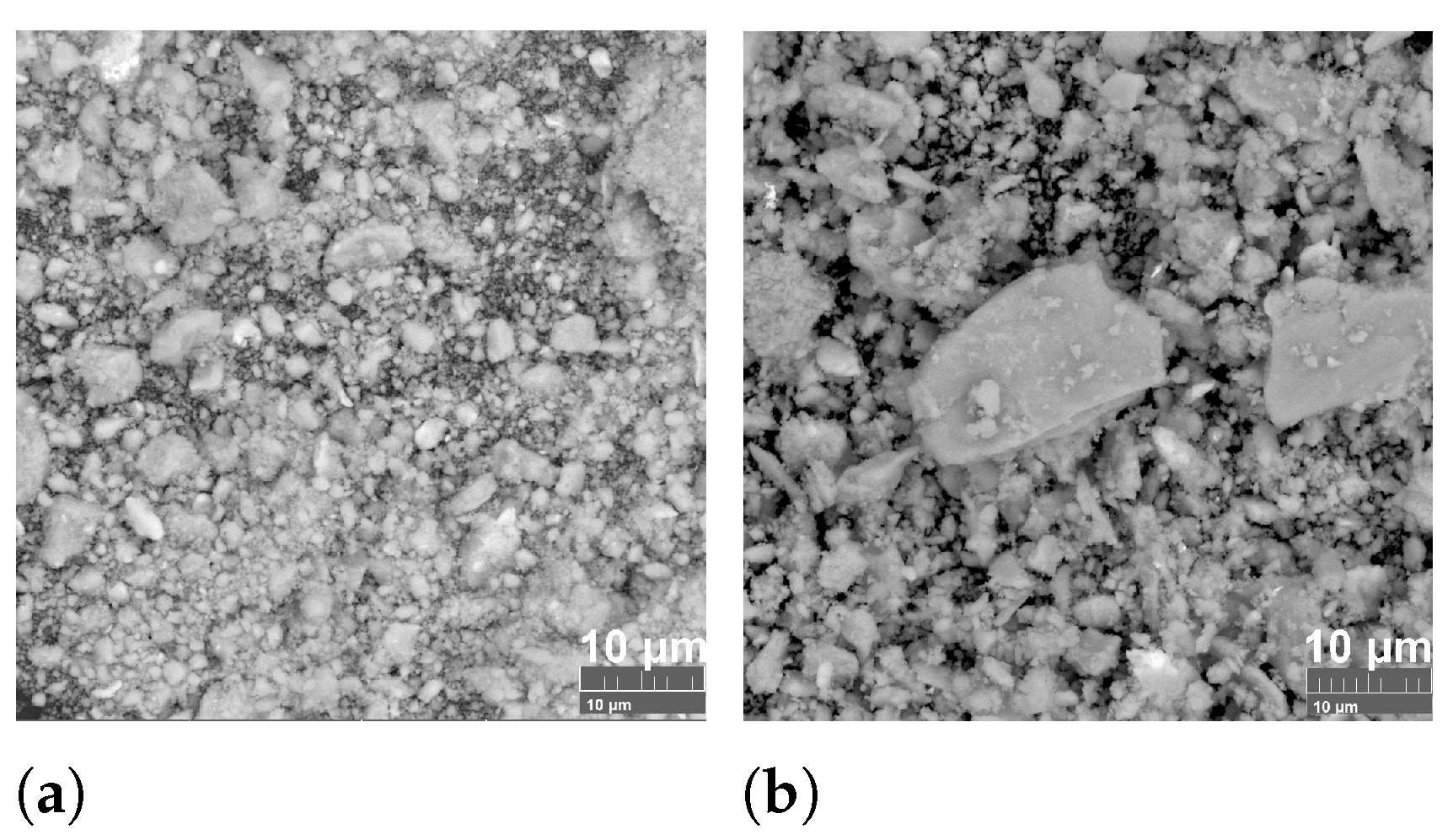
3.1. Structure
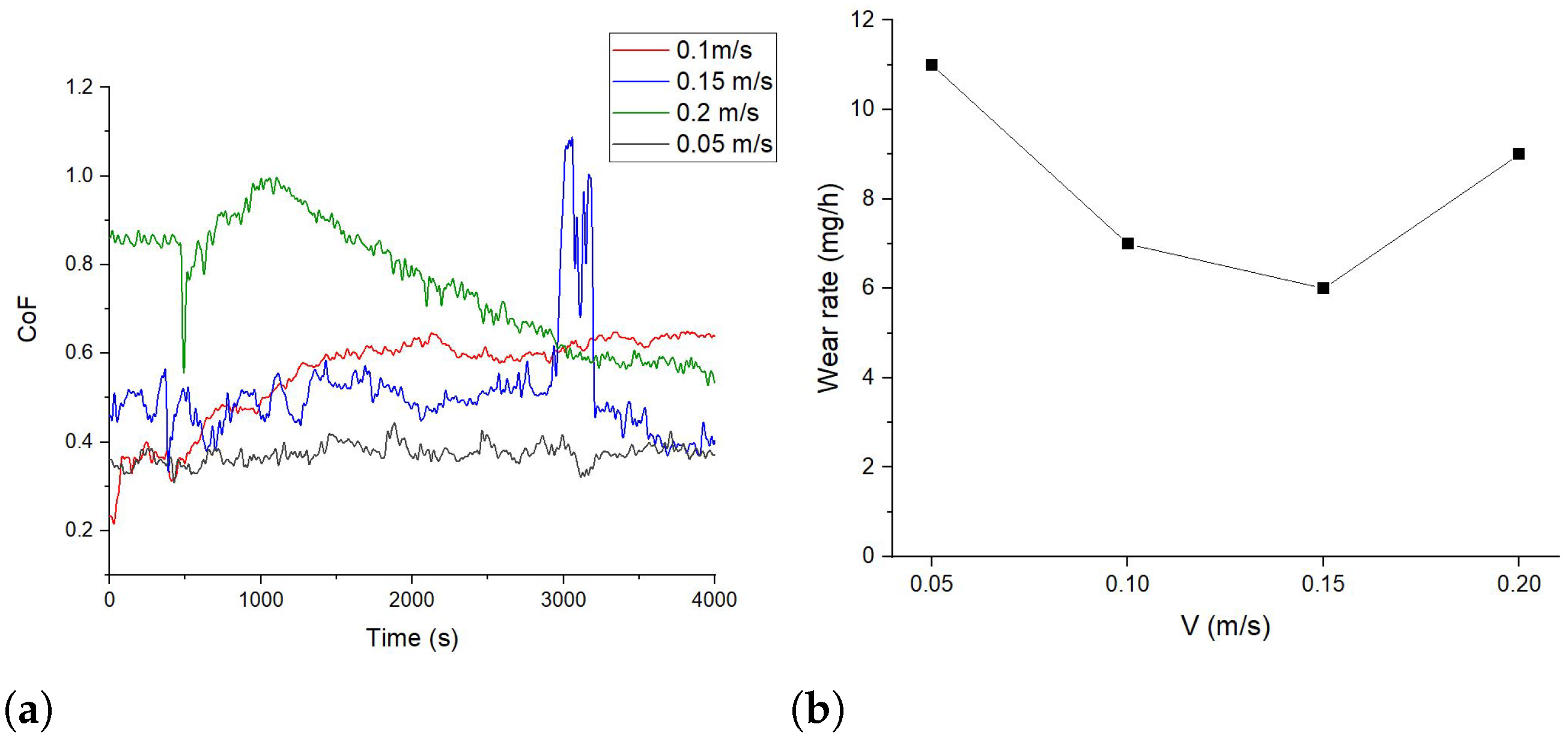
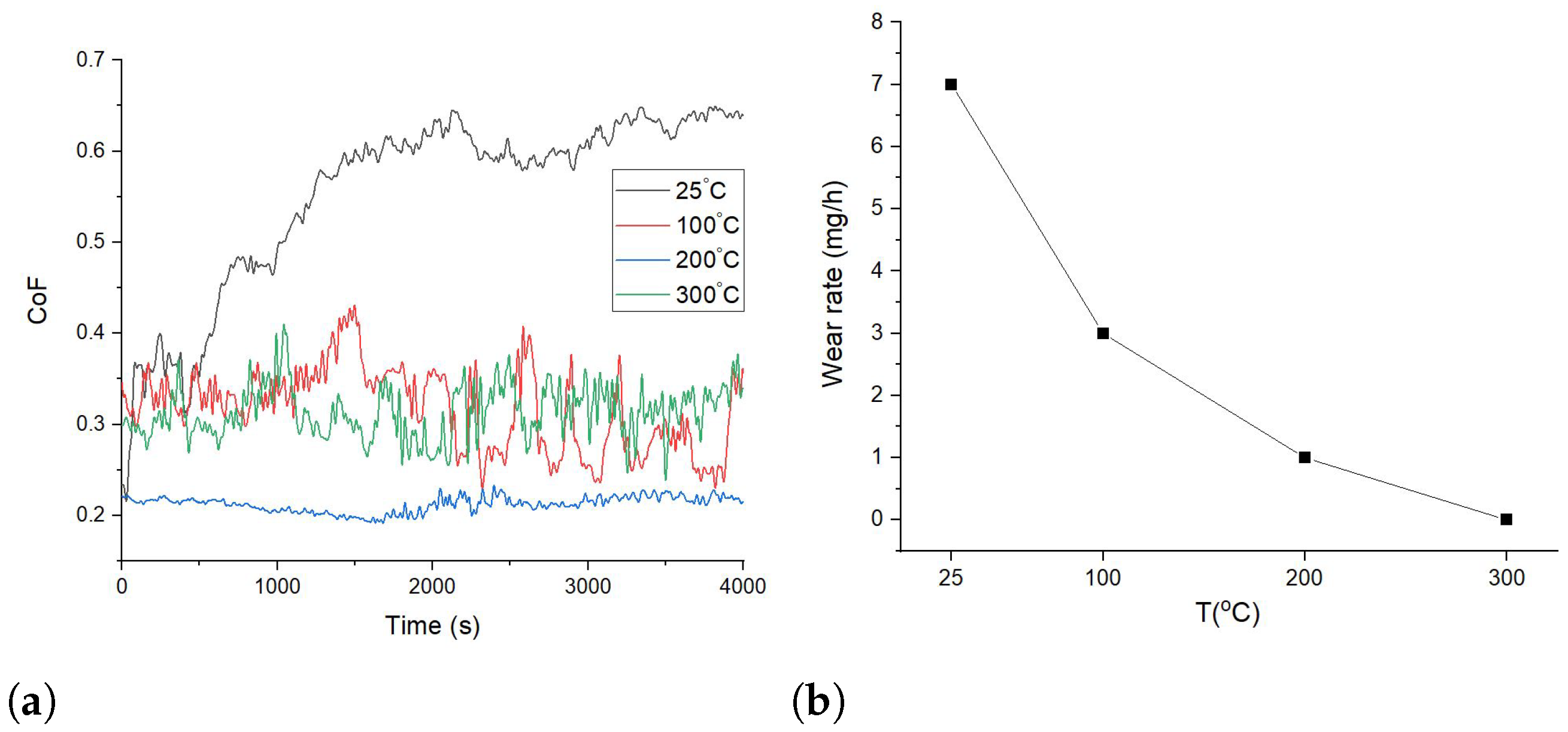
3.2. Tribological Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Radovic, M.; Barsoum, M.W. MAX phases: Bridging the gap between metals and ceramics. Am. Ceram. Soc. Bull. 2013, 92, 20–27. [Google Scholar]
- Barsoum, M.W.; El-Raghy, T. The MAX phases: Unique new carbide and nitride materials: Ternary ceramics turn out to be surprisingly soft and machinable, yet also heat-tolerant, strong and lightweight. Am. Sci. 2001, 89, 334–343. [Google Scholar] [CrossRef]
- Barsoum, M.; El-Raghy, T.; Rawn, C.; Porter, W.; Wang, H.; Payzant, E.; Hubbard, C. Thermal properties of Ti3SiC2. J. Phys. Chem. Solids 1999, 60, 429–439. [Google Scholar] [CrossRef]
- Perevislov, S.; Sokolova, T.; Stolyarova, V. The Ti3SiC2 max phases as promising materials for high temperature applications: Formation under various synthesis conditions. Mater. Chem. Phys. 2021, 267, 124625. [Google Scholar] [CrossRef]
- Gorai, S.; Bhattacharya, C. Structural and mechanical properties, phase stability and shock induced behaviour of Ti3SiC2 based on first principles. Phys. B Condens. Matter 2024, 673, 415444. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Z.; Zhou, Y. Cu/Ti3SiC2 composite: A new electrofriction material. Mater. Res. Innov. 1999, 3, 80–84. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Zheng, Y.; Li, H.; Li, S. Interface structure and wetting behaviour of Cu/Ti3SiC2 system. Adv. Appl. Ceram. 2015, 114, 39–44. [Google Scholar] [CrossRef]
- Dang, W.; Ren, S.; Zhou, J.; Yu, Y.; Li, Z.; Wang, L. Influence of Cu on the mechanical and tribological properties of Ti3SiC2. Ceram. Int. 2016, 42, 9972–9980. [Google Scholar] [CrossRef]
- Wu, Z.; Jiang, X.; Li, Y.; Christian, P.; Sun, H.; Zhang, Y.; Fang, Y.; Shu, R. Microstructures and properties of graphene nanoplatelets reinforced Cu/Ti3SiC2/C nanocomposites with efficient dispersion and strengthening achieved by high-pressure torsion. Mater. Charact. 2022, 193, 112308. [Google Scholar] [CrossRef]
- Nai, X.; Chen, H.; Zhao, S.; Wang, Q.; Li, W. Designing a low-melting Sn6Ag7Ni4Co2Ti high entropy alloy filler to optimize microstructure and mechanical properties of Ti3SiC2/Cu joint. Mater. Charact. 2023, 196, 112573. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, F.; Tulugan, K. Self-lubricating behavior caused by tribo-oxidation of Ti3SiC2/Cu composites in a wide temperature range. Ceram. Int. 2022, 48, 15504–15515. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Sefene, E.M. State-of-the-art of selective laser melting process: A comprehensive review. J. Manuf. Syst. 2022, 63, 250–274. [Google Scholar] [CrossRef]
- Gao, B.; Zhao, H.; Peng, L.; Sun, Z. A review of research progress in selective laser melting (SLM). Micromachines 2022, 14, 57. [Google Scholar] [CrossRef]
- Liu, W.; Song, J.; Li, W.; Ma, Z.; Liu, H.; Liu, B.; Xia, Y.; Wang, Z.; Huang, Y.; Liu, Y.; et al. Structure and properties study of in-situ TiC reinforced 316L materials prepared by laser melting deposition based on Ti3SiC2 decomposition. Ceram. Int. 2024, 50, 48448–48461. [Google Scholar] [CrossRef]
- Tan, Q.; Zhuang, W.; Attia, M.; Djugum, R.; Zhang, M. Recent progress in additive manufacturing of bulk MAX phase components: A review. J. Mater. Sci. Technol. 2022, 131, 30–47. [Google Scholar] [CrossRef]
- Gonzalez-Julian, J. Processing of MAX phases: From synthesis to applications. J. Am. Ceram. Soc. 2021, 104, 659–690. [Google Scholar] [CrossRef]
- Carrijo, M.M.; Caro, L.G.; Lorenz, H.; Greil, P.; Travitzky, N.; Rambo, C.R. Ti3SiC2-based inks for direct ink-jet printing technology. Ceram. Int. 2017, 43, 820–824. [Google Scholar] [CrossRef]
- Krinitcyn, M.; Fu, Z.; Harris, J.; Kostikov, K.; Pribytkov, G.A.; Greil, P.; Travitzky, N. Laminated Object Manufacturing of in-situ synthesized MAX-phase composites. Ceram. Int. 2017, 43, 9241–9245. [Google Scholar] [CrossRef]
- Nai, X.; Zhang, H.; Zhao, S.; Wang, P.; Chen, H.; Wang, P.; Vairis, A.; Li, W. Strengthening Ti3SiC2/Cu brazed joint assisted with cold spray additive manufacturing: Lower brazing temperature through interdiffusion and graded reinforcement for stress relaxation. J. Mater. Process. Technol. 2024, 331, 118530. [Google Scholar] [CrossRef]
- Eusébio, J.V.C. Additive Manufacturing for Metallic Materials Using Fused Filament Fabrication and Fused Granular Fabrication. Master’s Thesis, Faculdade de Engenharia da Universidade do Porto, Porto, Portugal, 2024. [Google Scholar]
- Arora, P.; Dehgahi, S.; Butt, S.U.; Nobes, D.S.; Qureshi, A.J. Fabrication of sacrificial wax pattern through large-scale fused granulated fabrication (FGF-AM) hybrid manufacturing system. Prog. Addit. Manuf. 2025, 10, 1325–1339. [Google Scholar] [CrossRef]
- Krinitcyn, M.; Kopytov, G.; Ryumin, E. Additive Manufacturing of Ti3AlC2/TiC and Ti3AlC2/SiC Ceramics Using the Fused Granules Fabrication Technique. J. Manuf. Mater. Process. 2024, 8, 123. [Google Scholar] [CrossRef]
- Eldho, A. Market and Application Review of large SCALE Additive Manufacturing. Master’s Thesis, Aalto University, Espoo, Finland, 2024. [Google Scholar]
- ISO 1133; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics. ISO International Standart: Geneva, Switzerland, 2022.
- ASTM E290; Standard Test Methods for Bend Testing of Material for Ductility. ASTM International: West Conshohocken, PA, USA, 2022.
- Krinitcyn, M.; Toropkov, N. Structure, Phase Composition, and Properties of Ti3AlC2—Nano-Cu Powder Composites. Coatings 2022, 12, 1928. [Google Scholar] [CrossRef]
- El-Raghy, T.; Barsoum, M.W. Diffusion kinetics of the carburization and silicidation of Ti3SiC2. J. Appl. Phys. 1998, 83, 112–119. [Google Scholar] [CrossRef]
- Qin, J.; He, D. Phase stability of Ti3SiC2 at high pressure and high temperature. Ceram. Int. 2013, 39, 9361–9367. [Google Scholar] [CrossRef]
- Oo, Z.; Low, I.; O’Connor, B. Dynamic study of the thermal stability of impure Ti3SiC2 in argon and air by neutron diffraction. Phys. B Condens. Matter 2006, 385, 499–501. [Google Scholar] [CrossRef]
- Pang, W.K.; Oo, Z.; Peterson, V.K.; Low, I.M. Phase and thermal stability in Ti3SiC2 and Ti3SiC2/TiC/TiSi2 systems. In Advances in Science and Technology of Mn+ 1axn Phases; Elsevier: Amsterdam, The Netherlands, 2012; pp. 389–413. [Google Scholar]
- Zhou, Y.; Gu, W. Chemical reaction and stability of Ti3SiC2 in Cu during high-temperature processing of Cu/Ti3SiC2 composites. Int. J. Mater. Res. 2021, 95, 50–56. [Google Scholar]
- Kulagina, V.; Chaplygina, A.; Popova, L.; Starostenkov, M.; Potekaev, A.; Klopotov, A. Structural phase transformations in alloys of the Cu–Pt system during ordering. Russ. Phys. J. 2012, 55, 814–824. [Google Scholar] [CrossRef]
- Olesinski, R.; Abbaschian, G. The Cu- Si (copper-silicon) system. Bull. Alloy Phase Diagrams 1986, 7, 170–178. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Cano, S.; Ecker, J.V.; Kitzmantel, M.; Arbeiter, F.; Kukla, C.; Holzer, C. Bending properties of lightweight copper specimens with different infill patterns produced by material extrusion additive manufacturing, solvent debinding and sintering. Appl. Sci. 2021, 11, 7262. [Google Scholar] [CrossRef]




| Series 1 | Series 2 | Series 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V = 0.05 m/s, T = 25 °C | = 8 N, T = 25 °C | V = 0.1 m/s, = 8 N | ||||||||||||
| , N | 2 | 4 | 6 | 8 | V, m/s | 0.05 | 0.1 | 0.15 | 0.2 | T, °C | 25 | 100 | 200 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krinitcyn, M.; Ryumin, E.; Kopytov, G.; Novitskaya, O. Composites Cu–Ti3SiC2 Obtained via Extrusion-Based Additive Manufacturing: Structure and Tribological Properties. Metals 2025, 15, 493. https://doi.org/10.3390/met15050493
Krinitcyn M, Ryumin E, Kopytov G, Novitskaya O. Composites Cu–Ti3SiC2 Obtained via Extrusion-Based Additive Manufacturing: Structure and Tribological Properties. Metals. 2025; 15(5):493. https://doi.org/10.3390/met15050493
Chicago/Turabian StyleKrinitcyn, Maksim, Egor Ryumin, Georgy Kopytov, and Olga Novitskaya. 2025. "Composites Cu–Ti3SiC2 Obtained via Extrusion-Based Additive Manufacturing: Structure and Tribological Properties" Metals 15, no. 5: 493. https://doi.org/10.3390/met15050493
APA StyleKrinitcyn, M., Ryumin, E., Kopytov, G., & Novitskaya, O. (2025). Composites Cu–Ti3SiC2 Obtained via Extrusion-Based Additive Manufacturing: Structure and Tribological Properties. Metals, 15(5), 493. https://doi.org/10.3390/met15050493