1. Introduction
Centrifugally cast heat-resistant alloys of the HP (ASTM A297/ASTM A297-21a [
1]) series are used for applications in the manufacture of high-temperature components in petrochemical systems, such as steam reformer tubes, radiation tubes, and ethylene cracking furnace tubes [
2,
3,
4,
5]. An HP alloy series, well known for its ASTM designation—where “H” signifies heat resistance and “P” denotes its nickel content—is primarily composed of Fe-25Cr-35Ni-0.4C (by weight) and is widely used in petrochemical furnace applications. Thanks to the unique mechanical properties under service at high temperatures, these alloys have good resistance to corrosion, high oxidation, carburization, and good creep resistance [
6,
7,
8,
9,
10]. The reformer furnace tubes made of the HP40 (ASTM A608 [
11]) alloy are designed for a nominal life of up to 100,000 h. However, the lifespan of these materials is influenced by various mechanisms, including creep, carburization, oxidation, thermal shock, and accidental overheating that occur due to the combined effects of the high service temperatures, mechanical loads, and the duration of service [
12,
13].
In recent years, there has been growing interest in the application of the centrifugally cast HP40Nb alloy, which has been developed by adding a small amount of niobium to the HP40 alloy [
14]. It should be mentioned that the addition of niobium to the HP alloy hinders the precipitation of chromium carbides and improves its mechanical properties, increasing creep strength and creep ductility, as well as carburization resistance [
12].
In the as-cast state, the microstructure of HP40Nb alloys features an austenitic matrix interspersed with intergranular and inter-dendritic eutectic-like primary Cr-rich carbides of the M
23C
6 type and Nb-rich carbides of the MC type. The microstructure of centrifugally cast HP40 reformer tubes is significantly affected by high-temperature service, leading to creep damage and the accumulation of microcracks [
15]. This damage is often characterized by the presence of carbides at grain boundaries [
16], coarsening of the grain boundaries due to carbide precipitation, and degradation of the mechanical properties [
17,
18]. These studies collectively underscore the importance of microstructural characterization in understanding the behavior of centrifugally cast steel furnace tubes, particularly in response to short or long high-temperature exposure. Thus, the formation of different carbide morphologies during processing and the expected structures in HP40Nb alloys during aging heat treatment can be understood through a combination of metallography, X-ray diffraction, and electron beam backscattered diffraction techniques [
19]. Skindaras et al. [
20] further explored the microstructure and mechanical properties of cast alloys with high chromium and nickel content after long-term high-temperature exposure, highlighting the emergence of carbides and other precipitates. The morphology and structure of the carbides in high-alloy steels, including HP40Nb alloys, are influenced by the aging time and secondary carbide precipitation [
21]. Specifically, the eutectic carbide morphology in HP alloys changes to more complex shapes with longer aging times, and the presence of secondary carbides can lead to the coalescence of eutectic carbides [
22]. The aging of HP40Nb alloys at 1000 °C under air results in the replacement of M
7C
3 carbides with M
23C
6 carbides, and the formation of a sub-surface zone depleted in chromium [
23]. The inter-dendritic niobium–titanium-rich carbides in HP-NbTi alloys can have blocky or nodular morphologies [
24]. The transformation of M
7C
3 to M
23C
6 and subsequent secondary carbide precipitation in austenitic matrices is a complex process influenced by various factors. The stability of the M
23C
6 phase is crucial, with its dissolution affecting the kinetics of the ferrite-to-austenite transformation [
25]. The formation of M
23C
6 from M
7C
3 is influenced by temperature and time, with the former being a key factor in the transformation [
26]. The presence of niobium and titanium in austenitic stainless steels can further complicate the process, with the formation of Z phase and the stability of the MX phase being important considerations [
27]. The transformation of M
7C
3 to M
23C
6 and the subsequent secondary carbide precipitation are thus influenced by a range of factors, including temperature, time, and the presence of other elements in the steel.
The microstructural development of HP40 alloy micro-alloyed with Nb after overheating at a high-temperature hold is, as stated before, a complex process influenced by various factors. These studies collectively suggest that the microstructural development of an HP40 alloy with Nb is a dynamic process and further research is needed to fully understand its behavior after short-term overheating. Moreover, findings that the content of inter-dendritic carbides is a key factor in its creep performance add to the importance of a better understanding of short-term exposure [
23].
Thermal instability results in accelerated microstructural aging, which unequivocally contributes to the premature and unexpected failure of tubes due to bending or cracking [
28]. It is essential to recognize that there is a significant gap in our understanding of the fitness for service of tubes that endure a temperature surge without displaying any cracking or visible damage. To ensure the safe and reliable operation of tube materials throughout their lifespan, it is essential to thoroughly verify their mechanical and metallurgical properties. This careful examination acts as a safeguard, confirming that the materials possess the necessary strength and durability to withstand the demands of their application [
29]. The evolution processes of carbides and other intermetallic compounds during creep exposure result in a reduction in ductility. This diminished ductility may lead to cracking due to thermal stresses occurring during shut-down and start-up operations, as well as during welding repairs [
30]. Enhancing the material’s performance significantly extends their lifespan, thereby reducing both capital costs and environmental impact for each unit of H
2 produced [
31].
Therefore, the aim of this study was to explore the effect of short-term overheating temperatures on the microstructure and creep behavior of an HP40Nb alloy. This was achieved by discussing the microstructural evolution of HP40Nb alloys during aging, with a focus on the formation of different carbides and transition zones.
The combination of presented techniques has provided new insight into how different carbide types and morphologies form during overheating, and further highlighted the significance of microstructure control and monitoring in utilization of the reformer tubes in extreme conditions.
4. Discussion
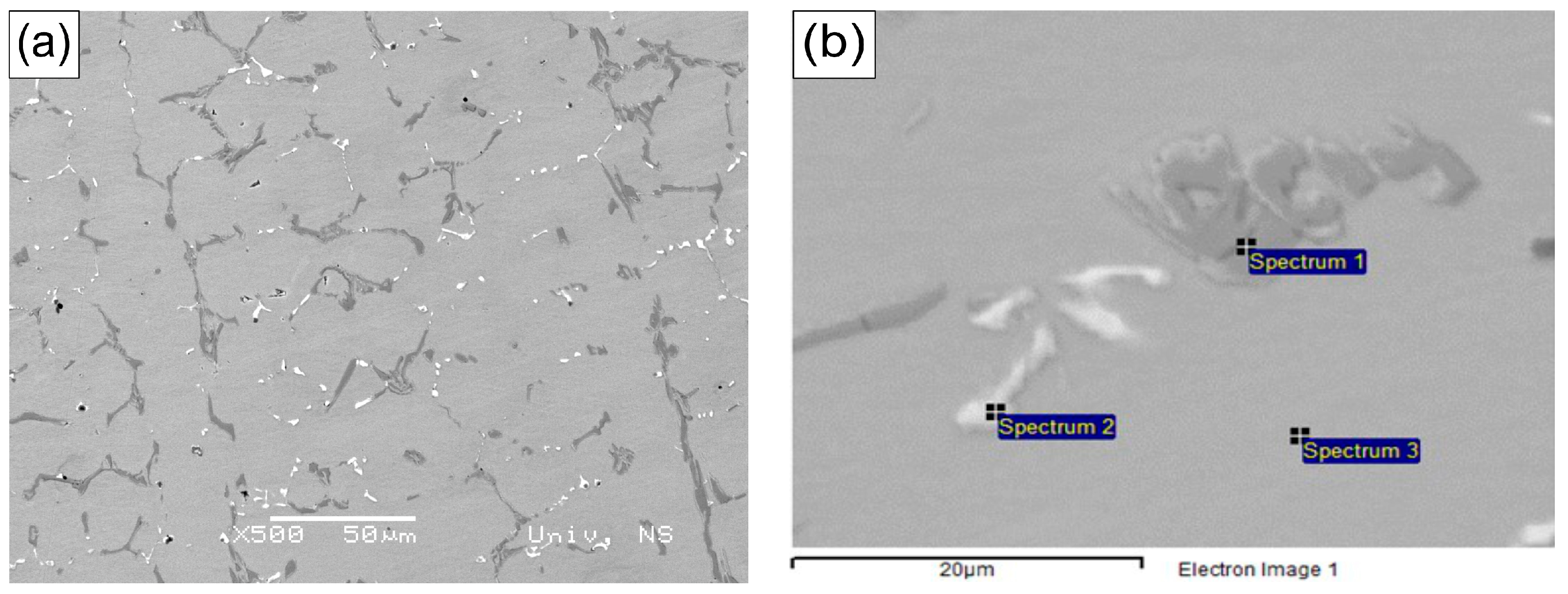
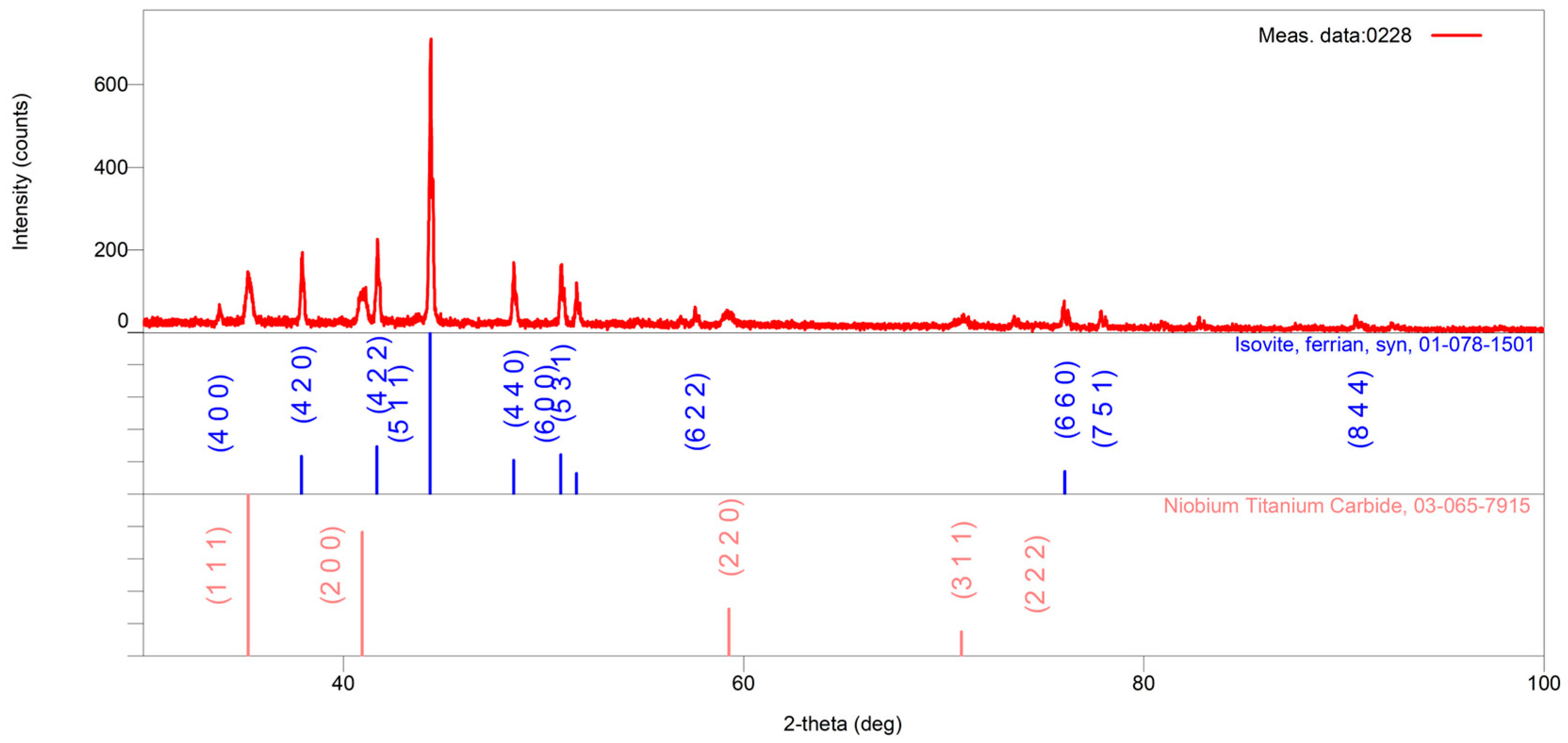
The microstructure of the as-received alloy consists of primary carbide networks dispersed within an austenitic matrix, as presented in
Figure 1. The dendritic morphology is indicative of a typical cast microstructure. Notably, no secondary carbide formation was observed, as shown in
Figure 2. SEM analysis further revealed that the primary carbide networks can be distinguished into two distinct phases, which exhibit dark and bright contrast. As illustrated in
Figure 1, the Cr-rich phases identified in the microstructure primarily correspond to M
23C
6 and M
7C
3 carbides, while the Nb-rich precipitate is characterized as NbC carbide. According to previous investigations [
35,
43,
44], niobium preferentially reacts with carbon during solidification at elevated temperatures, leading to the formation of NbC, with the Nb-to-C atomic ratio playing a crucial role in driving its precipitation. In the present study, the relatively low Nb-to-C ratio (0.2) was insufficient to fully incorporate all available free carbon into NbC [
35]. As a result, the remaining carbon in the matrix was preferentially bound by chromium, promoting the formation of Cr-rich carbides at lower solidification temperatures. Due to the high cooling rate, the complete transformation of M
7C
3 into M
23C
6 was inhibited.
The overheating temperature is an important factor that affects the morphology of carbides. On one hand, irregularly shaped carbides at the grain boundaries hinder the sliding of grain boundaries, while intragranular carbides prevent dislocation movement on the other hand. The precipitation of secondary carbides leads to a reduction in the carbon atom content in the matrix and decreases the effect of solid solution strengthening. During carbide precipitation, the ability of carbides to block dislocation slips is reduced. The morphology of carbides is an important factor influencing the creep properties of the HP40Nb alloy [
42,
45,
46].
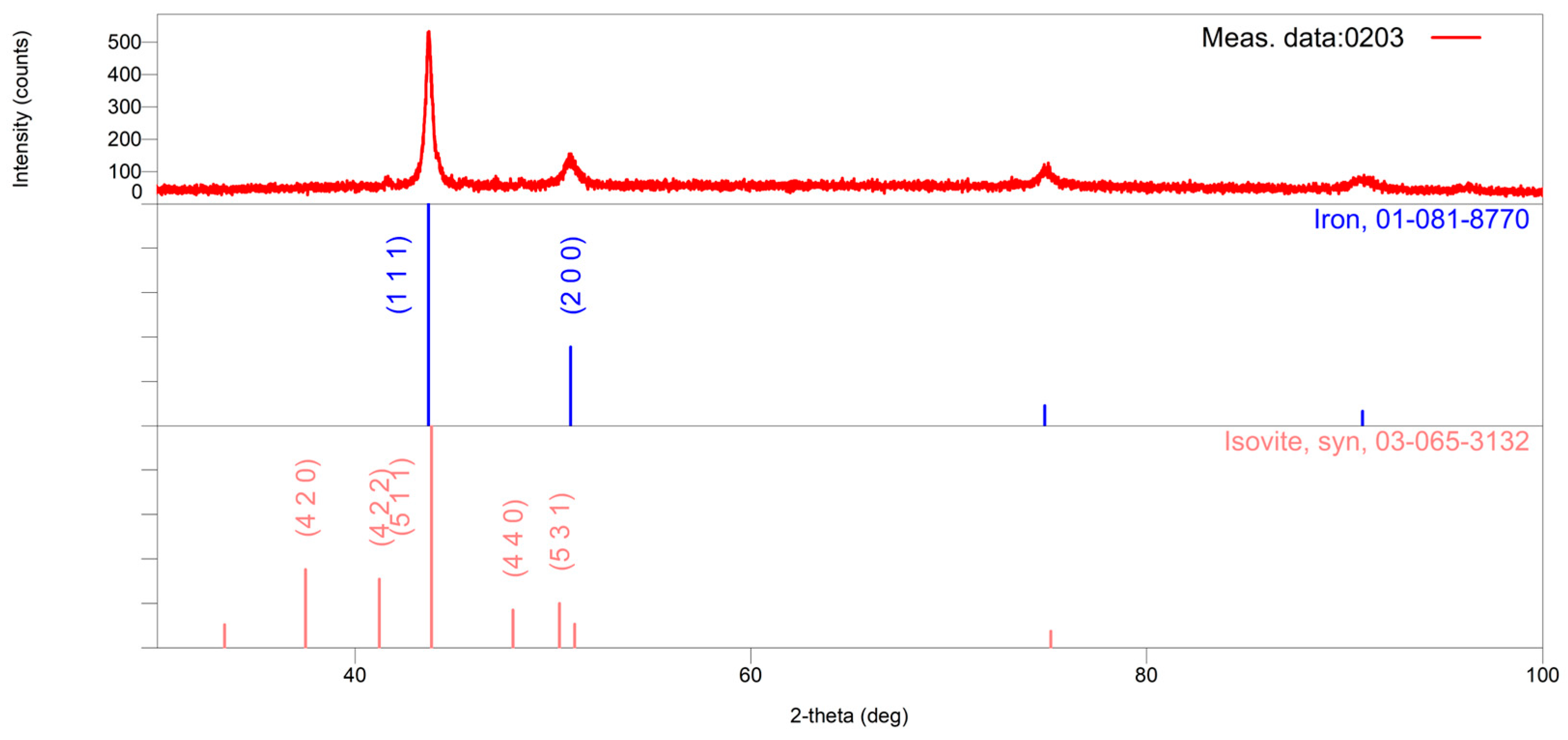
In comparison with the microstructure in its initial state, the microstructure of the HP40Nb alloy after overheating at 950 °C for 2 h (
Figure 10b) exhibits a markedly higher concentration of carbide in the austenitic region adjacent to the secondary carbide and within the austenitic matrix in the inter-dendritic region. Primary carbides with lamellar and plate-like morphologies are observed along the dendrites, forming discontinuous chains. Additionally, a significant amount of finely precipitated secondary carbides is visible within the austenitic matrix and near the boundaries of the dendrites. On the basis of the XRPD pattern presented in
Figure 4, the Cr-rich carbide is identified as an M
23C
6-type carbide, whereas the Nb-rich phase is classified as an NbC phase.
At an overheating temperature of 1050 °C, in addition to secondary carbides, a quantity of needle-like M23C6 type carbides (where M = Cr, Ni, Fe) was formed within the austenite matrix. The amount of secondary carbides increased as the overheating temperature rose to 1050 °C.
The results of the microstructure analysis of the HP40Nb alloy after overheating at 1050 °C for 2 h are consistent with the findings of Sourmail [
27], Fuyang et al. [
41], and the work of Martin C. H. Tse [
31]. Fuyang et al. [
41] also observed the transformation of M
7C
3-type carbides into M
23C
6 due to the exposure of the HP40Nb alloy to temperatures ranging from 700 to 990 °C for 1 to 6000 h. This transformation is driven by the thermodynamic instability of M
7C
3 at elevated temperatures, resulting in its dissolution and the subsequent precipitation of the more stable M
23C
6 phase. Furthermore, Tse’s research [
31] offers valuable insights into the metallurgical advancements in reformer tube alloys, which further enhance the understanding of carbides’ evolution under high-temperature conditions.
Liu et al. [
17] analyzed the changes in the microstructure and mechanical properties of the HP40Nb alloy during aging at 900 °C from 0 to 2016 h and observed the transformation of M
7C
3-type carbides into M
23C
6-type carbides.
Almeida et al. [
42] studied the influence of niobium content on the microstructural changes in the HP40Nb alloy after aging at 700, 900, and 1100 °C for 1000 h and also observed the transformation of M
7C
3-type carbides into M
23C
6-type carbides. Wang et al. [
35] investigated the effect of overheating the HP40Nb alloy at temperatures ranging from 900 to 1250 °C for 100 h on its microstructure and observed the transformation of M
7C
3-type carbides into M
23C
6-type carbides.
Zones without secondary carbides appeared in the inter-dendritic space (
Figure 10c,d). Near the primary carbides are many finely dispersed secondary carbides in the austenitic matrix.
The quantity of secondary carbides diminished, but their coarsening is evident [
34]. Overheating at a temperature of 1150 °C for 2 h resulted in the formation of globular-shaped secondary carbides that separated the regions between the dendrites.
Fuyang et al. [
41] also observed the dissolution of secondary carbides with increasing overheating temperature while studying the microstructural changes in the HP series of alloys containing 0.6% C, 1.34% Nb, and Mo during aging at temperatures between 750 and 950 °C across intervals of 1 to 1800 h.
Liu [
4] stated that the carbide shape present in the microstructure of the tested material is highly sensitive to the overheating temperature. The overheating of the alloy accelerates the dissolution of smaller carbide particles and the enlargement of larger ones through coagulation, accompanied by morphological changes in carbides that ultimately result in material failure. Coagulation requires a difference in the particle size of the carbide phase, resulting in the solid austenite solution becoming undersaturated and affecting smaller particles under these conditions.
As a result, the smaller particles dissolve, while carbon atoms and alloying elements are transferred via diffusion and incorporated on the surface of the larger particles.
According to the XRPD pattern of the phases present, it can be concluded that the phases present in the original material are the Cr-rich carbide M
7C
3 and the Nb carbide NbC; after overheating, the phases present in the material are Cr-rich carbide M
23C
6 and the Nb-rich carbide NbC. The difference between the Cr-rich phase in the original material and the superheated material is that the furnace tube is manufactured using a centrifugal casting process. The cooling rate of the original material is relatively fast during the manufacturing process. The metastable M
7C
3 carbide that crystallizes first does not have time to be converted into M
23C
6 carbide. After overheating, the metastable M
7C
3 carbide becomes stable. The state M
7C
3 carbide is completely transformed into the stable M
23C
6 carbide [
35]. The phase type, grain size, and degree of dispersion of secondary carbides in the microstructure affect the mechanical properties of the material [
20].
The difference between samples in the content of intragranular carbide and the degree of grain boundary carbide coarsening increases with longer superheating times.
After exposure for 8 h at 1050 °C (high temperature), secondary chromium carbides precipitate inside the grains (
Figure 14b). The appearance of needles (carbides), indicating carbides (dark gray) with gray edges, white carbides, and small gray carbides at the base, can be noticed in
Figure 14c. Fewer secondary carbides are present, and these have solidified. The change in the carbide contents can be attributed to the dynamic equilibrium between the dissolution and growth of carbides and the carbon diffusion.
As the temperature rises to 1150 °C for 8 h, the content of secondary carbides decreases due to carbide dissolution. The appearance of carbide needles becomes noticeable; dark gray carbides with gray edges are evident. Small, tiny white carbides are found at the base (as seen in
Figure 14c).
Liu and Chen [
17] also reported that the content of inter-dendritic carbides increases with longer aging times at a constant temperature of 900 °C.
Extending the overheating time to 8 h at 1150 °C (
Figure 14c) results in a reduction in the quantity of secondary carbides and the formation of carbide-free zones within the microstructure. The results indicate that with a prolonged soaking time, chromium carbide decomposes, and the carbon combines with niobium, leading to an increase in niobium-rich carbides. These findings are consistent with the study by Wang et al. [
4], which found that the carbide content decreased at a temperature of 1250 °C.
According to the microstructure results of the HP40Nb alloy samples after all overheating treatments (
Figure 10 and
Figure 14) and the hardness measurement results (
Table 11), it can be concluded that the presence of a large number of secondary carbides in the austenitic matrix contributes to the increased hardness of the alloy, as observed by other authors [
39,
41].
Lanz et al. [
39] investigated the effect of overheating temperatures ranging from 750 to 950 °C over various time intervals (1–1800 h) on a modified HP alloy containing 0.6% S and 1.34% Nb. They observed an increase in the alloy’s hardness after aging due to the precipitation of needle-like secondary carbides. However, as the aging time and temperature increased, the coarsening of these carbides led to a reduction in hardness. Picasso et al. [
33] studied a nickel-based alloy subjected to aging treatments at temperatures between 750 and 900 °C for durations of 1000–4000 h. Their research indicated an increase in hardness values due to aging compared with the as-cast state. However, when the aging time exceeded 500 h, the hardness value began to decline.
The maximum hardness observed in the sample treated at 1150 °C for 8 h is attributed to the dissolution of secondary chromium carbides and the diffusion of carbon into the austenitic matrix.