Abstract
In this investigation, it was found that the co-addition of Er and B causes both the modification and refinement of the Al-7Si alloy. The B element mainly forms a small amount of the AlB2 phase within the alloy, which can be used as a nucleation site for grains during casting, so the addition of B can significantly reduce the grain size of the Al-7Si alloy. However, the number density of AlB2 phase is too low, so its effect on improving the eutectic Si size and reducing the secondary dendrite arm spacing (SDAS) is not significant. The addition of 0.1 wt% Er can form a large amount of Al3Er phase within the alloy, which mainly serves as a nucleation site for eutectic Si during casting, so the addition of Er can significantly reduce the SDAS, eutectic Si size and morphology of Al-7Si alloys. However, due to the existence of a certain degree of mismatch between the Al matrix and the Al3Er phase, the relative grain refinement effect of Al3Er is not significant. In addition to this, we also observe the enrichment of Er at eutectic Si, which suggests that Er can interact with Si and thus inhibit the growth of eutectic Si. Therefore, Er can modulate eutectic Si through the Al3Er phase and the enrichment of Er. The co-addition of Er and B to Al-7Si alloys has better refining and modification effects than the addition of Er or B alone, mainly due to the modification effect of the Er element and the refining effect of the AlB2 phase. Unlike the Er-containing alloys, where the Al3Er phase plays a modifying role, the modification of the co-addition Er and B alloys is mainly caused by the enrichment of Er within the alloy.
1. Introduction
Al-Si alloys are widely used because of their good flowability, castability, and wear resistance [1,2,3]. Casting is a simple, economical, and effective process, that is capable of competently preparing Al alloy parts, ranging from precision to ingots weighing several tons. Solidification is one of the most important aspects in the casting process of an Al alloy [1]. However, the alloys are subject to casting process parameters, environmental parameters, elemental bias, and other influencing factors, resulting in alloy castings with non-uniform microstructures [2] and compositions [4], with equiaxial crystals in the core, columnar crystals in the middle, and fine crystals on the edges of the castings [5,6]. The properties of cast Al alloys can be improved by obtaining fine equiaxial crystals to achieve better formability and mechanical properties [7,8]. The grain refiners used for casting alloys are generally Al-Ti-B [9,10,11] and Al-Ti-C-B [12,13,14] intermediate alloys, but in Al-Si alloys, the Ti in the refiner will react with Si to generate Ti-Si compounds, which will have a poisoning effect on the Al-Si eutectic structure [15,16,17]. In addition, the refining effect of intermediate alloys on Al alloy ingots can be further improved by an annealing treatment [18,19,20], which is mainly due to the fact that more diffuse phases precipitate from the ingots.
It was found that rare-earth elements (RE) and transition elements can also refine the α-Al grains of aluminium castings, improve the microstructure, and obtain the equiaxial crystal structure [21,22,23,24,25,26]. At the same time, rare-earth elements and transition elements can be combined with Al in the annealing to form Al3M intermetallic compounds. The addition of microalloying can not only improve the microstructure of aluminium alloys, but also enhance the mechanical properties, cutting and machining properties, high-temperature mechanical properties, and heat resistance of the alloys [25,27,28,29,30]. Er, as a rare-earth element, has a lower price and higher reserves compared with Sc. Previous studies have found that Al-Er intermediate alloys can be used as a refining agent to refine grains [31]. The addition of Zr and Ti to Al-Er intermediate alloys further improved the refining ability [32]. Although the refiners in Al-Er intermediate alloys are less effective than Al-Ti-B alloys in refining pure aluminium, they have better refining effect in Al-Cu and Al-Mg alloys [33]. In addition, since Er can act as a microalloying element in aluminium alloys to reduce alloy casting defects, holes, and elemental segregation, and it can precipitate Al3Er phases in homogenization (annealing), Al-Er alloys have a wider prospect for application in alloys than Al-Ti-B.
The addition of a small amount of rare-earth elements in the Al-Si alloy melt can not only change the morphology and size of eutectic Si [34], but also play a role in removing slag and gas and impurities [35], so that the processing performance of casting Al-Si alloys has been greatly improved. And the rare-earth elements of the modification effect are long-lasting and stable. By adding rare-earth elements to Al-Si alloys, the rare-earth elements maintain their modifying effect even when the melting time is several hours long. The addition of rare-earth elements can make the needle-flake eutectic silicon turn into short rods or granules, and it can form Al3M phase with Al, so that the size of the blocky primary silicon can be refined to a certain extent [35]. The addition of the rare-earth element Ce to Al-Si alloys can effectively modify the eutectic Si [34]. The addition of no less than 0.2 wt% of Er can modify the eutectic Si into short rods or granules [36,37,38], but the modification effect of Er on eutectic Si was not observed in this study [39]. The rare-earth elements La and Ce can form intermetallic compounds with B, which can not only refine the grains of Al-Si alloys, but also have a modification effect on eutectic Si [40,41,42].
Experiments have shown that the addition of more than 0.2 wt% of rare-earth elements to the Al-Si alloy can produce a modification effect [36,37,38], and the modification effect of the alloy gradually deteriorates as the amount of rare-earth elements added decreases. Usually, the content of rare-earth microalloying elements added to aluminium alloys does not exceed 0.1 wt%. Therefore, in this experiment, the modification and refinement effects of adding 2 wt% of Al-5Er intermediate alloy (0.1 wt% Er), Al-B intermediate alloy, and Al-5Er-B intermediate alloy in Al-7Si alloy were investigated by metallurgical microscope and scanning electron microscope with the expectation of obtaining the microalloyed Al-Si alloys with fine grains and eutectic Si transformation.
2. Materials and Methods
2.1. Material Preparation
The Al-6Er alloy, Al-8B alloy, and pure Al, with a calculated composition ratio, were put into a VBF-1200X pit-type resistance furnace (Hefei Kejing Material Technology Compant, LTD, Hefei, China) and heated up to 1050 °C for melting. After the alloys were completely melted, the molten liquid was poured into iron moulds, in which the thickness needed to be controlled to be at around 3 mm to obtain the Al-5Er intermediate alloy, the Al-B intermediate alloy, and the Al-5Er-B intermediate alloy. The actual composition of the experimental alloys is shown in Table 1.

Table 1.
The chemical compositions of experimental alloys (wt%).
The modification and refinement test was carried out in a pit-type resistance furnace. Firstly, the total weight of about 750 g of pure aluminium and Al-20Si intermediate alloy were placed in a graphite crucible, and the temperature was increased to 800 °C at a rate of 10 °C/min, the raw materials were melted, and the temperature was retained for 5 min to complete the melting, and then cooled down to 705 °C and kept warm for 3 min to ensure the temperature of the melt was accurate and stable; three kinds of intermediate alloys were added in accordance with the weight ratio of 2 wt%, and stirred for 20 s, sampled at certain time intervals (10, 30, 60 min), and casted into a steel mould. The samples were then cast into steel moulds to obtain φ 60 × 20 mm3 cylindrical samples.
2.2. Characterisation of Refinement Effect
The cast cylindrical samples were cut at 5 mm from the bottom and ground using 180# sandpaper. The samples were corroded with the Borel reagent with the ratio of 120 mL HCl + 60 mL HNO3 + 10 mL HF + 10 mL H2O for 0.5~2 min. After that, the specimens were taken out, and the surface was cleaned with pure water and dried with a hair dryer, and the macroscopic morphology of the alloy was photographed.
In addition, samples were taken from the middle of the cylindrical samples, which were ground with 2000# sandpaper, polished with W1.5 polishing paste, and treated with anodic cladding to obtain polarised metallographic samples so as to observe the grain size. The anodic coating voltage was set at 25 V, the current was set at 0.8 A, and the coating time was 60 s.
2.3. Microstructure Characterisation
The ground and polished specimens were placed under a metallurgical microscope to observe the size of eutectic Si and α-Al of the alloy. The morphology and elemental distribution of eutectic Si inside the alloy were observed under a Gemini SEM 300 scanning electron microscope (Zeiss, Oberkochen, Germany). The role of Al3Er inside the alloy on the modification of the alloy is investigated.
3. Results and Discussion
3.1. Grain Refinement Effect of Er and B Addition on Al-7Si Alloys
Figure 1 shows the macroscopic morphology of various intermediate alloys added to Al-7Si alloy at 10, 30, and 60 min before casting. It can be seen that compared with Al-7Si alloy, Al-7Si-0.1Er alloy has no obvious refining effect on the alloy, but the finest grain size can be obtained by adding Al-5Er intermediate alloy for 30 min after casting. The addition of Al-B intermediate alloy can refine the Al-7Si alloy, and the time of intermediate alloy addition has little effect on the grain size of the alloy. Similarly to Al-7Si-0.02B alloy, the addition of Al-5Er-B intermediate alloy can refine Al-7Si alloy, and the finest grains can be obtained by casting after 30 min of addition. The addition of a mixture of Al-5Er and Al-B intermediate alloy has a similar effect on the refinement of Al-7Si alloy as the addition of Al-5Er-B intermediate alloy. It indicates that AlB2 intermetallic compounds mainly play the role of grain refinement in the alloy, and the addition of Er has no significant effect on the refinement effect of AlB2.
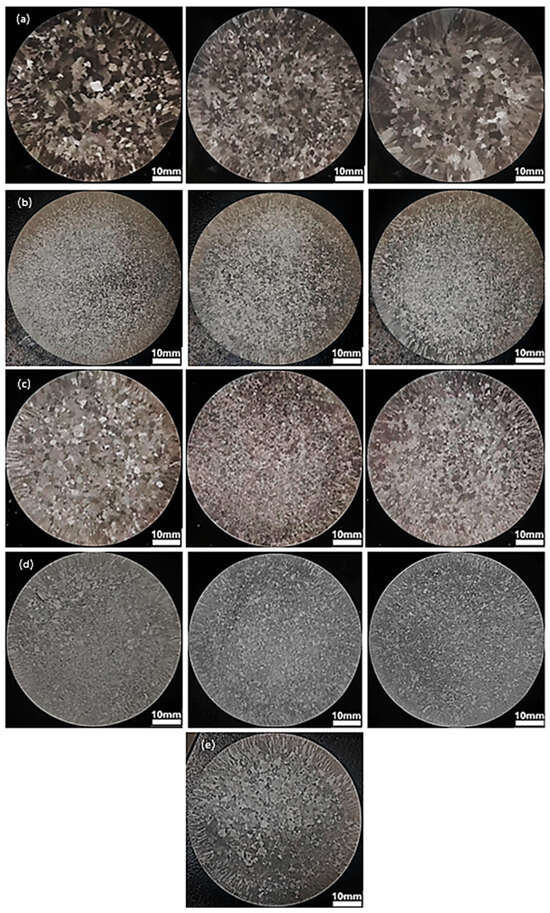
Figure 1.
Macroscopic morphology of Al-7Si alloy after adding 2 wt% of various intermediate alloys at 10, 30 and 60 min before casting: (a) added Al-5Er intermediate alloy; (b) added Al-B intermediate alloy; (c) added Al-5Er-B intermediate alloy; (d) mixed addition of Al-5Er and Al-B intermediate alloys; (e) Al-7Si alloy.
As can be seen from Figure 1, the optimum addition time of the intermediate alloy is 30 min before casting in order to obtain the finest grain size. Therefore, the polarised microstructure of Al-7Si alloy after the addition of various intermediate alloy is observed in Figure 2. As shown in Figure 2, the Al-7Si-0.1Er alloy is not as effective as the Al-7Si-0.02B alloy for grain refinement. As shown in Figure 2e, the addition of B has no significant effect on the reduction in the secondary dendrite arm spacing (SDAS) of the alloy. The SDAS of Al-7Si-0.1Er alloy is much smaller than that of Al-7Si-0.02B alloy. The smaller the SDAS, the more concentrated the size distribution, the higher the mechanical properties of the Al-7Si alloy. The Al-7Si-0.1Er-0.02B alloy can not only to achieve a relatively small grain, but also the alloy’s SDAS is the smallest, and the size distribution of the eutectic Si is also the most concentrated. It indicates that the co-addition of Er and B can have a synergistic effect on Al-Si alloys.
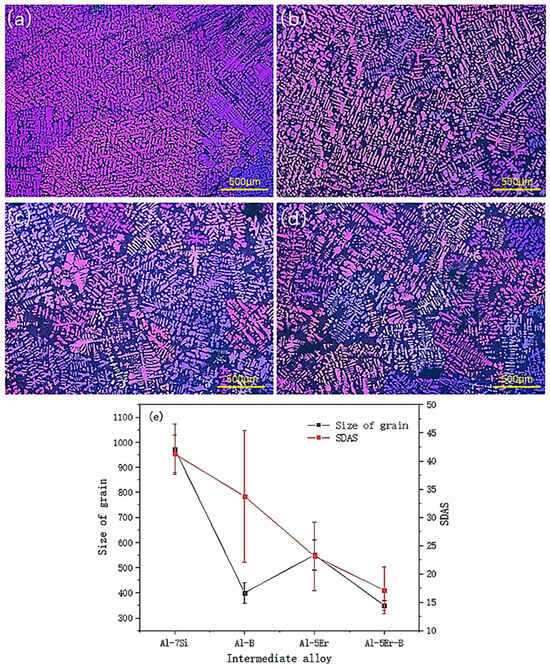
Figure 2.
Polarised microstructure of Al-7Si alloy and its grain size statistics after the addition of various intermediate alloys 30 min before casting: (a) Al-7Si alloy; (b) Al-7Si-0.1Er alloy; (c) Al-7Si-0.02B alloy; (d) Al-7Si-0.1Er-0.02B alloy; (e) grain and SDAS size statistics of Al-Si alloys after the addition of various intermediate alloys.
3.2. Modification Effect of Er and B Addition on Al-7Si Alloys
Figure 3 shows the morphology of eutectic Si after adding 2 wt% of various intermediate alloys to the Al-7Si alloy. Figure 3a shows that the eutectic Si in the Al-7Si alloy is in the shape of a long rod with a large length and aspect ratio, which can impair the properties of the alloy. Figure 3b illustrates that the addition of B does not change the morphology of eutectic Si much, but it can reduce the length and aspect ratio of eutectic Si. Figure 3c,d illustrate that the addition of Er can transform the morphology of eutectic Si to obtain a finer and shorter rod-like eutectic Si, while the addition of Er can make the width of eutectic Si larger and the size distribution of Si more concentrated, which is similar to the role of Sr in Al-Si alloys. Therefore, the addition of Er can have a modification effect on Al-Si alloys. Compared with Figure 3c, the modification effect of eutectic Si in Figure 3f is poor. This indicates that an annealing time too long leads to a poorer modification effect of eutectic Si, which further indicates that 30 min is the optimal time for intermediate alloy addition.
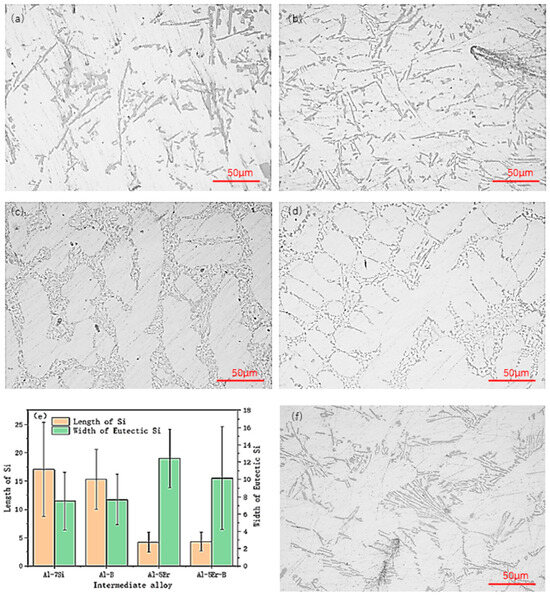
Figure 3.
Modification effect of various intermediate alloys added 30 min before casting on the eutectic microstructure of Al-7Si alloy: (a) Al-7Si alloy; (b) Al-7Si-0.02B alloy; (c) Al-7Si-0.1Er alloy; (d) Al-7Si-0.1Er-0.02B alloy; (e) dimensional statistics of eutectic Si; (f) Al-5Er intermediate alloy added 60 min before casting.
Figure 3e shows the statistical results of the length and width of eutectic Si after the addition of various intermediate alloys, and it is found that the addition of Er can make the length of eutectic Si decrease significantly, and the width of eutectic Si increase. The shorter the length and the wider the width of eutectic Si, the stronger the modification effect of this element on eutectic Si.
3.3. Refining Effect of B on Al-Si Alloys
B, a prevalent aluminium alloy deburring element, can have the effect of purifying the melt [8,15,43]. Experiments show that B can form the AlB2 phase in aluminium alloys [44], which can be used as a heterogeneous nucleation site to refine the alloy grains during alloy casting [8]. Therefore, the AlB2 phase can refine the Al-7Si alloy grains [43].
Figure 4 shows the SEM image and its correspondent mapping of the Al-7Si-0.02B alloy as-cast microstructure. It can be seen that after the addition of B to the Al-7Si alloy, a large amount of long-bar Si still exists inside the alloy, and a few AlB2 phases precipitate inside the grains. The AlB2 phase can be used as a heterogeneous nucleation site for the eutectic Si, but this role is not obvious due to its low number density (red circle). This is the reason why the secondary dendrite arm spacing (SDAS) of the alloy remains large after the addition of the Al-B intermediate alloy in Figure 2d.
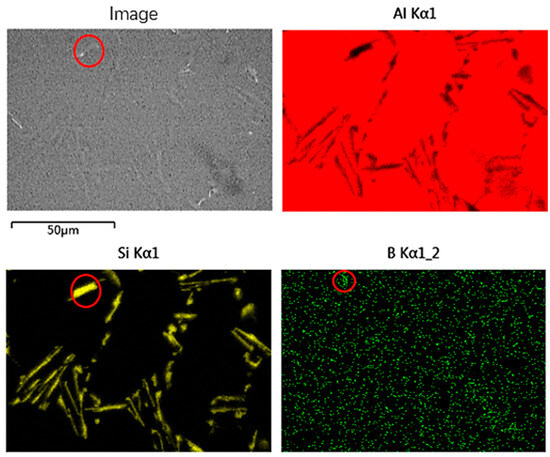
Figure 4.
SEM image of Al-7Si-0.02B alloy and its correspondent mapping.
3.4. Modification Effect of Er on Al-Si Alloys
Er, as a rare-earth element, can purify the aluminium melt and form a co-lattice Al3Er phase with Al [31,33]. The XRD and SEM organisation of the Al-5Er intermediate alloy is shown in Figure 5. It can be seen from the XRD peaks that there are more Al3Er phases inside the alloy. In addition, there is a eutectic microstructure in the intermediate alloy as shown in Figure 5c, and the Al3Er phases are laminar structures with a spacing of 0.26 μm. These laminar Al3Er phases are primary phases, which will melt and re-precipitate during the Al-7Si melting process.
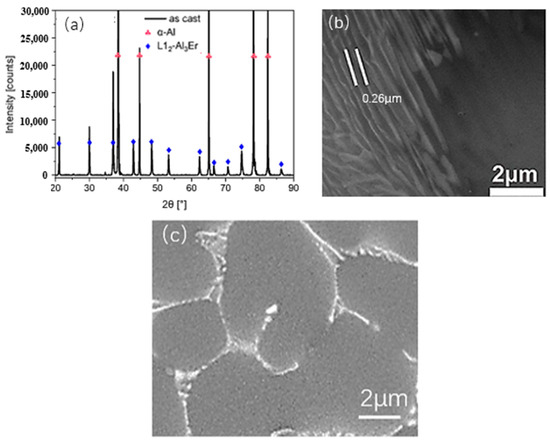
Figure 5.
X-ray diffraction peaks and SEM images of Al-5Er intermediate alloy. (a) XRD peaks of Al-5Er alloy; (b) Al-5Er alloy sheet spacing; (c) SEM image of Al-5Er alloy.
Figure 6 shows the SEM image of the Al-7Si-0.1Er alloy. There is a mismatch between the Al matrix and the Al3Er phase [33], and therefore, unlike the AlB2 phase, the Al3Er phase does not have a significant effect on grain refinement. However, due to the existence of a large number of micron-sized Al3Er phases (red circles) inside the alloy, and the Al3Er phases are uniformly distributed at the boundary between the eutectic Si and the aluminium matrix, the Al3Er phases can serve as the heterogeneous nucleation sites of the eutectic Si to promote the precipitation of the eutectic Si, which leads to the transformation of the eutectic Si from a long rod shape to a fine and homogeneous Si particles, and at this time the growth of eutectic Si is controlled by the distribution of the Al3Er phase. Meanwhile, the Al3Er phase precipitates along the boundary of the aluminium matrix, which can better regulate the precipitation of eutectic Si. This is also the reason why the fine dispersed Al3Er phase causes the reduction in the secondary dendrite arm spacing (SDAS) of the alloy.
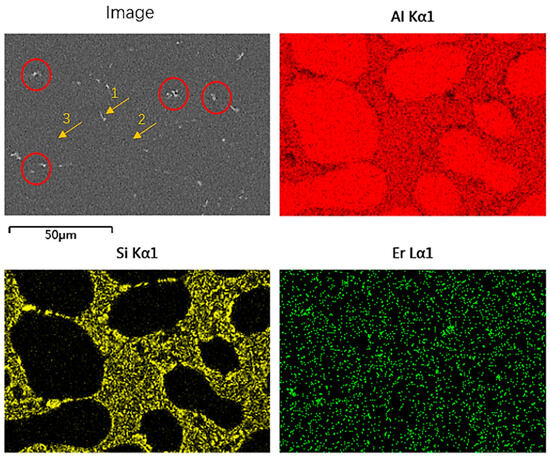
Figure 6.
SEM image of Al-7Si-0.1Er alloy and its correspondent mapping.
Table 2 shows the results of the spot scanning at the positions indicated by the arrows in Figure 6. It can be seen that arrow 3 contains mainly Al and a small amount of solid solution of Si exists in it. Arrow 1 contains mainly Al and Er with a solid solution of Si, confirming the interaction between Si and Er. The white precipitated phase is the Al3Er phase. Arrow 2 contains Al, Er, and Si, indicating an interaction between elemental Er and Si, which also inhibits the growth of Si. The growth of eutectic Si in this region is controlled by the enrichment of Er.

Table 2.
Spot scanning results in Al-7Si-0.1Er alloy.
In summary, the addition of Er to Al-7Si alloys not only forms the Al3Er phase as a heterogeneous nucleation site for eutectic Si, but also interacts with Si to inhibit the growth of eutectic Si.
3.5. Modification and Refinement Effects of Er and B on Al-7Si Alloys
Figure 4 and Figure 6 confirm that the AlB2 phase can play a role in grain refinement and the Al3Er phase can play a role in improving eutectic Si, respectively. Therefore, the mixed addition of Al-5Er and Al-B intermediate alloys can achieve the above two effects, which finally realises the modification and refinement of the alloy.
Figure 7 shows the observation of a SEM microstructure of the Al-5Er-B intermediate alloy. Compared to Figure 5c, the primary phase inside the intermediate alloy is finer and more discontinuous, and the lamellar features of the Al3Er phase are weakened. By mapping the alloy, it is found that the segregation of Er elements inside the alloy shows discontinuous distribution with ellipsoidal morphology for the Al3Er phase. The B element is not efficiently recognised due to its too light atomic mass. However, by comparing the SEM images and the distribution of Er elements, it can be found that there is a continuous flake distribution (red circle) of B elements, which is the AlB2 phase. Therefore, it is proved that the primary phases inside the Al-5Er-1B intermediate alloy are the Al3Er phase and the AlB2 phase, which are synergistically precipitated.
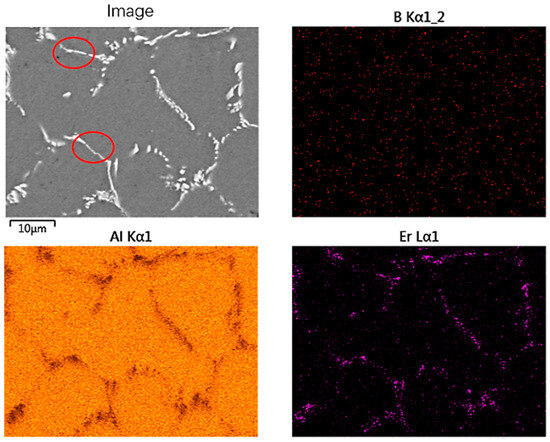
Figure 7.
SEM images of Al-5Er-B intermediate alloy and its correspondent mapping.
Figure 8 shows the SEM image of the Al-7Si-0.1Er-0.02B alloy. The AlB2 phase is a refractory high temperature phase that does not melt at 705 °C [8,15]. The long rod-like phase (red circle) is the AlB2 phase, which is consistent with the results of Figure 7. Unlike the results in Figure 6, the addition of the Al-5Er-B intermediate alloy to the Al-7Si alloy did not precipitate the diffusion and refinement of the Al3Er phase, but the modification effect of Er and B composite addition on eutectic Si is still significant.
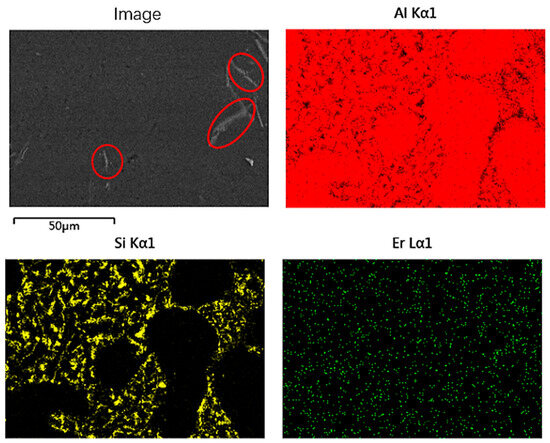
Figure 8.
SEM image of Al-7Si-0.1Er-0.02B alloy and its correspondent mapping.
Therefore, the composite addition of Er and B can not only refine the grain, but also modify the eutectic Si. However, its modification mechanism is different from that of the Al-5Er intermediate alloy. In the Er and B co-addition alloy, the modification effect occurs mainly due to the enrichment of Er.
Table 3 summarises the role of adding Al-7Si alloys to several common intermediate alloys. From the table, it can be seen that Al-Ti-B intermediate alloys have the best refining effect, but they do not modify eutectic Si. Al-Sr intermediate alloys have the best modification effect, but they do not refine the grains. The Al-5Er-B intermediate alloy proposed in this paper balances the grain refinement and eutectic Si modification effect, and obtains an alloy with both refinement and modification effects.

Table 3.
The effect of various intermediate alloys added to Al-7Si alloys.
3.6. Microhardness of Er and B Addition on Al-7Si Alloys
Figure 9 shows the evolution of the hardness of the Al-7Si alloy after the addition of various intermediate alloys. It can be seen that the addition of Er or B can enhance the hardness of the alloy, which occurs mainly due to the generation of Al3Er phase and AlB2 phase inside the alloy. The co-addition of Er and B further enhances the hardness of the alloy, which verifies the synergistic effect of Er and B.
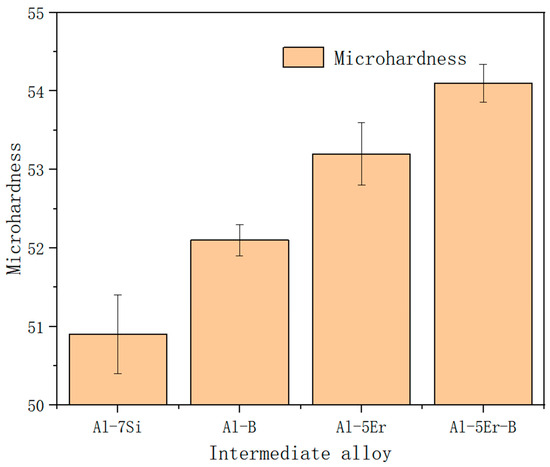
Figure 9.
Microhardness of Al-7Si alloy after adding 2 wt% of various intermediate alloys.
4. Conclusions
In this investigation, an Al-7Si-0.1Er-0.02B alloy with good modification and refinement capabilities was found. The impact of Er and B on the grain size and the eutectic Si of the alloy was examined by optical microscopy (OM), scanning electron microscopy (SEM), and X-Ray diffraction (XRD), and the modification mechanism of Er was put forth. Based on the experimental results, the main conclusions can be summarised as follows:
- The B element mainly forms a small amount of the AlB2 phase within the alloy, which can be used as a nucleation site for grains during casting, so the addition of B can significantly reduce the grain size of the Al-7Si alloy. However, the number density of the AlB2 phase is too low, so its effect on improving the eutectic Si size and reducing the secondary dendrite arm spacing (SDAS) is not significant.
- The addition of 0.1 wt% Er can form a large amount of Al3Er phase within the alloy, which mainly serves as a nucleation site for eutectic Si during casting, so the addition of Er can significantly reduce the SDAS, eutectic Si size, and morphology of Al-7Si alloys. However, due to the existence of a certain degree of mismatch between the Al matrix and the Al3Er phase, the relative grain refinement effect of Al3Er is not significant.
- We observed the enrichment of Er at eutectic Si, which suggests that Er can interact with Si and thus inhibit the growth of eutectic Si. Therefore, Er can modulate eutectic Si through interfacial step mechanism and heterogeneous nucleation mechanism.
- The co-addition of Er and B to Al-7Si alloys has better refining and modification effects than the addition of Er or B alone, which is mainly due to the modification effect of the element Er and the refining effect of the AlB2 phase.
- Unlike the modification mechanism of the Er-containing alloy, the modification of the alloy with the co-addition of Er and B is mainly controlled by the interfacial step mechanism.
Author Contributions
Conceptualization, Y.L. and S.W.; methodology, Y.L.; software, K.G. and H.H.; validation, Z.N.; formal analysis, Y.L., S.W., W.W., X.W., K.G. and H.H.; investigation, Y.L.; resources, S.W. and Z.N.; data curation, Z.N.; writing—original draft, Y.L. and W.W.; writing—review and editing, Y.L., S.W., W.W. and X.W.; supervision, Z.N.; project administration, S.W. and Z.N.; funding acquisition, S.W. and Z.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China [2021YFB3700902, 2021YFB3704204 and 2021YFB3704205]; the Beijing Natural Science Foundation [2202009]; the National Natural Science Foundation of China [51621003]; the Basic Research Program of Jiangsu Province 25 (NSF) [BK20191148]; the Beijing Lab Project for Modern Transportation Metallic Materials and Processing Technology; and the Jiangsu Key Laboratory for Clad Materials [BM2014006].
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Sun, J. Heterogeneous Nucleation Mechanism of Aluminum on Substrates. Ph.D. Thesis, Shanghai University, Shanghai, China, 2018. [Google Scholar]
- Zhao, J.T. Effect of Refiner (Al-Ti-Mg-Ce) on the Microstructure and Mechanical Properties of Aluminum Alloy. Ph.D. Thesis, Harbin Engineering University, Harbin, China, 2019. [Google Scholar]
- Malekan, M.; Shabestari, S.G. Effect of Grain Refinement on the Dendrite Coherency Point during Solidification of the A319 Aluminum Alloy. Metall. Mater. Trans. A 2009, 40, 3196–3203. [Google Scholar] [CrossRef]
- Sigworth, G.K.; Kuhn, T.A. Grain Refinement of Aluminum Casting Alloys. In Casting; ASM International: Almere, The Netherlands, 2008. [Google Scholar]
- Murty, B.S.; Kori, S.A.; Chakraborty, M. Grain refinement of aluminium and its alloys by heterogeneous nucleation and alloying. Int. Mater. Rev. 2002, 47, 3–29. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.R.; Thompson, G.E.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al–Ti–B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Cibula, A. The Grain Refinement of Aluminum Alloy Castings by Addition of Titanium and Boron. J. Inst. Met. 1951, 80, 1–16. [Google Scholar]
- Wang, T.M.; Chen, Z.N.; Fu, H.W.; Xu, J.; Fu, Y.; Li, T. Grain refining potency of Al–B master alloy on pure aluminum. Scr. Mater. 2011, 64, 1121–1124. [Google Scholar] [CrossRef]
- Jones, G.P.; Pearson, J. Factors affecting the grain-refinement of aluminum using titanium and boron additives. Metall. Trans. B 1976, 7, 223–234. [Google Scholar] [CrossRef]
- Arnberg, L.; Bäckerud, L.; Klang, H. Evidence of metastable phase in Al–Ti–(B) system. Met. Sci. J. 2013, 9, 14–17. [Google Scholar] [CrossRef]
- Abdel-Reihim, M.; Hess, N.; Reif, W.; Birch, M.E.J. Effect of solute content on the grain refinement of binary alloys. J. Mater. Sci. 1987, 22, 213–218. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, D.; Wei, Z.; Huang, L.; Song, W.; Chen, Y. The Refinement Effect of Al-Ti-C-RE Master Alloy Prepared by Adding Ce2O3 on Pure Al. Adv. Mater. Res. 2011, 139–141, 227–234. [Google Scholar] [CrossRef]
- Zhao, H.; Song, Y.; Li, M.; Guan, S. Grain refining efficiency and microstructure of Al-Ti-C-RE master alloy. J. Alloys Compd. 2010, 508, 206–211. [Google Scholar] [CrossRef]
- Cong, X.U.; Xiao, W.; Zhao, W.; Wang, W.; Shuji, H.; Hiroshi, Y.; Chaoli, M.A. Microstructure and formation mechanism of grain-refining particles in Al-Ti-C-RE grain refiners. J. Rare Earth 2015, 33, 553–560. [Google Scholar]
- Birol, Y. Performance of AlTi5B1, AlTi3B3 and AlB3 master alloys in refining grain structure of aluminium foundry alloys. Mater. Sci. Technol. 2012, 28, 481–486. [Google Scholar] [CrossRef]
- Birol, Y. Effect of silicon content in grain refining hypoeutectic Al-Si foundry alloys with boron and titanium additions. Mater. Sci. Technol. 2012, 28, 385–389. [Google Scholar] [CrossRef]
- Qiu, D.; Taylor, J.A.; Zhang, M.X.; Kelly, P.M. A mechanism for the poisoning effect of silicon on the grain refinement of Al-Si alloys. Acta Mater. 2007, 55, 1447–1456. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Murty, B.S.; Chakraborty, M. Effect of hot rolling and heat treatment of Al–5Ti 1B master alloy on the grain refining efficiency of aluminium. Mater. Sci. Eng. A 2001, 301, 180–186. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Hosoda, S.; Kim, I.S.; Watanabe, Y. Grain refining performance for Al and Al–Si alloy casts by addition of equal-channel angular pressed Al–5mass% Ti alloy. Mater. Sci. Eng. A 2006, 425, 55–63. [Google Scholar] [CrossRef]
- Rathi, S.K.; Sharma, A.; Sabatino, M.D. Effect of heat treatment for enhancing performance of Al 5Ti–1B master alloy on mechanical and hot tearing properties of Al–7Si–3Cu alloy. Mater. Res. Express 2019, 6, 86550. [Google Scholar] [CrossRef]
- Marcantonio, J.; Mondolfo, L. Nucleation of aluminium by several intermetallic compounds. J. Inst. Met. 1970, 98, 23–27. [Google Scholar]
- Wang, F.; Qiu, D.; Liu, Z.L.; Taylor, J.A.; Easton, M.A.; Zhang, M.X. The grain refinement mechanism of cast aluminium by zirconium. Acta Mater. 2013, 61, 5636–5645. [Google Scholar] [CrossRef]
- Norman, A.F.; Prangnell, P.B.; Mcewen, R.S. The solidification behaviour of dilute aluminium scandium alloys. Acta Mater. 1998, 46, 5715–5732. [Google Scholar] [CrossRef]
- Yang, J.J.; Nie, Z.R.; Jin, T.N.; Xu, G.; Mou, S.; Yin, Z. Existence form and refinement mechanism of rare earth Er in Al-Zn-Mg alloys. Chin. J. Nonferrous Met. 2004, 4, 620–626. [Google Scholar]
- Wen, S.P.; Xing, Z.B.; Huang, H.; Li, B.L.; Wang, W.; Nie, Z.R. The effect of erbium on the microstructure and mechanical properties of Al–Mg–Mn–Zr alloy. Mater. Sci. Eng. A 2009, 516, 42–49. [Google Scholar] [CrossRef]
- Yang, J.J.; Nie, Z.R.; Jin, T.N.; Xu, G.F.; Fu, J.B.; Ruan, H.Q.; Zuo, T.Y. Effect of trace rare earth element Er on high pure Al. Trans. Nonferrous Met. Soc. China 2003, 13, 1035–1039. [Google Scholar]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Criteria for developing castable, creep-resistant aluminum-based alloys—A review. Ztschrift Met. 2006, 97, 246–265. [Google Scholar] [CrossRef]
- Wen, S.P.; Gao, K.Y.; Li, Y.; Huang, H.; Nie, Z.R. Synergetic effect of Er and Zr on the precipitation hardening of Al Er–Zr alloy. Scr. Mater. 2011, 65, 592–595. [Google Scholar] [CrossRef]
- Nie, Z.R.; Jin, T.N.; Fu, J.B.; Zou, J.X.; Yang, J.J.; Zuo, T.Y. Development on research of advanced rare-earth aluminum alloy. Trans. Nonferrous Met. Soc. China 2003, 13, 509–514. [Google Scholar]
- Bai, S.; Liu, Z.Y.; Gu, Y.X.; Li, Y.; Hou, Y.; Chen, X. Microstructures and fatigue fracture behavior of an Al–Cu–Mg–Ag alloy with addition of rare earth Er. Mater. Sci. Eng. A 2010, 527, 1806–1814. [Google Scholar] [CrossRef]
- Lei, Z.G.; Wen, S.P.; Yang, G.; Ma, J.R.; Huang, H.; Nie, Z.R. Study on refining grain and microalloying effect of Al-5Er master alloy. Phys. Met. Metallogr. 2024, 125, 1672–1679. [Google Scholar] [CrossRef]
- Lei, Z.G.; Wen, S.P.; Yang, G.; Wei, W.; Huang, H.; Nie, Z. Study on microstructure and refining effect of deformed Al-4.5Er-1Zr-1.5Ti master alloy. J. Alloys Metall. Syst. 2024, 7, 100084. [Google Scholar]
- Lei, Z.G. Study on the Grain Refinement Effect of Al-Er-Zr Master Alloys and Its Application. Ph.D. Thesis, Beijing University of Technology, Beijing, China, 2024. [Google Scholar]
- Nogita, K.; McDonald, S.D.; Dahle, A.K. Eutectic modification of Al-Si alloys with rare earth metals. Mater. Trans. 2004, 45, 323–326. [Google Scholar] [CrossRef]
- Kowata, T.; Horie, H.; Hiratsuka, S.; Chida, A. Influence of Rare-earth Elements on Refinement of Primary Silicon Crystals in a Hypereutectic Al-Si Alloy. J. Jpn. Foundry Eng. Soc. 1994, 66, 803–808. [Google Scholar]
- Weiss, J.C., Jr.; Loper, C.R. Primary Silicon in Hypereutectic Aluminum-silicon Casting Alloys. Trans. Am. Fish. Soc. 1987, 32, 51–62. [Google Scholar]
- Li, Q.; Xia, T.; Lan, Y.; Li, P.; Fan, L. Effects of rare earth Er addition on microstructure and mechanical properties of hypereutectic Al–20% Si alloy. J. Alloys Compd. 2013, 588, 97–102. [Google Scholar] [CrossRef]
- Colombo, M.; Gariboldi, E.; Morri, A. Er addition to Al-Si-Mg-based casting alloy: Effects on microstructure, room and high temperature mechanical properties. J. Alloys Compd. Interdiscip. J. Mater. Sci. Solid State Chem. Phys. 2017, 708, 1234–1244. [Google Scholar] [CrossRef]
- He, Y.; Xi, H.; Ming, W.; Shao, Q.; Shen, R.; Lai, Y.; Wu, C.; Chen, J. Thermal stability and precipitate microstructures of AlSiMgEr alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 1–10. [Google Scholar] [CrossRef]
- Jing, L.; Pan, Y.; Lu, T.; Chai, W. Refinement effect of two rare earth borides in an Al-7Si-4Cu alloy: A comparative study. Mater. Charact. 2018, 145, 664–670. [Google Scholar] [CrossRef]
- Jing, L.; Pan, Y.; Lu, T.; Li, C.; Pi, J.; Sheng, N. Application of Al-2La-1B Grain Refiner to Al-10Si-0.3Mg Casting Alloy. J. Mater. Eng. Perform. 2018, 27, 2838–2843. [Google Scholar] [CrossRef]
- Jing, L.; Pan, Y.; Lu, T.; Pi, J.; Gu, T. Nucleation potency prediction of LaB6 with E2EM model and its influence on microstructure and tensile properties of Al-7Si-0.3Mg alloy. Trans. Nonferrous Met. Soc. China 2018, 28, 1687–1694. [Google Scholar] [CrossRef]
- Song, D.; Zhao, Y.; Jia, Y.; Huang, G.; Zhang, Z.; Zhou, N.; Li, X.; Zheng, K.; Fu, Y.; Zhang, W. Effect of B addition on the formation of Fe-rich phases in Al-Si-Fe alloys. J. Alloys Compd. 2023, 930, 167426. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Wang, Y.; Liu, Y. Microstructure and Combustion Characteristics of Aluminum-boron Alloy Powders. J. Mater. Sci. Eng. 2021, 192, 568–574. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).