Enhanced Corrosion Protection as a Sustainable Approach for Nickel Using Novel FeL Salen Complex: Electrochemical Investigation and DFT Insights
Abstract
1. Introduction
2. Experimental
2.1. Reagents
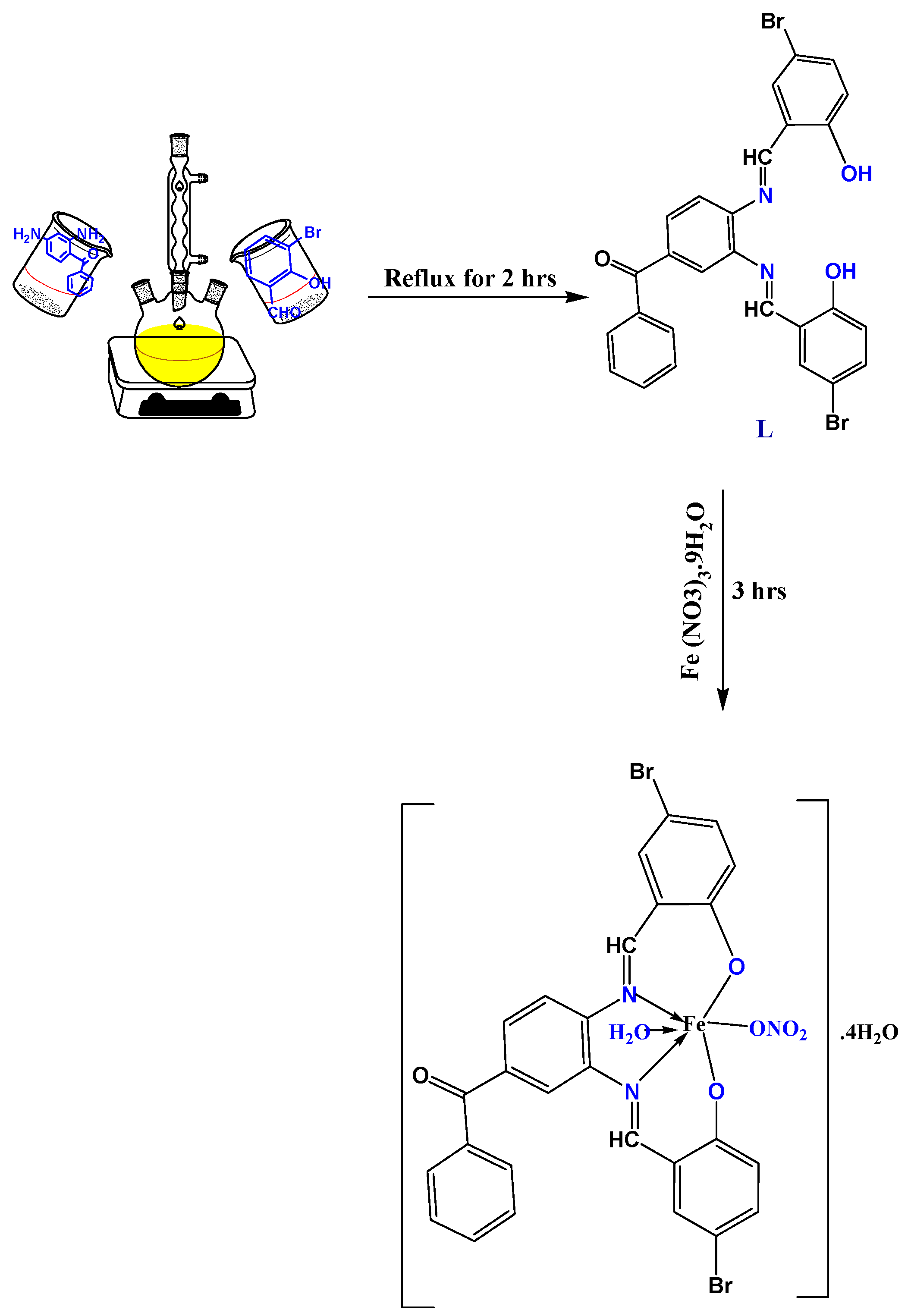
2.2. Synthesis of L Imine Ligand
2.3. Preparation of LFe Imine Complex
2.4. Equipments
2.5. Stoichiometry and Formation Constants of Complexes in Solution
2.6. Materials and Methods Utilized in Corrosion Inhibition
2.7. Computational Approach
3. Results and Discussion
3.1. Physicochemical Properties of the Prepared L Imine Ligand and Its Fe (III) Complex
3.1.1. NMR and IR of the Investigated Imine Ligand and Its FeL the Complex
3.1.2. Elemental Analyses and Conductivity Measurement Studies
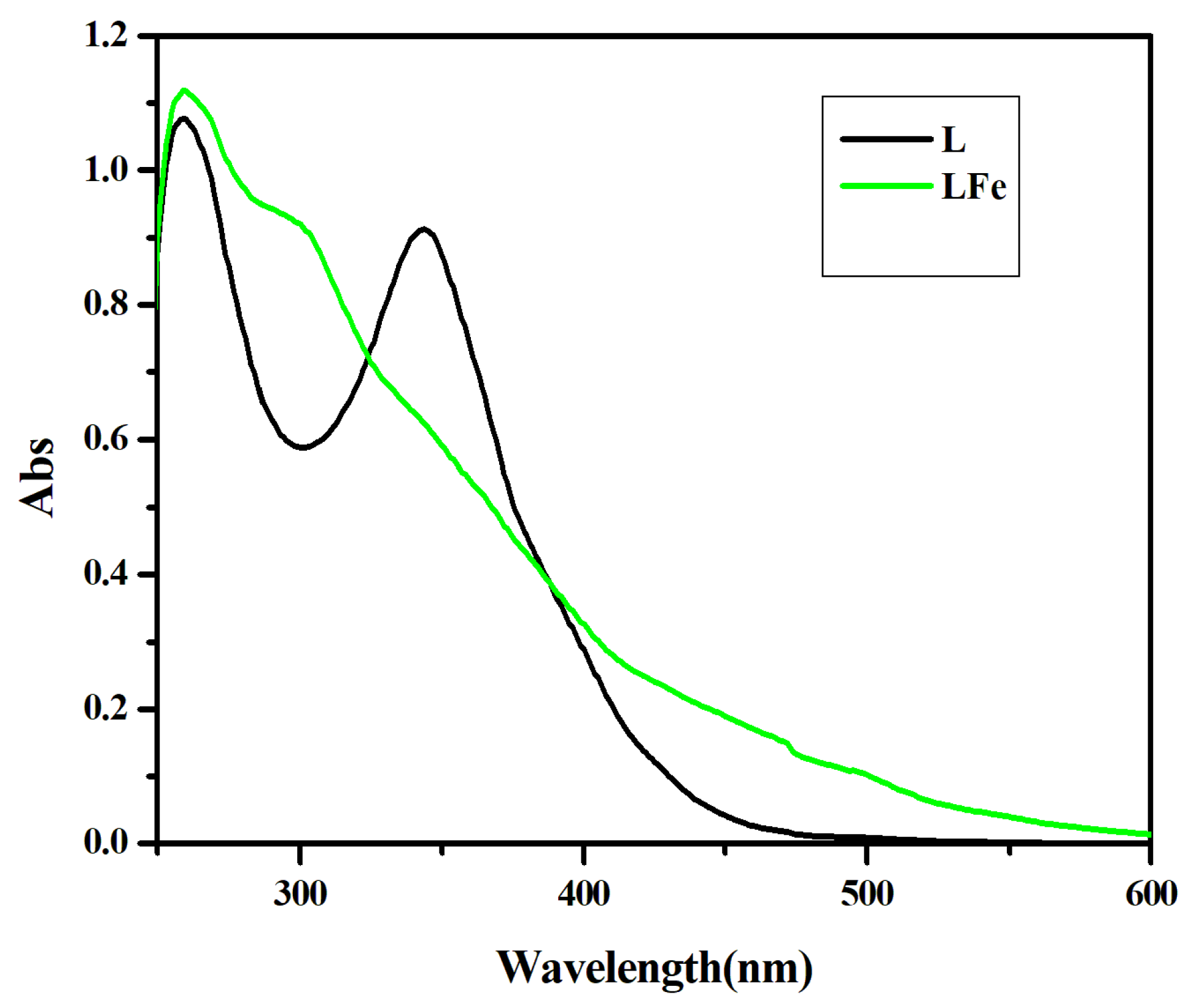
3.1.3. Electronic Spectrum
3.1.4. Moment of Magnetism
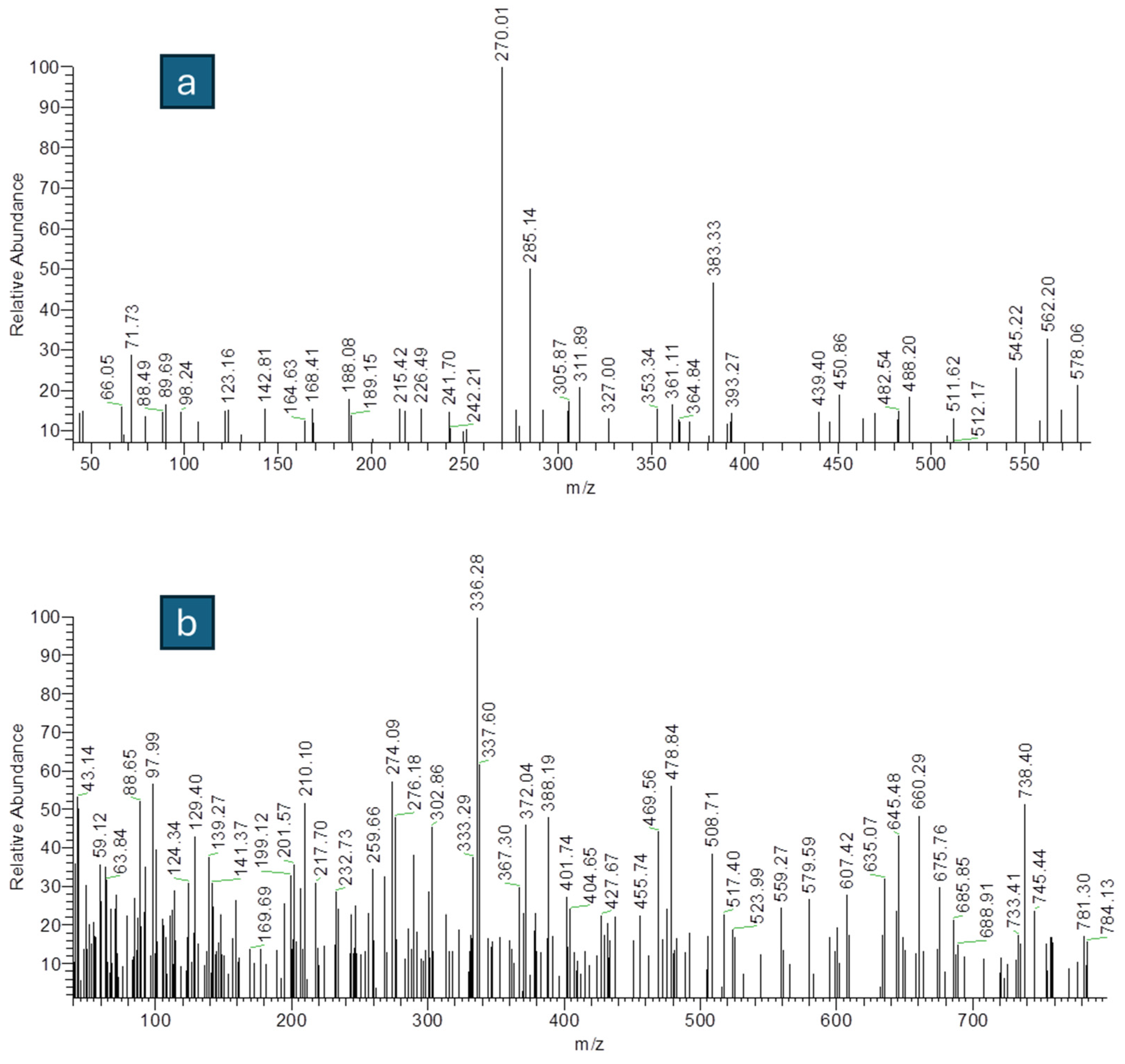
3.1.5. Mass Spectra
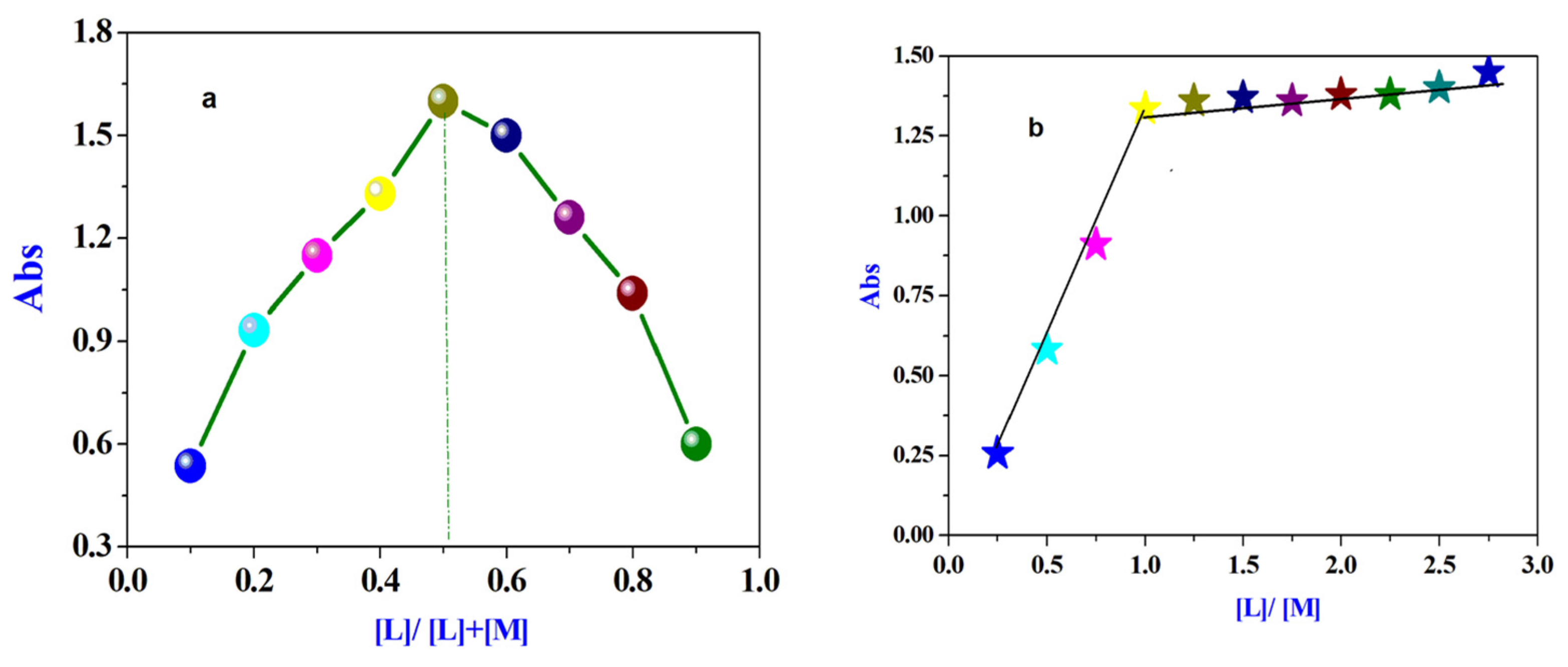
3.1.6. Spectrophotometric Determination of the Stoichiometry of the Prepared Complex
3.2. Impact of Concentration of the Inhibitor
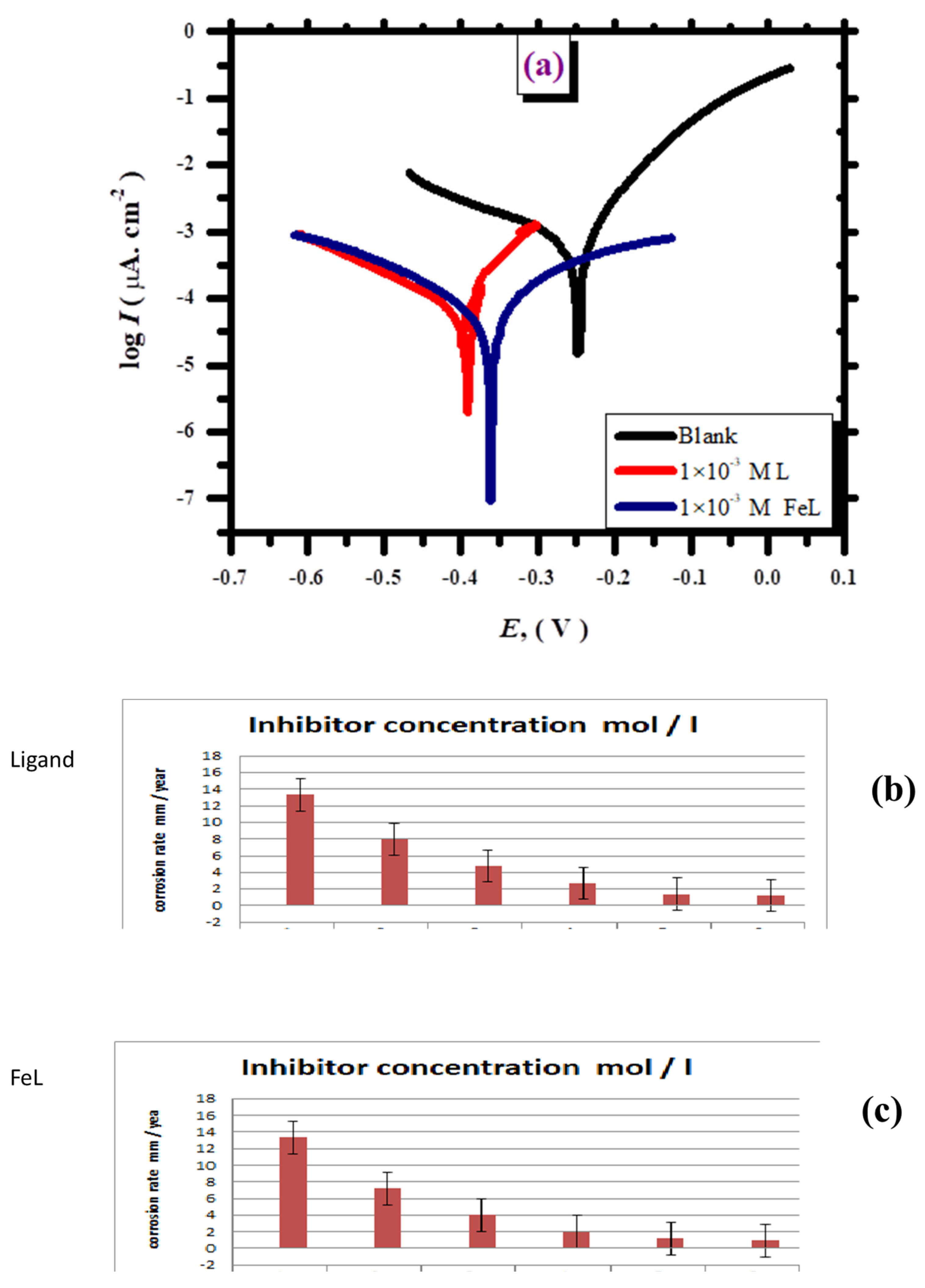
3.2.1. Tafel Plot
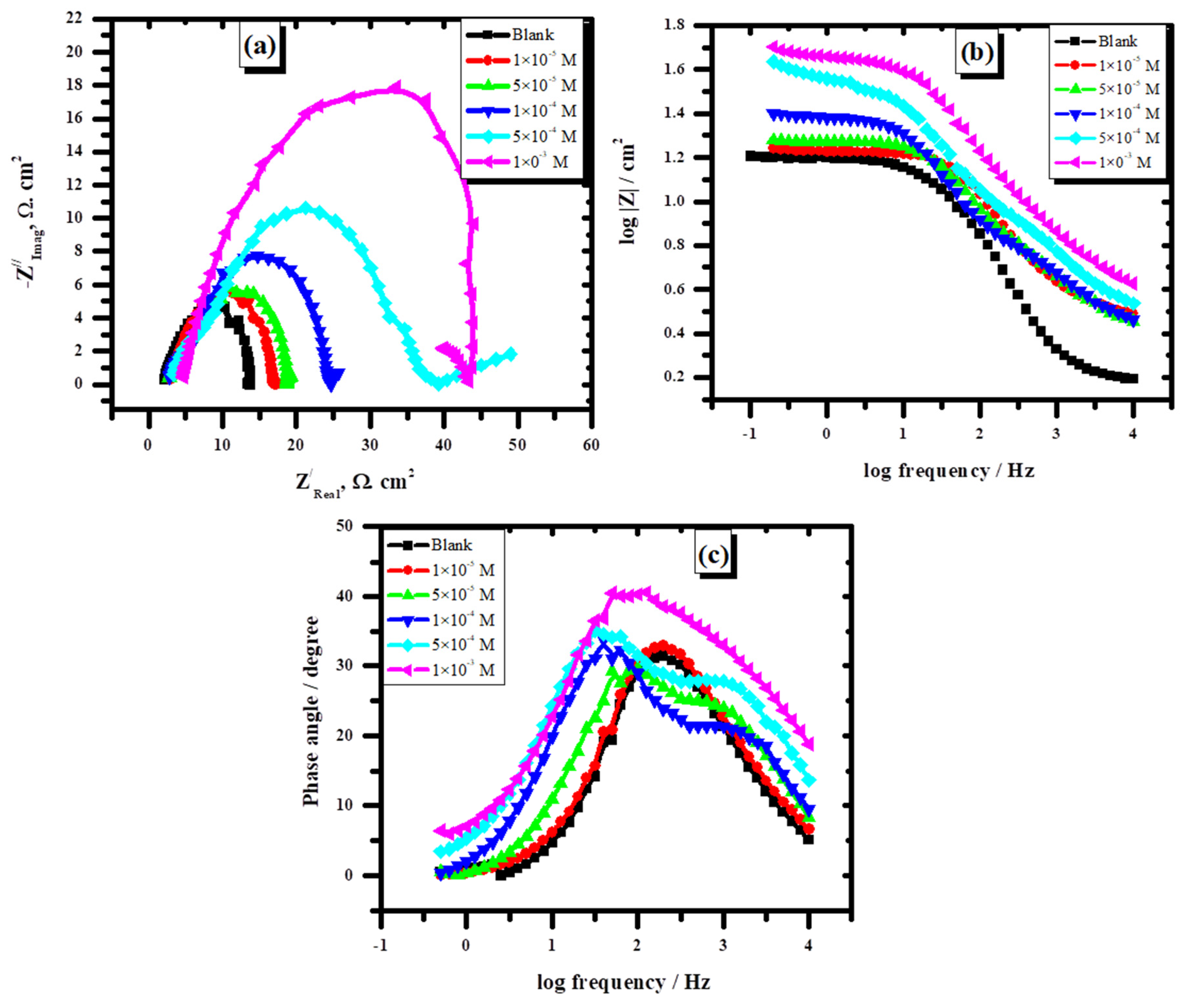
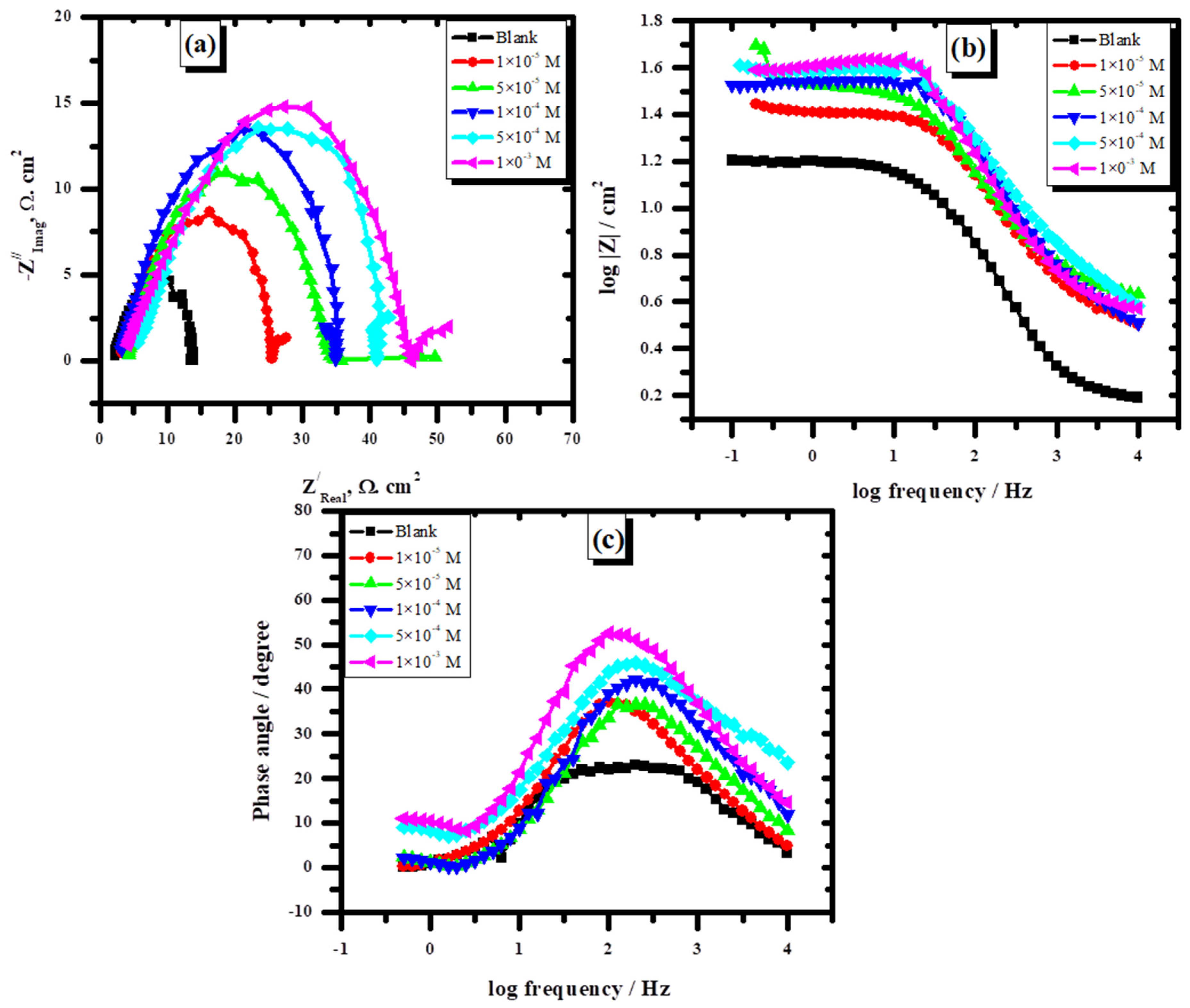
3.2.2. Electrochemical Impedance Spectroscopy
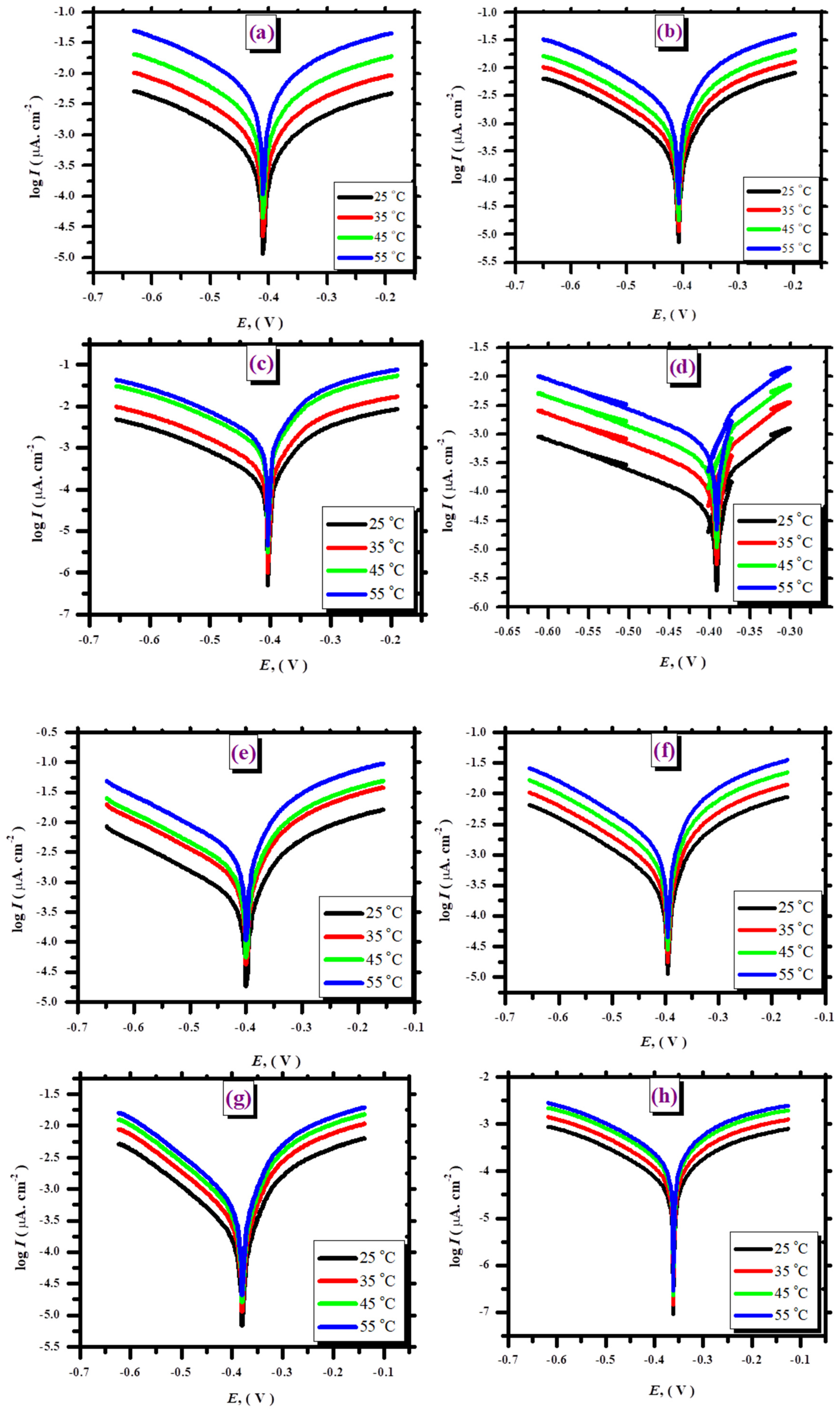
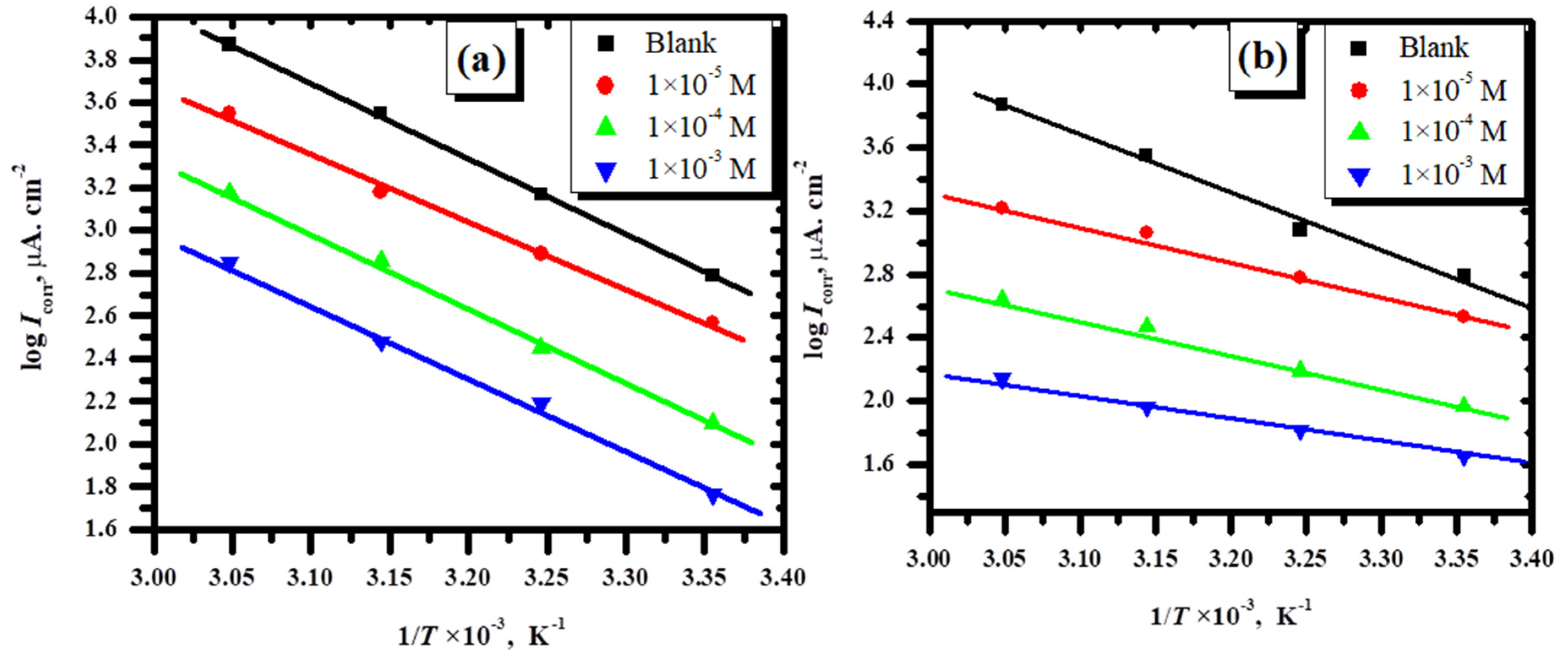
3.2.3. Effect of Temperature
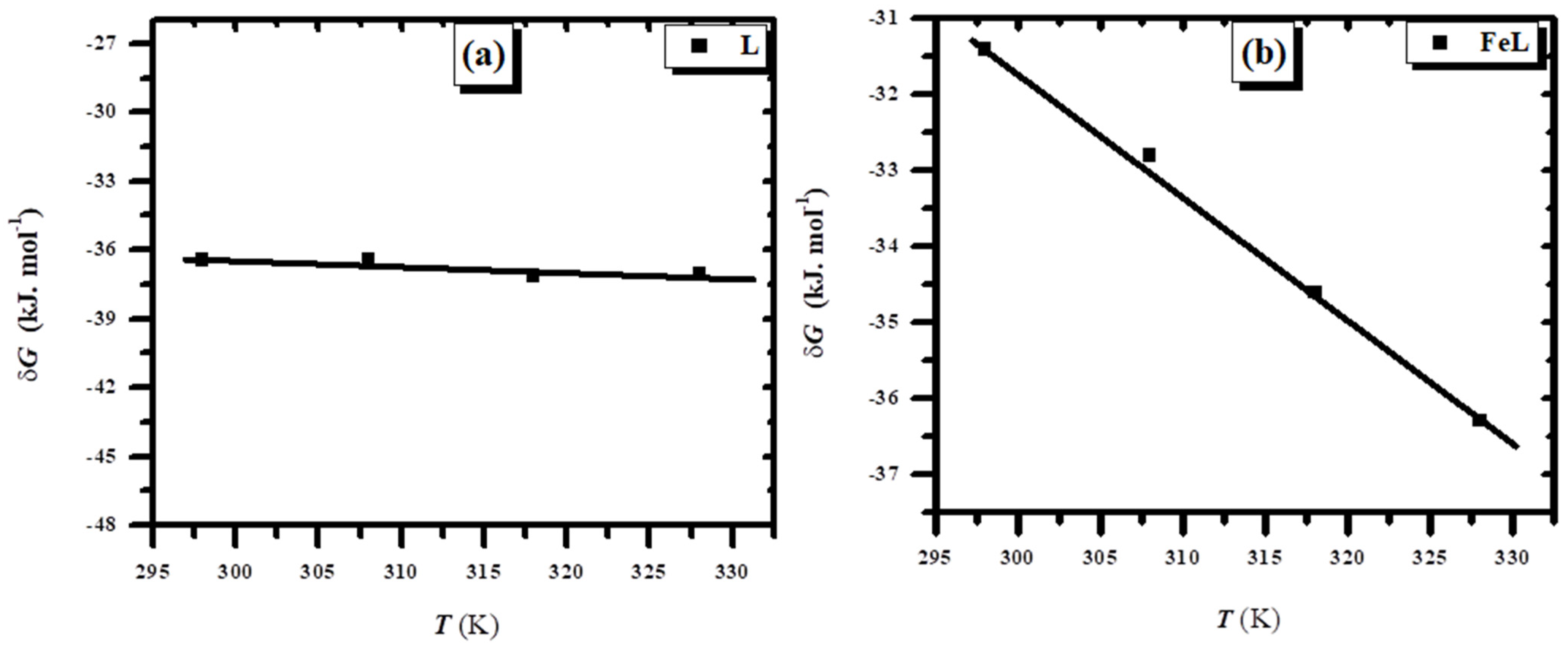
3.2.4. Thermodynamic Constants and the Adsorption Isotherm for the Corrosion Process
3.2.5. Surface Characterization
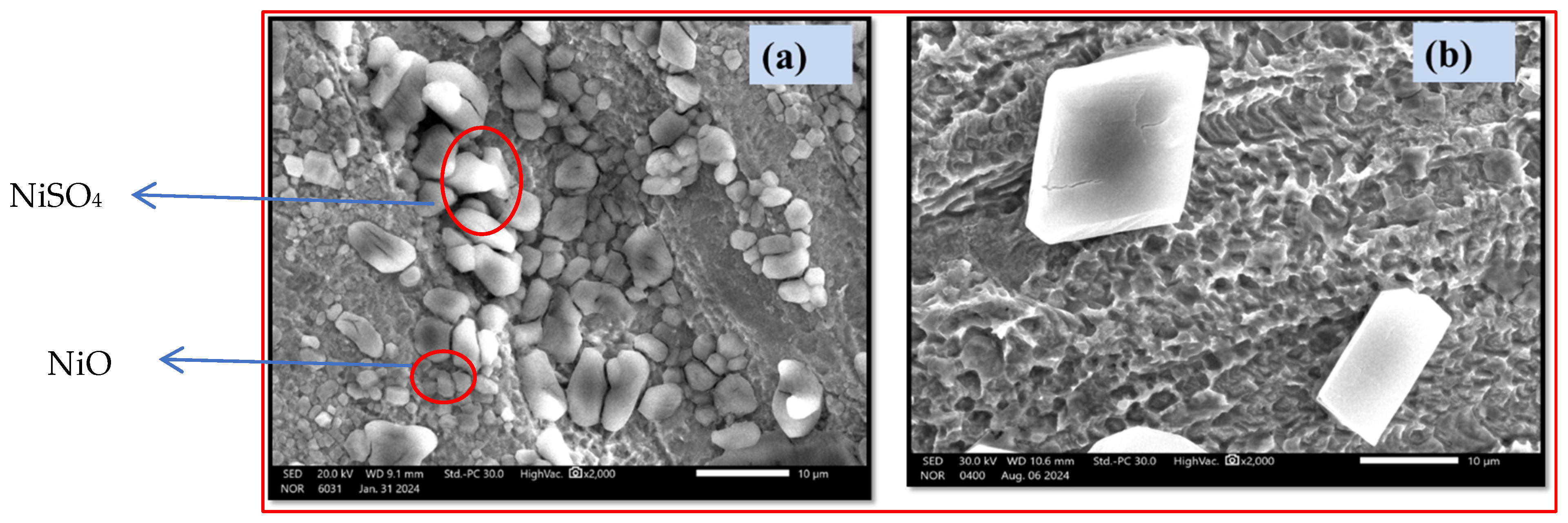
Scanning Electron Microscope (SEM)
Energy-Dispersive X-Ray Spectroscopy (EDX)
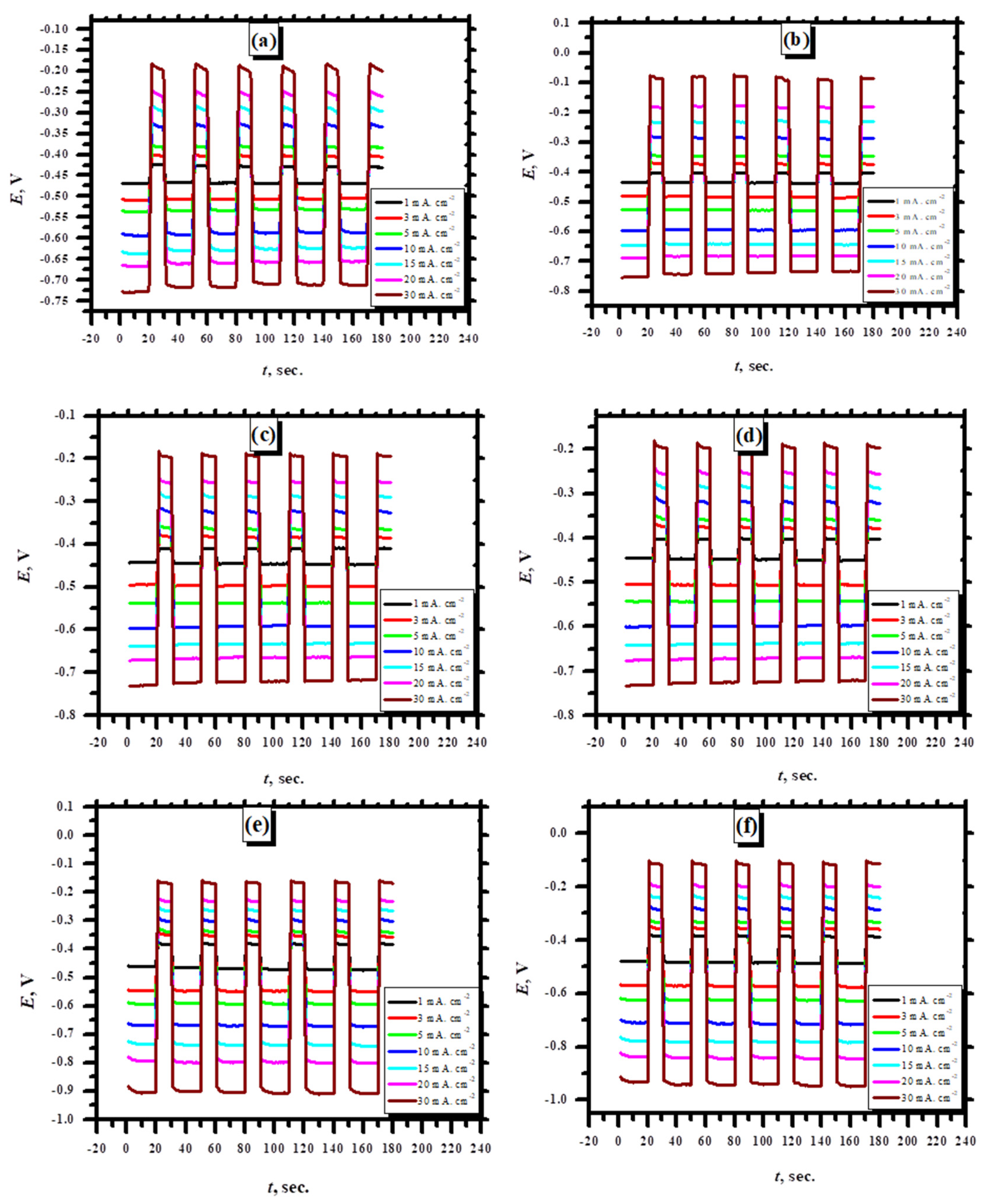
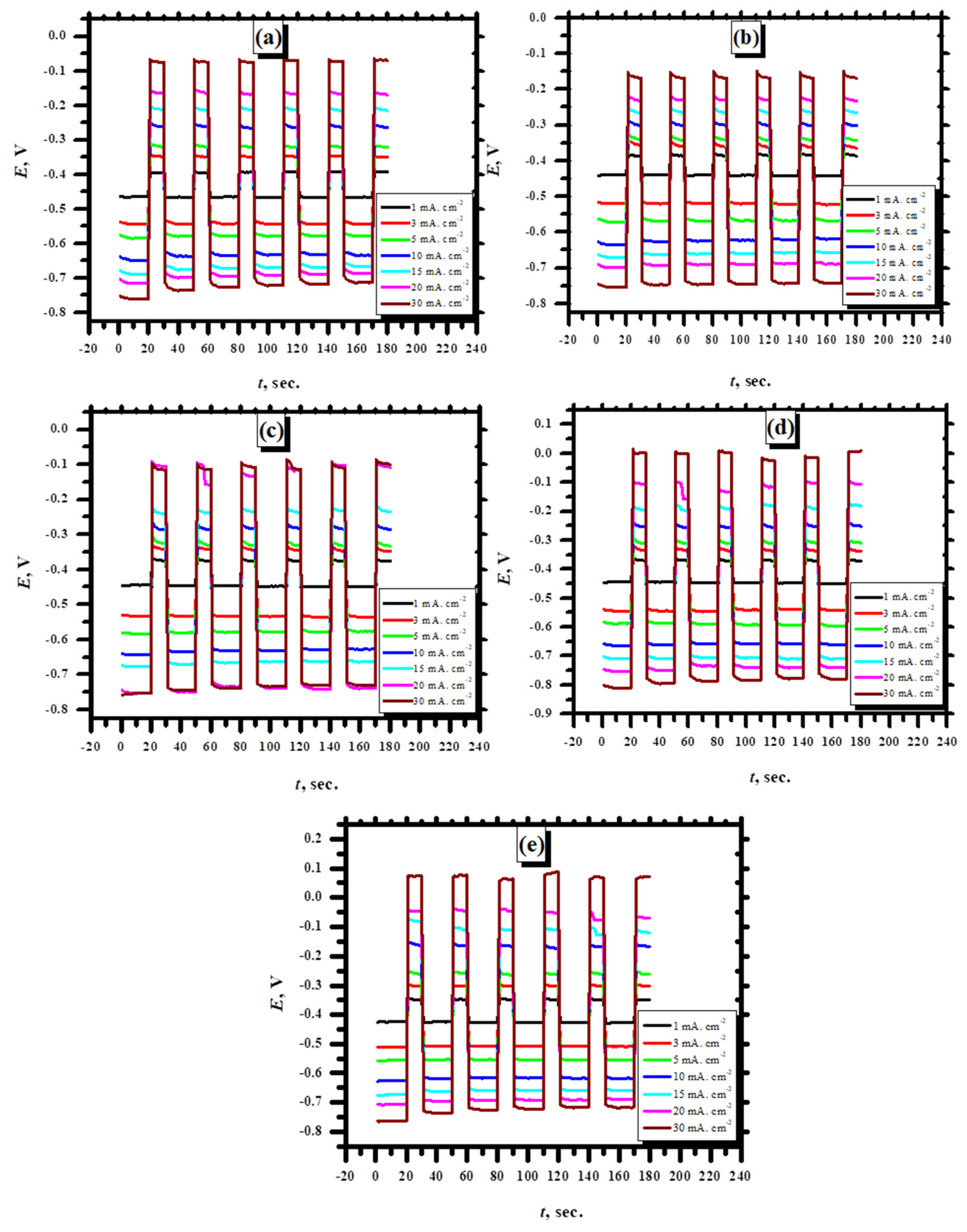
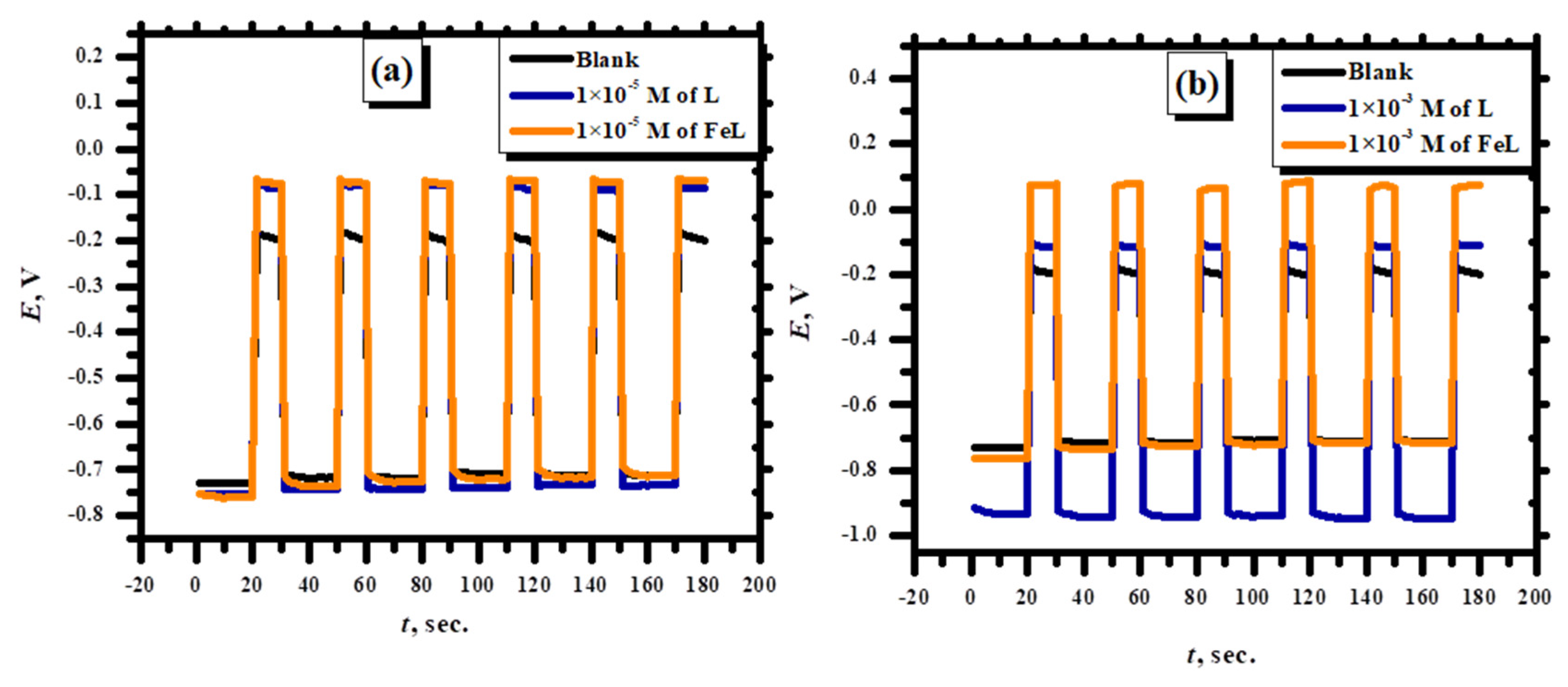
3.2.6. Charge Discharge Studies
3.3. Computational Details
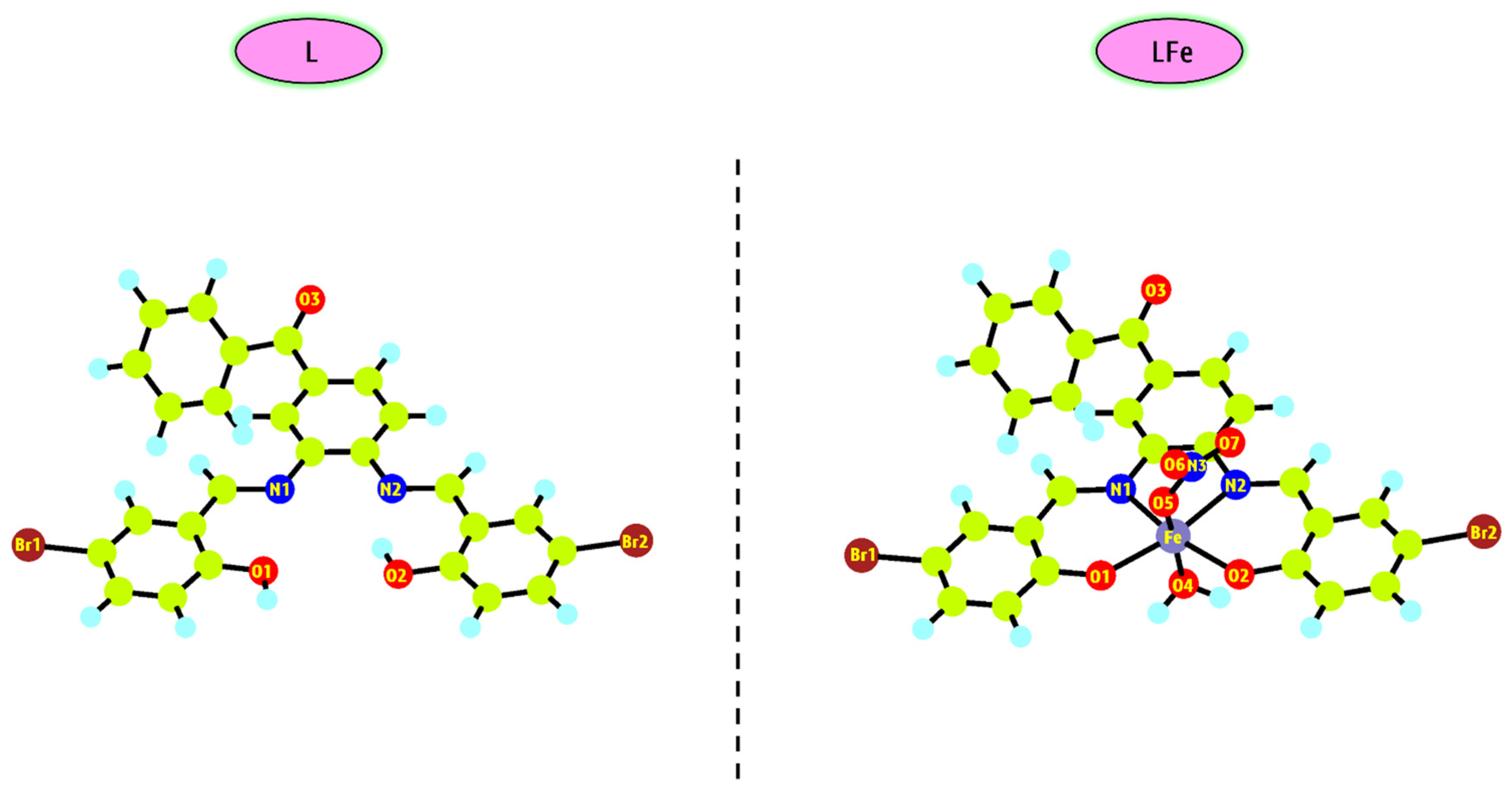
3.3.1. Optimized Structure
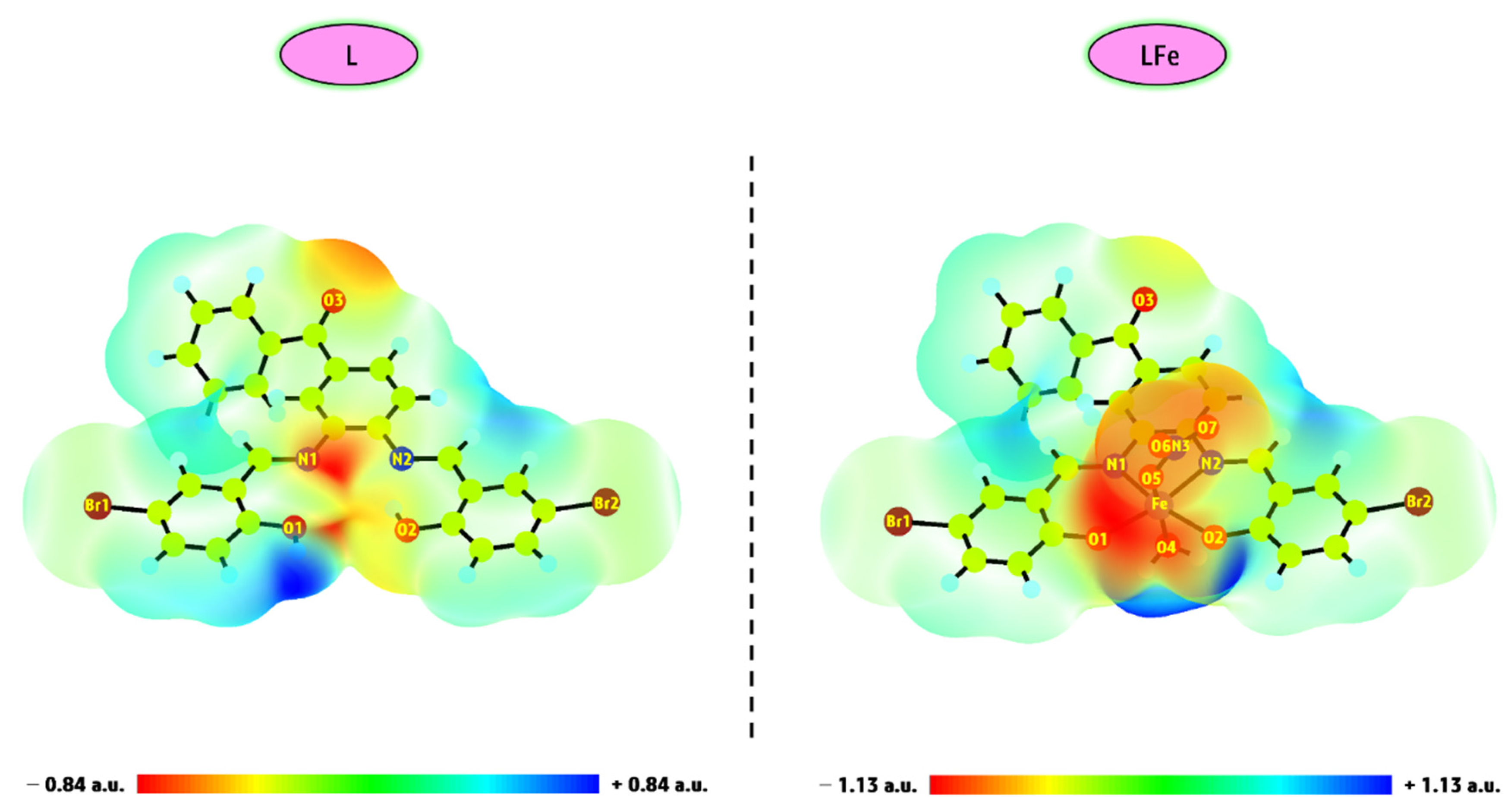
3.3.2. MEP Mapping
3.3.3. Mulliken Atomic Charge
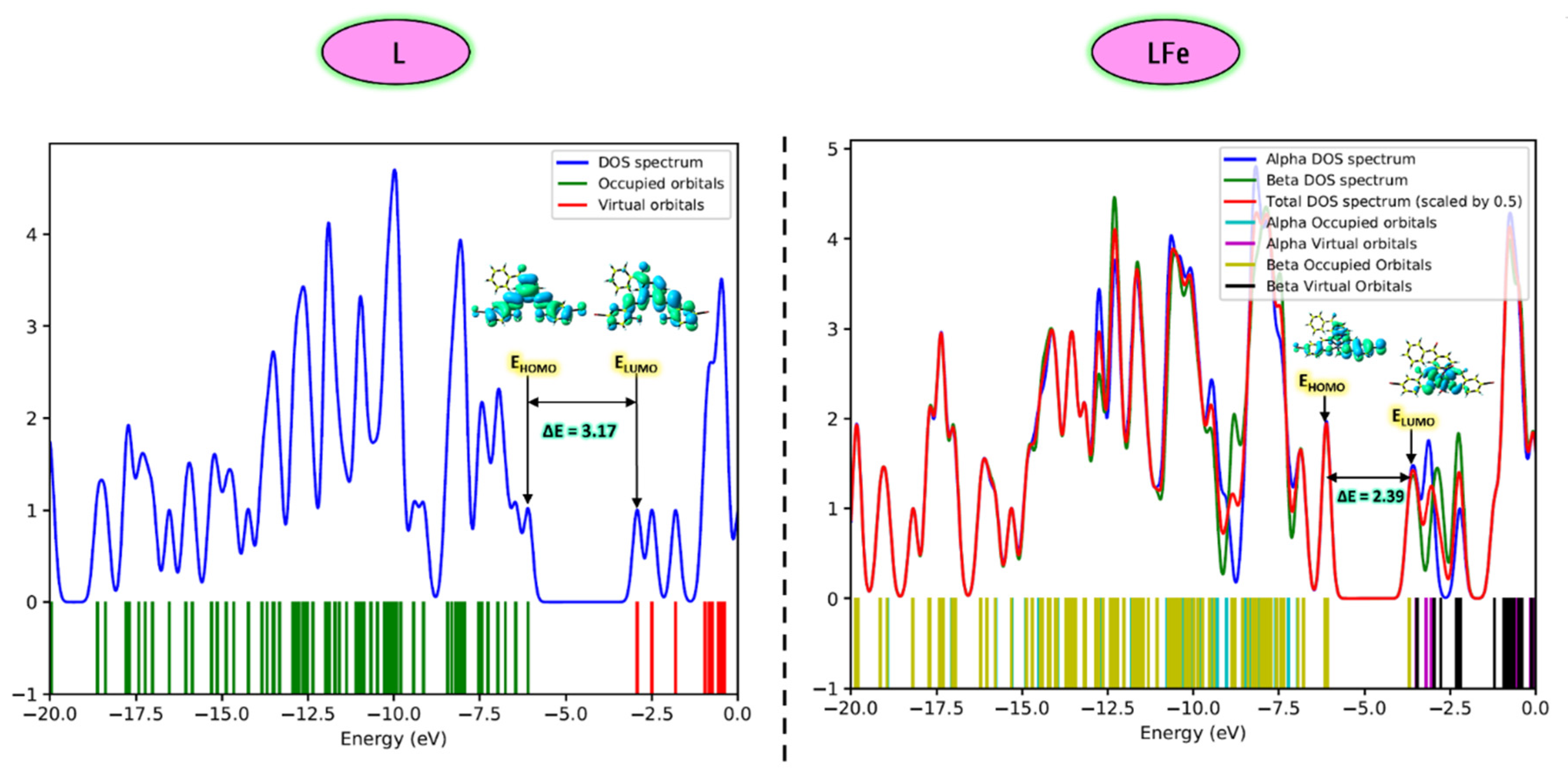
3.3.4. HOMO-LUMO Analysis
3.3.5. Inhibition of Corrosion and Quantum Reactivity
3.3.6. Density of States (DOS) Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selembo, P.A.; Merrill, M.D.; Logan, B.E. The use of stainless steel and nickel alloys as low-cost cathodes in microbial electrolysis cells. J. Power Sources 2009, 190, 271–278. [Google Scholar]
- Khaled, K.F. Molecular modeling and electrochemical investigations of the corrosion inhibition of nickel using some thiosemicarbazone derivatives. J. Appl. Electrochem. 2011, 41, 423–433. [Google Scholar]
- Arslan, T.; Kandemirli, F.; Ebenso, E.E.; Love, I.; Alemu, H. Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros. Sci. 2009, 51, 35. [Google Scholar] [CrossRef]
- Shokry, H.; Mabrouk, E.M. Computational and electrochemical investigation for corrosion inhibition of nickel in molar sulfuric acid by dihydrazide derivatives. Part II. Arab. J. Chem. 2017, 10, S3402–S3411. [Google Scholar]
- Shilkamy, H.A.E.S.; El-Khatib, R.M.; Feizi-Dehnayebi, M.; Alharas, M.M.; AL-Faze, R.; Abu-Dief, A.M. Green corrosion inhibitors for nickel in acidic media utilizing novel imine ligand and its Zn (II) metal chelate supported by DFT calculation. J. Mol. Liq. 2024, 416, 126468. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Mohamad, A.D.M.; Shehata, M.R.; Abu-Dief, A.M. Targeted synthesis of two iron (III) tetradentate dibasic chelating Schiff base complexes toward inhibition of acidic induced steel corrosion: Empirical and DFT insights. Appl. Organomet. Chem. 2022, 36, e6718. [Google Scholar]
- El-Lateef, H.M.A.; Abu-Dief, A.M.; Mohamed, M.A. Corrosion inhibition of carbon steel pipelines by some novel Schiff base compounds during acidizing treatment of oil wells studied by electrochemical and quantum chemical methods. J. Mol. Struct. 2017, 1130, 522–542. [Google Scholar]
- El-Lateef, H.M.A.; Abu-Dief, A.M.; Abdel-Rahman, L.H.; Sañudo, E.C.; Aliaga-Alcalde, N. Electrochemical and theoretical quantum approaches on the inhibition of C1018 carbon steel corrosion in acidic medium containing chloride using some newly synthesized phenolic Schiff bases compounds. J. Electroanal. Chem. 2015, 743, 120–133. [Google Scholar]
- Gowri, S.; SathiyabamA, J.; Rajendran, S. Corrosion inhibition by amino acids—An over review. Eur. Chem. Bull. 2012, 11, 470–476. [Google Scholar]
- El Attari, H.; Chefira, K.; Siniti, M.; El Kihel, A.; Rchid, H. Adsorption and corrosion inhibition performance of 1,3-dihydro- 2Hbenzimidazole-2-thione for mild steel C35 E in sulfuric acid solution. Int. J. Recent Trends Eng. Res. (IJRTER) 2017, 03, 2455–2457. [Google Scholar]
- Taleb, H.I.; Mohamed, A.Z. Corrosion inhibition of mild steel using fig leaves extract in hydrochloric acid solution. Int. J. Electrochem. Sci. 2011, 6, 6442–6455. [Google Scholar]
- Tian, H.; Li, W.; Cao, K.; Hou, B. Potent inhibition of copper corrosion in neutral chloride media by novel non-toxic thiadiazole derivatives. Corros. Sci. 2013, 73, 281–291. [Google Scholar] [CrossRef]
- Ismail, K.M. Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions. Electrochim. Acta 2007, 52, 7811–7819. [Google Scholar] [CrossRef]
- Saifi, H.; Bernard, M.C.; Joiret, S.; Rahmouni, K.; Takenouti, H.; Talhi, B. Corrosion inhibitive action of cysteine on Cu–30Ni alloy in aerated 0.5 M H2SO4. Mater. Chem. Phys. 2010, 120, 661–669. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O.; Umoren, S.A. Antifungal drugs as corrosion inhibitors for aluminium in 0.1 M HCl. Surf. Rev. Lett. 2008, 15, 277. [Google Scholar]
- Ebenso, E.E.; Alemu, H.; Umoren, S.A.; Obot, I.B. Inhibition of mild steel corrosion in sulphuric acid using alizarin yellow GG dye and synergistic iodide additive. Int. J. Electrochem. Sci. 2008, 3, 1325. [Google Scholar] [CrossRef]
- Bouklah, M.; Hammouti, B.; Lagrenee, M.; Bentiss, F. The inhibited effect of some tetrazolic compounds towards the corrosion of brass in nitric acid solution. Corros. Sci. 2006, 48, 2831. [Google Scholar] [CrossRef]
- Magaji, L.; Ameh, P.O.; Eddy, N.O.; Uzairu, A.; Siaka, A.A.; Habib, S.; Ayuba, A.M.; Gumel, S.M. Ciprofloxacin as corrosion inhibitors for mild steel—Effects of concentration and temperature. Int. J. Mod. Chem. 2012, 2, 64. [Google Scholar]
- Alahmadi, M.; Mohamed, W.S.; Zhukov, A.; Salaheldeen, M.; Alsaedi, W.H.; Alhashmialameer, D.; Al-Ghamdi, K.; Abu Dief, A.M. One-step hydrothermal synthesis of flower-like MoS2/VS2 nanocomposite for biomedical applications. Inorg. Chem. Commun. 2023, 157, 111336. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; El-Khatib, R.M.; Abdel-Fatah, S.M. Some new nano-sized Fe(II), Cd(II) and Zn(II) Schiff base complexes as precursor for metal oxides: Sonochemical synthesis, characterization, DNA interaction, in vitro antimicrobial and anticancer activities. Bioorg. Chem. 2016, 69, 140. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; El-Metwaly, N.M.; Alzahrani, S.O.; Alkhatib, F.M.; Abualnaja, M.; El-Dabea, T.; El-Remaily, M.A.A. Synthesis and characterization of Fe (III), Pd (II) and Cu (II)-thiazole complexes; DFT, pharmacophore modeling, in-vitro assay and DNA binding studies. J. Mol. Liq. 2021, 326, 115277. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xu, Y. Modified asynchronous orthogonal sample design scheme @ Job’s method to determine the stoichiometric ratio in the molecular association system. J. Mol. Struct. 2020, 1206, 127757. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Basha, M.; Abdel-Mawgoud, A.A.H. Three novel Ni(II), VO(II) and Cr(III) mononuclear complexes encompassing potentially tridentate imine ligand: Synthesis, structural characterization, DNA interaction, antimicrobial evaluation and anticancer activity. Appl. Organomet. Chem. 2017, 31, 3750. [Google Scholar] [CrossRef]
- Abdel-Samad, H.; El-Jemni, M.A.; Abd El Rehim, S.S.; Hassan, H.H. Simply Prepared α-Ni(OH)2-Based Electrode for Efficient Electrocatalysis of EOR and OER. Electrochim. Acta 2024, 503, 144896. [Google Scholar] [CrossRef]
- Van Mourik, T.; Bühl, M.; Gaigeot, M.P. Density functional theory across chemistry, physics and biology. Philos. Trans. R. Soc. A 2014, 372, 20120488. [Google Scholar] [CrossRef]
- Alaghaz, A.N.M.; Zayed, M.E.; Alharbi, S.A.; Ammar, R.A.; Chinnathambi, A. Synthesis, spectroscopic identification, thermal, potentiometric and antibacterial activity studies of 4-amino-5-mercapto-S-triazole Schiff’s base complexes. J. Mol. Struct. 2015, 1087, 60–67. [Google Scholar] [CrossRef]
- Tassaoui, K.; Damej, M.; Molhi, A.; Berisha, A.; Errili, M.; Ksama, S.; Mehmeti, V.; El Hajjaji, S.; Benmessaoud, M. Contribution to the corrosion inhibition of Cu–30Ni copper–nickel alloy by 3-amino-1,2,4-triazole-5-thiol (ATT) in 3% NaCl solution. Experimental and theoretical study (DFT, MC and MD). Int. J. Corros. Scale Inhib. 2022, 11, 221–244. [Google Scholar]
- Abu-Dief, A.M.; El-Khatib, R.M.; Aljohani, F.S.; Alzahrani, S.O.; Mahran, A.; Khalifa, M.E.; El-Metwaly, N.M. Synthesis and intensive characterization for novel Zn (II), Pd (II), Cr (III) and VO (II)-Schiff base complexes; DNA-interaction, DFT, drug-likeness and molecular docking studies. J. Mol. Struct. 2021, 1242, 130693. [Google Scholar] [CrossRef]
- Hosny, S.; Shehata, M.R.; Aly, S.A.; Alsehli, A.H.; Salaheldeen, M.; Abu-Dief, A.M.; Abu-El-Wafa, S.M. Designing of novel nano-sized coordination compounds based on Spinacia oleracea extract: Synthesis, structural characterization, molecular docking, computational calculations, and biomedical applications. Inorg. Chem. Commun. 2024, 160, 111994. [Google Scholar] [CrossRef]
- Emara, A.A. Structural, spectral and biological studies of binuclear tetradentate metal complexes of N3O Schiff base ligand synthesized from 4, 6-diacetylresorcinol and diethylenetriamine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 117–125. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Pahontu, E.; Usataia, I.; Graur, V.; Chumakov, Y.; Petrenko, P.; Gudumac, V.; Gulea, A. Synthesis, characterization, crystal structure of novel Cu (II), Co (III), Fe (III) and Cr (III) complexes with 2-hydroxybenzaldehyde-4-allyl-S-methylisothiosemicarbazone: Antimicrobial, antioxidant and in vitro antiproliferative activity. Appl. Organomet. Chem. 2018, 32, e4544. [Google Scholar] [CrossRef]
- Singh, B.K.; Mishra, P.; Prakash, A.; Bhojak, N. Spectroscopic, electrochemical and biological studies of the metal complexes of the Schiff base derived from pyrrole-2-carbaldehyde and ethylenediamine. Arab. J. Chem. 2017, 10, S472–S483. [Google Scholar] [CrossRef]
- Li, D.; Cui, X.; Wen, X.; Jin, G.; Liu, J.; Zheng, W. Effects of graphene-based corrosion inhibition materials on the microstructure and corrosion resistance of Ni-based coating on Mg-Li alloys. Corros. Sci. 2024, 233, 112116. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Hamdan, S.K.; Seleem, A.A. Nano structure Iron (II) and Copper (II) Schiff base complexes of a NNO-tridentate ligand as new antibiotic agents: Spectral, thermal behaviors and DNA binding ability. Int. J. Nano Chem. 2015, 1, 65–77. [Google Scholar]
- Thakur, A.; Kaya, S.; Kumar, A. Recent trends in the characterization and application progress of nano-modified coatings in corrosion mitigation of metals and alloys. Appl. Sci. 2023, 13, 730. [Google Scholar] [CrossRef]
- Danaee, I.; Ghasemi, O.; Rashed, G.R.; Avei, M.R.; Maddahy, M.H. Effect of hydroxyl group position on adsorption behavior and corrosion inhibition of hydroxybenzaldehyde Schiff bases: Electrochemical and quantum calculations. J. Mol. Struct. 2013, 1035, 247–259. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Abbasov, V.M.; Aliyeva, L.I.; Khalaf, M.M. Novel naphthenate surfactants based on petroleum acids and nitrogenous bases as corrosion inhibitors for C1018-type mild steel in CO2-saturated brine. Egypt. J. Pet. 2015, 24, 175–182. [Google Scholar] [CrossRef]
- Victoria, S.N.; Prasad, R.; Manivannan, R. Psidium guajava leaf extract as green corrosion inhibitor for mild steel in phosphoric acid. Int. J. Electrochem. Sci. 2015, 10, 2220–2238. [Google Scholar] [CrossRef]
- Avci, G. Corrosion inhibition of indole-3-acetic acid on mild steel in 0.5 M HCl. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 730–736. [Google Scholar] [CrossRef]
- Belayachi, M.; Serrar, H.; Zarrok, H.; El Assyry, A.; Zarrouk, A.; Oudda, H.; Boukhris, S.; Hammouti, B.; Ebenso, E.E.; Geunbour, A. New pyrimidothiazine derivative as corrosion inhibitor for carbon steel in acidic media. Int. J. Electrochem. Sci. 2015, 10, 3010–3025. [Google Scholar]
- Al-Gorair, A.S.; Al-Habal, T.; El-Sayed, R.; Al-Juaid, S.S.; Hameed, R.A.; Abdallah, M. Investigations of non-ionic surfactants derived from triazoles and pyrroles as potent corrosion inhibitors for carbon steel in hydrochloric acid. Int. J. Electrochem. Sci. 2023, 18, 100269. [Google Scholar]
- Verma, C.; Thakur, A.; Ganjoo, R.; Sharma, S.; Assad, H.; Kumar, A.; Quraishi, M.A.; Alfantazi, A. Coordination bonding and corrosion inhibition potential of nitrogen-rich heterocycles: Azoles and triazines as specific examples. Coord. Chem. Rev. 2023, 488, 215177. [Google Scholar]
- El-Lateef, H.M.A. Corrosion inhibition characteristics of a novel salycilidene isatin hydrazine sodium sulfonate on carbon steel in HCl and a synergistic nickel ions additive: A combined experimental and theoretical perspective. Appl. Surf. Sci. 2020, 501, 144237. [Google Scholar] [CrossRef]
- Deyab, M.A.; Alghamdi, M.M.; El-Zahhar, A.A. Impact of some inorganic anions on the corrosion of nickel in a solution containing Na2SO4 and NaClO4. Sci. Rep. 2024, 14, 1853. [Google Scholar] [CrossRef]
- Noorbakhsh Nezhad, A.H.; Davoodi, A.; Mohammadi Zahrani, E.; Arefinia, R. The effects of an inorganic corrosion inhibitor on the electrochemical behavior of superhydrophobic micro-nano structured Ni films in 3.5% NaCl solution. Surf. Coat. Technol. 2020, 395, 125946. [Google Scholar] [CrossRef]
- Feng, Z.; She, X.; Peng, J.; Qiang, Y.; Zhang, S. Robust corrosion protection of Ni-thiolate coordination polymer/Mg(OH)2 coating on magnesium alloy AZ31. J. Mater. Res. Technol. 2023, 26, 2407–2418. [Google Scholar]
- Abd El-Lateef, H.M.; Elrouby, M.; Shilkamy, H.A.S. Influence of phytic acid on corrosion behavior and charge-discharge processes of Pb-2% In alloy anode for lead-acid battery applications. J. Mol. Struct. 2024, 1306, 137938. [Google Scholar]
- Goulart, C.M.; Esteves-Souza, A.; Martinez-Huitle, C.A.; Rodrigues, C.J.F.; Maciel, M.A.M.; Echevarria, A. Experimental and theoretical evaluation of semicarbazones and thiosemicarbazones as organic corrosion inhibitors. Corros. Sci. 2013, 67, 281–291. [Google Scholar]
- Fouda, A.E.A.S.; Nageeb, M.; Gaber, G.A.; Ahmed, A.S.; El-Hossiany, A.A.; Atia, M.F. Carob fruit extract as naturally products corrosion inhibitor for copper-nickel alloys in brine solutions. Sci. Rep. 2024, 14, 29290. [Google Scholar]
- Majidi, R.; Farhadi, A.; Danaee, I.; Panah, N.B.; Zarei, D.; Nikmanesh, S. Investigation of synthesized planar Cu-MOF and spherical Ni-MOF nanofillers for improving the anti-corrosion performance of epoxy coatings. Prog. Org. Coat. 2023, 183, 107803. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choi, S.R.; Ko, S.J.; Kim, J.G. Effect of benzotriazole on the prevention of electroless nickel–immersion gold treated copper corrosion. J. Phys. Chem. Solids 2023, 176, 111226. [Google Scholar] [CrossRef]
- Anadebe, V.C.; Chukwuike, V.I.; Nayak, K.C.; Ebenso, E.E.; Barik, R.C. Combined electrochemical, atomic scale-DFT and MD simulation of Nickel based metal organic framework (Ni-MOF) as corrosion inhibitor for X65 pipeline steel in CO2-saturated brine. Mater. Chem. Phys. 2024, 312, 128606. [Google Scholar] [CrossRef]
- Tassaoui, K.; Al-Shami, A.; Damej, M.; Molhi, A.; Mounkachi, O.; Benmessaoud, M. Contribution to the corrosion inhibitors of copper-nickel (Cu-30Ni) in 3% NaCl solution by two new molecules of triazole: Electrochemical and theoretical studies. J. Mol. Struct. 2023, 1291, 135836. [Google Scholar] [CrossRef]
- Abbasov, V.; El-Lateef, H.M.A.; Aliyeva, L.; Qasimov, E.; Ismayilov, I.; Khalaf, M.M. A study of the corrosion inhibition of mild steel C1018 in CO2-saturated brine using some novel surfactants based on corn oil. Egypt. J. Pet. 2013, 22, 451–470. [Google Scholar] [CrossRef]
- Ismayilov, I.T.; El-Lateef, H.M.A.; Abbasov, V.M.; Aliyeva, L.I.; Efremenko, E.N.; Mamedxanova, S.A. Adsorption and corrosion inhibitive properties of novel surfactants in the series of fatty acids based on palm oil on carbon steel in CO2-containing solution. Int. Res. J. Pure Appl. Chem. 2014, 4, 299–314. [Google Scholar] [CrossRef]
- Ituen, E.; Singh, A.; Yuanhua, L. Synthesis of bio-based nickel nanoparticles composite, characterization and corrosion in hibition in simulated oilfield microbial and acidizing environments. J. Adhes. Sci. Technol. 2021, 35, 15–34. [Google Scholar] [CrossRef]
- Li, J.; Lin, O.; Cheng, C.; Wang, W.; Xu, C.; Ren, L. Fabrication of a Ni/SiC composite coating on steel surface with excellent corrosion inhibition performance. J. Mater. Process. Technol. 2021, 290, 116987.59. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; Luo, J.L. Probing the corrosion resistance of a smart electroless Ni-P composite coating embedded with pH-responsive corrosion inhibitor-loaded nanocapsules. Chem. Eng. J. 2021, 421, 127752. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A. Carboxymethyl cellulose/silver nanoparticles composite: Synthesis, characterization and application as a benign corrosion inhibitor for St37 steel in 15% H2SO4 medium. ACS Appl. Mater. Interfaces 2017, 9, 6376–6389. [Google Scholar] [CrossRef]
- Hussin, M.H.; Kassim, M.J. Electrochemical, thermodynamic and adsorption studies of (+)-catechin hydrate as natural mild steel corrosion inhibitor in 1 M HCl. Int. J. Electrochem. Sci. 2011, 6, 1396–1414. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Ma, C.; Tang, Q.; Li, H.; Chen, S.; Shen, J. Corrosion inhibition of Ga-based thermal interface materials with Ni coating on Cu substrate. Surf. Coat. Technol. 2024, 493, 131286. [Google Scholar] [CrossRef]
- Ukaga, I.; Okafor, P.; Onyeachu, I.B.; Ikeuba, A.I.; Njoku, D.I. The inhibitive performance of 2, 3-pyrazine dicarboxylic acid and synergistic impact of KI during acid corrosion of 70/30 and 90/10 copper–nickel alloys. Mater. Chem. Phys. 2023, 296, 127313. [Google Scholar] [CrossRef]
- Dardeer, H.M.; Abbas, S.A.; Ghobashy, M.M.; Gaber, G.A.; Aly, M.F. Synthesis and characterization of novel chitosan-sodium pyruvate polymer and its derivatives for corrosion feature evaluation of Cu-Ni alloy. Inorg. Chem. Commun. 2023, 157, 111308. [Google Scholar] [CrossRef]
- Akhavan-Bahabadi, Z.; Zare, H.R.; Mohammadpour, Z.; Mirjalili, B.B.F. Improvement of Anti-Corrosion Performance of a Cu-Ni Bimetallic Organic Framework Smart Coating Using Amino-Substitution Triazole. ChemistrySelect 2023, 8, e202300363. [Google Scholar] [CrossRef]
- Ituen, E.; Singh, A.; Yuanhua, L. Inhibitive effect of onion mesocarp extract-nickel nanoparticles composite on simultaneous hydrogen production and pipework corrosion in 1 M HCl. Int. J. Hydrogen Energy 2020, 45, 10814–10825. [Google Scholar] [CrossRef]
- Zhu, M.; Yi, H.; Lu, J.; Huang, C.; Zhang, H.; Bo, P.; Huang, J. Corrosion of Ni–Fe based alloy in chloride molten salts for concentrating solar power containing aluminum as corrosion inhibitor. Sol. Energy Mater. Sol. Cells 2022, 241, 111737. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, R.; Yadav, A.; Kumari, R. Synthesis, characterization and influence of reduced Graphene Oxide (rGO) on the performance of mixed metal oxide nano-composite as optoelectronic material and corrosion inhibitor. Chem. Data Collect. 2020, 29, 100527. [Google Scholar] [CrossRef]
- Elrouby, M.; Shilkamy, H.A.E.S.; El-Sayed, A.E.R. Breakdown of passivation for zinc-antimony alloy in alkaline batteries verification; galvanostatic, impedance spectra, and charge-discharge techniques. Korean J. Chem. Eng. 2023, 40, 572–583. [Google Scholar] [CrossRef]
- Sivasubramanian, M.; Saravanan, R.R.; Manimehan, I.; Panneerselvam, K. Crystal structure analysis, Hirshfeld surface analysis, spectral investigations (FT-IR, FT-R), DFT calculations, ADMET studies and molecular docking of 3H-Methyl-1H-pyrazole-1-carboxamide (3MPC). J. Ind. Chem. Soc. 2022, 99, 100402. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Feizi-Dehnayebi, M.; Nafady, A.; Almalki, M.A.; Kassem, A.M.; Al-Ola, K.A.A.; Abdel-Rahman, L.H. Fabrication, structural inspection, stability studies in solution and DFT calculations of some novel complexes drived from 4-(Benzothiazol-2-yliminomethyl)-phenol ligand: Pharmaceutical applications supported by molecular docking approach. J. Mol. Struct. 2025, 1328, 141284. [Google Scholar]
- Nirmala, T.; Kumar, M.; Swarnamughi, P.; Manikandan, P.; Geetha, E.; Manikandan, A.; Muthu, S. Spectroscopic, Molecular Structure, Electronic Topology Surface, and Pharmaceutical Investigation of 1-[(4-Chlorophenyl)Methyl]-1H-Indole-3-Carboxaldehyde by Quantum Computation—Prediction of Antitumor Activity. Chem. Phys. Impact 2024, 8, 100513. [Google Scholar]
- Hashim, K.M.; Manoj, E.; Kurup, M.P. A Novel Manganese (II) Bisthiocarbohydrazone Complex: Crystal Structures, Hirshfeld Surface Analysis, DFT and Molecular Docking Study with SARS-CoV-2. J. Mol. Struct. 2021, 1246, 131125. [Google Scholar] [PubMed]
- Mohammadi Ziarani, G.; Ebrahimi, D.; Feizi-Dehnayebi, M.; Badiei, A.; Abu-Dief, A.M. Tailored Silica-Based Sensors (SBA-Pr-Ald-MA) for Efficient Detection of Iron (III) Ions: A Comprehensive Theoretical and Experimental Viewpoint. Appl. Organomet. Chem. 2025, 39, e7917. [Google Scholar]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density functional theory of electronic structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar]
- Panahande, Z.; Mohammadi Ziarani, G.; Feizi-Dehnayebi, M.; Badiei, A.; Abu-Dief, A.M. Synthesis and DFT Calculation of Hg2+ Fluorescence Chemosensor Based on a New Hybrid Organic–Inorganic Nanoporous Material of SBA-Pr-Is-Hy. Appl. Organomet. Chem. 2025, 39, e7818. [Google Scholar]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Oguzie, E.E.; Adindu, C.B.; Enenebeaku, C.K.; Ogukwe, C.E.; Chidiebere, M.A.; Oguzie, K.L. Natural products for materials protection: Mechanism of corrosion inhibition of mild steel by acid extracts of Piper guineense. J. Phys. Chem. C 2012, 116, 13603–13615. [Google Scholar]
- Kumar, D.; Jain, V.; Rai, B. Imidazole Derivatives as Corrosion Inhibitors for Copper: A DFT and Reactive Force Field Study. Corros. Sci. 2020, 171, 108724. [Google Scholar] [CrossRef]
- Abdou, A. Synthesis, structural, molecular docking, DFT, vibrational spectroscopy, HOMO-LUMO, MEP exploration, antibacterial and antifungal activity of new Fe (III), Co (II) and Ni (II) hetero-ligand complexes. J. Mol. Struct. 2022, 1262, 132911. [Google Scholar]
- Lai, Z.; Wang, S.; Wang, C.; Hong, Y.; Zhou, G.; Chen, Y.; He, W.; Peng, Y.; Xiao, D. A Comparison of Typical Additives for Copper Electroplating Based on Theoretical Computation. Comput. Mater. Sci. 2018, 147, 95–102. [Google Scholar]
- Bourzi, H.; Oukhrib, R.; El Ibrahimi, B.; Oualid, H.A.; Abdellaoui, Y.; Balkard, B.; Hilali, M.; El Issami, S. Understanding of Anti-Corrosive Behavior of Some Tetrazole Derivatives in Acidic Medium: Adsorption on Cu (111) Surface Using Quantum Chemical Calculations and Monte Carlo Simulations. Surf. Sci. 2020, 702, 121692. [Google Scholar]
- Aayisha, S.; Renuga Devi, T.S.; Janani, S.; Muthu, S.; Raja, M.; Sevvanthi, S.; Raajaraman, B.R. Vibrational and computational analysis for molecular structure properties of N-(2-(trifluoromethyl) phenyl) acetamide: Density functional theory approach. Spectrosc. Lett. 2019, 52, 563–576. [Google Scholar]





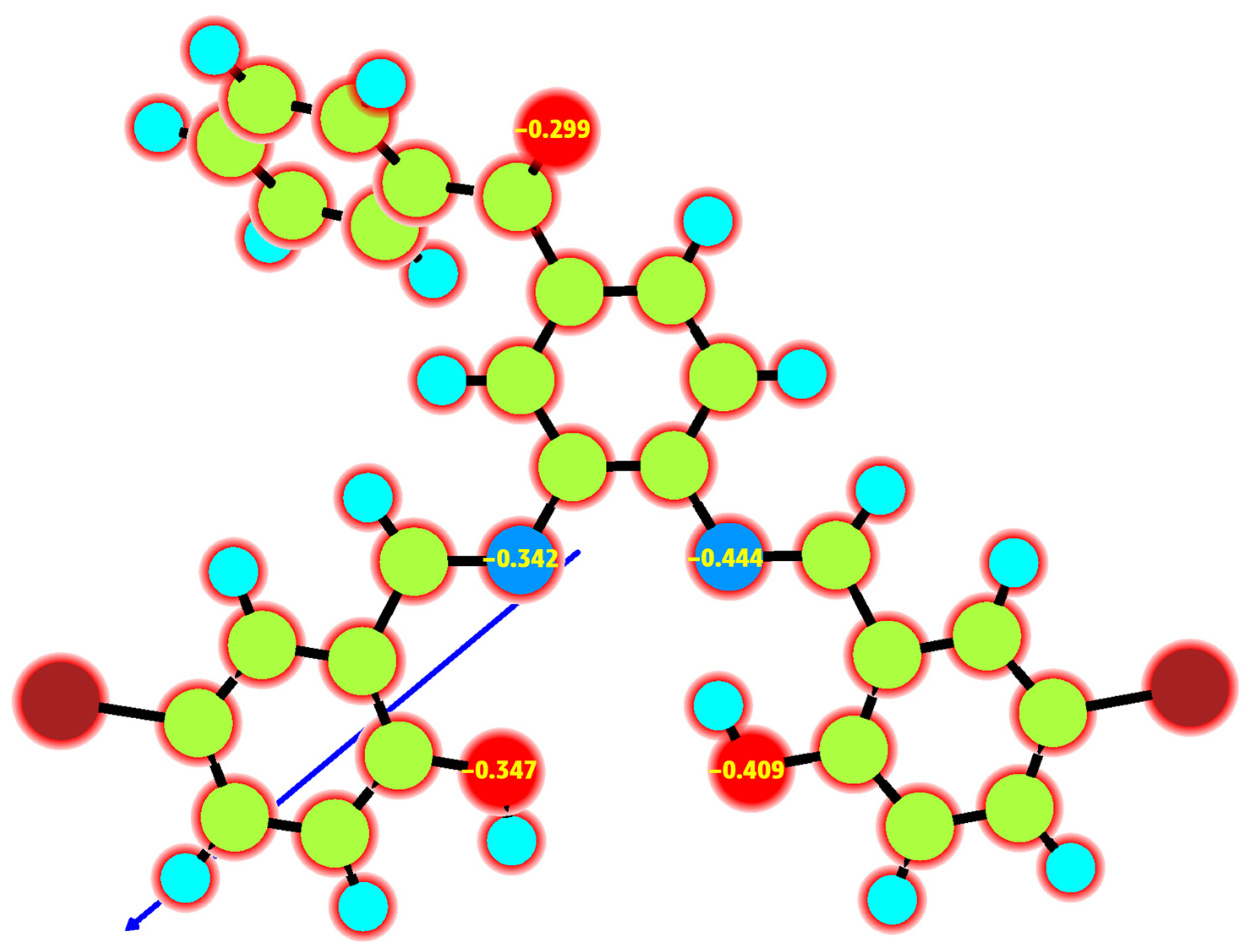

| Compound (Molecular Formula) | M. Weight | Yield | Color | Molar Conductance | μeff (BM) | M.p. and Dec.temp. (°C) | Analysis (%) Found (Calcd) | ||
|---|---|---|---|---|---|---|---|---|---|
| ᴧm, Ω−1 cm2 mol−1 | C | H | N | ||||||
| L C27H18Br2N2O3 | (578.25) | 95% | yellow | - | - | 210 | 56.15 (56.03) | 3.18 (3.11) | 4.76 (4.84) |
| LFe C27H26Br2FeN3O11 | (784.13) | 91% | bright brown | 10.75 | 5.52 | >300 | 41.28 (41.35) | 3.25 (3.34) | 5.41 (5.36) |
| Cinh. | Ecorr (V) | Icorr (µA cm−2) | βa (V/Decade) | βc (V/Decade) | Θ | η% |
|---|---|---|---|---|---|---|
| In the presence of L | ||||||
| Blank | −0.228 | 616.6 | 19.5 | −4.38 | - | - |
| 1 × 10−5 M | −0.377 | 371.5 | 23.3 | −4.53 | 0.397 | 39.7 |
| 5 × 10−5 M | −0.396 | 223.8 | 26.2 | −9.5 | 0.637 | 63.7 |
| 1 × 10−4 M | −0.394 | 125.3 | 31.1 | −5.3 | 0.796 | 79.6 |
| 5 × 10−4 M | −0.408 | 64.5 | 33.6 | −6.2 | 0.896 | 89.6 |
| 1 × 10−3 M | −0.408 | 58.3 | 33.4 | −4.75 | 0.905 | 90.5 |
| In the presence of FeL | ||||||
| 1 × 10−5 M | −0.387 | 338.3 | 33.4 | −4.6 | 0.451 | 45.1 |
| 5 × 10−5 M | −0.384 | 186.4 | 34.5 | −5.3 | 0.698 | 69.8 |
| 1 × 10−4 M | −0.370 | 93.0 | 33.1 | −5.1 | 0.849 | 84.9 |
| 5 × 10−4 M | −0.358 | 60.2 | 33.5 | −5.3 | 0.902 | 90.2 |
| 1 × 10−3 M | −0.344 | 44.67 | 29.3 | −4.3 | 0.927 | 92.7 |
| In the presence of L | |||
|---|---|---|---|
| Cinh. | Rs Ω | Rct Ω | Cdl F |
| Blank | 1.0 | 13.8 | 1.15 × 10−6 |
| 1 × 10−5 M | 1.0 | 17.0 | 9.36 × 10—7 |
| 5 × 10−5 M | 1.0 | 19.5 | 8.16 × 10−7 |
| 1 × 10−4 M | 1.0 | 24.6 | 6.46 × 10−7 |
| 5 × 10−4 M | 5.0 | 39.6 | 4.019 × 10−7 |
| 1 × 10−3 M | 5.0 | 43.7 | 3.6 × 10−7 |
| In the presence of FeL | |||
| 1 × 10−5 M | 1.0 | 25.4 | 6.3 × 10−7 |
| 5 × 10−5 M | 1.0 | 34.1 | 4.66 × 10−7 |
| 1 × 10−4 M | 3.0 | 35.2 | 4.52 × 10−7 |
| 5 × 10−4 M | 3.0 | 41.6 | 3.82 × 10−7 |
| 1 × 10−3 M | 1.0 | 46.5 | 3.42 × 10−7 |
| With the presence of 1 × 10−5 L | ||||||
|---|---|---|---|---|---|---|
| Temp. | Ecorr (V) | Icorr (µA cm−2) | βa (V/decade) | βc (V/decade) | Θ | η% |
| 25 °C | −0.377 | 371.5 | 23.3 | −4.53 | 0.397 | 39.7 |
| 35 °C | −0.388 | 776.3 | 17.01 | −4.14 | 0.354 | 35.4 |
| 45 °C | −0.391 | 1506.6 | 18.42 | −4.34 | 0.575 | 57.5 |
| 55 °C | −0.389 | 3548.13 | 20.87 | −4.47 | 0.521 | 52.1 |
| With the presence of 5 × 10−5 L | ||||||
| Temp. | Ecorr (V) | Icorr (µA cm−2) | βa (V/decade) | βc (V/decade) | Θ | η% |
| 25 °C | −0.396 | 223.8 | 26.2 | −9.5 | 0.637 | 63.7 |
| 35 °C | −0.418 | 302 | 13.8 | −5.6 | 0.75 | 75.0 |
| 45 °C | −0.415 | 432.5 | 28.2 | −5.5 | 0.878 | 87.8 |
| 55 °C | −0.427 | 1738 | 23.3 | −4.9 | 0.765 | 76.5 |
| With the presence of 1 × 10−4 L | ||||||
| Temp. | Ecorr (V) | Icorr (µA cm−2) | βa (V/decade) | βc (V/decade) | Θ | η% |
| 25 °C | −0.394 | 125.3 | 31.1 | −5.3 | 0.796 | 79.6 |
| 35 °C | −0.393 | 281.8 | 25.9 | −5.46 | 0.766 | 76.6 |
| 45 °C | −0.391 | 1071.6 | 20.81 | −4.83 | 0.698 | 69.8 |
| 55 °C | −0.388 | 1513.6 | 21.1 | −5.22 | 0.795 | 79.5 |
| With the presence of 1 × 10−3 L | ||||||
| Temp. | Ecorr (V) | Icorr (µA cm−2) | βa (V/decade) | βc (V/decade) | Θ | η% |
| 25 °C | −0.408 | 58.3 | 33.4 | −4.75 | 0.906 | 90.6 |
| 35 °C | −0.392 | 154.8 | 30.1 | −5.6 | 0.872 | 87.2 |
| 45 °C | −0.387 | 301.9 | 32.2 | −4.7 | 0.914 | 91.4 |
| 55 °C | −0.389 | 703.1 | 32.1 | −5.2 | 0.905 | 90.5 |
| With the presence of 1 × 10−5 FeL | ||||||
|---|---|---|---|---|---|---|
| Temp. | Ecorr (V) | Icorr (µA cm−2) | βa (V/decade) | βc (V/decade) | Θ | η% |
| 25 °C | −0.387 | 338.3 | 33.4 | −4.6 | 0.451 | 45.1 |
| 35 °C | −0.385 | 981.7 | 29.5 | −4.8 | 0.184 | 18.4 |
| 45 °C | −0.384 | 1161.4 | 27.6 | −4.65 | 0.673 | 67.3 |
| 55 °C | −0.389 | 1663.4 | 27.9 | −4.9 | 0.775 | 77.5 |
| With the presence of 5 × 10−5 FeL | ||||||
| Temp. | Ecorr (V) | Icorr (µA cm−2) | βa (V/decade) | βc (V/decade) | Θ | η% |
| 25 °C | −0.384 | 186.4 | 34.5 | −5.3 | 0.698 | 69.8 |
| 35 °C | −0.413 | 407.4 | 28.1 | −5.3 | 0.66 | 66.1 |
| 45 °C | −0.418 | 783.4 | 25.3 | −5.2 | 0.78 | 78.0 |
| 55 °C | −0.414 | 1148.2 | 26.1 | −6.6 | 0.844 | 84.4 |
| With the presence of 1 × 10−4 FeL | ||||||
| Temp. | Ecorr (V) | Icorr (µA cm−2) | βa (V/decade) | βc (V/decade) | Θ | η% |
| 25 °C | −0.370 | 93.0 | 33.1 | −5.1 | 0.85 | 85.0 |
| 35 °C | −0.363 | 301.9 | 27.1 | −6.3 | 0.748 | 74.8 |
| 45 °C | −0.363 | 297.2 | 28.2 | −6.4 | 0.916 | 91.6 |
| 55 °C | −0.366 | 436.5 | 28.6 | −6.3 | 0.942 | 94.2 |
| With the presence of 1 × 10−3 FeL | ||||||
| Temp. | Ecorr (V) | Icorr (µA cm−2) | βa (V/decade) | βc (V/decade) | Θ | η% |
| 25 °C | −0.344 | 44.67 | 29.3 | −4.3 | 0.927 | 92.7 |
| 35 °C | −0.340 | 89.2 | 28.2 | −4.5 | 0.925 | 92.5 |
| 45 °C | −0.354 | 91.8 | 28.1 | −4.9 | 0.974 | 97.4 |
| 55 °C | −0.348 | 138.1 | 28.8 | −3.77 | 0.981 | 98.1 |
| Temperature | Kads M−1 (L) | Kads M−1 (FeL) | −∆Goads. kJ·mol−1 (L) | −∆Goads. kJ·mol−1 (FeL) |
|---|---|---|---|---|
| 298 | 4.4 × 104 | 5.9 × 103 | 36.44 | 31.4 |
| 308 | 2.7 × 104 | 6.6 × 103 | 36.45 | 32.8 |
| 318 | 2.2 × 104 | 9.02 × 103 | 37.11 | 34.6 |
| 328 | 1.4 × 104 | 1.06 × 104 | 37.05 | 36.3 |
| L | |||
|---|---|---|---|
| Element | Line | Mass % | Atom % |
| N | K | 6.22 ± 0.10 | 9.73 ± 0.15 |
| O | K | 32.01 ± 0.16 | 37.92 ± 0.22 |
| C | K | 3.16 ± 0.01 | 2.06 ± 0.03 |
| S | K | 8.21 ± 0.04 | 1.92 ± 0.03 |
| Ni | K | 50.40 ± 0.11 | 48.37 ± 0.09 |
| Total | 100.00 | 100.00 | |
| FeL | |||
| O | K | 9.66 ± 0.07 | 15.73 ± 0.21 |
| C | K | 3.20 ± 0.01 | 3.05 ± 0.01 |
| N | K | 5.36 ± 0.02 | 7.2 ± 0.01 |
| S | K | 3.53 ± 0.03 | 1.23 ± 0.04 |
| Ni | K | 77.09 ± 0.11 | 71.73 ± 0.07 |
| Fe | K | 1.16 ± 0.01 | 1.06 ± 0.00 |
| Total | 100.00 | 100.00 | |
| Descriptor | L | FeL |
|---|---|---|
| EHOMO | −6.09 | −6.08 |
| ELUMO | −2.92 | −3.69 |
| ΔE(LUMO-HOMO) | 3.17 | 2.39 |
| χ | 4.50 | 4.88 |
| η | 1.58 | 1.19 |
| σ | 0.63 | 0.84 |
| Pi | −4.50 | −4.88 |
| ω | 6.41 | 10.00 |
| ΔNmax | 2.85 | 4.10 |
| In 0.5 M H2SO4 | |||
|---|---|---|---|
| Blank | |||
| Applied current density | tdis. | ΔV | S.C. (mA. hr−1) |
| 1 | 9.15 | 0.037 | 0.068 |
| 3 | 9.7 | 0.104 | 0.077 |
| 5 | 10.1 | 0.154 | 0.091 |
| 10 | 11.1 | 0.261 | 0.118 |
| 15 | 11.0 | 0.344 | 0.133 |
| 20 | 11.6 | 0.412 | 0.156 |
| 30 | 11.6 | 0.531 | 0.182 |
| In the presnce of 1 × 10−5 M of L | |||
| Applied current density | tdis. | ΔV | S.C. |
| 1 | 8.5 | 0.034 | 0.069 |
| 3 | 8.8 | 0.107 | 0.685 |
| 5 | 9.2 | 0.184 | 0.069 |
| 10 | 9.6 | 0.313 | 0.085 |
| 15 | 10.4 | 0.414 | 0.105 |
| 20 | 11.3 | 0.51 | 0.123 |
| 30 | 11.8 | 0.668 | 0.147 |
| In the presence of 5 × 10−5 M of L | |||
| Applied current density | tdis. | ΔV | S.C. |
| 1 | 9.0 | 0.035 | 0.714 |
| 3 | 9.2 | 0.119 | 0.064 |
| 5 | 10.2 | 0.177 | 0.08 |
| 10 | 10.6 | 0.28 | 0.105 |
| 15 | 11.2 | 0.353 | 0.132 |
| 20 | 11.3 | 0.148 | 0.422 |
| 30 | 11.4 | 0.174 | 0.544 |
| In the presence of 1 × 10−4 M of L | |||
| Applied current density | tdis. | ΔV | S.C. |
| 1 | 10.48 | 0.046 | 0.063 |
| 3 | 10.8 | 0.129 | 0.069 |
| 5 | 11.1 | 0.281 | 0.054 |
| 10 | 11.6 | 0.349 | 0.092 |
| 15 | 12.0 | 0.349 | 0.14 |
| 20 | 12.8 | 0.42 | 0.17 |
| 30 | 12.8 | 0.543 | 0.197 |
| In the presence of 5 × 10−4 M of L | |||
| Applied current density | tdis. | ΔV | S.C. |
| 1 | 9.8 | 0.079 | 0.0344 |
| 3 | 10.7 | 0.197 | 0.045 |
| 5 | 11.75 | 0.265 | 0.062 |
| 10 | 12.4 | 0.373 | 0.092 |
| 15 | 12.7 | 0.467 | 0.113 |
| 20 | 13.1 | 0.569 | 0.128 |
| 30 | 13.3 | 0.736 | 0.151 |
| In the presence of 1 × 10−3 M of L | |||
| Applied current density | tdis. | ΔV | S.C. |
| 1 | 9.8 | 0.096 | 0.028 |
| 3 | 11.5 | 0.22 | 0.043 |
| 5 | 11.7 | 0.28 | 0.568 |
| 10 | 12.0 | 0.42 | 0.079 |
| 15 | 12.6 | 0.532 | 0.098 |
| 20 | 13.0 | 0.639 | 0.113 |
| 30 | 13.2 | 0.822 | 0.134 |
| In the presence of FeL | |||
| In the existence of 1 × 10−5 M of FeL | |||
| Applied current density | tdis. | ΔV | S.C. |
| 1 | 10.6 | 0.074 | 0.039 |
| 3 | 11.0 | 0.194 | 0.047 |
| 5 | 12.0 | 0.266 | 0.063 |
| 10 | 12.3 | 0.383 | 0.089 |
| 15 | 12.8 | 0.475 | 0.112 |
| 20 | 12.9 | 0.549 | 0.131 |
| 30 | 12.9 | 0.690 | 0.155 |
| In the presence of 5 × 10−5 M of FeL | |||
| Applied current density | tdis. | ΔV | S.C. |
| 1 | 9.0 | 0.055 | 0.045 |
| 3 | 10.0 | 0.164 | 0.051 |
| 5 | 10.7 | 0.228 | 0.065 |
| 10 | 10.9 | 0.342 | 0.088 |
| 15 | 12.0 | 0.403 | 0.124 |
| 20 | 12.5 | 0.481 | 0.144 |
| 30 | 12.8 | 0.593 | 0.179 |
| In the presence of 1 × 10−4 M of FeL | |||
| Applied current density | tdis. | ΔV | S.C. |
| 1 | 9.8 | 0.079 | 0.034 |
| 3 | 11.0 | 0.187 | 0.049 |
| 5 | 11.1 | 0.265 | 0.058 |
| 10 | 12.1 | 0.388 | 0.086 |
| 15 | 12.6 | 0.433 | 0.121 |
| 20 | 12.8 | 0.644 | 0.110 |
| 30 | 13.1 | 0.644 | 0.170 |
| In the presence of 5 × 10−4 M of FeL | |||
| Applied current density | tdis. | ΔV | S.C. |
| 1 | 8.7 | 0.078 | 0.031 |
| 3 | 9.9 | 0.213 | 0.038 |
| 5 | 10.5 | 0.29 | 0.05 |
| 10 | 11.1 | 0.419 | 0.073 |
| 15 | 11.4 | 0.521 | 0.091 |
| 20 | 11.9 | 0.651 | 0.102 |
| 30 | 12.8 | 0.807 | 0.132 |
| In the presence of 1 × 10−3 M of FeL | |||
| Applied current density | tdis. | ΔV | S.C. |
| 1 | 9.82 | 0.073 | 0.037 |
| 3 | 10.5 | 0.206 | 0.042 |
| 5 | 11.1 | 0.299 | 0.052 |
| 10 | 11.7 | 0.465 | 0.698 |
| 15 | 12.0 | 0.596 | 0.084 |
| 20 | 12.6 | 0.303 | 0.231 |
| 30 | 13.3 | 0.690 | 0.161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilkamy, H.A.E.-S.; Salaheldeen, M.; Zhukov, A.; El-Kasaby, R.A.; Feizi-Dehnayebi, M.; Alharas, M.M.A.; Abo-Dief, H.M.; El-Khatib, R.M.; Abu-Dief, A.M. Enhanced Corrosion Protection as a Sustainable Approach for Nickel Using Novel FeL Salen Complex: Electrochemical Investigation and DFT Insights. Metals 2025, 15, 403. https://doi.org/10.3390/met15040403
Shilkamy HAE-S, Salaheldeen M, Zhukov A, El-Kasaby RA, Feizi-Dehnayebi M, Alharas MMA, Abo-Dief HM, El-Khatib RM, Abu-Dief AM. Enhanced Corrosion Protection as a Sustainable Approach for Nickel Using Novel FeL Salen Complex: Electrochemical Investigation and DFT Insights. Metals. 2025; 15(4):403. https://doi.org/10.3390/met15040403
Chicago/Turabian StyleShilkamy, Hoda Abd El-Shafy, Mohamed Salaheldeen, Arcady Zhukov, R. A. El-Kasaby, Mehran Feizi-Dehnayebi, Mona M. A. Alharas, Hala M. Abo-Dief, Rafat M. El-Khatib, and Ahmed M. Abu-Dief. 2025. "Enhanced Corrosion Protection as a Sustainable Approach for Nickel Using Novel FeL Salen Complex: Electrochemical Investigation and DFT Insights" Metals 15, no. 4: 403. https://doi.org/10.3390/met15040403
APA StyleShilkamy, H. A. E.-S., Salaheldeen, M., Zhukov, A., El-Kasaby, R. A., Feizi-Dehnayebi, M., Alharas, M. M. A., Abo-Dief, H. M., El-Khatib, R. M., & Abu-Dief, A. M. (2025). Enhanced Corrosion Protection as a Sustainable Approach for Nickel Using Novel FeL Salen Complex: Electrochemical Investigation and DFT Insights. Metals, 15(4), 403. https://doi.org/10.3390/met15040403










