Corrosion Behavior of Al-Mg Alloys with Different Alloying Element Contents in 3.5% NaCl Solution
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Electrochemical Measurements
2.3. Precipitate and Surface Analyses
3. Results
3.1. Intermetallic Phases
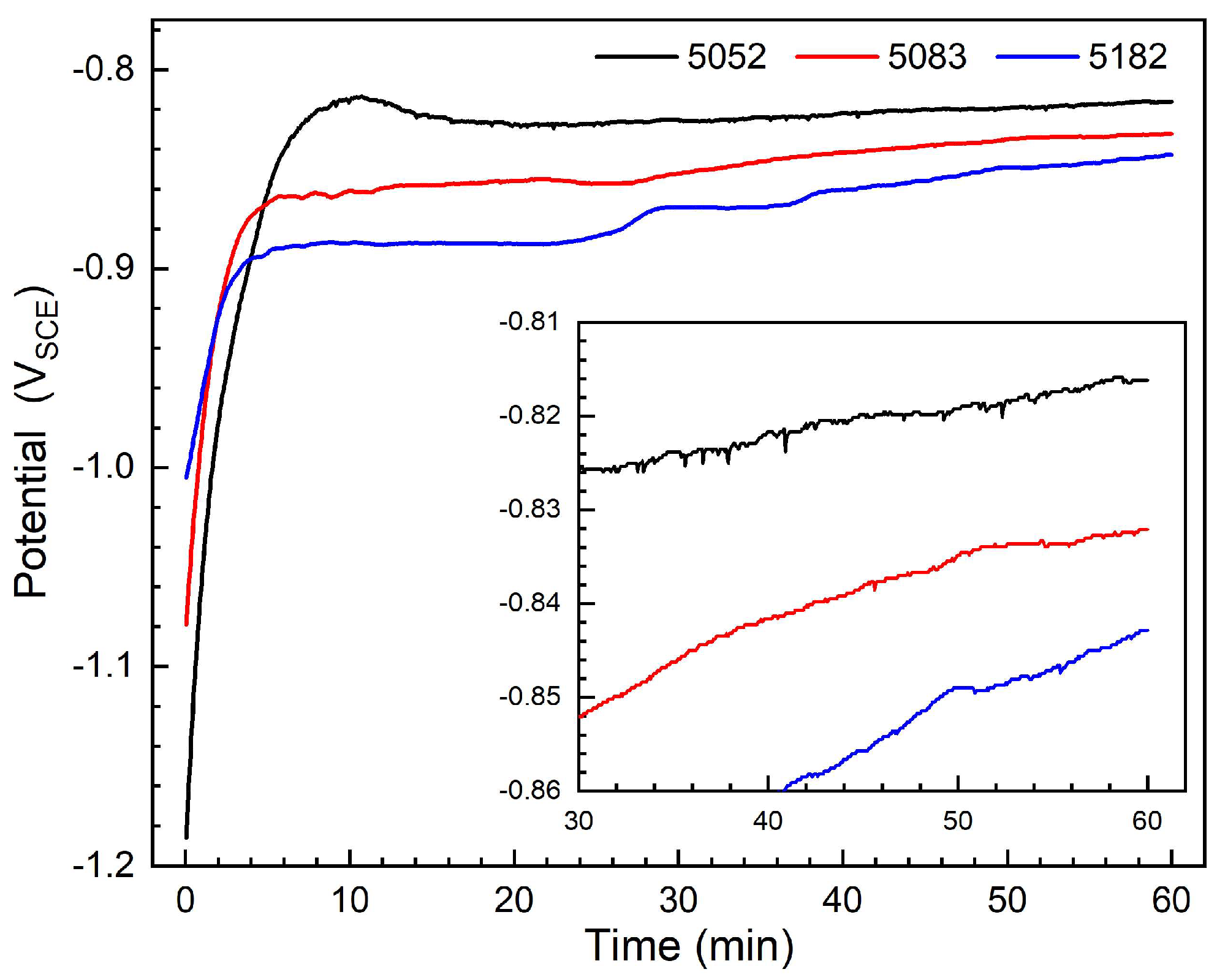
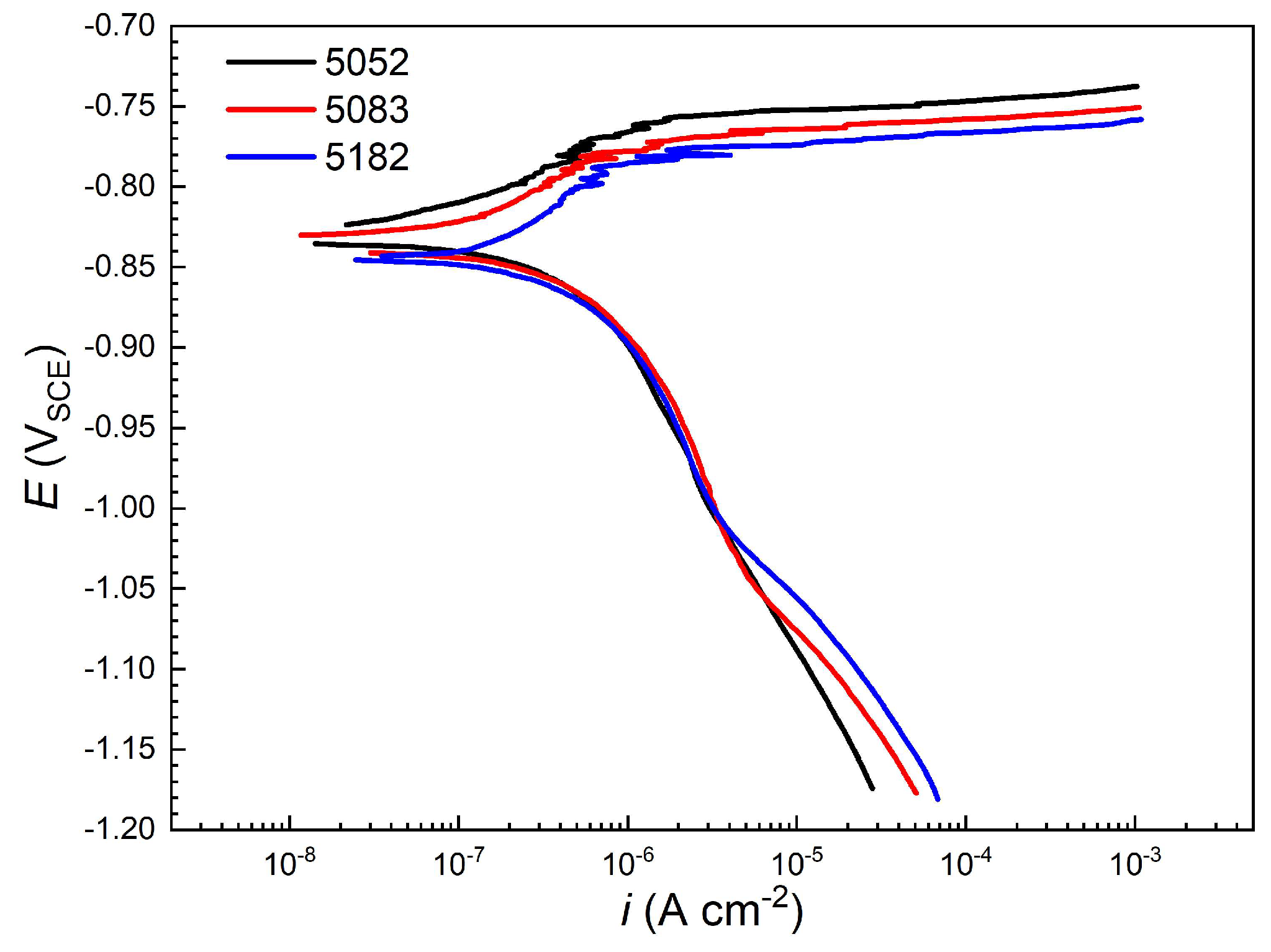
3.2. Potentiodynamic Polarization Characteristics
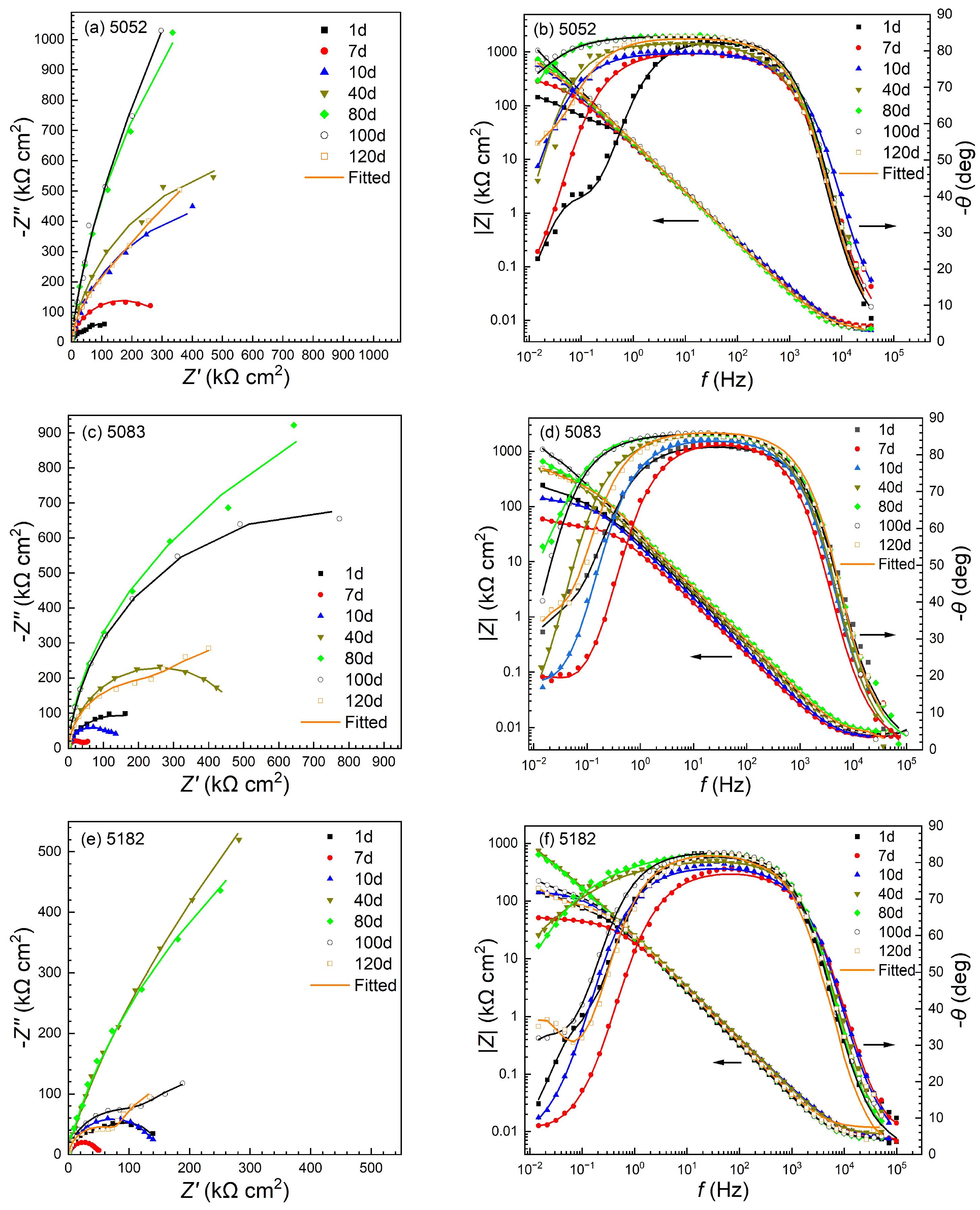
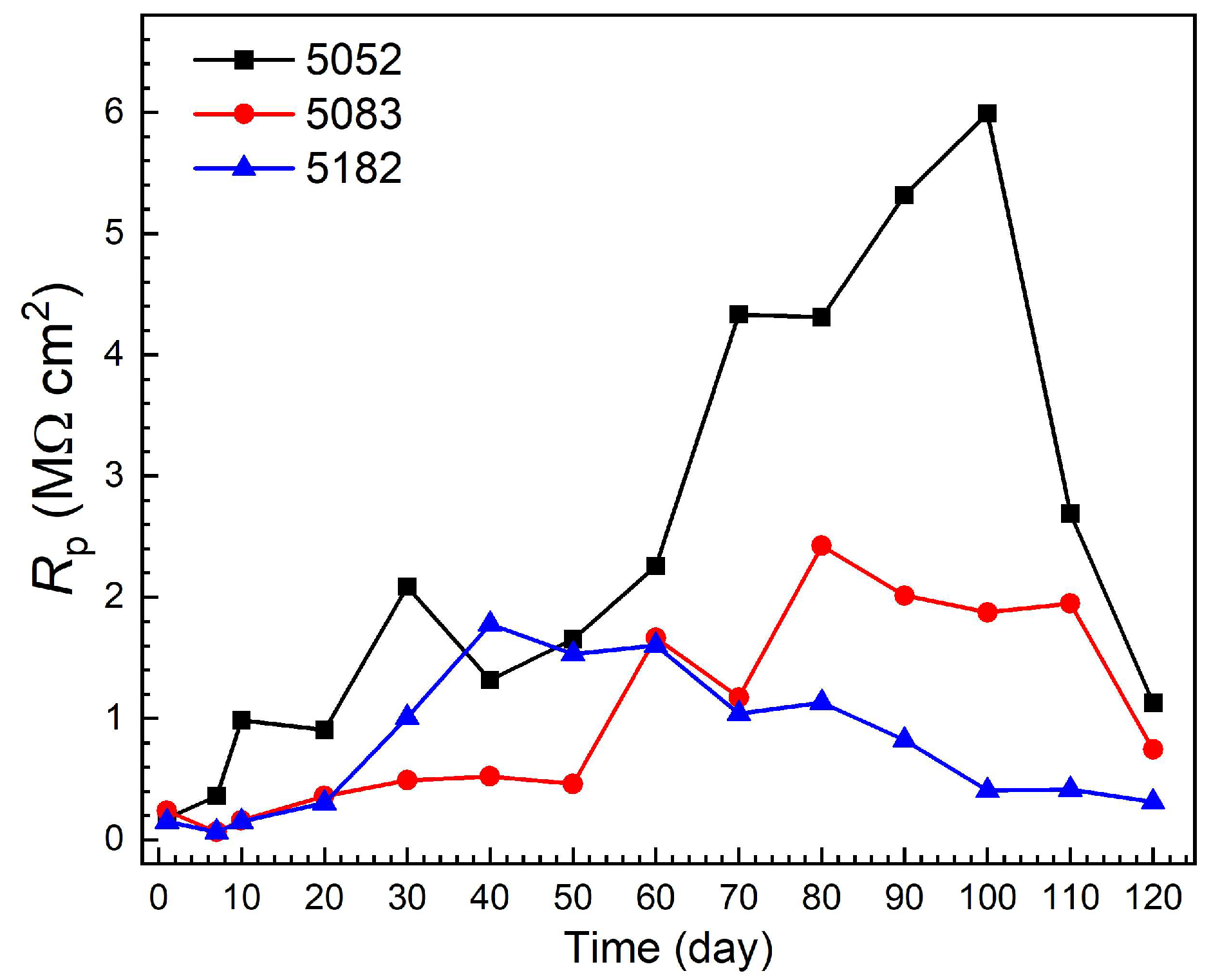
3.3. Impedance Evolution in the Corrosion Process
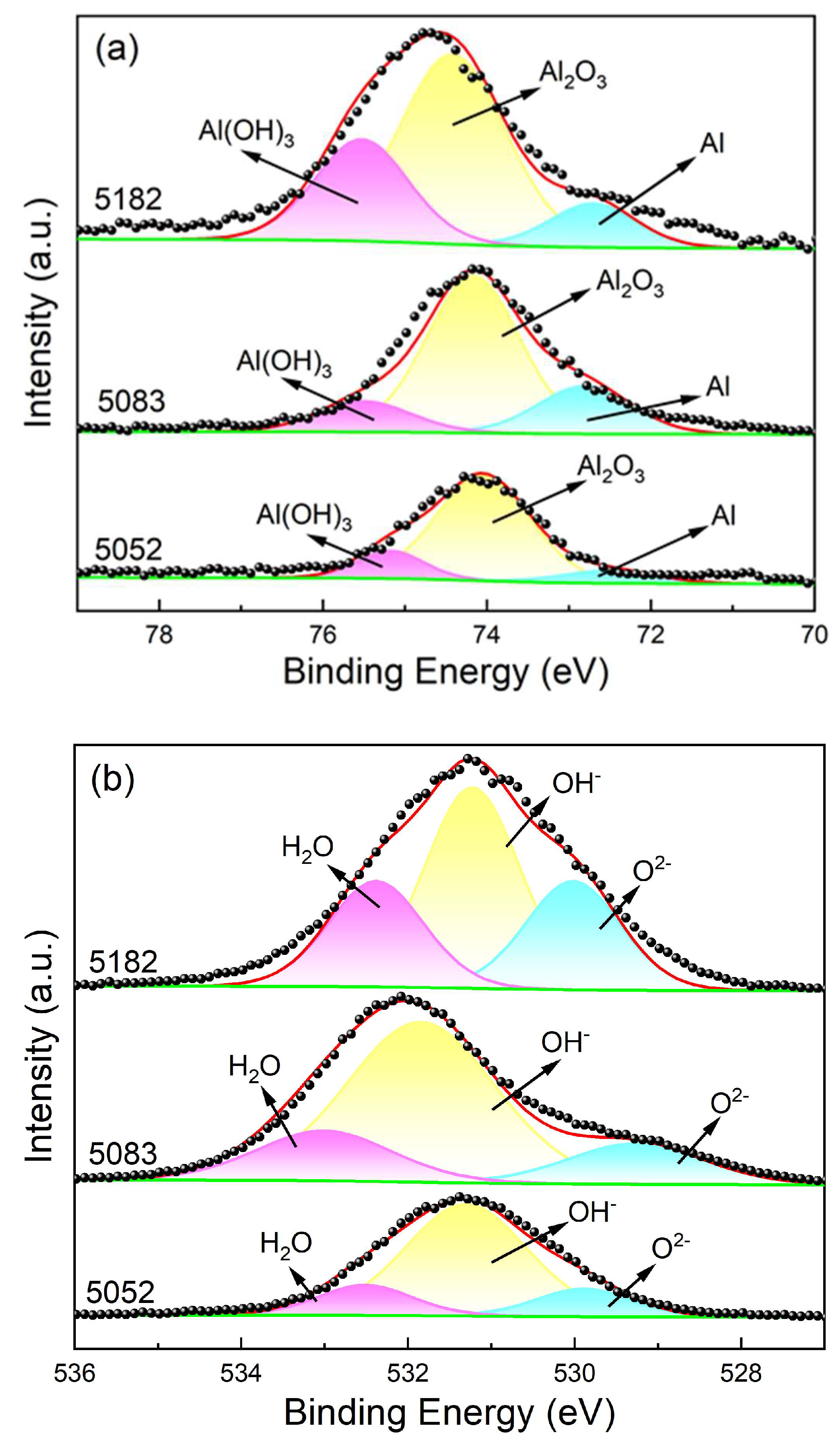
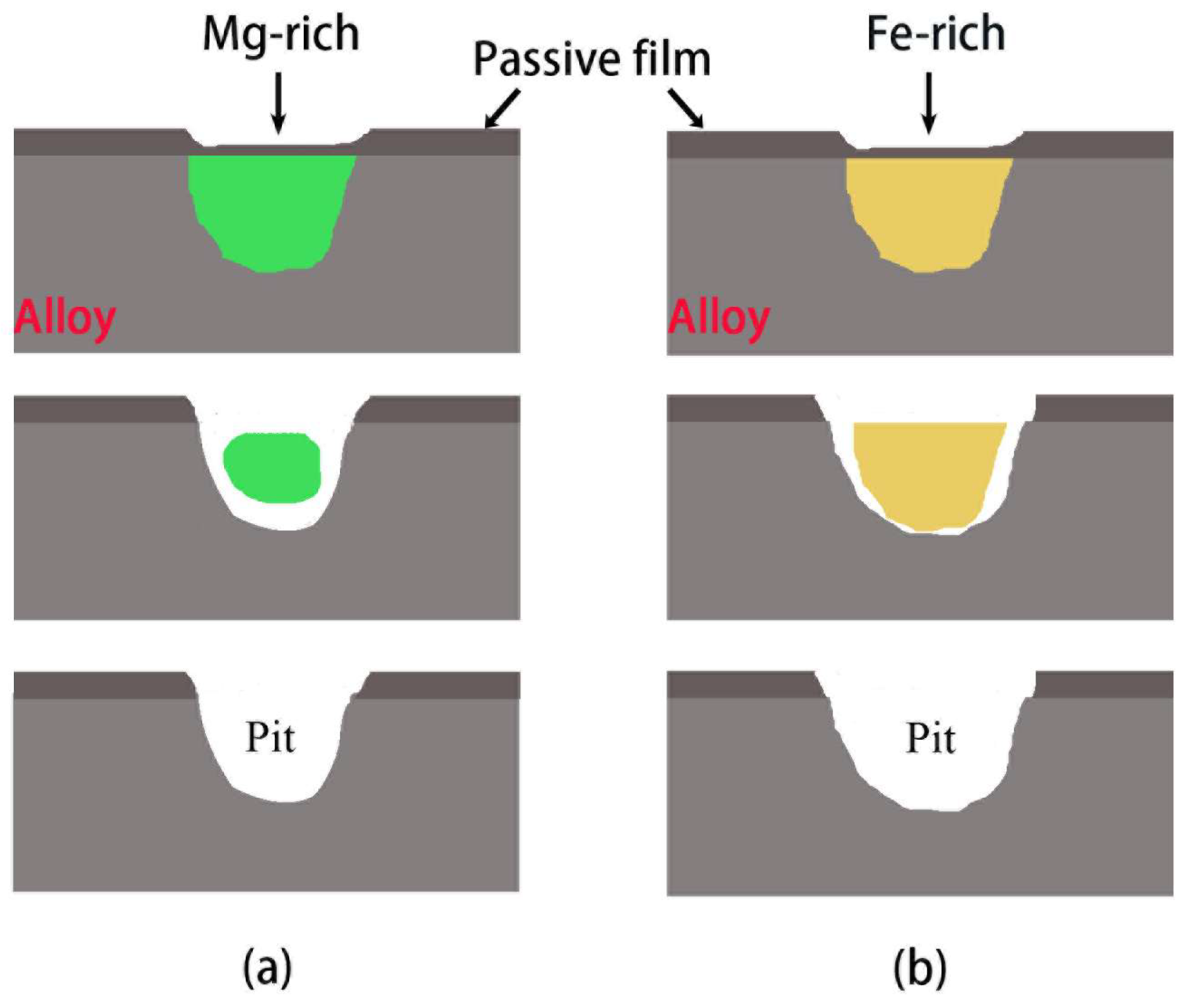
3.4. Surface Morphologies and Corrosion Products
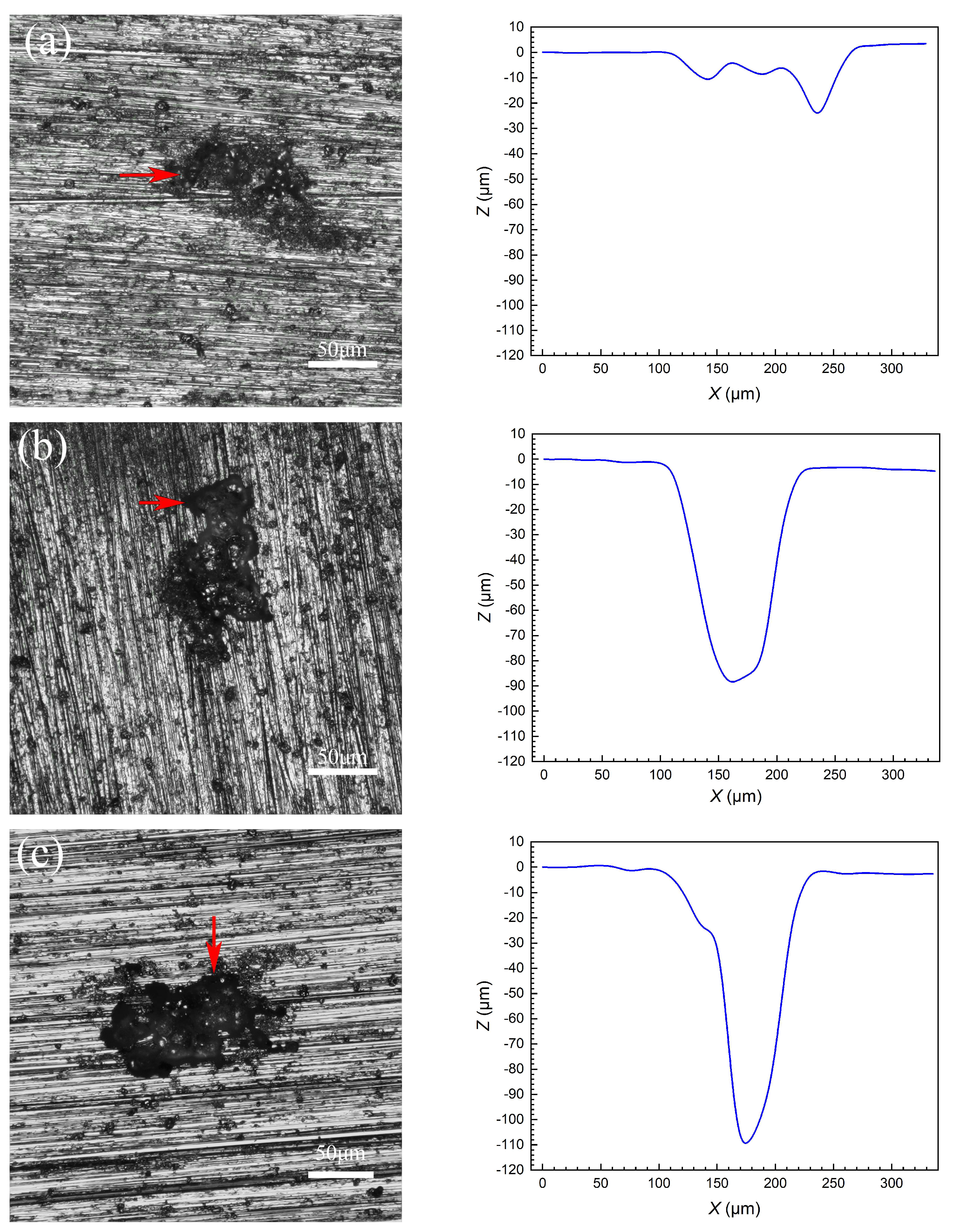
3.5. Pit Depth
4. Discussion
4.1. Interpretation for EIS Spectra
4.2. Effect of Alloying Elements on Corrosion
5. Conclusions
- (1)
- From 5052 alloy to 5083 and 5182 alloys, due to the increase in Mg content and the decrease in Cr content, the passive current density enlarges, while the pitting potential decreases slightly, indicating the slight increase in pitting susceptibility. However, the pitting potential differences are very small, less than about 22 mV.
- (2)
- The Rp values of the three alloys firstly tend to enlarge with the immersion time, and then start to reduce after about 40, 80 and 100 days for 5182, 5083 and 5052 alloys, respectively, with the occurrence of stable pitting corrosion. After 120 days of immersion, the corrosion resistance in the stable pits increases in the order of 5182 alloy < 5083 alloy < 5052 alloy, while the pit depth is exactly the opposite. But the pit depths of the three alloys are relatively small, approximately less than 110 μm.
- (3)
- The corrosion product films on the three alloy surfaces are mainly composed of Al2O3 and Al(OH)3, but the ratio of Al2O3/Al(OH)3 increases in the order of 5182 alloy < 5083 alloy < 5052 alloy. The intermetallic phases are the weak positions for the occurrence of pitting corrosion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Moubaraki, A.H.; Al-Rushud, H.H. The red sea as a corrosive environment: Corrosion rates and corrosion mechanism of aluminum alloys 7075, 2024, and 6061. Int. J. Corros. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Kuchariková, L.; Liptáková, T.; Tillová, E.; Kajánek, D.; Schmidová, E. Role of Chemical Composition in Corrosion of Aluminum Alloys. Metals 2018, 8, 581. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Cano, M.; Marcos, M. Influence of the cathodic intermetallics distribution on the reproducibility of the electrochemical measurements on AA5083 alloy in NaCl solutions. Corros. Sci. 2003, 45, 161–180. [Google Scholar] [CrossRef]
- Bethencourt, M.; Botana, F.J.; Cano, M.J.; Marcos, M.; Sánchez-Amaya, J.M.; González-Rovira, L. Using EIS to analyse samples of Al–Mg alloy AA5083 treated by thermal activation in cerium salt baths. Corros. Sci. 2008, 50, 1376–1384. [Google Scholar] [CrossRef]
- Engler, O.; Miller-Jupp, S. Control of second-phase particles in the Al-Mg-Mn alloy AA 5083. J. Alloys Compd. 2016, 689, 998–1010. [Google Scholar] [CrossRef]
- Glenn, A.M.; Muster, T.H.; Luo, C.; Zhou, X.; Thompson, G.E.; Boag, A.; Hughes, A.E. Corrosion of AA2024-T3 Part III: Propagation. Corros. Sci. 2011, 53, 40–50. [Google Scholar] [CrossRef]
- Arthanari, S.; Jang, J.C.; Shin, K.S. Corrosion performance of high pressure die-cast Al-Si-Mg-Zn alloys in 3.5 wt% NaCl solution. J. Alloys Compd. 2019, 783, 494–502. [Google Scholar] [CrossRef]
- Jebaraj, A.V.; Aditya, K.V.V.; Kumar, T.S.; Ajaykumar, L.; Deepak, C.R. Mechanical and corrosion behaviour of aluminum alloy 5083 and its weldment for marine applications. Mater. Today Proc. 2020, 22, 1470–1478. [Google Scholar] [CrossRef]
- Engler, O.; Kuhnke, K.; Westphal, K.; Hasenclever, J. Impact of chromium on the microchemistry evolution during solidification and homogenization of the Al-Mg alloy AA 5052. J. Alloys Compd. 2018, 744, 561–573. [Google Scholar] [CrossRef]
- Reboul, M.C.; Baroux, B. Metallurgical aspects of corrosion resistance of aluminium alloys. Mater. Corros. 2011, 62, 215–233. [Google Scholar] [CrossRef]
- Yang, Y.-K.; Allen, T. Direct visualization of β phase causing intergranular forms of corrosion in Al–Mg alloys. Mater. Charact. 2013, 80, 76–85. [Google Scholar] [CrossRef]
- Liu, M.; Schmutz, P.; Zanna, S.; Seyeux, A.; Ardelean, H.; Song, G.; Atrens, A.; Marcus, P. Electrochemical reactivity, surface composition and corrosion mechanisms of the complex metallic alloy Al3Mg2. Corros. Sci. 2010, 52, 562–578. [Google Scholar] [CrossRef]
- Li, Y.; Cai, J.M.; Guan, L.; Wang, G. pH-dependent electrochemical behaviour of Al3Mg2 in NaCl solution. Appl. Surf. Sci. 2019, 467–468, 619–633. [Google Scholar] [CrossRef]
- Zeng, F.-L.; Wei, Z.-L.; Li, J.-F.; Li, C.-X.; Tan, X.; Zhang, Z.; Zheng, Z.-Q. Corrosion mechanism associated with Mg2Si and Si particles in Al–Mg–Si alloys. Trans. Nonferrous Met. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Gao, Z.; Liu, Y.; Liu, X.; Guo, Q.; Yu, L.; Li, H. Corrosion behavior of Al–Mg2Si alloys with/without addition of Al–P master alloy. Mater. Charact. 2015, 110, 170–174. [Google Scholar] [CrossRef]
- Li, C.; Sun, J.; Li, Z.; Gao, Z.; Liu, Y.; Yu, L.; Li, H. Microstructure and corrosion behavior of Al–10%Mg 2 Si cast alloy after heat treatment. Mater. Charact. 2016, 122, 142–147. [Google Scholar] [CrossRef]
- Gupta, R.K.; Zhang, R.; Davies, C.H.J.; Birbilis, N. Influence of Mg Content on the Sensitization and Corrosion of Al-xMg(-Mn) Alloys. Corrosion 2013, 69, 1081–1087. [Google Scholar] [CrossRef]
- Shuaib, A.N. Mechanical properties of Al–2.5 Mg–0.1 Mn–Si–Cr–Fe alloys. Mater. Des. 2002, 23, 181–187. [Google Scholar] [CrossRef]
- Muller, I.L.; Galvele, J.R. Pitting potential of high purity binary aluminium alloys—II. AlMg and AlZn alloys. Corros. Sci. 1977, 17, 995–1007. [Google Scholar] [CrossRef]
- Brunner, J.G.; May, J.; Höppel, H.W.; Göken, M.; Virtanen, S. Localized corrosion of ultrafine-grained Al–Mg model alloys. Electrochim. Acta 2010, 55, 1966–1970. [Google Scholar] [CrossRef]
- Hajar, H.M.; Zulkifli, F.; Mohd Sabri, M.G.; Wan Nik, W.B. Protection against Corrosion of Aluminum Alloy in Marine Environment by Lawsonia inermis. Int. J. Corros. 2016, 2016, 4891803. [Google Scholar] [CrossRef]
- Xiao, W.; Peng, J.; Zhang, X.; Wu, K. Corrosion Resistance of Two Arc Spraying Coatings on EH36 Steel in Neutral Salt Spray Environment. J. Chin. Soc. Corros. Prot. 2023, 43, 1003–1014. [Google Scholar] [CrossRef]
- Darmawan, A.S.; Siswanto, W.A.; Purboputro, P.I.; Anggono, A.D.; Masyrukan; Hamid, A. Effect of Increasing Salinity to Corrosion Resistance of 5052 Aluminum Alloy in Artificial Seawater. Mater. Sci. Forum 2019, 961, 107–111. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, C.; Liu, Y.; Yan, Y.; Xu, P.; Jiang, Y.; Cao, X. Comparing the Corrosion Resistance of 5083 Al and Al2O3/5083 Al Composite in a Chloride Environment. Materials 2022, 16, 86. [Google Scholar] [CrossRef]
- Zhou, P.; Deng, L.; Guo, P.; Rao, W.; Wang, X.; Zhang, M. Influence of Microstructure Heterogeneity on the Corrosion Resistance and Microhardness of 5052 Al-Mg Alloy. JOM 2020, 72, 4305–4314. [Google Scholar] [CrossRef]
- Brillas, E.; Cabot, P.L.; Centellas, F.; Garrido, J.A.; Pérez, E.; Rodríguez, R.M. Electrochemical oxidation of high-purity and homogeneous Al–Mg alloys with low Mg contents. Electrochim. Acta 1998, 43, 799–812. [Google Scholar] [CrossRef]
- ISO 8407-2021; Corrosion of Metals and Alloys—Removal of Corrosion Products from Corrosion Test Specimens. International Organization for Standardization: Geneva, Switzerland, 2021.
- Liang, W.J.; Rometsch, P.A.; Cao, L.F.; Birbilis, N. General aspects related to the corrosion of 6xxx series aluminium alloys: Exploring the influence of Mg/Si ratio and Cu. Corros. Sci. 2013, 76, 119–128. [Google Scholar] [CrossRef]
- Delijić, K.; Markoli, B.; Naglič, I. The Influence of the Chemical Composition on the Corrosion Performances of Some Al-Fe-Si, Al-Mg-Si and Al-Mg-Mn Type of Alloys. Metall. Mater. Eng. 2014, 20, 217–234. [Google Scholar] [CrossRef]
- ISO 15158-2014; Corrosion of Metals and Alloys—Method of Measuring the Pitting Potential for Stainless Steels by Potentiodynamic Control in Sodium Chloride Solution. International Organization for Standardization: Geneva, Switzerland, 2014.
- Frankel, G.S.; Fajardo, S.; Lynch, B.M. Introductory lecture on corrosion chemistry: A focus on anodic hydrogen evolution on Al and Mg. Faraday Discuss 2015, 180, 11–33. [Google Scholar] [CrossRef]
- Ubeda, C.; Garces, G.; Adeva, P.; Llorente, I.; Frankel, G.S.; Fajardo, S. The role of the beta-Mg17Al12 phase on the anomalous hydrogen evolution and anodic dissolution of AZ magnesium alloys. Corros. Sci. 2020, 165, 108384. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- Scotto-Sheriff, S.; Darque-Ceretti, E.; Plassart, G.; Aucouturier, M. Physico-chemical characterisation of native air-formed oxide films on Al-Mg alloys at low temperature. Influence of water. J. Mater. Sci. 1999, 34, 5081–5088. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Zeng, R.; Song, L.; Guo, L.; Huang, X. Corrosion Resistance of the Superhydrophobic Mg(OH)2/Mg-Al Layered Double Hydroxide Coatings on Magnesium Alloys. Metals 2016, 6, 85. [Google Scholar] [CrossRef]
- Wu, G.; Dash, K.; Galano, M.L.; O’Reilly, K.A.Q. Oxidation studies of Al alloys: Part II Al-Mg alloy. Corros. Sci. 2019, 155, 97–108. [Google Scholar] [CrossRef]
- Tan, Z.; Xu, R.; Bi, H.; Zhang, Z.; Li, M. Effects of potential on corrosion behavior and contact resistance of 446 stainless steel in simulated proton exchange membrane fuel cell cathode environment. J. Solid State Electrochem. 2023, 27, 1993–2003. [Google Scholar] [CrossRef]
- Wang, L.; Zong, Q.; Sun, W.; Yang, Z.; Liu, G. Chemical modification of hydrotalcite coating for enhanced corrosion resistance. Corros. Sci. 2015, 93, 256–266. [Google Scholar] [CrossRef]
- Trdan, U.; Grum, J. SEM/EDS characterization of laser shock peening effect on localized corrosion of Al alloy in a near natural chloride environment. Corros. Sci. 2014, 82, 328–338. [Google Scholar] [CrossRef]
- Li, M.; Luo, S.; Wu, P.; Shen, J. Photocathodic protection effect of TiO2 films for carbon steel in 3% NaCl solutions. Electrochim. Acta 2005, 50, 3401–3406. [Google Scholar] [CrossRef]
- Abodi, L.C.; DeRose, J.A.; Van Damme, S.; Demeter, A.; Suter, T.; Deconinck, J. Modeling localized aluminum alloy corrosion in chloride solutions under non-equilibrium conditions: Steps toward understanding pitting initiation. Electrochim. Acta 2012, 63, 169–178. [Google Scholar] [CrossRef]
- Seri, O.; Kido, Y. Corrosion Phenomenon and Its Analysis of 6063 Aluminum Alloy in Ethyl Alcohol. Mater. Trans. 2009, 50, 1433–1439. [Google Scholar] [CrossRef]
- Wang, J.; Yang, H.; Du, M.; Hou, J.; Peng, W.; Lin, C. Corrosion Mechanism of 5083 Aluminum Alloy in Seawater Containing Phosphate. J. Ocean Univ. China 2021, 20, 372–382. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Mohanavel, V.; Kaladgi, A.R.; Kumar, V.; Ravichandran, M.; Arunkumar, G.; Basheer, D. The investigation of the effect of nano particles on dry sliding wear and corrosion behavior of Al-Mg/Al2O3 composites. Surf. Topogr. Metrol. Prop. 2021, 9, 045046. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.-T.; Song, Y.-F.; Ge, F.; Zhang, Y.; Meng, X.-J.; Ge, H.-H.; Zhao, Y.-Z. Effect of Al2O3 nanoparticles on the corrosion behavior of aluminum alloy in simulated vehicle coolant. J. Alloys Compd. 2021, 874, 159807. [Google Scholar] [CrossRef]
- Teng, H.-T.; Lee, T.-Y.; Chen, Y.-K.; Wang, H.-W.; Cao, G. Effect of Al(OH)3 on the hydrogen generation of aluminum–water system. J. Power Sources 2012, 219, 16–21. [Google Scholar] [CrossRef]
- Brady, M.P.; Rother, G.; Anovitz, L.M.; Littrell, K.C.; Unocic, K.A.; Elsentriecy, H.H.; Song, G.L.; Thomson, J.K.; Gallego, N.C.; Davis, B. Film Breakdown and Nano-Porous Mg(OH)2 Formation from Corrosion of Magnesium Alloys in Salt Solutions. J. Electrochem. Soc. 2015, 162, C140–C149. [Google Scholar] [CrossRef]
- Li, B.; Wang, T.; Li, P.; Wang, S.; Wang, L. Selective Laser Melting of 316L Stainless Steel: Influence of Co-Cr-Mo-W Addition on Corrosion Resistance. Metals 2021, 11, 597. [Google Scholar] [CrossRef]
- Li, J.; Shen, W.; Lin, P.; Wang, F.; Yang, Z. Effect of Solution Treatment Temperature on Microstructural Evolution, Precipitation Behavior, and Comprehensive Properties in UNS S32750 Super Duplex Stainless Steel. Metals 2020, 10, 1481. [Google Scholar] [CrossRef]
- McCafferty, E. Sequence of steps in the pitting of aluminum by chloride ions. Corros. Sci. 2003, 45, 1421–1438. [Google Scholar] [CrossRef]
- Zaid, B.; Saidi, D.; Benzaid, A.; Hadji, S. Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corros. Sci. 2008, 50, 1841–1847. [Google Scholar] [CrossRef]
- Ikeuba, A.I.; Njoku, C.N.; Ekerenam, O.O.; Njoku, D.I.; Udoh, I.I.; Daniel, E.F.; Uzoma, P.C.; Etim, I.-I.N.; Okonkwo, B.O. A review of the electrochemical and galvanic corrosion behavior of important intermetallic compounds in the context of aluminum alloys. RSC Adv. 2024, 14, 31921–31953. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 2005, 152, B140. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Z.; Sun, J.; Zhu, M.; Yao, Q.; Zhang, D.; Zhang, B.; Xiao, K.; Wu, J. Corrosion Behaviors of AA5083 and AA6061 in Artificial Seawater: Effects of Cl−, HSO3− and Temperature. Int. J. Electrochem. Sci. 2020, 15, 1218–1229. [Google Scholar] [CrossRef]
- Kosaba, T.; Nishimoto, M.; Muto, I. Elucidation of the trigger for trenching around Al6(Fe, Mn) on AA5083 aluminum alloy in diluted synthetic seawater. Corros. Sci. 2024, 239, 112362. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Zhang, M.; Wang, H.; Gong, W.; Lai, R.; Li, Y.; Teng, J. The corrosion behavior and mechanical properties of 5083 Al-Mg alloy manufactured by additive friction stir deposition. Corros. Sci. 2023, 213, 110972. [Google Scholar] [CrossRef]
- Wei, R.P. A model for particle-induced pit growth in aluminum alloys. Scr. Mater. 2001, 44, 2647–2652. [Google Scholar] [CrossRef]
- Ahmad, Z.; Aleem, A. Effect of heat treatment on the corrosion resistance of modified aluminum-magnesium alloys in seawater. J. Mater. Eng. Perform. 1993, 2, 739–744. [Google Scholar] [CrossRef]
- Zamin, M. The Role of Mn in the Corrosion Behavior of Al-Mn Alloys. Corrosion 1981, 37, 627–632. [Google Scholar] [CrossRef]




| Alloy | Mg | Cr | Mn | Si | Ti | Fe | Al |
|---|---|---|---|---|---|---|---|
| 5052 | 2.44 | 0.22 | 0.03 | 0.12 | 0.014 | 0.17 | Bal. |
| 5083 | 4.63 | 0.077 | 0.46 | 0.11 | 0.019 | 0.25 | Bal. |
| 5182 | 4.76 | 0.01 | 0.41 | 0.082 | 0.019 | 0.18 | Bal. |
| Alloy | Ecorr (VSCE) | Icorr (μA cm−2) | Epit (VSCE) |
|---|---|---|---|
| 5052 | −0.829 | 0.41 | −0.752 |
| 5083 | −0.838 | 0.55 | −0.764 |
| 5182 | −0.845 | 0.67 | −0.774 |
| Position | O | Na | Mg | Al | Si | Cl | Cr | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|
| A | 48.80 | 0.68 | 0.27 | 18.65 | 13.55 | 0.59 | 2.51 | 0.14 | 14.81 |
| B | 38.30 | 0.19 | 0.35 | 37.26 | 1.56 | 0.87 | 1.95 | 2.49 | 17.03 |
| C | 40.07 | 0.09 | 0.57 | 46.89 | 0.09 | 0.78 | 0.07 | 0.13 | 11.31 |
| Alloy | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 5052 | 28.1 | 27.8 | 22.8 | 14.2 | |||
| 5083 | 89.1 | 83.6 | 36.2 | 33.8 | 29.0 | 28.9 | 26.4 |
| 5182 | 110.5 | 99.8 | 96.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Xue, F.; Li, M. Corrosion Behavior of Al-Mg Alloys with Different Alloying Element Contents in 3.5% NaCl Solution. Metals 2025, 15, 327. https://doi.org/10.3390/met15030327
Zhou W, Xue F, Li M. Corrosion Behavior of Al-Mg Alloys with Different Alloying Element Contents in 3.5% NaCl Solution. Metals. 2025; 15(3):327. https://doi.org/10.3390/met15030327
Chicago/Turabian StyleZhou, Weitao, Fei Xue, and Moucheng Li. 2025. "Corrosion Behavior of Al-Mg Alloys with Different Alloying Element Contents in 3.5% NaCl Solution" Metals 15, no. 3: 327. https://doi.org/10.3390/met15030327
APA StyleZhou, W., Xue, F., & Li, M. (2025). Corrosion Behavior of Al-Mg Alloys with Different Alloying Element Contents in 3.5% NaCl Solution. Metals, 15(3), 327. https://doi.org/10.3390/met15030327






