Atmospheric Corrosion Behavior of Typical Aluminum Alloys in Low-Temperature Environment
Abstract
1. Introduction
2. Experimental
2.1. Preparation and Treatment of Corrosion Samples
2.2. Weight Loss Testing
2.3. Surface Characterization
3. Results
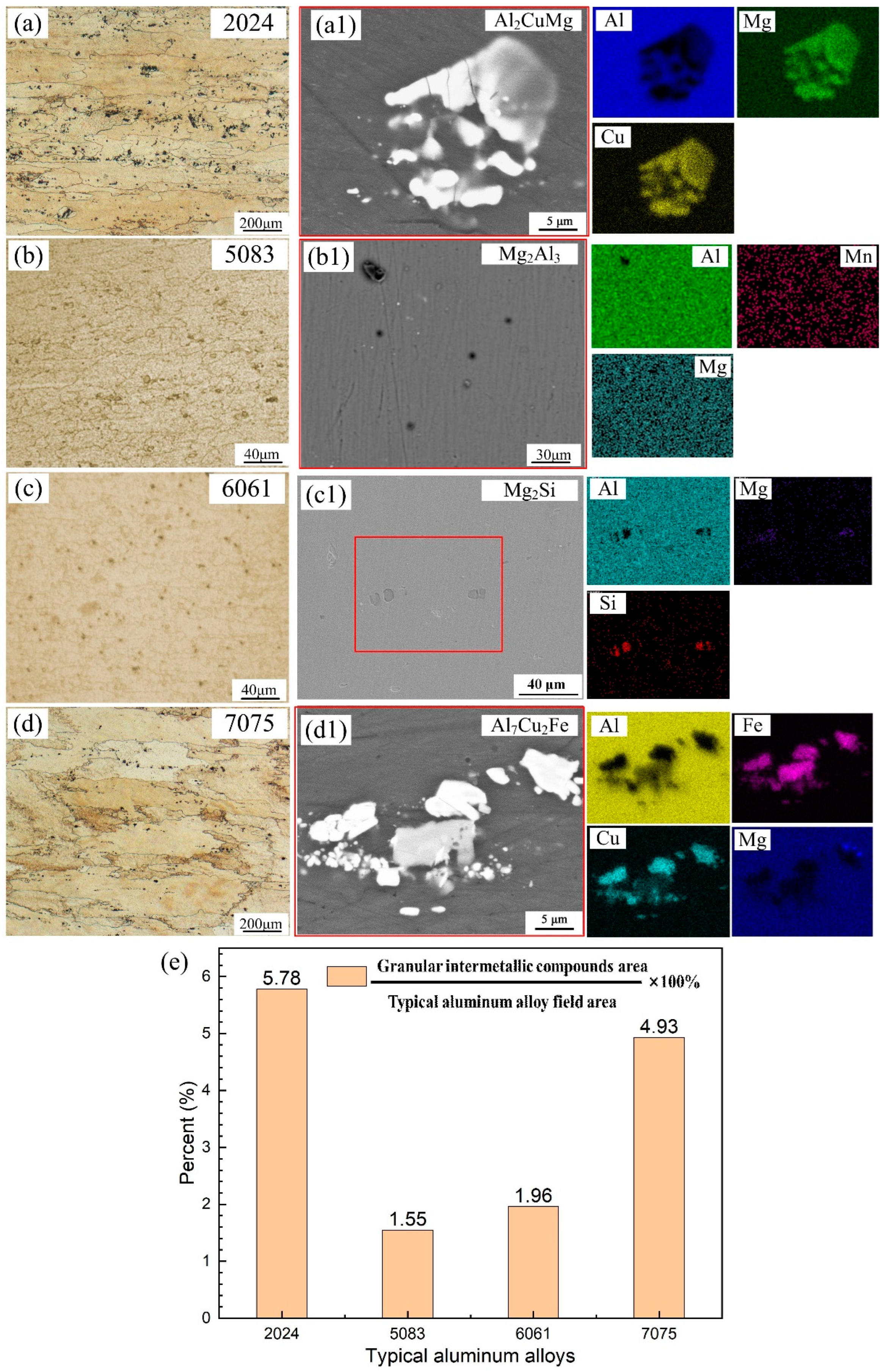
3.1. Microstructure and Environmental Conditions of the Exposure Site
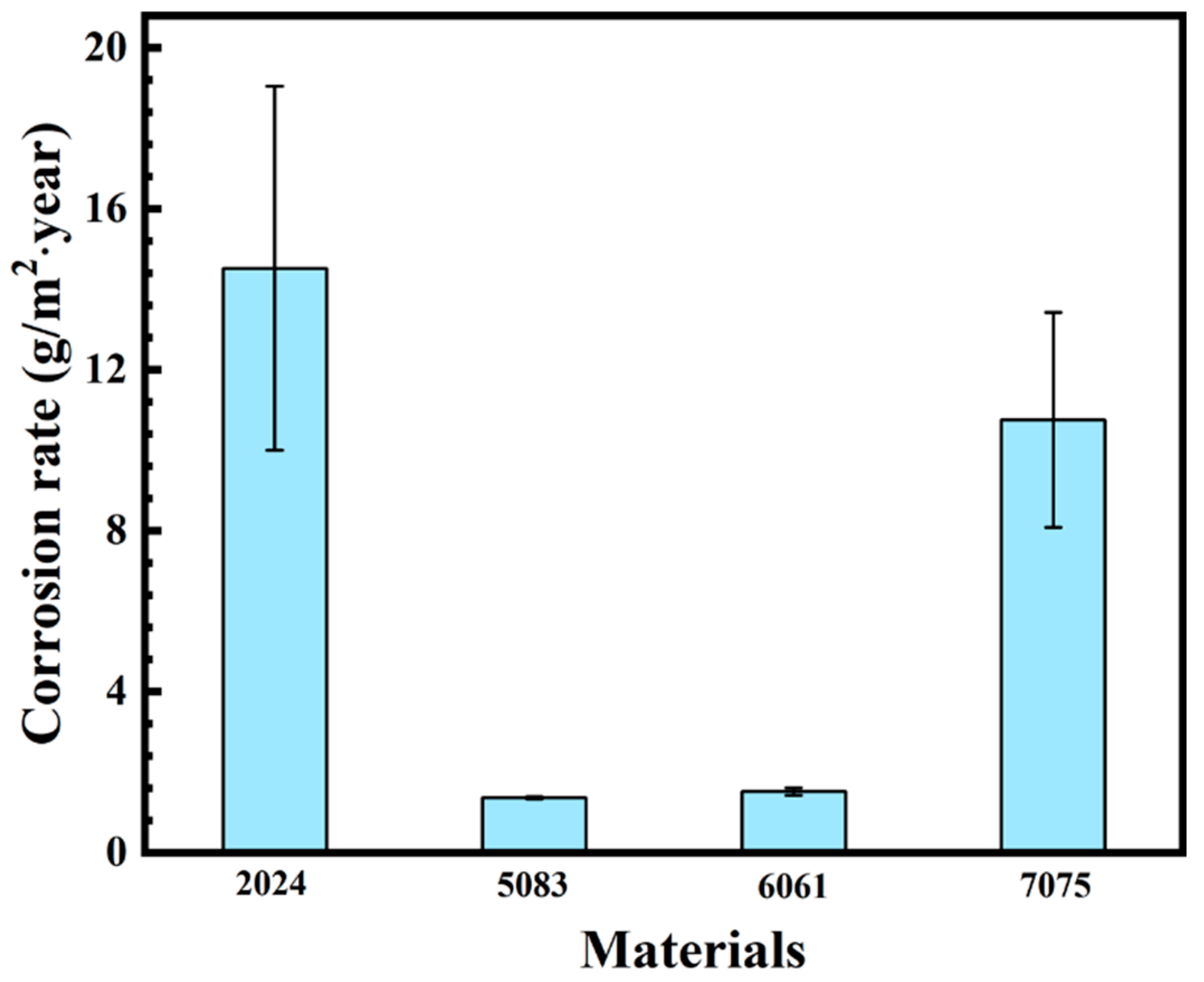
3.2. Determination of Corrosion Rate
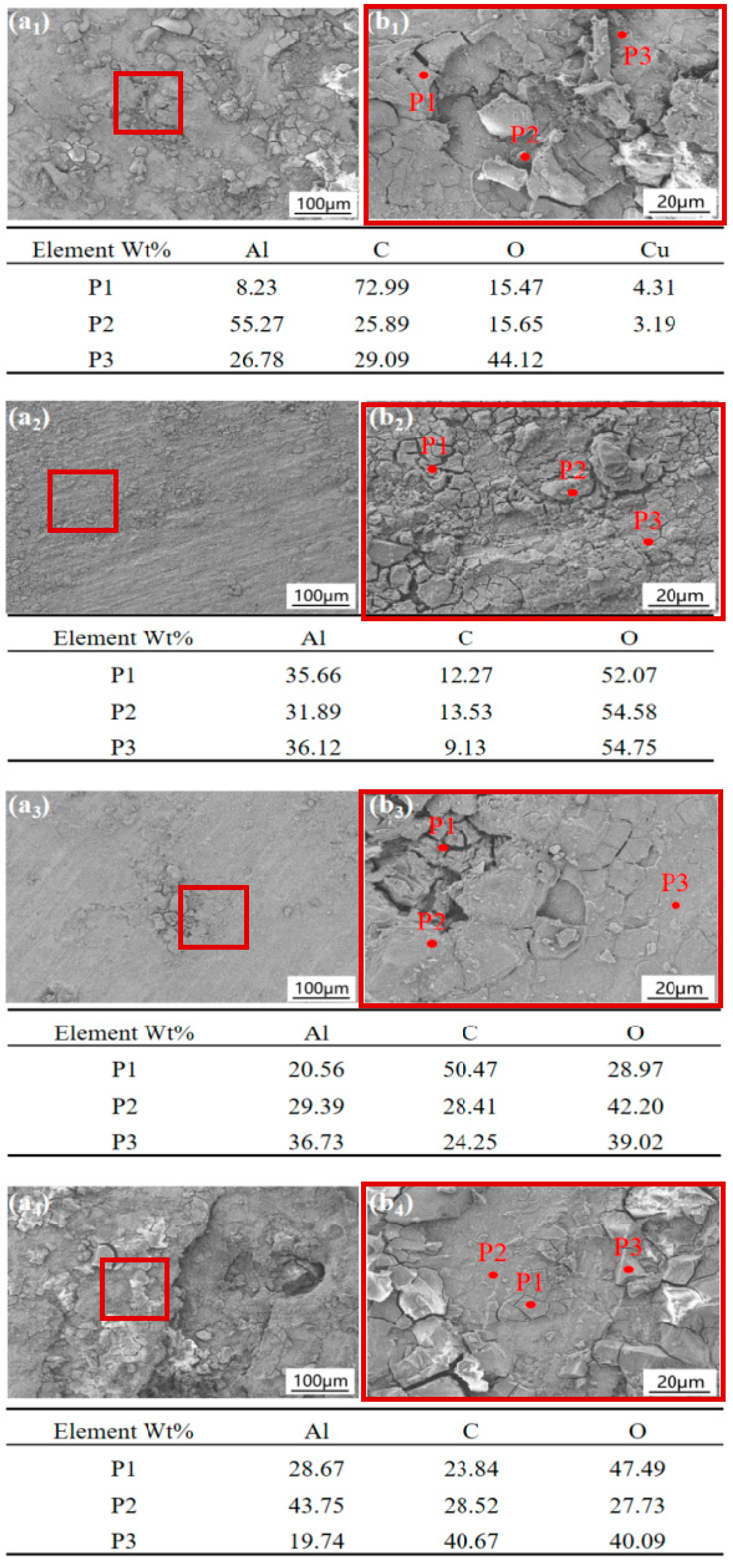
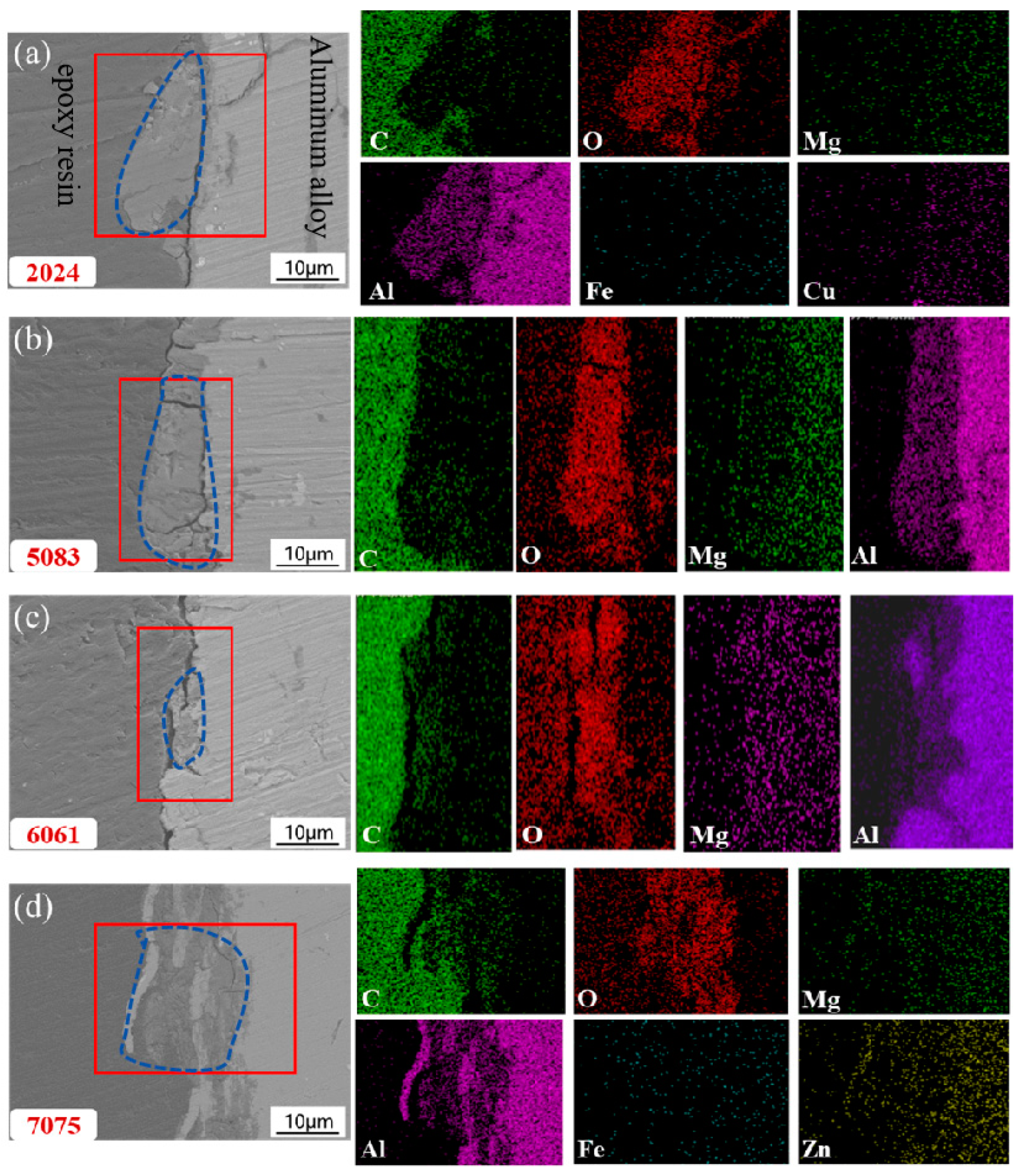
3.3. Corrosion Product Observation
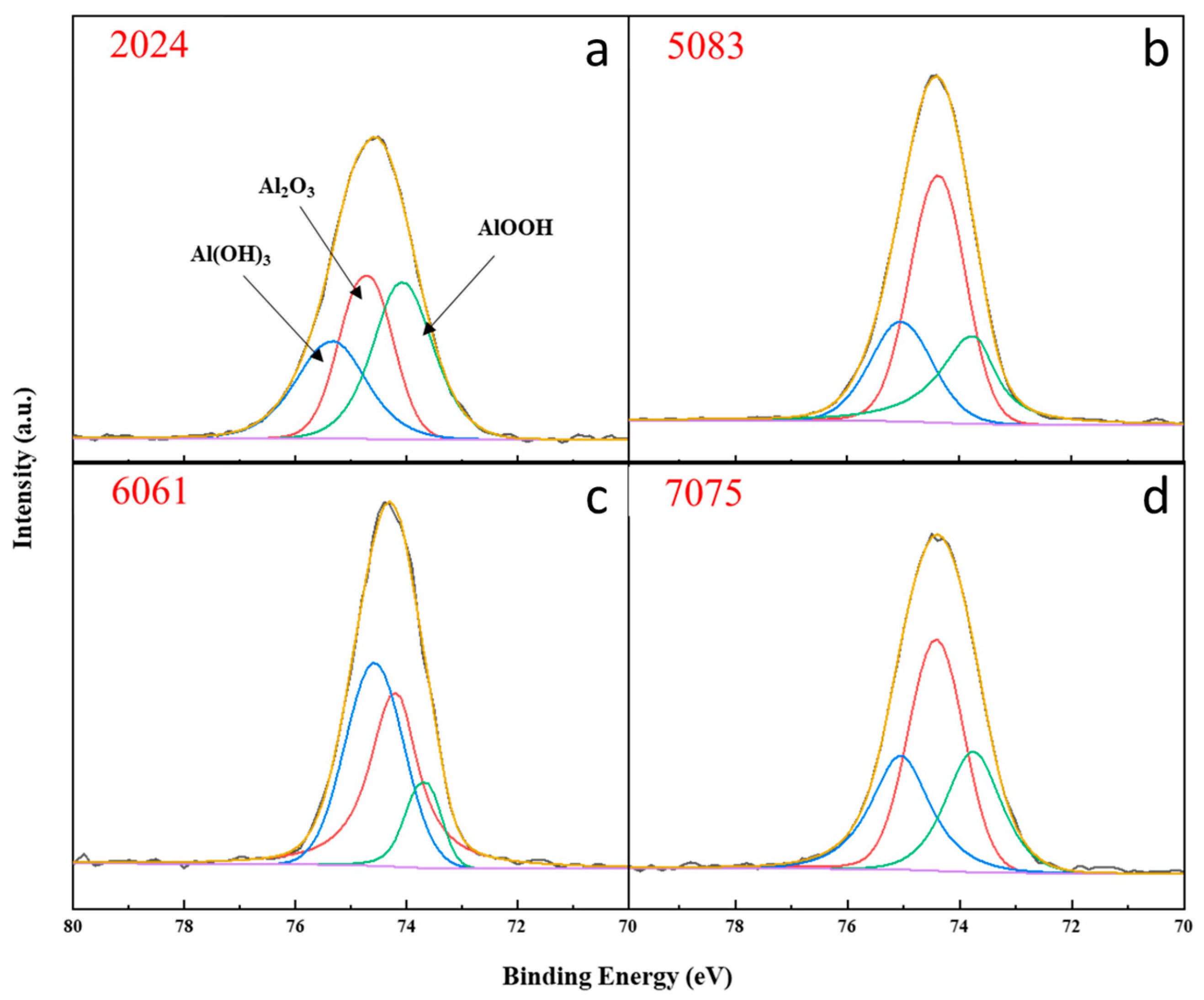
3.4. XPS Analysis
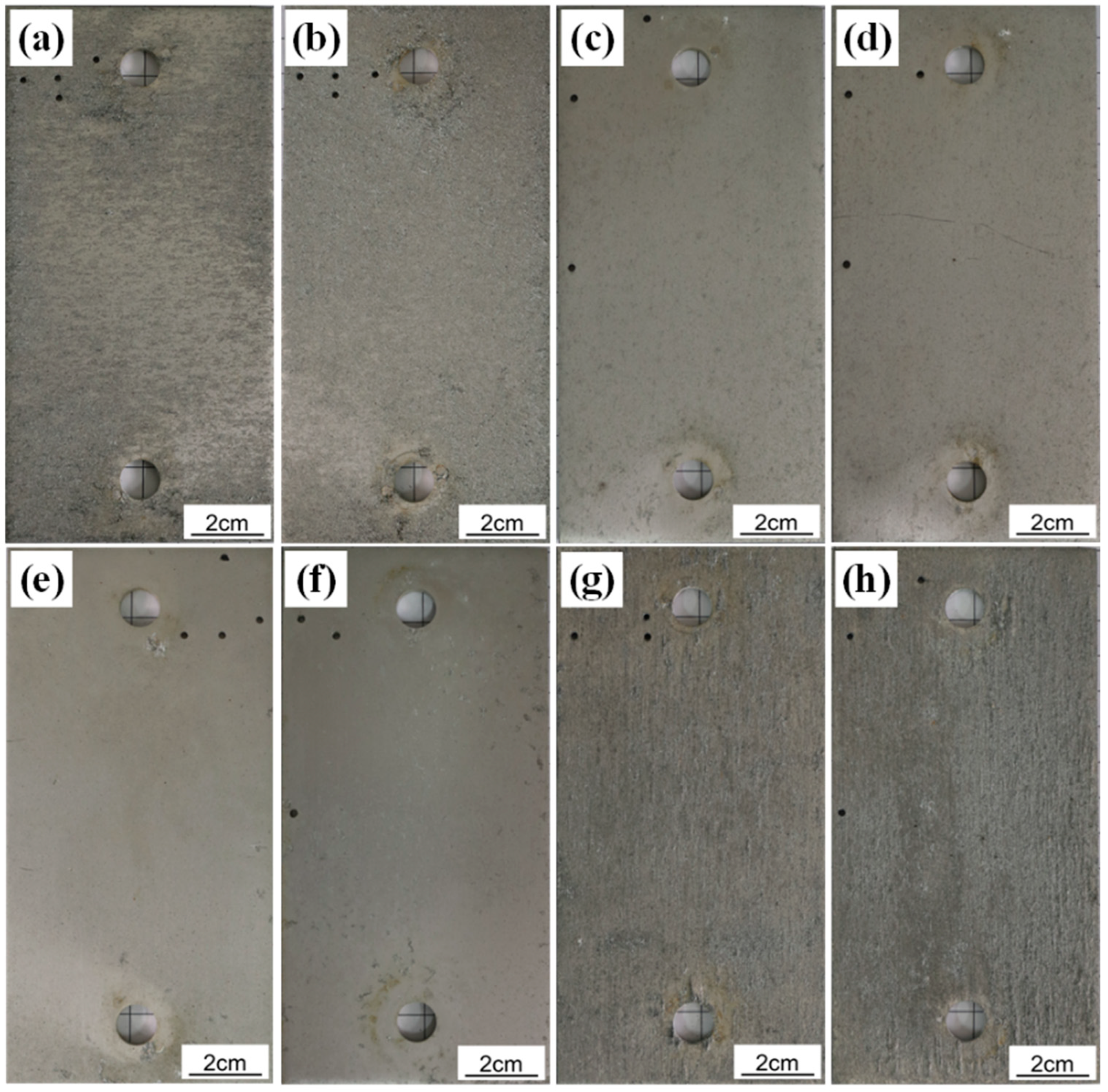
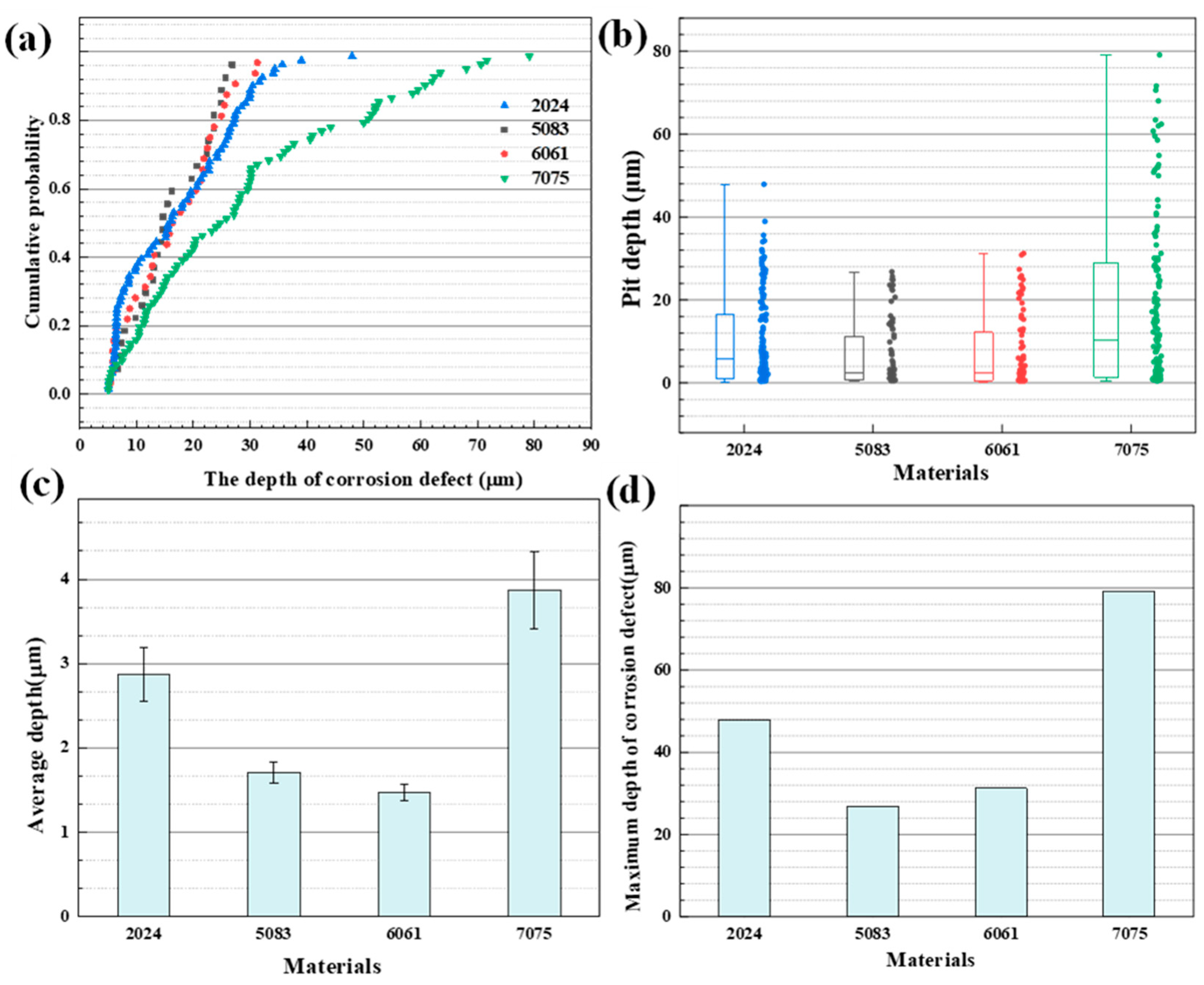
3.5. Surface Morphology and Topography Observation
4. Discussion
4.1. Effect of Aluminum Alloy Composition on Atmospheric Corrosion Behavior
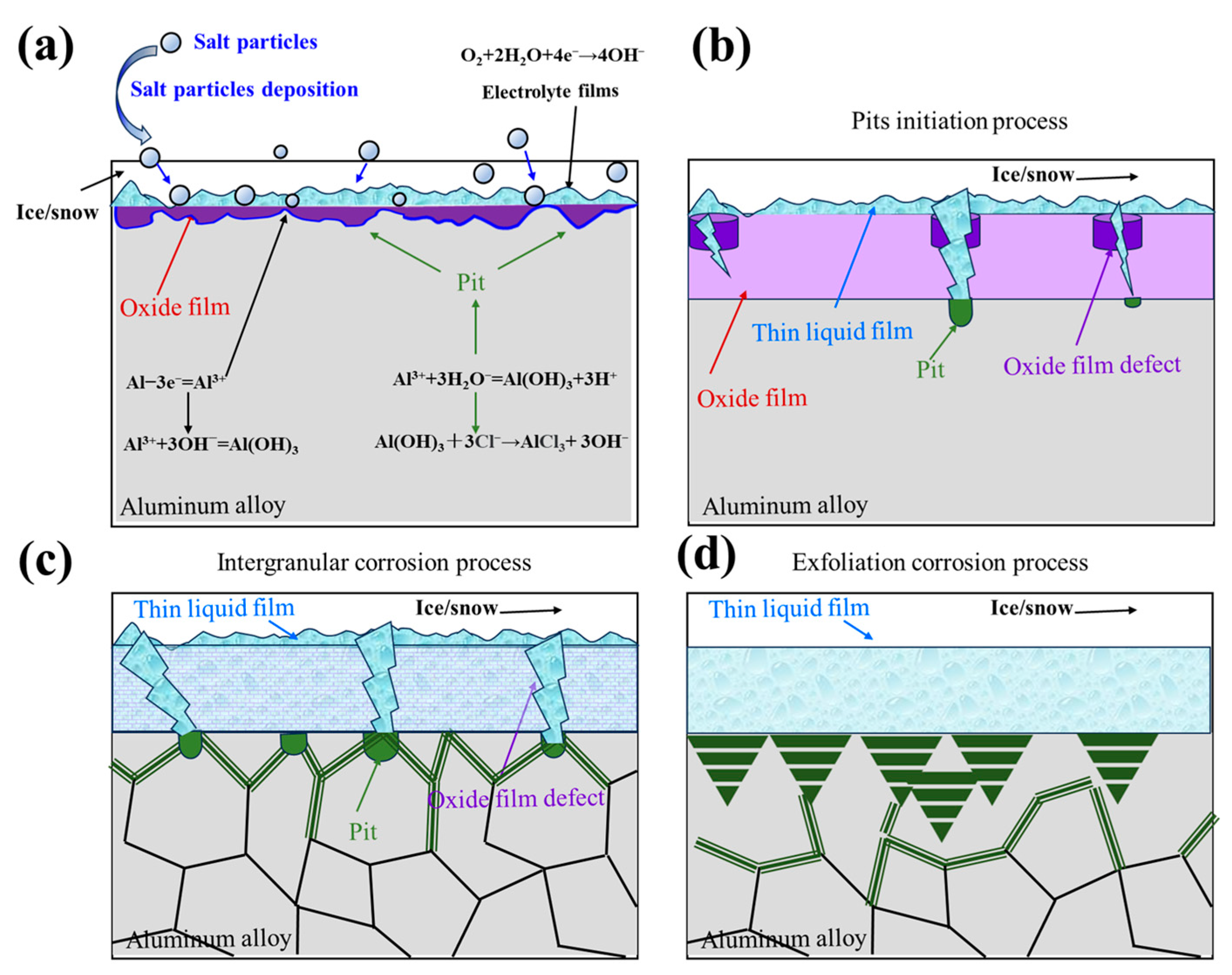
4.2. Corrosion Mechanism Analysis of the Aluminum Alloys in Antarctic Environment
5. Conclusions
- (1)
- The corrosion rate of the 2024, 5083, 6061, 7075 aluminum alloys exposed to the Antarctic environment was 14.5 g/(m2·year), 1.36 g/(m2·year), 1.5 g/(m2·year), and 10.8 g/(m2·year), respectively.
- (2)
- The corrosion products formed on the 2024, 5083, 6061, and 7075 aluminum alloys exposed to the Antarctic environment were primarily composed of AlOOH and Al2O3.
- (3)
- For the 7075 aluminum alloy, the average pit depth and the maximum pit depth were the largest, and the maximum pit depth of the 5083 aluminum alloy was the smallest.
- (4)
- In the Antarctic environment, the initiation and growth of aluminum alloy pits are facilitated by freeze–thaw cycles and salt deposition. Due to peculiar alloy composition and microstructure, the grain boundary of aluminum alloys has high activity and grain boundary corrosion occurs. The freeze–thaw cycle causes the corrosion products at the grain boundary to expand, and aluminum alloy exfoliation corrosion occurs due to the wedge effect.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.; Shi, Y. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2019, 35, 270–284. [Google Scholar] [CrossRef]
- Dan, Z.; Takigawa, S.; Muto, I.; Hara, N. Applicability of constant dew point corrosion tests for evaluating atmospheric corrosion of aluminium alloys. Corros. Sci. 2011, 53, 2006–2014. [Google Scholar] [CrossRef]
- Pathak, S.; Mendon, S.; Blanton, M.; Rawlins, J. Magnesium-based sacrificial anode cathodic protection coatings (Mg-rich primers) for aluminum alloys. Metals 2012, 2, 353–376. [Google Scholar] [CrossRef]
- Atalah, J.; Blamey, L.; Köhler, H.; Alfaro-Valdés, H.; Galarce, C.; Alvarado, C.; Sancy, M.; Páez, M.; Blamey, J. Study of an Antarctic thermophilic consortium and its influence on the electrochemical behavior of aluminum alloy 7075-T6. Bioelectrochemistry 2020, 133, 107450. [Google Scholar] [CrossRef]
- Shi, Z.; Cui, Z.; Li, J.; Hu, B.; An, Y.; Wang, X.; Cui, H. Atmospheric corrosion of AZ31B magnesium alloy in the Antarctic low-temperature environment. Acta. Metall. Sin. (Engl. Lett.) 2023, 36, 1421–1432. [Google Scholar] [CrossRef]
- Alvarado, C.; Sancy, M.; Blamey, J.; Galarce, C.; Monsalve, A.; Pineda, F.; Vejar, N.; Páez, M. Electrochemical characterization of aluminum alloy AA2024-T3 influenced by bacteria from Antarctica. Electrochim. Acta 2017, 247, 71–93. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Yang, D.; Liu, Y.; Terryn, H.; Cui, Z. Study on the roles of bisulfite in the stress corrosion cracking of 7050-T7451 aluminum alloy in the thin electrolyte layer environment. Corros. Sci. 2023, 215, 111030. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, H.; Chang, W.; Yu, Z.; Qiao, Q.; Zhou, M.; Guo, D.; Zhang, D.; Kwok, C.; Tam, L. Microstructure and localized corrosion properties of 2219 aluminum alloy manufactured by additive friction stir deposition. Corros. Sci. 2024, 240, 112508. [Google Scholar] [CrossRef]
- Song, H.; Zhao, C.; Bai, H.; Ren, X.; Shao, H.; Chi, J.; Dong, G.; Bi, J.; Tan, C. Investigation of grain boundary segregation evolution and corrosion behavior in 7050 aluminum alloy under oscillating laser melting. J. Alloys Compd. 2025, 1010, 177524. [Google Scholar] [CrossRef]
- Ao, R.; Liu, J.; Ren, X.; Liu, Y.; Jia, Y. Corrosion inhibition performance of two plant extracts and sodium silicate compound on AA2024-T3 aluminum alloy in NaOH medium. J. Alloys Compd. 2025, 1010, 177977. [Google Scholar] [CrossRef]
- Yang, C.; Sun, M.; Tao, Y.; Huang, A.; Chen, P.; Zhou, C.; Chu, P.; Zeng, X. Optimization of tribological properties and corrosion resistance of MAO coatings on LY12 aluminum alloy by co-doping with graphite particles and in situ formation of zinc phosphate. Ceram. Int. 2024, 50, 46018–46031. [Google Scholar] [CrossRef]
- Morcillo, M.; Chico, B.; Fuente, D.; Almeida, E.; Joseph, G.; Rivero, S.; Rosales, B. Atmospheric corrosion of reference metals in Antarctic sites. Cold. Reg. Sci. Technol. 2004, 40, 165–178. [Google Scholar] [CrossRef]
- Sun, S.; Zheng, Q.; Li, D.; Wen, J. Long-term atmospheric corrosion behaviour of aluminium alloys 2024 and 7075 in urban, coastal and industrial environments. Corros. Sci. 2009, 51, 719–727. [Google Scholar] [CrossRef]
- Díaz, I.; Fuentes, M.; Fuente, D.; Chico, B.; Jiménez, J.; Morcillo, M. Corrosion del aluminium 1050 an atmosferas costeras. Rev. Metal. 2019, 55, e153. [Google Scholar] [CrossRef]
- Li, Y. Investigation of Long-term Corrosion of AA2024 and AA5119 Aluminum Alloys under chromate coating in Marine Atmosphere by Electrochemical Impedance Technology. Int. J. Electrochem. Sci. 2022, 17, 22014. [Google Scholar] [CrossRef]
- Hu, X.; Han, F.; Niu, L.; Zhou, S.; Qian, Z.; Zhao, M.; Chen, J. Characteristics and influence factors of atmospheric corrosion of aluminum in marine and coastal environment. Corros. Prot. 2011, 32, 849–853. [Google Scholar]
- Cao, M.; Liu, L.; Fan, L.; Yu, Z.; Li, Y.; Oguzie, E.; Wang, F. Influence of temperature on corrosion behavior of 2A02 Al alloy in marine atmospheric environments. Materials 2018, 11, 235. [Google Scholar] [CrossRef]
- Paez, M.; Foong, T.; Ni, C.; Thompson, G.; Shimizu, K.; Habazaki, H.; Skeldon, P.; Wood, G. Barrier-type anodic film formation on an Al-3.5 wt% Cu alloy. Corros. Sci. 1996, 38, 59–72. [Google Scholar] [CrossRef]
- Tian, H.; Cui, Z.; Zhang, B.; Yang, W.; Yang, Y.; Cui, H. Atmospheric corrosion and mechanical property degradation of 2524-T3 aluminum alloy in marine environments. Corros. Sci. 2024, 239, 112398. [Google Scholar] [CrossRef]
- Buchheit, G.; Wall, F.; Stoner, G.; Moran, J. Anodic dissolution-based mechanism for the rapid cracking, preexposure, phenomenon demonstrated by aluminim-lithium-copper alloys. Corrosion 1995, 51, 417–428. [Google Scholar] [CrossRef]
- Buchheit, R.; Moran, J.; Stoner, G. Electrochemical behavior of the T-1 (Al2CuLi) intermetallic compuond and its role in localized corrosion of Al2-percent-Li-3-percent-Cu alloys. Corrosion 1994, 50, 120–130. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Zhou, J.; Liu, C.; Liu, J.; Gao, G. Influence of ultraviolet illumination on the corrosion behavior of 7A04 aluminum alloy in salt solutions with different pH values. J. Mater. Res. Technol. 2024, 32, 3921–3936. [Google Scholar] [CrossRef]
- ISO 9223; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation. International Organization for Standardization: London, UK, 2012.
- Tian, H.; Cui, Z.; Qi, X.; Man, C.; Cui, H. Effect of pH and applied potential on tribocorrosion behavior of 7075-T651 high strength aluminum alloy in chloride environment. Corros. Sci. 2025, 246, 112765. [Google Scholar] [CrossRef]
- Brass, G. Freezing point depression by common salts: Implications for corrosion in cold climates. In Cold Climate Corrosion: Special Topics; Perrigo, L., Byars, H., Divine, J., Eds.; NACE International: Houston, TX, USA, 1999; pp. 29–35. [Google Scholar]
- Tian, F.; Song, G.; Zheng, D. Corrosion damage in frozen 3.5 wt.% NaCl solution. Mater. Corros. 2021, 72, 1396–1409. [Google Scholar]
- Frankel, G. Pitting corrosion of metals—A review of the critical factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Xue, W.; Wang, Y.; Wu, S.; Zhang, B.; Zhang, Z.; Zhuo, X.; Zuo, P.; Lu, S.; Xia, J.; Li, X.; et al. Influence of impurity content on corrosion behavior of Al-Zn-Mg-Cu alloys in a tropical marine atmospheric environment. Corros. Sci. 2024, 237, 112319. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Gao, J.; Wang, W. Enhancing corrosion resistance of AZ31B magnesium alloy substrate in 3.5% NaCl solution with Al-Si coating prepared by cold metal transfer-based wire arc additive manufacturing. Int. J. Electrochem. Sci. 2024, 19, 100763. [Google Scholar] [CrossRef]
- Wang, L.; Liang, L.; Cheng, J.; Cheng, L.; Cui, Z. Quantitative study of the corrosion evolution and stress corrosion cracking of high strength aluminum alloys in solution and thin electrolyte layer containing Cl. Corros. Sci. 2021, 178, 109076. [Google Scholar] [CrossRef]
- Xia, D.; Ji, Y.; Zhang, R.; Mao, Y.; Behnamian, Y.; Hu, W.; Birbilis, N. On the localized corrosion of AA5083 in a simulated dynamic seawater/air interface—Part 1: Corrosion initiation mechanism. Corros. Sci. 2023, 213, 110985. [Google Scholar] [CrossRef]
- Michailidou, E.; Visser, P.; Mol, J.; Kosari, A.; Terryn, H.; Baert, K.; Gonzalez-Garcia, Y. The effect of pH on the corrosion protection of aluminum alloys in lithium-carbonate-containing NaCl solutions. Corros. Sci. 2023, 210, 110851. [Google Scholar] [CrossRef]
- Pyun, S.; Moon, S.; Ahn, S.; Kim, S. Effects of Cl−, NO3− and SO42− ions on anodic dissolution of pure aluminum in alkaline solution. Corros. Sci. 1999, 41, 653–667. [Google Scholar] [CrossRef]
- Bereket, G.; Yurt, A. The inhibition effect of amino acids and hydroxy carboxylic acids on pitting corrosion of aluminum alloy 7075. Corros. Sci. 2001, 43, 1179–1195. [Google Scholar] [CrossRef]
- Ma, H.; Tang, J.; Geng, P.; Bandaru, A.; Qin, G.; Luo, R.; Ma, N.; Yip, W.; To, S. Effect of grain orientation angles and compressive parameters on the deformation characteristics and corrosion property of 6061 Al alloy. Mater. Charact. 2024, 213, 114006. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Wang, Y.; Tian, H.; Xin, Y.; Hou, J.; Cui, Z. Comparative study of the corrosion behavior of base metal and welded joint of Ti-6Al-3Nb-2Zr-1Mo alloy in the acidic chloride environment. Corros. Sci. 2025, 244, 112649. [Google Scholar] [CrossRef]



| Materials | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| 2024 | 0.50 | 0.50 | 3.8–4.9 | 0.30–0.9 | 1.2–1.8 | 0.10 | 0.25 | 0.15 | Bal. |
| 5083 | 0.40 | 0.40 | 0.10 | 0.40–1.0 | 4.0–4.9 | 0.05–0.25 | 0.25 | 0.15 | Bal. |
| 6061 | 0.40–0.8 | 0.7 | 0.15–0.40 | 0.15 | 0.8–1.2 | 0.04–0.15 | 0.25 | 0.15 | Bal. |
| 7075 | 0.40 | 0.50 | 1.2–2.0 | 0.30 | 2.1–2.9 | 0.18–0.28 | 5.1–6.1 | 0.20 | Bal. |
| Month | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average temperature/°C | 0.4 | −2.6 | −8.6 | −12.0 | −15.2 | −15.1 | −15.6 | −13.1 | −13.0 | −10.0 | −4.3 | 0.2 |
| Maximum temperature/°C | 8.7 | 3.3 | 0.8 | −3.5 | −3.8 | −5.2 | −2.1 | −3.9 | −2.8 | 1.2 | 6.3 | 9.6 |
| Minimum temperature/°C | −5.9 | −10.5 | −22.4 | −21.3 | −33.7 | −33.2 | −32.1 | −36.4 | −26.7 | −27.3 | −17.4 | −6.8 |
| Number of freeze–thaw cycles/day | 8 | 5 | - | - | - | - | - | - | - | - | 6 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, T.; Wu, J.; Song, J.; Meng, D.; Jin, X.; Tian, H.; Cui, Z. Atmospheric Corrosion Behavior of Typical Aluminum Alloys in Low-Temperature Environment. Metals 2025, 15, 277. https://doi.org/10.3390/met15030277
Cui T, Wu J, Song J, Meng D, Jin X, Tian H, Cui Z. Atmospheric Corrosion Behavior of Typical Aluminum Alloys in Low-Temperature Environment. Metals. 2025; 15(3):277. https://doi.org/10.3390/met15030277
Chicago/Turabian StyleCui, Tengfei, Jianguo Wu, Jian Song, Di Meng, Xiaoli Jin, Huiyun Tian, and Zhongyu Cui. 2025. "Atmospheric Corrosion Behavior of Typical Aluminum Alloys in Low-Temperature Environment" Metals 15, no. 3: 277. https://doi.org/10.3390/met15030277
APA StyleCui, T., Wu, J., Song, J., Meng, D., Jin, X., Tian, H., & Cui, Z. (2025). Atmospheric Corrosion Behavior of Typical Aluminum Alloys in Low-Temperature Environment. Metals, 15(3), 277. https://doi.org/10.3390/met15030277






