Improving the Clinical Performance of Dental Implants Through Advanced Surface Treatments: The Case of Ti and ZrO2 Coatings
Abstract
1. Introduction
2. Conventional Surface Treatments for Dental Implants
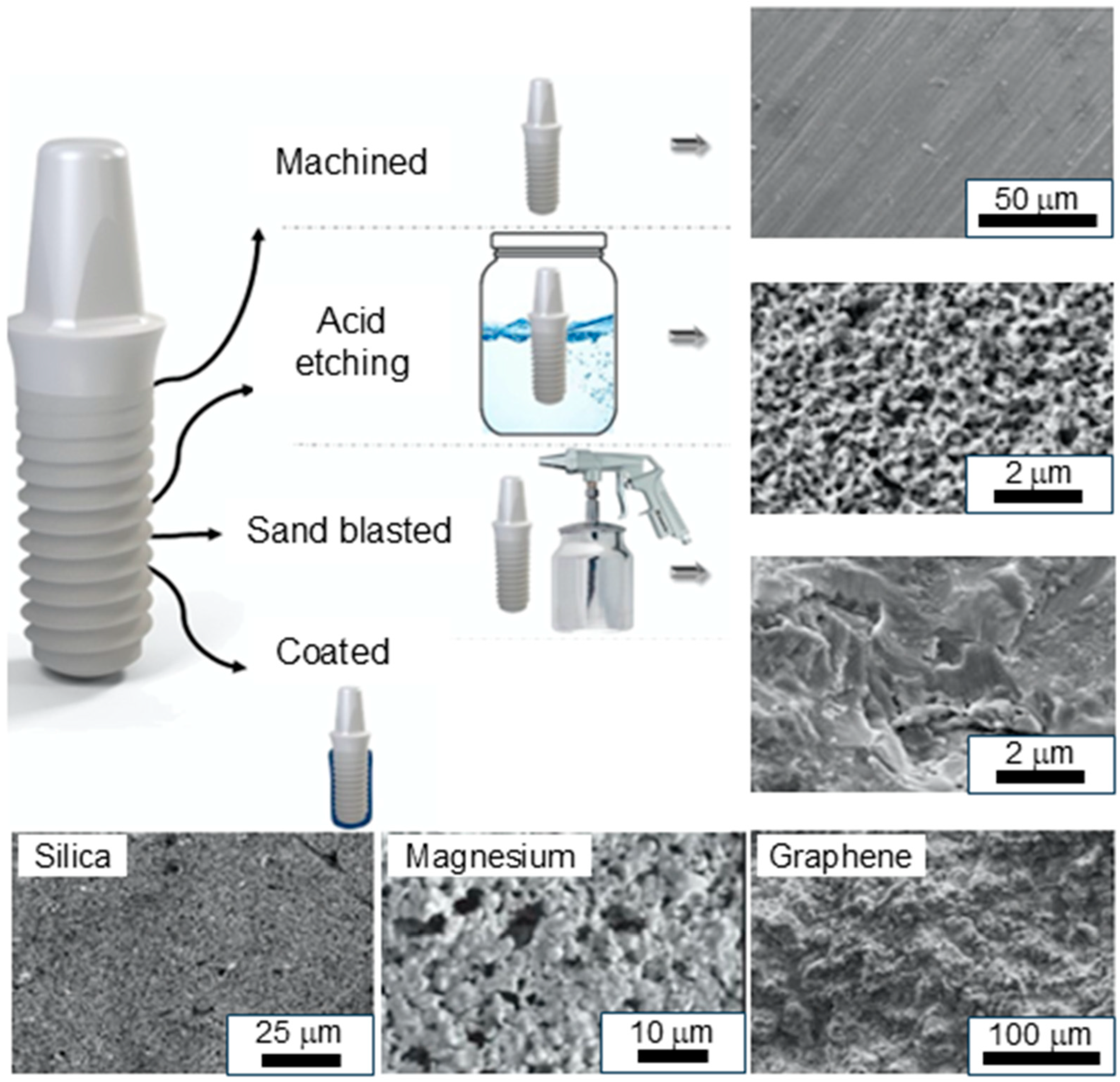
2.1. Machining
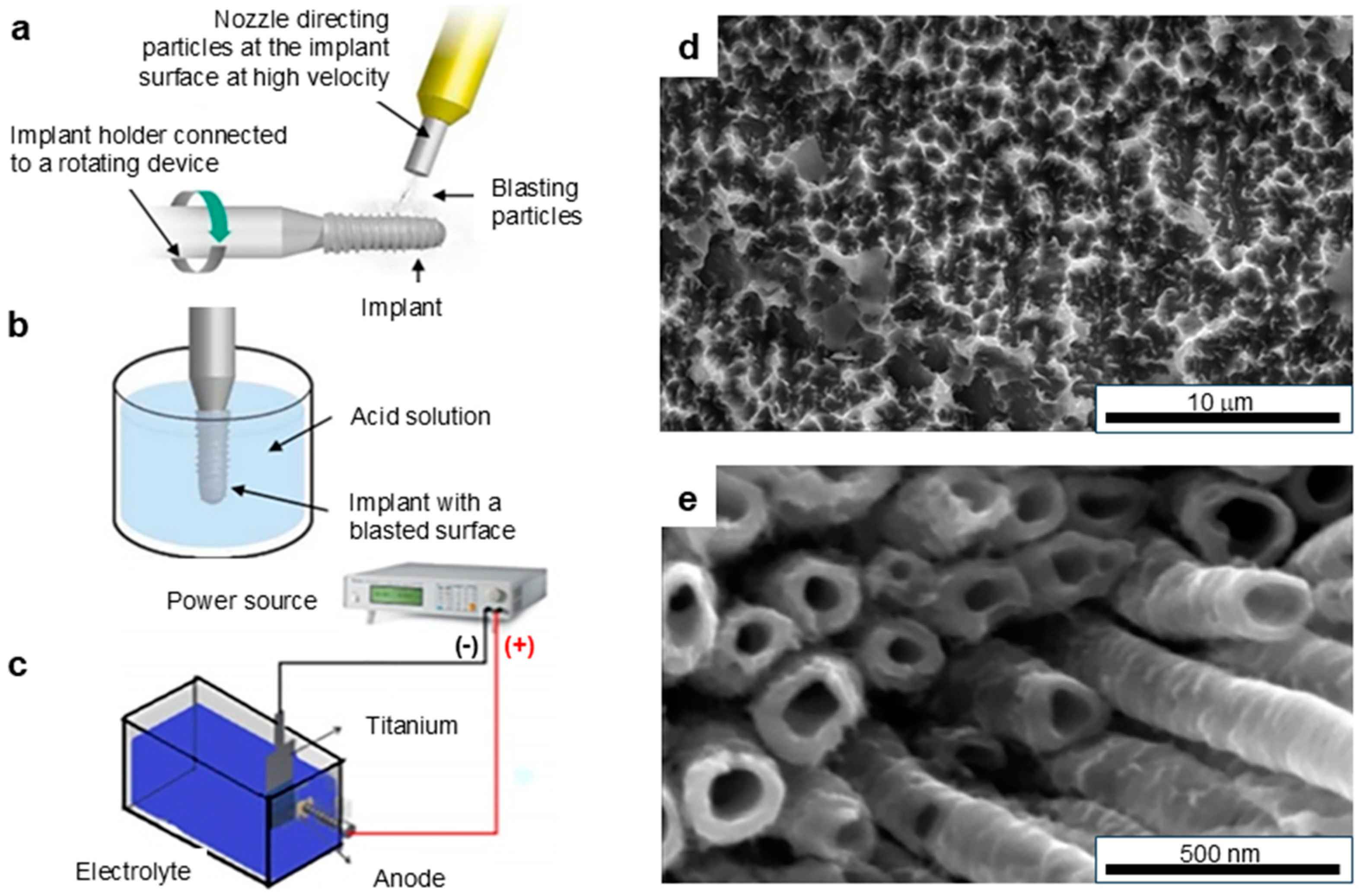
2.2. Grit Blasting
2.3. Acid Etching
2.4. Grit Blasting–Acid Etching
2.5. Anodizing
3. Recent Surface Treatment Methods Applied to Ti-Based Implants
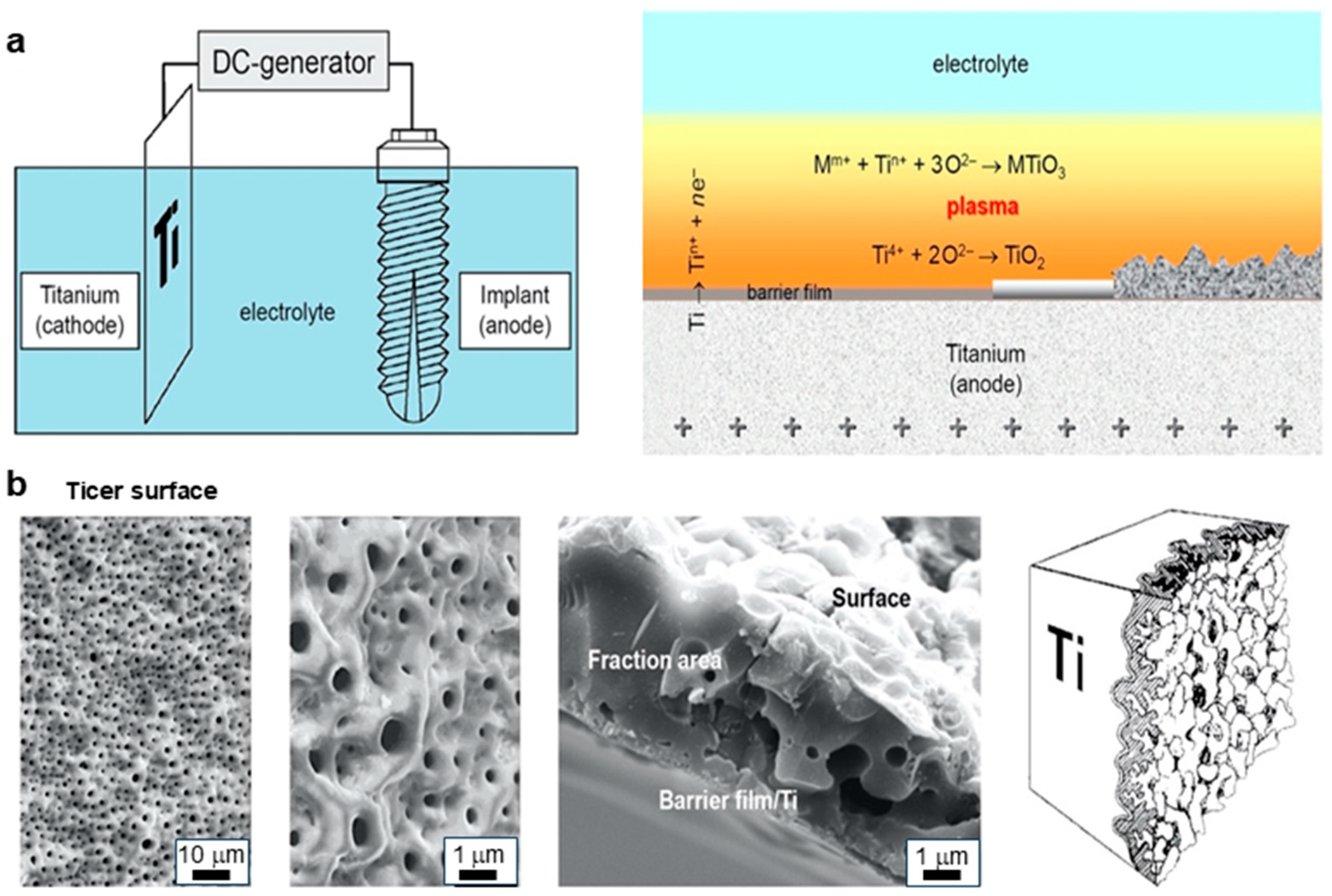
3.1. Anodic Spark Deposition Technique
3.2. Atomic Layer Deposition Technique
3.3. Plasma Spraying and Plasma Electrolytic Oxidation
4. Recent Surface Treatment for ZrO2-Based Implants
4.1. Laser Treatment
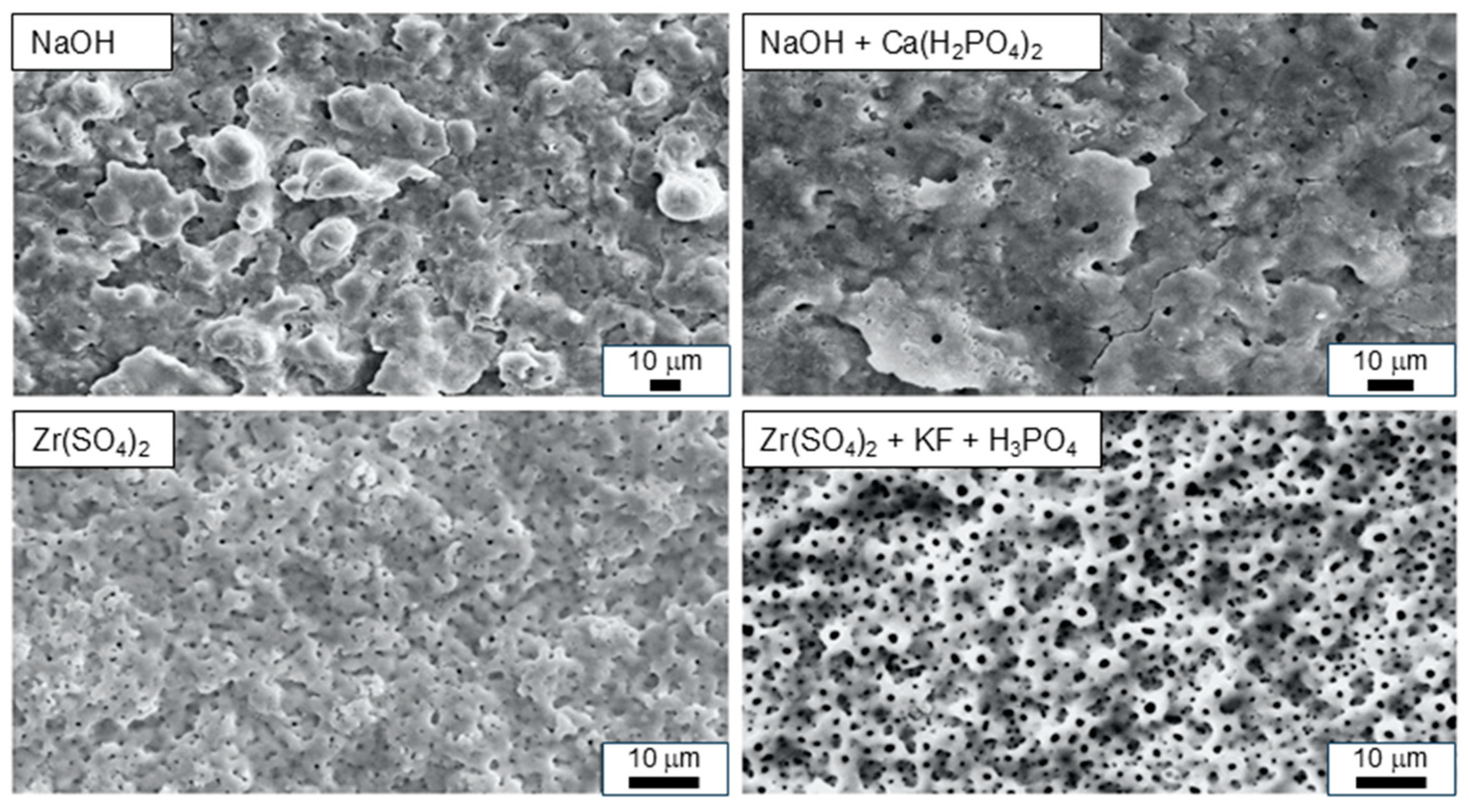
4.2. ZrO2-Based Coatings
4.2.1. Magnesium, Nitrogen, and Carbon Coatings
4.2.2. Hydroxyapatite and Calcium Phosphate Coatings
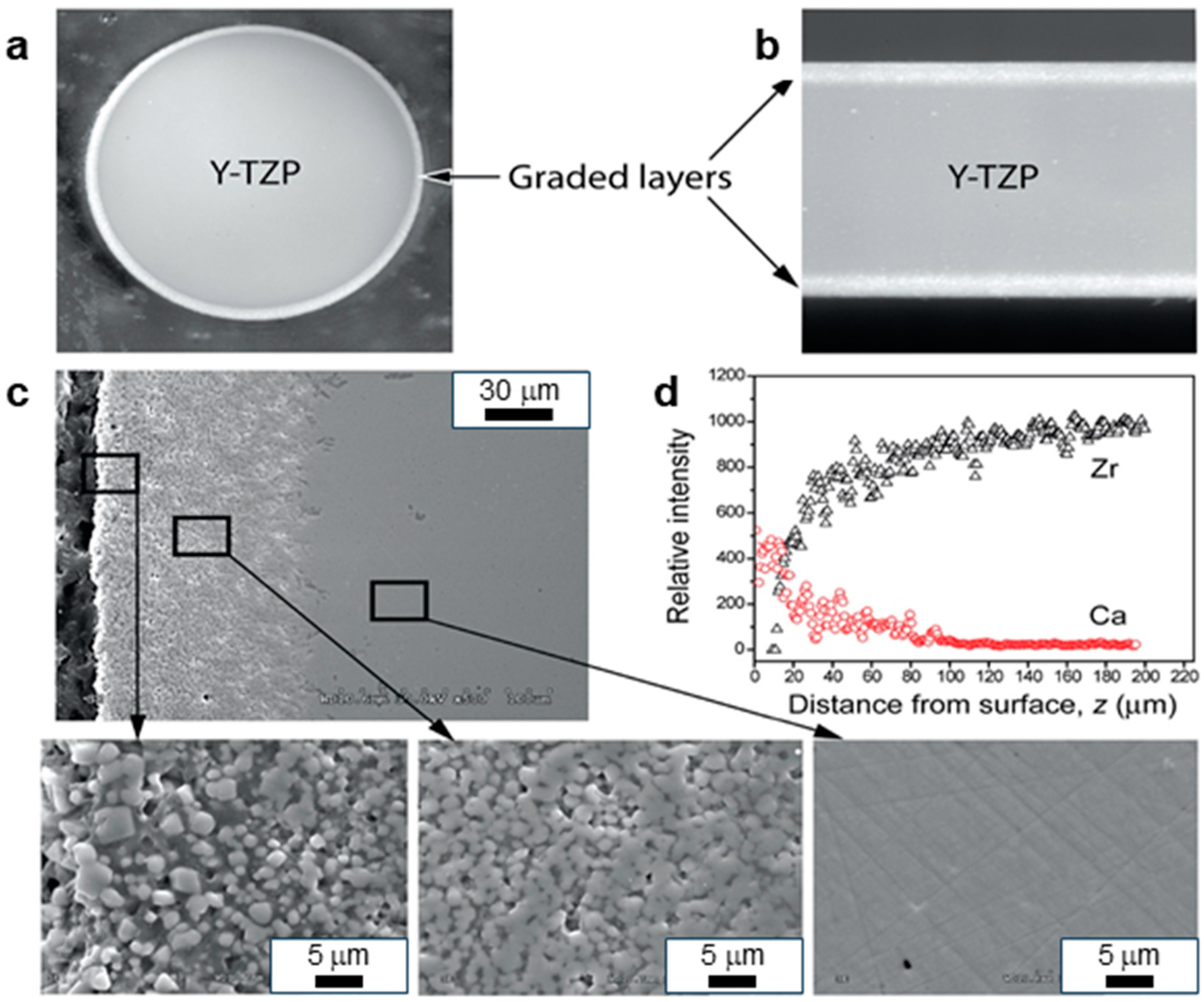
4.2.3. Graded ZrO2
5. Challenges and Limitations in Current Technologies
6. Prospective Approaches for Dental Implant Surface Engineering
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safaee, S.; Nesabi, M.; Nesabi, M.; Mamizad Moghtader, F. Osseointegration Dynamics: Insights into the Dental Bone-Implant Interface. J. Appl. Tissue Eng. 2023, 9, 1–9. [Google Scholar] [CrossRef]
- Ruggiero, A.; De Stefano, M. On the Biotribological Surfaces of Dental Implants: Investigation in the Framework of Osseointegration. Biotribology 2023, 35–36, 100254. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Nowińska, D.; Maszybrocka, J. Developments in Dental Implant Surface Modification. Coatings 2025, 15, 109. [Google Scholar] [CrossRef]
- Albrektsson, T.; Tengvall, P.; Amengual, L.; Coli, P.; Kotsakis, G.A.; Cochran, D. Osteoimmune Regulation Underlies Oral Implant Osseointegration and Its Perturbation. Front. Immunol. 2023, 13, 1056914. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.; Rokaya, D.; Bhattarai, B.P. Contemporary Concepts in Osseointegration of Dental Implants: A Review. Biomed. Res. Int. 2022, 2022, 6170452. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface Engineering of Biomaterials in Orthopedic and Dental Implants: Strategies to Improve Osteointegration, Bacteriostatic and Bactericidal Activities. Biotechnol. J. 2021, 16, e2000116. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Fang, S.; Zhong, Q.; Qi, S. Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment. Coatings 2022, 12, 1654. [Google Scholar] [CrossRef]
- Lin, D.-J.; Fuh, L.-J.; Chen, W.-C. Nano-Morphology, Crystallinity and Surface Potential of Anatase on Micro-Arc Oxidized Titanium Affect Its Protein Adsorption, Cell Proliferation and Cell Differentiation. Mater. Sci. Eng. C 2020, 107, 110204. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Vargas, A.L.M.; Sesterheim, P.; Teixeira, E.R.; Hubler, R. Extension of Hydrophilicity Stability by Reactive Plasma Treatment and Wet Storage on TiO2 Nanotube Surfaces for Biomedical Implant Applications. J. R. Soc. Interface 2020, 17, 20200650. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Jambhulkar, N.; Jaju, S.; Raut, A. Surface Modification Techniques for Different Materials Used in Dental Implants: A Review. Mater. Today Proc. 2022, 60, 2266–2269. [Google Scholar] [CrossRef]
- Sharma, P.; Mishra, V.; Murab, S. Unlocking Osseointegration: Surface Engineering Strategies for Enhanced Dental Implant Integration. ACS Biomater. Sci. Eng. 2025, 11, 67–94. [Google Scholar] [CrossRef]
- Fangaia, S.I.G. Tribological Study of Metal Alloys Subject to Dental Wear. Ph.D. Thesis, University of Coimbra, Coimbra, Portugal, 2022. Available online: https://estudogeral.uc.pt/handle/10316/101749 (accessed on 10 March 2025).
- Li, J.; Zhou, P.; Attarilar, S.; Shi, H. Innovative Surface Modification Procedures to Achieve Micro/Nano-Graded Ti-Based Biomedical Alloys and Implants. Coatings 2021, 11, 647. [Google Scholar] [CrossRef]
- Körtvélyessy, G. The Impact of Different Cone-Angle Implant-Abutment Relationships on the Long-Term Success of Implant Restorations. Ph.D. Thesis, University of Szeged, Szeged, Hungary, 2023. Available online: https://doktori.bibl.u-szeged.hu/id/eprint/11803/ (accessed on 10 March 2025).
- Tramontana, D. Custom Abutments for Dental Implants, Their Evolution and Uses: A Narrative Review. Ph.D. Thesis, Universidade Fernando Pessoa, Porto, Portugal, 2022. Available online: https://bdigital.ufp.pt/entities/publication/9d7f967d-48b5-49e1-8ef0-d83a4e7ad52c (accessed on 10 March 2025).
- Gasik, M.; Lambert, F.; Bacevic, M. Biomechanical Properties of Bone and Mucosa for Design and Application of Dental Implants. Materials 2021, 14, 2845. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tsoi, J.K.H.; Lam, W.Y.H.; Pow, E.H.N. Implant Framework Misfit: A Systematic Review on Assessment Methods and Clinical Complications. Clin. Implant Dent. Relat. Res. 2021, 23, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.; Lapinska, B.; Nissan, J.; Lukomska-Szymanska, M. Factors Influencing Marginal Bone Loss Around Dental Implants: A Narrative Review. Coatings 2021, 11, 865. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, M.; Yeo, I.-S.L. Three Interfaces of the Dental Implant System and Their Clinical Effects on Hard and Soft Tissues. Mater. Horiz. 2022, 9, 1387–1411. [Google Scholar] [CrossRef]
- Malmqvist, S.; Erdenborg, J.; Johannsen, G.; Johannsen, A. Patient’s Experiences of Dental Implants, Peri-Implantitis, and Its Treatment—A Qualitative Interview Study. Int. J. Dent. Hyg. 2024, 22, 530–539. [Google Scholar] [CrossRef]
- Cesar, P.F.; Miranda, R.B.P.; Santos, K.F.; Scherrer, S.S.; Zhang, Y. Recent Advances in Dental Zirconia: 15 Years of Material and Processing Evolution. Dent. Mater. 2024, 40, 824–836. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Gupta, S.; Lorusso, F.; Scarano, A.; Noumbissi, S. Oral Tissue Interactions and Cellular Response to Zirconia Implant-Prosthetic Components: A Critical Review. Materials 2021, 14, 2825. [Google Scholar] [CrossRef]
- Sun, J.; Ding, Q.; Chen, Y.; Li, J.; Wang, Z.; Wei, Z.; Ge, X.; Zhang, L. Effects and Underlying Mechanism of Micro-Nano-Structured Zirconia Surfaces on Biological Behaviors of Human Gingival Fibroblasts Under Inflammatory Conditions. Acta Biomater. 2024, 183, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.d.J. Calcium Phosphate and Zirconia Toughened Alumina Reinforced Titanium and CoCrMo Matrix Structures for Articulating Surfaces of Load-Bearing Implants. Ph.D. Thesis, Washington State University, Pullman, WA, USA, 2021. [Google Scholar] [CrossRef]
- Jain, V.K.; Patel, D.S.; Ramkumar, J.; Bhattacharyya, B.; Doloi, B.; Sarkar, B.R.; Ranjan, P.; Sankar, E.S.S.; Jayal, A.D. Micro-Machining: An Overview (Part II). J. Micromanuf. 2022, 5, 46–73. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Muradás, T.C.; Penha, N.; Campos, M.M. Innovative Surfaces and Alloys for Dental Implants: What About Biointerface-Safety Concerns? Dent. Mater. 2021, 37, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wan, K.; Lu, J.; Yuan, C.; Cui, Y.; Duan, R.; Yu, J. Research Progress on Surface Modification of Titanium Implants. Coatings 2025, 15, 229. [Google Scholar] [CrossRef]
- García, I.; Trobajo, C.; Amghouz, Z.; Alonso-Guervos, M.; Díaz, R.; Mendoza, R.; Mauvezín-Quevedo, M.; Adawy, A. Ag- and Sr-Enriched Nanofibrous Titanium Phosphate Phases as Potential Antimicrobial Cement and Coating for a Biomedical Alloy. Mater. Sci. Eng. C 2021, 126, 112168. [Google Scholar] [CrossRef] [PubMed]
- Minguela Díaz, J. Surface Characterization and Cell Instructive Properties of Superficially Modified Dental Zirconia. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2021. [Google Scholar] [CrossRef]
- Pereira, R.M.; Ribas, R.G.; Montanheiro, T.L.D.A.; Schatkoski, V.M.; Rodrigues, K.F.; Kito, L.T.; Kobo, L.K.; Campos, T.M.B.; Bonfante, E.A.; Gierthmuehlen, P.C.; et al. An Engineering Perspective of Ceramics Applied in Dental Reconstructions. J. Appl. Oral Sci. 2023, 31, e20220421. [Google Scholar] [CrossRef]
- Poznyak, A.; Pligovka, A.; Salerno, M. Anodizing of Hydrogenated Titanium and Zirconium Films. Materials 2021, 14, 7490. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Sun, Y.; Yuan, J.C.-C.; Mathew, M.T.; Sukotjo, C.; Takoudis, C.G. In Vitro Corrosion Behavior of Coated Ti6Al4V with TiO2, ZrO2, and TiO2/ZrO2 Mixed Nanofilms Using Atomic Layer Deposition for Dental Implants. Surf. Coat. Technol. 2022, 444, 128686. [Google Scholar] [CrossRef]
- Hadzik, J.; Kubasiewicz-Ross, P.; Gębarowski, T.; Waloszczyk, N.; Maciej, A.; Stolarczyk, A.; Gedrange, T.; Dominiak, M.; Szajna, E.; Simka, W. An Experimental Anodized Titanium Surface for Transgingival Dental Implant Elements—Preliminary Report. J. Funct. Biomater. 2023, 14, 34. [Google Scholar] [CrossRef]
- Hussain, S.; Sabiruddin, K. Surface Modification Methods of Titanium (Ti)-Based Implant Materials for Biomedical Applications. In Bioimplants Manufacturing, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024; p. 22. ISBN 9781003509943. [Google Scholar]
- Saha, S.; Roy, S. Metallic Dental Implants Wear Mechanisms, Materials, and Manufacturing Processes: A Literature Review. Materials 2023, 16, 161. [Google Scholar] [CrossRef]
- Huang, S.; Wei, H.; Li, D. Additive Manufacturing Technologies in the Oral Implant Clinic: A Review of Current Applications and Progress. Front. Bioeng. Biotechnol. 2023, 11, 1100155. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.B.; Silva, N.; Marques, J.F.; Mata, A.; Silva, F.S.; Caramês, J. Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review. Biomimetics 2022, 7, 74. [Google Scholar] [CrossRef]
- Tahan, F.I.; Abdelhamid, A.M.; Osman, E.; Harouny, R.; Hachem, R.; Aly, Y.M. The Effect of Nitric Acid–Hydrofluoric Acid Etching Solution on the Shear Bond Strength and Mode of Failure of Resin Cement to Zirconia (In Vitro Study). Ann. Stomatol. 2024, 15, 85–93. [Google Scholar] [CrossRef]
- Abdelraouf, R. Introduction to Materials–Tissue Interface. Biomater. Sci. 2022, 1, 35–52. [Google Scholar]
- Assaf, A.; Azer, S.S.; Sfeir, A.; Al-Haj Husain, N.; Özcan, M. Risk Factors with Porcelain Laminate Veneers Experienced During Cementation: A Review. Materials 2023, 16, 4932. [Google Scholar] [CrossRef] [PubMed]
- Osak, P.; Skwarek, S.; Łukowiec, D.; Przeliorz, G.; Łosiewicz, B. Preparation and Characterization of Oxide Nanotubes on Titanium Surface for Use in Controlled Drug Release Systems. Materials 2024, 17, 3753. [Google Scholar] [CrossRef] [PubMed]
- Benčina, M.; Resnik, M.; Starič, P.; Junkar, I. Use of Plasma Technologies for Antibacterial Surface Properties of Metals. Molecules 2021, 26, 1418. [Google Scholar] [CrossRef]
- Aljafery, A.M.A.; Fatalla, A.A.; Haider, J. Cytotoxicity, Corrosion Resistance, and Wettability of Titanium and Ti-TiB2 Composite Fabricated by Powder Metallurgy for Dental Implants. Metals 2024, 14, 538. [Google Scholar] [CrossRef]
- Sapiro, I. Comparative Roughness Analysis of Dental Implants and Comparison of Methods Using Scanning Electron Microscopy and White Light Interferometry. Ph.D. Thesis, Charité—Universitätsmedizin Berlin, Berlin, Germany, 2024. Available online: https://refubium.fu-berlin.de/handle/fub188/41881 (accessed on 8 March 2025).
- Ming, X.; Wu, Y.; Zhang, Z.; Li, Y. Micro-Arc Oxidation in Titanium and Its Alloys: Development and Potential of Implants. Coatings 2023, 13, 2064. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Biocompatibility of Plasma Electrolytic Oxidation Coated Titanium Alloy for Biomedical Applications. BioNanoScience 2025, 15, 232. [Google Scholar] [CrossRef]
- Kyrylenko, S.; Warchoł, F.; Oleshko, O.; Husak, Y.; Kazek-Kęsik, A.; Korniienko, V.; Deineka, V.; Sowa, M.; Maciej, A.; Michalska, J.; et al. Effects of the Sources of Calcium and Phosphorus on the Structural and Functional Properties of Ceramic Coatings on Titanium Dental Implants Produced by Plasma Electrolytic Oxidation. Mater. Sci. Eng. C 2021, 119, 111607. [Google Scholar] [CrossRef]
- Mishchenko, O.; Volchykhina, K.; Maksymov, D.; Manukhina, O.; Pogorielov, M.; Pavlenko, M.; Iatsunskyi, I. Advanced Strategies for Enhancing the Biocompatibility and Antibacterial Properties of Implantable Structures. Materials 2025, 18, 822. [Google Scholar] [CrossRef] [PubMed]
- Golalipour, S.; Jalalian, E.; Koosha, S.; Khorshidi, S.; Torshabi, M.; Sayyari, M. In Vitro Effect of Anodization on Surface Roughness and Bacterial Adhesion to Titanium Abutments. J. Prosthet. Dent. 2025, 133, 291.e1–291.e8. [Google Scholar] [CrossRef] [PubMed]
- Vattanasup, C.; Kuntiyaratana, T.; Rungsiyakull, P.; Chaijareenont, P. Color Formation on Titanium Surface Treated by Anodization and the Surface Characteristics: A Review. Chiang Mai Dent. J. 2023, 44, 13–21. Available online: https://www.dent.cmu.ac.th/cmdj/frontend/web/?r=site/viewarticle&id=199 (accessed on 8 March 2025). [CrossRef]
- Meng, F.; Yin, Z.; Ren, X.; Geng, Z.; Su, J. Construction of Local Drug Delivery System on Titanium-Based Implants to Improve Osseointegration. Pharmaceutics 2022, 14, 1069. [Google Scholar] [CrossRef] [PubMed]
- Sotova, C.; Yanushevich, O.; Kriheli, N.; Grigoriev, S.; Evdokimov, V.; Kramar, O.; Nozdrina, M.; Peretyagin, N.; Undritsova, N.; Popelyshkin, E.; et al. Dental Implants: Modern Materials and Methods of Their Surface Modification. Materials 2023, 16, 7383. [Google Scholar] [CrossRef]
- Kandavalli, S.R.; Wang, Q.; Ebrahimi, M.; Gode, C.; Djavanroodi, F.; Attarilar, S.; Liu, S. A Brief Review on the Evolution of Metallic Dental Implants: History, Design, and Application. Front. Mater. 2021, 8, 646383. [Google Scholar] [CrossRef]
- Al-Amin, M.D. Effects of Multiple Metal Oxides on Properties of ZTA-5TiO2-5MgO Composites. Ph.D. Thesis, University of Dhaka, Dhaka, Bangladesh, 2023. Available online: http://reposit.library.du.ac.bd:8080/xmlui/handle/123456789/2656 (accessed on 8 March 2025).
- Hichem, N.; Hadjer, Z.; Fateh, S.; Feriel, L.; Wang, Z. The Potential Exposure and Hazards of Zirconia Nanoparticles: A Review. Ecotoxicol. Environ. Contam. 2022, 17, 1–21. [Google Scholar] [CrossRef]
- Gautam, S.; Bhatnagar, D.; Bansal, D.; Batra, H.; Goyal, N. Recent Advancements in Nanomaterials for Biomedical Implants. Biomed. Eng. Adv. 2022, 3, 100029. [Google Scholar] [CrossRef]
- Losic, D. Advancing Titanium Medical Implants by Surface Engineering: Recent Progress and Challenges. Expert Opin. Drug Deliv. 2021, 18, 1355–1378. [Google Scholar] [CrossRef]
- Steblevskaya, N.I.; Belobeletskaya, M.V.; Yarovaya, T.P.; Nedozorov, P.M. Preparation and Luminescence Properties of the Composite of Mixed Europium and Tantalum Oxides/ZrO2 + TiO2/Ti. Russ. J. Inorg. Chem. 2022, 67, 415–423. [Google Scholar] [CrossRef]
- Baishya, K.; Bacova, J.; Al Chimali, B.; Capek, J.; Michalicka, J.; Gautier, G.; Le Borgne, B.; Rousar, T.; Macak, J.M. Ultrathin ALD Coatings of Zr and V Oxides on Anodic TiO2 Nanotube Layers: Comparison of the Osteoblast Cell Growth. ACS Appl. Mater. Interfaces 2025, 17, 739–749. [Google Scholar] [CrossRef]
- Hayashi, T.; Asakura, M.; Koie, S.; Hasegawa, S.; Mieki, A.; Aimu, K.; Kawai, T. In Vitro Study of Zirconia Surface Modification for Dental Implants by Atomic Layer Deposition. Int. J. Mol. Sci. 2023, 24, 10101. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Webster, T.J. Metallic Nanoscaffolds as Osteogenic Promoters: Advances, Challenges and Scope. Metals 2021, 11, 1356. [Google Scholar] [CrossRef]
- Puspasari, V.; Ridhova, A.; Hermawan, A.; Amal, M.I.; Khan, M.M. ZnO-Based Antimicrobial Coatings for Biomedical Applications. Bioprocess Biosyst. Eng. 2022, 45, 1421–1445. [Google Scholar] [CrossRef] [PubMed]
- Jadon, M.S.; Kumar, S. Fabrication of TiO2 Nanoparticle Coating on Stainless Steel 316L and Its Assessment for Orthopaedic Applications. Int. J. Online Biomed. Eng. 2024, 20, 47–63. [Google Scholar] [CrossRef]
- Gürsoy, E.; Yılmaz, H. Nanoparticles and Their Application in Prosthetic Dentistry. Clin. Exp. Health Sci. 2023, 13, 685–695. [Google Scholar] [CrossRef]
- Itoh, H.; Yanagishita, T. Effects of Pore Arrangement of TiO2-Coated Porous Alumina Membranes on Photocatalytic Properties. J. Phys. Chem. C 2024, 128, 6478–6486. [Google Scholar] [CrossRef]
- Hashemi Astaneh, S. Surface Functionalization and Enhancement of Properties of Biomaterials Through Atomic Layer Deposition. Ph.D. Thesis, University of Illinois Chicago, Chicago, IL, USA, 2021. [Google Scholar] [CrossRef]
- Rudolf, R.; Majerič, P.; Lazić, V.; Raić, K.T. Advanced Dental Metallic Materials; Springer: Berlin/Heidelberg, Germany, 2024; ISBN 9783031473500. [Google Scholar]
- Momesso, G.A.C.; de Souza Santos, A.M.; Fonseca e Santos, J.M.; da Cruz, N.C.; Okamoto, R.; Barão, V.A.R.; Siroma, R.S.; Shibli, J.A.; Perez Faverani, L. Comparison Between Plasma Electrolytic Oxidation Coating and Sandblasted Acid-Etched Surface Treatment: Histometric, Tomographic, and Expression Levels of Osteoclastogenic Factors in Osteoporotic Rats. Materials 2020, 13, 1604. [Google Scholar] [CrossRef]
- Sen, A.; Banerjee, N.; Biswas, A.R.; Ghosh, T.K.; Samanta, A.; Kumar, M.; Das, S.; Srivastava, U.; Sengupta, D.; Maity, S.R. Standards of Mechanical, Physical, Chemical, and Biological Properties of Bioceramics. In Advanced Bioceramics; CRC Press: Boca Raton, FL, USA, 2023; pp. 1–17. ISBN 9781003258353. [Google Scholar]
- Paul, S.; Hanisch, O.; Nesic, D. Human Gingival Fibroblast Proliferation on Materials Used for Dental Implant Abutments: A Systematic Review. Int. J. Prosthodont. 2021, 34, 811–828. [Google Scholar] [CrossRef]
- van Oirschot, B.A.J.A.; Zhang, Y.; Alghamdi, H.S.; Cordeiro, J.M.; Nagay, B.E.; Barao, V.A.R.; de Avila, E.D.; van den Beucken, J.J.J.P. Surface Engineering for Dental Implantology: Favoring Tissue Responses Along the Implant. Tissue Eng. Part A 2022, 28, 555–572. [Google Scholar] [CrossRef]
- Abdulla, M.A.; Hasan, R.H.; Al-Hyani, O.H. Radiographic and Histologic Assessment of Osseointegration for Surface-Treated Titanium Dental Implants: An Experimental Study in Dogs. J. Dent. Res. Dent. Clin. Dent. Prospects 2024, 18, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia Surface Modifications for Implant Dentistry. Mater. Sci. Eng. C 2019, 12, 1294–1305. [Google Scholar] [CrossRef]
- Kunrath, M.F. Customized Dental Implants: Manufacturing Processes, Topography, Osseointegration and Future Perspectives of 3D Fabricated Implants. Bioprinting 2020, 20, e00107. [Google Scholar] [CrossRef]
- Ispas, A.; Iosif, L.; Murariu-Măgureanu, C.; Craciun, A.; Constantiniuc, M. Zirconia in Dental Medicine: A Brief Overview of Its Properties and Processing Techniques. Hum. Vet. Med. 2021, 13, 33–39. [Google Scholar]
- Agut López, R. Microstructural Design of a New Zirconia-Based Material for Dental Implants. Bachelor’s Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2021. [Google Scholar]
- Eftekhar Ashtiani, R.; Alam, M.; Tavakolizadeh, S.; Abbasi, K. The Role of Biomaterials and Biocompatible Materials in Implant-Supported Dental Prosthesis. Evid. Based Complement. Alternat. Med. 2021, 2021, 3349433. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Wu, W.; Hou, M.; Liu, T.; Wang, T.; Yang, H. Review of Zirconia-Based Biomimetic Scaffolds for Bone Tissue Engineering. J. Mater. Sci. 2021, 56, 8309–8333. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Raffaele-Esposito, A.; Carvalho, O.; Silva, F.; Özcan, M.; Henriques, B. Surface Modification of Zirconia or Lithium Disilicate-Reinforced Glass Ceramic by Laser Texturing to Increase the Adhesion of Prosthetic Surfaces to Resin Cements: An Integrative Review. Clin. Oral Investig. 2023, 27, 3331–3345. [Google Scholar] [CrossRef]
- Rodríguez Montaño, Ó. Influence of the Micro-Geometry of a Scaffold for Bone Regeneration on the Stresses of the Newly Formed Tissue. Ph.D. Thesis, National University of Colombia, Bogotá, Colombia, 2021. [Google Scholar]
- Carvalho, A.; Grenho, L.; Fernandes, M.H.; Daskalova, A.; Trifonov, A.; Buchvarov, I.; Monteiro, F.J. Femtosecond Laser Microstructuring of Alumina Toughened Zirconia for Surface Functionalization of Dental Implants. Ceram. Int. 2020, 46, 1383–1389. [Google Scholar] [CrossRef]
- Migliorini, F.; Schenker, H.; Betsch, M.; Maffulli, N.; Tingart, M.; Hildebrand, F.; Lecouturier, S.; Rath, B.; Eschweiler, J. Silica Coated High-Performance Oxide Ceramics Promote Greater Ossification Than Titanium Implants: An In Vivo Study. J. Orthop. Surg. Res. 2023, 18, 31. [Google Scholar] [CrossRef]
- Drevet, R.; Fauré, J.; Benhayoune, H. Electrophoretic Deposition of Bioactive Glass Coatings for Bone Implant Applications: A Review. Coatings 2024, 14, 1084. [Google Scholar] [CrossRef]
- Avelino, S.O.M.; Alvares Sobral-Silva, L.; Thim, G.P.; de Almeida-Silva, L.A.; Dos Santos Lupp, J.; Campos, T.M.B.; de Vasconcellos, L.M.R. Development, Characterization, and Biological Study of Bioglass Coatings 45S5 and BioK on Zirconia Implant Surfaces. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35380. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Pradhan, L.; Yenurkar, D.; Kumar, K.; Mukherjee, S. Advancement in Ceramic Biomaterials for Dental Implants. Int. J. Appl. Ceram. Technol. 2024, 21, 2796–2817. [Google Scholar] [CrossRef]
- Liang, J.; Lu, X.; Zheng, X.; Li, Y.R.; Geng, X.Y.; Sun, K.X.; Cai, H.X.; Jia, Q.; Jiang, H.B.; Liu, K. Modification of Titanium Orthopedic Implants with Bioactive Glass: A Systematic Review of In Vivo and In Vitro Studies. Front. Bioeng. Biotechnol. 2023, 11, 1269223. [Google Scholar] [CrossRef] [PubMed]
- Amghouz, Z.; García, J.R.; Adawy, A. A Review on the Synthesis and Current and Prospective Applications of Zirconium and Titanium Phosphates. Eng 2022, 3, 161–174. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Z.-Y.; Chen, W. The Fabrication and In Vitro Bioactivity of HA/ZrO2/MgO Gradient Composite Coatings. Ceram. Int. 2024, 50, 1814–1825. [Google Scholar] [CrossRef]
- Guo, S.; Liu, N.; Liu, K.; Li, Y.; Zhang, W.; Zhu, B.; Gu, B.; Wen, N. Effects of Carbon and Nitrogen Plasma Immersion Ion Implantation on Bioactivity of Zirconia. RSC Adv. 2020, 10, 35917–35929. [Google Scholar] [CrossRef]
- Fróis, A.; Francisco, R.; Morais, P.V.; Santos, L.F.; Peres, M.; Lorenz, K.; Santos, A.C.; Louro, C.S. The Influence of Low Nitrogen Doping on Bacterial Adhesion of Sputtered a-C:H Coatings. Diam. Relat. Mater. 2024, 147, 111309. [Google Scholar] [CrossRef]
- Rogala-Wielgus, D.; Zieliński, A. Preparation and Properties of Composite Coatings, Based on Carbon Nanotubes, for Medical Applications. Carbon Lett. 2024, 34, 565–601. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.-H.; Kim, N.-K.; Kang, I.-K. Bioactive Surface of Zirconia Implant Prepared by Nano-Hydroxyapatite and Type I Collagen. Coatings 2022, 12, 1335. [Google Scholar] [CrossRef]
- Rathee, G.; Bartwal, G.; Rathee, J.; Mishra, Y.K.; Kaushik, A.; Solanki, P.R. Emerging Multimodal Zirconia Nanosystems for High-Performance Biomedical Applications. Adv. NanoBiomed Res. 2021, 1, 2100039. [Google Scholar] [CrossRef]
- Kim, J.; Kang, I.G.; Cheon, K.H.; Lee, S.; Park, S.; Kim, H.E.; Han, C.M. Stable Sol-Gel Hydroxyapatite Coating on Zirconia Dental Implant for Improved Osseointegration. J. Mater. Sci. Mater. Med. 2021, 32, 81. [Google Scholar] [CrossRef]
- Aboushelib, M.N.; Shawky, R. Osteogenesis Ability of CAD/CAM Porous Zirconia Scaffolds Enriched with Nano-Hydroxyapatite Particles. Int. J. Implant Dent. 2017, 3, 21. [Google Scholar] [CrossRef]
- Ding, S.J.; Chu, Y.H.; Chen, P.T. Mechanical Biocompatibility, Osteogenic Activity, and Antibacterial Efficacy of Calcium Silicate-Zirconia Biocomposites. ACS Omega 2021, 6, 7106–7118. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted Hydroxyapatite Coatings of Bone Implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- García, I.; Trobajo, C.; Amghouz, Z.; Adawy, A. Nanolayered Metal Phosphates as Biocompatible Reservoirs for Antimicrobial Silver Nanoparticles. Materials 2021, 14, 1481. [Google Scholar] [CrossRef]
- Lalzawmliana, V.; Mukherjee, P.; Roy, S.; Roy, M.; Nandi, S.K. Ceramic Biomaterials in Advanced Biomedical Applications. In Functional Biomaterials; Jana, S., Jana, S., Eds.; Springer: Singapore, 2022; pp. 239–254. [Google Scholar] [CrossRef]
- Sarasati, A.; Wihadmadyatami, H.; Ana, I.D. Carbonate Apatite Nanoparticles: A Novel Nano-Adjuvant for Oral Mucosal Vaccines and Immunomodulator. OpenNano 2023, 12, 100149. [Google Scholar] [CrossRef]
- Schünemann, F.H. Development of Bioactive-Zirconia Surfaces for Biomedical Applications. Ph.D. Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2023. Available online: https://repositorio.ufsc.br/handle/123456789/249886 (accessed on 8 March 2025).
- He, Q.; Zhang, W.; Zhan, X.; Qin, Y.; Ye, J. Enhanced Bioactivity and Hydrothermal Aging Resistance of Y-TZP Ceramics for Dental Implant. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 1824–1839. [Google Scholar] [CrossRef]
- Parekh, A.; Moore, M.; Janorkar, A.V.; Roach, M.D. Mg-Doped Carbonated Hydroxyapatite and Tricalcium Phosphate Anodized Coatings on Titanium Implant Alloys. Appl. Sci. 2024, 14, 11831. [Google Scholar] [CrossRef]
- Sari, M.; Kristianto, N.A.; Chotimah, A.I.D.; Yusuf, Y. Carbonated Hydroxyapatite-Based Honeycomb Scaffold Coatings on a Titanium Alloy for Bone Implant Application—Physicochemical and Mechanical Properties Analysis. Coatings 2021, 11, 941. [Google Scholar] [CrossRef]
- Kengtanyakich, S.; Peampring, C. An Experimental Study on Hydrothermal Degradation of Cubic-Containing Translucent Zirconia. J. Adv. Prosthodont. 2020, 12, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Oh, E.J.; Lim, M.J.; Lee, K.W. Change of Phase Transformation and Bond Strength of Y-TZP with Various Hydrofluoric Acid Etching. Restor. Dent. Endod. 2021, 46, e54. [Google Scholar] [CrossRef]
- Li, K.; Rao, J.; Ning, C. Optimized Sintering and Mechanical Properties of Y-TZP Ceramics for Dental Restorations by Adding Lithium Disilicate Glass Ceramics. J. Adv. Ceram. 2021, 10, 1326–1337. [Google Scholar] [CrossRef]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-Bone-Interface: Reviewing the Impact of Titanium Surface Modifications on Osteogenic Processes In Vitro and In Vivo. Bioeng. Transl. Med. 2021, 1, 10239. [Google Scholar] [CrossRef]
- Gaur, P.; Muthukumar, C.; Parthasarathy, V. Friction and Wear Properties of Biocomposites for Dental, Orthopedic, and Biomedical Applications. In Tribological Properties, Performance and Applications of Biocomposites; Muthukumar, C., Krishnasamy, S., Thiagamani, S.M., Chinnachamy, G., Eds.; Wiley-VCH: New York, NY, USA, 2023; Chapter 12. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Verma, V.; Hazari, P.; Verma, P. Do Implants Made of Polyetheretherketone and Its Composites Have Reduced Stress Shielding Effects Compared to Other Dental Implant Materials? A Systematic Review. Evid. Based Dent. 2023, 24, 193–194. [Google Scholar] [CrossRef]
- Manfredini, M.; Poli, P.P.; Giboli, L.; Beretta, M.; Maiorana, C.; Pellegrini, M. Clinical Factors on Dental Implant Fractures: A Systematic Review. Dent. J. 2024, 12, 200. [Google Scholar] [CrossRef]
- Esteves, G.M.; Esteves, J.; Resende, M.; Mendes, L.; Azevedo, A.S. Antimicrobial and Antibiofilm Coating of Dental Implants—Past and New Perspectives. Antibiotics 2022, 11, 235. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, P.; Gao, W. Microbial Dysbiosis in Periodontitis and Peri-implantitis: Pathogenesis, Immune Responses, and Therapeutics. Front. Cell. Infect. Microbiol. 2025, 15, 1517154. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Li, J.; Xiang, X.; Wu, J.; Zeng, S. Stimuli-Responsive Materials in Oral Diseases: A Review. Clin. Oral Investig. 2024, 28, 497. [Google Scholar] [CrossRef] [PubMed]
- Abdulghafor, M.A.; Mahmood, M.K.; Tassery, H.; Tardivo, D.; Falguiere, A.; Lan, R. Biomimetic Coatings in Implant Dentistry: A Quick Update. J. Funct. Biomater. 2024, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Kocak-Oztug, N.A.; Ravali, E.I. Titanium Dental Implants in Compromised Conditions: Need for Enhanced Bioactivity and Therapy. In Surface Modification of Titanium Dental Implants; Gulati, K., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Khaohoen, A.; Sornsuwan, T.; Chaijareenont, P.; Poovarodom, P.; Rungsiyakull, C.; Rungsiyakull, P. Biomaterials and Clinical Application of Dental Implants in Relation to Bone Density—A Narrative Review. J. Clin. Med. 2023, 12, 6924. [Google Scholar] [CrossRef]
- Karmakar, R.; Dixit, M.; Rengan, A.K.; Pati, F. Nanostructures Using 3D Printing. In Advances in Nanostructures; Gautam, S., Singh, J.P., Rai, D.P., Kumar, A., Mishra, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 195–229. [Google Scholar] [CrossRef]
- Hakim, L.K.; Yari, A.; Nikparto, N.; Mehraban, S.H.; Cheperli, S.; Asadi, A.; Darehdor, A.A.; Nezaminia, S.; Dortaj, D.; Nazari, Y.; et al. The Current Applications of Nano and Biomaterials in Drug Delivery of Dental Implants. BMC Oral Health 2024, 24, 126. [Google Scholar] [CrossRef]
- Cicha, I.; Priefer, R.; Severino, P.; Souto, E.B.; Jain, S. Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules 2022, 12, 1198. [Google Scholar] [CrossRef]
- Meng, M.; Wang, J.; Huang, H.; Liu, X.; Zhang, J.; Li, Z. 3D Printing Metal Implants in Orthopedic Surgery: Methods, Applications and Future Prospects. J. Orthop. Transl. 2023, 42, 94–112. [Google Scholar] [CrossRef]
- Raheem, A.A.; Hameed, P.; Whenish, R.; Elsen, R.S.; G, A.; Jaiswal, A.K.; Prashanth, K.G.; Manivasagam, G. A Review on Development of Bio-Inspired Implants Using 3D Printing. Biomimetics 2021, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Maintz, M.; Tourbier, C.; de Wild, M.; Cattin, P.C.; Beyer, M.; Seiler, D.; Honigmann, P.; Sharma, N.; Thieringer, F.M. Patient-Specific Implants Made of 3D Printed Bioresorbable Polymers at the Point-of-Care: Material, Technology, and Scope of Surgical Application. 3D Print. Med. 2024, 10, 13. [Google Scholar] [CrossRef]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, G. Surface Modification of Ti6Al4V Alloy via Advanced Coatings: Mechanical, Tribological, Corrosion, Wetting, and Biocompatibility Studies. J. Alloys Compd. 2024, 989, 174418. [Google Scholar] [CrossRef]
- Varma, M.V.; Kandasubramanian, B.; Ibrahim, S.M. 3D Printed Scaffolds for Biomedical Applications. Mater. Chem. Phys. 2020, 255, 123642. [Google Scholar] [CrossRef]
- Dixit, S.; Gupta, S.; Sharma, A. Surgical Devices for Biomedical Implants. In Additive Manufacturing for Biomedical Applications; Dixit, A., Kumar, A., Pathak, D.K., Eds.; Biomedical Materials for Multi-Functional Applications; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
| # | Search Query | Number of Results |
|---|---|---|
| 1 | Surface Treatment * OR Surface Modification * OR Dental Implant Coating * OR Nano-textured Surface * OR Surface Roughness * OR Plasma Spraying * OR Acid Etching * OR Anodization* OR Grit Blasting * OR Atomic Layer Deposition* (Title) | 31,556 |
| 2 | Titanium Dental Implant * OR Zirconia Dental Implant * OR Ti-based Implant* OR ZrO2 Implant * OR Hybrid Dental Implant * OR Ti/ZrO2 Coating * OR ZrO2 Bioactivity * (Title) | 1308 |
| 3 | Titanium Dental Implant * OR Zirconia Dental Implant * OR Ti-based Implant* OR ZrO2 Implant * OR Hybrid Dental Implant * OR Ti/ZrO2 Coating * OR ZrO2 Bioactivity * (Title) | 709 |
| 4 | #5 AND #2 AND #3 | 100 |
| Technique | Material | Process | Surface Scale | Ref. |
|---|---|---|---|---|
| Machining | Ti, ZrO2, Ti alloys | Mechanical lathe | Macro/micro | [6,26,27] |
| Grit blasting | Ti, ZrO2, ceramics, Ti alloys | Particle blasting with sand, Al, Ti, alumina, silica, hydroxyapatite | Micro/nano | [10,12,28] |
| Acid etching | Ti, ZrO2, ceramics, Ti alloys | Acid solutions of varying concentrations, temperatures, and application times | Micro/nano | [6,29] |
| Grit blasting–acid etching | Ti, ZrO2, ceramics, Ti alloys | Several kinds of grit blasting and acid etching | Micro/nano | [10,11,30,31] |
| Anodizing | Ti, ZrO2, Ti alloys | Electrochemical methods using various electrolyte solutions, temperatures, times, and voltages | Micro/nano | [32,33,34] |
| Plasma spraying | Ti, ZrO2, Ti alloys | Creation of thin films over the surface using a plasma torch under vacuum | Micro/nano | [7,35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aissi, M.; Tayyaba, Q.; Er-Ramly, A.; Hermawan, H.; Merzouk, N. Improving the Clinical Performance of Dental Implants Through Advanced Surface Treatments: The Case of Ti and ZrO2 Coatings. Metals 2025, 15, 320. https://doi.org/10.3390/met15030320
Aissi M, Tayyaba Q, Er-Ramly A, Hermawan H, Merzouk N. Improving the Clinical Performance of Dental Implants Through Advanced Surface Treatments: The Case of Ti and ZrO2 Coatings. Metals. 2025; 15(3):320. https://doi.org/10.3390/met15030320
Chicago/Turabian StyleAissi, Mohamed, Qanita Tayyaba, Azzedine Er-Ramly, Hendra Hermawan, and Nadia Merzouk. 2025. "Improving the Clinical Performance of Dental Implants Through Advanced Surface Treatments: The Case of Ti and ZrO2 Coatings" Metals 15, no. 3: 320. https://doi.org/10.3390/met15030320
APA StyleAissi, M., Tayyaba, Q., Er-Ramly, A., Hermawan, H., & Merzouk, N. (2025). Improving the Clinical Performance of Dental Implants Through Advanced Surface Treatments: The Case of Ti and ZrO2 Coatings. Metals, 15(3), 320. https://doi.org/10.3390/met15030320








