Effects of Heat Treatment on Microstructures and Corrosion Properties of AlxCrFeNi Medium-Entropy Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Microstructural Characterization and Composition Analysis
2.3. Microhardness Test
2.4. Corrosion Properties
2.4.1. Electrochemical Tests
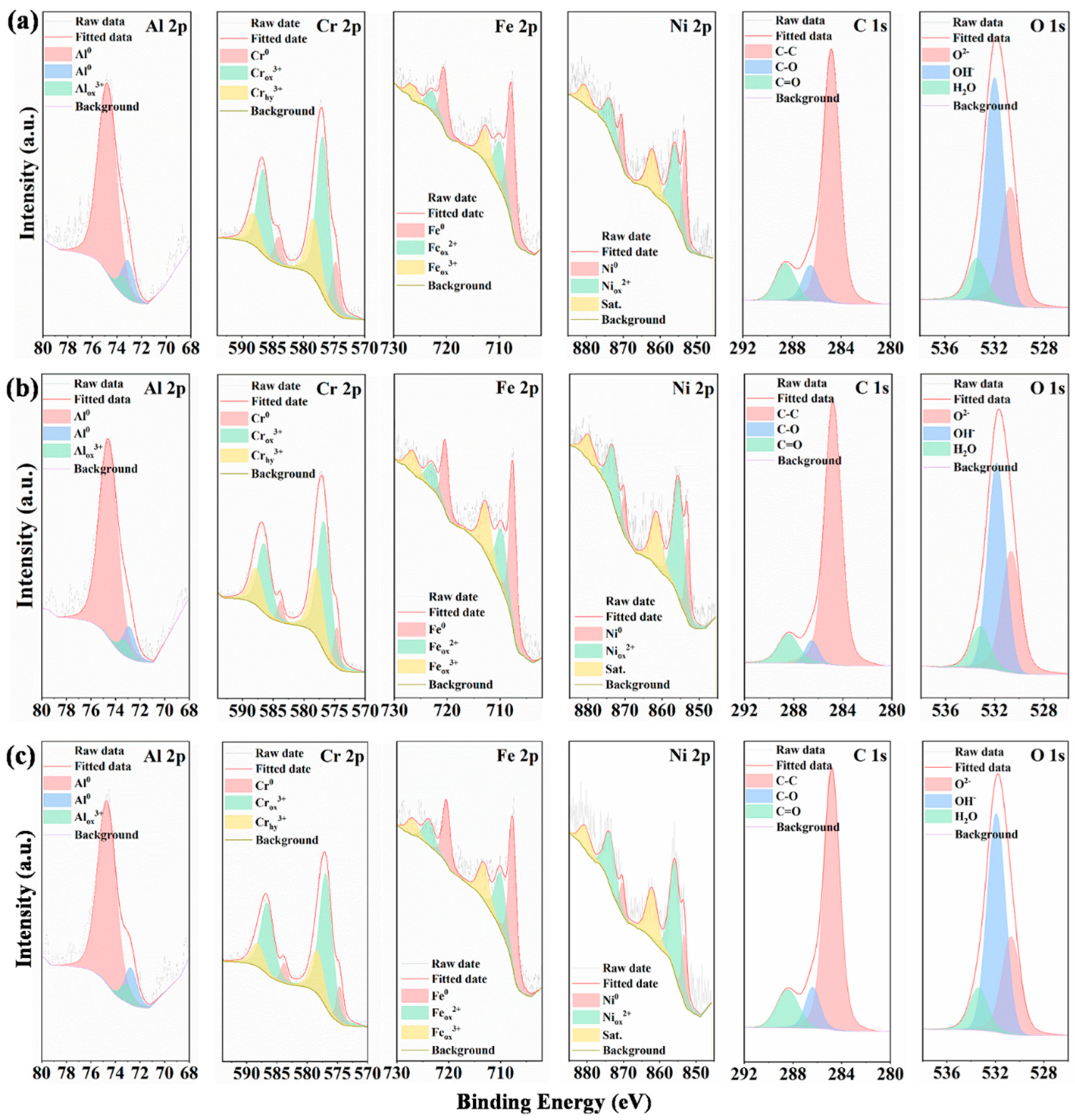
2.4.2. In-Situ Immersion Corrosion Tests and XPS Characterizations
3. Results and Discussion
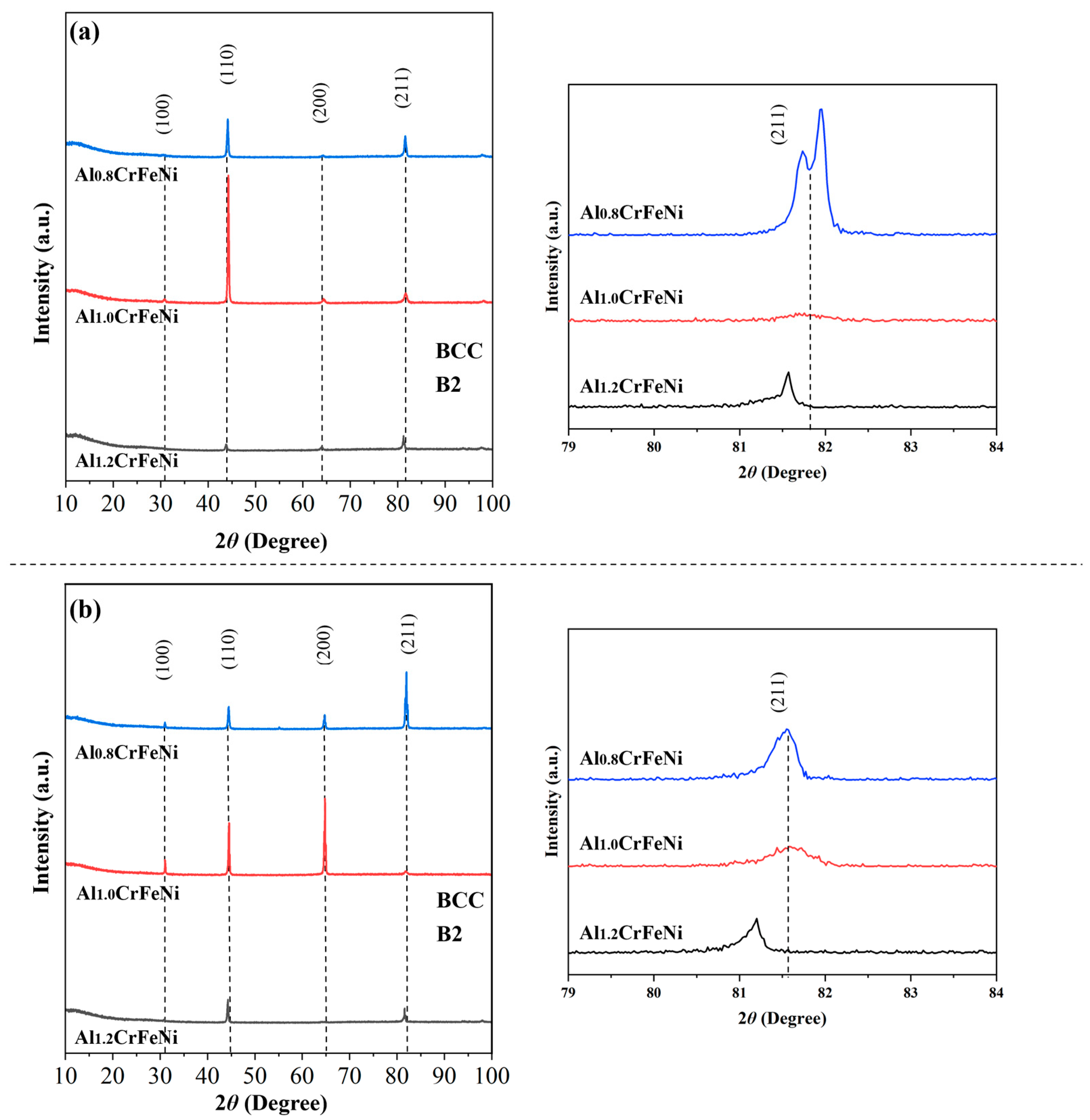
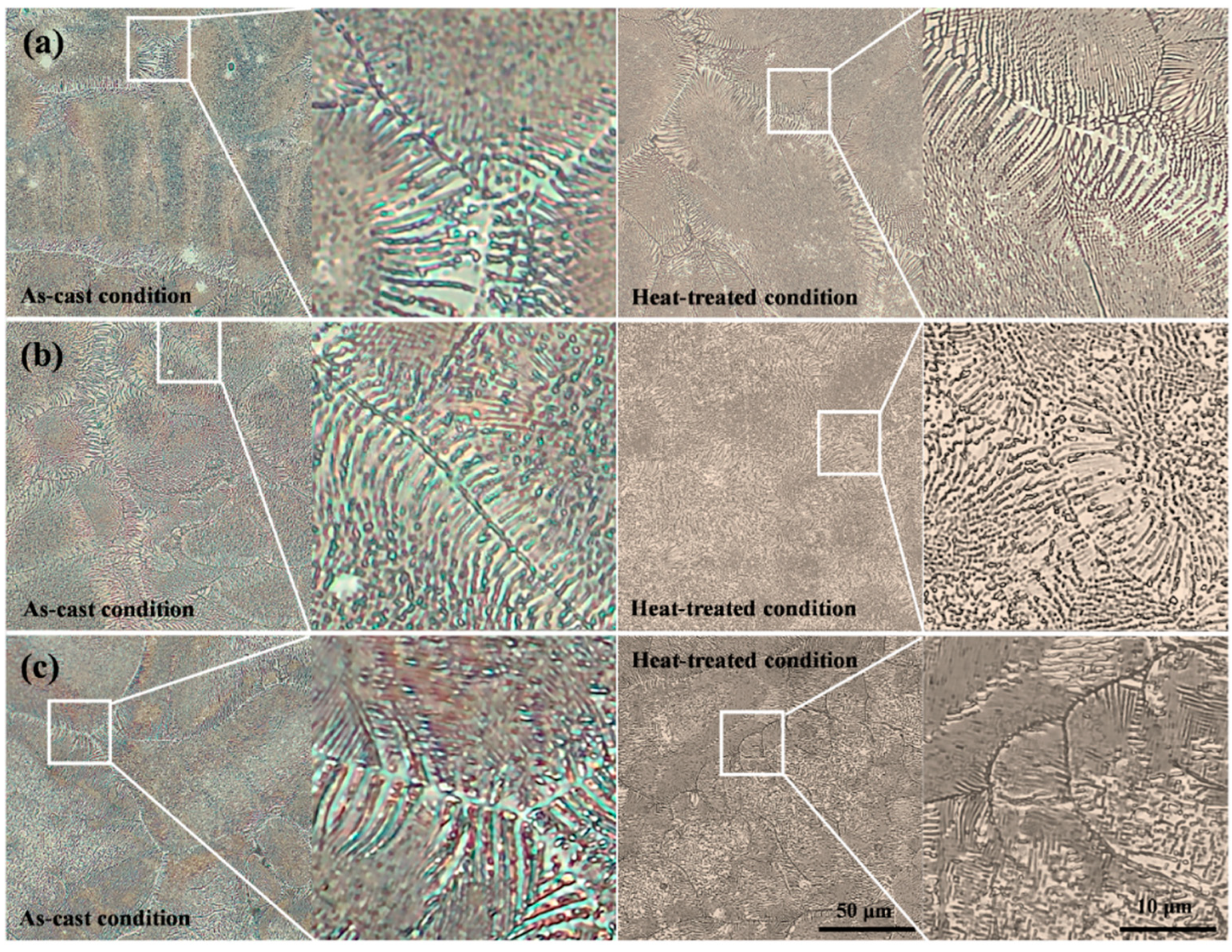
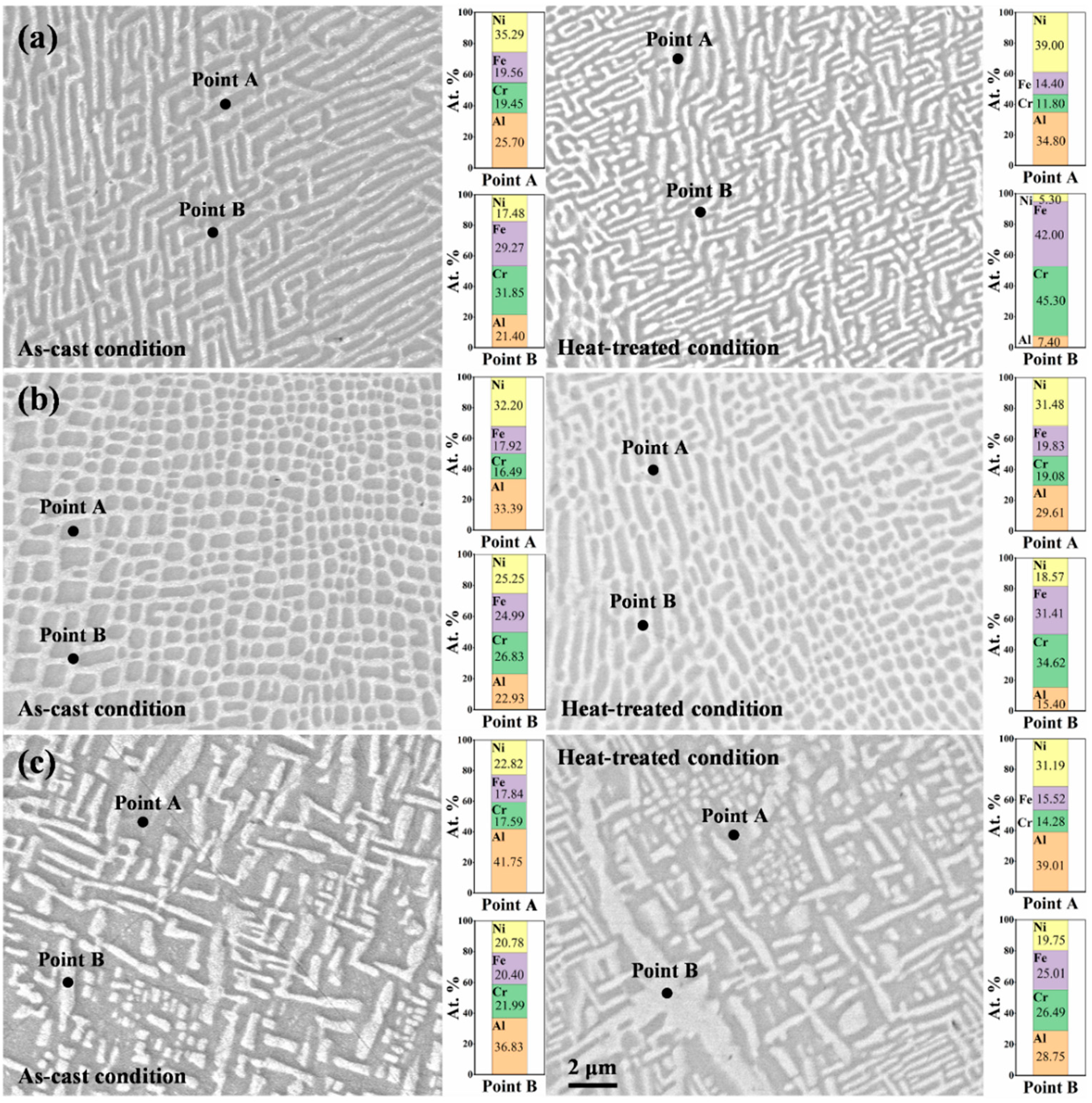
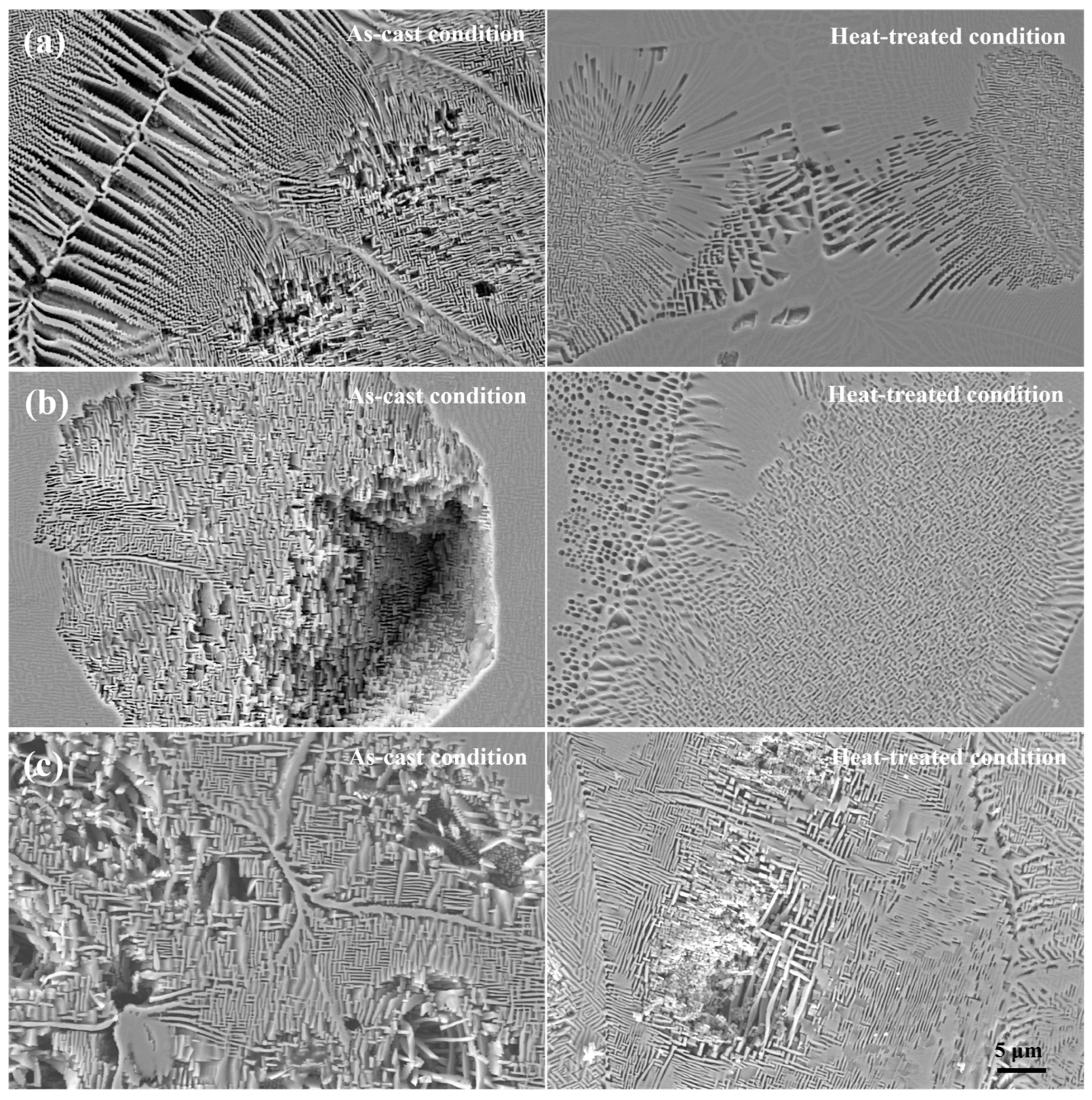
3.1. Microstructural Characteristics
3.2. Microhardness Property
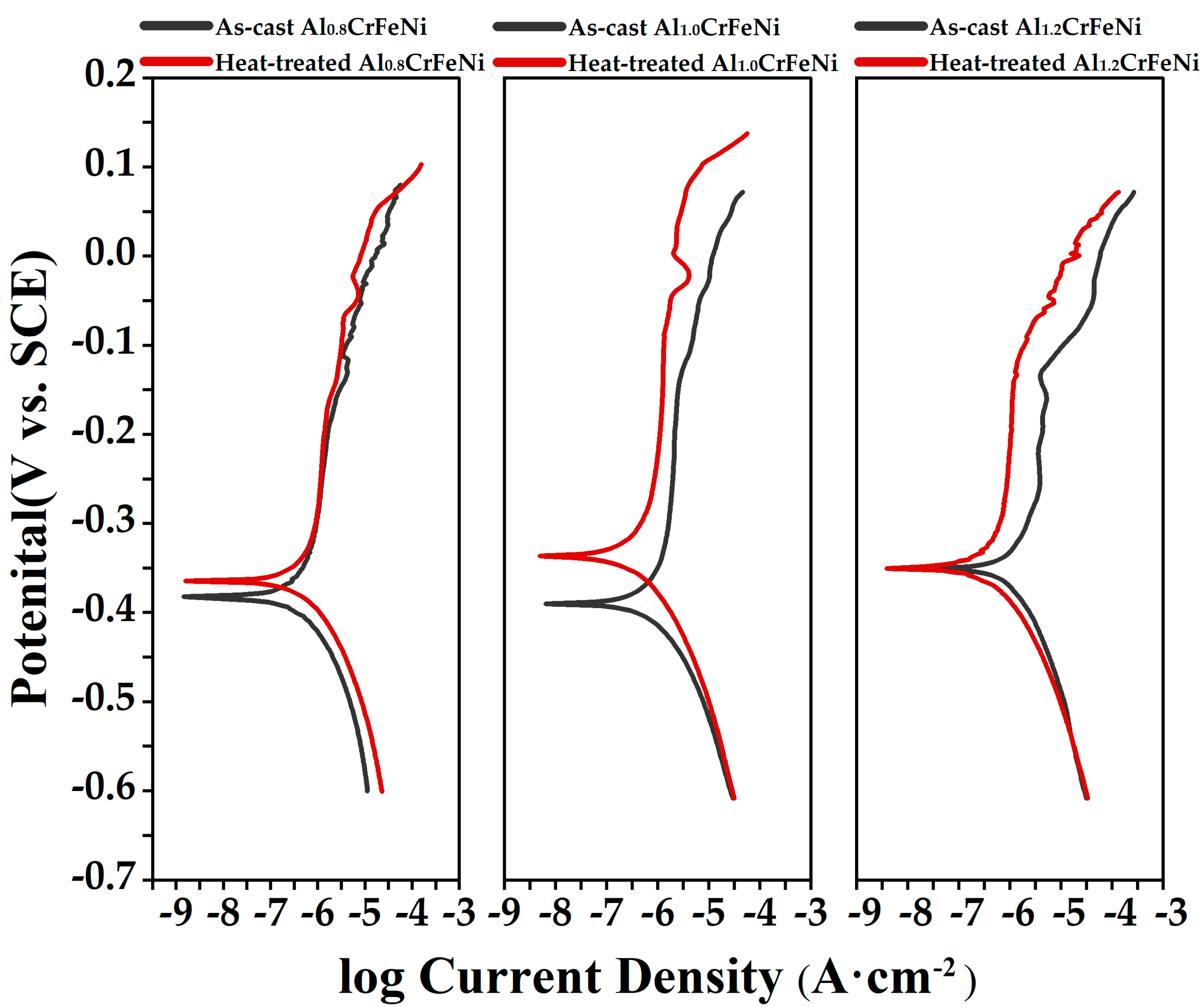
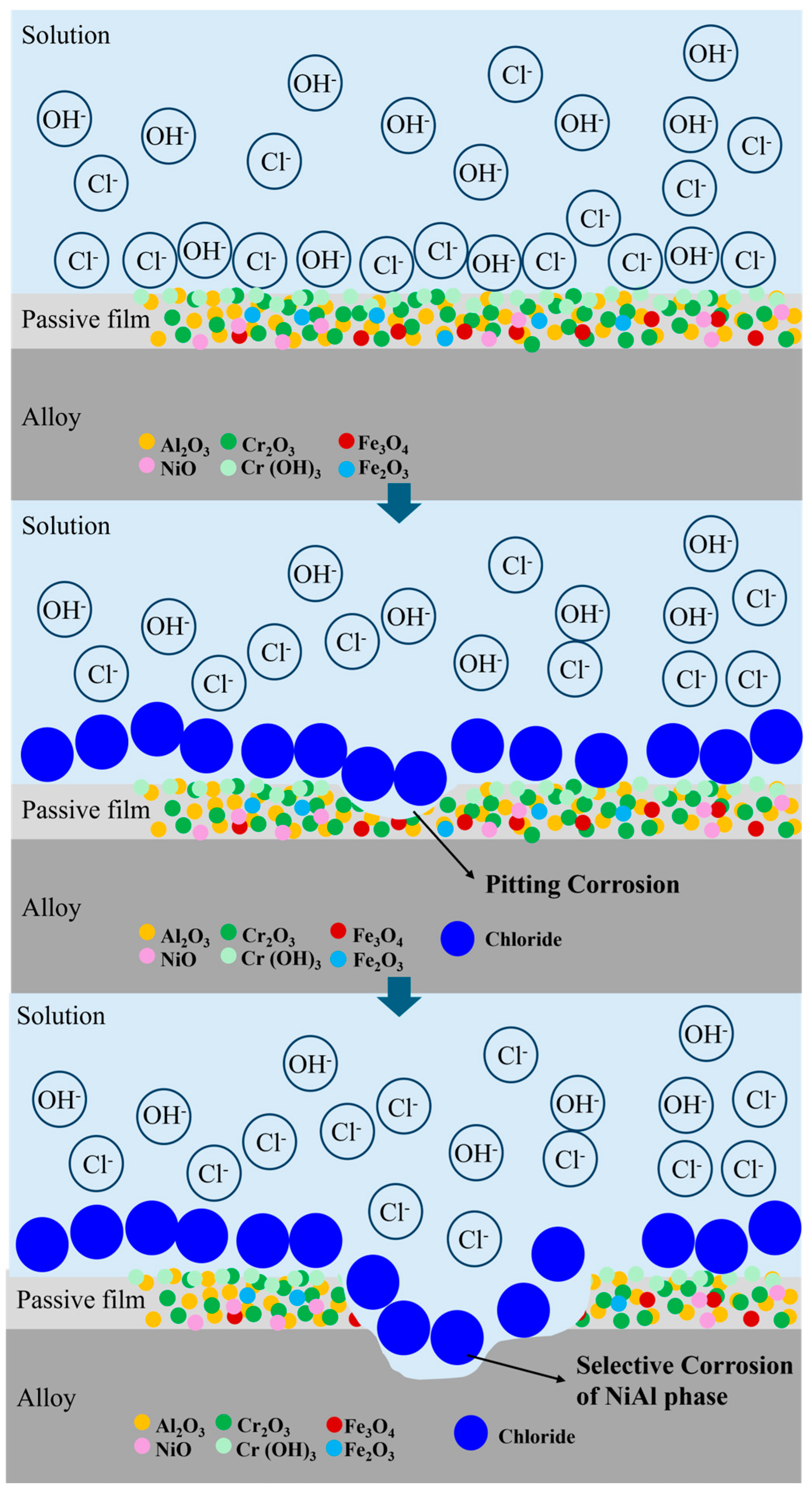
3.3. Corrosion Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Castro, D.; Jaeger, P.; Baptista, A.C.; Oliveira, J.P. An Overview of High-Entropy Alloys as Biomaterials. Metals 2021, 11, 648. [Google Scholar] [CrossRef]
- Coury, F.G.; Zepon, G.; Bolfarini, C. Multi-principal element alloys from the CrCoNi family: Outlook and perspectives. J. Mater. Res. Technol.-JmrT 2021, 15, 3461–3480. [Google Scholar] [CrossRef]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and Development of High Entropy Alloys for Structural Applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Tsai, M.-H.; Yeh, J.-W.; Yang, C.-C. Effect of temperature on mechanical properties of Al0.5CoCrCuFeNi wrought alloy. J. Alloys Compd. 2010, 490, 160–165. [Google Scholar] [CrossRef]
- Shi, T.; Lei, P.-H.; Yan, X.; Li, J.; Zhou, Y.-D.; Wang, Y.-P.; Su, Z.-X.; Dou, Y.-K.; He, X.-F.; Yun, D.; et al. Current development of body-centered cubic high-entropy alloys for nuclear applications. Tungsten 2021, 3, 197–217. [Google Scholar] [CrossRef]
- Todai, M.; Nagase, T.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar] [CrossRef]
- Aly, H.A.; Abdelghafar, K.A.; Gaber, G.A.; Mohamed, L.Z. Fabrication, Characterization, and Corrosion Behavior of a New Cu40Mn25Al20Fe5Co5Ni5 High-Entropy Alloy in HNO3 Solution. J. Mater. Eng. Perform. 2021, 30, 1430–1443. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90, 2734517. [Google Scholar] [CrossRef]
- Hillel, G.; Natovitz, L.; Salhov, S.; Haroush, S.; Pinkas, M.; Meshi, L. Understanding the Role of the Constituting Elements of the AlCoCrFeNi High Entropy Alloy through the Investigation of Quaternary Alloys. Metals 2020, 10, 1275. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, W.; Xia, Z.; Xiong, W.; Fu, Z. Influence of synthesis method on microstructure and mechanical behavior of Co-free AlCrFeNi medium-entropy alloy. Intermetallics 2019, 108, 45–54. [Google Scholar] [CrossRef]
- Liu, M.; Zuo, L.; Li, X.; Li, R.; Zhang, T. Microstructure and Mechanical Properties of Al25−xCr25+0.5xFe25Ni25+0.5x (x = 19, 17, 15 at%) Multi-Component Alloys. Adv. Eng. Mater. 2018, 20, 201701057. [Google Scholar] [CrossRef]
- Wang, M.; Wen, Z.; Ma, B.; Liu, J.; Zou, Z.; Zhao, Y. Enhancing the strength of AlCrFeNi HEAs via tailoring aluminum content and optimal aging treatment. J. Alloys Compd. 2022, 893, 162242. [Google Scholar] [CrossRef]
- Chen, X.; Qi, J.Q.; Sui, Y.W.; He, Y.Z.; Wei, F.X.; Meng, Q.K.; Sun, Z. Effects of aluminum on microstructure and compressive properties of Al-Cr-Fe-Ni eutectic multi-component alloys. Mater. Sci. Eng. Struct. Mater. Prop. Microstruct. Process. 2017, 681, 25–31. [Google Scholar] [CrossRef]
- Wang, M.; Wen, Z.; Liu, J.; Ma, B.; Wang, M.; Zou, Z.; Zhao, Y. Labyrinthine structure AlxCrFeNi (x ≥ 1) eutectic high entropy alloys with duplex reinforced phases. J. Alloys Compd. 2022, 918, 165441. [Google Scholar] [CrossRef]
- Wang, W.-R.; Wang, W.-L.; Wang, S.-C.; Tsai, Y.-C.; Lai, C.-H.; Yeh, J.-W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Bjorklund, S.; Donaghey, L.F.; Hillert, M. Effect of alloying elements on rate of ostwald ripening of cementite in steel. Acta Metall. 1972, 20, 867. [Google Scholar] [CrossRef]
- Garip, Y.; Ergin, N.; Ozdemir, O. Resistance sintering of CoCrFeNiAlx (x = 0.7, 0.85, 1) high entropy alloys: Microstructural characterization, oxidation and corrosion properties. J. Alloys Compd. 2021, 877. [Google Scholar] [CrossRef]
- Qvarfort, R. Critical pitting temperature-measurements of stainless-steels with an improved electrochemical method. Corros. Sci. 1989, 29, 987–993. [Google Scholar] [CrossRef]
- Shi, Y.; Collins, L.; Feng, R.; Zhang, C.; Balke, N.; Liaw, P.K.; Yang, B. Homogenization of AlxCoCrFeNi high-entropy alloys with improved corrosion resistance. Corros. Sci. 2018, 133, 120–131. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting corrosion of metals—A review of the critical factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Engelhardt, G.R. The Point Defect Model for Bi-Layer Passive Films. In Proceedings of the Symposium on Corrosion General Session Held During the 217th Meeting of the Electrochemical-Society (ECS), Vancouver, BC, Canada, 25–30 April 2010; pp. 123–144. [Google Scholar]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control: An Introduction to Corrosion Science and Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Korchef, A.; Kahoul, A. Corrosion Behavior of Commercial Aluminum Alloy Processed by Equal Channel Angular Pressing. Int. J. Corros. 2013, 2013, 983261. [Google Scholar] [CrossRef]
- Li, J.S.; Mao, Q.Z.; Chen, M.; Qin, W.B.; Lu, X.K.; Liu, T.; She, D.S.; Kang, J.J.; Wang, G.; Zhu, X.B.; et al. Enhanced pitting resistance through designing a high-strength 316L stainless steel with heterostructure. J. Mater. Res. Technol. 2021, 10, 132–137. [Google Scholar] [CrossRef]
- Zhao, Q.; Pan, Z.; Wang, X.; Luo, H.; Liu, Y.; Li, X. Corrosion and passive behavior of AlxCrFeNi3-x (x = 0.6, 0.8, 1.0) eutectic high entropy alloys in chloride environment. Corros. Sci. 2022, 208, 110666. [Google Scholar] [CrossRef]
- Ming, J.; Wu, M.; Shi, J. Passive film modification by concrete carbonation: Re-visiting a corrosion-resistant steel with Cr and Mo. Cem. Concr. Compos. 2021, 123, 104178. [Google Scholar] [CrossRef]
- Wu, H.; Xie, J.; Yang, H.; Sheng, N.; Yang, Y.; Hou, G.; Li, J.; Zhou, Y.; Sun, X. Corrosion behaviors and passive film properties of a newly developed cost-effective AlCrFeNi eutectic high entropy alloy in different corrosive solutions. Mater. Today Commun. 2023, 37, 107602. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, G.-H.; Fan, X.-H.; Jin, J.; Zhang, L.; Du, Y.-X. Corrosion behavior and surface characterization of an equiatomic CoCrFeMoNi high-entropy alloy under various pH conditions. J. Alloys Compd. 2022, 900, 163432. [Google Scholar] [CrossRef]
- Nascimento, C.B.; Donatus, U.; Ríos, C.T.; Antunes, R.A. Passive film composition and stability of CoCrFeNi and CoCrFeNiAl high entropy alloysin chloride solution. Mater. Chem. Phys. 2021, 267, 124582. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Z.; Shi, W.; Huang, J. Corrosion behaviors of W-doped laser-cladded high-entropy alloy of AlCoCrFeNi in a simulated sulfur-containing seawater environment. Intermetallics 2024, 169, 108298. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, G.; Han, J.; Li, J.; Zhang, H.; Zhang, S.; Lin, L.; Chen, L.; Cao, X. Effect of Heat Treatment on the Corrosion Resistance of AlFeCoNiMo0.2 High-Entropy Alloy in NaCl and H2SO4 Solutions. Metals 2023, 13, 849. [Google Scholar] [CrossRef]



| Element | Al0.8CrFeNi | Al1.0CrFeNi | Al1.2CrFeNi | |||
|---|---|---|---|---|---|---|
| Nominal | Measured | Nominal | Measured | Nominal | Measured | |
| Al | 21.05 | 21.65 | 25 | 25.29 | 28.57 | 28.88 |
| Cr | 26.31 | 25.64 | 25 | 24.14 | 23.81 | 22.96 |
| Fe | 26.31 | 25.55 | 25 | 24.82 | 23.81 | 23.25 |
| Ni | 26.31 | 27.16 | 25 | 25.75 | 23.81 | 24.91 |
| Sample | Hardness (HV1.0) | Sample | Hardness (HV1.0) | ||
|---|---|---|---|---|---|
| As-cast condition | Al0.8CrFeNi | 457.9 | Heat-treated condition | Al0.8CrFeNi | 431.06 |
| Al1.0CrFeNi | 462.4 | Al1.0CrFeNi | 439.38 | ||
| Al1.2CrFeNi | 466.96 | Al1.2CrFeNi | 451.02 | ||
| Sample | Ecorr/(V) | Jcorr/(A/cm2) | Ep/(V) | Corrosion Rate (mm/year) | |
|---|---|---|---|---|---|
| As-cast condition | Al0.8CrFeNi | −0.38 | 4.7 × 10−7 | 0.11 | 0.011 |
| Al1.0CrFeNi | −0.39 | 6.1 × 10−7 | 0.10 | 0.015 | |
| Al1.2CrFeNi | −0.35 | 1.1 × 10−6 | 0.06 | 0.026 | |
| Heat-treated condition | Al0.8CrFeNi | −0.36 | 3.6 × 10−7 | 0.15 | 0.004 |
| Al1.0CrFeNi | −0.33 | 4.3 × 10−7 | 0.14 | 0.010 | |
| Al1.2CrFeNi | −0.42 | 4.8 × 10−7 | 0.07 | 0.012 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Pang, Y.; Zhang, Q.; Yang, L.; Song, Z.; Zhang, Y. Effects of Heat Treatment on Microstructures and Corrosion Properties of AlxCrFeNi Medium-Entropy Alloy. Metals 2025, 15, 292. https://doi.org/10.3390/met15030292
Guo P, Pang Y, Zhang Q, Yang L, Song Z, Zhang Y. Effects of Heat Treatment on Microstructures and Corrosion Properties of AlxCrFeNi Medium-Entropy Alloy. Metals. 2025; 15(3):292. https://doi.org/10.3390/met15030292
Chicago/Turabian StyleGuo, Pushan, Yuan Pang, Qingke Zhang, Lijing Yang, Zhenlun Song, and Yi Zhang. 2025. "Effects of Heat Treatment on Microstructures and Corrosion Properties of AlxCrFeNi Medium-Entropy Alloy" Metals 15, no. 3: 292. https://doi.org/10.3390/met15030292
APA StyleGuo, P., Pang, Y., Zhang, Q., Yang, L., Song, Z., & Zhang, Y. (2025). Effects of Heat Treatment on Microstructures and Corrosion Properties of AlxCrFeNi Medium-Entropy Alloy. Metals, 15(3), 292. https://doi.org/10.3390/met15030292









