Review of the Properties and Degradation Mechanisms of Refractories in Aluminum Reduction Cells
Abstract
1. Introduction
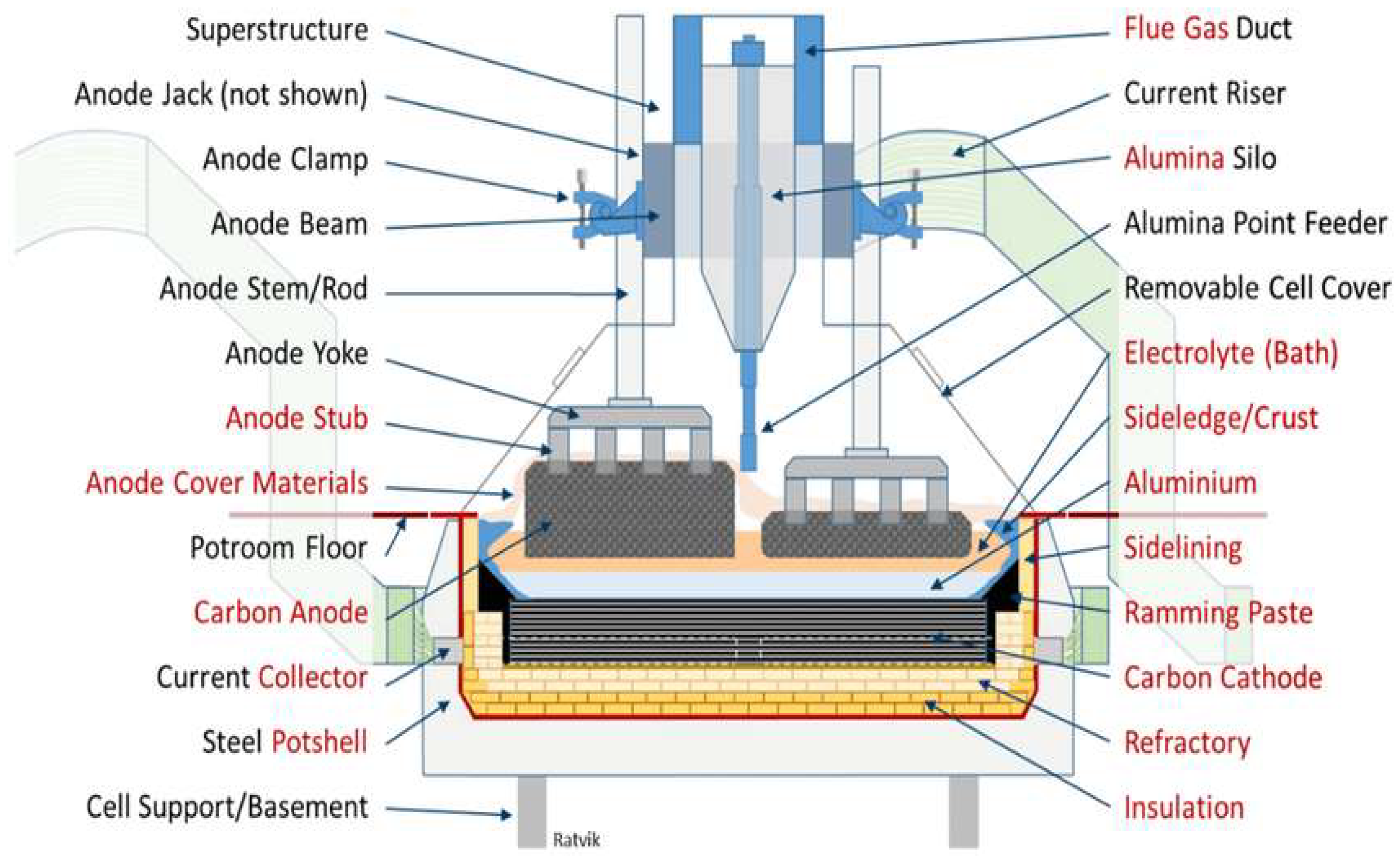
2. Production of Primary Aluminum
2.1. The Hall Héroult Process
2.2. The Electrolytic Bath
3. Generalities on Refractories
3.1. Definition
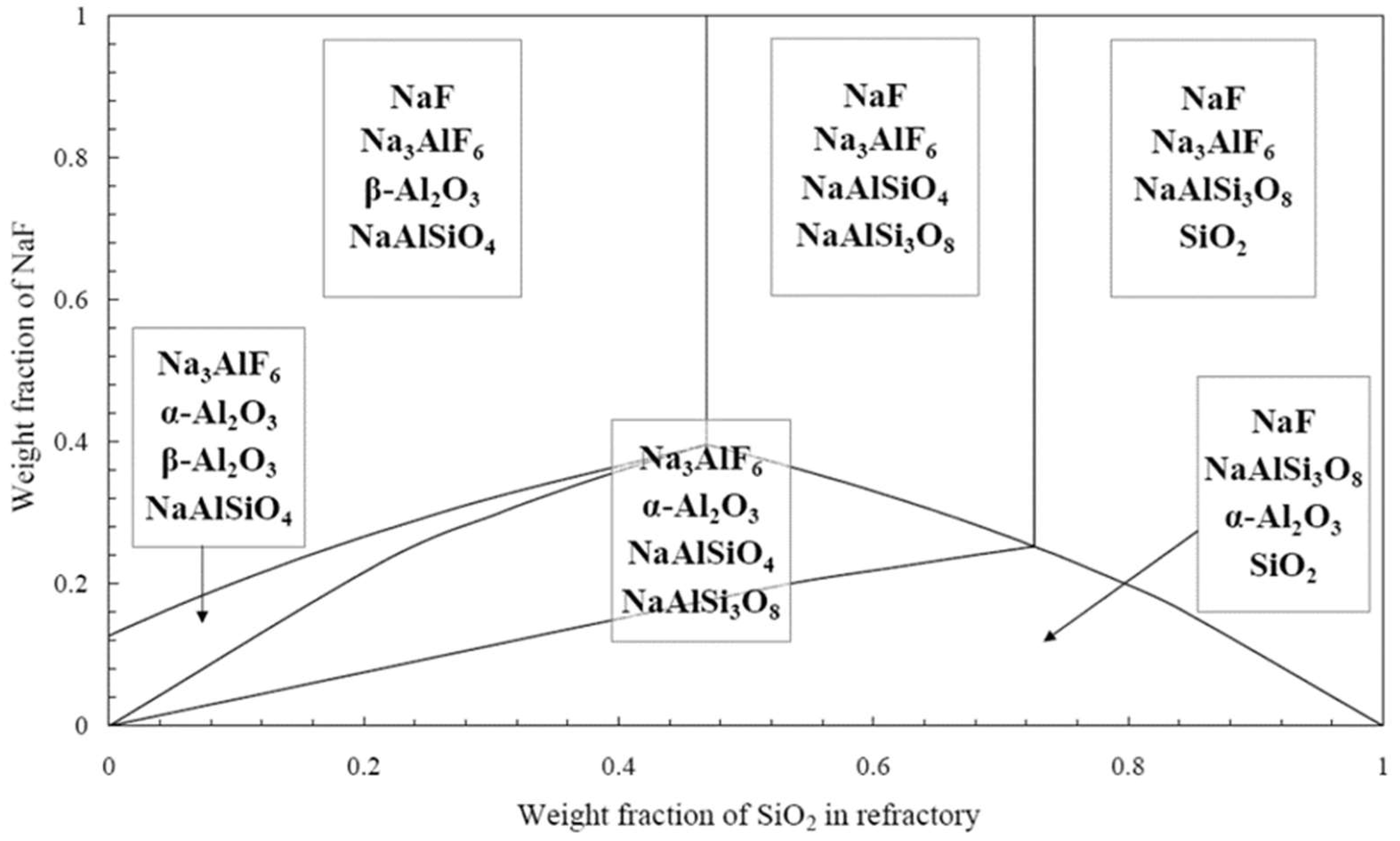
3.2. Impact of Alumina/Silica Ratio on Refractory Performance
3.3. Thermal Conductivity and Density
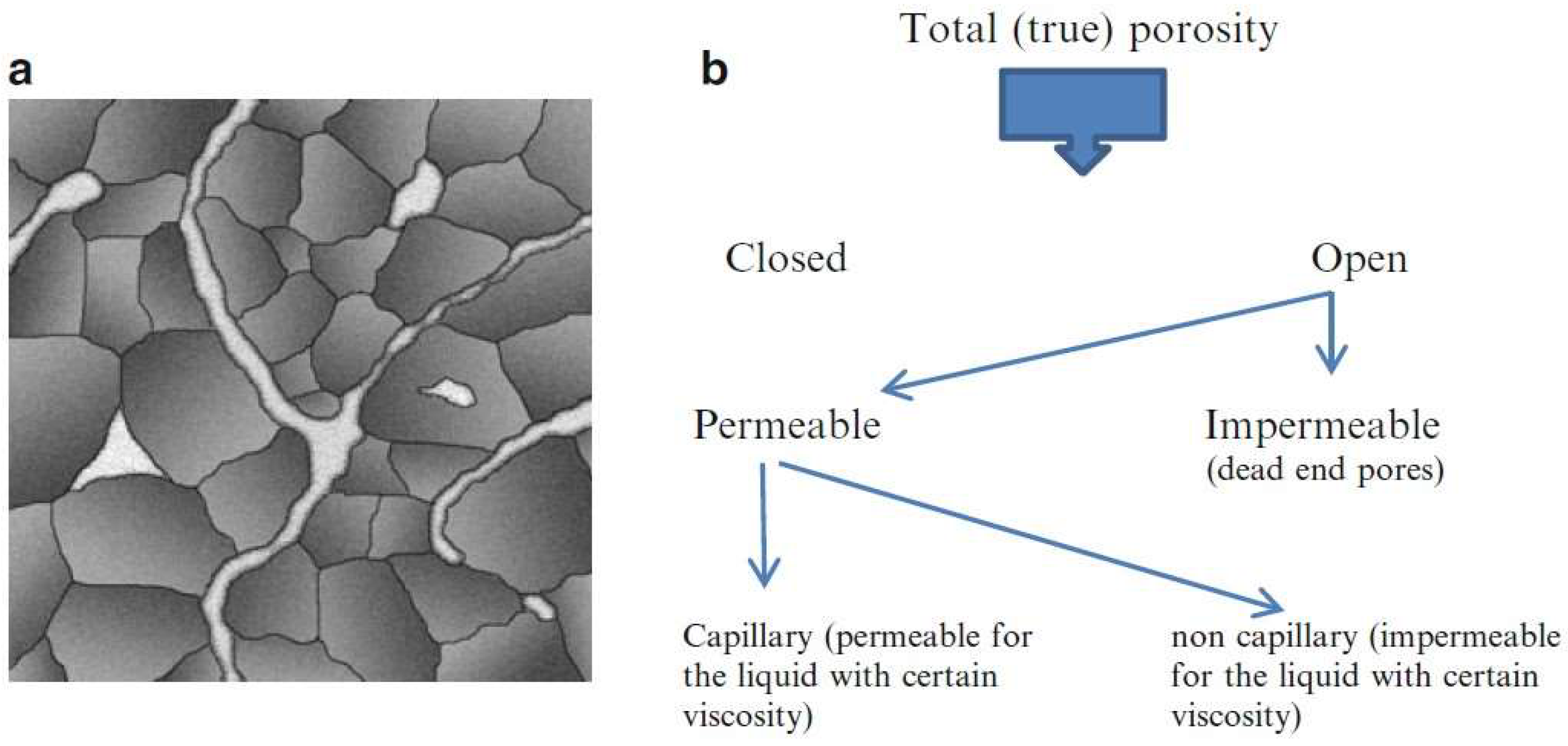
3.3.1. Measurements of Porosity and Density
3.3.2. Measurements of Thermal Conductivity
3.4. Thermomechanical Characteristics
3.4.1. Thermal Expansion
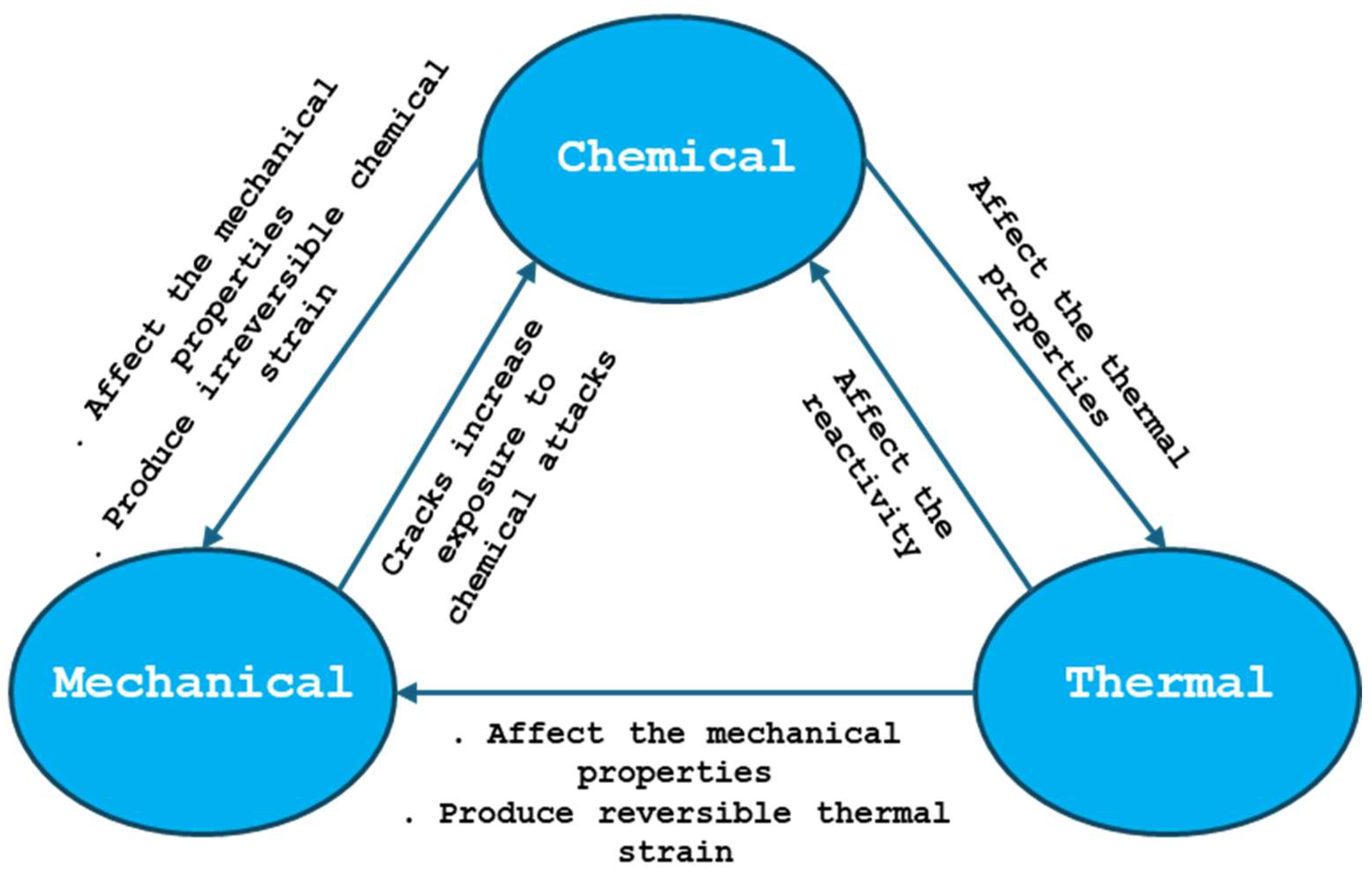
3.4.2. Thermal/Chemical/Mechanical Interactions on Refractories
3.5. Degradation of Refractories
3.5.1. Degradation of Ordinary Refractory Bricks
- Previous Works
- Recent Advances
3.5.2. On-Field Investigations
3.5.3. Laboratory-Scale Testing
4. Conclusions
5. Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yurkov, A. Refractories for Aluminium, Chapter 1; Springer: New York, NY, USA, 2015. [Google Scholar]
- Tabereaux, A. Super-high amperage prebake cell technologies in operation at worldwide aluminum smelters. Light Met. Age 2017, 75, 26–29. [Google Scholar]
- Chausse, P.; Laquerrière, M.; Dupont, J. Thermal Gradients in Reduction Cells. Trans. Electrochem. Soc. 2012, 3, 89–95. [Google Scholar]
- Siljan, J.; Hansen, B.; Pedersen, K. Effect of Sodium Vapor on Refractories. J. Mater. Sci. 2002, 40, 689–701. [Google Scholar]
- Tschöpe, A.; Schöning, C.; Grande, T. Mineralogical Transformations of Aluminosilicate Refractories. Refractory J. 2016, 7, 87–96. [Google Scholar]
- Proshkin, Y.; Ivanov, A.; Sidorov, K. Phase Transformation of Aluminosilicate Refractories. In Proceedings of the TMS Annual Meeting & Exhibition, New Orleans, LA, USA, 9–13 March 2008; Springer: Cham, Switzerland, 2008; pp. 78–85. [Google Scholar]
- Solheim, A.; Schoning, C. Sodium vapour degradation of refractories used in aluminium cells. In Proceedings of the TMS Annual Meeting & Exhibition, New Orleans, LA, USA, 9–13 March 2008; Springer: Cham, Switzerland, 2008. [Google Scholar]
- Ben Salem, M.H.; Soucy, G.; Marceau, D.; Godefroy, A.; Charest, S. Exploring Refractory Material Degradation in Aluminium Electrolysis Cell. In Proceedings of the ICSOBA 2024, Lyon, France, 27–31 October 2024; pp. 1043–1050. [Google Scholar]
- Pelletier, R.; Allaire, C.; Tremblay, F. The corrosion of potlining refractories: A unified approach. JOM 2001, 53, 18–22. [Google Scholar] [CrossRef]
- Ben Salem, M.H.; Soucy, G.; Marceau, D.; Godefroy, A.; Charest, S. Study of the degradation of ordinary refractory bricks in an aluminum reduction cell. In Proceedings of the TMS Annual Meeting & Exhibition, Orlando, FL, USA, 3–7 March 2024; Springer Nature: Cham, Switzerland, 2024; pp. 664–670. [Google Scholar]
- Roberts, M.; Miller, T.; Johnson, R. High-Temperature Stability of Alumina-Silica Mixtures. J. Am. Ceram. Soc. 2008, 89, 451–457. [Google Scholar]
- Polmear, I.J. Light Alloys: Metallurgy of Aluminum and Magnesium Alloys, 4th ed.; Elsevier: Oxford, UK, 2017. [Google Scholar]
- Haupin, W.E. Electrochemistry of the Hall-Heroult process for aluminum smelting. J. Chem. Educ. 1983, 60, 279. [Google Scholar] [CrossRef]
- Allard, F.; Soucy, G.; Rivoaland, L. Formation of deposits on the cathode surface of aluminum electrolysis cells. Metall. Mater. Trans. B 2014, 45, 2475–2485. [Google Scholar] [CrossRef]
- Lillebuen, B.; Bugge, M.; Høie, H. Alumina dissolution and current efficiency in Hall-Héroult cells. In Proceedings of the TMS Annual Meeting & Exhibition, San Francisco, CA, USA, 15–19 February 2009; Springer: Cham, Switzerland, 2009; pp. 389–394. [Google Scholar]
- Liu, X.; Tang, J.; Zhou, W. Visualization of alumina dissolution in cryolitic melts. In Proceedings of the TMS Annual Meeting & Exhibition, San Francisco, CA, USA, 27 February–3 March 1994; Springer: Cham, Switzerland, 1994; pp. 359–364. [Google Scholar]
- Brilliot, P.; Martin, L.; Dupuis, J. Penetration and Chemical Reactions in Carbon Cathodes during Aluminium Electrolysis. Metall. Trans. B 1993, 24B, 75–89. [Google Scholar] [CrossRef]
- Ratvik, A.P.; Solheim, A.; Grande, T. Aluminium production process: From Hall–Héroult to modern smelters. ChemTexts 2022, 8, 10. [Google Scholar] [CrossRef]
- Solheim, A. Towards a proper understanding of sideledge facing the metal in aluminium cells. In Proceedings of the TMS Annual Meeting & Exhibition, San Antonio, TX, USA, 12–16 March 2006; Springer: Cham, Switzerland, 2006; pp. 439–443. [Google Scholar]
- Andersen, D.H.; Zhang, Z.L. Fracture and physical properties of carbon anodes for the aluminum reduction cell. Eng. Fract. Mech. 2011, 78, 2998–3016. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Zhang, T. Refractory Performance in Modern Reduction Cells. In Proceedings of the TMS Annual Meeting & Exhibition, Orlando, FL, USA, 15–19 March 2015; Springer: Cham, Switzerland, 2015; pp. 195–204. [Google Scholar]
- Yang, Y.; Sun, H.; Zhou, L.; Wang, J. Effect of physiochemical properties and bath chemistry on alumina dissolution rate in cryolite electrolyte. JOM 2015, 67, 973–983. [Google Scholar] [CrossRef]
- Gerbeau, J.-F. Problèmes Mathématiques et Numériques Posés Par la Modélisation de L’électrolyse de L’aluminium. Ph.D. Thesis, Ecole des Ponts ParisTech, Paris, France, 1998. [Google Scholar]
- Pelletier, G.; Allaire, C.; Houle, M. Energy Efficiency in Aluminum Reduction Cells. J. Electrochem. Sci. 2013, 16, 101–112. [Google Scholar]
- Schøning, C.; Grande, T.; Siljan, O.-J. Cathode-Refractory Materials for Aluminium Reduction Cells. In Proceedings of the TMS Annual Meeting & Exhibition, San Diego, CA, USA, 28 February–4 March 1999; Springer: Cham, Switzerland, 1999; pp. 231–238. [Google Scholar]
- Foster, P.A. Phase Equilibria in the System Na3AlF6−AlF3. J. Am. Ceram. Soc. 1970, 53, 598–600. [Google Scholar] [CrossRef]
- Solheim, A.; Sterten, Å. Activity Data for the System NaF-AlF3. In Proceedings of the TMS Annual Meeting & Exhibition, Orlando, FL, USA, 9–13 February 1997; Springer: Cham, Switzerland, 1997; p. 225. [Google Scholar]
- Holm, J.; Holm, B. Structural Properties of Refractory Materials. Acta Chem. Scand. 1973, 27, 1410–1415. [Google Scholar] [CrossRef]
- Brisson, P.-Y.; Houde, R.; Tremblay, F. Revisiting Sodium and Bath Penetration in the Carbon Lining of Aluminum Electrolysis Cell. In Proceedings of the TMS Annual Meeting & Exhibition, San Francisco, CA, USA, 13–17 February 2005; Springer: Cham, Switzerland, 2005; pp. 727–732. [Google Scholar]
- Arndt, K.; Wagner, H.; Müller, J. Thermochemical Properties of Refractory Materials. Electrochemistry 1924, 78. [Google Scholar]
- AFNOR Documentation. Memento de la nomenclature, classification et normalisation des produits réfractaires. Rev. Métall. 1966, 110, 110–115. [Google Scholar]
- Mason, P.; Hightower, S.; Browning, J. Thermal Conductivity in High-Alumina Bricks. J. Appl. Phys. 2007, 91, 211–222. [Google Scholar]
- Brosnan, D.A. Alumina-Silica Brick. In Mechanical Engineering; Marcel Dekker, CRC Press/Taylor and Francis: New York, NY, USA, 2004; pp. 178–179. [Google Scholar]
- Litovsky, E.Y.; Puchkelevitch, N.A. Thermophysical Properties of Materials; Metallurgia: Moscow, Russia, 1982; 150p. (In Russian) [Google Scholar]
- Routschka, G.; Wuthnow, H. Handbook of Refractory Materials: Design, Properties, Testing; Vulkan Verlag: Essen, Germany, 2012; 355p. [Google Scholar]
- Strelov, K.K. Structure and Properties of Refractories; Metallurgia: Moscow, Russia, 1982; 207p. (In Russian) [Google Scholar]
- ASTM C20-00; Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM C134-95; Standard Test Methods for Size, Dimensional Measurements, and Bulk Density of Refractory Brick and Insulating Firebrick. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM C182-88; Standard Test Method for Thermal Conductivity of Refractories by the Hot Wire (Platinum Resistance Thermometer Technique). ASTM International: West Conshohocken, PA, USA, 1998.
- ASTM C417-93; Standard Test Method for Thermal Conductivity of Unfired Refractories. ASTM International: West Conshohocken, PA, USA, 1998.
- ASTM C1113-99; Standard Test Method for Thermal Conductivity of Refractories by Hot Wire (Platinum Resistance Thermometer Technique). ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 8894-1:2010; Refractory materials—Determination of thermal conductivity—Part 1: Hot-wire methods (Cross-array). International Organization for Standardization: Geneva, Switzerland, 2010.
- ASTM E1461-13; Standard Test Method for Thermal Diffusivity by the Flash Method. ASTM International: West Conshohocken, PA, USA, 2022.
- McGinnis, J.; Johnson, T.; Walker, R. Chemical Resistance of Aluminosilicate Refractories. In Proceedings of the TMS Annual Meeting & Exhibition, Orlando, FL, USA, 11–15 March 2012; Springer: Cham, Switzerland, 2012; pp. 455–462. [Google Scholar]
- International Aluminum Institute. Guidelines for Refractory Applications in Electrolysis Cells; IAI Standards: Maharashtra, India, 2019. [Google Scholar]
- Siljan, J.; Martin, R.; Thompson, L. Thermal Aging of Alumina-Silica Bricks. J. Ceram. 2010, 12, 189–201. [Google Scholar]
- Tschöpe, K.; Schøning, C.; Grande, T. Autopsies of Spent Pot Linings—A Revised View. In Proceedings of the TMS Annual Meeting & Exhibition, San Francisco, CA, USA, 15–19 February 2009; Springer: Cham, Switzerland, 2009; pp. 1085–1090. [Google Scholar]
- Kvam, K.R.; Øye, H.A. Homogeneity and degradation of SiC sidelinings. In Proceedings of the Ninth International Symposium on Light Metals Production, Trondheim, Norway, 18–21 August 1997; p. 225. [Google Scholar]
- Solheim, A.; Sterten, Å.; Støre, T. Thermal Behavior of Sidewall Materials. In Proceedings of the TMS Annual Meeting & Exhibition, Las Vegas, NV, USA, 12–16 February 1995; Springer: Cham, Switzerland, 1995; pp. 73–82. [Google Scholar]
- Pelletier, R.; Allaire, C. Corrosion in potlining refractories: Effect of cathode material interpreted using a unified approach. JOM 2003, 55, 58–62. [Google Scholar] [CrossRef]
- Tschöpe, K.; Rutlin, J.; Grande, T. Chemical Degradation Map for Sodium Attack in Refractory Linings. In Essential Readings in Light Metals; Springer: Cham, Switzerland, 2016; pp. 978–983. [Google Scholar]
- Luneng, R.; Wang, Z.; Ratvik, A.P.; Grande, T. Thermogravimetric Analysis of Thermal Insulating Materials Exposed to Sodium Vapor. In Light Metals; Chesonis, C., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Schøning, C.; Grande, T. The Stability of Refractory Oxides in Sodium-Rich Environments. JOM 2006, 58, 58–61. [Google Scholar] [CrossRef]
- Rutlin, J.; Grande, T. Phase Equilibria in Subsystems of the Quaternary Reciprocal System Na2O–SiO2–Al2O3–NaF–SiF4–AlF3. J. Am. Ceram. Soc. 1999, 82, 2538–2544. [Google Scholar] [CrossRef]
- Lambotte, G.; Chartrand, P. A Thermodynamic Approach to the Corrosion of the Cathode Refractory Lining in Aluminium Electrolysis Cell: Modelling of the Al2O3-Na2O-SiO2-AlF3-NaF-SiF4 System. In Proceedings of the TMS Annual Meeting & Exhibition, San Antonio, TX, USA, 3–7 March 2013; Springer: Cham, Switzerland, 2013; pp. 909–916. [Google Scholar]
- Brunk, F. A Barrier Concept Based on a Double Layer of Dense Refractory Bricks for Protecting the Cell-Bottom Lining. In Advances in Refractories for the Metallurgical Industries III; CIM: Quebec, QC, Canada, 1999. [Google Scholar]
- Jasim, A.; Ahli, Y.; Mukhanov, A.; Almualla, S.F. Impact of Lining Materials Degradation on Reduction Cell Performance. In ICSOBA 2024 Proceedings; ICSOBA: Québec, QC, Canada, 2024; pp. 1337–1342. [Google Scholar]



| Composition | Description | Melting Point (°C) |
|---|---|---|
| Na3AlF6 (Cryolite) | Congruent melting compound at 25 mol% AlF3 | 1010 |
| Na5Al3F14 (Chiolite) | Incongruent melting compound at 37 mol% AlF3 | 734 |
| Eutectic Point 1 | Located at 13.8 mol% AlF3 | 888 |
| Eutectic Point 2 | Located at 45.2 mol% AlF3 | 695 |
| Peritectic Point | Results from the incongruent melting of chiolite, positioned between 39.4 and 40.8 mol% AlF3 | 734 |
| Category | Description |
|---|---|
| Chamotte Bricks (High-Silica Bricks) | Alumina content between 10 and 45 wt%; balanced in cost, thermal stability, and resistance to stress. |
| High-Alumina Bricks | Alumina content exceeding 45 wt%; offers enhanced performance in high-temperature environments. |
| Shaped Refractories | Delivered in their final usable form, such as bricks. |
| Unshaped Refractories | Supplied as loose materials requiring shaping, such as concretes and ramming pastes. |
| Symbol | Parameter | Definition |
|---|---|---|
| Total Porosity | Ratio of the combined volume of open and closed pores to the total material volume, including solids. | |
| Open Porosity | Ratio of the volume of open pores to the total material volume. | |
| Closed Porosity | Ratio of the volume of closed pores to the total material volume. |
| Material | Thermal Conductivity at °C, W/m· | |||||||
|---|---|---|---|---|---|---|---|---|
| 20 | 200 | 400 | 500 | 600 | 800 | 1000 | 1200 | |
| Fireclay | 1.16 | - | - | 1.34 | - | 1.47 | 1.51 | 1.55 |
| Silica | 1.16 | - | - | 1.4 | - | 1.5 | 1.63 | - |
| High alumina (85% Al2O3) | - | 2.33 | - | 2.2 | 2.1 | 2.1 | 2.1 | 2.1 |
| Porosity, % | <10 | 10–15 | 15–20 | 20–25 |
|---|---|---|---|---|
| 1.5 | 2.0 | 2.4 | 2.6 |
| Material | ×106 K−1 |
|---|---|
| Fireclay (15% Al2O3) | 7–9 |
| Fireclay (30% Al2O3) | 4, 5–6 |
| Type of Strains | Description |
|---|---|
| Mechanical | Instantaneous elastic (reversible), plastic (irreversible), and time-dependent viscoelastic (reversible) and viscoplastic strains due to compression, tension, and bending, which can cause structural damage. |
| Thermal | Caused by thermal shock and differential thermal expansion due to fluctuating temperatures (reversible). |
| Chemical | Corrosion resulting from interactions with the cryolitic bath, sodium vapor, and other aggressive agents (irreversible). |
| Phase | Formation Mechanism | Impact on Refractory Material |
|---|---|---|
| Nepheline | Reaction of cryolitic bath with alumina and silica. | Weakens structural integrity. |
| Albite | Interaction with bath components, especially in high-SiO2 conditions. | Reduces thermal stability and increases porosity. |
| Factor | Description |
|---|---|
| Bath Composition | Relative proportions of Na2O, SiO2, and Al2O3 in the cryolitic bath. |
| Refractory Properties | Includes porosity, silica-to-alumina ratio, and mineralogical structure of the material. |
| Environmental Conditions | Temperature gradients, surrounding gas composition (e.g., sodium vapor, CO2), and vapor pressure of the bath. |
| Aspect | Observation | Significance |
|---|---|---|
| Thermal and Chemical Analysis | Thermogravimetry and Differential Thermal Analysis (TG/DTA) revealed mass loss at ~730 °C due to the melting of chiolite (Na5Al3F14) and calcium cryolite (Na2Ca3Al2F14). | The transition to a liquid state facilitated chemical interaction between the bath and ORBs. |
| Contamination and Phase Formation | At 700 °C, a thin reaction layer formed. At 960 °C, multiple layers were observed—heavily degraded outer zone, intermediate dense layer, and inner unaffected zone. | XRD confirmed the formation of nepheline, albite (NaAlSi3O8), and other sodium-rich phases, consistent with autopsy findings. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Salem, M.H.; Soucy, G.; Marceau, D.; Godefroy, A.; Charest, S. Review of the Properties and Degradation Mechanisms of Refractories in Aluminum Reduction Cells. Metals 2025, 15, 278. https://doi.org/10.3390/met15030278
Ben Salem MH, Soucy G, Marceau D, Godefroy A, Charest S. Review of the Properties and Degradation Mechanisms of Refractories in Aluminum Reduction Cells. Metals. 2025; 15(3):278. https://doi.org/10.3390/met15030278
Chicago/Turabian StyleBen Salem, Mohamed Hassen, Gervais Soucy, Daniel Marceau, Antoine Godefroy, and Sébastien Charest. 2025. "Review of the Properties and Degradation Mechanisms of Refractories in Aluminum Reduction Cells" Metals 15, no. 3: 278. https://doi.org/10.3390/met15030278
APA StyleBen Salem, M. H., Soucy, G., Marceau, D., Godefroy, A., & Charest, S. (2025). Review of the Properties and Degradation Mechanisms of Refractories in Aluminum Reduction Cells. Metals, 15(3), 278. https://doi.org/10.3390/met15030278





