Effect of Leaching of Ionic Rare Earth Ores on the Permeability Coefficient of Mineral Soil and Its Correlation with the State Parameter
Abstract
1. Introduction
2. Materials and Methods
2.1. Properties of Experimental Materials
2.2. Experimental Process
3. Result
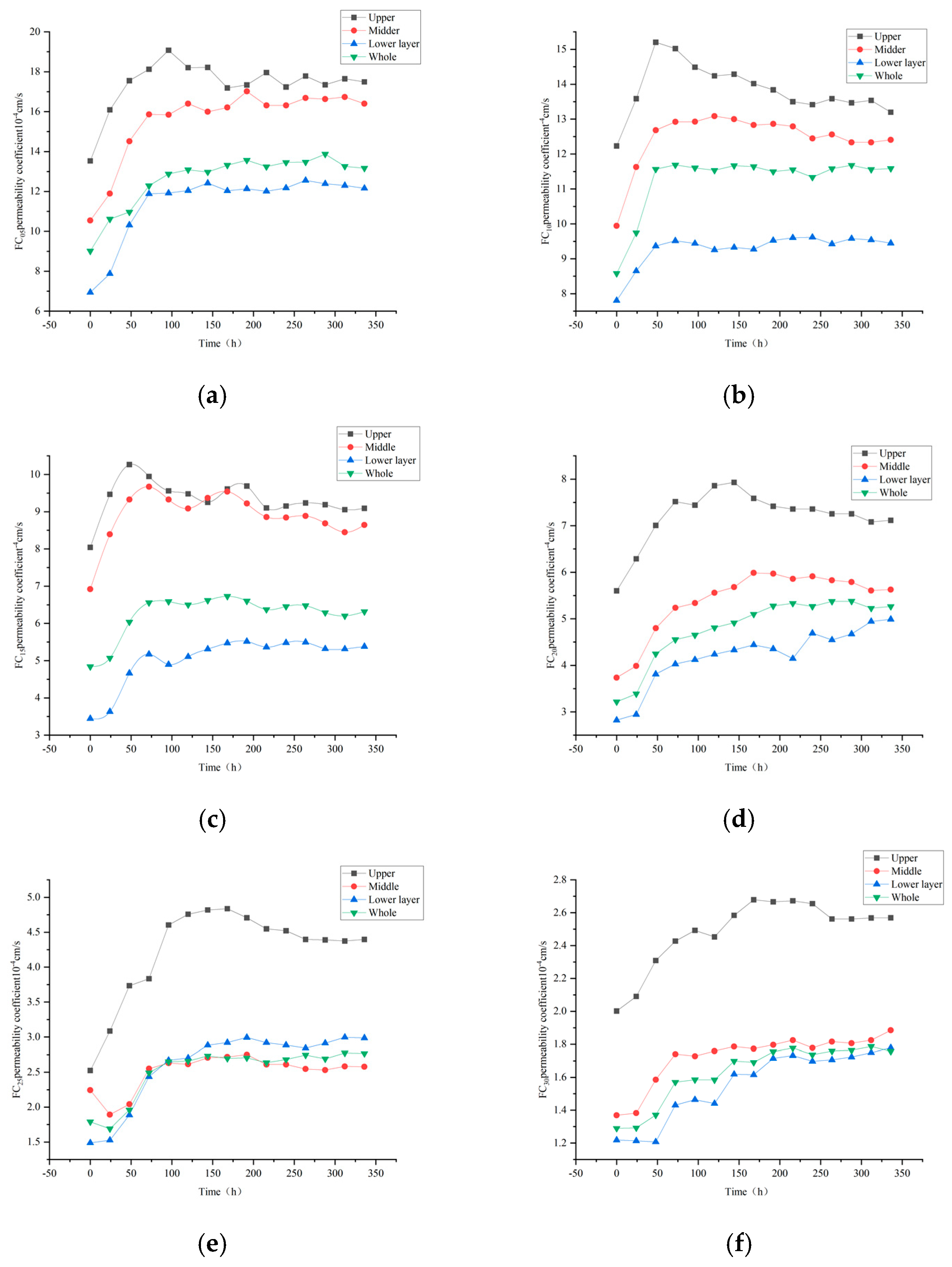
3.1. Effect of Clear Water Seepage on Permeability Coefficient of Different Layers of Rare Earth Ore with Different Fines Content
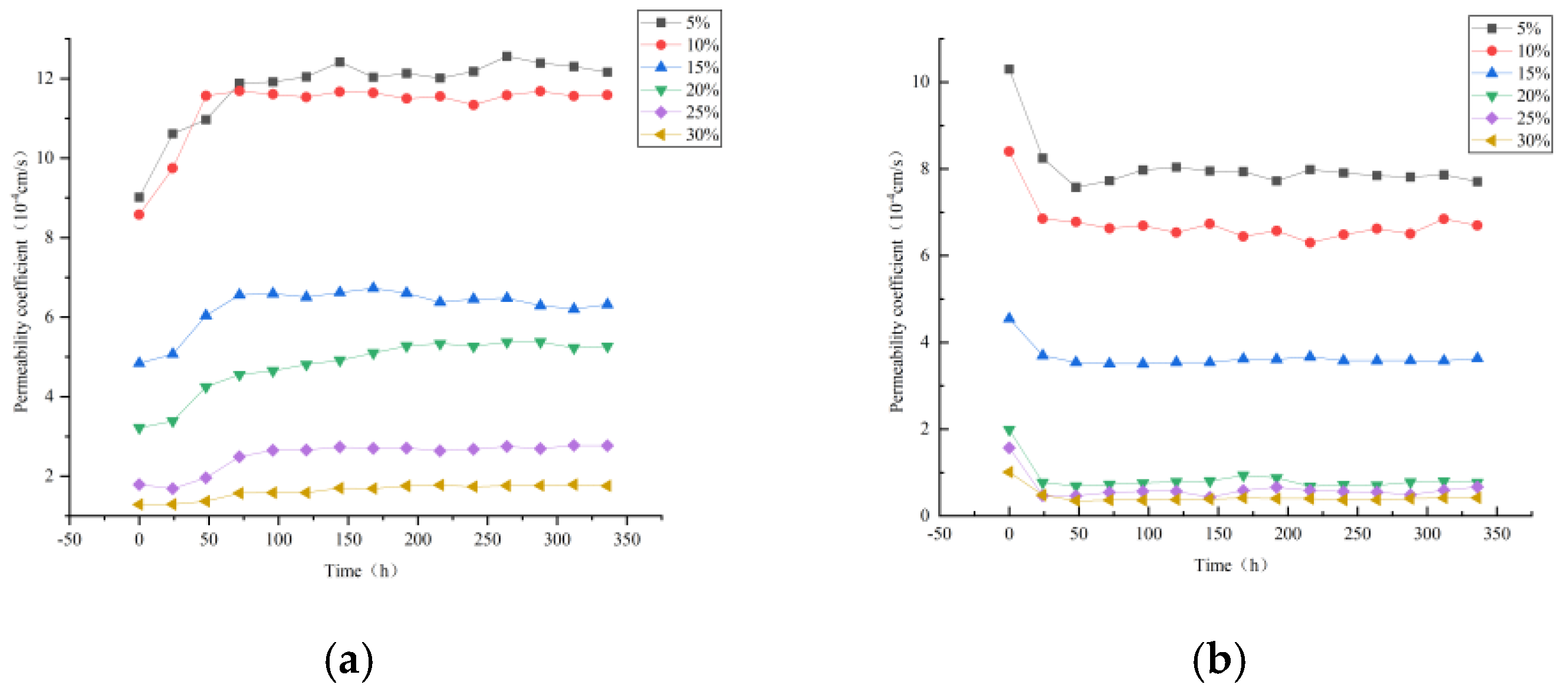
3.2. Influence of Magnesium Sulfate Leaching on the Permeability Coefficient of Each Layer of Rare Earth Ores with Different Fines Content
3.3. Variation of the Overall Permeability Coefficient of the Sample
4. Discussion
4.1. Correlation Between Fine Grain Content and Initial Permeability Coefficient
4.2. Correlation of Inhomogeneity Coefficient with Initial Permeability Coefficient
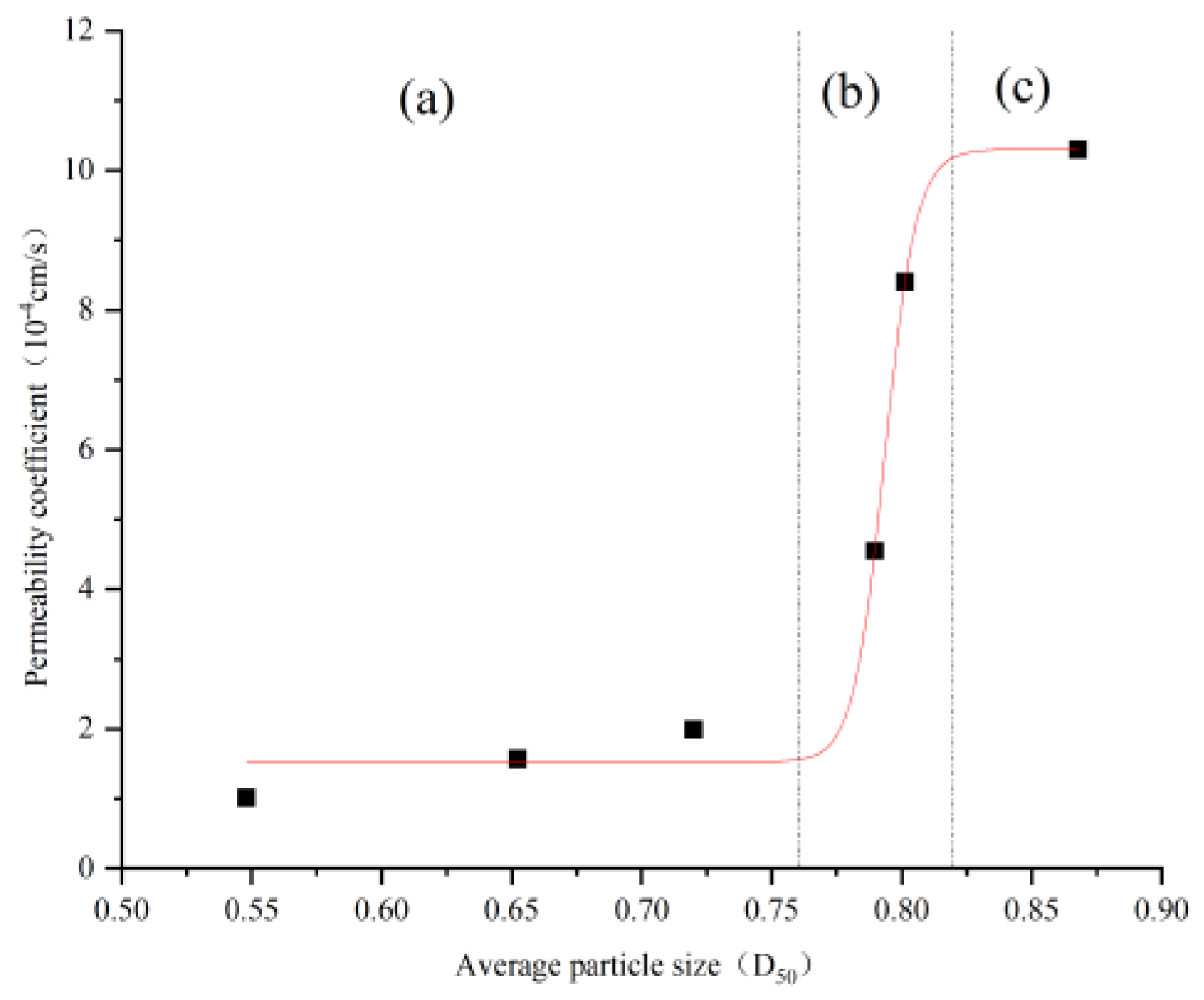
4.3. Correlation Between Average Particle Size D50 and Initial Permeability Coefficient
4.4. Correlation of Intergranular Porosity with Initial Permeability Coefficient
- e = Porosity ratio
- es = Intergranular porosity ratio
- fc = Fine grain volume (cm3)
- 1 − fc = Coarse Grain Volume (cm3)
5. Conclusions
- (1)
- Experimental results indicate that during clear water infiltration, the permeability coefficients of the various layers of rare earth ores follow the order: upper layer > middle layer > overall > lower layer. This phenomenon can be attributed to the higher hydraulic gradient present in the upper layer, which enhances the migration of fine particles and improves pore connectivity. Consequently, as the fine particle content increases, particularly when it exceeds 10%, the permeability coefficients of the middle and lower layers become increasingly similar, indicating a significant suppressive effect of fine particles on pore connectivity.
- (2)
- Under magnesium sulfate leaching conditions, the permeability coefficients of each layer exhibit a similar hierarchical relationship as observed with clear water. However, during the initial 1 to 2 days of treatment, permeability coefficients decrease rapidly and subsequently stabilize. This variation is closely associated with the chemical exchange reactions between the magnesium sulfate solution and the rare earth particles. Such reactions alter the surface charge distribution of the particles, thereby affecting the electrostatic repulsion among particles and the pore structure, leading to a reduction in overall permeability.
- (3)
- A comparison of the initial permeability coefficients of different fine particle samples reveals a significant negative correlation between increasing fine particle content and permeability coefficients. Specifically, when the fine particle content is below 10%, the permeability coefficient decreases gradually; however, once the fine particle content reaches the range of 10% to 20%, the permeability coefficient declines sharply, demonstrating strong sensitivity. Additionally, an increase in the uniformity coefficient suggests that an improved particle size distribution can limit permeability, despite the fact that a high uniformity coefficient generally indicates better particle grading.
- (4)
- To accurately assess the permeability performance when fine particle content exceeds 20%, this study introduces the concept of inter-particle void ratio (es). The analysis indicates that an increase in the inter-particle void ratio leads to a significant reduction in the permeability coefficient of the ore body, especially when es values are between 1.29 and 1.89, at which point a drastic decline in permeability is observed. This reflects changes in the connectivity of internal pores within the ore. Therefore, the inter-particle void ratio effectively captures the spatial relationships among fine particles and provides new insights into understanding permeability variations.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, G.D.; Wang, K.; Chen, Q.S.; Zhang, Y.F.; Xing, J.Y.; Long, T.; Dong, Y.T.; Ni, J.P. The change of world rare earth industrial structure and the problems faced by China’s rare earth industry. Acta Geol. Sin. 2021, 42, 265–272. [Google Scholar]
- Kong, L.; Li, X.; Tian, H. Correlation between fine-grained effect of permeability coefficient and state parameters in sandy soils. Geotechnics 2011, 32 (Suppl. S2), 21–26. [Google Scholar]
- He, S.; Xianmu, S.-A.; Rong, H. Experimental study on the effect of particle migration and ion adsorption on permeability of coarse-grained soil. Nonferrous Met. Sci. Eng. 2018, 9, 80–85. [Google Scholar]
- Luo, Z.; Huang, Q.; Wang, G. Experimental study on the change rule of permeability during leaching of ionic rare earths. Nonferrous Met. Sci. Eng. 2014, 5, 95–99. [Google Scholar] [CrossRef]
- Yin, S.; Qi, Y.; Xie, F. Permeability characteristics of rare earths in weathered crust leachate with different pore structures. Chin. J. Nonferrous Met. 2018, 28, 1043–1049. [Google Scholar]
- Li, S.; Liu, Z.; Mong, J. Test on engineering characteristics of red clay under the action of ammonium carbonate solution. J. Henan Univ. Sci. Technol. Nat. Sci. Ed. 2019, 36, 57–63. [Google Scholar]
- Liang, J.; Fang, Y.; Gu, R. Analysis of micro electric field effects on the seepage of fine particles clay. Rock Soil Mech. 2010, 31, 3043–3050. [Google Scholar]
- Chen, X.; He, Q.; Chen, J.F.; Huang, L.; Tan, C.M.; Yin, Y.Q.; Jiao, Y.F.; Xiao, Y.F. Development of leaching technology and theory of ion-adsorption type rare earth ore. J. Chin. Soc. Rare Earths 2022, 40, 936–947. [Google Scholar]
- Feng, J.; Zhou, F.; Chi, R.A.; Liu, X.; Xu, Y.L.; Liu, Q. Effect of a novel compound on leaching process of weathered crust elution deposited rare earth ore. Miner. Eng. 2018, 129, 63–70. [Google Scholar] [CrossRef]
- Liu, D.F.; Zhang, Z.Y.; Chi, R.A. Seepage mechanism during in-situ leaching process of weathered crust elution-deposited rare earth ores with magnesium salt. Physicochem. Probl. Miner. Process. 2020, 56, 349–361. [Google Scholar] [CrossRef]
- Deng, Z.X.; Qin, L.; Wang, G.S.; Luo, S.H.; Peng, C.L.; Li, Q. Metallogenic process of ion adsorption REE ore based on the occurrence regularity of La in kaolin. Ore Geol. Rev. 2019, 112, 103022. [Google Scholar] [CrossRef]
- Peng, L.; Chen, B.; Zou, X.; Zou, Z.; Xiao, W.; Que, X. New scheme for rare earth allotment characteristics and type classification of ionic rare earth ores. Chin. J. Rare Earths 2021, 39, 624. [Google Scholar]
- Zhou, F.; Liu, Q.; Feng, J.; Su, J.X.; Liu, X.; Chi, R.A. Role of initial moisture content on the leaching process of weathered crust elution-deposited rare earth ores. Sep. Purif. Technol. 2019, 217, 24–30. [Google Scholar] [CrossRef]
- Qiu, T.; Yan, H.; Li, J.; Liu, Q.; Ai, G. Response surface method for optimization of leaching of a low-grade ionic rare earth ore. Powder Technol. 2018, 330, 330–338. [Google Scholar] [CrossRef]
- Han, M.; Wang, D.; Rao, Y.; Xu, W.; Nie, W. An experimental study on the kinetics of leaching ion-adsorbed REE deposits with different concentrations of magnesium sulfate. Metals 2023, 13, 1906. [Google Scholar] [CrossRef]
- Luo, S.H.; Luo, T.; Wang, G.S.; Liu, J.; Hu, S.L.; Zhu, D.M. Effect of heterogeneity of leaching solution on leaching rate in ionic rare earth ore body. Soils 2018, 50, 421–427. [Google Scholar]
- Tian, J.; Yin, J.Q.; Chi, R.A.; Rao, G.H.; Jiang, M.T.; Ouyang, K.X. Kinetics on leaching rare earth from the weathered crust elution-deposited rare earth ores with ammonium sulfate solution. Hydrometallurgy 2010, 101, 166–170. [Google Scholar]
- Zou, H.L.; Zhang, Z.Y.; Chen, Z.; Liu, D.F.; Chai, X.W.; Zhang, H.; Chi, R.A. Seepage process on weathered crust elution-deposited rare earth ores with ammonium carboxylate solution. Physicochem. Probl. Miner. Process. 2020, 56, 90–102. [Google Scholar]
- Wei, D.D.; Wang, H.N.; Yang, Z.Z. Experimental research on the leaching of ionic rare earth ore with weak permeability. Min. Res. Dev. 2016, 36, 55–58. [Google Scholar]
- Sun, R.; Zhang, Y.; Fan, K.; Shi, Y. Molecular simulation of adsorption properties of clay minerals in shale. J. Chem. Eng. 2015, 66, 2118–2122. [Google Scholar]
- Li, F.J.; Xiao, Z.; Zeng, J.Y.; Chen, J.Q.; Sun, X.Q. Recovery of REEs from leaching liquor of ion-adsorbed-type rare earth ores using ionic liquid based on cooking oil. Hydrometallurgy 2020, 196, 105449. [Google Scholar] [CrossRef]
- Yang, L.F.; Wang, D.S.; Li, C.C.; Sun, Y.Y.; Zhou, X.Z.; Li, Y.X. Searching for a high efficiency and environmentally benign reagent to leach ion-adsorption rare earths based on the zeta potential of clay particles. Green Chem. 2018, 20, 4528–4536. [Google Scholar] [CrossRef]
- Gao, Z.; Rao, Y.; Shi, L.; Xiang, R.; Yang, Z. Effect of magnesium sulfate solution on pore structure of ionic rare earth ore during leaching process. Minerals 2023, 13, 294. [Google Scholar] [CrossRef]
- Long, P.; Wang, G.; Zhang, C.; Yang, Y.; Cao, X.; Shi, Z. Kinetics model for leaching of ion-adsorption type rare earth ores. J. Rare Earths 2020, 38, 1354–1360. [Google Scholar] [CrossRef]
- Xie, F.F.; Wang, G.S.; Wen, T. Discussion on reasons for difference of ionic phase rare earth grade distribution in different grain size fractions. Chin. J. Nonferr. Met. 2023, 33, 1672–1681. [Google Scholar]
- Yan, D.R.; Ro, S.; Sunam, O.; Kim, S. On the global rare earth elements utilization and its supply-demand in the future. IOP Conf. Ser. Earth Environ. Sci. 2020, 508, 012084. [Google Scholar] [CrossRef]
- Sen, T.K.; Khilar, K.C. Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv. Colloid Interface Sci. 2006, 119, 71–96. [Google Scholar]
- Zhang, X.; Gao, Z.; Rao, Y.; Shi, L.; Xu, W. Evolutionary law of pore structure of ion-adsorbed rare earth ore leaching process. Minerals 2023, 13, 322. [Google Scholar] [CrossRef]
- Wu, K.X.; Zhang, L.; Zhu, P.; Chen, L.K.; Tian, Z.F.; Xing, X.Y. Research on particle size distribution and its variation of ion-adsorption type rare earth ore. Chin. Rare Earths 2016, 37, 67–74. [Google Scholar]
- Thevanayagam, S.; Mohan, S. Intergranular State Variables and Stress-Strain Behaviour of Silty Sands. Geotechnique 2000, 50, 1–23. [Google Scholar] [CrossRef]
- Xenaki, V.C.; Athanasopoulos, G.A. Liquefaction resistance of sand-silt mixtures: An experimental investigation of the effect of fines. Soil Dyn. Earthq. Eng. 2003, 23, 183–194. [Google Scholar] [CrossRef]






| Parameter Type | Value |
|---|---|
| moisture content (%) | 20.47 |
| dry density (g/cm3) | 1.35 |
| specific gravity of particles | 2.70 |
| porosity ratio | 0.995 |
| porosity (%) | 49.87 |
| Particle Size (mm) | >5 | 5~2 | 2~1 | 1~0.5 | 0.5~0.25 | 0.25~0.075 | <0.075 |
|---|---|---|---|---|---|---|---|
| percentage content (%) | 5 | 24 | 15 | 18 | 15 | 10 | 13 |
| Oxide | Na2O | MgO | AL2O3 | SiO2 | SO3 | K2O | CaO |
|---|---|---|---|---|---|---|---|
| Content (%) | 0.146 | 0.127 | 34.975 | 48.251 | 0.029 | 1.847 | 0.098 |
| Oxide | TiO2 | MnO | Fe2O3 | NiO | ZnO | Ga2O3 | Rb2O |
| Content (%) | 0.037 | 0.080 | 1.994 | 0.004 | 0.019 | 0.009 | 0.041 |
| Oxide | Y2O3 | ZrO2 | Nd2O3 | Yb2O3 | WO3 | PbO | ThO2 |
| Content (%) | 0.097 | 0.028 | 0.031 | 0.014 | 0.007 | 0.041 | 0.007 |
| Fines Content (%) | Pore Ratio (e) | Average Particle Size D50 (mm) | Curvature Coefficient Cc | Coefficient of Inhomogeneity Cu |
|---|---|---|---|---|
| 5 | 0.97 | 0.87 | 0.93 | 9.15 |
| 10 | 1.06 | 0.80 | 1.13 | 12.20 |
| 15 | 1.23 | 0.79 | 3.59 | 45.41 |
| 20 | 1.31 | 0.72 | 3.84 | 53.93 |
| 25 | 1.37 | 0.65 | 2.42 | 60.29 |
| 30 | 1.45 | 0.55 | 0.77 | 63.12 |
| Fine Grain Content (%) | Initial Permeability Coefficient (10−4 cm/s) |
|---|---|
| 5 | 10.30 |
| 10 | 8.40 |
| 15 | 4.55 |
| 20 | 1.99 |
| 25 | 1.57 |
| 30 | 1.01 |
| Unevenness Coefficient Cu | Initial Permeability Coefficient (10−4 cm/s) |
|---|---|
| 9.15 | 10.30 |
| 12.20 | 8.40 |
| 45.41 | 4.55 |
| 53.93 | 1.99 |
| 60.29 | 1.57 |
| 63.12 | 1.01 |
| Average Particle Size D50 (mm) | Initial Permeability Coefficient (10−4 cm/s) |
|---|---|
| 0.87 | 10.30 |
| 0.80 | 8.40 |
| 0.79 | 4.55 |
| 0.72 | 1.99 |
| 0.65 | 1.57 |
| 0.55 | 1.01 |
| Intergranular Porosity Ratio es | Initial Permeability Coefficient (10−4 cm/s) |
|---|---|
| 1.07 | 10.30 |
| 1.29 | 8.40 |
| 1.62 | 4.55 |
| 1.89 | 1.99 |
| 2.16 | 1.57 |
| 2.50 | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, R.; Rao, Y.; Zhang, J.; Zhang, L.; Yang, Z. Effect of Leaching of Ionic Rare Earth Ores on the Permeability Coefficient of Mineral Soil and Its Correlation with the State Parameter. Metals 2025, 15, 258. https://doi.org/10.3390/met15030258
Xiang R, Rao Y, Zhang J, Zhang L, Yang Z. Effect of Leaching of Ionic Rare Earth Ores on the Permeability Coefficient of Mineral Soil and Its Correlation with the State Parameter. Metals. 2025; 15(3):258. https://doi.org/10.3390/met15030258
Chicago/Turabian StyleXiang, Run, Yunzhang Rao, Jiyong Zhang, Laiye Zhang, and Zhihua Yang. 2025. "Effect of Leaching of Ionic Rare Earth Ores on the Permeability Coefficient of Mineral Soil and Its Correlation with the State Parameter" Metals 15, no. 3: 258. https://doi.org/10.3390/met15030258
APA StyleXiang, R., Rao, Y., Zhang, J., Zhang, L., & Yang, Z. (2025). Effect of Leaching of Ionic Rare Earth Ores on the Permeability Coefficient of Mineral Soil and Its Correlation with the State Parameter. Metals, 15(3), 258. https://doi.org/10.3390/met15030258






