Abstract
The interaction between the high-entropy alloy CoCrCu2FeNi and diamond, as well as the graphitization of diamond, were investigated using in situ transmission electron microscopy in the temperature range of 20–900 °C. To ensure the absence of interaction between diamond and the HEA at the initial stage of the experiment, the test sample was prepared by magnetron sputtering of the CoCrCu2FeNi coating on a diamond single crystal. The following stages of the interaction of diamond with the CoCrCu2FeNi alloy were discovered. A partial transformation of FCC to BCC crystal lattice occurs in CoCrCu2FeNi HEA at 500 °C. At a temperature of 700 °C, the process of diffusion of Fe, Co, Ni, and Cu over the diamond surface commences. These elements catalyze the transformation of diamond into graphite at a temperature of 800 °C. Carbon in graphite interacts with chromium from the HEA to form Cr7C3 carbide. At 900 °C, a secondary copper-based phase with an FCC lattice is formed within the CoCrCu2FeNi coating.
1. Introduction
Diamond is a unique, natural, and man-made material, having no rival in terms of thermal conductivity and hardness. It is used as a component in metal matrix composites that are applied as abrasive tools and thermally conductive devices. Adhesion at the diamond/metal interface resulting from chemical bonding between the constituents is critical for such materials. High-entropy alloys (HEAs) with FCC structures, which contain elements within the Co-Cr-Cu-Fe-Mn-Mo-Ni-Ti system, are promising materials to be used as a matrix of diamond-based composites. These HEAs are characterized by a combination of high strength, hardness, ductility, and wear resistance [1,2,3,4]. They are easily manufacturable and can be applied in powder technology for fabricating metal matrix diamond composites [5,6]. These HEAs simultaneously contain components responsible for high thermal conductivity (Cu) and adhesion to diamond (Cr, Ti, Co) [7,8,9]. Co-Cr-Cu-Fe-Ni HEAs are of particular interest. It has been demonstrated previously [10,11,12] that these HEAs are characterized by a good combination of mechanical properties (UTS~1000 MPa, hardness~100 HRB) and can ensure strong adhesion to diamond due to the formation of carbide layers at the interface.
Studies focusing on the nature of interactions at the metal–diamond interface in simple systems (Ni–diamond [13], Cr–diamond [14,15], and NiCr–diamond [16]) have been published recently. They demonstrate that the Ni–Cr alloy is more reactive with respect to diamond compared to single-component matrices. Adding chromium to nickel results in the formation of a coherent interface with diamond and slows down the diffusion of carbon by accelerating the formation of interphase carbides. These studies were conducted post-mortem, and the results may not reflect all the features that occur during the heating and/or consolidation of such systems.
Studies devoted to first-principles calculations in Co-Cr-Cu-Fe-Mn-Mo-Ni-Ti–diamond systems are of great value [17,18,19,20], but the results require experimental validation.
In situ transmission electron microscopy (TEM) is a novel method that can shed light on many phenomena occurring upon the interaction between multicomponent alloys and diamond, as well as study all the processes over time [21,22,23,24]. This method is a powerful tool that allows one to determine the temperature intervals of structural and phase transformations with high accuracy, while simultaneously identifying them using diffraction or spectral methods.
This study aimed to in situ investigate the evolution of structural and phase transformations at the interface between the CoCrCu2FeNi HEA and single crystal diamond upon vacuum heating in the temperature range of 20–900 °C. The increased copper content in the alloy compared to the equiatomic one was chosen to ensure more effective solid solution hardening of the matrix [25] and the formation of a ductile copper-based sublayer at the interface, favorably affecting the ductility of the entire composite [12]. Understanding the processes taking place at the HEA–diamond interface allows us to optimize the manufacturing regimes of metal–diamond composites. It will be possible to improve the performance of HEA-based diamond cutting tools, ensure the high bonding strength of diamond to binders, and protect diamond from excessive degradation.
2. Materials and Methods
The studies were carried out using a model sample: a single crystal of diamond with a magnetron coating (CoCrCu2FeNi HEA) sputtered onto the (100) face. It allowed us to identify the sequence and characteristic temperatures of the main structural transformations within a broad temperature range (20–900 °C), as well as characterize the initial stage of the processes. It poses a challenge to post-mortem studies of annealed samples. This approach to obtaining the test sample provides good contact at the interface and minimal interaction between the components at the initial stage of the experiment. The maximum test temperature of 900 °C was selected as it is the typical temperature for the hot pressing or pressureless sintering of metal–diamond composite materials, specifically segments for diamond tools, which contain copper in substantial quantities [7,26,27,28].
The CoCrCu2FeNi coating was deposited onto a diamond substrate by magnetron sputtering on a UHV-2M setup with two magnetrons and a slot-type ion source for substrate cleaning, equipped with a Pinnacle+ Advanced Energy DC power supply unit, a TruPlasma RF 1003 Trumpf Hüttinger high-frequency unit, a TruPlasma Highpulse 4002 Trumpf Hüttinger unit for high-power impulse magnetron sputtering, and a PlasmaScope Horiba JY optical spectrometer for plasma diagnostics. Figure 1a shows a schematic of the setup.
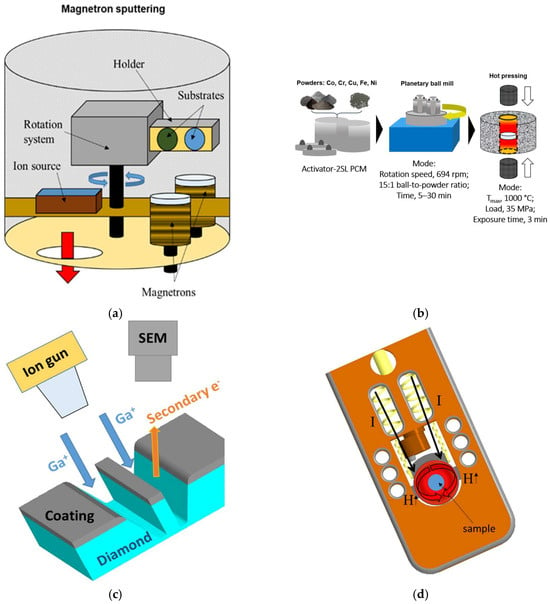
Figure 1.
A schematic of the equipment and procedures used in this study: (a) a magnetron sputtering setup; (b) fabrication of the target using the powder metallurgy methods; (c) preparing the sample using a focused ion beam; and (d) the operating part of a TEM heating holder.
The CoCrCu2FeNi target for magnetron sputtering was fabricated using powder metallurgy methods. The starting materials were as follows: carbonyl iron powder of VK-3 grade (JSC “Sintez CIP”, Dzerzhinsky, Russia; average particle size d = 9 μm, impurity content ≤ 0.3 wt.%), carbonyl nickel powder of PNK-UT3 grade (JSC “Kola Mining and Metallurgical Company”, Monchegorsk, Russia; d = 10 μm, impurity content ≤ 0.06 wt.%), reduced cobalt powder of EFS-1 grade (Hanrui Cobalt Co., Ltd., Nanjing, China; d = 1.2 μm, impurity content ≤ 0.03 wt.%); electrolytic chromium powder of PM-ERKh grade (JSC “Polema”, Tula, Russia; d = 80 μm, impurity content 0.05 wt.%), and electrolytic copper powder of PMS-1 grade (JSC “Uralelectromed”, V. Pyshma, Russia; d = 24 μm, impurity content 0.12 wt.%). Powder mixtures were prepared using an Activator-2SL planetary ball mill (PBM) (OJSC “Chemical Machine Building Plant”, Novosibirsk, Russia) in the following mode: rotational speed of jars, 694 rpm; centrifugal factor, 90× g; duration, 30 min; and ball-to-powder ratio, 15:1. The mixture was subjected to additional treatment with 10 wt.% of isopropyl alcohol added to the jar to ensure powder refinement. Compact targets shaped as cylinders 50 mm in diameter and 5 mm high were obtained with the hot pressing (HP) of powder mixtures on a DSP-515 SA machine (Dr. Fritsch, Fellbach, Germany). Vacuum hot pressing was conducted at a maximum temperature of 1000 °C, a compacting pressure of 35 MPa, and a duration of isobaric exposure of 3 min. The flowchart is shown in Figure 1b.
The sample (lamella) for the in situ TEM studies was prepared using the focused ion beam technique with a FEI Quanta 200 3D dual-beam microscope (FEI Company, Hillsboro, OR, USA) (Figure 1c). The CoCrCu2FeNi–diamond lamella had dimensions of ~7 × 2 × 0.1 μm.
The in situ investigation of the microstructure upon lamella heating was carried out using a JEM-2100 transmission electron microscope (Jeol, Tokyo, Japan); the residual pressure in the microscope column was 10−6 Pa. The lamella was heated in a Gatan heating holder model 652 (Gatan, Pleasanton, CA, USA) by passing an electric current through a ring-shaped tantalum heater surrounding the lamella (Figure 1d). The heating rate was (50 ± 5) °C/min; temperatures of 20, 500, 600, 700, 800, and 900 °C were selected to study the alloy structure. Structure imaging was conducted at fixed temperatures after exposure for 15–20 min, which was needed to equalize the temperature gradients causing sample drift. The TEM microstructure images were used to estimate the crystallite size using the linear intercept method.
Diamond samples with CoCrCu2FeNi HEA coatings were annealed in a Thermionic T1 vacuum furnace (OJSC “Thermionic”, Podolsk, Russia) at temperatures of 700 and 900 °C, an exposure time of 15 min, and a residual pressure in the furnace chamber of 10−5 Pa.
The phase composition of the samples after annealing was studied by X-ray diffraction analysis on a D2 Phaser diffractometer (Bruker, Billerica, MA, USA), using CuKα radiation in the Bragg–Brentano geometry in the 2θ range of 10–120°. The Diffrac.EVA v. 4.3.1.2 software (Bruker, USA) was used for phase identification.
3. Results
Figure 2 shows an image of the CoCrCu2FeNi–diamond lamella at room temperature. The upper part of the lamella represents a single crystal diamond. In the lower part, there is the CoCrCu2FeNi HEA coating with a nanocrystalline structure. The coating is composed of FCC solid solution as evidenced by electron diffraction data (a characteristic set of diffraction rings from the (111), (200), and (220) planes, etc.).
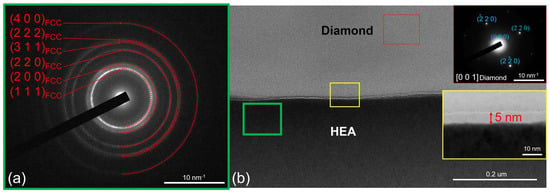
Figure 2.
The selected area electron diffraction (SAED) and (a) structure of the CoCrCu2FeNi–diamond interface (b) at room temperature.
The lamella has a linear interface with an intermediate amorphous carbon (AC) layer that is 4–5 nm thick, which is an artifact of magnetron sputtering of the coating. There are no traces of carbide formation at the interface.
The CoCrCu2FeNi coating has a uniform chemical composition as determined using EDX according to the distribution of the intensity of characteristic X-ray radiation from elements present in the coating (Figure 3).
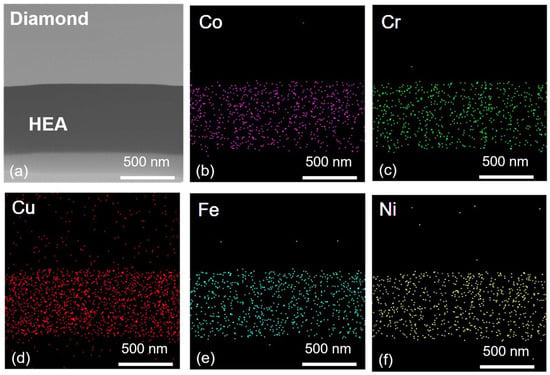
Figure 3.
Elemental mapping of CoCrCu2FeNi–diamond interface: scanning transmission electron microscopy image (a) and element distribution maps of Co (b), Cr (c), Cu (d), Fe (e), Ni (f).
No structural changes in the lamella were observed in the temperature range of 20–450 °C. At 500 °C, two new diffraction rings corresponding to interplanar spacings d = 0.1989 nm and d = 0.1400 nm appeared in the electron diffraction pattern from the coating (Figure 4). The determined interplanar spacings correspond to the (110) and (200) planes of the α-Fe phase and Fe-based BCC solid solutions (e.g., for the alloy with composition Cr0.325Fe0.457Co0.218 studied in ref. [29]). Partial FCC-to-BCC phase transition is caused by diffusionless phase transformation similar to the martensitic transformation. This phenomenon was previously observed and described in more detail in refs. [30,31], which focused on the thermal stability of CoCrCuFeNi coatings deposited by magnetron sputtering.
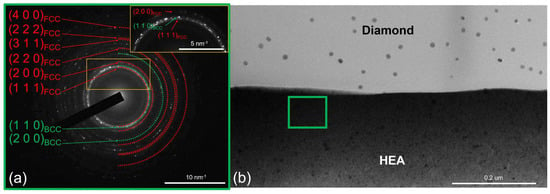
Figure 4.
The SAED from the CoCrCu2FeNi coating and (a) the structure of the CoCrCu2FeNi–diamond interface (b) at 500 °C.
Furthermore, it is important to note that nanoparticles 10–20 nm in size appeared on the diamond part of the lamella during the heating process (round areas of dark contrast). We believe their appearance at the very beginning of the heating experiment is due to the presence of small amounts of gallium on the surface of the lamella, which remained after ion etching.
Elevation of the annealing temperature to 600 °C was not accompanied by any significant changes at the CoCrCu2FeNi–diamond interface (Figure 5). No signs of diamond graphitization were observed; a thin amorphous carbon layer at the interface was retained. The electron diffraction pattern contained diffraction rings corresponding to the FCC and BCC solid solutions. Few low-intensity reflections from the planes with an interplanar spacing of d = 0.2430 nm additionally appeared; these reflections are likely to belong to the NiCrO3 phase, which was formed via the interaction of impure oxygen with nickel and chromium present in the coating. Annealing at 600 °C was accompanied by the growth of crystallites in the coating up to the values of ~25 nm.
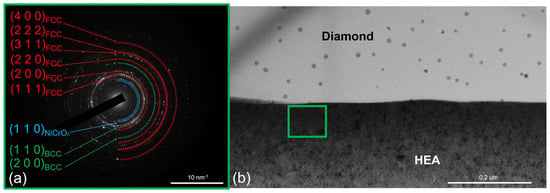
Figure 5.
The SAED from the CoCrCu2FeNi coating and (a) the structure of the CoCrCu2FeNi–diamond interface (b) at 600 °C.
An increase in temperature to 700 °C intensified crystallite growth in the coating (Figure 6). The average crystallite size was 40 nm; individual crystallites were as large as 80 nm. No changes in the phase composition of the coating were observed. Irregularly shaped regions with a characteristic size of ~20 nm appeared on the diamond surface near the interface during isothermal exposure at 700 °C (Figure 6, inset 2). These regions with a darker contrast were formed via surface diffusion of coating components over the diamond. Their chemical and phase compositions were subsequently identified at higher annealing temperatures.
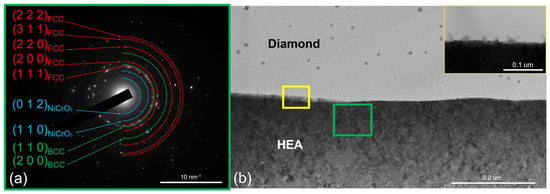
Figure 6.
The SAED from the CoCrCu2FeNi coating and (a) the structure of the CoCrCu2FeNi–diamond interface (b) at 700 °C.
Heating to 800 °C was accompanied by the intense diffusion of metals within the coating over the surface of the diamond part of the lamella. Figure 7 shows the resulting intermediate layer at the interface. In Supplementary Video S1, one can see how this process evolves during isothermal exposure.
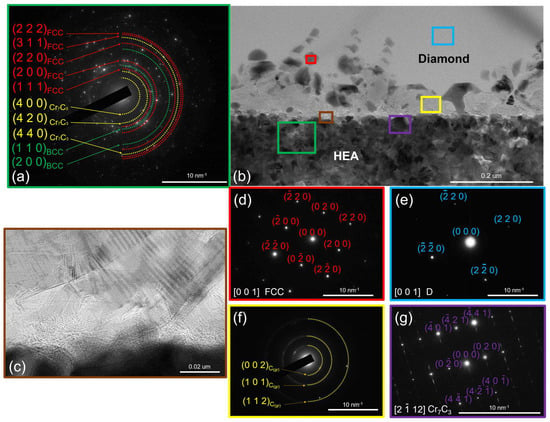
Figure 7.
The SAED from the CoCrCu2FeNi coating and (a) the structure of the CoCrCu2FeNi–diamond interface (b) at 800 °C, with a high-resolution image of graphite-like carbon (c), and the SAEDs from FCC droplets: (d) diamond (e), graphite-like carbon (f), and Cr7C3 (g).
Five typical regions can be differentiated at the CoCrCu2FeNi–diamond interface once the diffusion processes slow down: region 1—intact diamond (not subjected to graphitization); region 2—local regions (droplets) based on HEA components; region 3—graphite-like carbon; region 4—the Me7C3-type carbide phase; and region 5—the CoCrCu2FeNi coating with a chemical composition altered as some metal was consumed to form regions 2 and 4.
The droplets formed during the diffusion of metals consist of Fe, Co, Cu, and Ni. The average composition was determined according to the results of EDX analysis at ten points (30%Fe–28%Co–21%Ni–21%Cu). These droplets were crystallized on the diamond surface as FCC crystals coherent to the diamond lattice (220)diamond || (220)FeCoNiCu. Iron, cobalt, and nickel within these droplets strongly catalyze the diamond-to-graphite phase transition [13]. Their distribution on the diamond surface results in the formation of a layer made of polycrystalline graphite with a fiber-like shape. These crystallites were found to belong to graphite-like carbon according to the electron diffraction data ((002), (101), and (004) planes with the interplanar spacings d(002) = 0.3360 nm, d(101) = 0.2030 nm, and d(004) = 0.1678 nm, which corresponds to graphite ICDD card # 00-023-0064).
The type of the Me7C3 carbide phase was identified by analyzing the electron diffraction patterns recorded for several individual crystallites. Figure 7 shows an electron diffraction pattern corresponding to the crystal with a hexagonal structure (space group P63mc) typical of Cr7C3- or (Cr,Fe)7C3-type complex carbides. Superlattice reflections present in the electron diffraction patterns are also typical of Cr7C3 carbide. They are indicative of a special ordered arrangement of dissolved carbon atoms: at the centers of octahedral complexes [32].
The chemical composition of the HEA-based coating was substantially changed as a result of diffusion processes within the coating and the carbide formation upon the interaction between chromium and graphitized carbon. The average composition of the HEA-based coating (region 5 in Figure 7) was 27% Ni, 25% Co, 23% Fe, 15% Cu, and 10% Cr. The lack of chromium was attributed to its consumption in Me7C3 carbide formation. Copper concentration was also significantly lower than that in the as-deposited coating. This could be explained by the fact that copper is involved in the formation of catalytic “droplets” on the diamond surface at almost the same ratio as Fe, Co, and Ni.
An increase in annealing temperature to 900 °C did not cause any further changes in the phase composition at the CoCrCu2FeNi–diamond interface (Figure 8). The sample contained the same five characteristic regions as those observed at 800 °C. It is noteworthy that the thickness of the HEA-based coating was reduced and its integrity was disrupted at the sites where it contacts diamond. The coating thickness was reduced because of mass transfer via the surface diffusion mechanism.
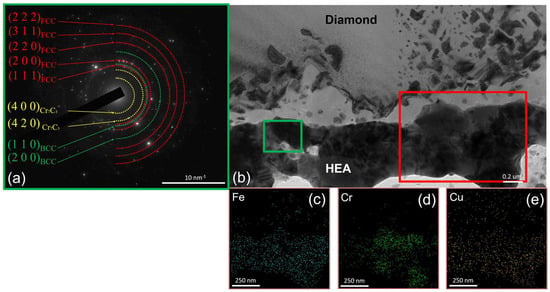
Figure 8.
The SAED from the CoCrCu2FeNi coating; (a) the structure of the CoCrCu2FeNi–diamond interface (b) at 900 °C and EDX elemental maps for Fe (c), Cr (d), Cu (e).
An EDX study of the chemical composition of the coating revealed that copper content was lower than that in the as-deposited coating (~14–16%). Since copper was involved in the formation of catalytic “droplets” on the diamond surface in the same degree as Fe, Co, and Ni, this shortcoming should be attributed to the formation of a secondary phase of the FCC2 copper-based solid solution phase, which was previously demonstrated in our studies [10,11] and other works focusing on CoCrCuFeNi HEA [33,34,35].
XRD and EDX studies were carried out to detect the FCC2 phase in the annealed CoCrCu2FeNi coating on a diamond substrate. The as-deposited coating had a nanocrystalline FCC structure as evidenced by a broad diffraction peak from the (111) planes (Figure 9). After annealing at 700 °C, the FCC phase crystallized in the coating (the BCC phase underwent a reverse BCC-to-FCC transformation upon cooling to temperatures below 500 °C), and a small amount of the NiCrO3 oxide phase was present, which was also detected in the electron diffraction patterns (Figure 5 and Figure 6). Peaks belonging to the FCC2 phase with a lattice parameter of a = 0.3597 nm, close to that of pure copper, were detected in the XRD pattern of the coating annealed at 900 °C.
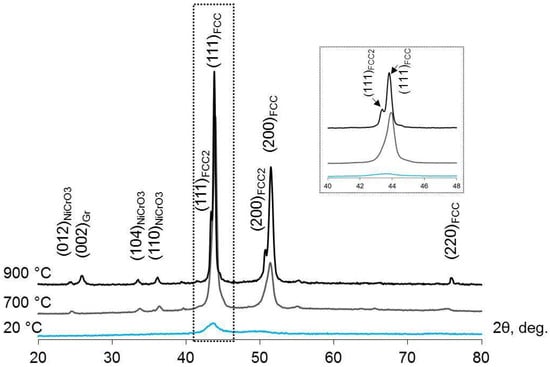
Figure 9.
XRD patterns recorded for the initial CoCrCu2FeNi coating on a diamond substrate and for the coating after vacuum annealing at 700 and 900 °C. The inset shows a zoomed-in region of the XRD pattern in the 2θ range of 40–48°.
The microstructure of the CoCrCu2FeNi coating on a diamond substrate after annealing at 900 °C was analyzed to study the FCC2 phase distribution. As shown in Figure 10, the FCC2 phase is formed as near-spherical grains, 1–5 µm in diameter, which are uniformly distributed over the surface. Upon formation of an intermediate layer, Co, Cr, Fe, and Ni atoms presumably repel Cu atoms away from the diamond interface due to higher adsorption energy [18]. This process is accompanied by a graphitizing effect of Fe, Co, and Ni with respect to diamond (which was observed in this study at temperatures above 800 °C), followed by the formation of Cr7C3 chromium carbide. The FCC2 copper-based phase is formed farther from diamond compared to Cr7C3 carbide, which was previously proved experimentally in ref. [12].
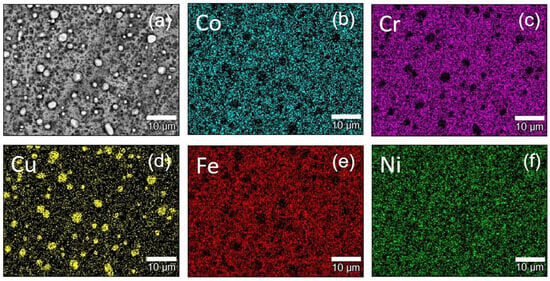
Figure 10.
The surface of the diamond substrate with CoCrCu2FeNi coating after annealing at 900 °C: scanning electron microscopy image (a) and element distribution maps of Co (b), Cr (c), Cu (d), Fe (e), Ni (f).
Hence, we have carried out an in situ TEM study on the evolution of the structure and phase transformation at the CoCrCu2FeNi HEA–diamond interface in the temperature range of 20 to 900 °C. The following features have been revealed: partial FCC-to-BCC transformation takes place in the coating based on CoCrCu2FeNi HEA at 500 °C; at 700 °C, diamond starts to interact with HEA, which manifests itself as a metal diffusion over the diamond surface; at 800 °C, diamond graphitization takes place and graphite interacts with the HEA to yield Cr7C3 carbide; at 900 °C, the secondary copper phase is precipitated as spherical grains in HEA. Figure 11 shows a schematic illustration of the process.
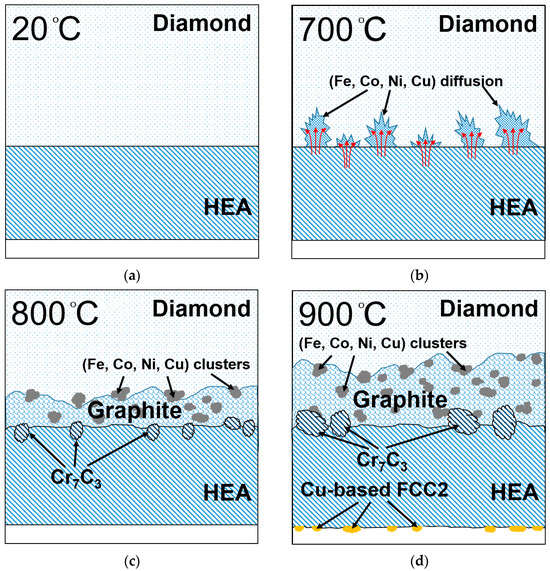
Figure 11.
Schematic illustration of structure changes at CoCrCu2FeNi—diamond interface at (a) 20 °C, (b) 700 °C, (c) 800 °C, and (d) 900 °C.
4. Conclusions
An in situ TEM study of the phase and structural transformations at the interface between CoCrCu2FeNi HEA and single crystal diamond was carried out upon vacuum heating in the temperature range of 20–900 °C. The following features have been revealed:
- No structural changes in the HEA or at the interface are observed in the temperature range of 20–450 °C. Above 500 °C, the coating consists of two phases (i.e., partial FCC-to-BCC phase transition is observed for CoCrCu2FeNi HEA).
- The interaction between CoCrCu2FeNi HEA and diamond starts at 700 °C and manifests itself as the surface diffusion of Fe, Co, Ni, and Cu on diamond.
- Intense diamond graphitization takes place at 800 °C. Large 30% Fe, 28% Co, 21% Ni, and 21% Cu clusters coherent to the diamond lattice (220)diamond || (220)FeCoNiCu are formed on the diamond surface under these conditions. They catalyze the transformation of diamond to graphite-like carbon with a crystallite size of 5–20 nm. Graphite interacts with chromium in the HEA, yielding Cr7C3 carbide.
- During annealing, copper is repelled away from the interface with diamond because of lower adsorption energy to be segregated in the FCC2 phase with spherical grains, 1–5 µm in size.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/met15030257/s1, Video S1 Diffusion of HEA over the diamond surface.
Author Contributions
Conceptualization, P.A.L. and A.D.F.; methodology, A.N.S. and E.M.E.; validation, A.N.S., E.M.E. and A.A.Z.; formal analysis, A.N.S.; investigation, P.A.L., A.D.F. and E.M.E.; writing—original draft preparation, P.A.L., A.D.F. and A.A.Z.; visualization, A.D.F.; supervision, E.A.L.; project administration, P.A.L.; funding acquisition, P.A.L. and E.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number “22-79-10144”.
Data Availability Statement
Data will be available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HEAs | High-entropy alloys |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscopy |
| PBM | Planetary ball mill |
| HP | Hot pressing |
| AC | Amorphous carbon |
| SAED | Selected area electron diffraction |
| EDX | Energy dispersive X-ray |
| BCC | Body-centered cubic lattice |
| FCC | Face-centered cubic lattice |
| XRD | X-ray diffraction analysis |
References
- Yang, Y.-F.; Hu, F.; Xia, T.; Li, R.-H.; Bai, J.-Y.; Zhu, J.-Q.; Xu, J.-Y.; Zhang, G.-F. High entropy alloys: A review of preparation techniques, properties and industry applications. J. Alloys Compd. 2025, 1010, 177691. [Google Scholar] [CrossRef]
- Olejarz, A.; Huo, W.Y.; Zieliński, M.; Diduszko, R.; Wyszkowska, E.; Kosińska, A.; Kalita, D.; Jóźwik, I.; Chmielewski, M.; Fang, F.; et al. Microstructure and mechanical properties of mechanically-alloyed CoCrFeNi high-entropy alloys using low ball-to-powder ratio. J. Alloys Compd. 2023, 938, 168196. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Z.; Chen, H.; Niu, M.; Cheng, J. Mechanical properties of CoCrFeNi-X (X = Ti,Sn) high entropy alloy and tribological properties in simulated seawater environment. Tribol. Int. 2025, 202, 110306. [Google Scholar] [CrossRef]
- Jiang, X.; Zeng, X.K.; Xie, W.; Liu, M.; Leng, Y. Optimizing substrate bias voltage to improve mechanical and tribological properties of ductile FeCoNiCu high entropy alloy coatings with FCC structure. J. Alloys Compd. 2024, 1004, 175972. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Mu, D.; Shen, G.; Liu, M.; Zhang, M.; Chan, S.L.I.; Liang, J.; Wang, J. Microstructure evolution and mechanical properties of CoCrFeNiAl0.3 high entropy alloy produced by ball milling in combination with thermomechanical consolidation. Mater. Charact. 2022, 187, 111833. [Google Scholar] [CrossRef]
- Yang, T.; Cai, B.; Shi, Y.; Wang, M.; Zhang, G. Preparation of nanostructured CoCrFeMnNi high entropy alloy by hot pressing sintering gas atomized powders. Micron 2021, 147, 103082. [Google Scholar] [CrossRef]
- Timmer, C.; Tillmann, W.; Wojarski, L.; Ferreira, M.P. Investigation of the applicability of Cu–Fe–Mn–Ni based high entropy and compositionally complex alloys as metal matrix composites for cobalt free hot-pressed diamond tools. J. Mater. Res. Technol. 2023, 26, 5518–5534. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Zhang, F.-L.; Pan, X.-Y.; Gao, P.; Zheng, C.-J.; Zhou, Y.-M.; Li, H.-B. Fabrication and evaluation of high-entropy alloy reinforced Fe bond diamond tool. J. Mater. Process. Technol. 2024, 328, 118419. [Google Scholar] [CrossRef]
- Shah, A.W.; Wang, K.; Siddique, J.A.; Li, W. A comprehensive review of diamond-reinforced metal matrix composites for thermal management in high-performance electronics. J. Mater. Res. Technol. 2024, 33, 8174–8197. [Google Scholar] [CrossRef]
- Mukanov, S.; Loginov, P.; Fedotov, A.; Bychkova, M.; Antonyuk, M.; Levashov, E. The Effect of Copper on the Microstructure, Wear and Corrosion Resistance of CoCrCuFeNi High-Entropy Alloys Manufactured by Powder Metallurgy. Materials 2023, 16, 1178. [Google Scholar] [CrossRef]
- Loginov, P.A.; Fedotov, A.D.; Mukanov, S.K.; Manakova, O.S.; Zaitsev, A.A.; Akhmetov, A.S.; Rupasov, S.I.; Levashov, E.A. Manufacturing of Metal–Diamond Composites with High-Strength CoCrCuxFeNi High-Entropy Alloy Used as a Binder. Materials 2023, 16, 1285. [Google Scholar] [CrossRef] [PubMed]
- Loginov, P.A.; Zaitsev, A.A.; Sidorenko, D.A.; Eganova, E.; Levashov, E. Interfacial interaction and evaluation of bonding strength between diamond and CoCrFeNi(Cu,Ti) high-entropy alloys. Diam. Relat. Mat. 2024, 151, 111849. [Google Scholar] [CrossRef]
- Tulić, S.; Waitz, T.; Čaplovičová, M.; Habler, G.; Vretenár, V.; Susi, T.; Skákalová, V. Catalytic graphitization of single-crystal diamond. Carbon 2021, 185, 300–313. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, W.; Mu, D.; Wu, Y.; Lin, Q.; Xu, X.; Huang, H. Influences of early-stage C diffusion on growth microstructures in solid-state interface reaction between CVD diamond and sputtered Cr. Mater. Charact. 2022, 196, 112603. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, W.; Mu, D.; Lin, Q.; Xu, X.; Huang, H. Growth mechanisms of interfacial carbides in solid-state reaction between single-crystal diamond and chromium. J. Mater. Sci. Technol. 2022, 144, 138–149. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, Z.; Lin, Q.; Huang, G.; Xu, X.; Huang, H.; Mu, D. Towards tailorable interface microstructure through Solid-state interface reaction between synthetic diamond grits and sputtered Ni-Cr binary alloy. Appl. Surf. Sci. 2022, 596, 153531. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Zhang, M.; Peng, P. First-principles calculations on brazed diamond with FeCoCrNi high entropy alloys doped with strong carbide-forming elements. Solid State Commun. 2022, 357, 114980. [Google Scholar] [CrossRef]
- Meng, W.; Lu, J.; Li, H.; Deng, Z.; Wang, B.; Ma, M. The interfacial reaction between diamond (100) surface and CuNi-based filler alloys containing Cr by first-principles calculations. Diam. Relat. Mat. 2023, 131, 109559. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, S.; Chen, W.; Lu, C.; Li, Y.; Li, H.; Cheng, Y.; Yang, J.; He, Y. First-principles comparative study on diamond/carbide combinations: Interfacial adhesion and bonding nature. Int. J. Refract. Hard Mater. 2024, 119, 106566. [Google Scholar] [CrossRef]
- Deng, A.; Lu, J.; Li, D.; Wang, Y. Exploring the activation energy of diamond reacting with metals and metal oxides by first-principle calculation. Diam. Relat. Mat. 2021, 118, 108522. [Google Scholar] [CrossRef]
- Sibirev, A.; Ubyivovk, E.; Belyaev, S.; Resnina, N. In situ transmission electron microscopy study of martensite boundaries movement on cooling and heating of the NiTi shape memory alloy. Mater. Lett. 2022, 319, 132267. [Google Scholar] [CrossRef]
- Resnina, N.; Sibirev, A.; Belyaev, S.; Ubyivovk, E. In situ TEM observation of the martensite interface movement on heating—Cooling—Heating of the pre-deformed NiTi shape memory alloy. Mater. Lett. 2023, 347, 134641. [Google Scholar] [CrossRef]
- Bazlov, A.I.; Tabachkova, N.Y.u.; Zolotorevsky, V.S.; Louzguine-Luzgin, D.V. Unusual crystallization of Al85Y8Ni5Co2 metallic glass observed in situ in TEM at different heating rates. Intermetallics 2018, 94, 192–199. [Google Scholar] [CrossRef]
- Shkodich, N.F.; Smoliarova, T.; Ali, H.; Eggert, B.; Rao, Z.; Spasova, M.; Tarasov, I.; Wende, H.; Ollefs, K.; Gault, B.; et al. Effect of high energy ball milling, heat treatment and spark plasma sintering on structure, composition, thermal stability and magnetism in CoCrFeNiGax (x = 0.5; 1) high entropy alloys. Acta Mater. 2025, 284, 120569. [Google Scholar] [CrossRef]
- Shim, S.H.; Pouraliakbar, H.; Lee, B.J.; Kim, Y.K.; Rizi, M.S.; Hong, S.I. Strengthening and deformation behavior of as-cast CoCrCu1.5MnNi high entropy alloy with micro-/nanoscale precipitation. Mater. Sci. Eng. A 2022, 853, 143729. [Google Scholar] [CrossRef]
- Ren, J.; Ma, Y.; Zhou, Q.; Sun, Z.; Liu, X.; Li, Y. Effect of basalt fiber particles on the holding strength and wear resistance of sintered Cu-based diamond composites. Ceram. Int. 2024, 50, 24979–24986. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, H.; Liu, B.; Liu, Y. Enhanced drilling performance of impregnated diamond bits by introducing a novel HEA binder phase. Int. J. Refract. Hard Mater. 2024, 118, 106449. [Google Scholar] [CrossRef]
- Cygan-Bączek, E.; Cygan, S.; Wyżga, P.; Novák, P.; Lapčák, L.; Romański, A. Improvement in Abrasive Wear Resistance of Metal Matrix Composites Used for Diamond–Impregnated Tools by Heat Treatment. Materials 2023, 16, 6198. [Google Scholar] [CrossRef]
- Dementyeva, G.P.; Eliokums, O.A.; Kavalerova, L.A.; Livshits, B.G.; Milyaev, I.M. Phase Transformation in an Fe-Cr-Co Alloy in the Temperature Range 600–1300 °C. Izv. vysshikh uchebnykh zavedeniy. Chernaya Metall. 1976, 5, 149–150. (In Russian) [Google Scholar]
- Arfaoui, M.; Kovács, V.K.; Radnóczi, G. Diffusionless FCC to BCC phase transformation in CoCrCuFeNi MPEA thin films. J. Alloys Compd. 2021, 863, 158712. [Google Scholar] [CrossRef]
- Arfaoui, M.; Radnóczi, G.; Kovács, V.K. Transformations in CrFeCoNiCu High Entropy Alloy Thin Films during In-Situ Annealing in TEM. Coatings 2020, 10, 60. [Google Scholar] [CrossRef]
- Eshed, E.; Choudhuri, D.; Osovski, S. M7C3: The story of a misunderstood carbide. Acta Mater. 2022, 235, 117985. [Google Scholar] [CrossRef]
- Kuptsov, K.A.; Antonyuk, M.N.; Sheveyko, A.N.; Bondarev, A.; Ignatov, S.; Slukin, P.; Dwivedi, P.; Fraile, A.; Polcar, T.; Shtansky, D. High-entropy Fe-Cr-Ni-Co-(Cu) coatings produced by vacuum electro-spark deposition for marine and coastal applications. Surf. Coat. Technol. 2023, 453, 129136. [Google Scholar] [CrossRef]
- Shkodich, N.F.; Kovalev, I.D.; Kuskov, K.V.; Kovalev, D.; Vergunova, Y.; Scheck, Y.; Vadchenko, S.; Politano, O.; Baras, F.; Rogachev, A. Fast mechanical synthesis, structure evolution, and thermal stability of nanostructured CoCrFeNiCu high entropy alloy. J. Alloys Compd. 2022, 893, 161839. [Google Scholar] [CrossRef]
- Poliakov, M.V.; Kovalev, D.Y.; Volkova, L.S.; Vadchenko, S.G.; Rogachev, A.S. Evolution of the Structure and Phase Composition of High-Entropy CoCrFeNiCu Alloy during Prolonged Annealing. Phys. Met. Metallogr. 2023, 124, 1005–1016. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).