Kinetics of Oxidation of Binary Ti-Cu Alloys in the 600–800 °C Temperature Range
Abstract
1. Introduction
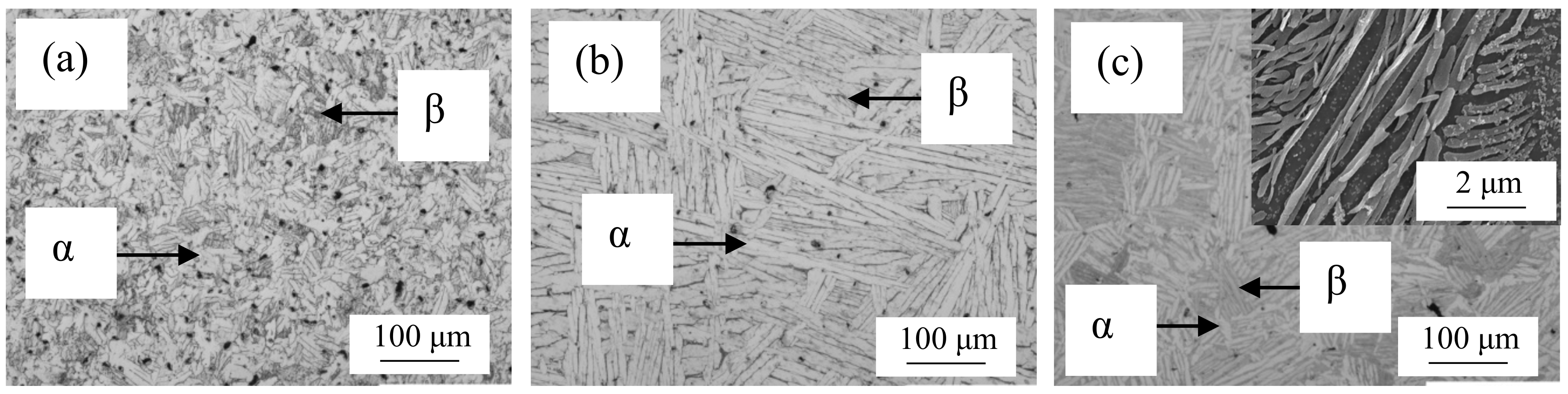
2. Materials and Methods
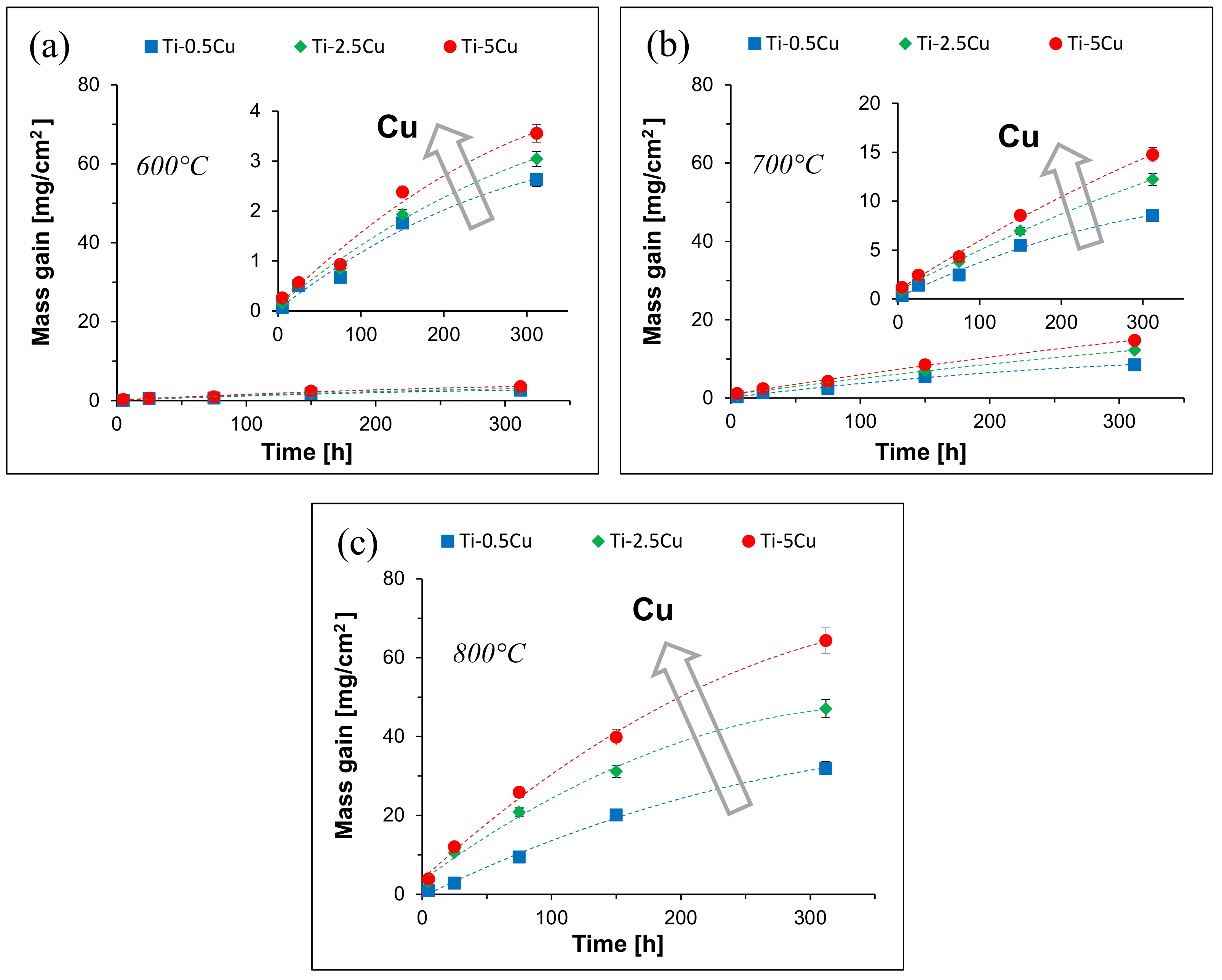
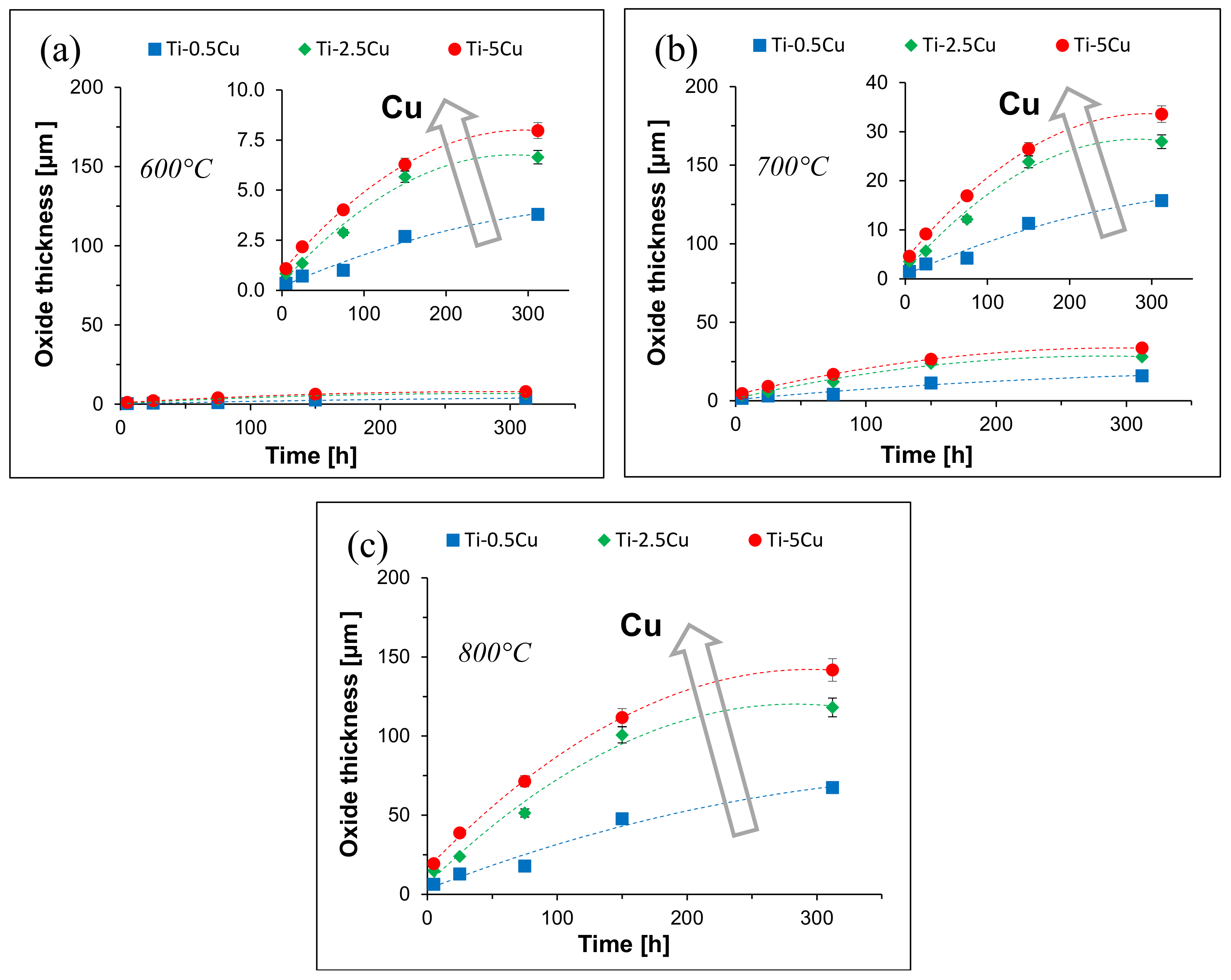
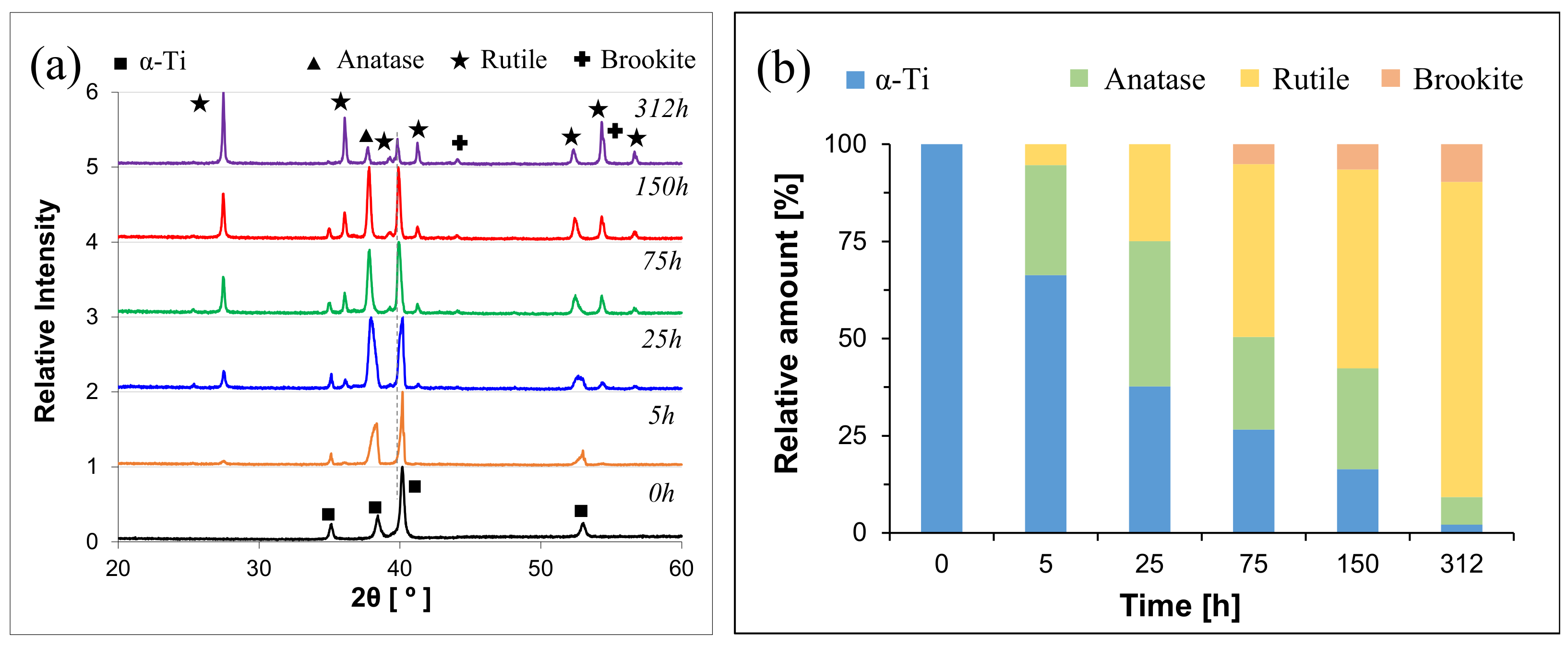
3. Results
4. Discussion
4.1. Isothermal Oxidation Kinetics
4.2. Oxide Layer Growth Kinetics
4.3. Oxidation Mechanism
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.-C.; Pinkerton, A.J.; Li, L. Fibre Laser Welding of Dissimilar Alloys of Ti-6Al-4V and Inconel 718 for Aerospace Applications. Int. J. Adv. Manuf. Technol. 2011, 52, 977–987. [Google Scholar] [CrossRef]
- Boyer, R.; Welsch, G.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Novelty, OH, USA, 1998. [Google Scholar]
- Qu, S.J.; Tang, S.Q.; Feng, A.H.; Feng, C.; Shen, J.; Chen, D.L. Microstructural Evolution and High-temperature Oxidation Mechanisms of a Titanium Aluminide Based Alloy. Acta Mater. 2018, 148, 300–310. [Google Scholar] [CrossRef]
- Imam, M.A.; Froes, F.H.; Reddy, R.G. Cost Effective Developments for Fabrication of Titanium Components. Key Eng. Mater. 2013, 551, 3–10. [Google Scholar] [CrossRef]
- Amherd Hidalgo, A.; Frykholm, R.; Ebel, T.; Pyczak, F. Powder Metallurgy Strategies to Improve Properties and Processing of Titanium Alloys: A Review. Adv. Eng. Mater. 2017, 19, 1600743. [Google Scholar] [CrossRef]
- Kitashima, T.; Liu, L.J.; Murakami, H. Numerical Analysis of Oxygen Transport in Alpha Titanium during Isothermal Oxidation. J. Electrochem. Soc. 2013, 160, C441. [Google Scholar] [CrossRef]
- Kitashima, T.; Kawamura, T. Prediction of Oxidation Behavior of Near-α Titanium Alloys. Scr. Mater. 2016, 124, 56–58. [Google Scholar] [CrossRef]
- Jung, I.H.; Eriksson, G.; Wu, P.; Pelton, A. Thermodynamic Modeling of the Al2O3-Ti2O3-TiO2 System and Its Applications to the Fe-Al-Ti-O Inclusion Diagram. ISIJ Int. 2009, 49, 1290–1297. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef]
- Siblani, M.; Ollivier, M.; Favergeon, L.; Chartrand, P. Experimental and Thermodynamic Modeling of Al2O3 Corundum and TiO2 Rutile Structures Forming on Ti-6Al-4V Powder during Oxidation. Acta Mater. 2023, 256, 119037. [Google Scholar] [CrossRef]
- Raynova, S.; Yang, F.; Bolzoni, L. Mechanical Behaviour of Induction Sintered Blended Elemental Powder Metallurgy Ti Alloys. Mater. Sci. Eng. A 2021, 799, 140157. [Google Scholar] [CrossRef]
- Bolzoni, L.; Esteban, P.G.; Ruiz-Navas, E.M.; Gordo, E. Mechanical Behaviour of Pressed and Sintered Titanium Alloys Obtained from Prealloyed and Blended Elemental Powders. J. Mech. Behav. Biomed. Mater. 2012, 14, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Reverte, E.; Tsipas, S.A.; Gordo, E. Oxidation and Corrosion Behavior of New Low-Cost Ti-7Fe-3Al and Ti-7Fe-5Cr Alloys from Titanium Hydride Powders. Metals 2020, 10, 254. [Google Scholar] [CrossRef]
- Garbacz, H.; Lewandowska, M. Microstructural Changes during Oxidation of Titanium Alloys. Mater. Chem. Phys. 2003, 81, 542–547. [Google Scholar] [CrossRef]
- Tsipas, S.A.; Gordo, E.; Jiménez-Morales, A. Oxidation and Corrosion Protection by Halide Treatment of Powder Metallurgy Ti and Ti6Al4V Alloy. Corros. Sci. 2014, 88, 263–274. [Google Scholar] [CrossRef]
- Jiang, H.; Hirohasi, M.; Imanari, H.; Lu, Y. The Relationship between the Morphology of the Scale and the Oxidation Kinetics at Elevated Temperatures in Ti-Al Alloys. Scr. Mater. 2001, 45, 253–259. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Z.; Liu, Y.; Liu, W. Influence of Thermal Oxidation Duration on the Microstructure and Fretting Wear Behavior of Ti6Al4V Alloy. Mater. Chem. Phys. 2015, 159, 139–151. [Google Scholar] [CrossRef]
- Aniołek, K.; Łosiewicz, B.; Kubisztal, J.; Osak, P.; Stróż, A.; Barylski, A.; Kaptacz, S. Isothermal Oxidation of Ti-6Al-7Nb Alloy. Vacuum 2015, 114, 114–118. [Google Scholar] [CrossRef]
- Du, H.L.; Datta, P.K.; Lewis, D.B.; Burnell-Gray, J.S. Air Oxidation Behaviour of Ti-6Al-4V Alloy between 650 and 850. Corros. Sci. 1994, 36, 631–642. [Google Scholar] [CrossRef]
- Latief, F.H.; Sherif, E.-S.M.; Wismogroho, A.S.; Widayatno, W.B.; Abdo, H.S. The Cyclic Oxidation and Hardness Characteristics of Thermally Exposed Titanium Prepared by Inductive Sintering-Assisted Powder Metallurgy. Crystals 2020, 10, 104. [Google Scholar] [CrossRef]
- Kofstad, P. High-temperature Oxidation of Titanium. J. Less Common Met. 1967, 12, 449–464. [Google Scholar] [CrossRef]
- Frangini, S.; Mignone, A.; de Riccardis, F. Various Aspects of the Air Oxidation Behaviour of a Ti6Al4V Alloy at Temperatures in the Range 600–700 °C. J. Mater. Sci. 1994, 29, 714–720. [Google Scholar] [CrossRef]
- Mungole, M.N.; Singh, N.; Mathur, N. Oxidation Behaviour of Ti6Al4V Titanium Alloy in Oxygen. Mater. Sci. Technol. 2002, 18, 111–114. [Google Scholar] [CrossRef]
- Kumar, S.; Narayanan, T.S.; Raman, S.G.S.; Seshadri, S. Thermal Oxidation of Ti6Al4V Alloy: Microstructural and Electrochemical Characterization. Mater. Chem. Phys. 2010, 119, 337–346. [Google Scholar] [CrossRef]
- Guleryuz, H.; Cimenoglu, H. Oxidation of Ti-6Al-4V Alloy. J. Alloys Comp. 2009, 472, 241–246. [Google Scholar] [CrossRef]
- Rajabi, A.; Mashreghi, A.R.; Hasani, S. Non-isothermal Kinetic Analysis of High Temperature Oxidation of Ti-6Al-4V Alloy. J. Alloys Comp. 2020, 815, 151948. [Google Scholar] [CrossRef]
- Gaddam, R.; Sefer, B.; Pederson, R.; Antti, M.L. Oxidation and Alpha-case Formation in Ti-6Al-2Sn-4Zr-2Mo Alloy. Mater. Charact. 2015, 99, 166–174. [Google Scholar] [CrossRef]
- Bolzoni, L.; Esteban, P.G.; Ruiz-Navas, E.M.; Gordo, E. Influence of Powder Characteristics on Sintering Behaviour and Properties of PM Ti Alloys Produced from Prealloyed Powder and Master Alloy. Powder Metall. 2011, 54, 543–550. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M.; Kaysser, W.A. Influence of Microstructure on Oxidation Behaviour of Near-α Titanium Alloys. Mater. Sci. Technol. 1996, 12, 213–218. [Google Scholar] [CrossRef]
- Otsuka, H.F.; Takahashi, H.K.; Mori, K. Development of Ti-Cu Alloy Sheets for Automobile Exhaust Systems. In Nippon Steel & Sumitomo Metal Technical Report no. 106; Nippon Steel: Tokyo, Japan, 2014; pp. 53–59. [Google Scholar]
- Zhu, K.Y.; Zhao, Y.Q.; Qu, H.L.; Wu, Z.L.; Zhao, X.M. Microstructure and Properties of Burn-resistant Ti-Al-Cu Alloys. J. Mater. Sci. 2000, 35, 5609–5612. [Google Scholar] [CrossRef]
- Kikuchi, M.; Takada, Y.; Kiyosue, S.; Yoda, M.; Woldu, M.; Cai, Z.; Okuno, O.; Okabe, T. Mechanical Properties and Microstructures of Cast Ti-Cu Alloys. Dent. Mater. 2003, 19, 174–181. [Google Scholar] [CrossRef]
- Zhang, E.; Ren, J.; Li, S.; Yang, L.; Qin, G. Optimization of Mechanical Properties, Biocorrosion Properties and Antibacterial Properties of As-cast Ti-Cu Alloys. Biomed. Mater. 2016, 11, 065001. [Google Scholar] [CrossRef]
- Yi, C.; Ke, Z.; Zhang, L.; Tan, J.; Jiang, Y.; He, Z. Antibacterial Ti-Cu Alloy with Enhanced Mechanical Properties as Implant Applications. Mater. Res. Exp. 2020, 7, 105404. [Google Scholar] [CrossRef]
- Zhang, E.; Li, S.; Ren, J.; Zhang, L.; Han, Y. Effect of Extrusion Processing on the Microstructure, Mechanical Properties, Biocorrosion Properties and Antibacterial Properties of Ti-Cu Sintered Alloys. Mater. Sci. Eng. C 2016, 69, 760–768. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Liu, C.; Wang, H.; Ren, B.; Yang, K.; Zhang, E. Effect of Cu Content on the Antibacterial Activity of Titanium-Copper Sintered Alloys. Mater. Sci. Eng. C 2014, 35, 392–400. [Google Scholar] [CrossRef]
- Alshammari, Y.; Yang, F.; Bolzoni, L. Low-cost Powder Metallurgy Ti-Cu Alloys as a Potential Antibacterial Material. J. Mech. Behav. Biomed. Mater. 2019, 95, 232–239. [Google Scholar] [CrossRef]
- Alshammari, Y.; Jia, M.; Yang, F.; Bolzoni, L. The Effect of α+ β Forging on the Mechanical Properties and Microstructure of Binary Titanium Alloys Produced via a Cost-effective Powder Metallurgy Route. Mater. Sci. Eng. A 2020, 769, 138496. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, X.; Chen, M.; Hou, B. Effect of the Existing Form of Cu Element on the Mechanical Properties, Bio-corrosion and Antibacterial Properties of Ti-Cu Alloys for Biomedical Application. Mater. Sci. Eng. C 2016, 69, 1210–1221. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Sun, Z.; Wang, H.; Ren, L.; Yang, K. Optimization of Mechanical Property, Antibacterial Property and Corrosion Resistance of Ti-Cu Alloy for Dental Implant. J. Mater. Sci. Technol. 2019, 35, 2336–2344. [Google Scholar] [CrossRef]
- Chen, H.; Mi, G.B.; Li, P.J.; Cao, C.X. Influence Mechanism of Cu on High Temperature Oxidation Behavior of Titanium Alloys. Mater. Sci. For. 2019, 944, 110–119. [Google Scholar] [CrossRef]
- Murray, J.L. Phase Diagrams of Binary Titanium Alloys, 1st ed.; ASM International: Novelty, OH, USA, 1987. [Google Scholar]
- Holden, F.C.; Watts, A.A.; Ogden, H.R.; Jaffee, R.I. Heat Treatment and Mechanical Properties of Ti-Cu Alloys. JOM 1955, 7, 117–125. [Google Scholar] [CrossRef]
- Williams, J.C.; Taggart, R.; Polonis, D.H. An Electron Microscopy Study of Modes of Intermetallic Precipitation in Ti-Cu Alloys. Metall. Trans. 1971, 2, 1139–1148. [Google Scholar] [CrossRef]
- Vaché, N.; Cadoret, Y.; Dod, B.; Monceau, D. Modeling the Oxidation Kinetics of Titanium Alloys: Review, Method and Application to Ti-64 and Ti-6242s Alloys. Corros. Sci. 2021, 178, 109041. [Google Scholar] [CrossRef]
- Kofstad, P.; Anderson, P.B.; Krudtaa, O.J. Oxidation of Titanium in the Temperature Range 800–1200 °C. J. Less Common Met. 1961, 3, 89–97. [Google Scholar] [CrossRef]
- Kofstad, P.; Hauffe, K. Oxydation von Titan. Mater. Corros. 1956, 7, 642–649. [Google Scholar] [CrossRef]
- Nakajima, H.; Koiwa, M. Diffusion in Titanium. ISIJ Int. 1991, 31, 757–766. [Google Scholar] [CrossRef]
- Gardner, H.M.; Gopon, P.; Magazzeni, C.M.; Radecka, A.; Fox, K.; Rugg, D.; Wade, J.; Armstrong, D.E.J.; Moody, M.P.; Bagot, P.A.J. Quantifying the Effect of Oxygen on Micro-mechanical Properties of a Near-alpha Titanium Alloy. J. Mater. Res. 2021, 36, 2529–2544. [Google Scholar] [CrossRef]
- Mi, G.; Yao, K.; Bai, P.; Cheng, C.; Min, X. High Temperature Oxidation and Wear Behaviors of Ti-V-Cr Fireproof Titanium Alloy. Metals 2017, 7, 226. [Google Scholar] [CrossRef]
- Pang, Y.; Xu, G.; Feng, Q.; Lv, J.; Qin, Y.; Zhang, Y.; Zheng, Z.; Wu, Y. Crystalline Orientation Preference for TiO2 Nanotube Arrays with Efficient Photoelectrochemical Properties. Phys. Lett. A 2018, 382, 2759–2762. [Google Scholar] [CrossRef]
- Stefanov, B.I.; Niklasson, G.A.; Granqvist, C.G.; Österlund, L. Quantitative Relation between Photocatalytic Activity and Degree of <001> Orientation for Anatase TiO2 Thin Films. J. Mater. Chem. A 2015, 3, 17369–17375. [Google Scholar]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Penn, R.L.; Banfield, J.F. Formation of Rutile Nuclei at Anatase {112} Twin Interfaces and the Phase Transformation Mechanism in Nanocrystalline Titania. Am. Miner. 1999, 84, 871–876. [Google Scholar] [CrossRef]
- Gouma, P.I.; Mills, M.J. Anatase-to-Rutile Transformation in Titania Powders. J. Am. Cer. Soc. 2001, 84, 619–622. [Google Scholar] [CrossRef]
- Shao, Y.; Tang, D.; Sun, J.; Lee, Y.; Xiong, W. Lattice Deformation and Phase Transformation from Nano-scale Anatase to Nano-scale Rutile TiO2 Prepared by a Sol-gel Technique. China Particuol. 2004, 2, 119–123. [Google Scholar] [CrossRef]
- Song, M.; Lu, Z.; Li, D. Phase transformations among TiO2 polymorphs. Nanoscale 2020, 12, 23183–23190. [Google Scholar] [CrossRef]
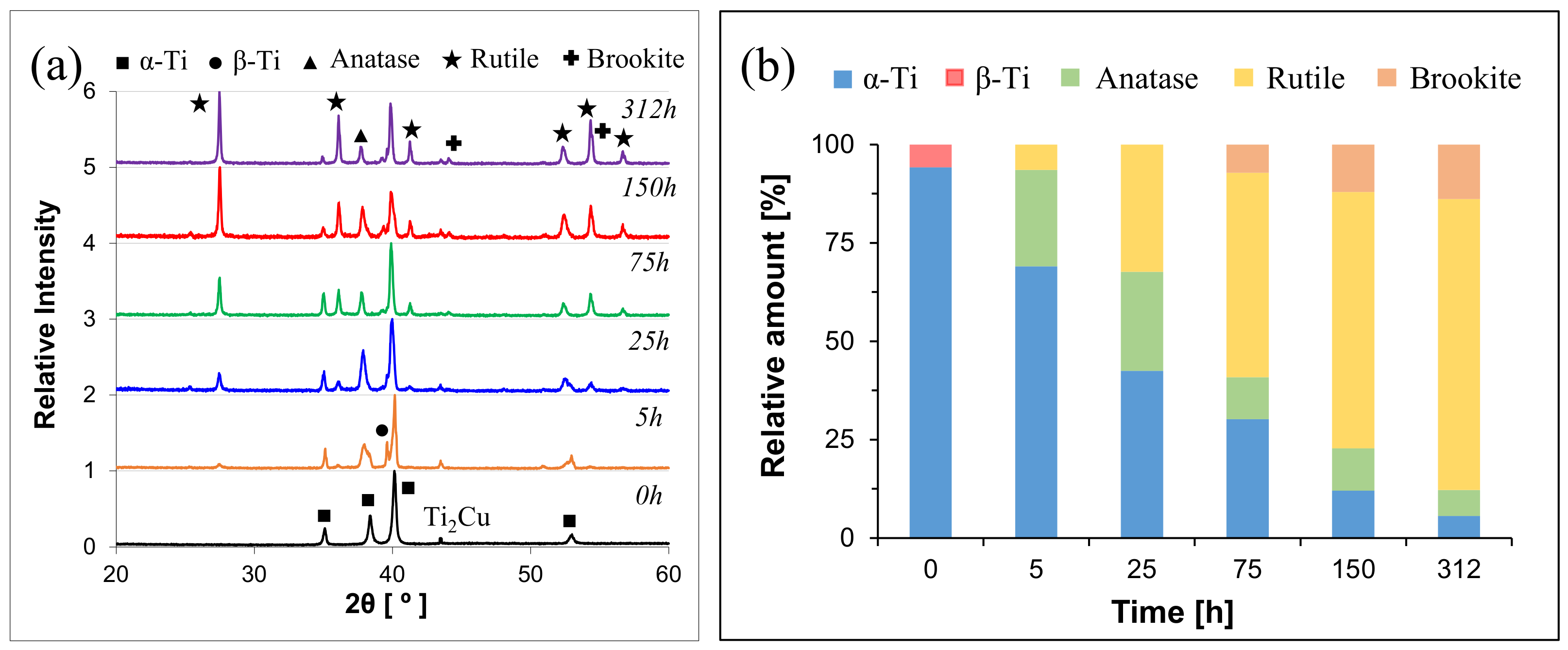
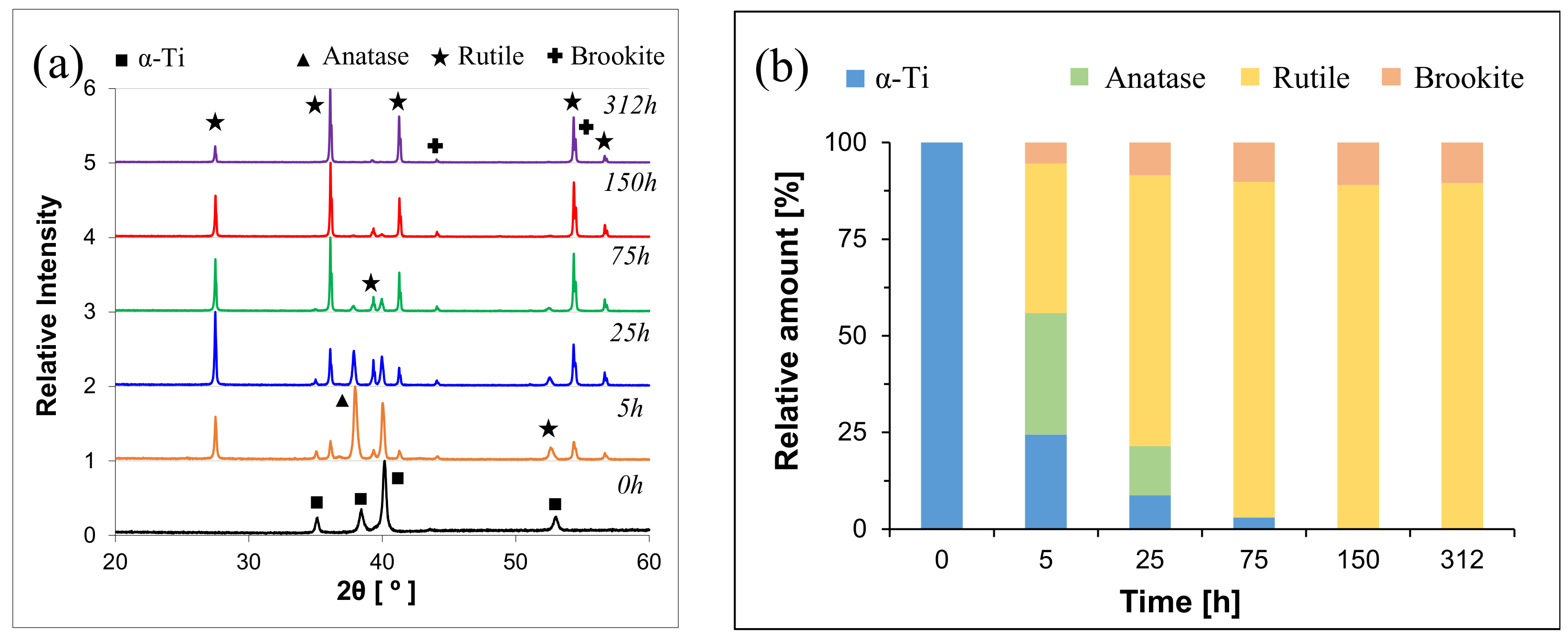

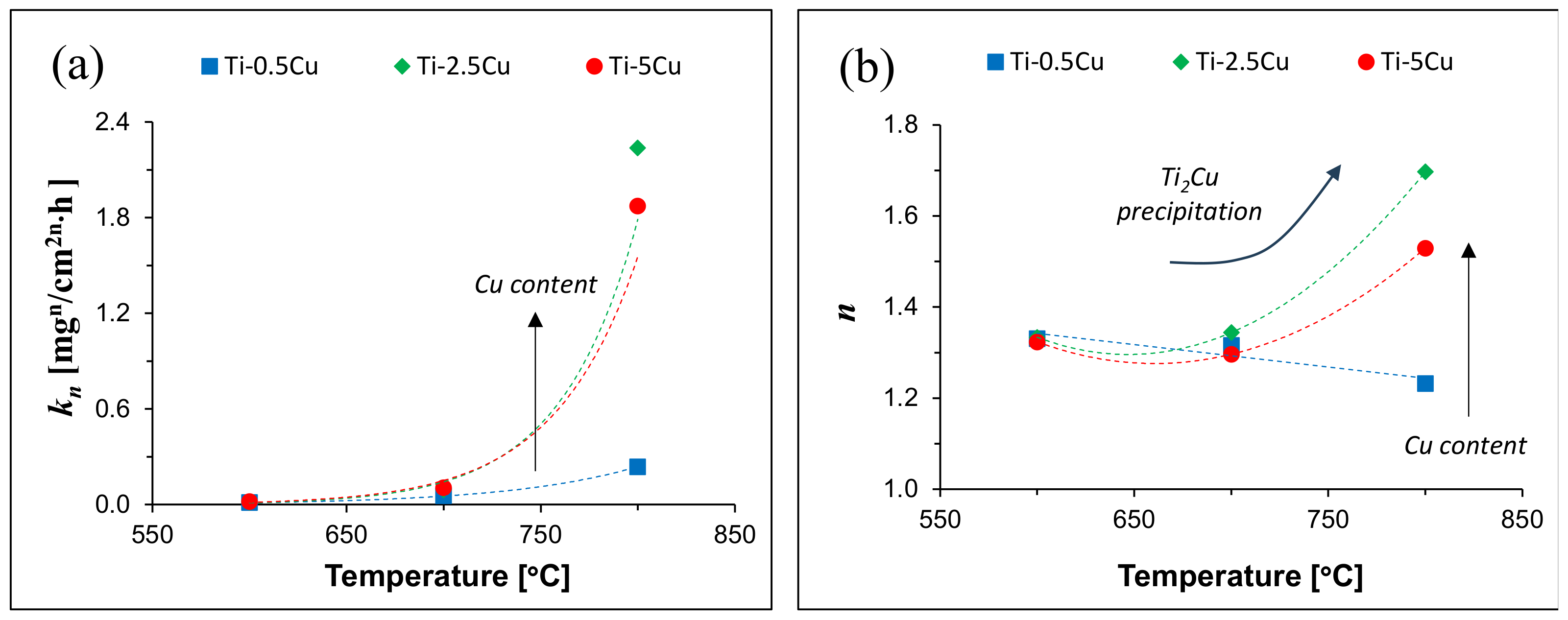
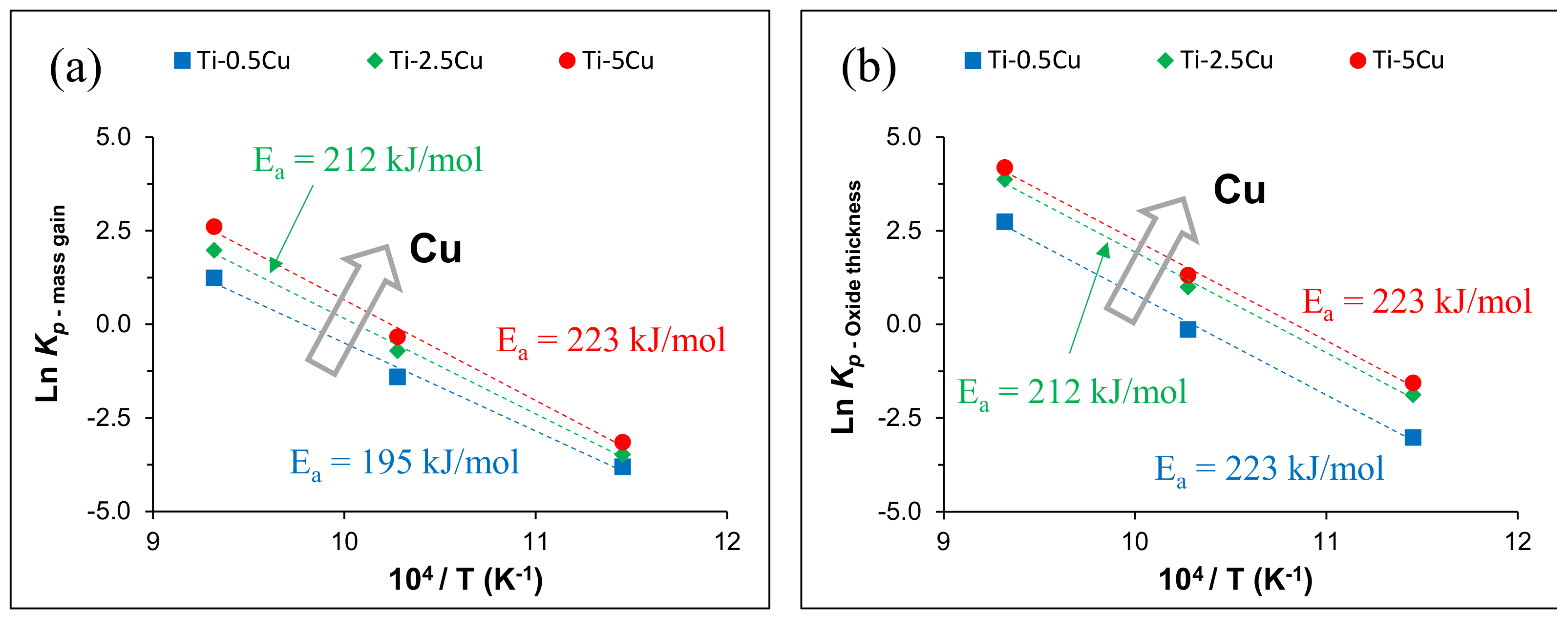
| Alloy | Temperature [°C] | kn [mgn/cm2n·h] | n | R2 |
|---|---|---|---|---|
| Ti-0.5Cu | 600 | 0.01 | 1.33 | 0.97 |
| 700 | 0.01 | 1.33 | 0.99 | |
| 800 | 0.02 | 1.32 | 0.99 | |
| Ti-2.5Cu | 600 | 0.06 | 1.32 | 0.99 |
| 700 | 0.09 | 1.34 | 0.99 | |
| 800 | 0.10 | 1.30 | 1.00 | |
| Ti-5Cu | 600 | 0.24 | 1.23 | 0.97 |
| 700 | 2.24 | 1.70 | 0.99 | |
| 800 | 1.87 | 1.53 | 1.00 |
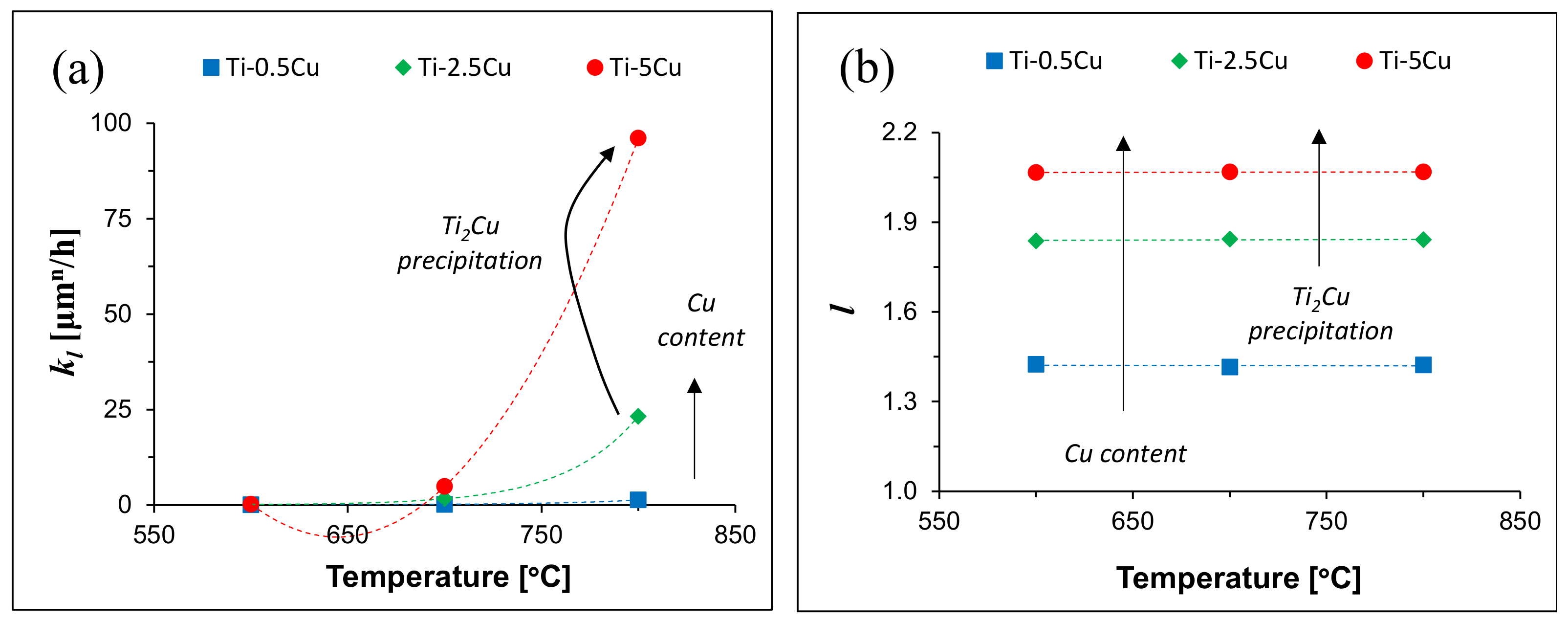
| Alloy | Temperature [°C] | kl [μmn/h] | l | R2 |
|---|---|---|---|---|
| Ti-0.5Cu | 600 | 0.02 | 1.42 | 0.96 |
| 700 | 0.17 | 1.42 | 0.96 | |
| 800 | 1.32 | 1.42 | 0.96 | |
| Ti-2.5Cu | 600 | 0.11 | 1.84 | 0.94 |
| 700 | 1.65 | 1.84 | 0.95 | |
| 800 | 23.21 | 1.84 | 0.95 | |
| Ti-5Cu | 600 | 0.25 | 2.07 | 0.99 |
| 700 | 4.88 | 2.07 | 0.99 | |
| 800 | 96.08 | 2.07 | 0.99 |
| Alloy | Alloy Type | T (°C) | 0 h | 5 h | 25 h | 75 h | 150 h | 312 h |
|---|---|---|---|---|---|---|---|---|
| Ti-0.5Cu | Near-α | 600 | α-Ti | α-Ti | α-Ti→Anatase | α-Ti→Anatase | Anatase→Rutile | Rutile |
| Ti-2.5Cu | α + β | α-Ti | α-Ti | α-Ti | α-Ti | Rutile | Rutile | |
| Ti-5Cu | α + β | α-Ti | α-Ti | α-Ti | α-Ti | α-Ti→Rutile | Rutile | |
| Ti-0.5Cu | Near-α | 700 | α-Ti | α-Ti→Anatase | Rutile | Rutile | Rutile | Rutile |
| Ti-2.5Cu | α + β | α-Ti | α-Ti→Anatase | Rutile | Rutile | Rutile | Rutile | |
| Ti-5Cu | α + β | α-Ti | α-Ti→Rutile | Rutile | Rutile | Rutile | Rutile | |
| Ti-0.5Cu | Near-α | 800 | α-Ti | Rutile | Rutile | Rutile | Rutile | Rutile |
| Ti-2.5Cu | α + β | α-Ti | Rutile | Rutile | Rutile | Rutile | Rutile | |
| Ti-5Cu | α + β | α-Ti | Rutile | Rutile | Rutile | Rutile | Rutile |
| Alloy | Alloy Type | T (°C) | 0 h | 5 h | 25 h | 75 h | 150 h | 312 h |
|---|---|---|---|---|---|---|---|---|
| Ti-0.5Cu | Near-α | 600 | α-Ti(101) | α-Ti(101) | a-TiO2(004) | r-TiO2(110) | r-TiO2(110) | r-TiO2(110) |
| Ti-2.5Cu | α + β | α-Ti(101) | α-Ti(101) | α-Ti(101) | r-TiO2(110) | r-TiO2(110) | r-TiO2(110) | |
| Ti-5Cu | α + β | α-Ti(101) | α-Ti(101) | α-Ti(101) | r-TiO2(110) | r-TiO2(110) | r-TiO2(110) | |
| Ti-0.5Cu | Near-α | 700 | α-Ti(101) | a-TiO2(004) | r-TiO2(110) | r-TiO2(101) | r-TiO2(101) | r-TiO2(101) |
| Ti-2.5Cu | α + β | α-Ti(101) | r-TiO2(110) | r-TiO2(110) | r-TiO2(110) | r-TiO2(101) | r-TiO2(101) | |
| Ti-5Cu | α + β | α-Ti(101) | r-TiO2(110) | r-TiO2(110) | r-TiO2(110) | r-TiO2(101) | r-TiO2(101) | |
| Ti-0.5Cu | Near-α | 800 | α-Ti(101) | r-TiO2(211) | r-TiO2(211) | r-TiO2(110) | r-TiO2(110) | r-TiO2(110) |
| Ti-2.5Cu | α + β | α-Ti(101) | r-TiO2(211) | r-TiO2(211) | r-TiO2(211) | r-TiO2(211) | r-TiO2(110) | |
| Ti-5Cu | α + β | α-Ti(101) | r-TiO2(211) | r-TiO2(211) | r-TiO2(211) | r-TiO2(211) | r-TiO2(110) |
| Alloy | Temperature (°C) | kp (mg2/cm4·h) | Ref. |
|---|---|---|---|
| Ti-0.5Cu (PM) | 600 | 0.02 | This work |
| 700 | 0.25 | ||
| 800 | 3.45 | ||
| Ti-2.5Cu (PM) | 600 | 0.03 | This work |
| 700 | 0.49 | ||
| 800 | 7.25 | ||
| Ti-5Cu (PM) | 600 | 0.04 | This work |
| 700 | 0.72 | ||
| 800 | 13.59 | ||
| Ti (PM) | 600 | 0.42 | [13] |
| Ti-6Al-4V (PM) | 600 | 0.004 | [15] |
| Ti-6Al-4V (W) | 600 | 0.004 | [22] |
| Ti-6Al-4V (W) | 650 | 0.015 | [22] |
| Ti-6Al-4V (W) | 700 | 0.12 | [22] |
| Ti-6Al-4V (W) | 800 | 86.37 | [50] |
| Ti-7Fe (PM) | 600 | 0.18 | [13] |
| Ti-7Fe-3Al (PM) | 600 | 0.11 | [13] |
| Ti-7Fe-5Cr (PM) | 600 | 0.41 | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqattan, F.; Yang, F.; Bolzoni, L. Kinetics of Oxidation of Binary Ti-Cu Alloys in the 600–800 °C Temperature Range. Metals 2025, 15, 222. https://doi.org/10.3390/met15020222
Alqattan F, Yang F, Bolzoni L. Kinetics of Oxidation of Binary Ti-Cu Alloys in the 600–800 °C Temperature Range. Metals. 2025; 15(2):222. https://doi.org/10.3390/met15020222
Chicago/Turabian StyleAlqattan, Fatemah, Fei Yang, and Leandro Bolzoni. 2025. "Kinetics of Oxidation of Binary Ti-Cu Alloys in the 600–800 °C Temperature Range" Metals 15, no. 2: 222. https://doi.org/10.3390/met15020222
APA StyleAlqattan, F., Yang, F., & Bolzoni, L. (2025). Kinetics of Oxidation of Binary Ti-Cu Alloys in the 600–800 °C Temperature Range. Metals, 15(2), 222. https://doi.org/10.3390/met15020222








