Extraction of Rhenium with Trialkylbenzylammonium Chloride
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
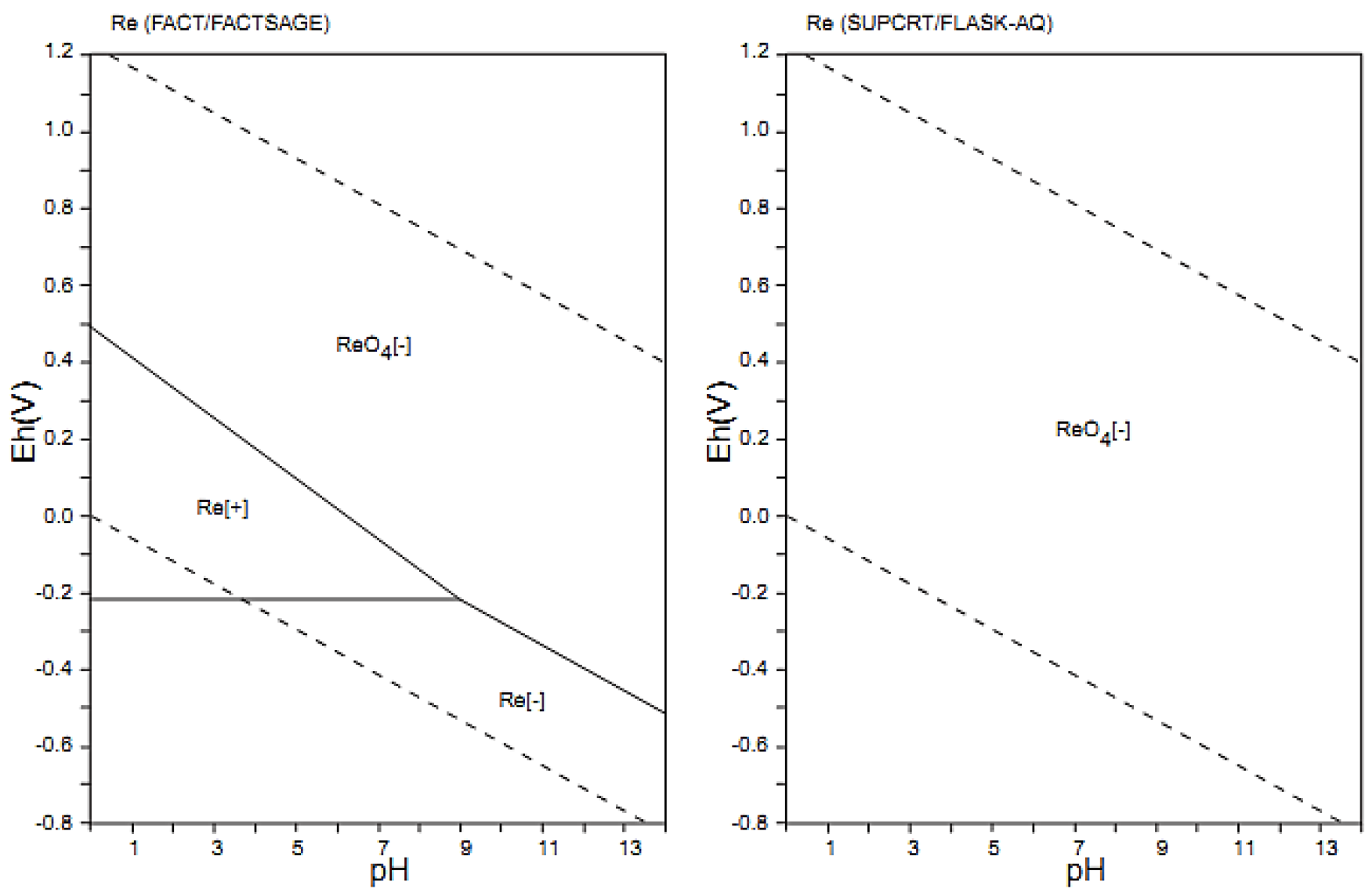
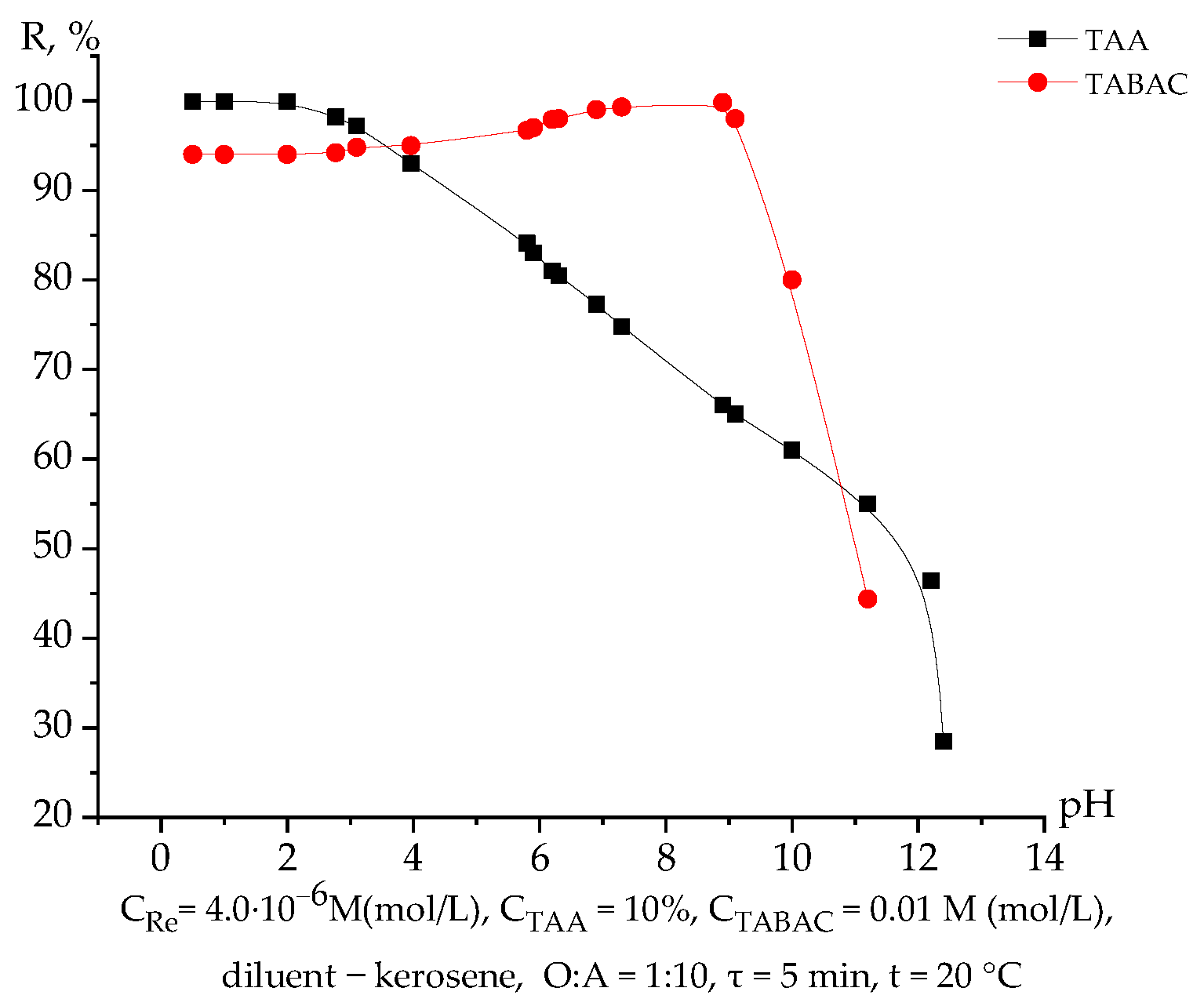
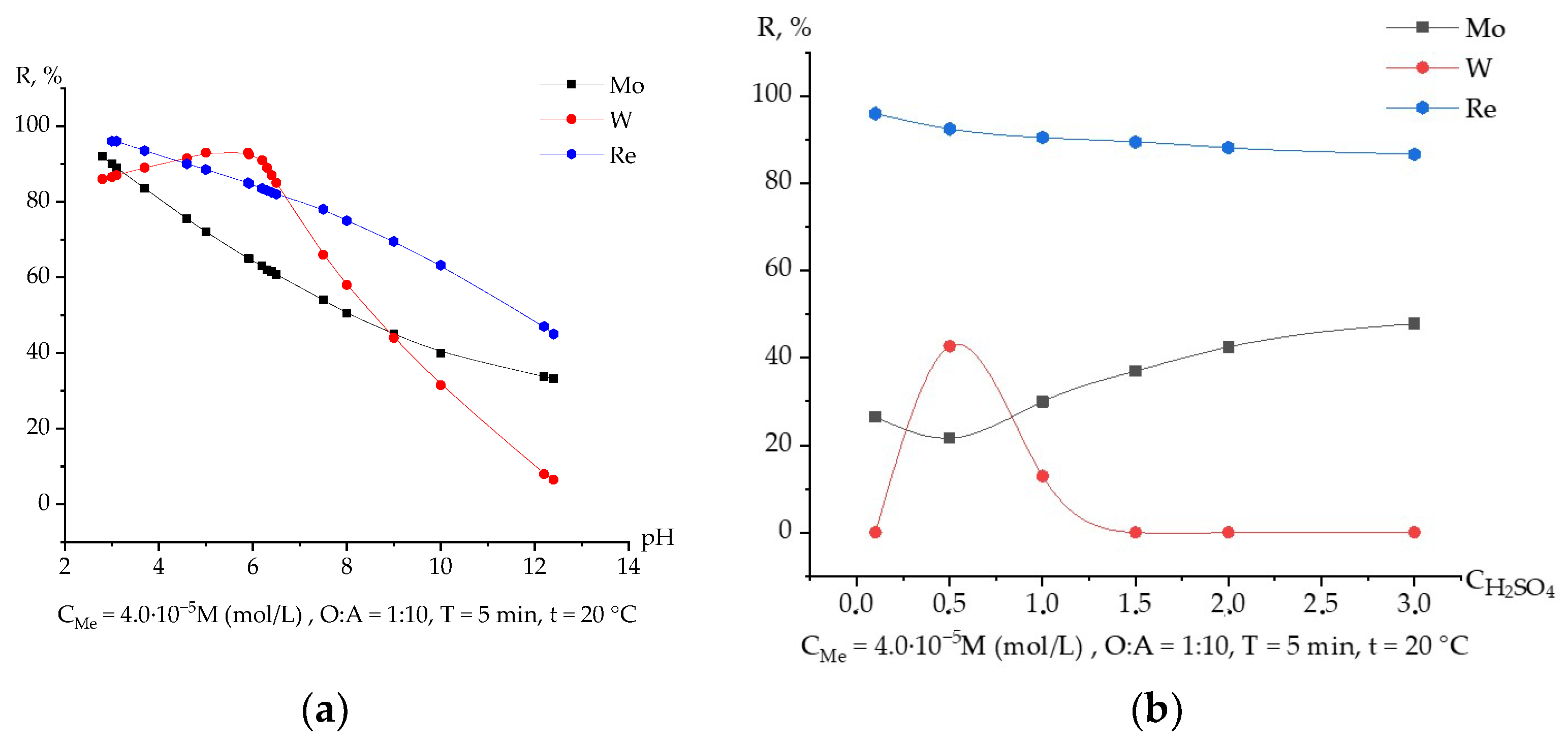
3.1. Extraction of Rhenium Depending on the pH
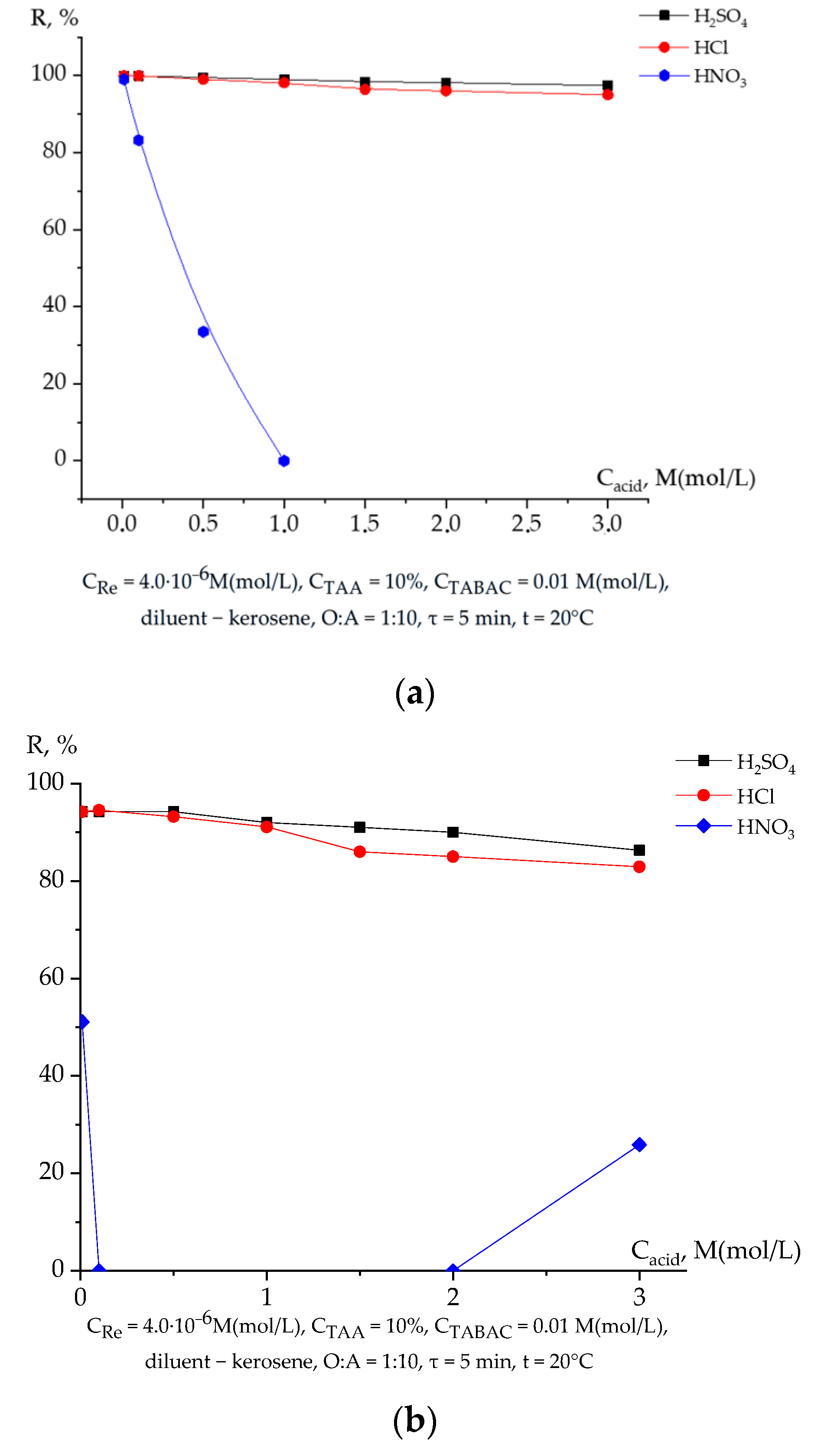
3.2. Extraction of Rhenium Depending on the Nature and Concentration of Mineral Acid
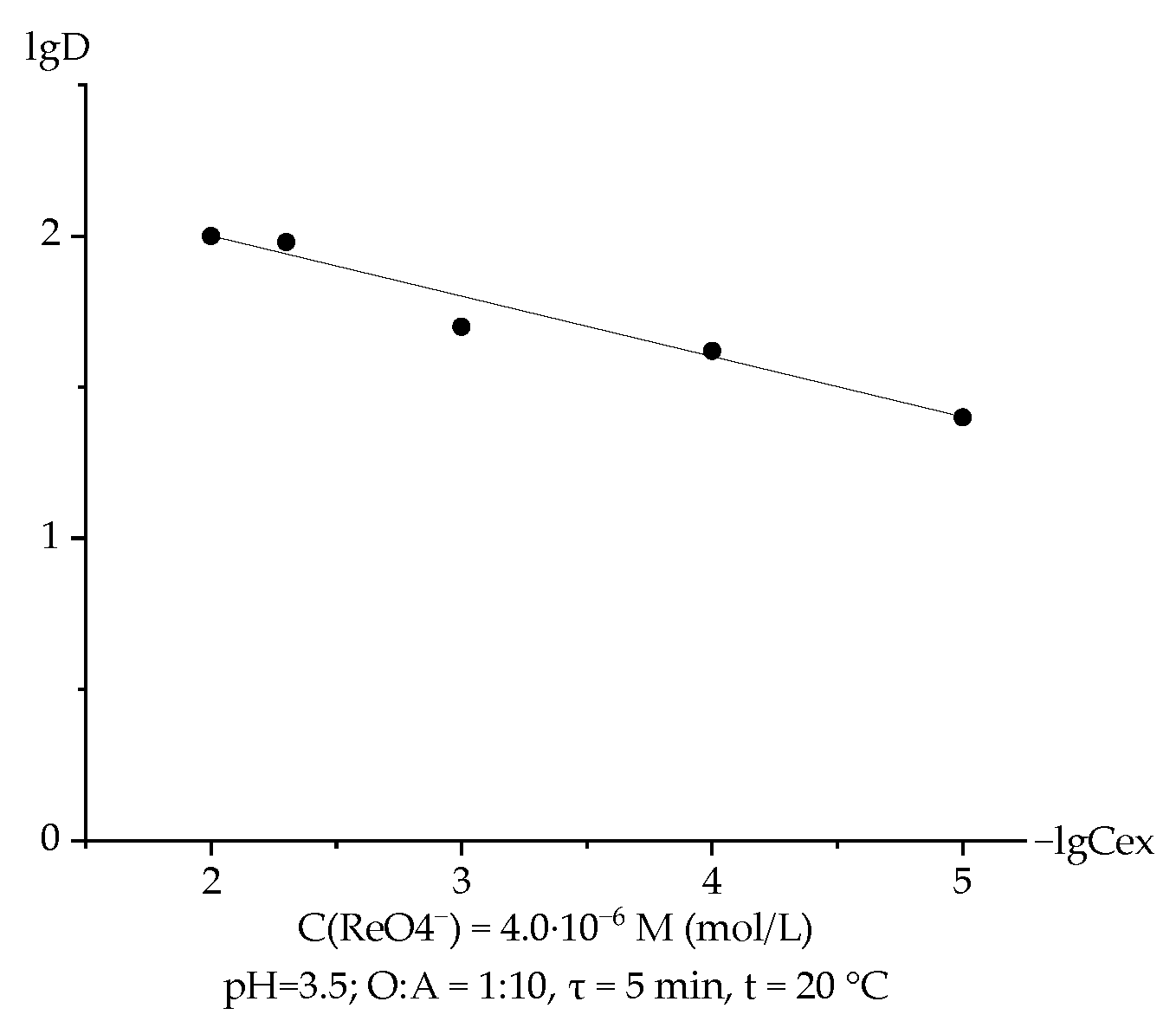
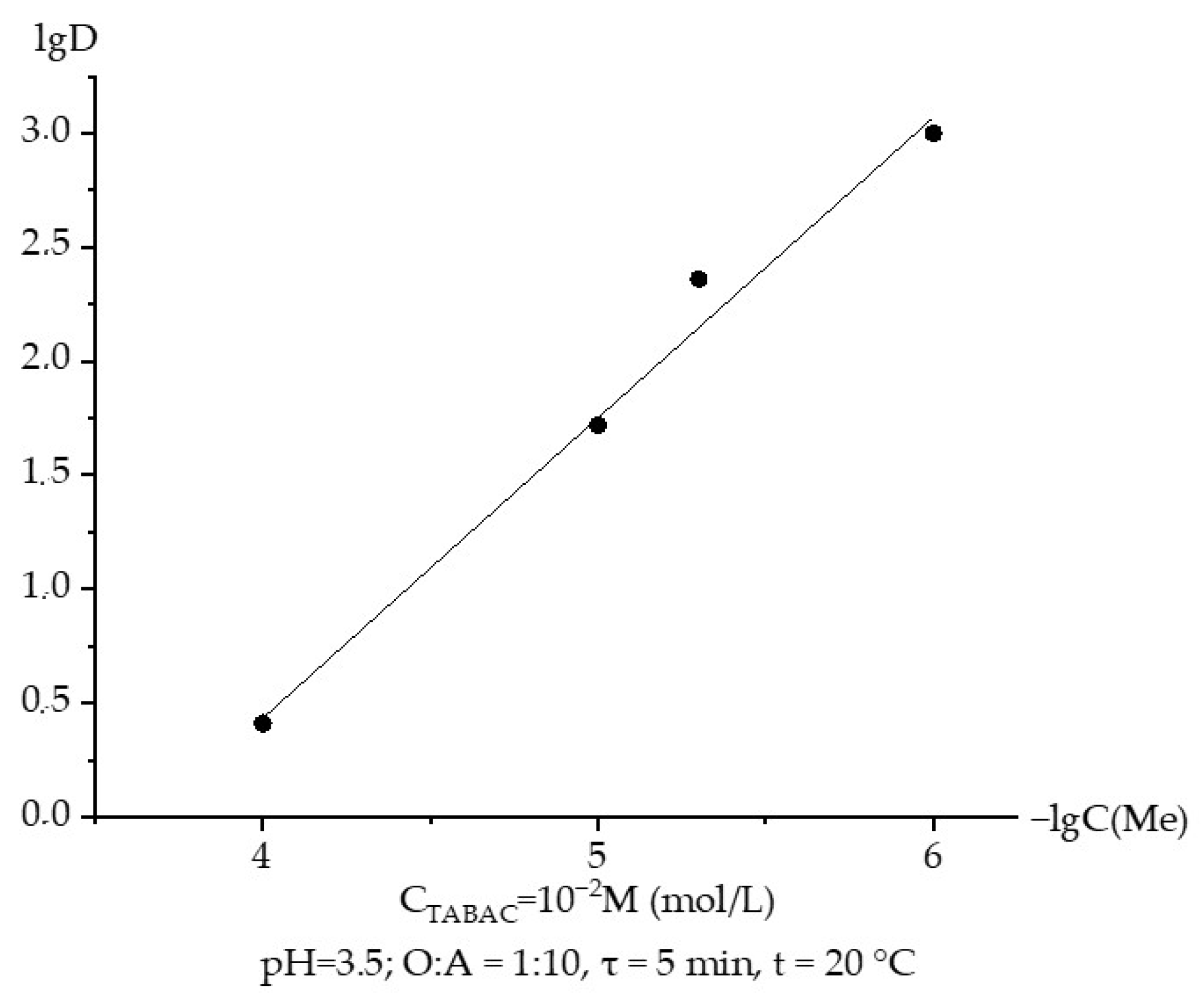
3.3. Extraction of Rhenium with TABAC-Kerosene Solution Depending on the Concentration of the Extractant
3.4. Extraction of Rhenium by TABAC-Kerosene Solution Depending on the Metal Concentrations
3.5. Study of the Possibility of Separating Molybdenum, Tungsten, and Rhenium Using the TABAC-Kerosene Solution
4. Conclusions
- Quantitative recovery (>99%) of rhenium is achieved within the pH range of 2–7 and in sulfuric and hydrochloric acid concentrations ranging from 0.1 to 3.0 M (mol/L).
- The extraction of rhenium from nitric acid solutions does not exceed 30%, which is attributed to the competing extraction of HNO3.
- The extraction efficiency of TABAC for rhenium is superior to that of the industrial extractant trialkylamine.
- Rhenium extraction by TABAC follows an anion exchange mechanism, resulting in the formation of complexes of the composition [R3R′NH]ReO4, which are extracted into the organic phase.
- Trialkylbenzylammonium chloride is a selective extractant for rhenium. Rhenium can be effectively separated from molybdenum and tungsten from solutions with a pH greater than 7, as well as from hydrochloric and sulfuric acid solutions.
- The separation factors for rhenium, molybdenum, and tungsten in TABAC extraction are higher than those in extraction using trialkylamine.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Palant, A.A.; Troshkina, I.D.; Chekmarev, A.M.; Kostylev, A.I. Tekhnologiya Reniya; Galeya-Print: Moscow, Russia; Russian Academy of Sciences: Moscow, Russia, 2015; 329p. [Google Scholar]
- USGS. Mineral Commodity Summaries 2024: Rhenium. 2024. Available online: https://pubs.usgs.gov/periodicals/mcs2024/mcs2024-rhenium.pdf (accessed on 15 December 2024).
- Melentyev, G.B.; Troshkina, I.D.; Zubkov, A.A. Resursno-ekologicheskie problemy sozdaniya proizvodstv reniya v Rossii i perspektivy ikh resheniya. Obs. Vopr. Ekol. Bezop. Okruz. Sredy 2011, 4, 1–15. [Google Scholar]
- Truong, H.T.; Nguyen, T.H.; Lee, M. Separation of molybdenum(VI), rhenium(VII), tungsten(VI), and vanadium(V) by solvent extraction. Hydrometallurgy 2017, 171, 298–305. [Google Scholar] [CrossRef]
- Naumov, A.V. Rhythms of rhenium. Russ. J. Non-Ferr. Met. 2007, 48, 418–423. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Kim, M.; Lee, J.; Ilyas, S. Liquid–liquid extraction of rhenium(VII) from an acidic chloride solution using Cyanex 923. Hydrometallurgy 2015, 157, 33–38. [Google Scholar] [CrossRef]
- Srivastava, R.R.; Lee, J.C.; Kim, M.S. Complexation chemistry in liquid–liquid extraction of rhenium. J. Chem. Technol. Biotechnol. 2015, 90, 1752–1764. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH Diagrams. Intercomparison of Thermodynamic Databases. Geological Survey of Japan Open File Report No. 419; National Institute of Advanced Industrial Science and Technology Research Center for Deep Geological Environments: Tsukuba, Japan, 2005. [Google Scholar]
- Nekovář, P.; Schrötterová, D. Extraction of V(V), Mo(VI) and W(VI) polynuclear species by primene JMT. Chem. Eng. J. 2000, 79, 229–233. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A review on the separation of molybdenum, tungsten, and vanadium from leach liquors of diverse resources by solvent extraction. Geosystem Eng. 2016, 19, 247–259. [Google Scholar] [CrossRef]
- Cheema, H.A.; Ilyas, S.; Masud, S.; Muhsan, M.A.; Mahmood, I.; Lee, J. Selective recovery of rhenium from molybdenite flue-dust leach liquor using solvent extraction with TBP. Sep. Purif. Technol. 2018, 191, 116–121. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Ranjbar, M.; Alizadeh, M. Effect of operational parameters and internal recycle on rhenium solvent extraction from leach liquors using a mixer-settler. Eng. Sci. Technol. Int. J. 2014, 17, 45–49. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Alizadeh, M.; Ranjbar, M. Optimization of Re solvent extraction from molybdenite roasting dust leaching solution and the performance evaluation of extraction in a multi-stage mixer–settler. Int. J. Miner. Process. 2014, 130, 88–94. [Google Scholar] [CrossRef]
- Travkin, V.F.; Antonov, A.V.; Kubasov, V.L.; Ishchenko, A.A.; Glubokov, Y.M. Extraction of rhenium(VII) and molybdenum(VI) with hexabutyltriamide of phosphoric acid from acid media. Russ. J. Appl. Chem. 2006, 79, 909–913. [Google Scholar] [CrossRef]
- Travkin, V.F.; Palant, A.A. Liquid extraction of rhenium(VII) and molybdenum(VI) with trialkylphosphine oxide from acidic solutions. Russ. Metall. 2012, 2012, 45–47. [Google Scholar] [CrossRef]
- Sato, T.; Sato, K. Liquid-liquid extraction of rhenium (VII) from hydrochloric acid solutions by neutral organophosphorus compounds and high molecular weight amines. Hydrometallurgy 1990, 25, 281–291. [Google Scholar] [CrossRef]
- Anderson, C.D.; Taylor, P.R.; Anderson, C.G. Extractive metallurgy of rhenium: A review. Min. Metall. Explor. 2013, 30, 59–73. [Google Scholar] [CrossRef]
- Abisheva, Z.S.; Zagorodnyaya, A.N.; Bekturganov, N.S. Review of technologies for rhenium recovery from mineral raw materials in Kazakhstan. Hydrometallurgy 2011, 109, 1–8. [Google Scholar] [CrossRef]
- Fang, D.; Shan, W.; Yan, Q.; Li, D.; Xia, L.; Zang, S. Extraction of rhenium from sulphuric acid solution with used amine N235. Fluid Phase Equilibria 2014, 383, 1–4. [Google Scholar] [CrossRef]
- Lou, Z.; Guo, C.; Feng, X.; Zhang, S.; Xing, Z.; Shan, W.; Xiong, Y. Selective extraction and separation of Re(VII) from Mo(VI) by TritonX-100/N235/iso-amyl alcohol/n-heptane/NaCl microemulsion system. Hydrometallurgy 2015, 157, 199–206. [Google Scholar] [CrossRef]
- Cao, Z.; Zhong, H.; Qiu, Z. Solvent extraction of rhenium from molybdenum in alkaline solution. Hydrometallurgy 2009, 97, 153–157. [Google Scholar] [CrossRef]
- Iatsenko Gerhardt, N.; Palant, A.A.; Petrova, V.A.; Tagirov, R.K. Solvent extraction of molybdenum (VI), tungsten (VI) and rhenium (VII) by diisododecylamine from leach liquors. Hydrometallurgy 2001, 60, 1–5. [Google Scholar] [CrossRef]
- Palant, A.A.; Petrova, V.A.; Yatsenko, N.A. Extraction of Rhenium(VII) from Solutions in Sulfuric Acid with Di(isododecyl)amine. Russ. J. Inorg. Chem. 1998, 43, 283–286. [Google Scholar]
- Fang, D.-W.; Song, Z.-R.; Zhang, S.-C.; Li, J.; Zang, S.-L. Solvent Extraction of Rhenium(VII) from Aqueous Solution Assisted by Hydrophobic Ionic Liquid. J. Chem. Eng. Data 2017, 62, 1094–1098. [Google Scholar] [CrossRef]
- Karagiozov, L.; Vasilev, C. Separation of molybdenum and rhenium by extraction with mixtures of trioctylamine and aliquat 336 followed by selective stripping. Hydrometallurgy 1979, 4, 51–55. [Google Scholar]
- Schmidt, V.S. Ekstraktsiya Aminami; Atomizdat: Moscow, Russia, 1980; 264p. [Google Scholar]
| Name | Formula | M, g/mol | Density, g/cm3 |
|---|---|---|---|
| Trialkylamine | R3N(R–C7–C9) | 354 | 0.81 |
| Trialkylbenzyl ammonium chloride | R3R′NCl(R–C7–C9;R′–C6H5CH2) | 552 | 0.91 |
| Kerosene | RH (R–C9–C16) | 151 | 0.80 |
| pH | C(H2SO4), M (mol/L) | D(Re)·D(Mo) | D(Re)·D(W) | ||
|---|---|---|---|---|---|
| 4.0 | 2.7 | 21,780 | 1.3 | 44,286 | |
| 6.0 | 20.9 | 23,853 | 1.6 | 322,750 | |
| 8.9 | 11.7 | 91 | 53.2 | 20 | |
| 11.2 | 9.2 | 11.1 | 19.1 | 1956 | |
| 0.10 | 66.8 | 865 | |||
| 0.50 | 58.4 | 445 | |||
| 1.0 | 17.3 | 401 | 55.8 | 124 | |
| 2.0 | 10.9 | 743 | 21.6 | 1203 | |
| 3.0 | 6.8 | 579 |
| Parameter | Trialkylamine (TAA) | Trialkylbenzylammonium Chloride (TABAC) |
|---|---|---|
| Extraction mechanism | Anion exchange, two-step process | Anion exchange, single-step process |
| Optimal pH range | 2–6 | 2–7 |
| Extraction efficiency | ~90–96% | >99% |
| Acid types for extraction | H2SO4, HCl, HNO3 | H2SO4, HCl, limited efficiency in HNO3 |
| Separation from Molybdenum and Tungsten | Limited selectivity | High selectivity |
| Compatibility with solvent | Kerosene | Kerosene |
| Efficiency in nitric acid | <30% | <30% |
| Formation of complexes | [R3NH]ReO4, [R3NH]HSO4 | [R4N]ReO4 |
| Industrial Use | Commonly used in acidic solutions | High potential due to high selectivity |
| Separation coefficient (Re/Mo) | Moderate | High |
| Separation coefficient (Re/W) | Moderate | High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalina, I.A.; Zlobina, E.V.; Bekishev, Z.Z.; Ismailova, A.G.; Tassibekov, K.S.; Iskakov, Z.A.; Toksanbayev, B.Z.; Kumarbekova, A.T.; Fomenko, A.S. Extraction of Rhenium with Trialkylbenzylammonium Chloride. Metals 2025, 15, 212. https://doi.org/10.3390/met15020212
Kalina IA, Zlobina EV, Bekishev ZZ, Ismailova AG, Tassibekov KS, Iskakov ZA, Toksanbayev BZ, Kumarbekova AT, Fomenko AS. Extraction of Rhenium with Trialkylbenzylammonium Chloride. Metals. 2025; 15(2):212. https://doi.org/10.3390/met15020212
Chicago/Turabian StyleKalina, I. A., E. V. Zlobina, Zh. Zh. Bekishev, A. G. Ismailova, Kh. S. Tassibekov, Z. A. Iskakov, B. Zh. Toksanbayev, A. T. Kumarbekova, and A. S. Fomenko. 2025. "Extraction of Rhenium with Trialkylbenzylammonium Chloride" Metals 15, no. 2: 212. https://doi.org/10.3390/met15020212
APA StyleKalina, I. A., Zlobina, E. V., Bekishev, Z. Z., Ismailova, A. G., Tassibekov, K. S., Iskakov, Z. A., Toksanbayev, B. Z., Kumarbekova, A. T., & Fomenko, A. S. (2025). Extraction of Rhenium with Trialkylbenzylammonium Chloride. Metals, 15(2), 212. https://doi.org/10.3390/met15020212






