Diffusion Behavior and Kinetics of the Iron–Nickel Interface During Annealing Treatment
Abstract
1. Introduction
2. Experimental Details
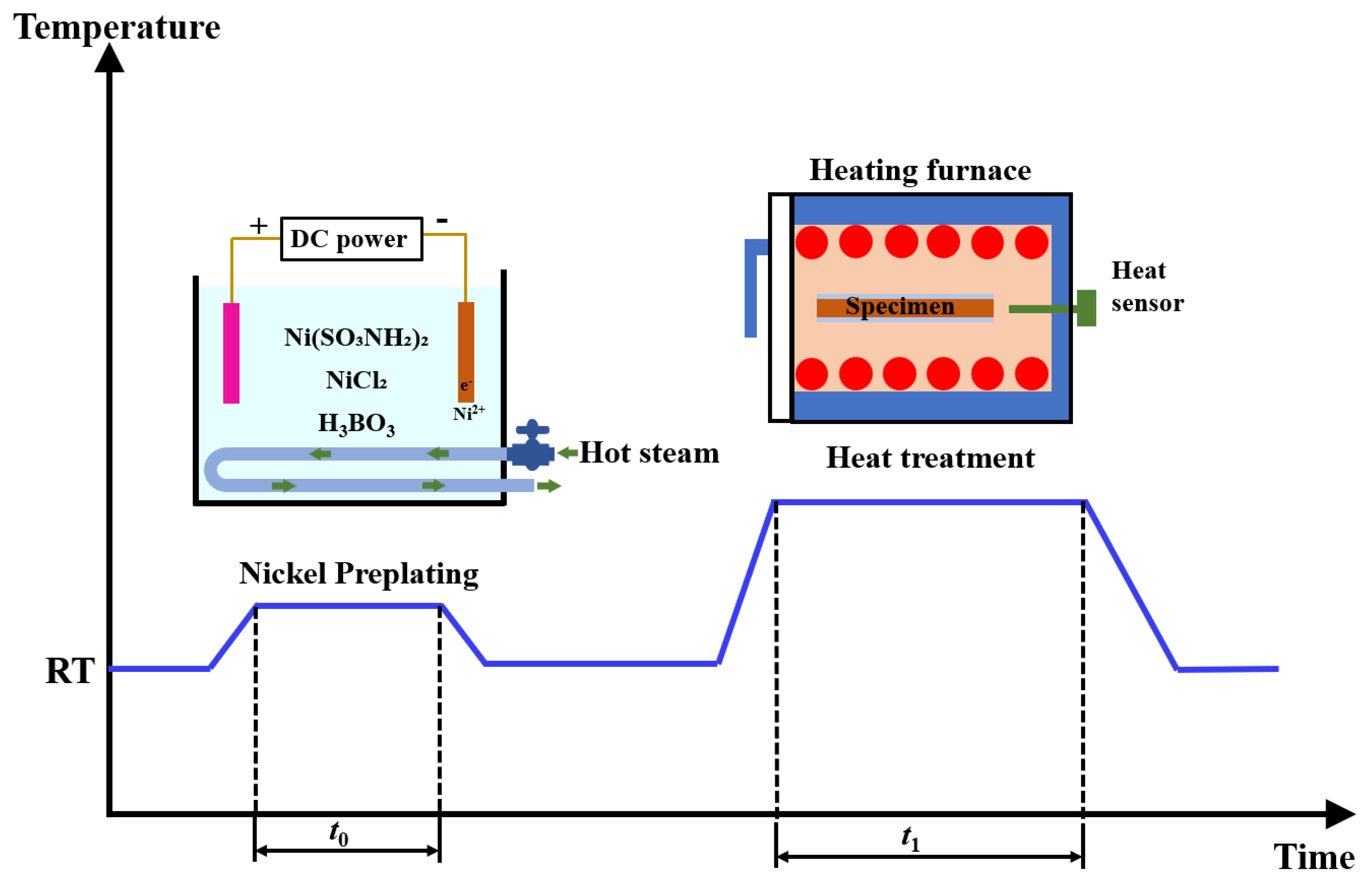
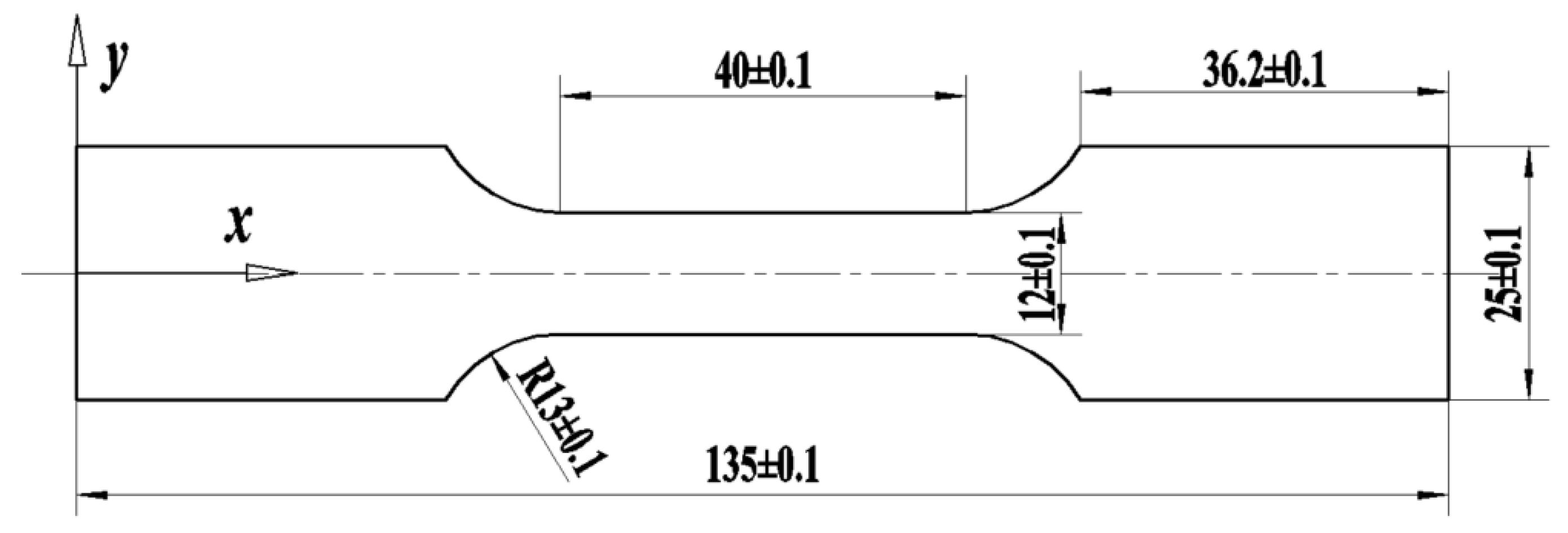
2.1. Materials and Treatment Process
2.2. Properties Test and Microstructure Observation
3. Results
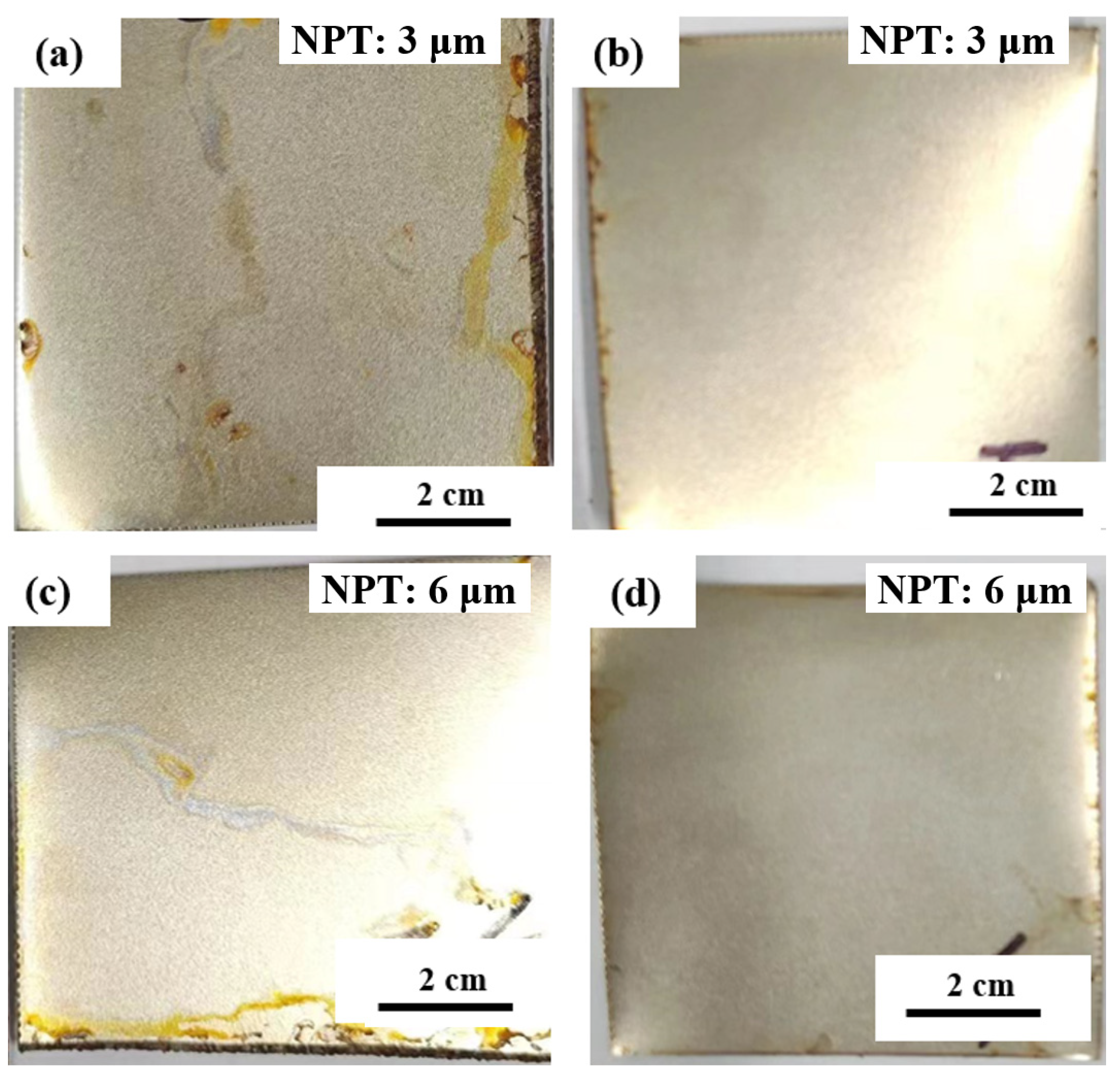
3.1. Mechanical Properties
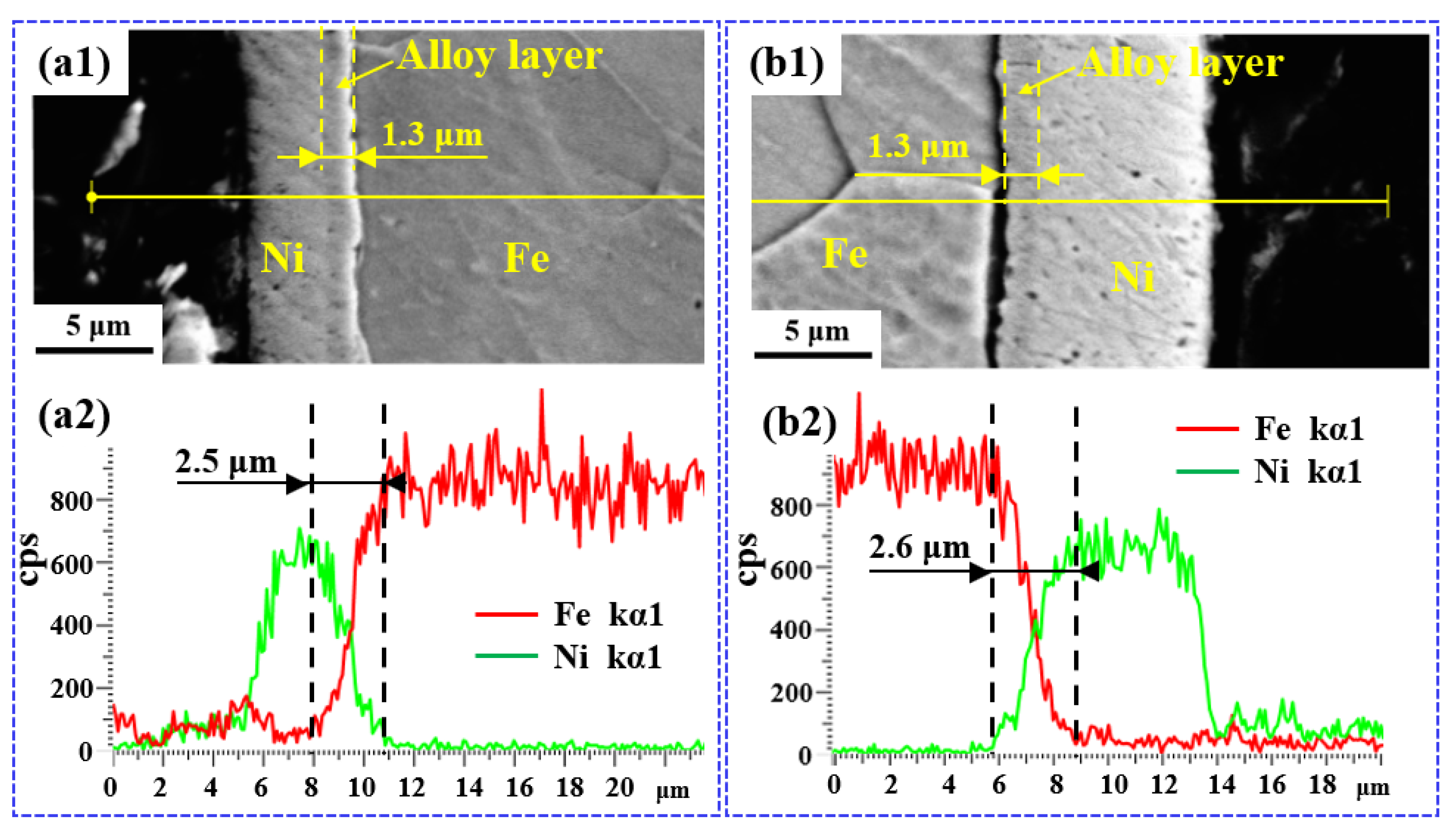
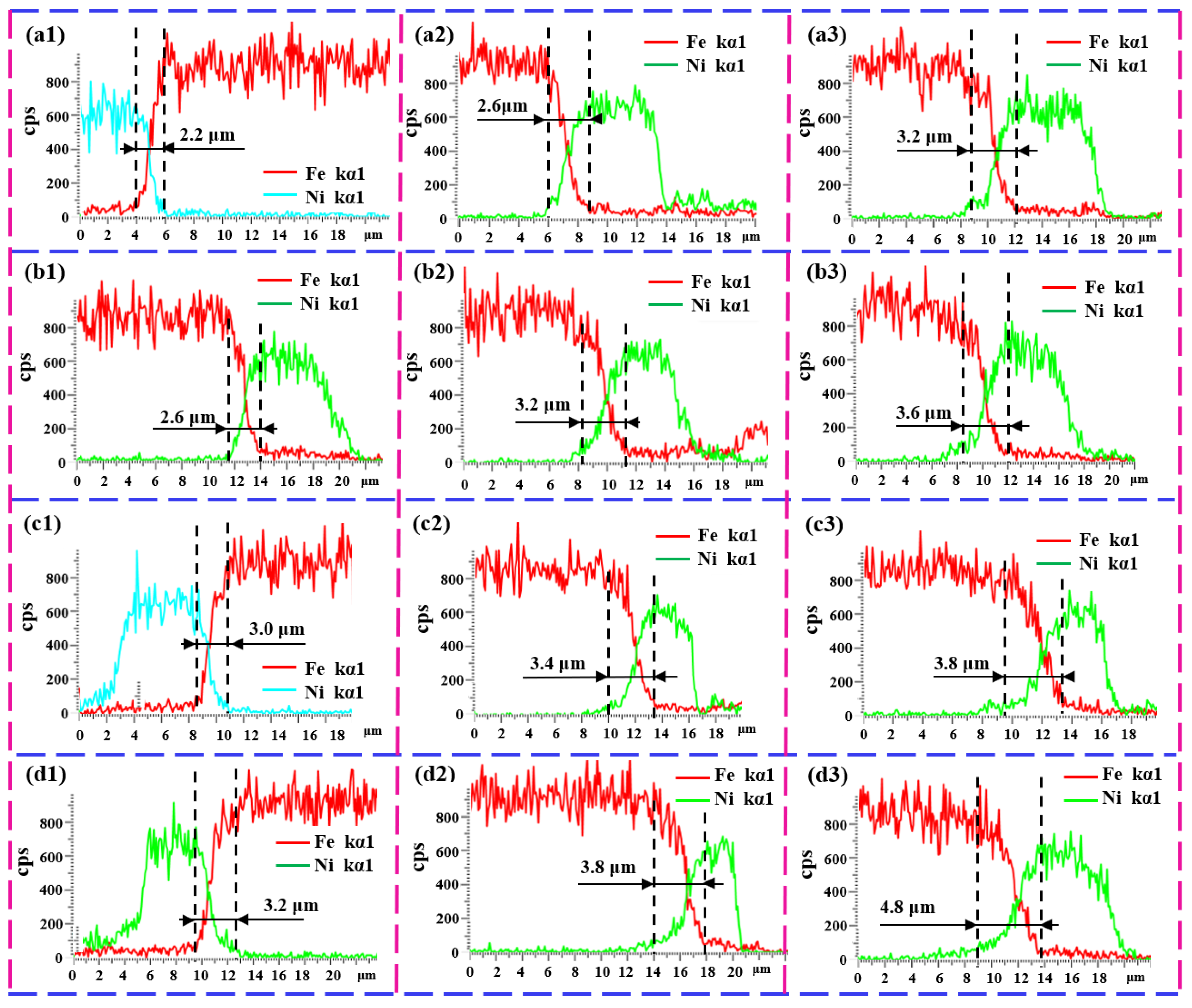
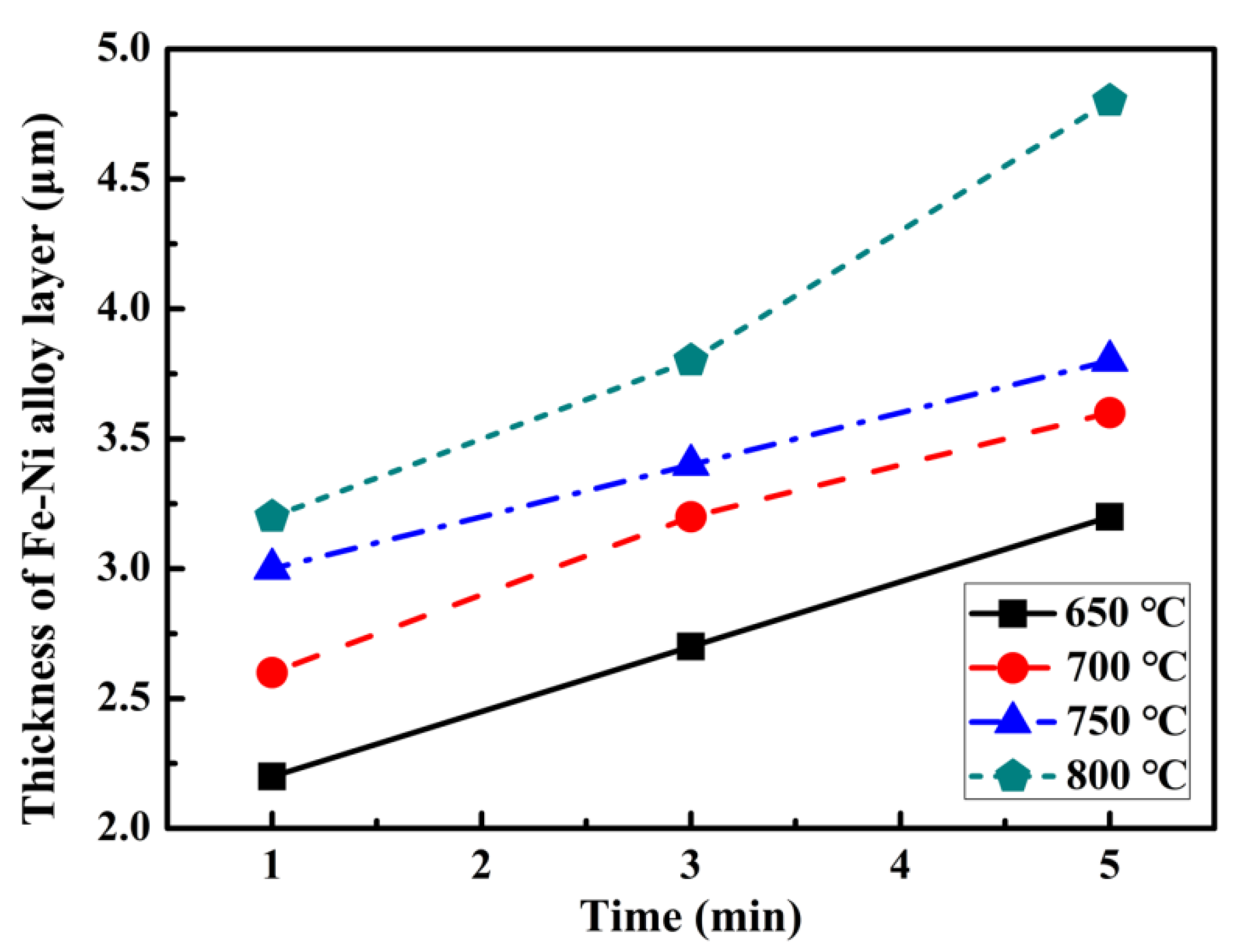
3.2. Microstructure Evolutions
4. Discussion
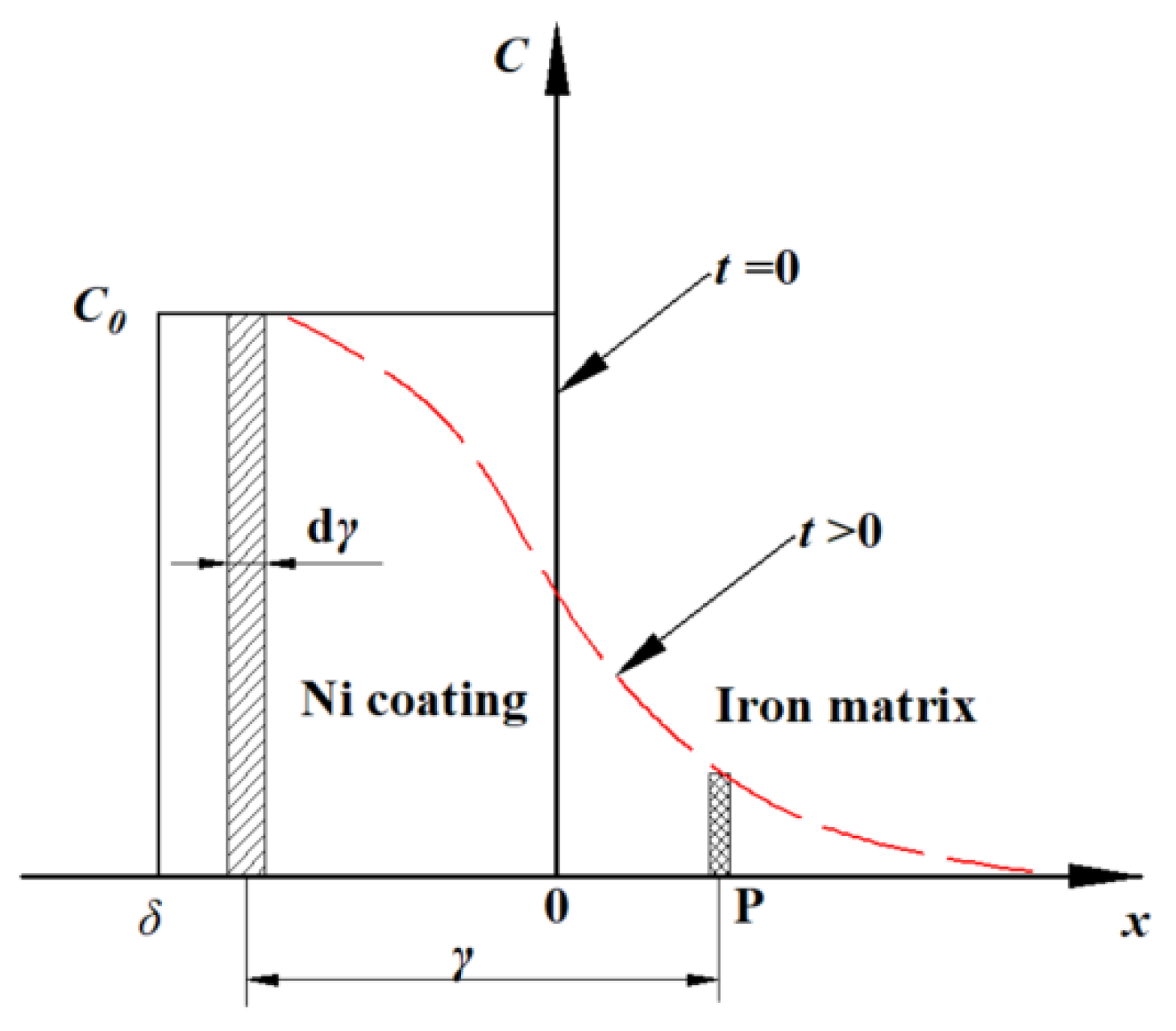
4.1. Physical Model for Annealing Diffusion at the Iron–Nickel Interface
4.2. Mathematical Model for Annealing Diffusion at the Iron–Nickel Interface
4.3. Mathematical Model Solving and Results Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kozłowska, A.; Grajcar, A.; Opara, J.; Kaczmarczyk, J.; Janik, A.; Radwański, K. Mechanical behaviour and micromechanical modelling of medium-Mn steel microstructure evolution. Int. J. Mech. Sci. 2022, 220, 107151. [Google Scholar] [CrossRef]
- Wang, Y. Study on Zn-Ni Electrodeposited coating on 40Mn Steel as Construction Material and its Corrosion Resistance in Simulated Concrete Pore Solution. Int. J. Electrochem. Sci. 2022, 17, 220752. [Google Scholar] [CrossRef]
- Ren, S.; Cui, M.; Chen, X.; Mei, S.; Qiang, Y. Comparative study on corrosion inhibition of N doped and N,S codoped carbon dots for carbon steel in strong acidic solution. J. Colloid Interface Sci. 2022, 628, 384–397. [Google Scholar] [CrossRef]
- Gao, Q.; Yu, X.; Jiang, H.; Zhang, S.; Yu, Q.; Wang, C.; Tang, Y.; Zhang, L.; Song, Y.; Li, L.; et al. Local corrosion behavior of pipeline steel under deposition layer in produced water of Alkali/ Surfactants/Polymers. Colloids Surf. Physicochem. Eng. Asp. 2023, 679, 132609. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Deng, Z.; Xu, S.; Xu, Y.; Zeng, L.; Li, D.; Yang, Y.; Zhong, Z. Effect of moisture on corrosion behavior of Q235 steel in bentonite clay. Int. J. Electrochem. Sci. 2023, 18, 100164. [Google Scholar] [CrossRef]
- Govindan, S.; Boopathi, M.; Venkatesh, A.P.; Maridurai, T.; Sathish, T.; Balakrishnan, E. Statistical analysis of electrodeposited nickel coating to S275JR grade mild steel. Mater. Today Proc. 2021, 46, 4159–4164. [Google Scholar] [CrossRef]
- Naderi, J.; Sarhan, A.A.D. Measure and evaluate the hardness of the electrodeposited Nickel-Phosphorous (Ni-P) thin film coating on carbon steel alloy for automotive applications. Measurement 2019, 139, 490–497. [Google Scholar] [CrossRef]
- Kumar, M.; Sidpara, A.; Racherla, V. Improving surface finish and wear resistance of electroless nickel plating using flexible abrasive tool. Wear 2024, 546–547, 205326. [Google Scholar] [CrossRef]
- Hao, J.-Z.; Xu, J.-J.; Cao, H.-L.; Miao, H.; Xu, S.-A. The effects of P and metal elements in electroless nickel-based alloys on the formation of Ti/Zr conversion coating. J. Alloys Compd. 2024, 982, 173761. [Google Scholar] [CrossRef]
- Awasthi, S.; De, S.; Pandey, S.K. Electrodeposited carbon nanostructured nickel composite coatings: A review. Heliyon 2024, 10, e26051. [Google Scholar] [CrossRef] [PubMed]
- Sudagar, J.; Lian, J.; Sha, W. Electroless nickel, alloy, composite and nano coatings—A critical review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Faraji, S.; Ani, F.N. The development supercapacitor from activated carbon by electroless plating—A review. Renew. Sustain. Energy Rev. 2015, 42, 823–834. [Google Scholar] [CrossRef]
- Zhou, L.Q.; Li, Y.P.; Zhou, Y.C. Numerical Analysis of Electrodeposited Nickel Coating in Multistage Drawing Processes. J. Eng. Mater. Technol. 2005, 127, 233–243. [Google Scholar] [CrossRef]
- Ren, Q.-Q.; Baik, S.-I.; An, D.; Isheim, D.; Zhu, M.; Krakauer, B.W.; Seidman, D.N. The effects of heat-treatment parameters on the mechanical properties and microstructures of a low-carbon dual-phase steel. Mater. Sci. Eng. A 2023, 888, 145801. [Google Scholar] [CrossRef]
- Santhosh, R.; Geetha, M.; Nageswara Rao, M. Recent Developments in Heat Treatment of Beta Titanium Alloys for Aerospace Applications. Trans. Indian Inst. Met. 2017, 70, 1681–1688. [Google Scholar] [CrossRef]
- Carvalho, A.L.M.; Renaudin, L.B.; Zara, A.J.; Martins, J.P. Microstructure analysis of 7050 aluminum alloy processed by multistage aging treatments. J. Alloys Compd. 2022, 907, 164400. [Google Scholar] [CrossRef]
- Ashrafizadeh, S.M.; Eivani, A.R.; Jafarian, H.R.; Zhou, J. Improvement of mechanical properties of AA6063 aluminum alloy after equal channel angular pressing by applying a two-stage solution treatment. Mater. Sci. Eng. A 2017, 687, 54–62. [Google Scholar] [CrossRef]
- Ma, W.; Wang, B.; Yang, L.; Tang, X.; Xiao, W. Influence of solution heat treatment on mechanical response and fracture behaviour of aluminium alloy sheets: An experimental study. Mater. Des. 2015, 88, 1119–1126. [Google Scholar] [CrossRef]
- Zhang, W.; Li, W.; Liu, H.; He, T.; Deng, S.; Tian, H.; Cao, W. Microstructure effects on improving rolling contact fatigue by a double-quenching heat treatment process for GCr15 steel. Tribol. Int. 2024, 194, 109519. [Google Scholar] [CrossRef]
- Jiang, P.; Xu, Z.; Li, Z.; Pan, H.; Zhang, Y. Microstructure, mechanical properties and wear resistances of 40CrNiMoA steel affected by cyclic quenching treatment. Mater. Today Commun. 2024, 40, 109799. [Google Scholar] [CrossRef]
- Ramana, M.V.; Rao, G.K.M.; Sagar, B.; Panthangi, R.K.; Kumar, B.V.R.R. Optimization of surface roughness and tool wear in sustainable dry turning of Iron based Nickel A286 alloy using Taguchi’s method. Clean. Eng. Technol. 2021, 2, 100034. [Google Scholar] [CrossRef]
- Singla, Y.K.; Maughan, M.R.; Arora, N.; Dwivedi, D.K. Enhancing the wear resistance of iron-based alloys: A comprehensive review of alloying element effects. J. Manuf. Process. 2024, 120, 135–160. [Google Scholar] [CrossRef]
- Prasad, M.J.N.V.; Chokshi, A.H. On the exothermic peak during annealing of electrodeposited nanocrystalline nickel. Scr. Mater. 2011, 64, 544–547. [Google Scholar] [CrossRef]
- Julie, S.; Dash, M.K.; Wasekar, N.P.; David, C.; Kamruddin, M. Effect of annealing and irradiation on the evolution of texture and grain boundary interface in electrodeposited nanocrystalline nickel of varying grain sizes. Surf. Coat. Technol. 2021, 426, 127770. [Google Scholar] [CrossRef]
- Singh, A.K.; Bhattacharya, B.; Biswas, S. High strength, ductility and sheet formability by normalizing and quenching of low carbon microalloyed dual-phase steel. Mater. Sci. Eng. A 2024, 890, 145848. [Google Scholar] [CrossRef]
- Murty, S.V.S.N.; Rao, B.N.; Kashyap, B.P. Improved ductile fracture criterion for cold forming of spheroidised steel. J. Mater. Process. Technol. 2004, 147, 94–101. [Google Scholar] [CrossRef]
- Vermolena, F.; Vuikb, K. A numerical method to compute the dissolution of second phases in ternary alloys. J. Comput. Appl. Math. 1998, 93, 123–143. [Google Scholar] [CrossRef]
- Wu, W.; Song, Y.; Zhou, P.; Lu, J.; Yu, Y.; Hua, L. Rapid dissolution kinetic of the second phases in high-strength aluminum alloy by electroshock treatment. J. Mater. Sci. 2024, 59, 17259–17277. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Zhao, X.; Li, Y. Diffusion mechanism and rules of nickel on surface of low carbon steel. Heat Treat. Met. 2023, 48, 121–128. [Google Scholar] [CrossRef]
- Sun, J.; Chen, D.; Ma, Y. Strain-Induced Diffusion of Nickel in Nanocrystalline Fe Produced by High Energy Shot Peening. Rare Met. Mater. Eng. 2017, 46, 596–600. [Google Scholar]
- Sun, J. Diffusion of Nickel in Nanocrytalline Iron. Mater. Rep. B 2014, 28, 159–162. [Google Scholar]




| Composition | C | Si | Mn | P | S | Al | Fe |
|---|---|---|---|---|---|---|---|
| wt.% | 0.082 | 0.011 | 0.215 | 0.0131 | 0.0079 | 0.076 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Zhong, F.; Zhou, P.; Lu, J.; Wang, F. Diffusion Behavior and Kinetics of the Iron–Nickel Interface During Annealing Treatment. Metals 2025, 15, 211. https://doi.org/10.3390/met15020211
Wu W, Zhong F, Zhou P, Lu J, Wang F. Diffusion Behavior and Kinetics of the Iron–Nickel Interface During Annealing Treatment. Metals. 2025; 15(2):211. https://doi.org/10.3390/met15020211
Chicago/Turabian StyleWu, Wenlin, Fei Zhong, Pu Zhou, Jue Lu, and Feng Wang. 2025. "Diffusion Behavior and Kinetics of the Iron–Nickel Interface During Annealing Treatment" Metals 15, no. 2: 211. https://doi.org/10.3390/met15020211
APA StyleWu, W., Zhong, F., Zhou, P., Lu, J., & Wang, F. (2025). Diffusion Behavior and Kinetics of the Iron–Nickel Interface During Annealing Treatment. Metals, 15(2), 211. https://doi.org/10.3390/met15020211






