High-Efficiency Extraction of Lithium and Aluminum from Coal Fly Ash Using Activation-Sintering Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Test Materials and Analysis
2.2. Extraction Instruments and Main Testing Methods
2.2.1. Test Instruments
2.2.2. Determination of Alumina Leaching Rate
- ω(Al2O3) is the titer of the standard EDTA solution for alumina (mg/mL);
- V is the volume of the standard acetic acid zinc solution consumed by the test solution (mL);
- m is the mass of the ash sample (g); and
- 0.638 is the factor for converting titanium dioxide to alumina.
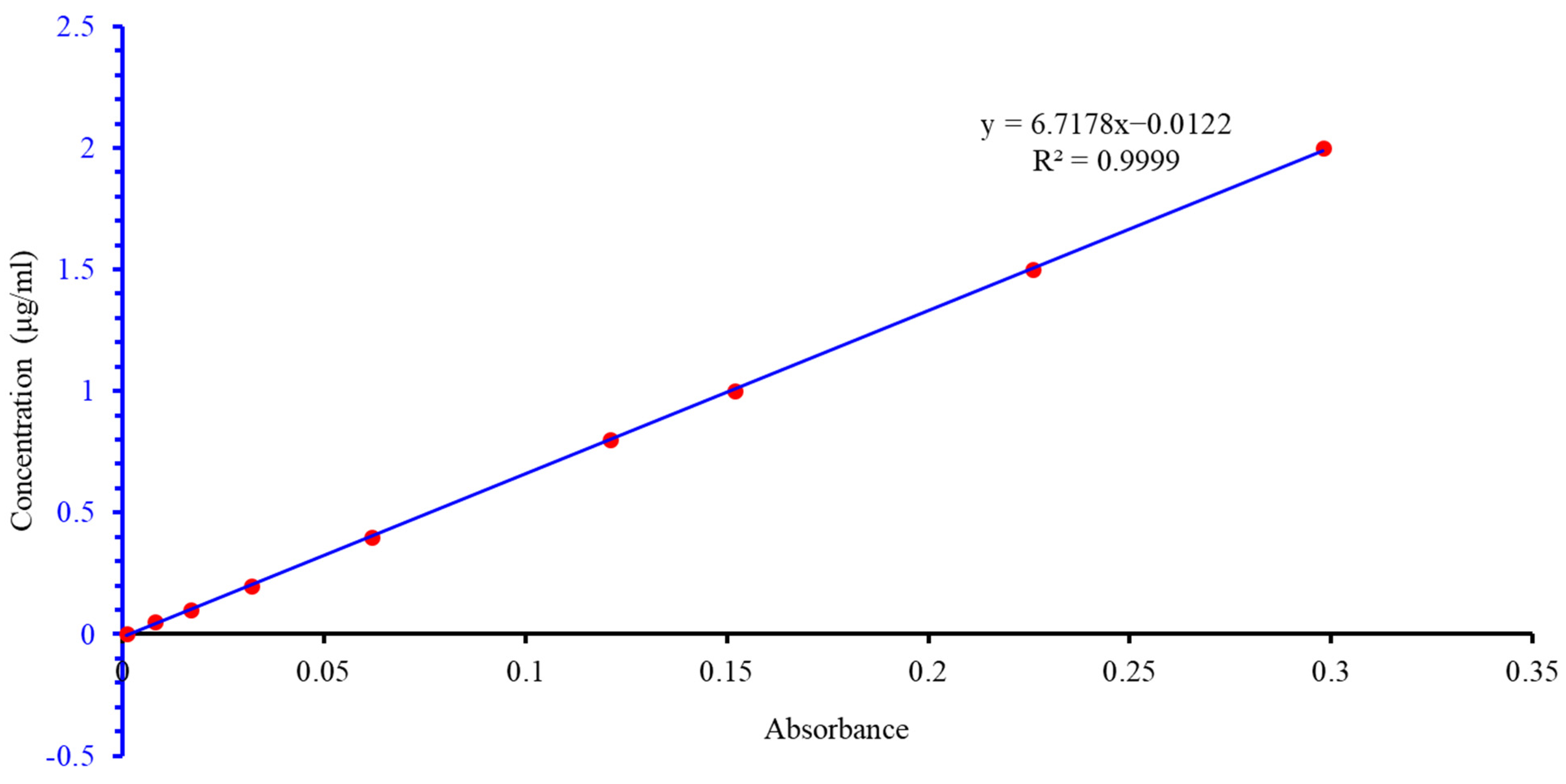
2.2.3. Determination of Lithium Leaching Rate
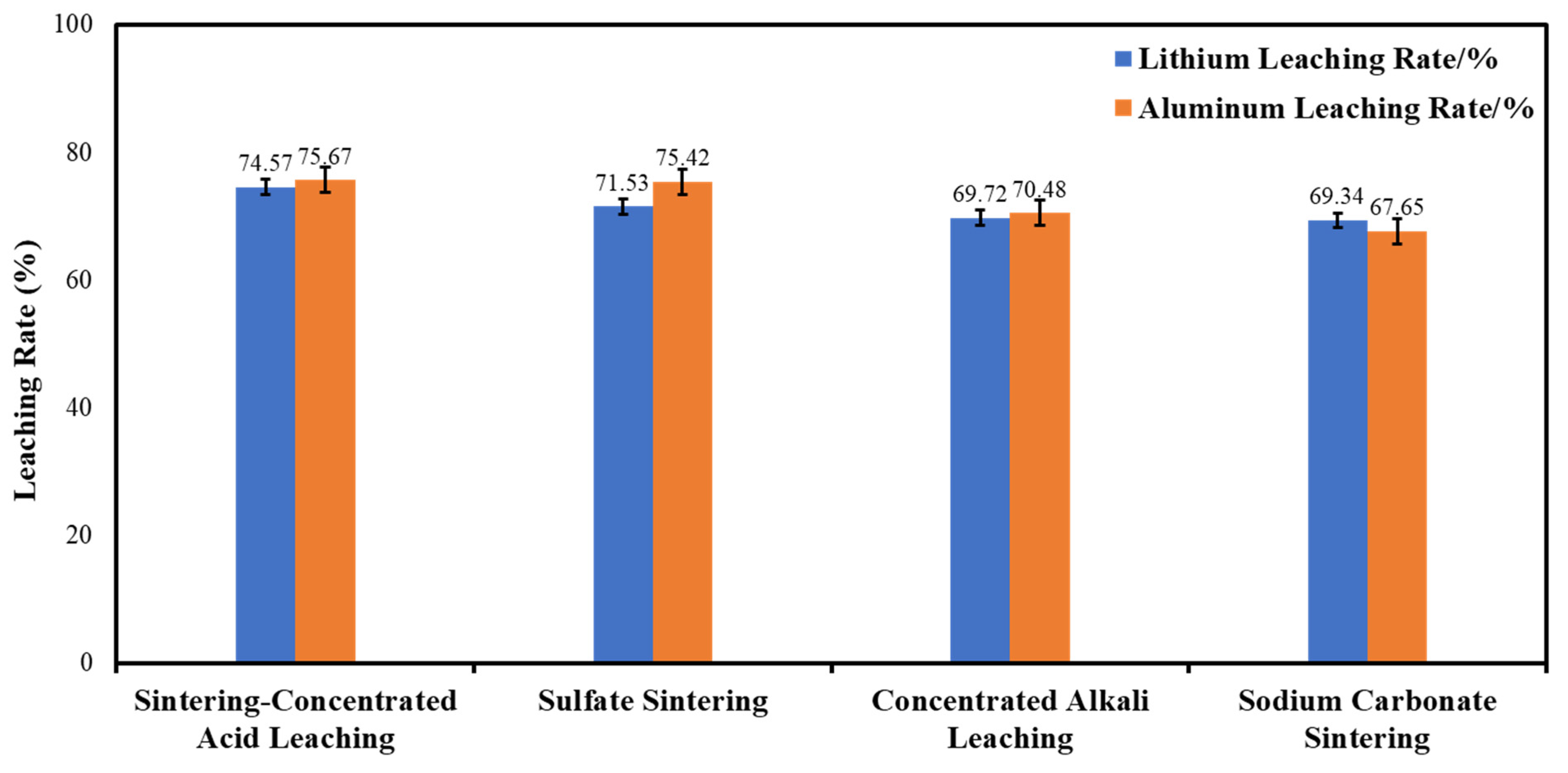
2.3. Research on Sintering-Leaching Scheme
2.4. Optimized Leaching Scheme
- (1)
- Ensure a high extraction rate. Through in-depth research and analysis of four experimental schemes, it is clear that increasing the leaching rate of metallic lithium is a key point. Since lithium is often trapped within the Si–Al lattice, to improve the leaching rate, it is necessary to release it first. As can be seen from the experimental results, adding a sintering agent can significantly increase the leaching rate of metallic lithium. Therefore, an appropriate amount of sintering agent should be added to the CFA for sintering before leaching.
- (2)
- Focus on low cost. Cost is an important indicator for evaluating the quality of a process. By studying different schemes, three main approaches to reducing the leaching costs of aluminum and lithium have been identified. First, maximize the recycling of materials to achieve material circular utilization. Second, minimize energy consumption. Third, on the premise of ensuring the extraction rate of the target product, extract other valuable energy sources as much as possible.
- (3)
- Ensure no pollution. The optimal extraction scheme should not only ensure a high lithium extraction rate and low cost but also guarantee no harm to the environment, achieving zero pollution.
- (1)
- Ignition Decomposition
- (2)
- Water Leaching and Dissolution
- (3)
- Determination of Aluminum and Lithium
2.5. Univariate Tests
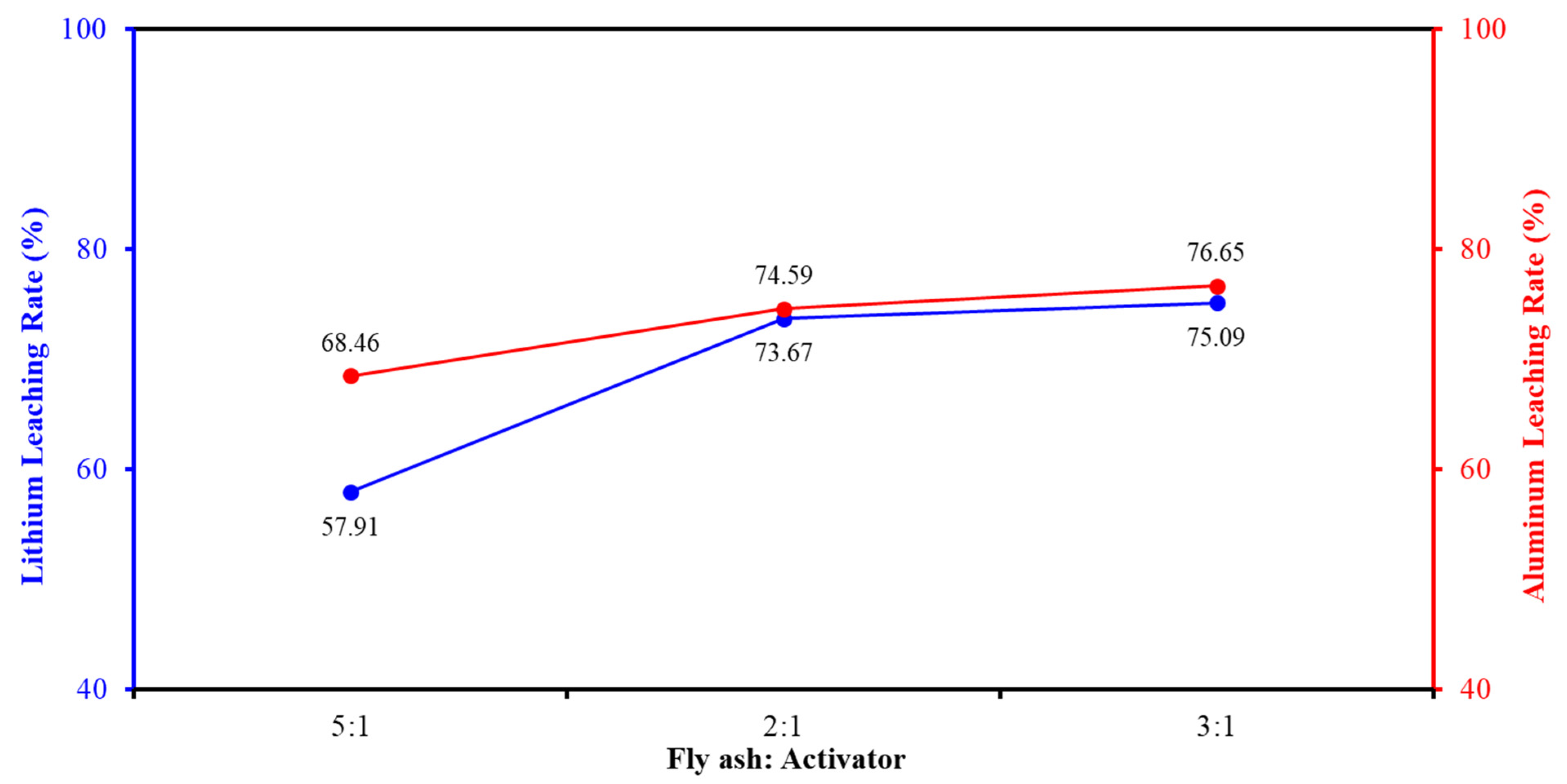
2.5.1. Activator and Sintering Agent
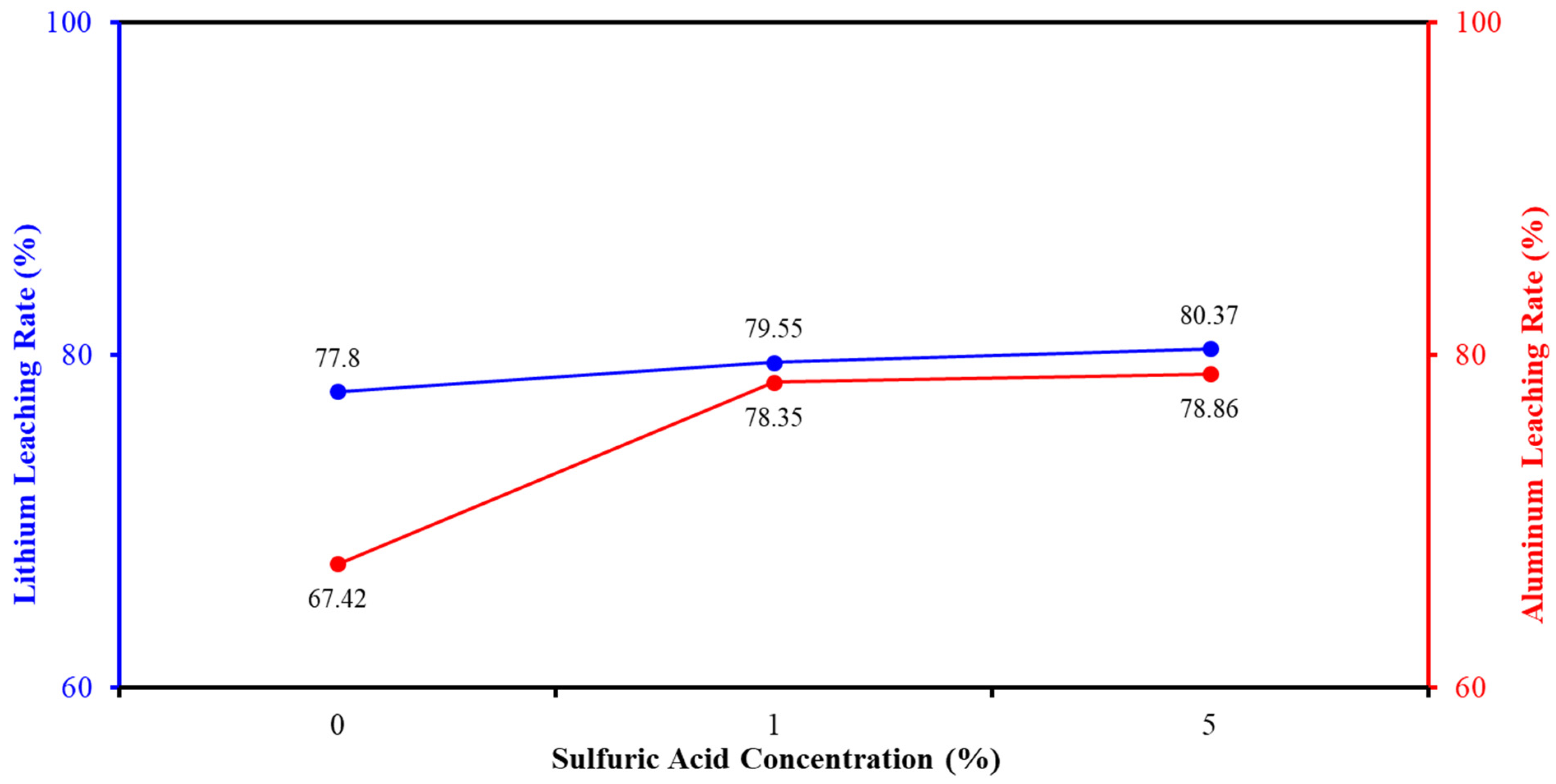
2.5.2. Determination of Leaching Agent Concentration
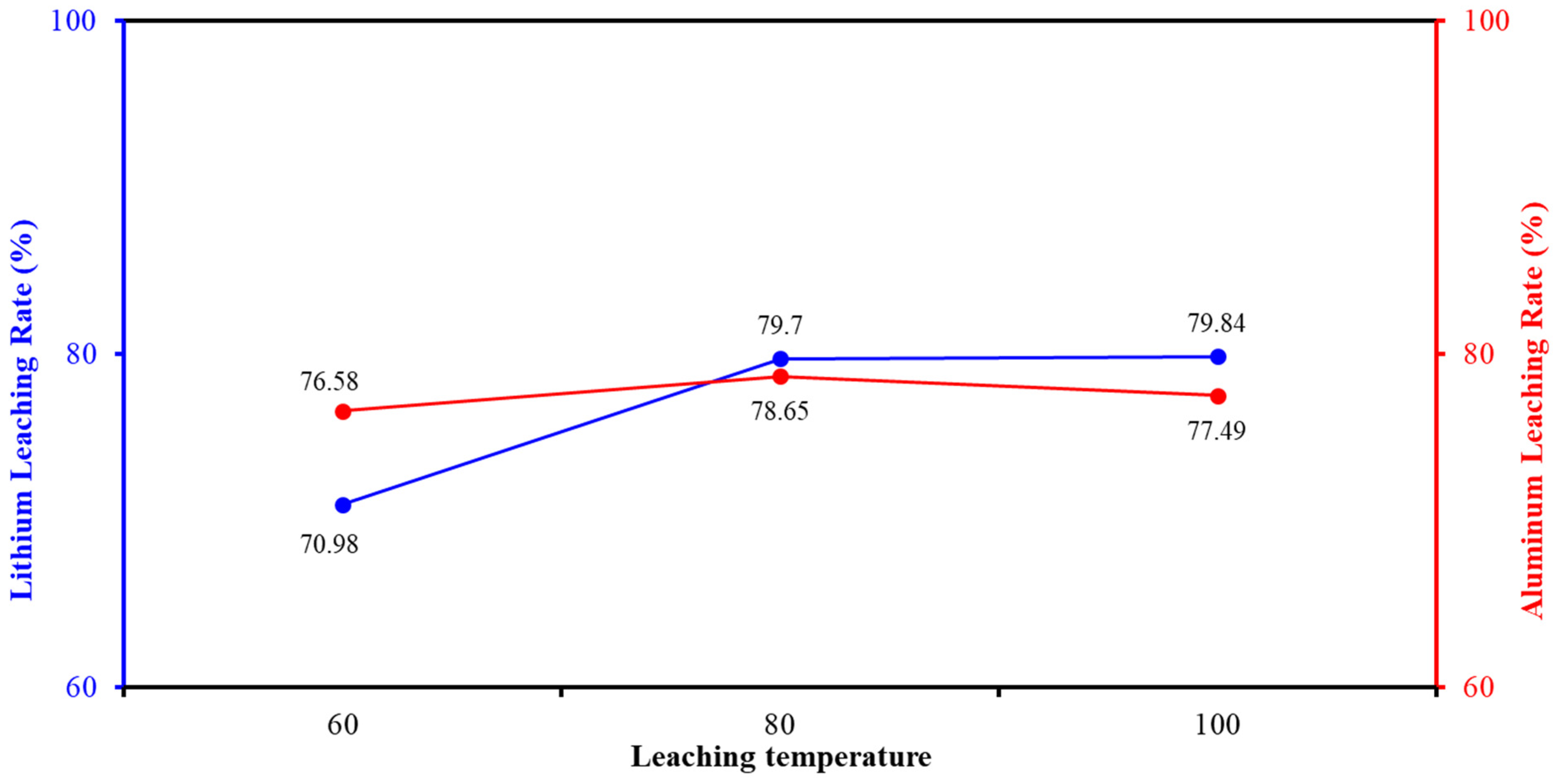
2.5.3. Determination of Leaching Temperature
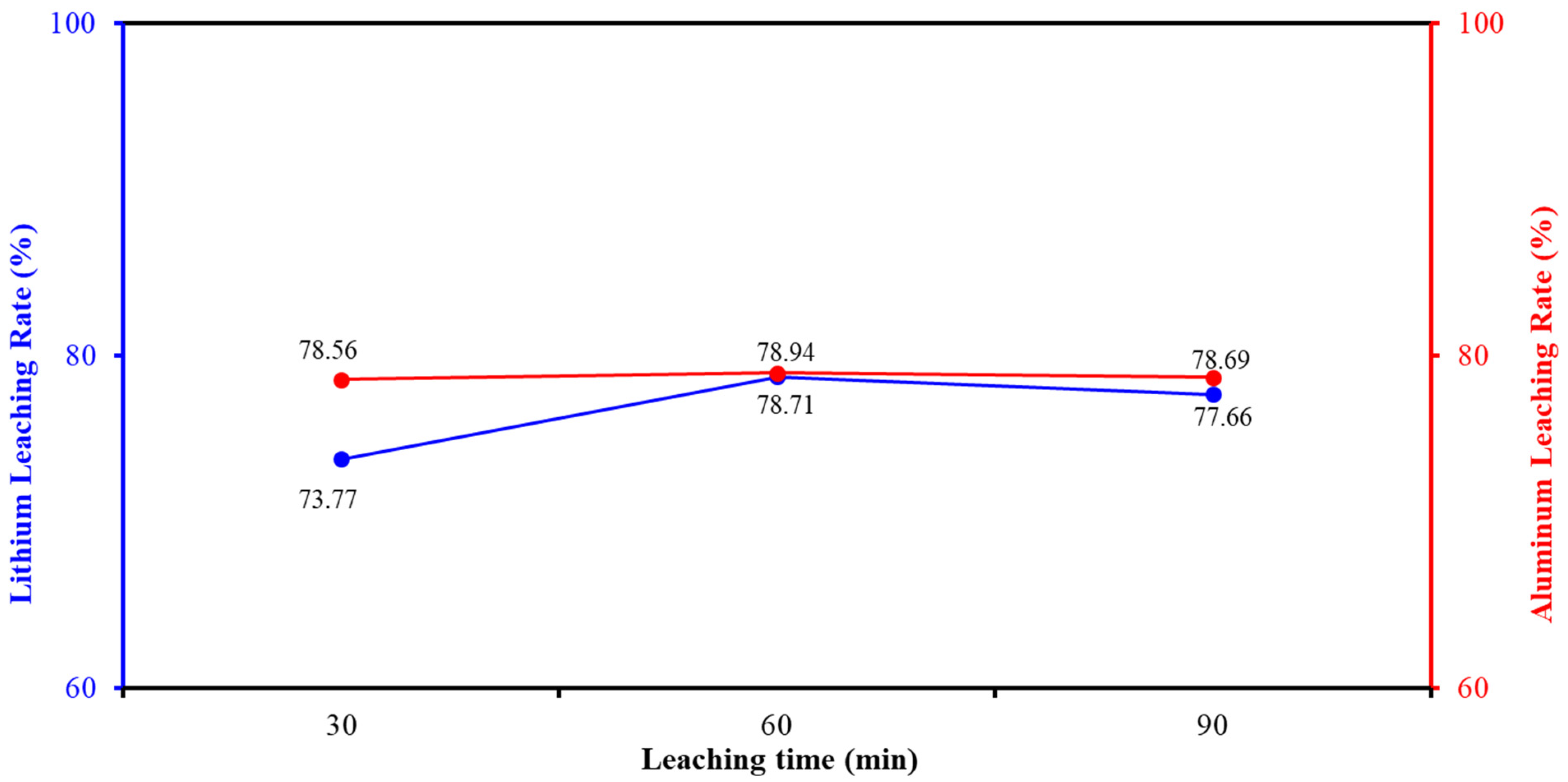
2.5.4. Determination of Leaching Time
2.6. Orthogonal Tests
3. Results and Discussion
3.1. Optimal Leaching Scheme
- (1)
- Pulverization and Sintering of CFA
- (2)
- Leaching of Samples
- (3)
- Precipitation of Lithium and Aluminum
- (4)
- Aluminum–Lithium Separation
- (5)
- Lithium Extraction
- (6)
- Aluminum Extraction
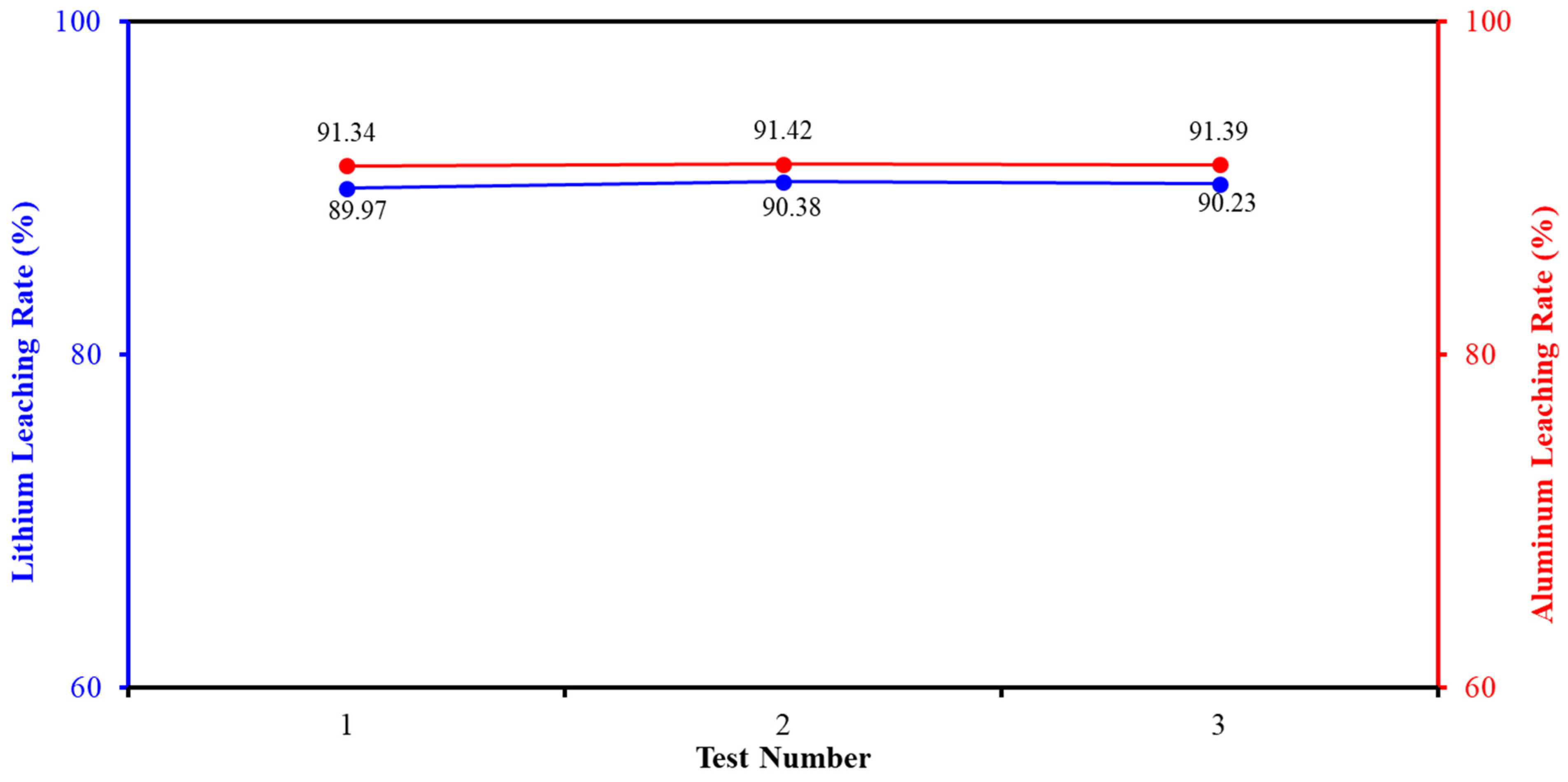
3.2. Validation of Test Results
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.-J.; Zhang, Y.-F.; Sun, J.-M.; Gong, Y.-B. Research Progress on Extraction of Alumina from High-Aluminum Fly Ash. Mod. Chem. Ind. 2022, 42, 66–70. [Google Scholar] [CrossRef]
- Hou, H.; Shao, S.; Ma, B.; Li, X.; Shi, S.; Chen, Y.; Wang, C. Sustainable Process for Valuable-Metal Recovery from Circulating Fluidized Bed Fly Ash Through Nitric Acid Pressure Leaching. J. Clean. Prod. 2022, 360, 132212. [Google Scholar] [CrossRef]
- Xu, F.; Qin, S.; Li, S.; Cui, L.; Wang, Y.; Qi, D.; Zhao, G. Research Progress on Geochemistry and Extraction of Lithium from Coal and Coal Ash. Coal Sci. Technol. 2021, 49, 220–229. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Zhao, C.L.; Li, Y.H.; Wang, J.X.; Lin, M.Y. Li Distribution and Mode of Occurrences in Li-Bearing Coal Seam 9 from Pingshuo Mining District, Ningwu Coalfield, Northern China. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2013, 31, 27–38. Available online: https://www.researchgate.net/publication/236965462 (accessed on 1 January 2025).
- Zhao, L.; Wang, X.; Dai, S. Lithium Resources in Coal-Bearing Strata: Occurrence, Mineralization, and Resource Potential. J. China Coal Soc. 2022, 47, 1750–1760. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Zhao, C.; Li, Y.; Wang, J.; Liu, S. Li Distribution and Mode of Occurrences in Li-Bearing Coal Seam # 6 from the Guanbanwusu Mine, Inner Mongolia, Northern China. Energy Explor. Exploit. 2012, 30, 109–130. [Google Scholar] [CrossRef]
- Fang, H.; Zhou, C.; Xu, S.; Shi, J.; Hu, Y.; Liu, G. High-Efficiency Extraction of Aluminum and Lithium from Coal Fly Ash Using a Novel Sodium Pyrosulfate Mechanochemical Activation-Sodium Persulfate Pressure Leaching Technology. J. Clean. Prod. 2023, 423, 138841. [Google Scholar] [CrossRef]
- Homa, R.; Sied, Z.S.; Hadi, A.; Alireza, S.; Sina, G. A Sustainable Method for Germanium, Vanadium and Lithium Extraction from Coal Fly Ash: Sodium Salts Roasting and Organic Acids Leaching. Fuel 2022, 312, 122844. [Google Scholar] [CrossRef]
- Dong, H.; Chen, J.; Li, X.Y.; Shao, L. The Effect of Activator on Lithium Extraction from Xinjiang Fly Ash. Chem. Ind. Eng. Prog. 2019, 38, 1538–1544. [Google Scholar] [CrossRef]
- Shan, X. Study on Distribution and Extraction Characteristics of Valuable Elements in Fly Ash. Master’s Thesis, Shanxi University, Taiyuan, China, 2019. [Google Scholar] [CrossRef]
- Rezaei, H.; Shafaei, S.Z.; Abdollahi, H.; Ghassa, S.; Boroumand, Z.; Nosratabad, A.F. Spent-Medium Leaching of Germanium, Vanadium and Lithium from Coal Fly Ash with Biogenic Carboxylic Acids and Comparison with Chemical Leaching. Hydrometallurgy 2023, 217, 106038. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, F.; Zheng, P.; Wang, Y.; Zhao, L.; Xu, C. Selective Recovery of Lithium from High-aluminum Fly Ash by Using Alkali-dissolution-assisted HBTA–TOPO Synergistic Extraction. J. Clean. Prod. 2024, 434, 139998. [Google Scholar] [CrossRef]
- Li, S.; Bo, P.; Kang, L.; Guo, H.; Gao, W.; Qin, S. Activation Pretreatment and Leaching Process of High-Alumina Coal Fly Ash to Extract Lithium and Aluminum. Metals 2020, 10, 893. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Mi, X.; Wang, Z.; Han, J.; Zeng, G.; Liu, P.; Guan, Q.; Ji, H.; Huang, S. Extraction of Lithium from Coal Fly Ash by Low-Temperature Ammonium Fluoride Activation—Assisted Leaching. Sep. Purif. Technol. 2021, 279, 119757. [Google Scholar] [CrossRef]
- Liping, W.; Chao, L. Research Progress on Resource Utilization of Aluminum and Silicon in Fly Ash. Multipurp. Util. Miner. Resour. 2024, 45, 13. [Google Scholar] [CrossRef]
- Li, W.; Zou, P.; Chi, J.; Liu, D.; Wu, Y. Leaching of Alumina From Circulating Fluidized Bed Fly Ash Using Hydrochloric Acid. Hydrometall. China 2020, 39, 110–113. [Google Scholar] [CrossRef]
- Shoppert, A.; Valeev, D.; Loginova, I. Kinetics of Aluminum and Scandium Extraction from Desilicated Coal Fly Ash by High-Pressure HCl Leaching. Metals 2023, 13, 1994. [Google Scholar] [CrossRef]
- Tripathy, A.K.; Behera, B.; Aishvarya, V.; Sheik, A.R.; Dash, B.; Sarangi, C.K.; Tripathy, B.C.; Sanjay, K.; Bhattacharya, I.N. Sodium Fluoride Assisted Acid Leaching of Coal Fly Ash for the Extraction of Alumina. Miner. Eng. 2019, 131, 140–145. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G.; Zhao, B.; Liu, P. Alumina Extraction from High-Alumina Fly Ash by Soda Lime Low-Calcium Sintering. Light Met. 2018, 12, 12–15. [Google Scholar] [CrossRef]
- Xing, L.; Li, X.; Cao, P.; Luo, J.; Jiang, H. Stepwise Extraction and Utilization of Silica and Alumina from Coal Fly Ash by Mild Hydrothermal Process. Process Saf. Environ. Prot. 2024, 182, 918–929. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Xakalashe, B.; Ndlovu, S.; Forsberg, K.; Friedrich, B. A Cleaner Approach for Recovering Al and Ti from Coal Fly Ash via Microwave-Assisted Baking, Leaching, and Precipitation. Hydrometallurgy 2021, 206, 105754. [Google Scholar] [CrossRef]
- Guo, C.; Zou, J.; Ma, S.; Yang, J.; Wang, K. Alumina Extraction from Coal Fly Ash via Low-Temperature Potassium Bisulfate Calcination. Minerals 2019, 9, 585. [Google Scholar] [CrossRef]
- Yu, W.; Rao, Z.; Yuan, H.; Wei, P.; Nyarko-Appiah, J.E.; Jiang, W. An Efficient and Environmental Friendly Strategy for Alumina Extraction and Fe–Si Alloys Production from Coal Fly Ash by Combining Vacuum Thermal Reduction, Alkali Dissolving, and Magnetic Separation. J. Clean. Prod. 2023, 408, 137129. [Google Scholar] [CrossRef]
- GB/T 482-2008; Sampling of Coal Seams. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Standardization Administration of the People’s Republic of China: Beijing, China, 2008; p. 16.
- GB/T 1574-2007; Test Method for Analysis of Coal Ash. Standardization Administration of the People’s Republic of China: Beijing, China, 2007; p. 36.
- DZ/T 0203-2002; Specifications for Rare Metal Mineral Exploration. Ministry of Land and Resources of the People’s Republic of China: Beijing, China, 2002; p. 46.
- Dai, S.; Zhao, L.; Peng, S.; Chou, C.-L.; Wang, X.; Zhang, Y.; Li, D.; Sun, Y. Abundances and Distribution of Minerals and Elements in High-Alumina Coal Fly Ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2009, 81, 320–332. [Google Scholar] [CrossRef]
- Wu, Y.; Xin, H.; Liu, J.; Xu, Y.; Ji, F. The Research on Extraction of Alumina from Aluminum Ammonium Sulfate Solution. Light Met. 2019, 11–14. [Google Scholar] [CrossRef]
- Wang, Z. Applied Basic Research of the Resource Utilization of Alumina-Extracted Residue from Coal Fly Ash by Sub-Molten Salt Method. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2019. Available online: https://kns.cnki.net/kcms2/article/abstract?v=ZkvsKdWJ3SQQg9atBBkyl1gwtpB0oZDQNkk4lQ6RryIbNum_a4FOzF12Yp89nDR_a4KpJv6-VpWu-VsFCehBQMXaQny_a1HsHn7nqNL8Nvv5xqMpM-HsHDB0ohkzsZ077EdtekiJexS60OCsjQYrJ-U0z8O9FEjym3-VGNVhAv6tcV9GPMl-tOAULX1TXCjXnYPHZJegVm0=&uniplatform=NZKPT&language=CHS (accessed on 23 June 2024).
- Feng, G. The Effect of Sodium Carbonate on Digestion Process of Sintered Alumina Grog and its Concentration Control. Conserv. Util. Miner. Resour. 2000, 32–35. [Google Scholar] [CrossRef]
- Zhu, S.; Mao, L.; Cao, B.; Li, M.; Qin, Y.; Wu, M.; Cao, L.; Wu, G. Multi-factor Analysis of Lithium Extraction Rate from Coal Ash Based on Orthogonal Experiment. Coal Technol. 2023, 42, 221–225. [Google Scholar] [CrossRef]
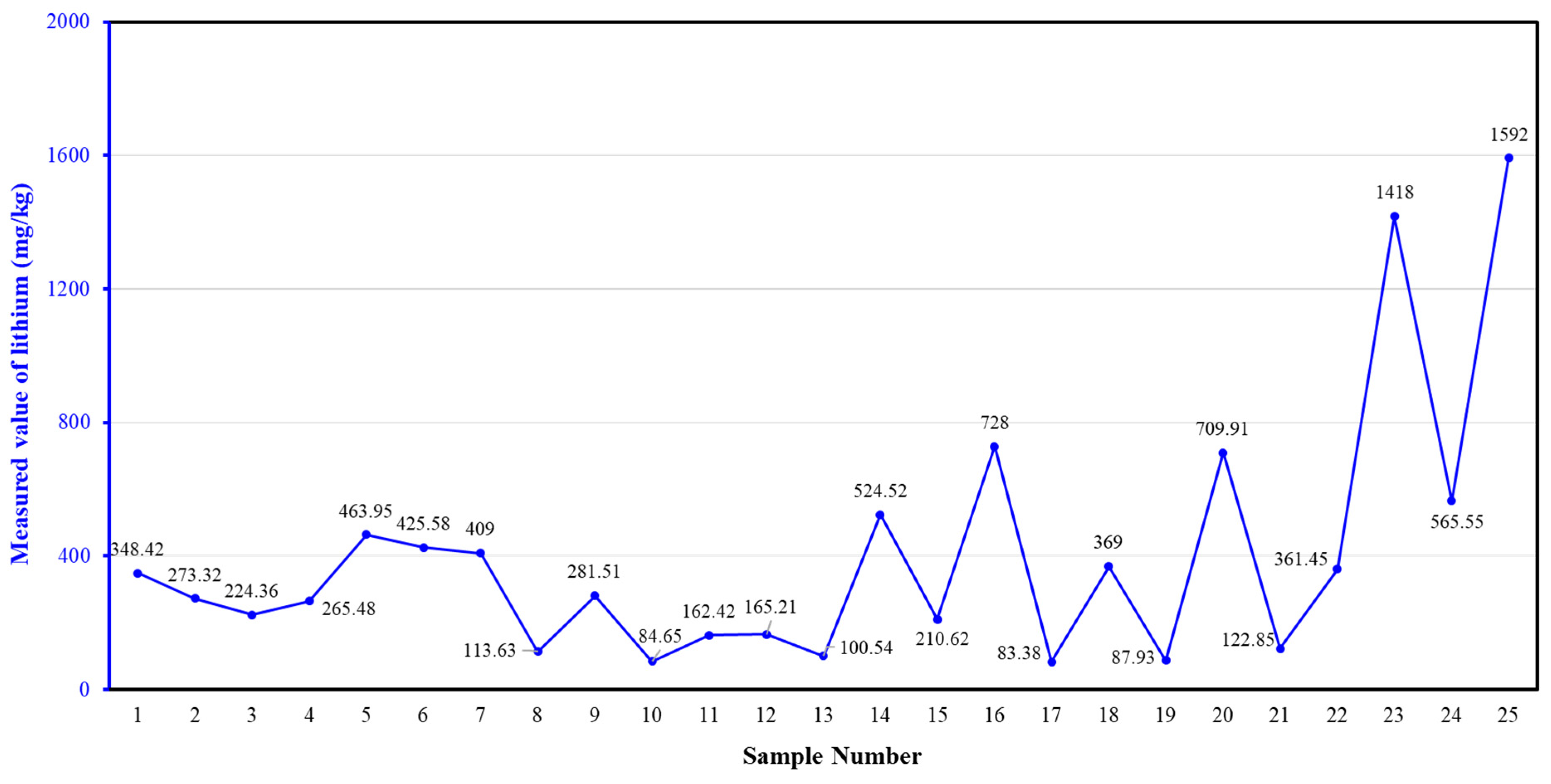




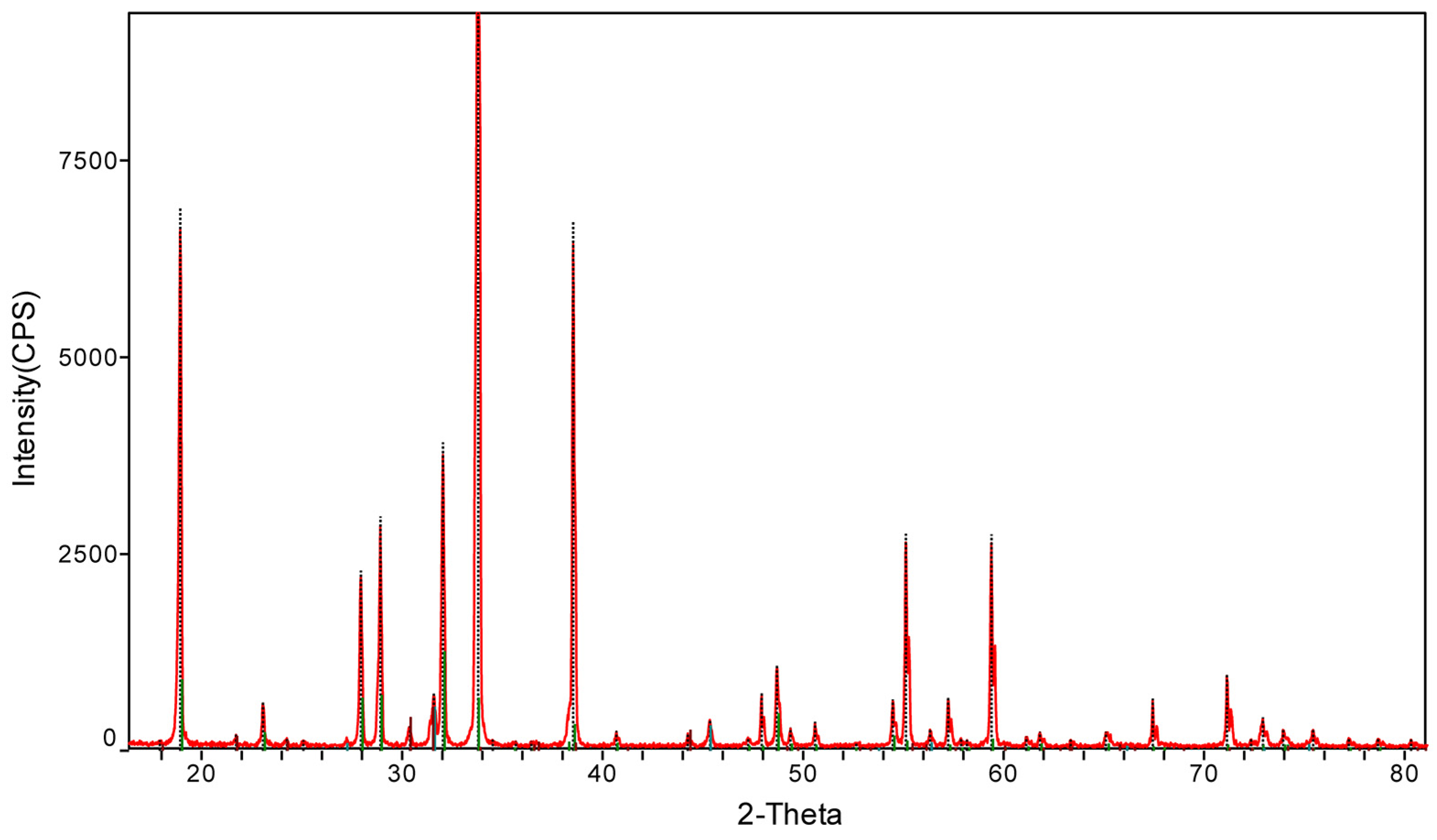
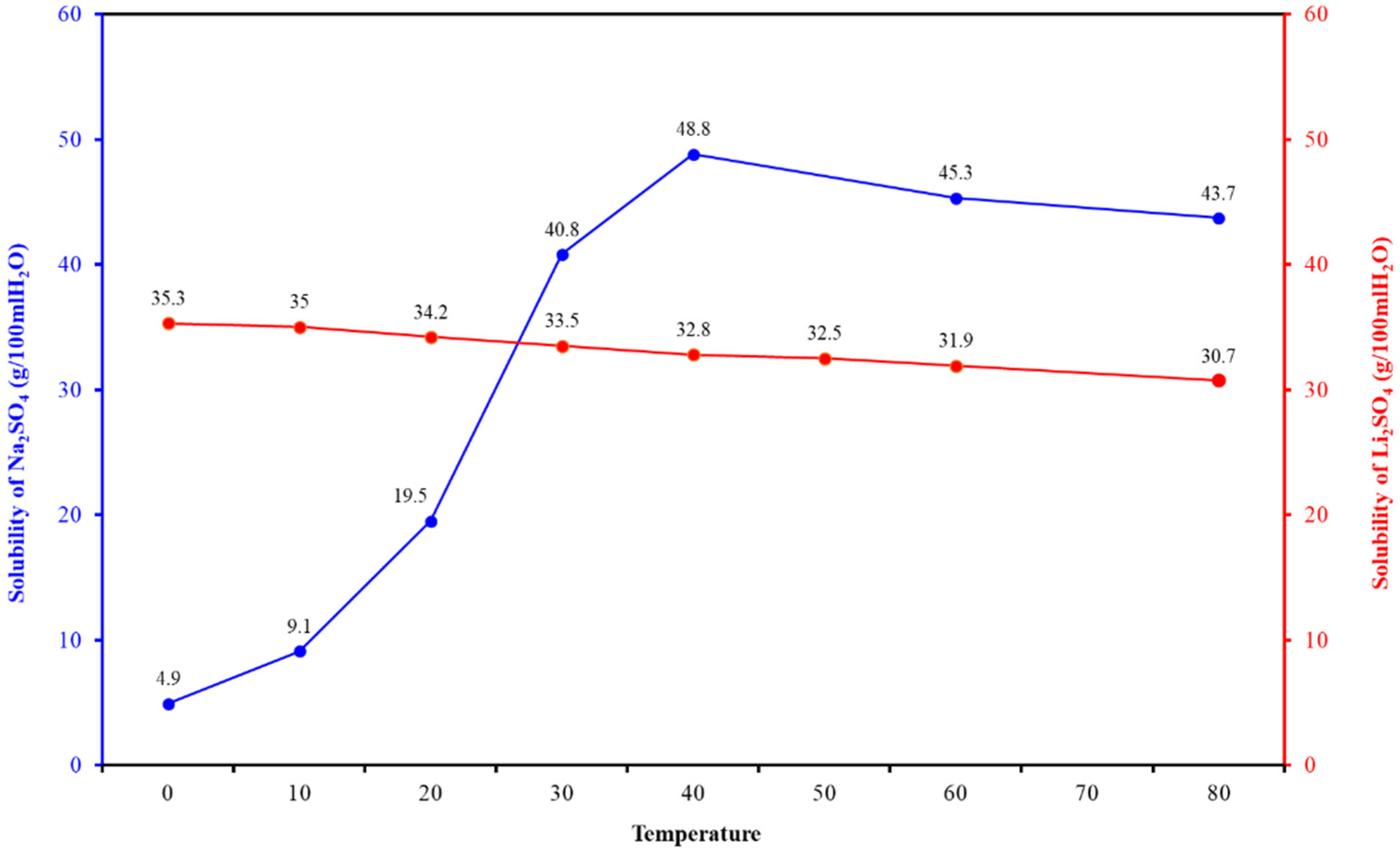

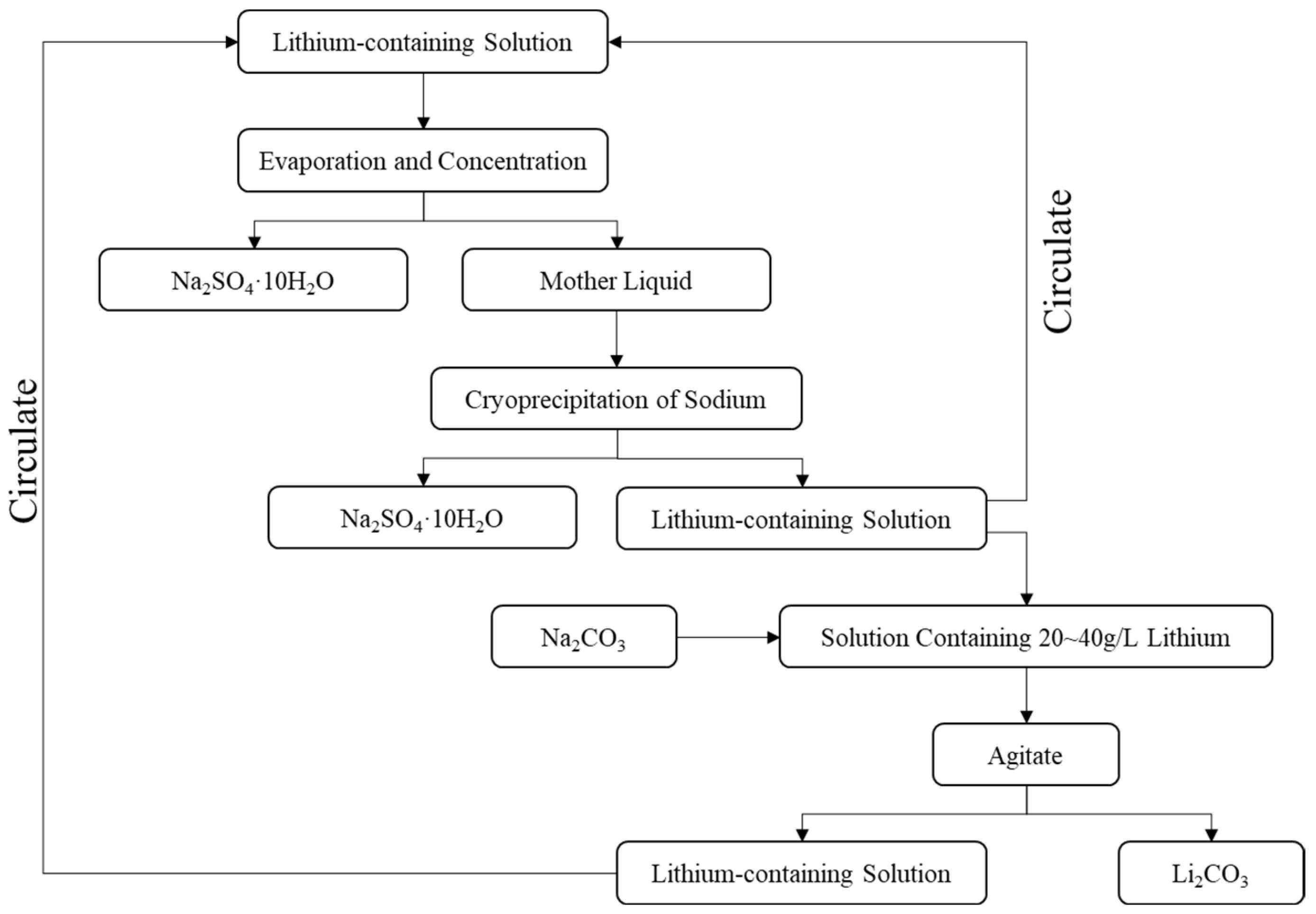
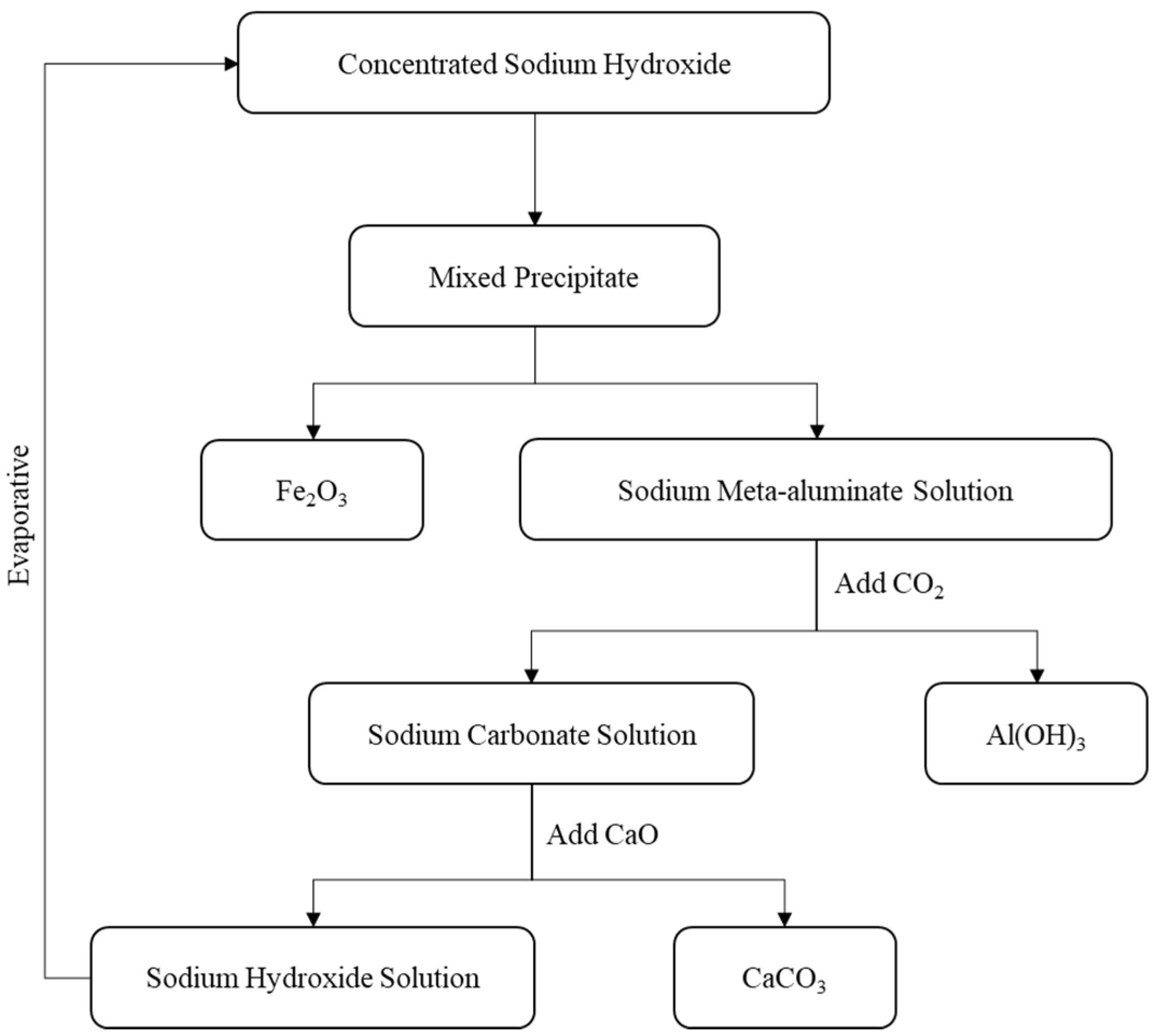

| Number | SiO2 (%) | Fe2O3 (%) | Al2O3 (%) | CaO (%) | MgO (%) |
|---|---|---|---|---|---|
| 1 | 47.41 | 5.92 | 37.93 | 5.00 | 3.74 |
| 2 | 52.22 | 3.39 | 43.20 | 0.84 | 0.35 |
| 3 | 42.74 | 6.69 | 41.69 | 3.53 | 5.35 |
| 4 | 13.92 | 4.41 | 53.77 | 11.34 | 16.56 |
| 5 | 57.67 | 6.66 | 32.13 | 0.41 | 3.13 |
| 6 | 51.31 | 4.19 | 42.96 | 0.79 | 0.75 |
| 7 | 42.14 | 4.07 | 50.40 | 0.58 | 2.81 |
| 8 | 20.92 | 20.24 | 17.46 | 11.68 | 29.70 |
| 9 | 44.13 | 10.90 | 40.96 | 1.70 | 2.30 |
| 10 | 25.53 | 24.89 | 34.56 | 12.84 | 2.19 |
| 11 | 32.12 | 12.86 | 33.11 | 7.00 | 14.91 |
| 12 | 31.85 | 14.78 | 36.22 | 7.85 | 9.30 |
| 13 | 21.09 | 5.47 | 53.22 | 6.90 | 13.31 |
| 14 | 48.44 | 3.42 | 40.01 | 0.23 | 7.90 |
| 15 | 8.86 | 11.87 | 23.00 | 43.01 | 13.26 |
| 16 | 37.90 | 2.63 | 40.78 | 0.33 | 18.36 |
| 17 | 22.93 | 13.34 | 42.84 | 9.72 | 11.17 |
| 18 | 50.31 | 0.96 | 39.26 | 0.01 | 9.45 |
| 19 | 14.24 | 4.81 | 47.87 | 32.09 | 0.99 |
| 20 | 35.29 | 4.27 | 41.60 | 0.55 | 18.29 |
| 21 | 13.54 | 8.81 | 56.07 | 2.05 | 19.53 |
| 22 | 24.25 | 3.17 | 68.40 | 2.00 | 2.19 |
| 23 | 44.00 | 4.88 | 47.86 | 0.24 | 3.02 |
| 24 | 16.40 | 1.95 | 66.71 | 0.51 | 14.43 |
| 25 | 51.16 | 2.09 | 46.33 | 0.41 | 0.02 |
| Level | Factors | Lithium Leaching Rate/% | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 600 | 30 | 400 | 30 | 74.53 |
| 2 | 600 | 60 | 500 | 60 | 75.99 |
| 3 | 600 | 90 | 600 | 90 | 57.89 |
| 4 | 800 | 30 | 500 | 90 | 70.41 |
| 5 | 800 | 60 | 600 | 30 | 63.69 |
| 6 | 800 | 90 | 400 | 60 | 72.59 |
| 7 | 1000 | 30 | 600 | 60 | 90.16 |
| 8 | 1000 | 60 | 400 | 90 | 76.99 |
| 9 | 1000 | 90 | 500 | 30 | 69.14 |
| K1 | 208.41 | 235.1 | 224.11 | 207.36 | T = 651.39 |
| K2 | 206.69 | 216.67 | 215.54 | 238.74 | |
| K3 | 236.29 | 199.62 | 211.74 | 205.29 | |
| K1/3 | 69.47 | 78.37 | 74.7 | 69.12 | Y = 72.38 |
| K2/3 | 68.9 | 72.22 | 71.85 | 79.58 | |
| K3/3 | 78.76 | 66.54 | 70.58 | 68.43 | |
| R | 29.6 | 35.48 | 12.37 | 33.45 | |
| Factors | B > D > A > C | ||||
| Optimal Solution | 1000 °C | 30 min | 400 °C | 60 min | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, Y.; Zhao, Q.; Xu, M.; Yao, B.; Zhang, P.; Ji, X.; Fan, J. High-Efficiency Extraction of Lithium and Aluminum from Coal Fly Ash Using Activation-Sintering Technology. Metals 2025, 15, 208. https://doi.org/10.3390/met15020208
Zhang C, Li Y, Zhao Q, Xu M, Yao B, Zhang P, Ji X, Fan J. High-Efficiency Extraction of Lithium and Aluminum from Coal Fly Ash Using Activation-Sintering Technology. Metals. 2025; 15(2):208. https://doi.org/10.3390/met15020208
Chicago/Turabian StyleZhang, Chenliang, Yanheng Li, Qiaojing Zhao, Mingjing Xu, Bin Yao, Pengpeng Zhang, Xuan Ji, and Jiawei Fan. 2025. "High-Efficiency Extraction of Lithium and Aluminum from Coal Fly Ash Using Activation-Sintering Technology" Metals 15, no. 2: 208. https://doi.org/10.3390/met15020208
APA StyleZhang, C., Li, Y., Zhao, Q., Xu, M., Yao, B., Zhang, P., Ji, X., & Fan, J. (2025). High-Efficiency Extraction of Lithium and Aluminum from Coal Fly Ash Using Activation-Sintering Technology. Metals, 15(2), 208. https://doi.org/10.3390/met15020208





