Abstract
Jarosite residue (JR), a hazardous solid waste generated in non-ferrous metallurgy, poses significant environmental challenges due to its large volume and poor storage stability. However, its high content of valuable metals (such as iron, zinc, gallium, indium, silver, …) makes its efficient recovery and comprehensive utilization highly significant. This study investigates the “oxidative roasting–reductive smelting” process for JR treatment. The reduction thermodynamics of JR-R (roasted JR) were analyzed, and the effects of smelting temperature, time, and slag basicity on the reduction and smelting process were examined. The results indicate that increasing slag basicity and temperature generally decreases slag viscosity. Thermodynamic calculations demonstrate that reductive smelting effectively enriches valuable metals (>1039 °C). The optimal conditions for reductive smelting of JR were determined to be as follows: smelting temperature of 1550 °C, smelting time of 60 min, and slag basicity of 0.9. Under these conditions, the process achieved an Fe grade of 92.87% in pig iron with a recovery rate of 90.66%, a Ga grade of 377 g/t with a recovery rate of 94.91%, and Zn and In volatilization rates of 99.91% and 83.36%, respectively. This study provides a feasible approach for the comprehensive recovery of valuable metals such as Ga, Fe, Zn, and In from JR, offering promising economic and social benefits.
1. Introduction
Jarosite residue (JR) is a solid waste produced during the iron removal process in zinc hydrometallurgy plants. It typically contains valuable metals such as Fe, Pb, Zn, and Cu, as well as trace amounts of high-value rare and precious metals like In and Ga, making it economically valuable for recovery. The residue generally consists of 25–30% Fe, 3–6% Zn, 0.5–2% Pb, and 100–300 g/t In [1,2,3,4]. According to the <<National Hazardous Waste List>> (2021 edition), JR is classified as solid waste with a waste code of 321-004-48, categorized under non-ferrous metal smelting waste. The most common method for handling jarosite residue is stockpiling [5,6,7,8]. While jarosite can remain stable under certain acidic conditions, it tends to hydrolyze or decompose when pH levels rise or under thermal conditions, leading to the leaching of heavy metal ions, which can pollute soil and water environments [9,10,11]. Therefore, the long-term storage of jarosite residue is not advisable, and timely treatment and proper utilization are essential. Otherwise, it would result in resource waste and potential environmental hazards. Direct disposal or stockpiling can lead to the leaching of heavy metal ions during natural accumulation, causing heavy metal contamination of soil and water bodies, as well as the loss of iron resources and other valuable metals [12]. Consequently, in the context of growing resource scarcity and increasingly stringent environmental regulations, developing an economically viable and environmentally friendly approach for the comprehensive treatment of large volumes of jarosite residue is a significant challenge facing the non-ferrous metallurgy industry, crucial for the sustainable development of the zinc metallurgical sector.
Currently, the disposal of jarosite residue can be categorized into two main approaches: harmless stabilization and the recovery of valuable metals. Harmless stabilization techniques for jarosite residue include high-temperature sintering, reduction roasting–magnetic separation, high-temperature hydrolysis, and solvent leaching [5,13]. However, these harmless disposal methods have not been widely adopted due to their inability to fully utilize the synergistic recovery of iron and other valuable metals, as well as their high operational costs. The existing methods for extracting valuable metals from jarosite residue mainly target specific metals, failing to achieve comprehensive utilization. Therefore, there is an urgent need to develop a method that enables the efficient recovery of valuable metal resources while being applicable to industrial-scale operations.
Significant progress has been made in the resource recovery and harmless treatment of jarosite residue, primarily reflected in the following three aspects: (1) Valuable elements such as lead, copper, cadmium, nickel, zinc, and silver can be extracted from jarosite residue using hydrometallurgical, pyrometallurgical, or combined pyro-hydrometallurgical methods, with recovery rates for certain metals exceeding 90%. (2) Jarosite residue can be used to manufacture construction materials such as cement and sintered bricks, which are then applied as cement additives in construction projects. (3) Jarosite residue can also be used in the preparation of other high-value-added materials, such as electrode materials for energy storage systems, from jarosite residue [14]. The hydrometallurgical process for metal recovery generally involves three main stages: leaching, solution concentration and purification, and metal recovery [15]. The main advantage of hydrometallurgy is its high metal recovery rate, making it an effective solution for metal extraction and suitable for processing complex and impure ores, making it a versatile option for different ore types. However, it is associated with high costs due to complex procedures and the need for chemicals, as well as challenges such as equipment corrosion, degradation, and environmental pollution [16,17]. Pyrometallurgical processes, known for their high processing efficiency and acceptable heavy metal recovery rates, have become increasingly widely adopted [18]. These methods primarily involve high-temperature roasting decomposition, where jarosite is first roasted at high temperatures to break down its crystal structure, followed by carbothermic reduction to convert it to metal. The metals are volatilized and then collected as oxides. Typically, this process is conducted at temperatures ranging from 1100 to 1300 °C, with coke or fine coal added to create a reducing atmosphere [19]. This approach is characterized by strong adaptability to raw materials, simplicity of the process, high operability, and high comprehensive metal recovery rates, making it a prevalent method for treating metallurgical waste both domestically and internationally [20,21]. However, current hydrometallurgical processes still face high reagent consumption in industrial applications [22].
In this study, jarosite residue was subjected to oxidative roasting and reduced through smelting with coke (used as a reducing agent) and flux. During the process, Zn and In in the material were reduced to metallic vapor and entered the gas phase, where they were oxidized to form oxides (ZnO and In2O3), ultimately concentrating in the dust. Ga, due to its strong affinity for iron, followed a similar path as iron during the reduction smelting process and accumulated in the smelted pig iron. This research provides a feasible pathway for the comprehensive recovery of dispersed metals such as In and Ga, as well as Fe and Zn resources, from jarosite residue. This approach enables the recovery of valuable metals while mitigating environmental hazards, demonstrating promising potential for both economic and social benefits.
2. Materials and Methods
2.1. Materials
The JR used in this study was sourced from a domestic smelting plant. The JR was pre-dried at 100 ± 5 °C for 10 h to remove free moisture before being subjected to chemical analysis. The chemical compositions of both the raw JR and roasted, pre-treated JR are shown in Table 1. (According to the corresponding national standards, chemical titration and X-ray fluorescence (XRF-PANalytical, Almelo, The Netherlands) analysis were employed for the determination of the chemical composition of JR). It can be observed that the primary valuable metals in the JR are Fe and Zn, with concentrations of 28.25% and 3.61%, respectively. The concentrations of the dispersed metals In and Ga are 490 g/t and 160 g/t, respectively, indicating significant potential for resource utilization. The contents of hazardous elements such as As and Cd are relatively low, while the loss on ignition (LOI) reaches values as high as 49.33%. According to previous studies [22,23,24,25], the decomposition of jarosite residue involves two distinct stages of significant mass loss as the temperature increases: dehydroxylation occurring between 280 and 450 °C, followed by the release of sulfur from sulfates as SO3 gas in the range of 580 to 730 °C.

Table 1.
The chemical composition of JR/wt.%.
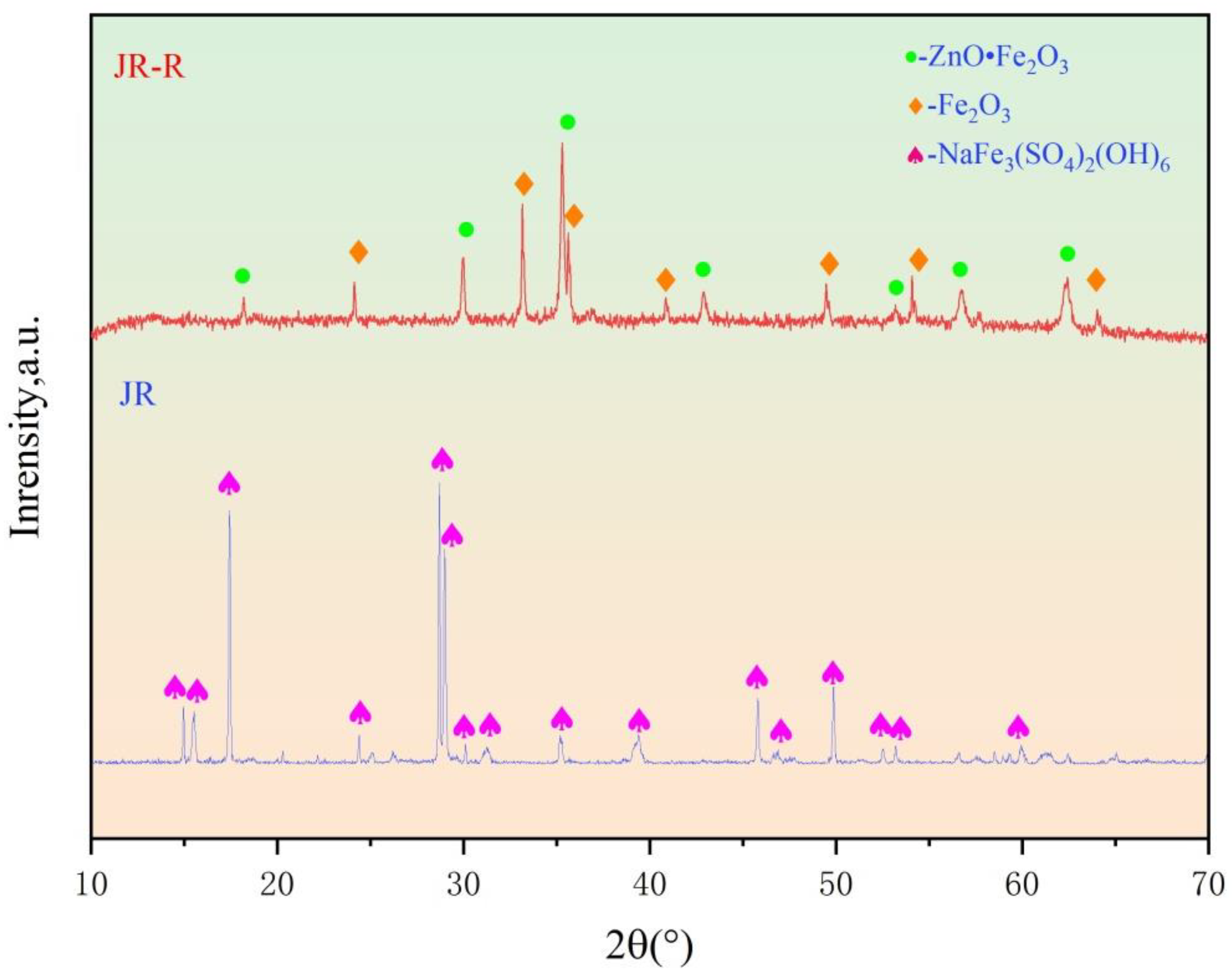
To further investigate the phase composition of the JR, X-ray diffraction (XRD) analysis was conducted on the sample. As shown in Table 1 and Figure 1, the primary phase present in the JR is sodium jarosite (NaFe3(SO4)2(OH)6). After the roasting pre-treatment, the main components of the JR are Fe2O3 and ZnO•Fe2O3.
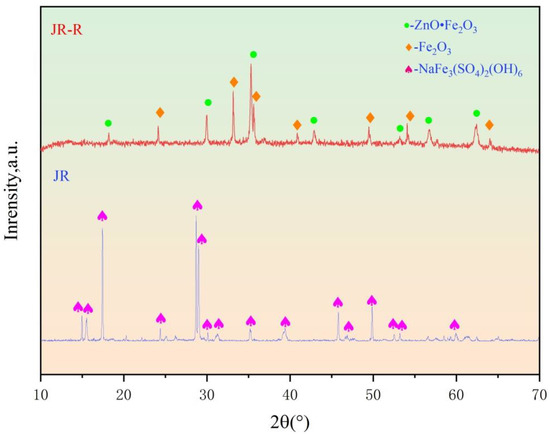
Figure 1.
XRD analyses of JR and JR-R.
Coke was selected as the reducing agent for the subsequent reduction smelting experiments. The coke was provided by a domestic steel plant. The results of the proximate analysis and the chemical composition of the ash are presented in Table 2 and Table 3, respectively.

Table 2.
Industrial analysis of reductant/wt.%.

Table 3.
Ash chemical composition of reductant/wt.%.
2.2. Methods
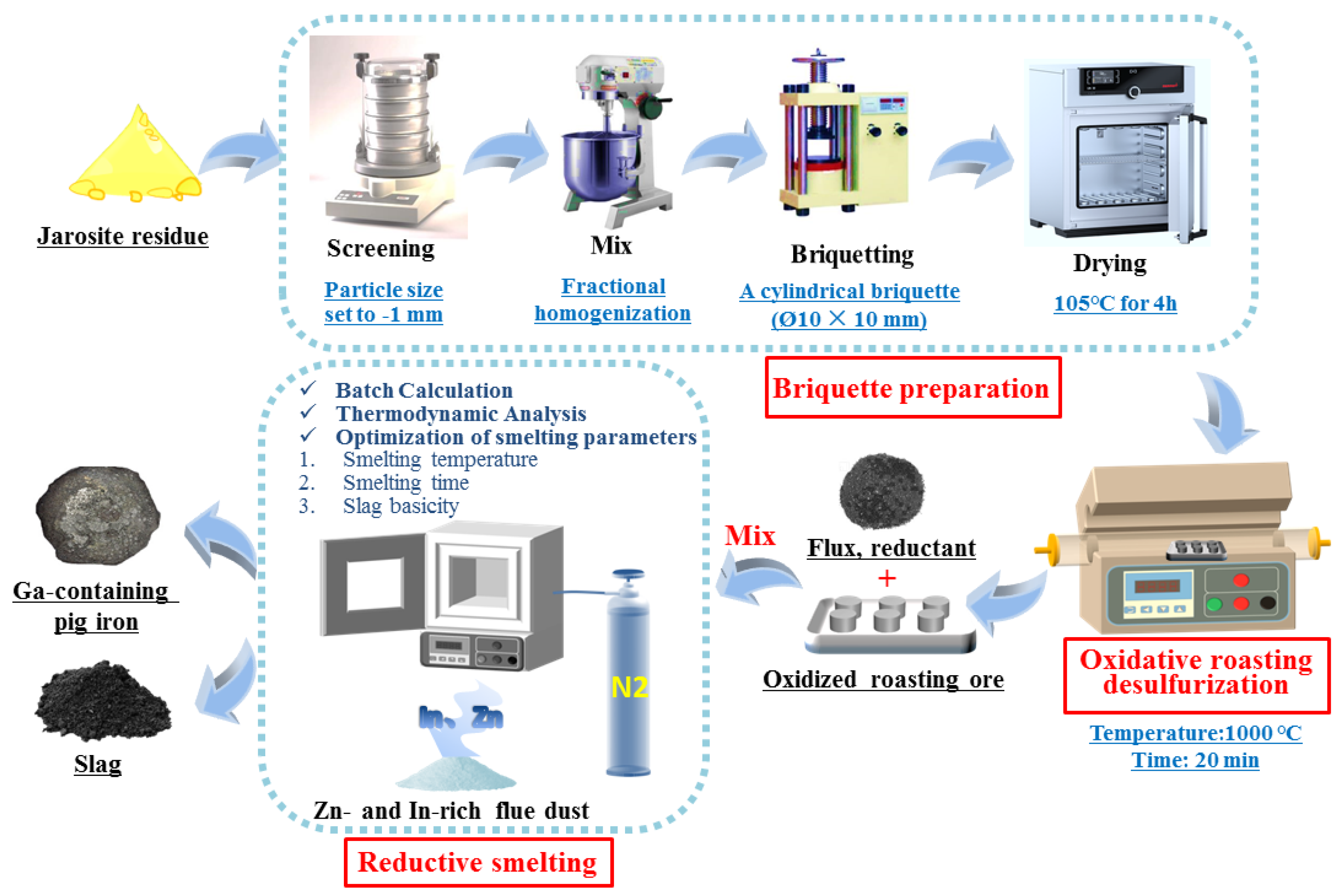
Figure 2 illustrates the experimental flowchart for the comprehensive recovery of valuable metals from JR. The detailed experimental procedure is described below.
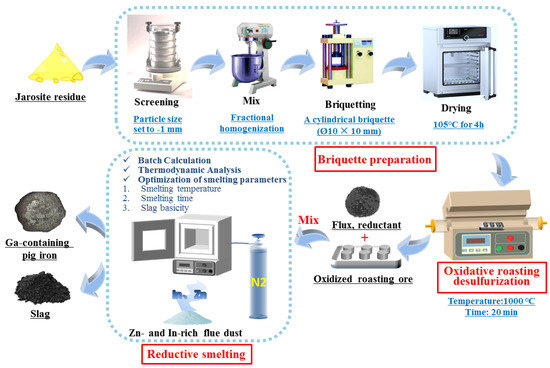
Figure 2.
Schematic diagram of the JR “roasting desulfurization briquetting–reduction smelting” process.
2.2.1. Jarosite Briquettes
The jarosite was dried to a moisture content of approximately 10%, then manually crushed to a particle size of −1 mm for later use. It was mixed uniformly in a mixer to achieve the homogenization of fractional proportions. Subsequently, a briquetting machine was used to press the material into cylindrical briquettes with a base diameter of Ø10 mm and a height of 10 mm. The briquettes were dried in a forced-air oven at 105 °C for 4 h for further use.
2.2.2. Roasting Desulfurization Test
The desulfurization roasting test of the dry briquettes was conducted in a controlled atmosphere horizontal tube furnace. Ten dried briquettes were weighed and placed into an alumina crucible. The briquettes were roasted for 20 min at 1300 °C, which allows for the rapid decomposition of sulfates [26,27] and ensures their adequate consolidation for sufficient mechanical strength; they were then rapidly removed and cooled using liquid nitrogen. The residual sulfur content in the roasted briquettes was measured, and the desulfurization rate was calculated.
The equation is as follows:
where
φ = desulfurization rate of JR oxidation roasting, %;
m1 = mass of the dry briquettes before roasting, g;
S1 = sulfur content in the JR before roasting, %;
m2 = mass of the briquettes after roasting, g;
S2 = sulfur content in the briquettes after roasting, %.
2.2.3. High-Temperature Smelting Test
The experiment was conducted in an SG-6-160 resistance furnace (380 V, 12 kW, 1650 °C). The smelting container used was a graphite crucible with the following specifications: bottom diameter of Φ50 mm, top diameter of Φ65 mm, and height of 100 mm. The slag for this experiment was designed using binary basicity (R = CaO%/SiO2%), and the amounts of roasted briquettes, coke (reducing agent), and other slag-forming agents (all of analytical grade) were weighed according to the batch calculation and mixed thoroughly. The mixed material was placed into the graphite crucible, which was then placed in the high-temperature chamber of the furnace that had reached the target temperature. The temperature was raised to the target value, and once the set temperature was reached, the heating was maintained for a specified period. After the set time, the graphite crucible was removed, covered, and buried in waste coal for cooling to room temperature. The pig iron was then removed and weighed, and the slag was collected. The contents of Fe, Ga, Zn, In, and other elements in the pig iron and slag samples were analyzed to calculate the corresponding technical parameters.
The recovery rates of Fe and Ga can be calculated as follows:
where
σ = Fe and Ga recovery rate, %;
m1 = mass of the oxidized roasted briquettes used for reduction smelting, g;
γ1 = Fe and Ga content in the oxidized roasted briquettes, wt.%;
m2 = mass of pig iron, g;
γ2 = Fe and Ga content in the pig iron, wt.%.
The recovery rates of Zn and In can be calculated as follows:
where
μ = volatility rate of Zn and In, %;
m1 = mass of the oxidized roasted briquettes before reduction smelting, g;
α1 = Zn and In content in the oxidized roasted briquettes, wt.%;
m2 = mass of pig iron, g;
α2 = Zn and In content in the pig iron, wt.%;
m3 = mass of slag, g;
α3 = Zn and In content in the slag, wt.%.
3. Results
3.1. Basic Research on the Reductive Smelting Process
3.1.1. Raw Material Properties and Batch Calculation
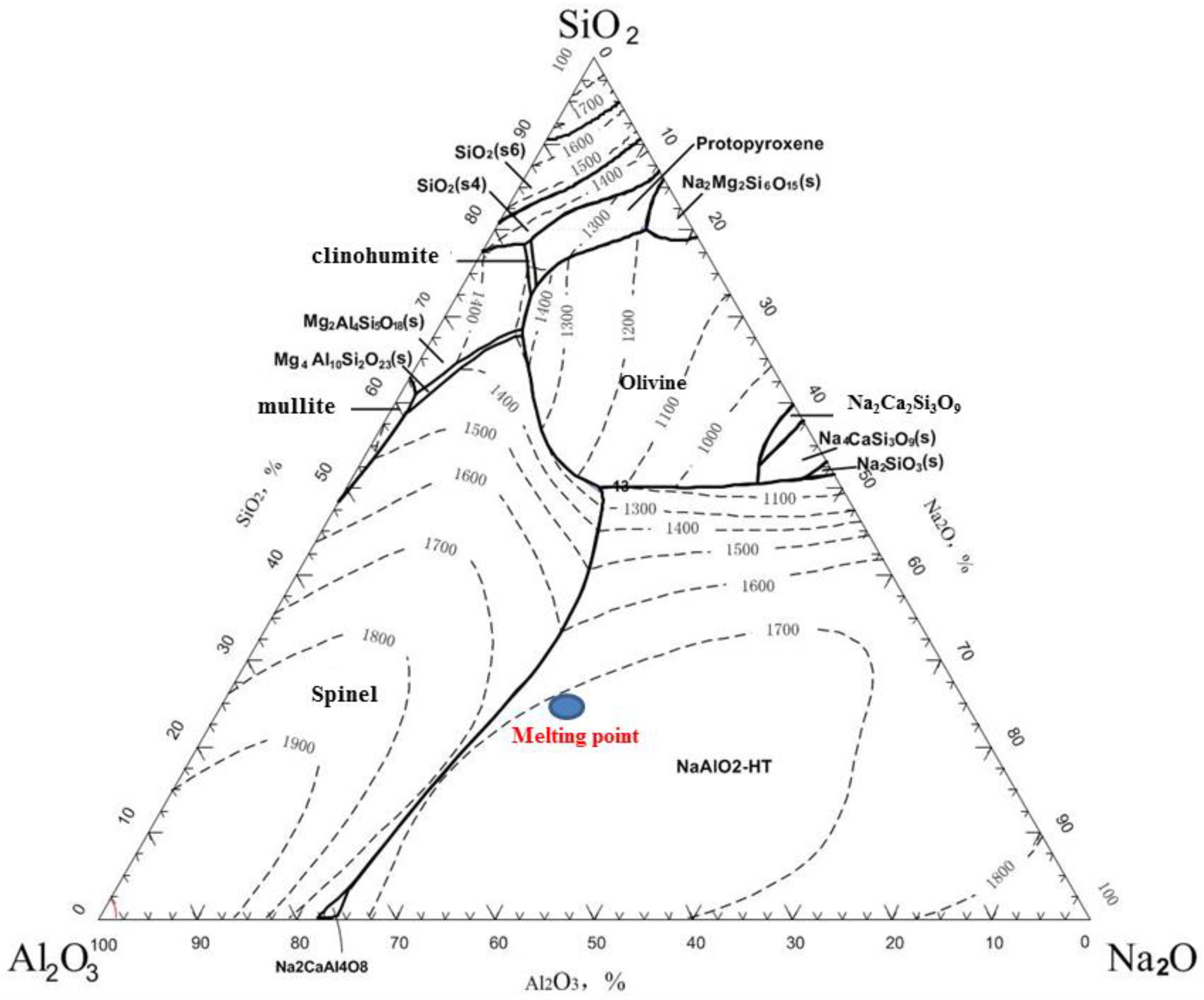
The reduction smelting experiment was conducted using the roasted briquettes of JR. The purpose of this process is to reduce Zn and In, causing them to volatilize and enter the smoke dust while separating them from the slag, and to ensure that Ga enters the pig iron. It is assumed that the slag and iron are completely separated, meaning that Fe and Ga all enter the hot metal and that SiO2, Al2O3, CaO, MgO, and Na2O from the roasted briquettes all enter the slag phase. In this case, the contents of SiO2, Al2O3, CaO, MgO, and Na2O in the slag are as follows: SiO2 = 18.31%, Al2O3 = 27.71%, CaO = 1.75%, MgO = 15.61%, and Na2O = 36.62%, with a basicity of 0.096. The SiO2—Al2O3—Na2O phase diagram (by using FactSage software 8.0) for the fixed slag, with CaO = 1.75% and MgO = 15.61%, is shown in Figure 3. It can be seen that the melting point of this slag is approximately 1700 °C, which is too high and exceeds the requirements for conventional electric furnace smelting. Therefore, adjustments to the slag system are necessary to reduce the melting point and viscosity of the slag as much as possible to meet the requirements of conventional electric furnace smelting.
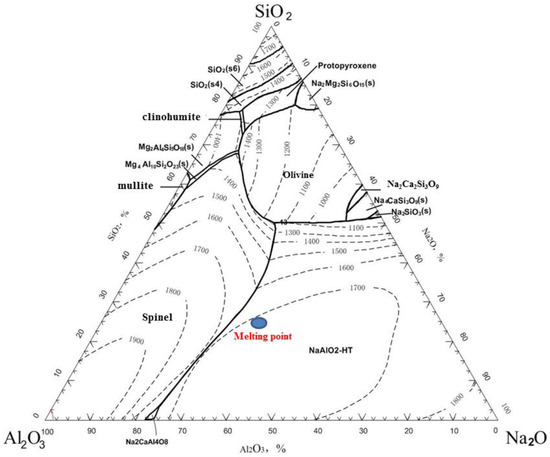
Figure 3.
Phase diagram of SiO2—Al2O3—Na2O with CaO = 1.75% and MgO = 15.61%.
Assuming a reduction smelting test with 100 kg of roasted briquette, the calculation for the charge materials is as follows:
The required carbon amount for reducing iron oxide is calculated based on the reaction: 3C + Fe2O3 = 2Fe + 3CO. The carbon amount required is
The required carbon amount for reducing zinc oxide is calculated based on the reaction: C + ZnO = Zn + CO. The required carbon amount is
The amount of carbon required to reduce 100 kg of roasted briquette is M1 + M2 = 19.48 kg. Assuming a coke utilization rate of 95%, the required amount of coke is = 23.96 kg.
The masses of SiO2, Al2O3, CaO, MgO and Na2O in 100 kg of roasted briquette are as follows:
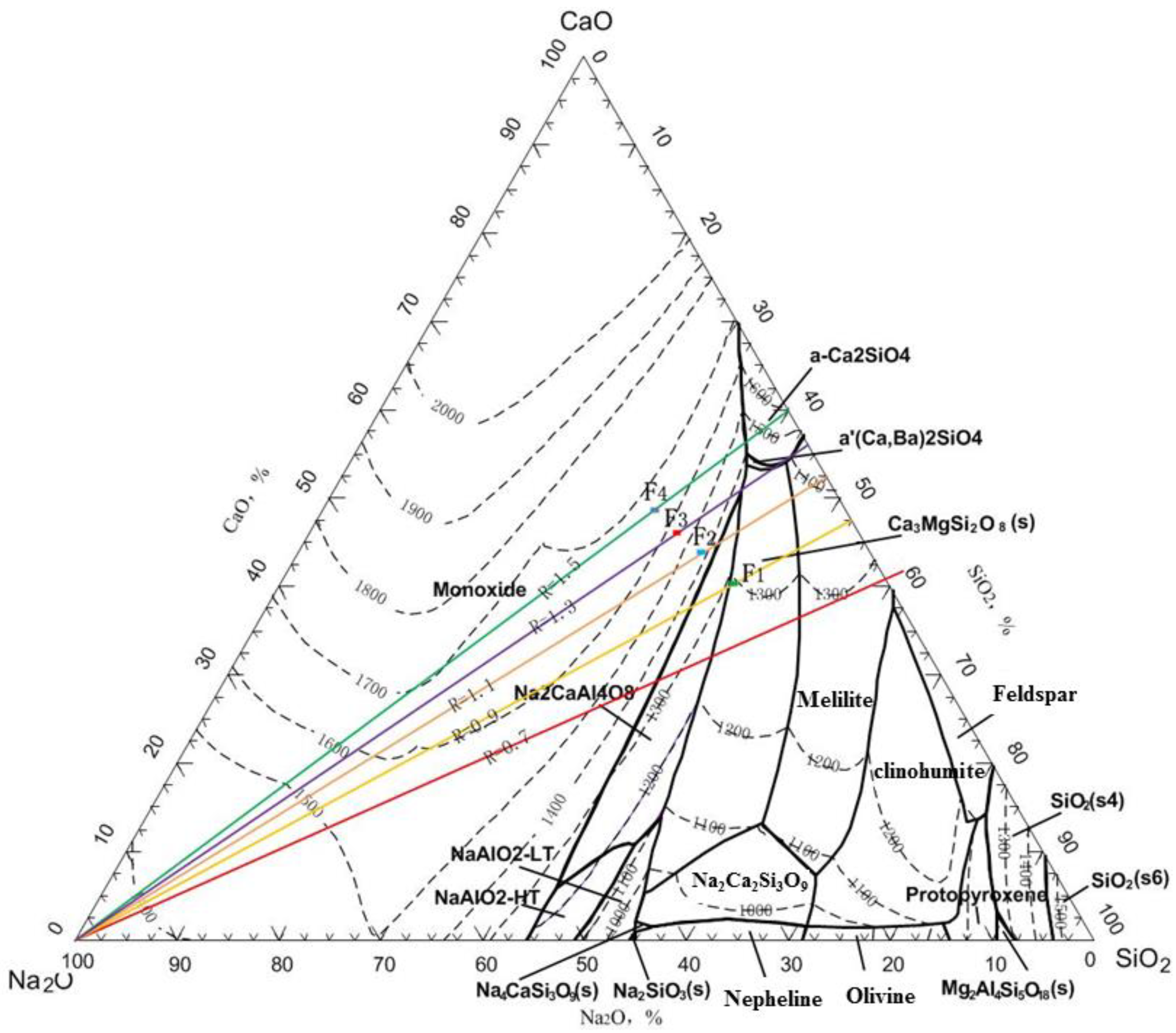
The typical high furnace slag contains 15% Al2O3, 8.0% MgO, and a slag basicity of 1.0–1.1 [28,29,30]. Therefore, the designed slag basicity is 1.1, Al2O3 content is 15.0%, MgO content is 8.0%, and the slag composition is 31.55% CaO, 28.62% SiO2, 15.0% Al2O3, 8.0% MgO, and 16.83% Na2O. According to the CaO-SiO2-Na2O-Al2O3-MgO five-component slag phase diagram, the melting point of the slag composition is 1450–1500 °C (see point F2 in Figure 14). Therefore, the additional mass of SiO2, Al2O3, CaO, and MgO fluxes added to 100 kg of roasted briquette (considering the SiO2, Al2O3, CaO, and MgO brought in by the coke ash) is as follows: MSiO2 = 2.76 kg, MAl2O3 = 0.31 kg, MCaO = 4.2 kg, MMgO = 0.11 kg.
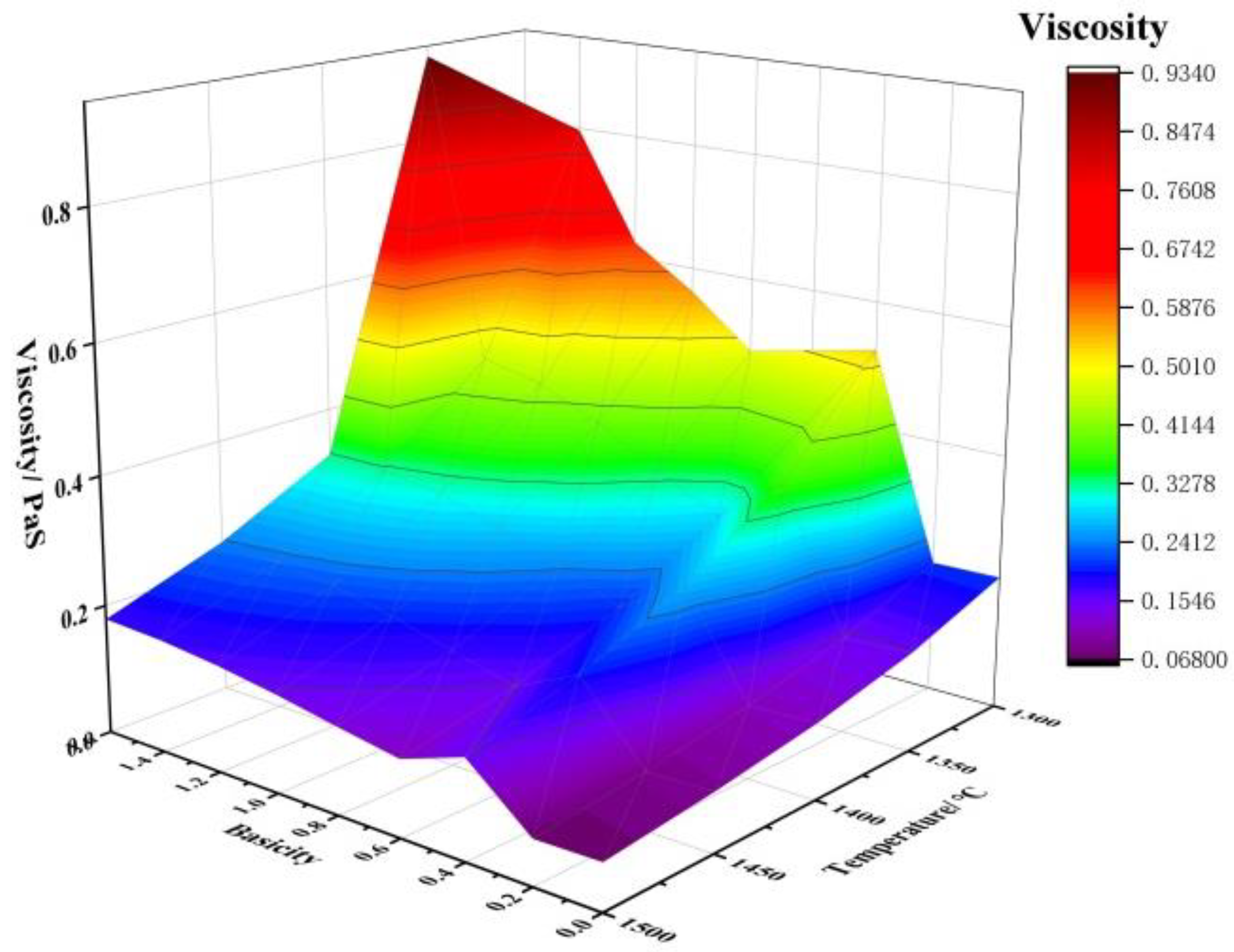
Apart from the melting point, viscosity is an important property of slag, which affects whether the smelting process can proceed smoothly and whether the metal can adequately settle and separate through the slag layer [31]. Therefore, the smelting process requires slag with appropriate viscosity. The liquid phase amount and viscosity of the initial slag during the smelting separation of JR briquettes at 1300–1500 °C under different basicities were calculated using the Equilib and Viscosity modules of the thermodynamic software FactSage 8.0. The Viscosity module in the software applies to fully liquid phases, whereas the initial slag at lower temperatures is not entirely liquid, necessitating correction using the Einstein–Roscoe equation, as shown below [8]:
where
η = η0(1 − c)−2.5
η—viscosity of the slag, Pa∙S;
η0—viscosity of the slag in a fully liquid phase, Pa∙S;
c—solid phase content, %.
The changes in the liquid phase amount and viscosity of the initial slag during the smelting separation of JR briquettes at 1300–1500 °C under different basicities are shown in Figure 4. As seen in Figure 4, with the increase in basicity and temperature, the slag viscosity generally shows a downward trend. Therefore, to achieve suitable fluidity, the temperature and basicity can be appropriately increased.
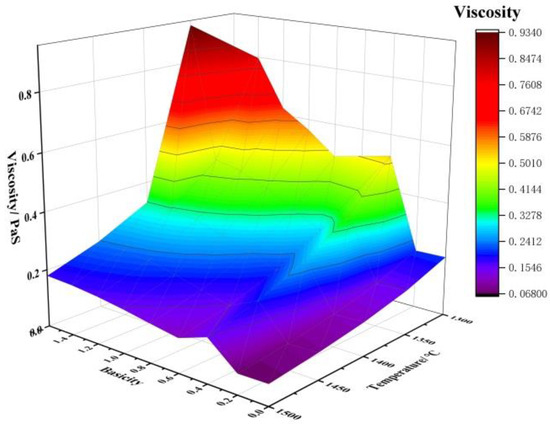
Figure 4.
Effect of basicity and temperature on viscosity of slag.
3.1.2. Thermodynamic Analysis of the Reduction of Roasted JR
The standard Gibbs free energy of a chemical reaction serves as the criterion for determining whether a reaction can proceed. When ΔrGΘm = 0, the reaction reaches equilibrium; when ΔrGΘm < 0, the reaction proceeds in the forward direction; and when ΔrGΘm > 0, the reaction proceeds in the reverse direction. In this study, the calculation of the standard Gibbs free energy for the relevant chemical reactions was performed using the FactSage software.
The roasted product of JR primarily contains hematite and zinc ferrite as the main phases, with the rare metals indium and gallium existing in oxide forms. Table 4 presents the melting and boiling points of Fe, Zn, In, and gallium oxides and their elemental forms. It can be observed that metallic iron and its oxides have higher melting and boiling points, while Zn, In, and Ga have lower melting points. Zinc is volatile, indium has a relatively low vapor pressure and is difficult to volatilize, and gallium has an extremely low vapor pressure, also making it difficult to volatilize. Through thermodynamic analysis, the possibility of reducing the oxides of iron, zinc, indium, and gallium in the roasted JR is assessed, as well as the forms of the reduction products, to reveal the reduction behavior of each metal oxide during the reduction process of the roasted JR.

Table 4.
The material melting boiling point of roasted ore.
(1) Reduction behavior of Fe
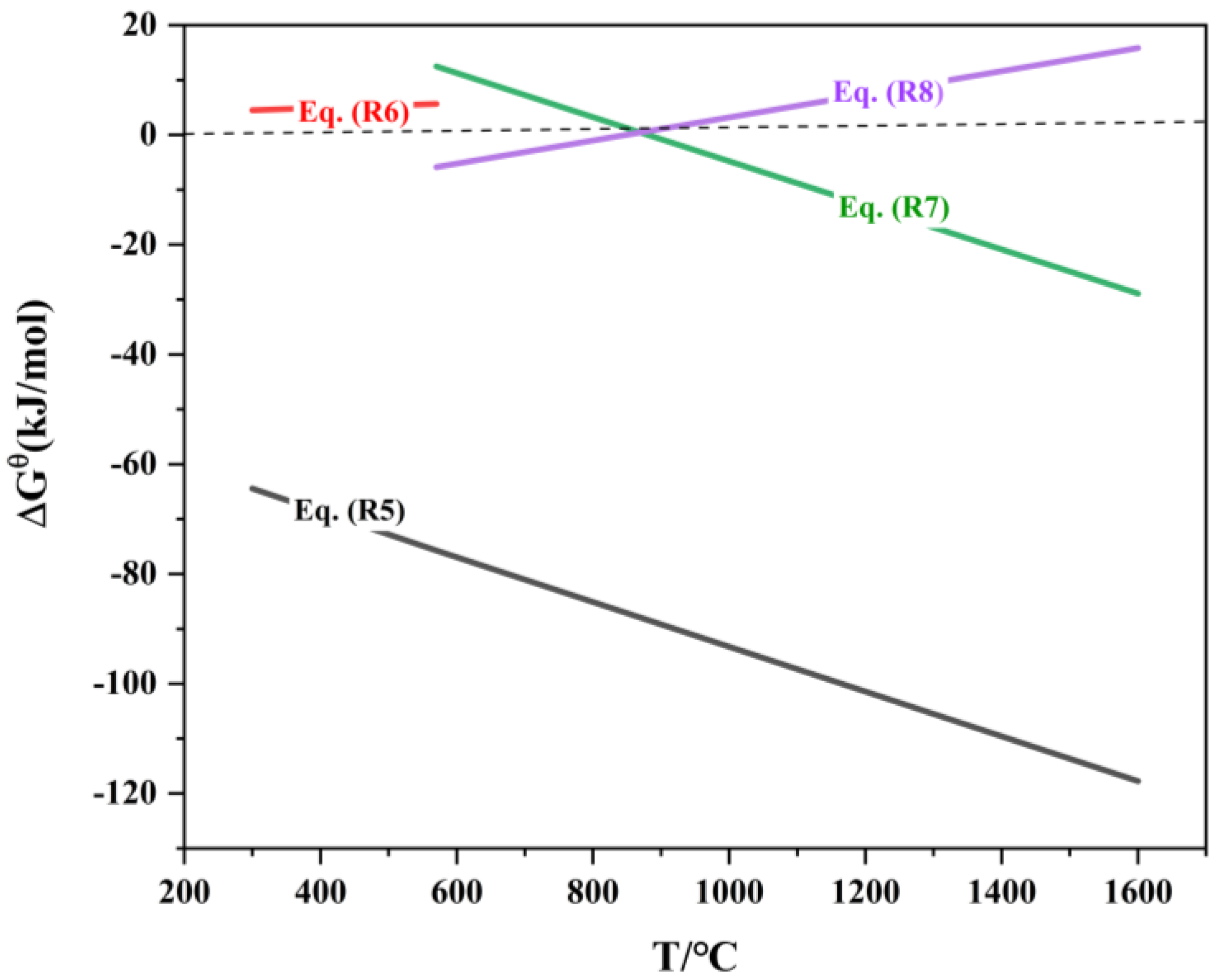
The reduction of iron oxides follows the principle of stepwise reduction from higher-valent oxides to lower-valent oxides. In a coal-based direct reduction system, the relationship between the standard Gibbs free energy of the iron oxide reduction reaction and temperature is shown in Table 5 and Figure 5.

Table 5.
Reactions and corresponding Iron oxide.
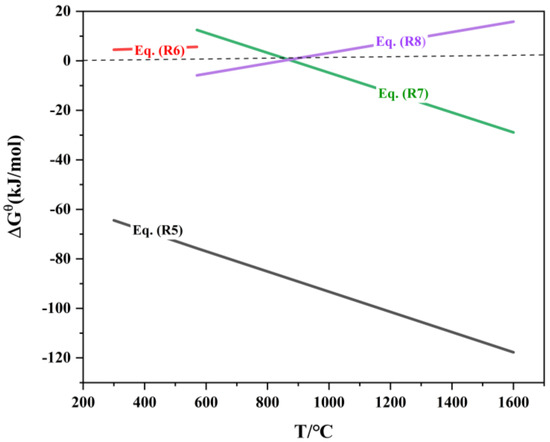
Figure 5.
ΔrGΘm-T of reduction reaction of iron oxide.
The relationship between the equilibrium constant of the above reaction and the gaseous equilibrium components is expressed as
ΔrGΘm = −RTlnKΘ KΘ = PCO2/PCO PCO = 1/(1 + KΘ)
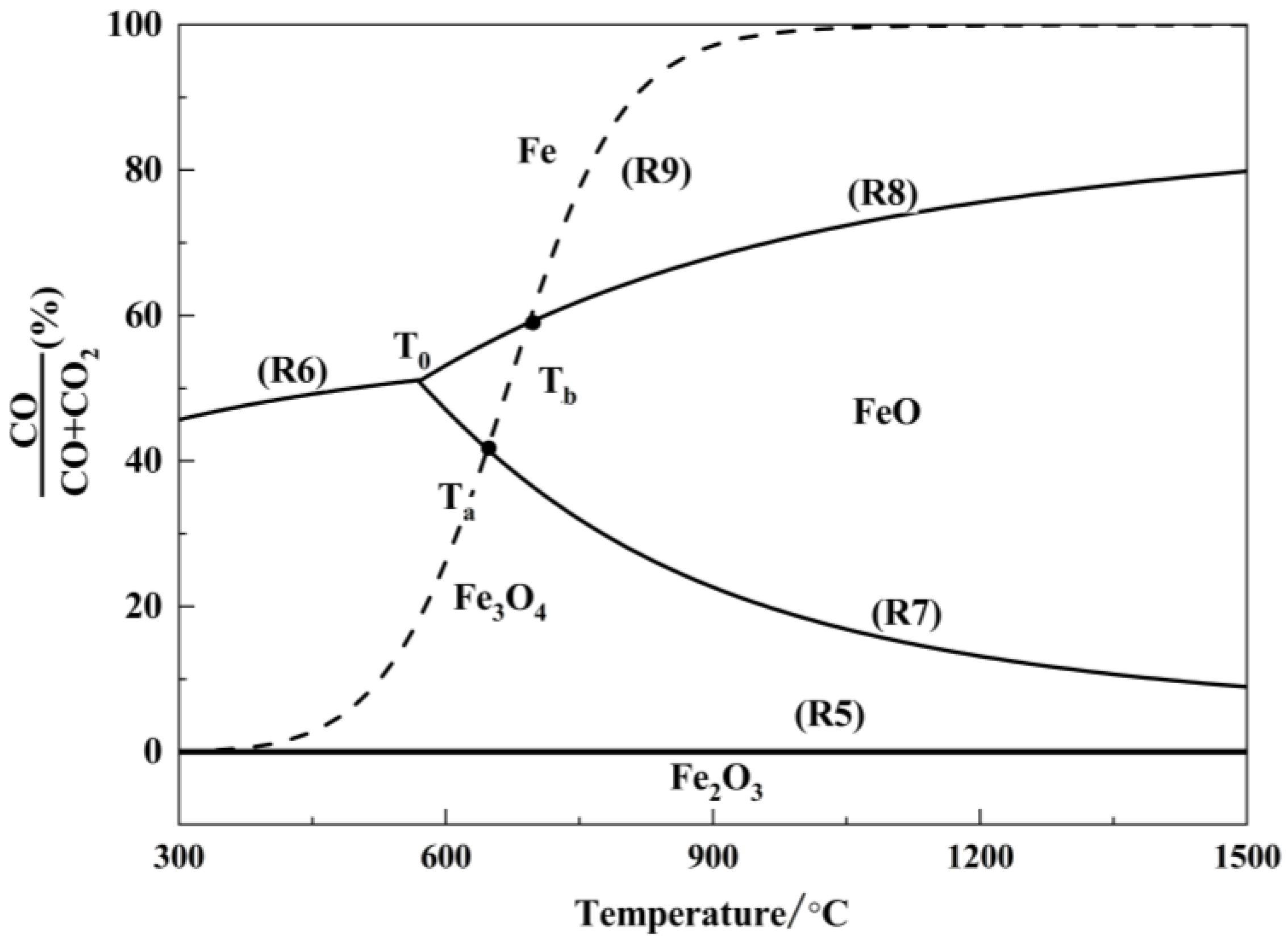
Using Equations (R10), the equilibrium gaseous composition of reactions (R5) to (R9) as a function of temperature is calculated and plotted in Figure 6, alongside the equilibrium composition of the carbon gasification reaction.
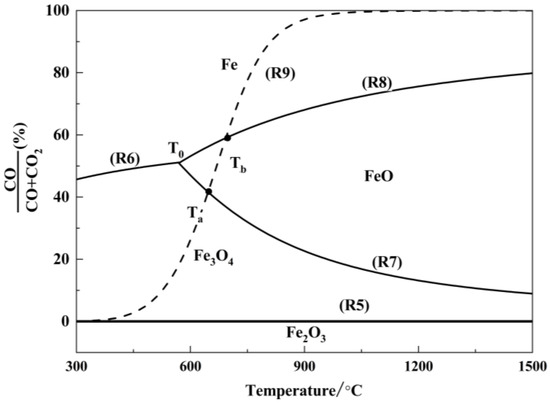
Figure 6.
Restore balance of iron oxides.
As shown in Figure 6, the equilibrium curve for the reaction between Fe2O3 and CO is close to the x-axis, indicating that the equilibrium CO concentration, φ(CO), is approximately 0. This implies that even trace amounts of CO in the system can reduce Fe2O3 to Fe3O4. The equilibrium curve of the carbon gasification reaction intersects with the equilibrium curves of the indirect reduction reactions of Fe3O4 and FeO at points Ta (683 °C) and Tb (718 °C), respectively. When T < Ta, the CO concentration in the system is lower than the equilibrium CO concentration for the indirect reduction reactions of Fe3O4 and FeO, and Fe3O4 is the stable phase in the equilibrium system. Within the temperature range Ta < T < Tb, the CO concentration in the system exceeds the equilibrium CO concentration for the indirect reduction reaction of Fe3O4, and FeO becomes the stable phase in the equilibrium system. When T > Tb, the CO concentration in the system is higher than the equilibrium CO concentration for the indirect reduction reaction of FeO, and metallic Fe becomes the stable phase in the equilibrium system. Thus, to ensure the reduction of high-valence iron oxides to metallic iron, it is theoretically sufficient to provide an adequate amount of reductant and maintain the reaction system at a temperature above 718 °C.
(2) Reduction behavior of Zn
The melting and boiling points of zinc are 420 °C and 907 °C, respectively, while ZnO has higher melting and boiling points of 1975 °C and 2360 °C. The relationship between the standard Gibbs free energy of the zinc oxide reduction reaction and temperature is shown in Table 6 and Figure 7. From the ΔrGΘm expression of reaction (R11), it is evident that the reaction can spontaneously occur at a minimum temperature of 348 °C. Within the temperature range of 420–907 °C, if ZnO is reduced to Zn, the metallic zinc will exist in a liquid state, with partial volatilization occurring as zinc vapor. When the reduction temperature exceeds 907 °C, the reduced metallic zinc will exist in a gaseous state.

Table 6.
Reactions and corresponding Zinc oxide.
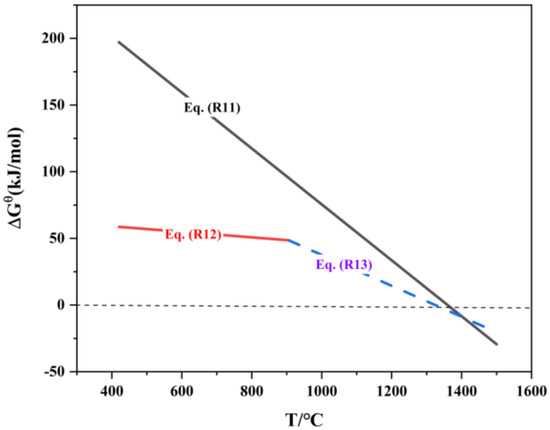
Figure 7.
ΔrGΘm-T of reduction reaction of zinc oxide.
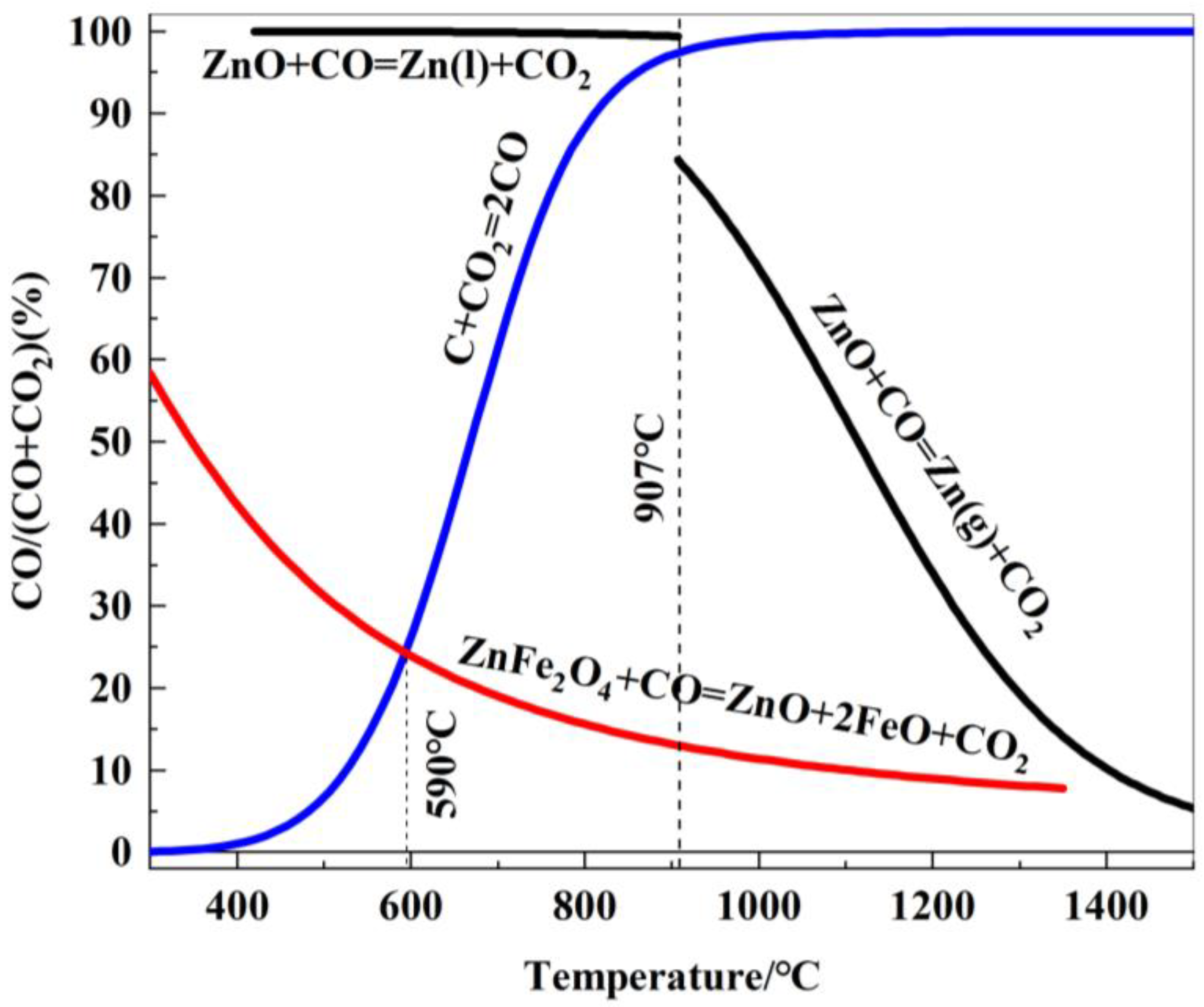
The relationship between the gaseous equilibrium composition and temperature for the carbon gasification reaction and the various related reduction reactions of zinc ferrite is plotted in Figure 8.
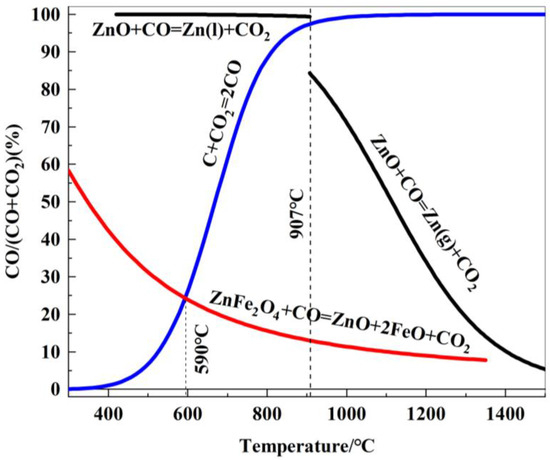
Figure 8.
Relationship between gas equilibrium composition and reduction temperature of zinc oxides reduced by carbon.
As shown in Figure 8, zinc ferrite is difficult to reduce at temperatures below 590 °C. When the reduction temperature exceeds 590 °C, zinc ferrite is reduced to ZnO and FeO, and the reduction process becomes increasingly favorable as the temperature rises. Below 907 °C (the boiling point of zinc), ZnO cannot be reduced by CO. However, at temperatures above 907 °C, ZnO is reduced by CO to form gaseous metallic zinc, and the reduction becomes more favorable with increasing temperature.
(3) Reduction behavior of In
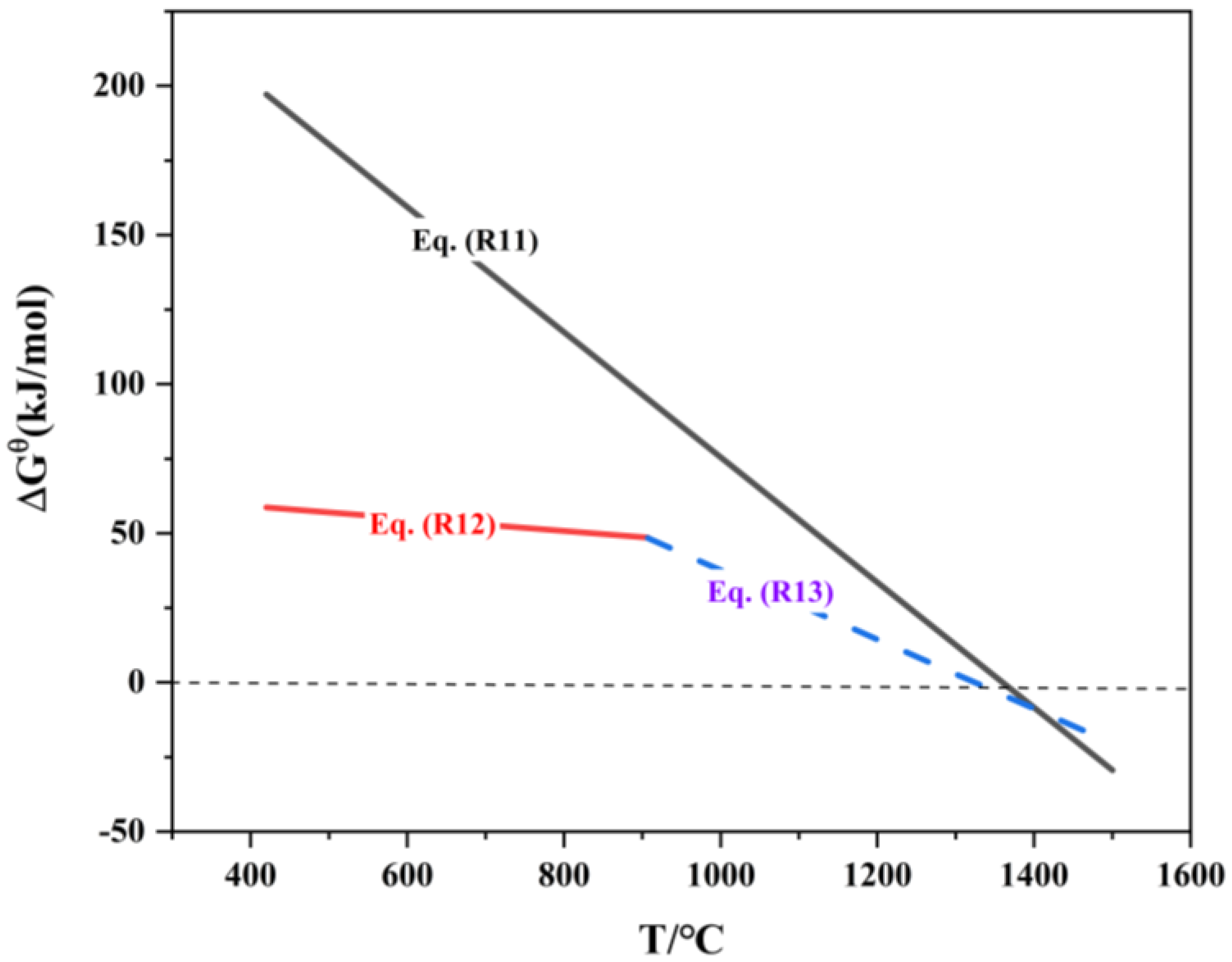
After high-temperature roasting of JR, indium exists in the form of In2O3. Given the low indium content in the roasted ore and the fact that carbon can only contact the surface of the briquette during the reduction process, In2O3 is mainly reduced through indirect reduction reactions. The relationship between the standard Gibbs free energy of the indium oxide reduction reaction and temperature is shown in Table 7.

Table 7.
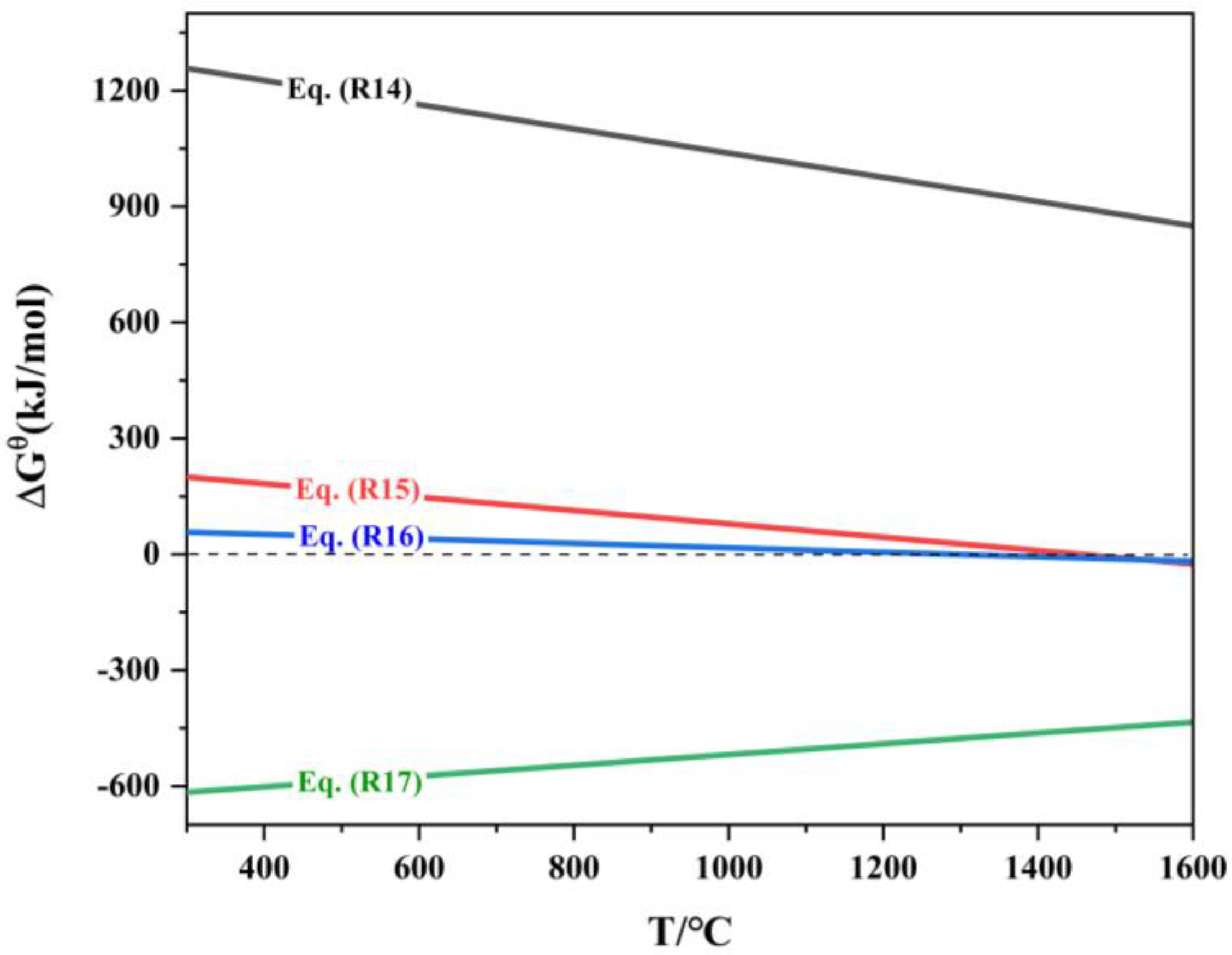
Reactions and corresponding Indium oxide.
The thermodynamic diagram plotted based on reactions (R14) to (R17) is shown in Figure 9. Analysis of Figure 9 indicates that the Gibbs free energy ΔrGΘm for reaction (R14) is greater than 0, meaning that this reaction cannot proceed in the forward direction. The minimum temperatures for the spontaneous forward reactions (R15) and (R16) are 1197 °C and 1020 °C, respectively. Therefore, during the reduction process, In2O3 is reduced by CO to form In2O(g) and In(l). Studies have pointed out [32] that In2O(g) can volatilize significantly at temperatures above 800 °C, while indium itself has a relatively low vapor pressure and is difficult to volatilize, with a vapor pressure of only 106.66 Pa at 1200 °C. However, it can be easily carried by zinc vapor into the flue gas collection system. The above analysis shows that when the reduction temperature exceeds 907 °C, ZnO is easily reduced to form zinc vapor, which facilitates the volatilization of indium.
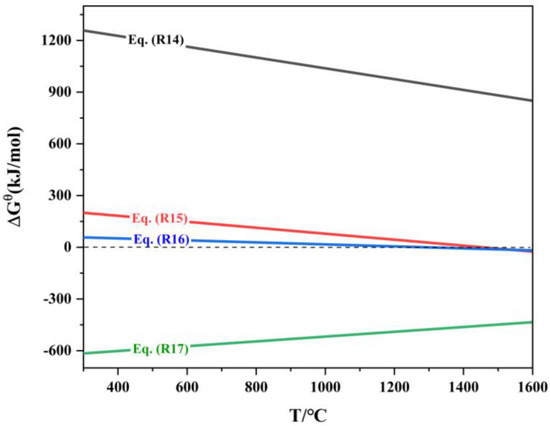
Figure 9.
ΔrGΘm-T of reduction reaction of indium oxide.
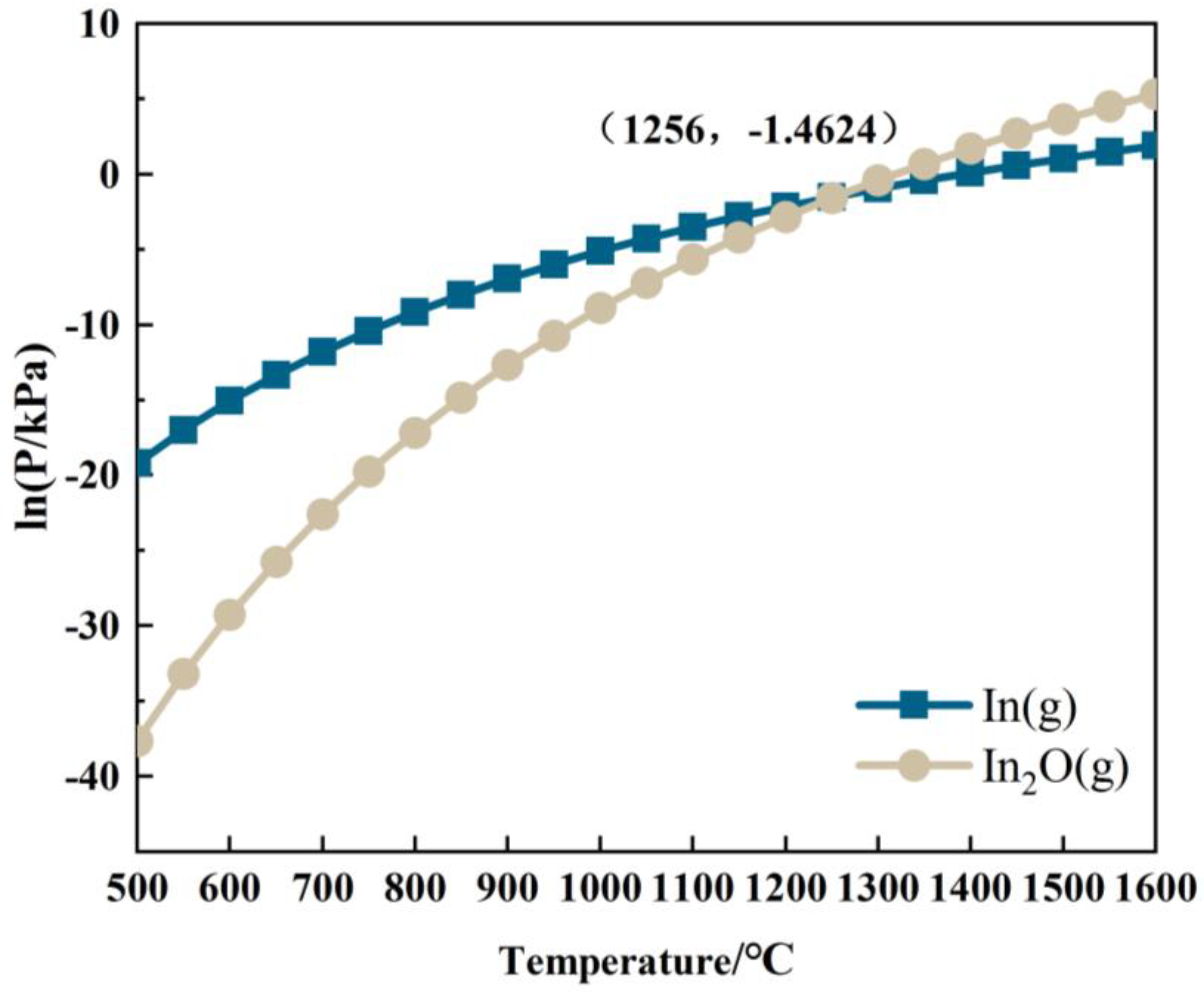
The relationship between the partial pressures of In(g) and In2O(g) and temperature in the In2O3 reduction system is expressed in Equations (R18) and (R19) [33].
lnP In(g) = −27,723.1/T + 16.669
lnP In2O(g) = −56,548.0/T + 35.521
Based on Equations (R18) and (R19), the relationship between the partial pressures of In(g) and In2O(g) with temperature in the In-C-O system is shown in Figure 10. As depicted in Figure 10, the partial pressures of In(g) and In2O(g) gradually increase with rising temperature. When the temperature is below 1256 °C, the partial pressure of In(g) exceeds that of In2O(g), and the main reduction product of In2O3 in the system is In(g). Conversely, at temperatures above 1256 °C, the main reduction product of In2O3 shifts to In2O(g).
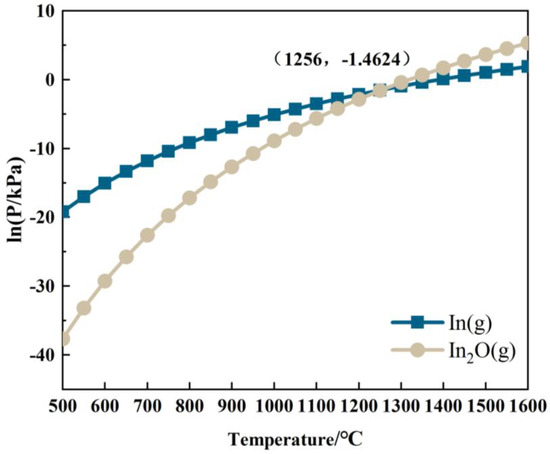
Figure 10.
Curves of lnP In(g)-T and lnP In2O(g)-T(4) reduction behavior of Ga.
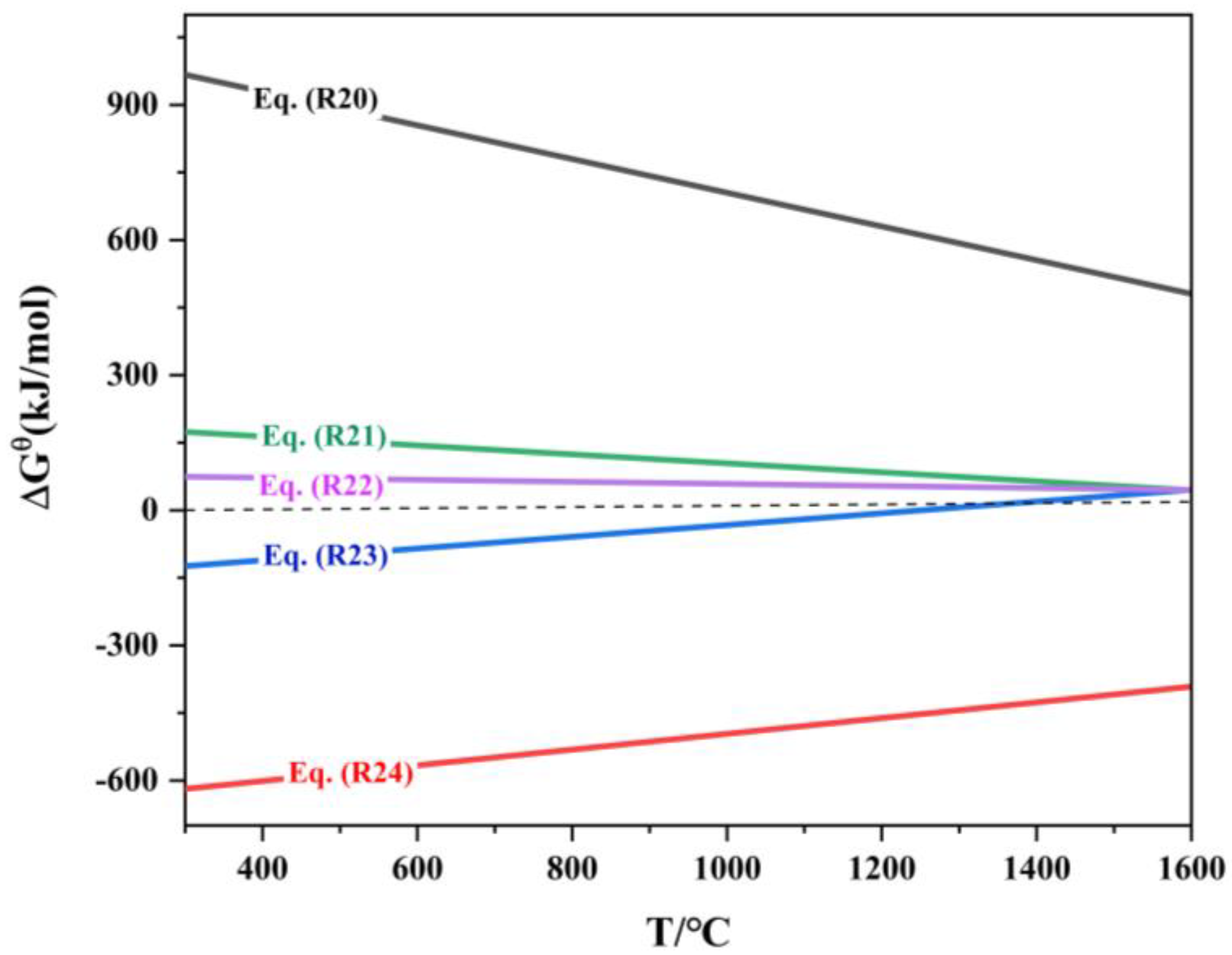
Gallium in the roasted jarosite briquette exists in the form of oxides, and during the reduction process, carbon can only contact the surface of the briquette. The reduction of gallium oxides primarily occurs through indirect reduction by CO. The order of reducibility of gallium oxides, from easiest to most difficult, is as follows [7]: Ga2O3 → GaO → Ga2O → Ga. The relationship between the standard Gibbs free energy of the indium oxide reduction reaction and temperature is shown in Table 8 and Figure 11.

Table 8.
Reactions and corresponding gallium oxide.
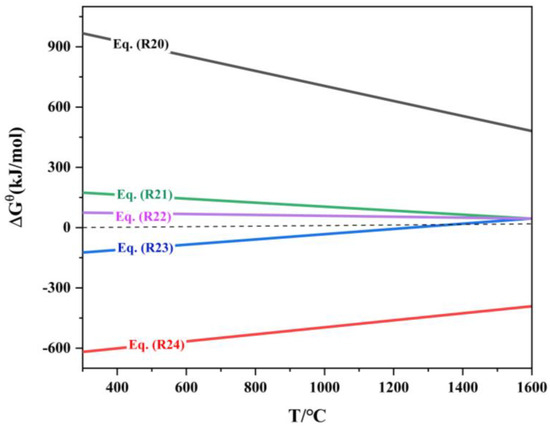
Figure 11.
ΔrGΘm-T of reduction reaction of gallium oxide.
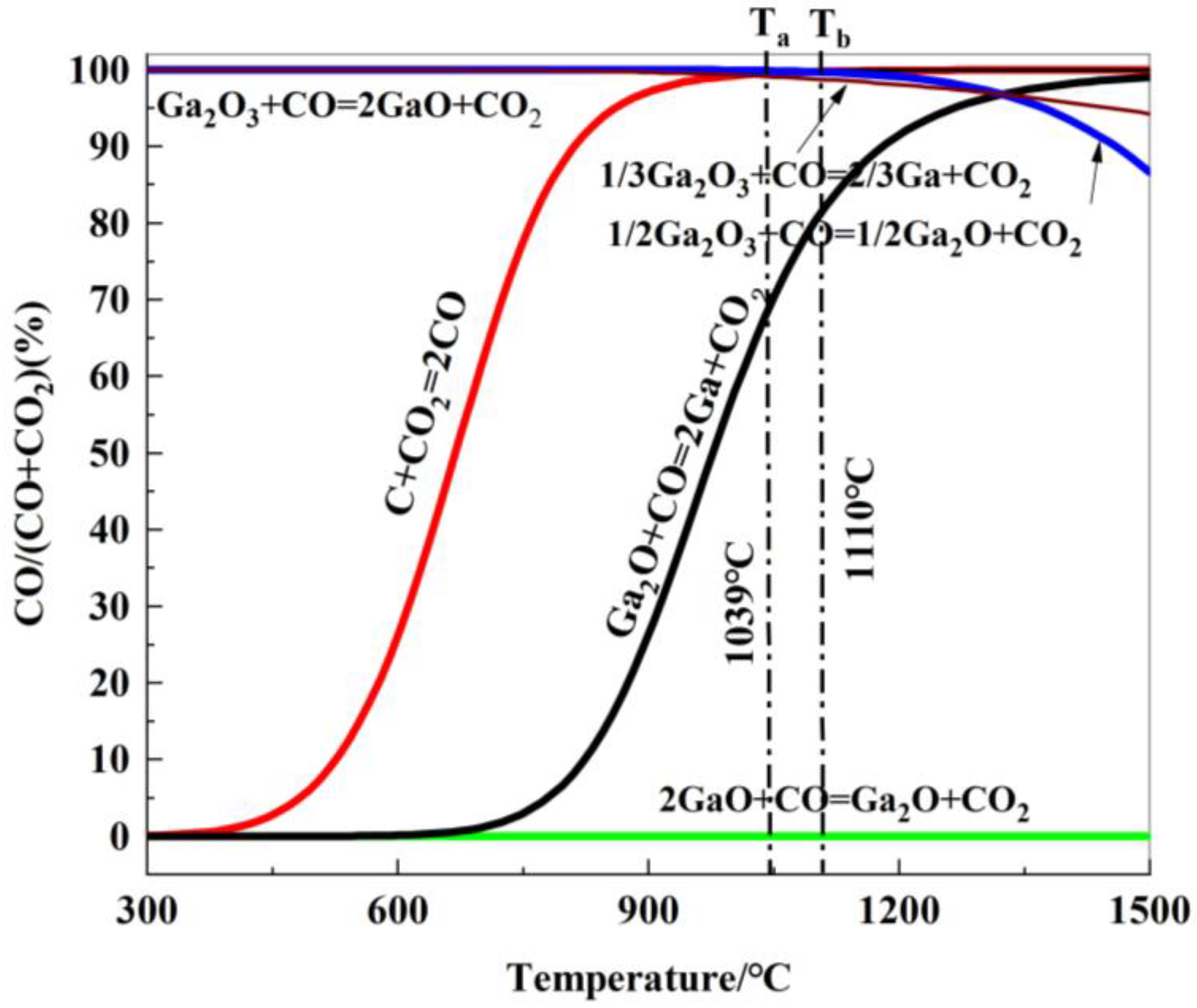
Based on the thermodynamic calculations for reactions (R20) to (R24), the relationship between the equilibrium gaseous composition of the carbon gasification reaction and the reduction reaction of gallium oxide with temperature is shown in Figure 12.
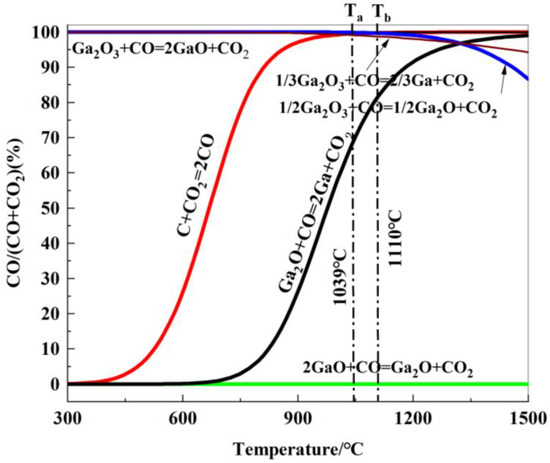
Figure 12.
Relationship between gas equilibrium composition and reduction temperature of gallium oxides reduced by carbon.
As seen in Figure 12, the equilibrium curve for the reaction between Ga2O3 and CO is close to the 100-axis, indicating that the equilibrium CO concentration, φ(CO), is approximately 100. This means that Ga2O3 cannot be reduced by CO to form GaO. The equilibrium curve for the carbon gasification reaction intersects with the indirect reduction reaction curves for the formation of Ga and Ga2O from Ga2O3 at points Ta (1039 °C) and Tb (1110 °C), respectively. The equilibrium curve for the reduction of Ga2O by CO lies below the equilibrium curve for the carbon gasification reaction, suggesting that Ga2O can be reduced to Ga.
In conclusion, when T > 1039 °C, gallium oxide is reduced by CO to form liquid metallic gallium, and the reduction reaction becomes increasingly favorable as the temperature rises.
From the thermodynamic analysis of carbon reduction of Fe, Zn, In, and Ga roasted jarosite briquette, it is evident that conducting direct reduction experiments on roasted jarosite briquette can result in the reduction and volatilization of Zn and In, which become concentrated in the flue dust, while Fe and Ga are reduced and concentrated in the pig iron.
3.2. Study on the Reductive Smelting Process of JR
3.2.1. Study on the Reduction Smelting Process of JR
A single-factor experimental method was used to investigate the effects of three smelting parameters—smelting temperature, smelting time, and slag basicity—on the results of reductive smelting.
3.2.2. The Effect of Smelting Temperature on Smelting Performance
The experimental conditions were smelting time of 40 min and slag basicity R(CaO/SiO2) = 1.1, with slag containing 15.0% Al2O3 and 8.0% MgO. The impact of smelting temperature on the recovery indicators of valuable metals is shown in Table 9. At 1450 °C, the slag and iron did not fully separate due to insufficient heat in the system, which prevented the complete melting of the charge and resulted in high slag viscosity, hindering the separation of slag and iron. When the temperature was increased to 1500 °C and 1550 °C, slag and iron separation was achieved. At 1500 °C, the Fe and Ga contents in the hot metal were 90.28% and 342 g/t, respectively, with recovery rates of 90.49% for Fe and 89.05% for Ga. The hot metal yield was 57.28%, and the volatilization rates of Zn and In were 99.78% and 82.84%, respectively. At 1550 °C, the Fe and Ga contents in the hot metal increased to 92.93% and 355 g/t, while the recovery rates of Fe and Ga and the volatilization rates of Zn and In remained essentially unchanged, but the hot metal yield decreased to 55.66%. Considering all factors, a smelting temperature of 1550 °C was selected. Generally, for the smelting reduction process, extending the smelting time can facilitate the equilibrium of chemical reactions at the slag–iron interface, thereby improving metal recovery rates.

Table 9.
Effects of smelting temperature on production indexes.
3.2.3. The Effect of Smelting Time on Smelting Performance
The experimental conditions were smelting temperature of 1550 °C, slag basicity R(CaO/SiO2) = 1.1, with slag containing 15.0% Al2O3 and 8.0% MgO. The effect of smelting time on the recovery indicators of valuable metals is shown in Table 10. It can be seen that at a smelting time of 20 min, the materials were not fully melted, and slag and iron could not be completely separated. When the smelting time was extended to 40 min, slag and iron separation were achieved, with Fe and Ga grades in the hot metal at 92.93% and 355 g/t, and Fe and Ga recovery rates at 90.51% and 89.82%, respectively. The volatilization rates of Zn and In were 99.82% and 82.39%, and the hot metal yield was 55.66%. Further extending the smelting time to 60 and 80 min increased the Ga content in hot metal to 367 g/t, with Ga recovery exceeding 93.5%, while other smelting indicators showed little change. Therefore, an optimal smelting time of 60 min was selected.

Table 10.
Effects of smelting time on production indexes.
3.2.4. The Effect of Slag Basicity on Smelting Performance
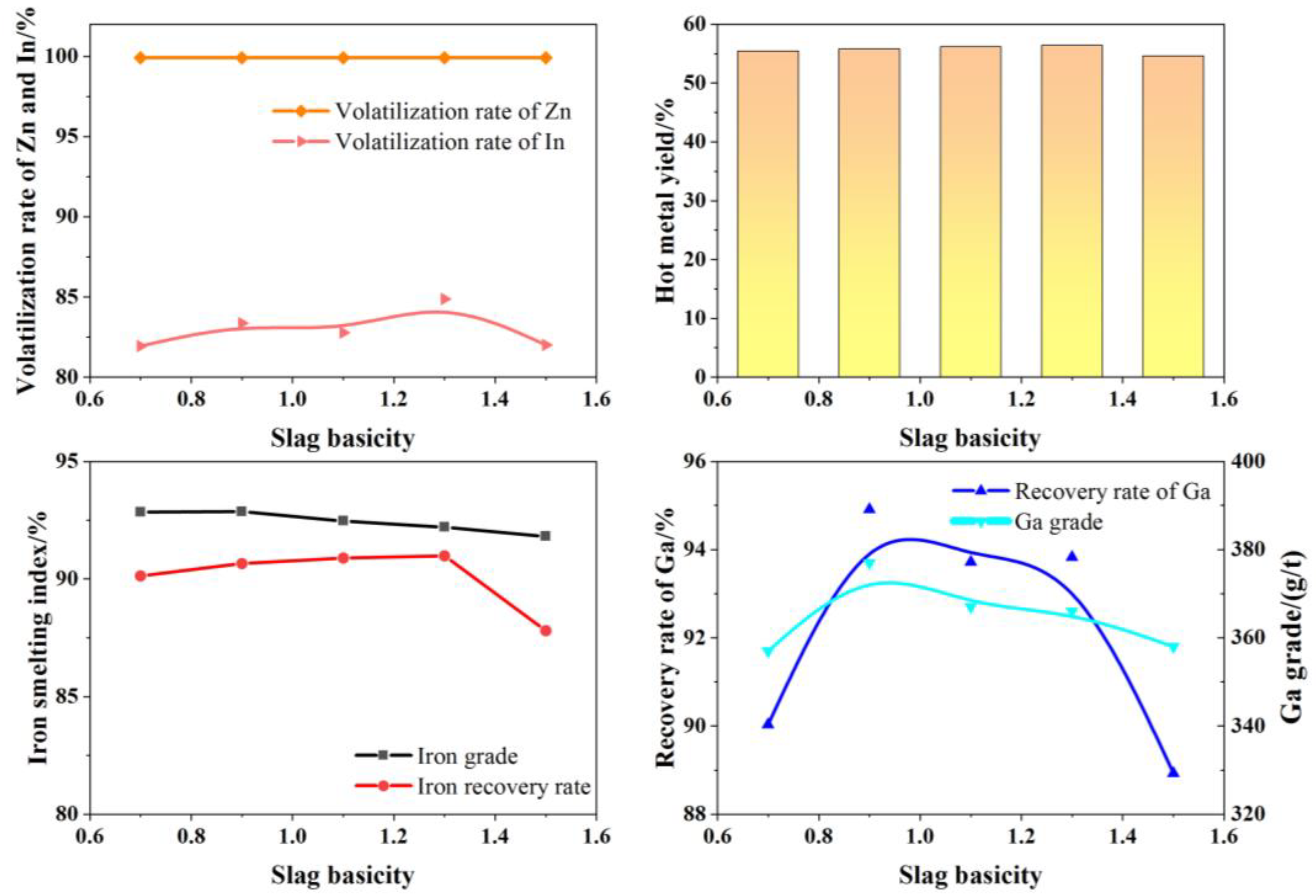
Under fixed experimental conditions—smelting temperature of 1550 °C, smelting time of 60 min, and slag composition with 15.0% Al2O3 and 8.0% MgO—the effect of slag basicity on smelting performance was investigated. The results are shown in Figure 13.
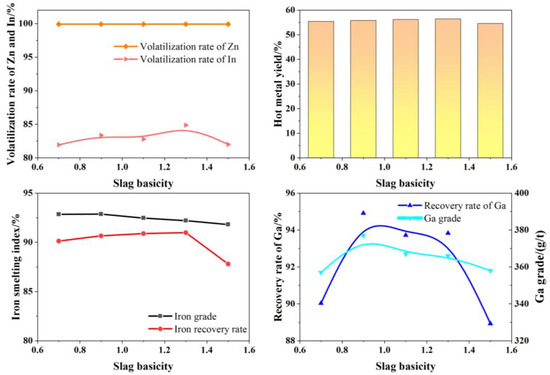
Figure 13.
Effects of basicity of slag on production indexes.
As shown in Figure 14, within a slag basicity range of 0.9–1.3, the basicity has little impact on the smelting indicators. The iron content in the hot metal ranges from 92.20% to 92.87%, with an iron recovery rate of 90.66% to 90.99%; the Ga content ranges from 366 g/t to 377 g/t, with a Ga recovery rate of 93.72% to 95.61%; the Zn volatilization rate is 99.90%, and the In volatilization rate ranges from 82.77% to 84.87%. When the slag basicity is further increased to 1.5, the recovery indicators for valuable metals show a decline. Within an appropriate range of basicity, the slag exhibits good fluidity, facilitating the settling and separation of iron and gallium from the slag. When the slag basicity is within 0.9–1.3, the slag melting point is low (points F1, F2, and F3 in Figure 14). When the slag basicity exceeds 1.3, the melting point becomes too high (point F4 in Figure 14), reducing slag overheating and increasing viscosity, which worsens the slag–iron separation. Considering all smelting indicators, the optimal slag basicity is 0.9.
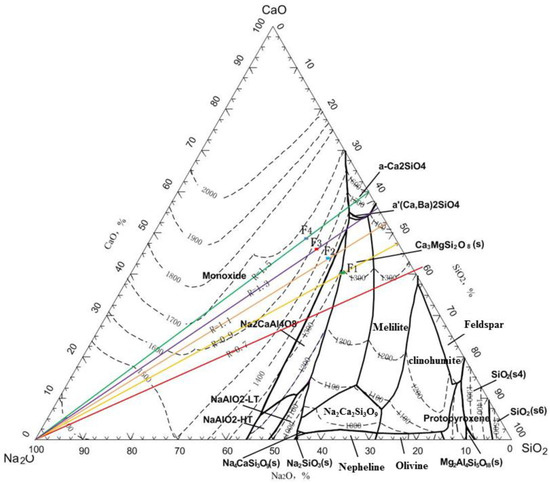
Figure 14.
Phase diagram of CaO—SiO2—Na2O with Al2O3 = 15.0% and MgO = 8.0%.
In summary, the optimal smelting conditions are a smelting temperature of 1550 °C, smelting time of 60 min, and slag basicity of 0.9, with Al2O3 content of 15.0% and MgO content of 8.0% in the slag. Under these conditions, the iron content in the hot metal is 92.87%, with an iron recovery rate of 90.66%; Ga content is 377 g/t, with a Ga recovery rate of 94.91%; the volatilization rates of Zn and In are 99.91% and 83.36%, respectively; and the hot metal yield is 55.79%. The chemical analysis results of the hot metal and slag are shown in Table 11 and Table 12.

Table 11.
Chemical composition of gallium pig iron/wt.%.

Table 12.
Chemical composition of slag/wt.%.
3.3. Economic Benefit Analysis of Valuable Metal Recovery
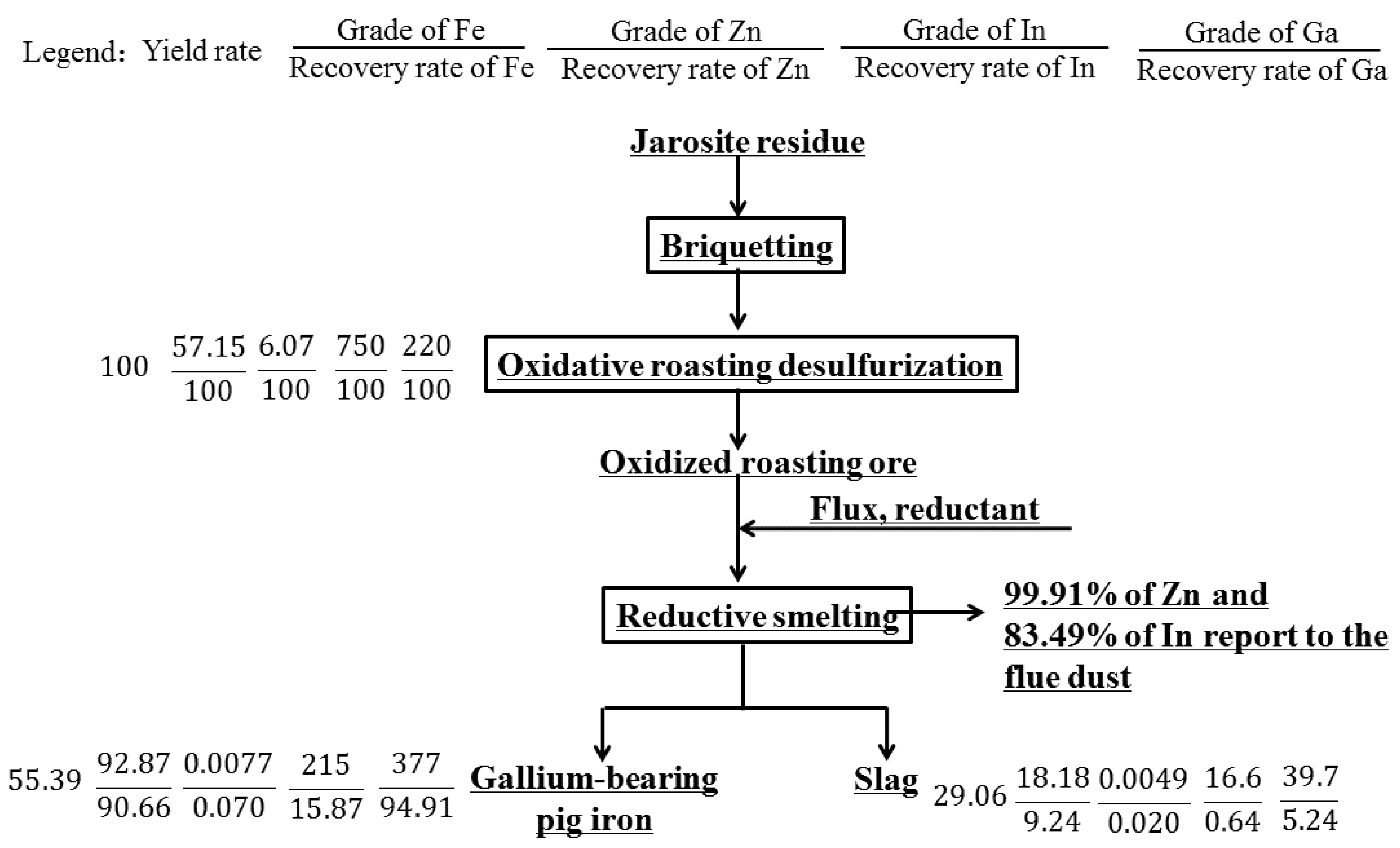
After conducting economic and technical analysis, this process achieves a comprehensive recovery and utilization of valuable metals such as gallium, iron, zinc, and indium, with good economic benefits and promising prospects for promotion. The theoretical metal balance diagram is shown in Figure 15, from which the economic benefits of valuable metal recovery from jarosite residue can be estimated.
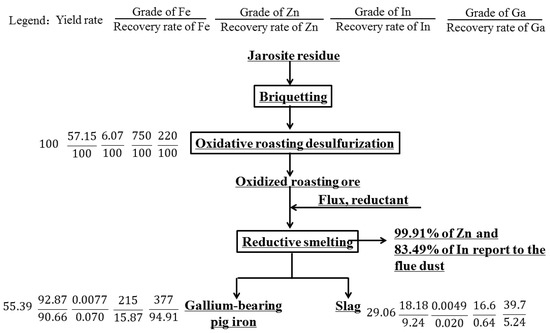
Figure 15.
Elements balance in the full flowsheet of processing jarosite residues.
Ga is used in the manufacturing of the semiconductor gallium nitride, gallium arsenide, gallium phosphide, and germanium semiconductor dopants; pure gallium and low-melting alloys can serve as heat exchange media for nuclear reactions and as filling materials for high-temperature thermometers. If this process is applied to an annual production of 100 wt jarosite residue, it is estimated that 2 tons of gallium can be recovered, with an estimated economic benefit of approximately CNY 36 million.
Fe, as a key raw material in industry, is widely used in the fields of machinery, metallurgy, chemicals, and aerospace materials. If this process is applied to an annual production of 100 wt jarosite residue, it is estimated that 47 wt of iron can be recovered, with an estimated economic benefit of CNY 1.5 billion.
Zn is an important industrial metal material, primarily used in the production of hot-dip galvanized steel sheets, galvanized steel pipes, alloys, electronic components, and chemical raw materials. It has excellent corrosion resistance and electrical conductivity, making it widely used in industries such as steel, construction, electronics, and metallurgy. If this process is applied to an annual production of 100 wt jarosite residue, it is estimated that 6 wt of zinc can be recovered, with an estimated economic benefit of CNY 1.3 billion.
In, due to its strong optical permeability and conductivity, is primarily used in the production of ITO targets (used for manufacturing liquid crystal displays and flat-panel screens) and can also be applied in the electronics semiconductor field. If this process is applied to an annual production of 100 wt jarosite residue, it is estimated that 63 tons of indium can be recovered, with an estimated economic benefit of CNY 900 million.
4. Conclusions
1. Increasing the smelting temperature can increase the superheating degree of slag, reduce slag viscosity, facilitate the settlement and aggregation of metal particles, and reduce mechanical inclusion losses. Extending the smelting time helps promote the slag–iron interface reaction towards an equilibrium, thereby improving the metal recovery rate. Within an appropriate range of basicity, the good fluidity of the slag favors the separation of iron and gallium from the slag.
2. The optimal process conditions for the desulfurization roasting and reduction smelting of JR are a coke powder ratio of 23.96%, a smelting temperature of 1550 °C, a smelting time of 60 min, and a slag basicity of 0.9, where the slag contains 15.0% Al2O3 and 8.0% MgO. Under these conditions, the pig iron achieved an iron grade of 92.87%, with an iron recovery rate of 90.66%; the gallium grade was 377 g/t, with a gallium recovery rate of 94.91%; and the volatilization rates of Zn and In were 99.91% and 83.36%, respectively.
3. Through economic and technical analysis, this process realizes the comprehensive recovery and utilization of valuable metals such as gallium, iron, zinc, and indium, showing promising economic benefits and prospects for promotion. For an annual production of 100 wt JR, this process can recover 2 tons of Ga, 470,000 tons of Fe, 60,000 tons of Zn, and 63 tons of In, with an estimated total economic benefit of CNY 4.7 billion.
Author Contributions
Conceptualization, Q.Z.; methodology, Q.Z., Z.G. and C.Y.; software, Q.Z.; validation, D.Z., J.P., Z.G., S.L. and C.Y.; formal analysis, Q.Z., S.L. and X.X.; investigation, Q.Z. resources, Z.G., S.L. and C.Y.; data curation, Q.Z.; writing—original draft preparation, Q.Z.; writing—review and editing, J.P. and X.X.; visualization, J.P.; supervision, D.Z.; project administration, J.P. and D.Z.; funding acquisition, J.P. and D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (No. 52004339), the Key Research and Development Project of Hunan Province, China (No. 2022SK2075), and the China Baowu Low Carbon Metallurgy Innovation Foudation (BWLCF202216).
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to some information that could compromise the privacy of research participants.
Acknowledgments
The authors would like to acknowledge the Analytical and Testing Center of Central South University which supplied the facilities for the measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gao, L.; Dai, Z.; Zhang, K.; Liu, Z.; Dong, Y. Study on Roasting and Acid Leaching of Ammonium Jarosite Residue. Nonferrous Met. Extr. Metall. 2016, 10, 9–11+57. [Google Scholar]
- Cui, R.; Li, D.; Wang, G.; Li, X.; Zhao, J.; Wang, Z.; Gui, H. Thermal decomposition reaction and kinetic analysis of ammonium jarosite residues. Chin. J. Nonferrous Met. 2024, 34, 2452–2462. [Google Scholar]
- Zeng, Y.; Zhang, W.; Wu, J.; Wei, Y.; Ke, Y.; Shi, M.; Yan, X.; Lin, Z. Simultaneous recovery of Fe2O3 and PbCl2 from hazardous jarosite residues via hydrothermal phase transformation with NaCl. Hydrometallurgy 2023, 221, 106150. [Google Scholar] [CrossRef]
- Xie, S.; Liao, C.; Zhao, B. Kinetic and thermodynamic studies on lead-rich slag reduction at various CaO/SiO2 ratios. JOM 2022, 74, 3625–3633. [Google Scholar] [CrossRef]
- Steinlechner, S.; Höber, L. CO2-Optimized Recovery of Special Metals from Precipitation Residue by Selective Chlorination. Rare Met. Technol. 2022, 237–244. [Google Scholar]
- Hage, J.L.T.; Schuiling, R.D.; Vriend, S.P. Production of magnetite from sodiumjarosite under reducing hydrothermal conditions. The Reduction of FeIII to FeII with Cellulose. Can. Metall. Q. 1999, 38, 267–276. [Google Scholar] [CrossRef]
- Pappu, A.; Saxena, M.; Asolekar, S.R. Jarosite characteristics and its utilisation potentials. Sci. Total Environ. 2006, 359, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Rincón, J.M. Microstructural characterization of a goethite waste from zinc hydrometallurgical process. Mater. Lett. 1997, 31, 67–73. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, B.; Lu, X.; Dai, X. Oxygen-Enriched Enhanced Reduction Volatilization Smelting of Jarosite Residue and Leaching Toxicity of Smelting Final Slag. Nonferrous Met. Extr. Metall. 2022, 9, 150–155. [Google Scholar]
- Calla-Choque, D.; Lapidus, G.T. Acid decomposition and silver leaching with thiourea and oxalate from an industrial jarosite sample. Hydrometallurgy 2020, 192, 105289. [Google Scholar] [CrossRef]
- Palden, T.; Regadio, M.; Onghena, B.; Binnemans, K. Selective Metal Recovery from Jarosite Residue by Leaching with Acid-Equilibrated Ionic Liquids and Precipitation-Stripping. ACS Sustain. Chem. Eng. 2019, 7, 4239–4246. [Google Scholar] [CrossRef]
- Calla-Choque, D.; Nava-Alonso, F.; Fuentes-Aceituno, J.C. Acid decomposition and thiourea leaching of silver from hazardous jarosite residues: Effect of some cations on the stability of the thiourea system. J. Hazard. Mater. 2016, 317, 440–448. [Google Scholar] [CrossRef]
- Asokan, P.; Saxena, M.; Asolekar, S.R. Hazardous jarosite use in developing non-hazardous product for engineering application. J. Hazard. Mater. 2006, 137, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, Z.; Li, S.; Wang, Q.; Wang, D.; Li, H. Research Progress in Resource Utilization of Industrial Output Jarosite Residues. Mater. Rep. 2022, 36, 163–171. [Google Scholar]
- Ju, S.; Zhang, Y.; Zhang, Y.; Xue, P.; Wang, Y. Clean hydrometallurgical route to recover zinc, silver, lead, copper, cadmium and iron from hazardous jarosite residues produced during zinc hydrometallurgy. J. Hazard. Mater. 2011, 192, 554–558. [Google Scholar] [CrossRef]
- Kumar Singh, V.; Manna, S.; Kumar Biswas, J.; Pugazhendhi, A. Recovery of residual metals from jarosite waste using chemical and biochemical processes to achieve sustainability: A state-of-the-art review. J. Environ. Manag. 2023, 343, 118221. [Google Scholar] [CrossRef]
- Wu, J.; Chai, L.; Lin, Z.; Wei, Y.; Shi, M.; Peng, J.; Peng, N.; Yan, X. Fe(II)-induced transformation of Jarosite residues generated from zinc hydrometallurgy: Influence on metals behaviors during acid washing. Hydrometallurgy 2021, 200, 105523. [Google Scholar] [CrossRef]
- Zhu, D.; Yang, C.; Pan, J.; Guo, Z.; Li, S. New pyrometallurgical route for separation and recovery of Fe, Zn, In, Ga and S from jarosite residues. J. Clean. Prod. 2018, 205, 781–788. [Google Scholar] [CrossRef]
- Qin, S.; Wang, H.; Su, L. Comprehensive treatment and utilization of jarosite of nonferrous metallurgy. Min. Metall. 2018, 27, 110–113. [Google Scholar]
- Liu, C.; Ju, S.H.; Zhang, L.B.; Srinivasakannan, C.; Peng, J.H.; Le, T.Q.X.; Guo, Z.Y. Recovery of valuable metals from jarosite by sulphuric acid roasting using microwave and water leaching. Can. Metall. Q. 2017, 56, 1–9. [Google Scholar] [CrossRef]
- Mocellin, J.; Mercier, G.; Morel, J.L.; Blais, J.F.; Simonnot, M.O. Factors influencing the Zn and Mn extraction from pyrometallurgical sludge in the steel manufacturing industry. J. Environ. Manag. 2015, 158, 48–54. [Google Scholar] [CrossRef]
- Cao, X.E.; Hong, L.K.; Zhou, H.M.; Tang, X.F.; Yuan-Hong, Q.I. Thermal decomposition process of jarosite residue. Min. Metall. Eng. 2016, 36, 67–70. [Google Scholar]
- Desborough, G.A.; Smith, K.S.; Lowers, H.A.; Swayze, G.A.; Hammarstrom, J.M.; Diehl, S.F.; Leinz, R.W.; Driscoll, R.L. Mineralogical and chemical characteristics of some natural jarosites. Geochim. Cosmochim. Acta 2010, 74, 1041–1056. [Google Scholar] [CrossRef]
- Frost, R.; Wills, R.-A.; Kloprogge, J.; Martens, W. Thermal decomposition of ammonium jarosite (NH4)Fe3(SO4)2(OH)6. J. Therm. Anal. Calorim. 2006, 84, 489–496. [Google Scholar] [CrossRef]
- Rämä, M.; Nurmi, S.; Jokilaakso, A.; Klemettinen, L.; Taskinen, P.; Salminen, J. Thermal Processing of Jarosite Leach Residue for a Safe Disposable Slag and Valuable Metals Recovery. Metals 2018, 8, 744. [Google Scholar] [CrossRef]
- Picazo-Rodríguez, N.G.; Carrillo-Pedroza, F.R.; Soria-Aguilar, M.D.J.; Baltierra, G.; González, G.; Martinez-Luevanos, A.; Almaguer Guzmán, I. Use of Thermally Modified Jarosite for the Removal of Hexavalent Chromium by Adsorption. Crystals 2022, 12, 80. [Google Scholar] [CrossRef]
- Xu, C.; Xie, Q.; Xu, F.; Zhou, Y.; Wang, H.; Chen, T.; Peng, S. Preparation of Monoclinic Pyrrhotite by Thermal Decomposition of Jarosite Residues and Its Heavy Metal Removal Performance. Minerals 2021, 11, 267. [Google Scholar] [CrossRef]
- Xiong, Y.; Shu, W.; Dong, Z. Smelting Practice in No.6 BF of WISCO with High Al2O3 in slag. Ironmaking 2009, 28, 17–21. [Google Scholar]
- Fan, J.; Cai, M.; Zhang, H. Study on Effect of Al2O3 Content and MgO Content on Melting Temperature at BF Slag. Shanxi Metall. 2007, 3, 22–23+44. [Google Scholar]
- Chen, D.; Sun, S.; Zhao, S. Influence Slag with High Content of Al2O3 on Blast Furnace Production. Shandong Metall. 2005, 1, 12–14. [Google Scholar]
- Hage, J.L.T.; Schuiling, R.D. Comparative column elution of jarosite waste and its autoclaved product—Evidence for the immobilization of deleterious elements in jarosite. Miner. Eng. 2000, 13, 287–296. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, S.; Lin, X.; Li, Y.; Li, J. Experimental Study on the Comprehensive Recovery of Indium, Germanium, Lead, and Silver from Zinc Smelting Slag. Non-Ferr. Metall. 2001, 2, 34–35+38. [Google Scholar]
- Zhang, Y.; Chen, Z.; Ning, S. Thermodynamical calculation of deoxidization and volatilization of In2O3. Chin. J. Nonferrous Met. 2002, 12, 592–595. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).