Recovering Battery-Grade LiOH·H2O from Spent Lithium-Containing Sagger Crucible by Thermal Dehydration and BaSO4-Driven Double Decomposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
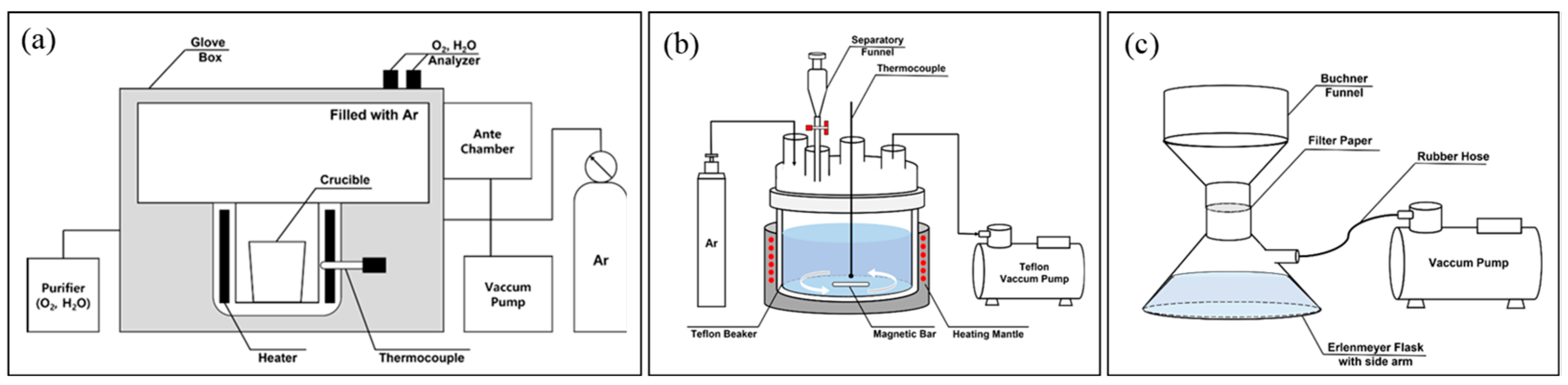
2.2. Experimental Apparatus
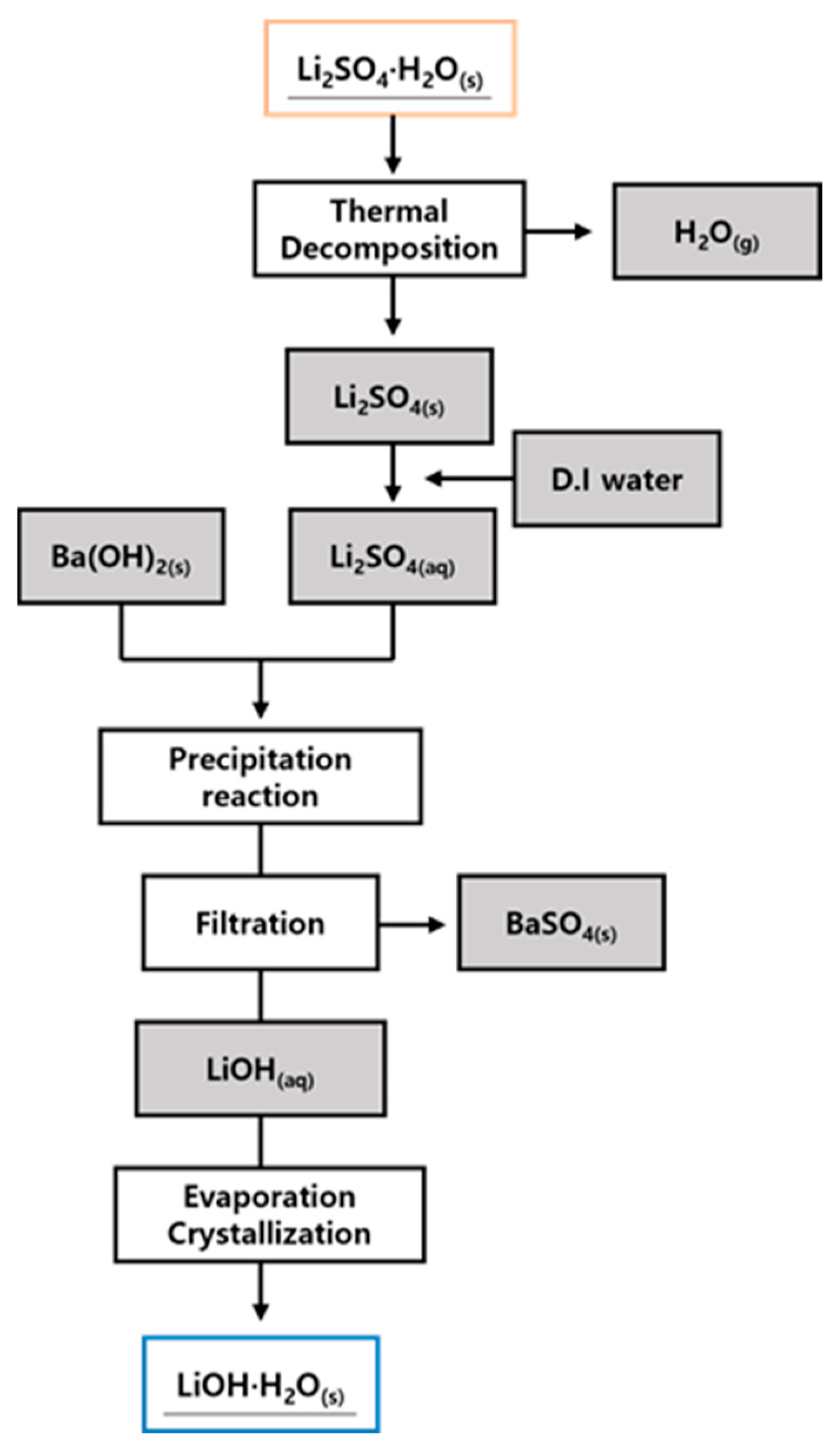
2.3. Overall Process Flow
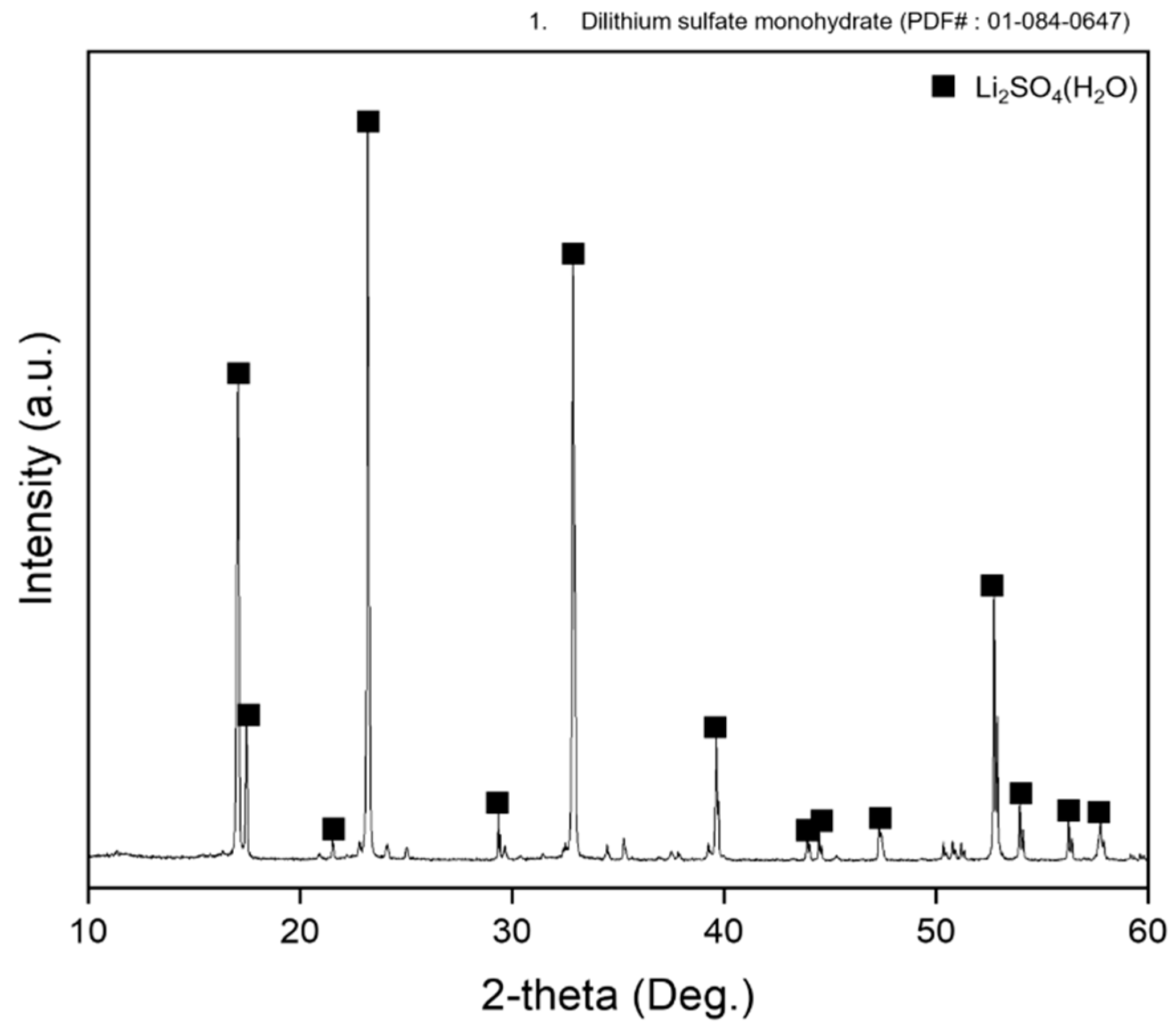
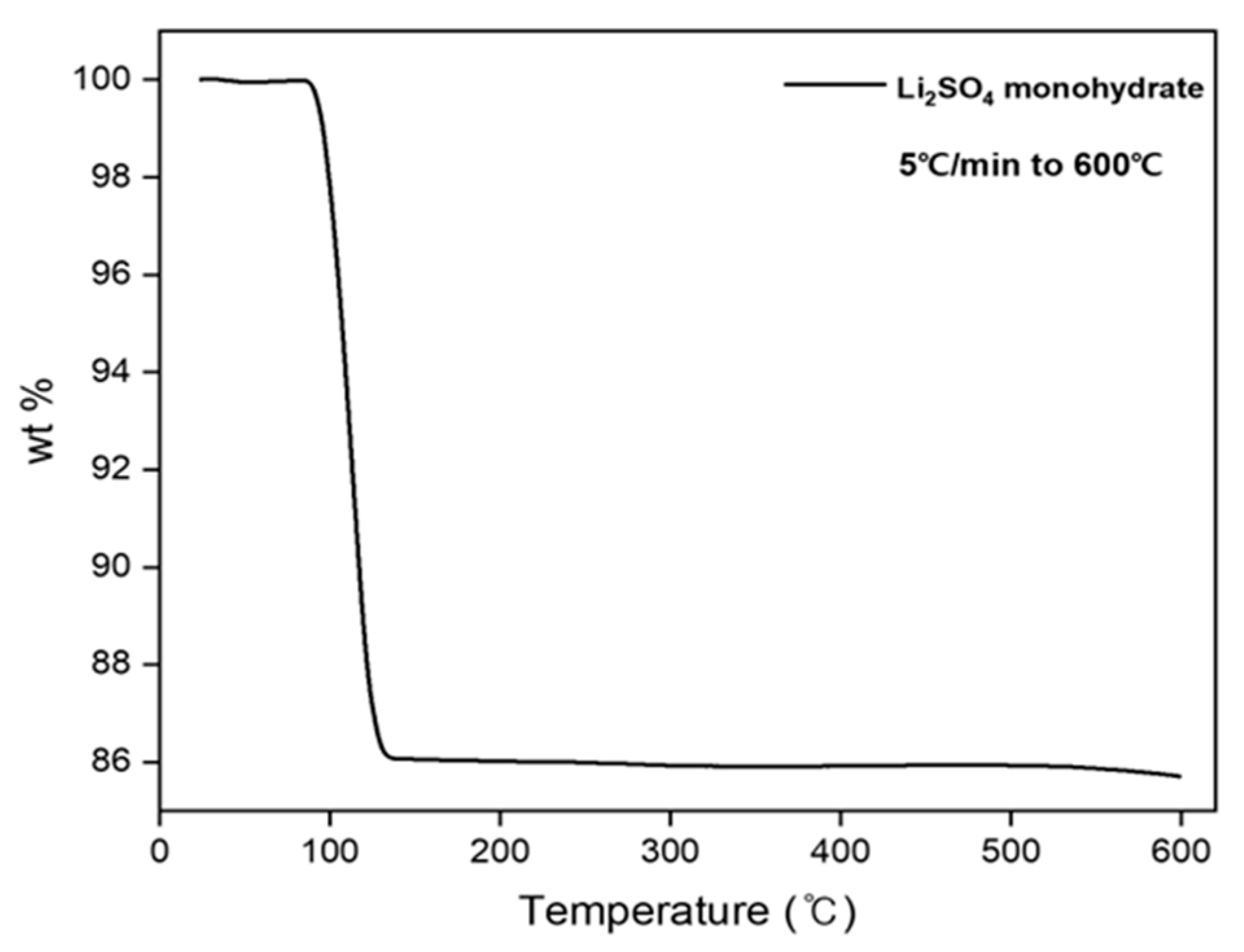
2.4. Thermal Dehydration of Li2SO4·H2O
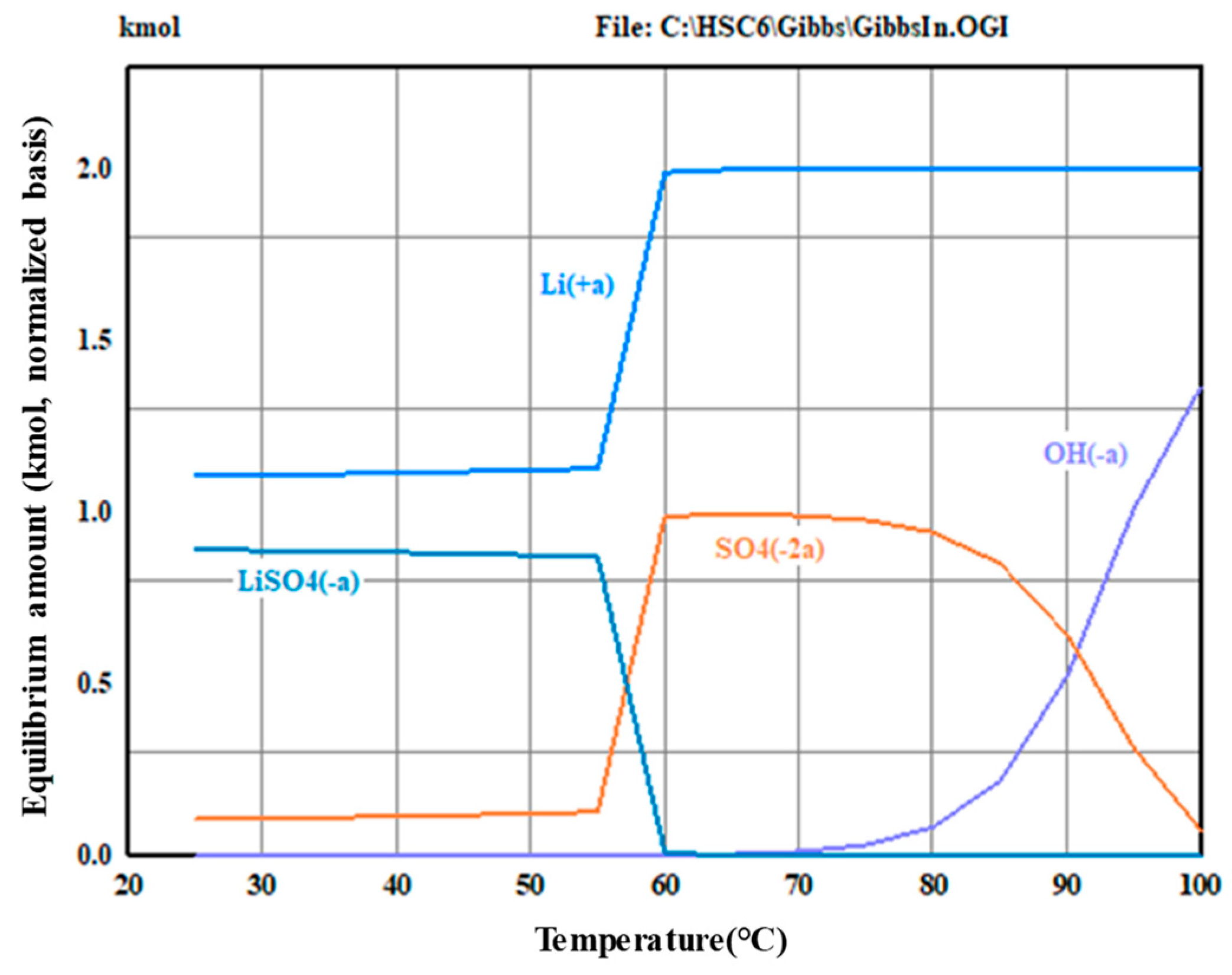
2.5. Ba(OH)2 Double Decomposition Experiments
2.6. Ba(OH)2 Double Decomposition Experiments
3. Results and Discussion
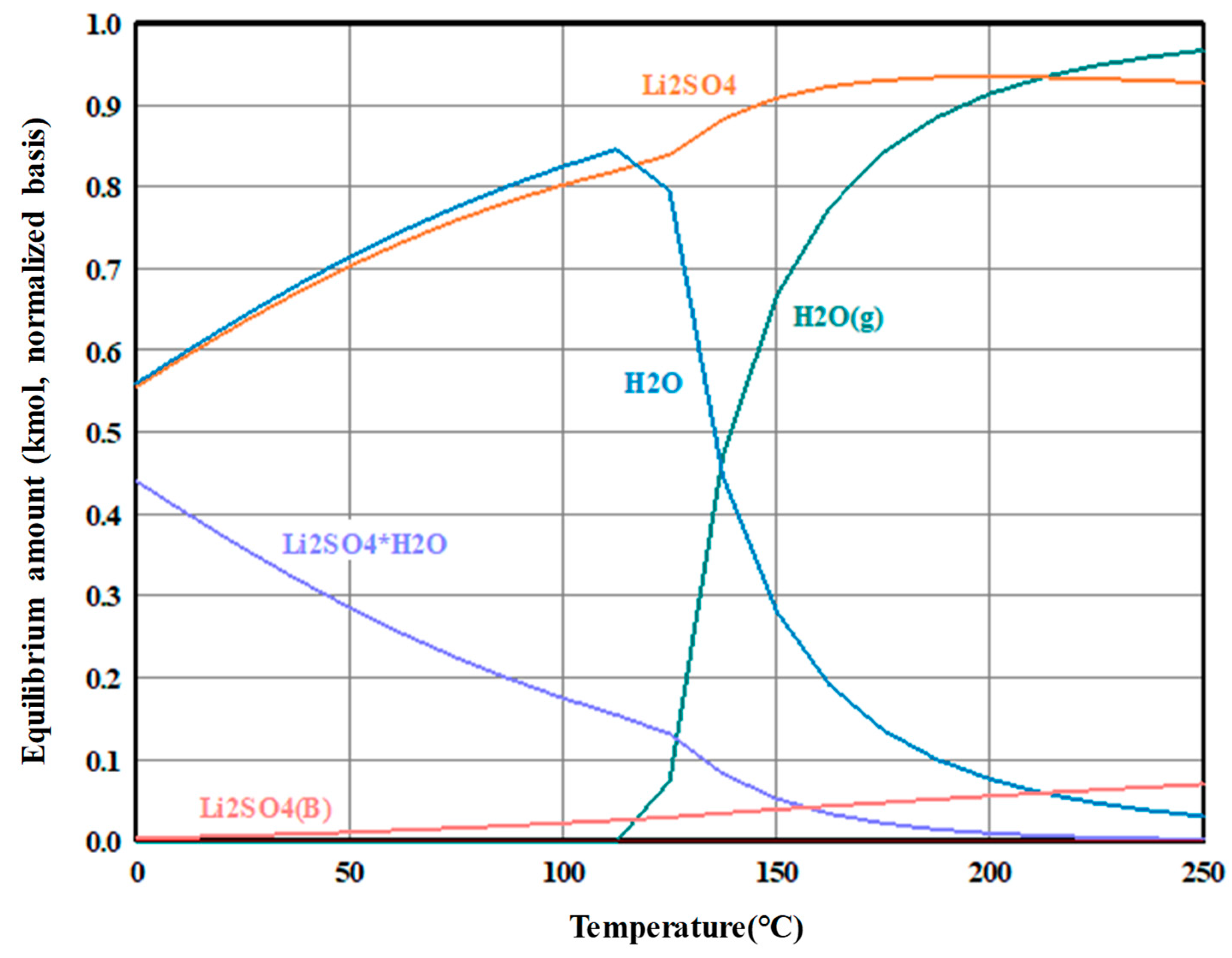
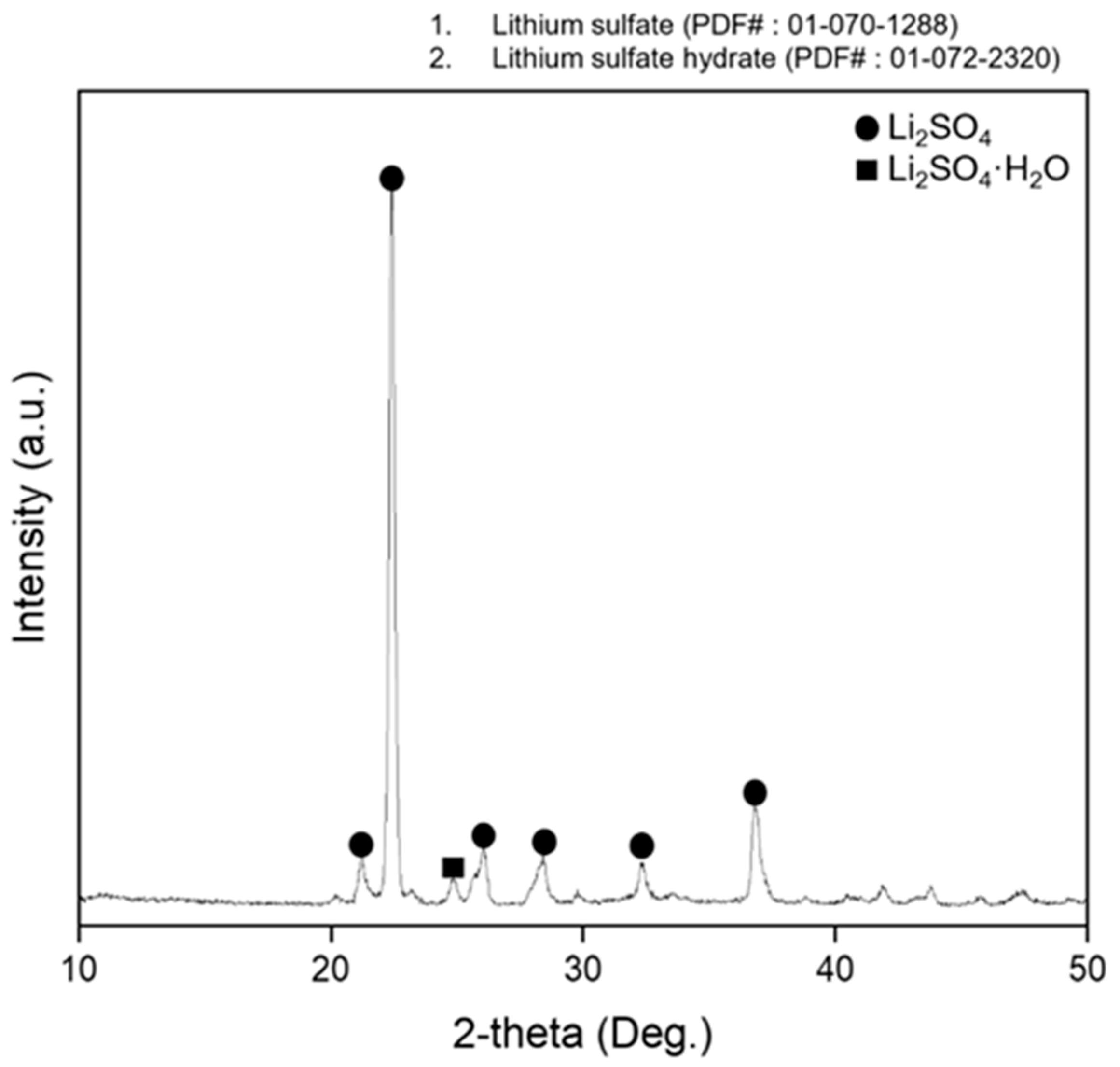
3.1. Thermal Decomposition of Lithium Sulfate Monohydrate
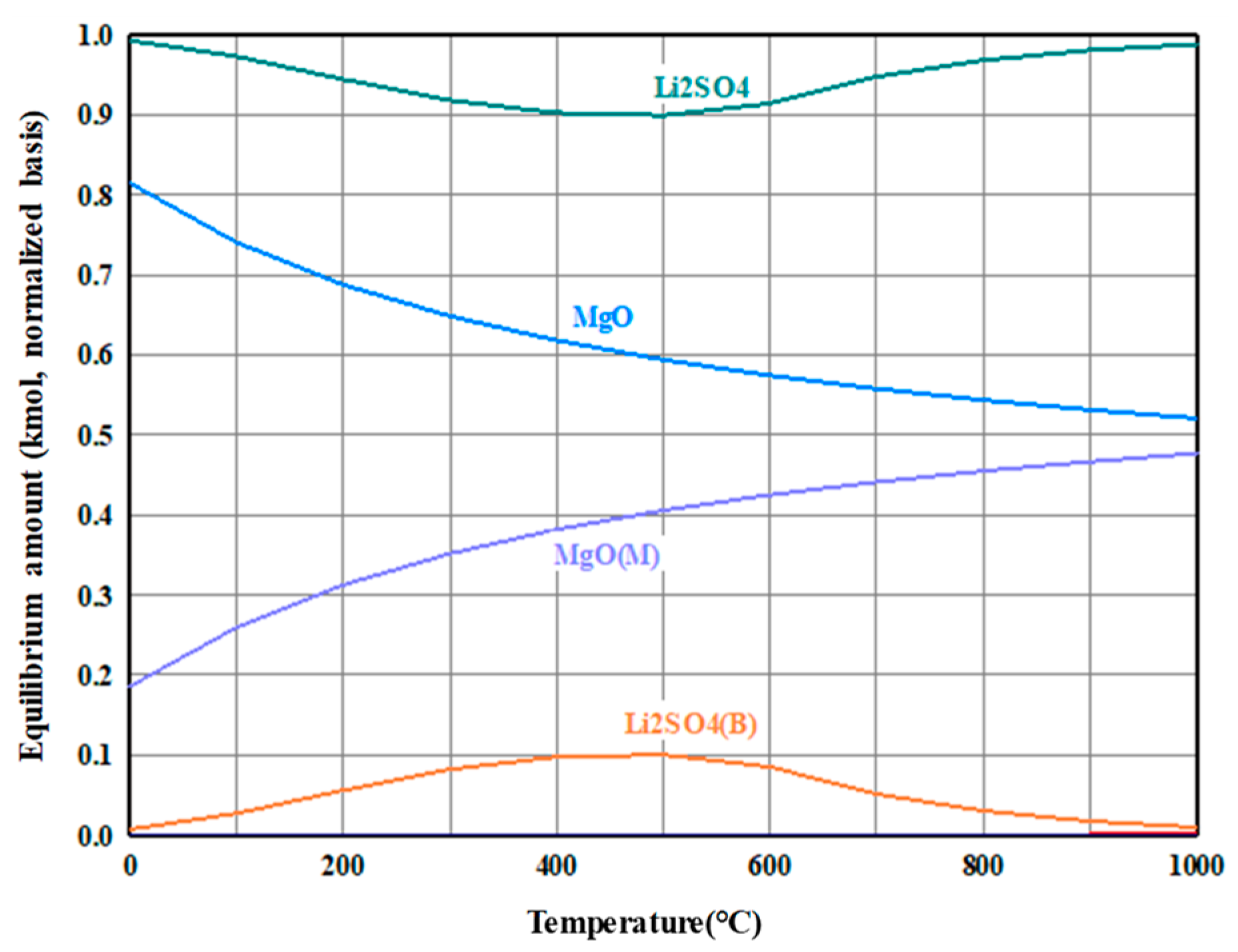
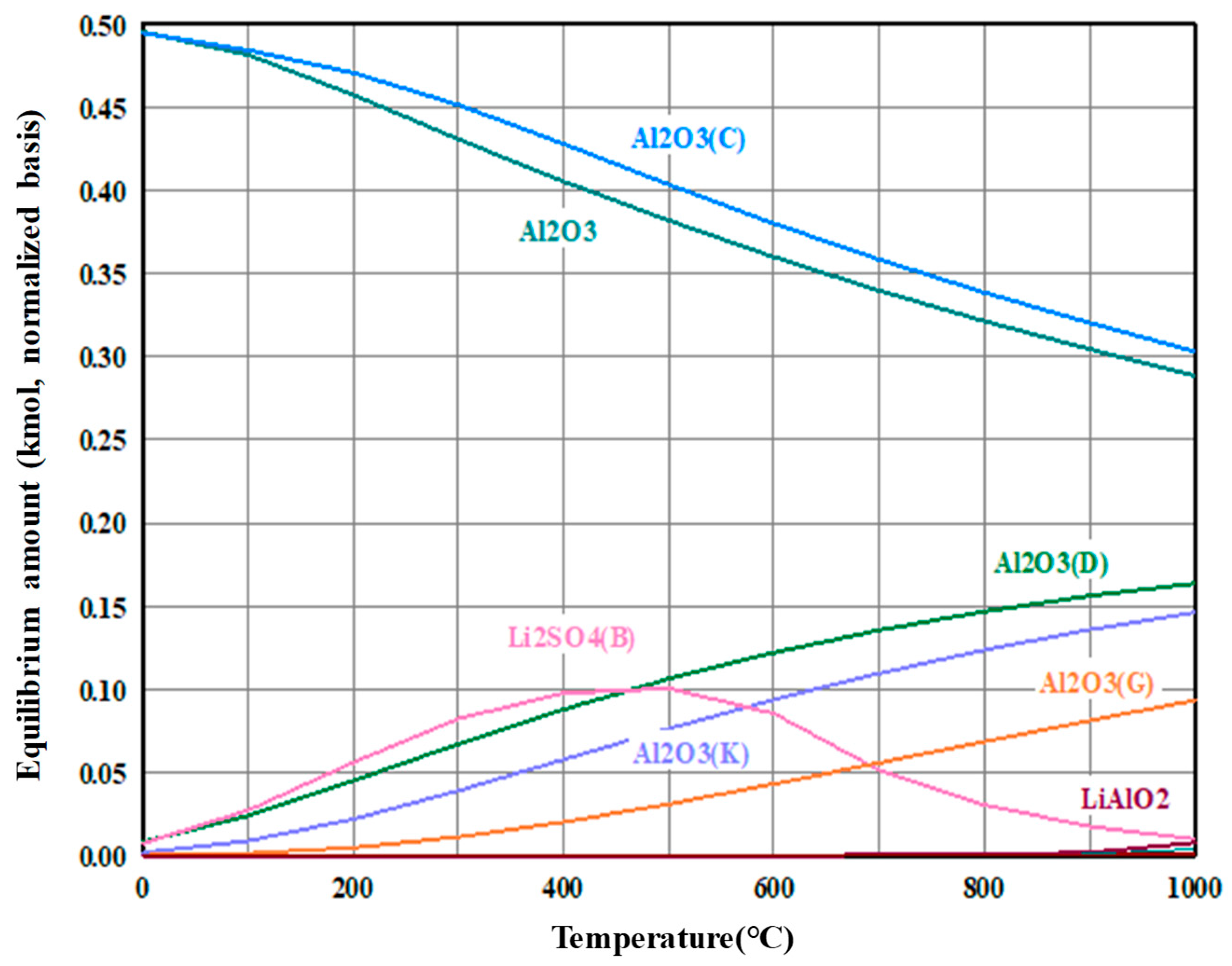
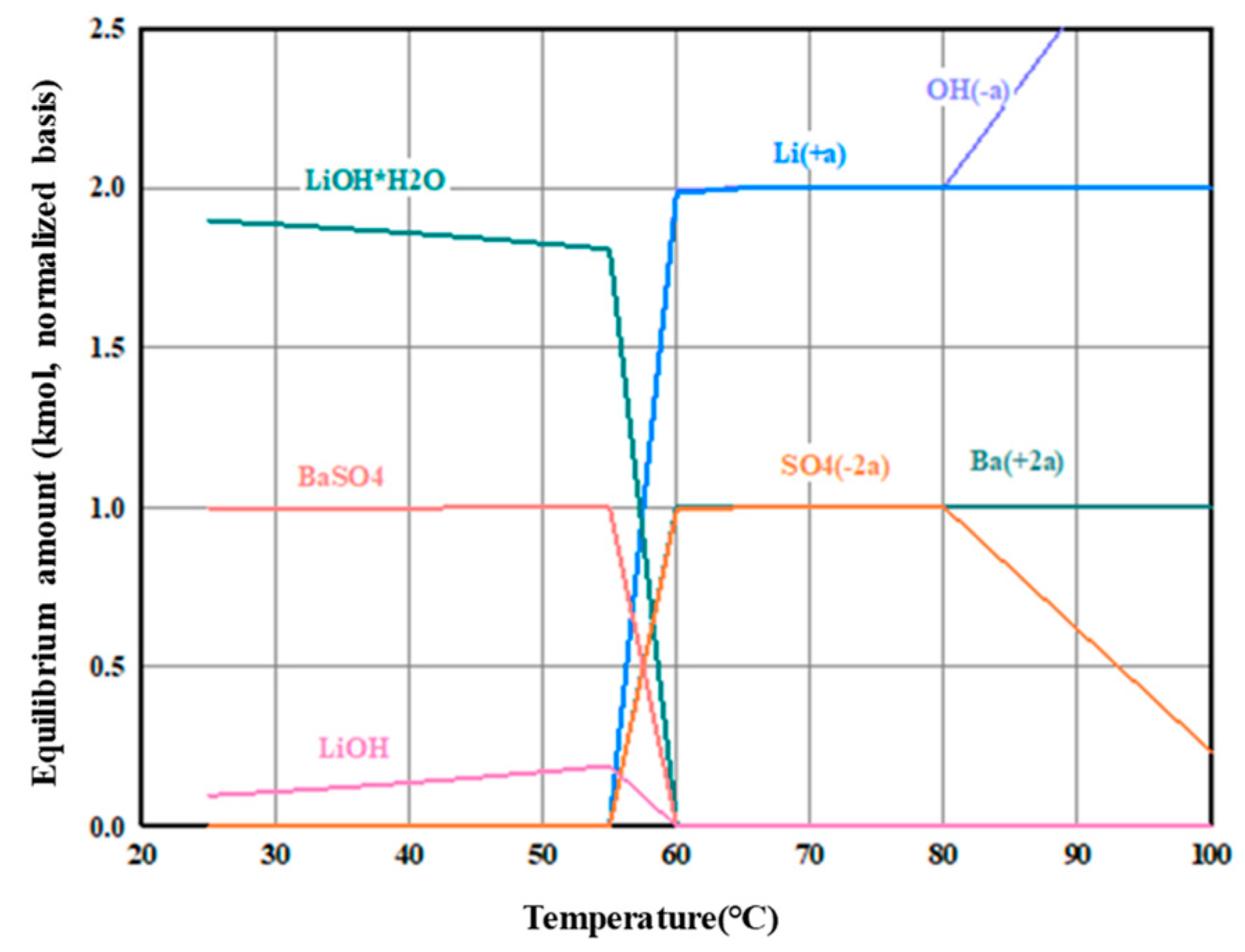
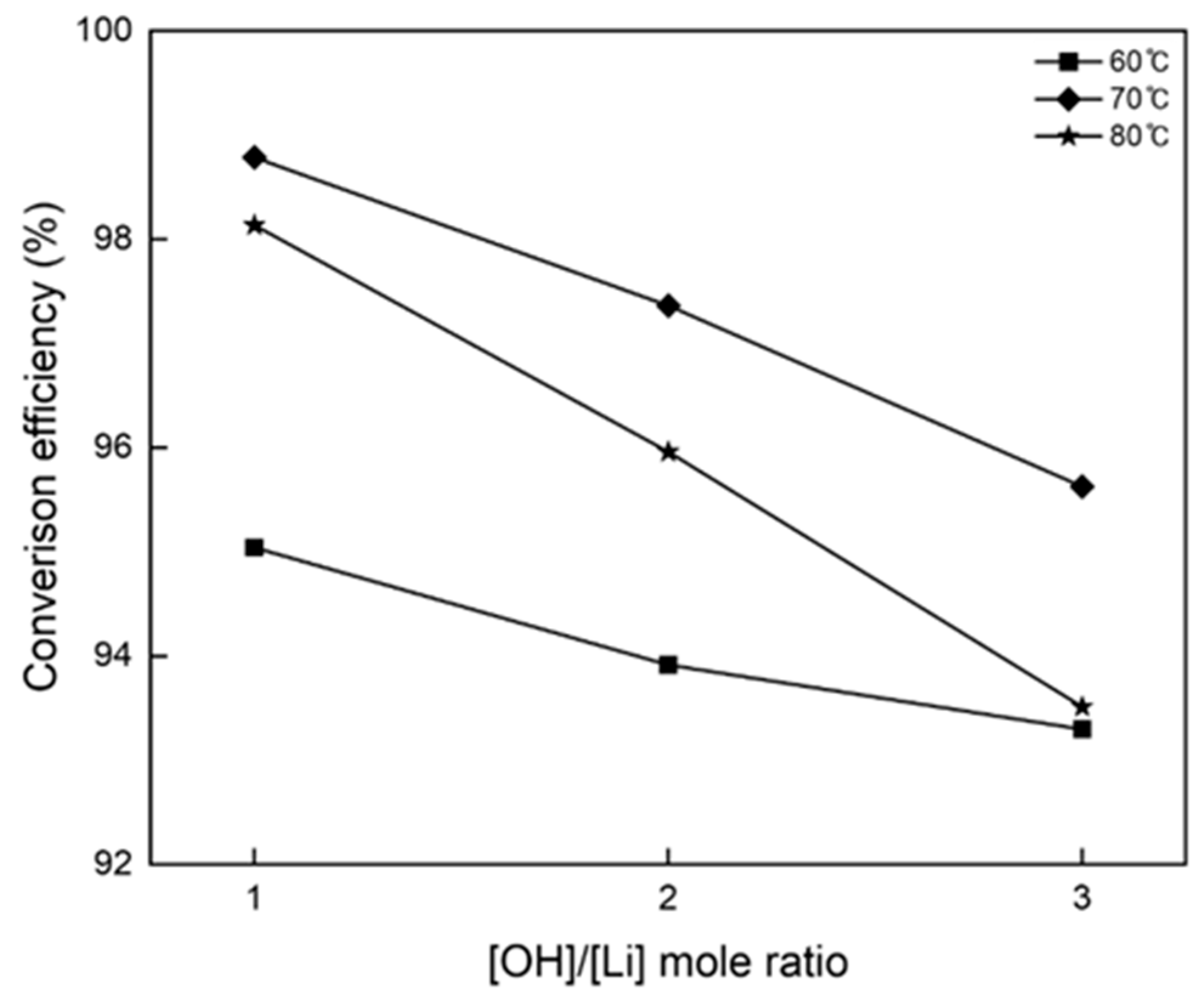
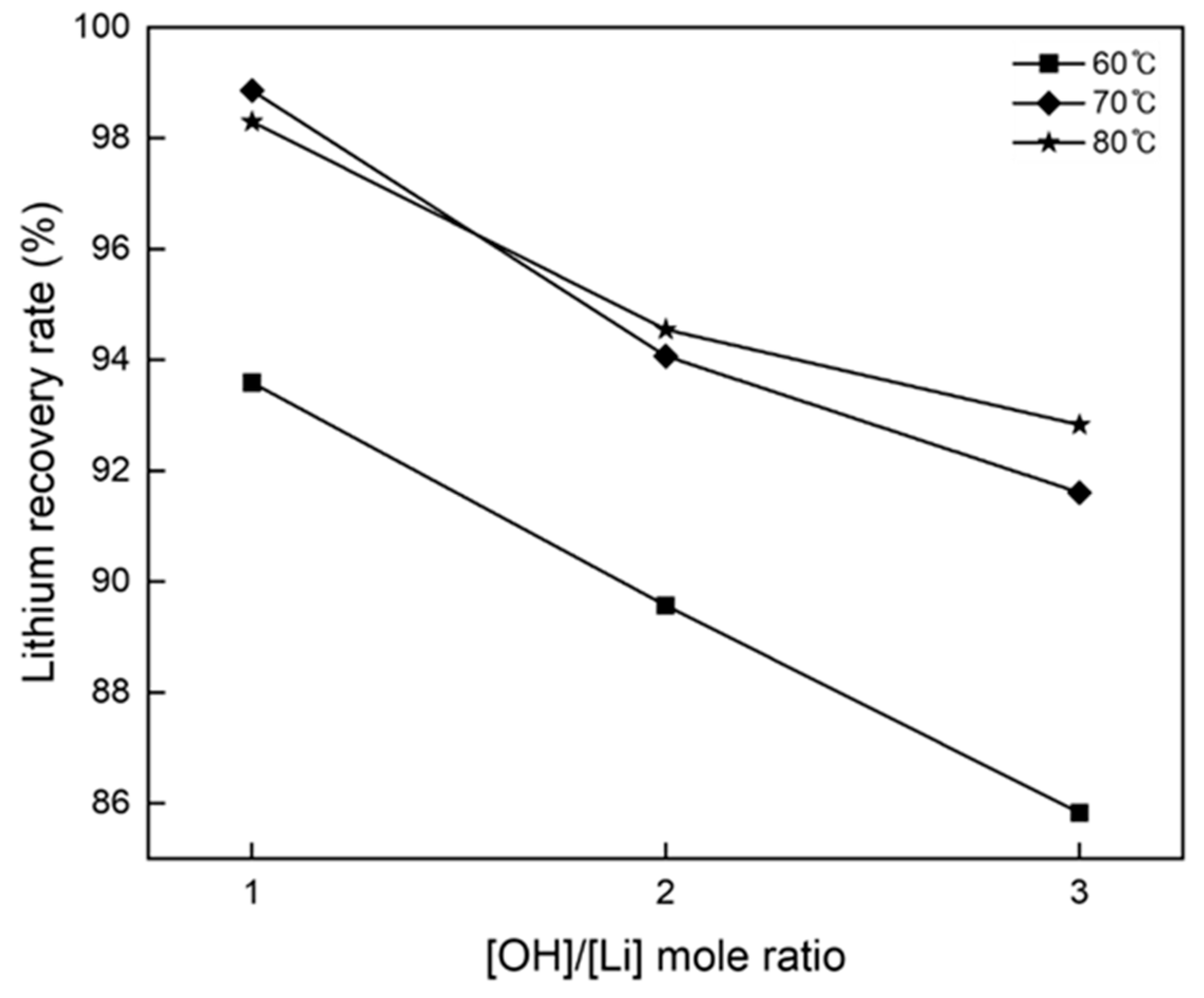
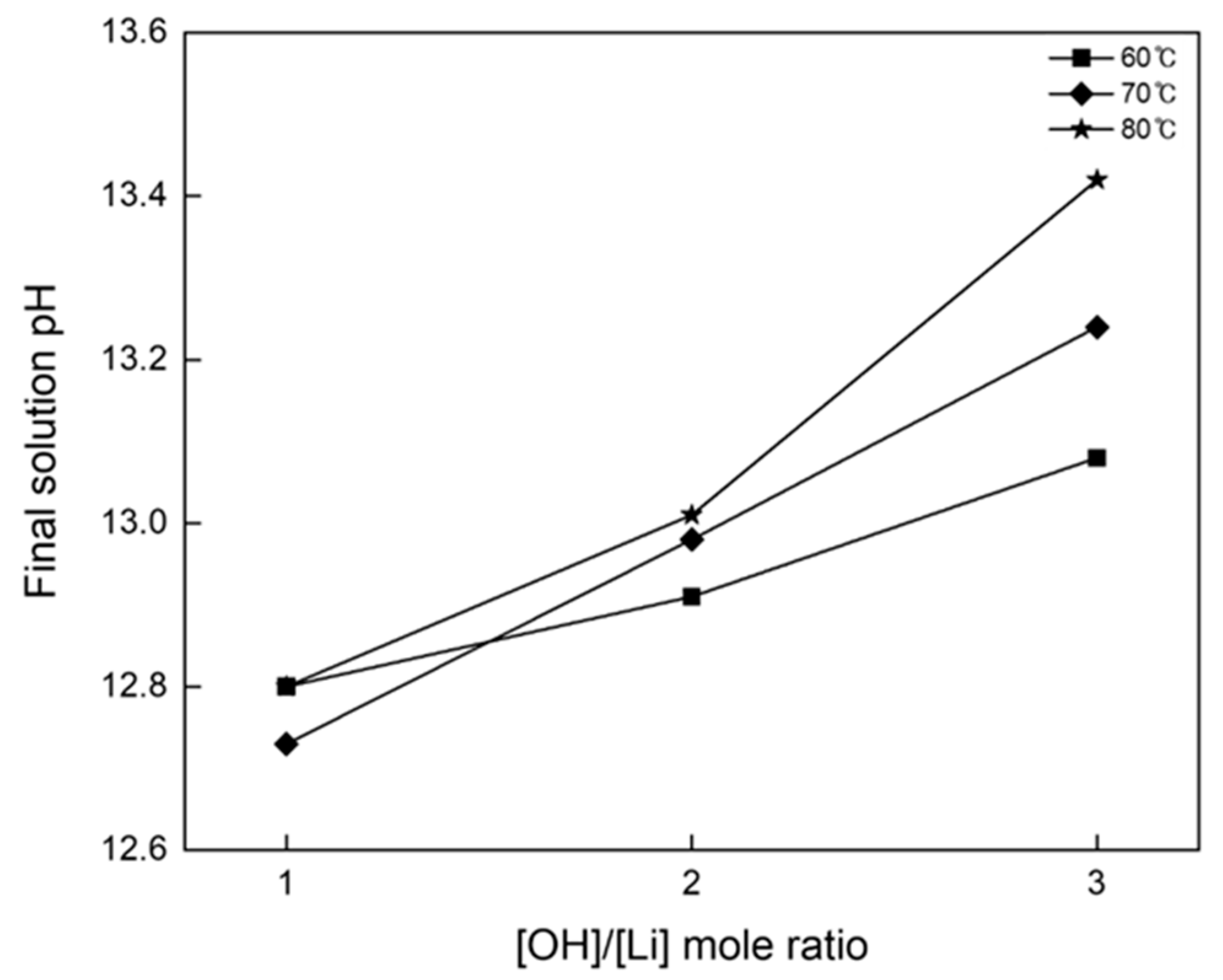
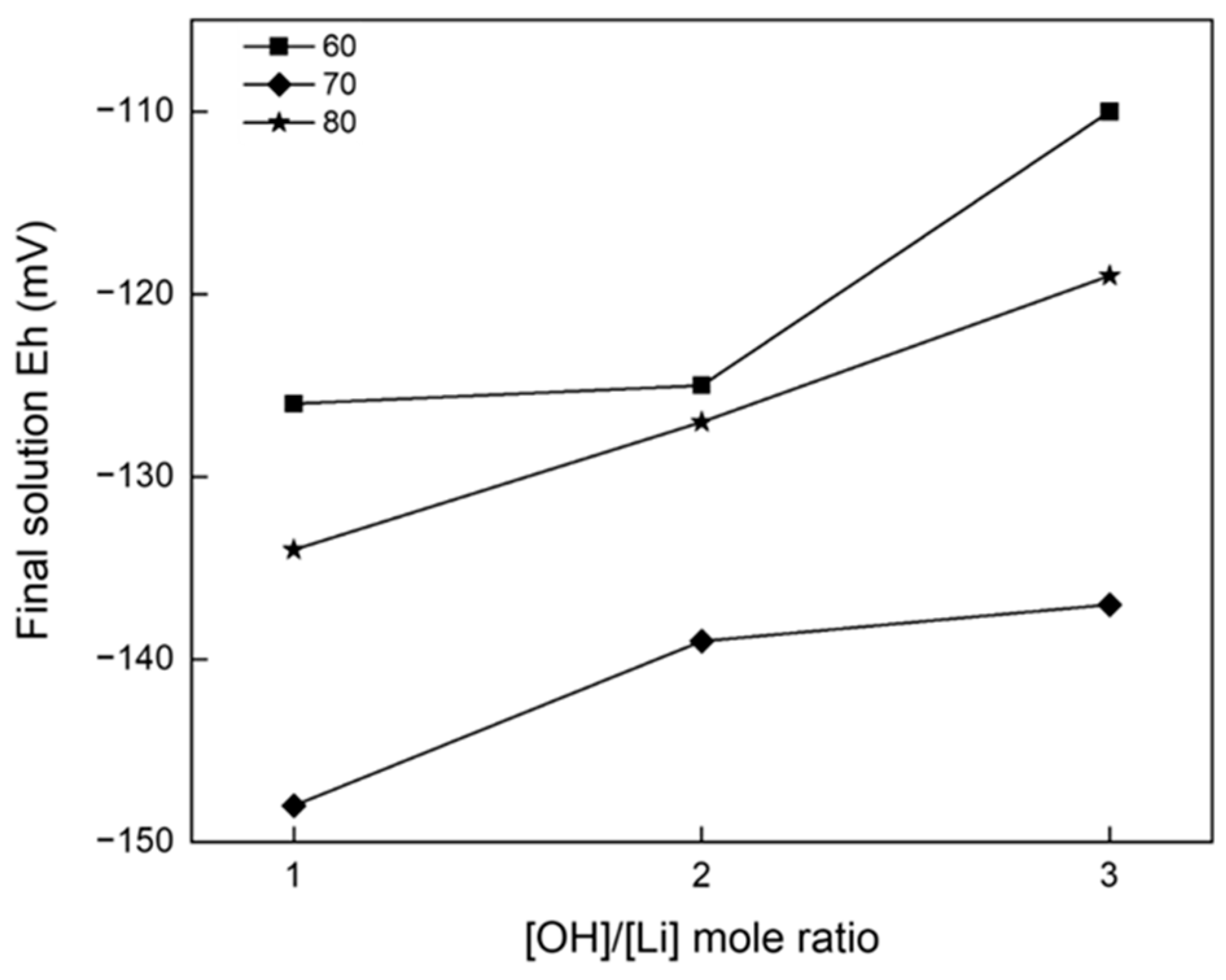
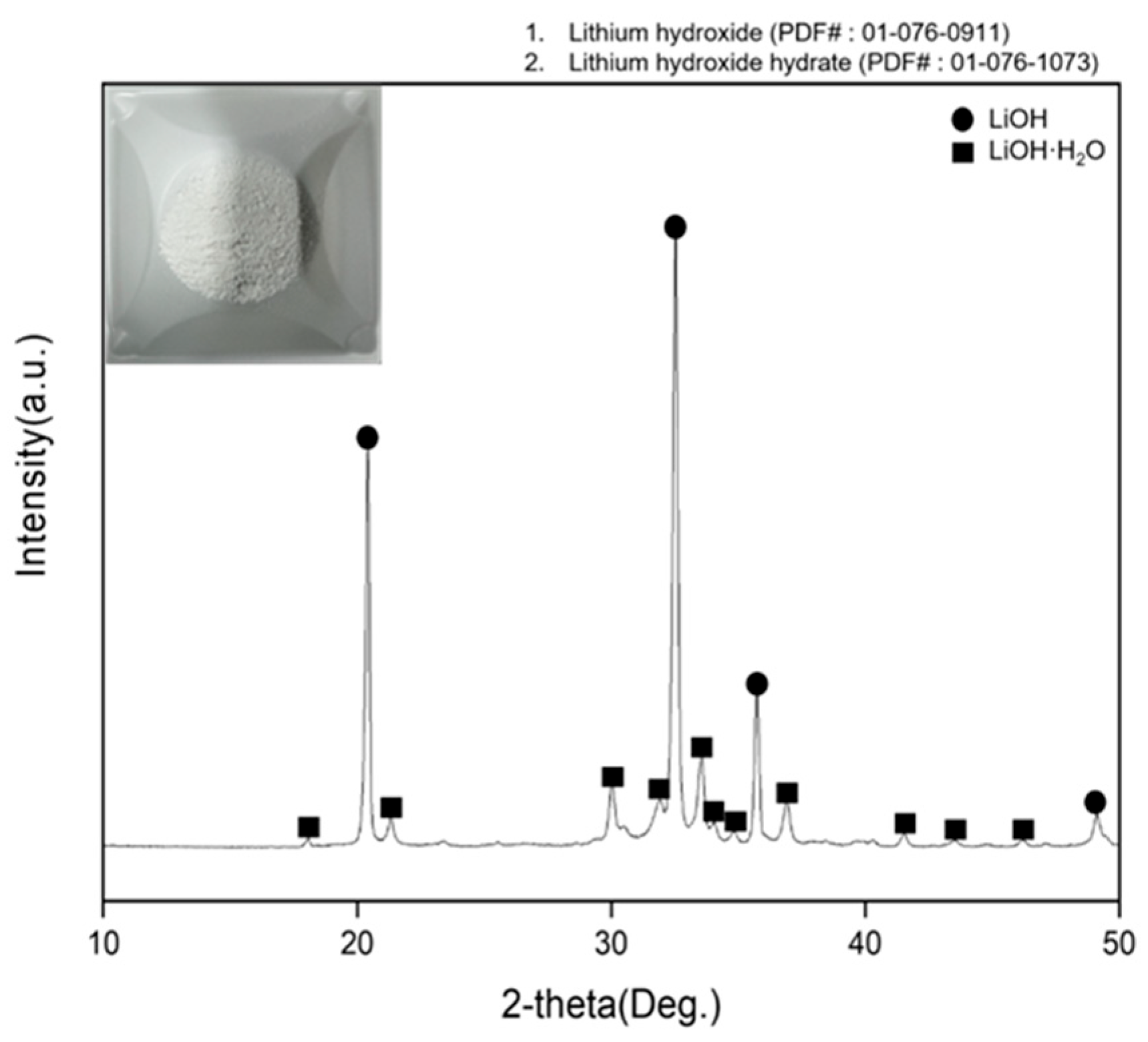
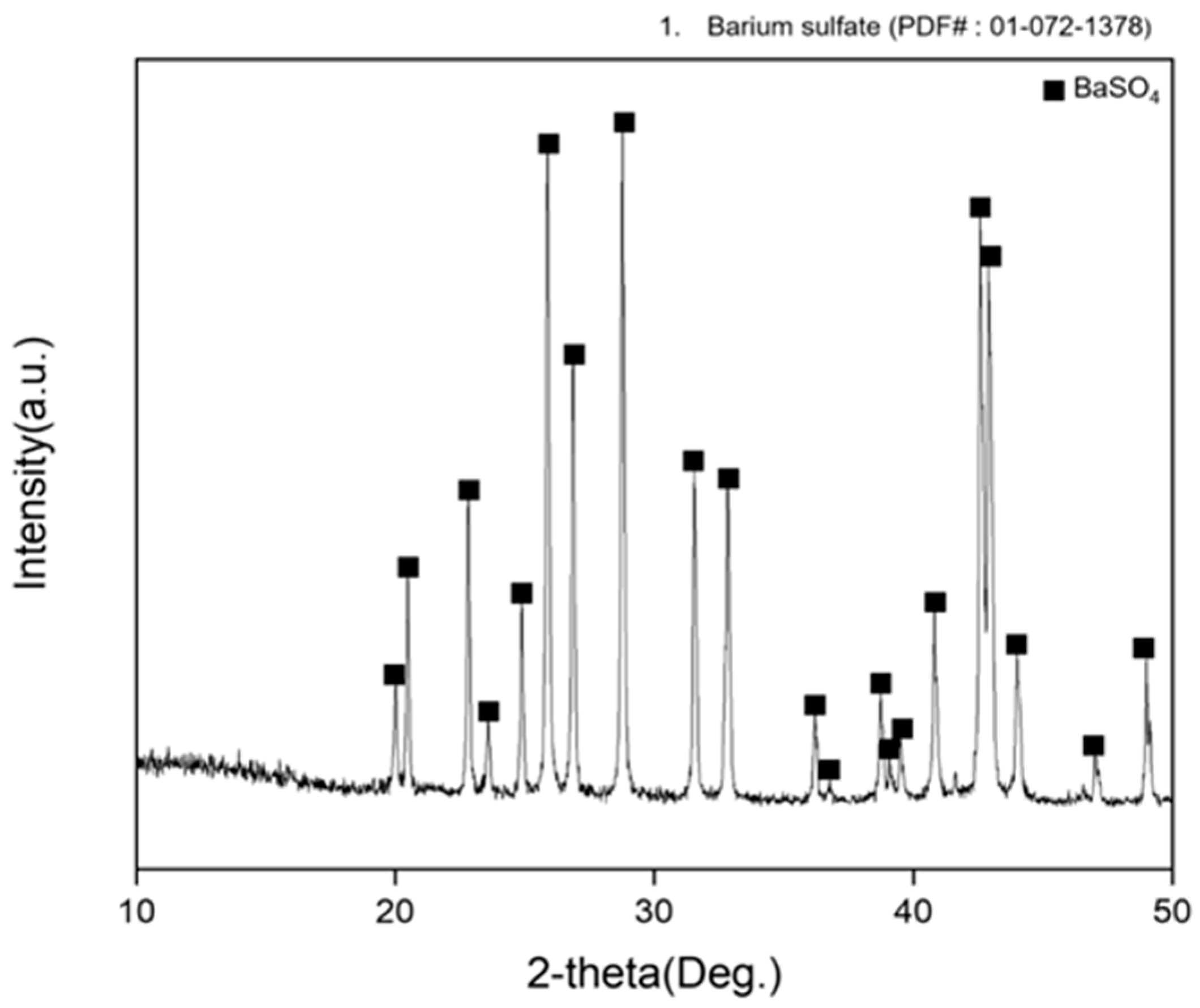
3.2. Precipitation and Double Decomposition Reaction
3.3. Thermal Decomposition of Lithium Sulfate Monohydrate
3.4. Precipitation and Double Displacement Reaction
3.5. Overall Advantages, Limitations, and Comparison with Prior Routes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grageda, M.; Gonzalez, A.; Quispe, A.; Ushak, S. Analysis of a Process for Producing Battery Grade Lithium Hydroxide by Membrane Electrodialysis. Membranes 2020, 10, 198. [Google Scholar] [CrossRef]
- Kim, S.Y.; Choi, S.H.; Lee, E.J.; Kim, J.S. Synthesis and electrochemical performance of Ni-rich NCM cathode materials for lithium-ion batteries. J. Korean Electrochem. Soc. 2017, 20, 67. [Google Scholar] [CrossRef]
- Ngala, J.K.; Chernova, N.A.; Ma, M.; Mamak, M.; Zavalij, P.Y.; Whittingham, M.S. The synthesis, characterization and electrochemical behavior of the layered LiNi0.4Mn0.4Co0.2O2 compound. J. Mater. Chem. 2004, 14, 214. [Google Scholar] [CrossRef]
- Kim, D.W.; Park, J.R.; Ahn, N.K.; Choi, G.M.; Jin, Y.H.; Yang, J.K. A review on the recovery of the lithium carbonate powders from lithium-containing substances. J. Korean Cryst. Growth Cryst. Technol. 2019, 29, 91. [Google Scholar] [CrossRef]
- Hien-Dinh, T.T.; Luong, V.T.; Giere, R.; Tran, T. Extraction of lithium from lepidolite via iron sulphide roasting and water leaching. Hydrometallurgy 2015, 153, 154. [Google Scholar] [CrossRef]
- Ryabtsev, A.D.; Nemkov, N.M.; Kotsupalo, N.P.; Serikova, L.A. Preparation of high-purity lithium hydroxide monohydrate from technical-grade lithium carbonate by membrane electrolysis. Russ. J. Appl. Chem. 2004, 77, 1108–1116. [Google Scholar] [CrossRef]
- Kim, G.J. Recovery of lithium hydroxide from spent lithium carbonate using crystallizations. Sep. Sci. Technol. 2008, 43, 420–430. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, J.; Cai, W.; Yang, Y.; Yi, M.; Xiang, L. Effects of temperature on conversion of Li2CO3 to LiOH in Ca(OH)2 suspension. Particuology 2017, 34, 97–102. [Google Scholar] [CrossRef]
- Joo, S.Y.; Kang, Y.B.; Shim, H.W.; Byun, S.H.; Kim, Y.H.; Lee, C.G.; Kim, D.G. Study on preparation of high purity lithium hydroxide powder with 2-step precipitation process using lithium carbonate recovered from Waste LIB battery. J. Korean Inst. Resour. Recycl. 2019, 28, 60–67. [Google Scholar] [CrossRef]
- Kumar, A.; Fukuda, H.; Hatton, T.A. Lithium Recovery from Oil and Gas Produced Water: A Need for a Growing Energy Industry. ACS Energy Lett. 2019, 4, 1471–1474. [Google Scholar] [CrossRef]
- Tam, T.; Luong, V.T. Ch. 3 Lithium Production Process. In Lithium Process Chemistry- Resources, Extraction, Batteries, and Recycling; Chagnes, A., Swiaatowska, J., Eds.; Elsevier Inc.: New York, NY, USA, 2015; p. 99. [Google Scholar]
- Chon, U.; Han, G.; Kim, K. Current Status of Lithium Resources. J. Korean Inst. Resour. Recycl. 2010, 19, 3–8. Available online: https://koreascience.kr/article/JAKO201029848352569.page (accessed on 18 November 2025).
- Meng, F.; McNeice, J.; Zadeh, S.S. Review of Lithium Production and Recovery from Minerals, Brines, and Lithium-Ion Batteries. Miner. Process. Extr. Metall. Rev. 2021, 42, 123–141. [Google Scholar] [CrossRef]
- Yanjia, Z.; Xiaodong, T.; Dayong, Q.; Jingjing, L.; Hong, W. Research progress of technology of lithium extraction. Separ. Purif. Tech. 2025, 359, 130561. [Google Scholar] [CrossRef]
- Rioyo, J.; Tuset, S.; Grau, R. Lithium extraction from spodumene by the traditional sulfuric acid process: A review. Miner. Process. Extr. Metall. Rev. 2020, 46, 1–10. [Google Scholar] [CrossRef]
- Garrett, D.E. Handbook of Lithium and Natural Calcium Chloride; Elsevier Science & Technology: Amsterdam, The Netherlands, 2004; p. 164. [Google Scholar]
- David, L. Experimental phase equilibria in the system LiAlSiO4-SiO2-H2O: A petrogenetic grid for lithium- rich pegmatites. Am. Mineral. 1984, 69, 995–1004. [Google Scholar]
- Zilberman, P. The CO2 Absorber Based on LiOH. Acta Med. Marisiensis. 2015, 61, 4–6. [Google Scholar] [CrossRef]
- Liu, H.; Azimi, G. Production of battery grade lithium hydroxide monohydrate using barium hydroxide causticizing agent. Resour. Conserv. Recycl. 2022, 179, 106115. [Google Scholar] [CrossRef]
- Neuman, S.P. Theoretical derivation of Darcy’s law. Acta Mech. 1977, 25, 153–170. [Google Scholar] [CrossRef]
- Li, J.; Zeng, T.; Kobayashi, N.; Wu, R.; Xu, H.; Deng, L.; He, Z.; Huang, H. Carbonation Reaction of Lithium Hydroxide during Low-Temperature Thermal Energy Storage. J. Renew. Mater. 2021, 9, 1621–1630. [Google Scholar] [CrossRef]
- Aprilianto, D.R.; Perdana, I.; Rochmadi Petrus, H.T.B.M. Effect of Sulfate and Carbonate Ions on Lithium Carbonate Precipitation. Ind. Eng. Chem. Res. 2024, 63, 18262–18273. [Google Scholar] [CrossRef]
- Vera, M.L.; Torres, W.R.; Galli, C.I.; Chagnes, A.; Flexer, V. Environmental impact of direct lithium extraction from brines. Nat. Rev. Earth Environ. 2023, 4, 675–690. [Google Scholar] [CrossRef]
- Wei, G.; Wang, M.; Lin, C.; Xu, C.; Gao, J. Optimizing Operational Parameters for Lithium Hydroxide Production by Bipolar Membrane Electrodialysis. Separations 2024, 11, 146. [Google Scholar] [CrossRef]
- Peeters, N.; Tijink, M. Conversion of Lithium Chloride into Lithium Hydroxide: A Literature Review. J. Sustain. Metall. 2024, 10, 1127–1145. [Google Scholar] [CrossRef]


| Element/Parameter | Concentration (mg·L−1) or Value |
|---|---|
| pH (25 °C) | 9.48 |
| Li | 1373 |
| Na | 1830.44 |
| Ca | 21.638 |
| Mg | 3.042 |
| Si | 21.159 |
| Fe | 2.017 |
| Li2SO4 Conc. | Initial Li Conc. | Initial pH | Temperature, °C | [OH]:[Li] |
|---|---|---|---|---|
| 0.1 M | 1373 ppm | 9.48 | 60 | 1:0.1 |
| 2:0.1 | ||||
| 3:0.1 | ||||
| 70 | 1:0.1 | |||
| 2:0.1 | ||||
| 3:0.1 | ||||
| 80 | 1:0.1 | |||
| 2:0.1 | ||||
| 3:0.1 |
| Element | Raw Li2SO4·H2O, wt.% | Thermal Decomposition Li2SO4, wt.% |
|---|---|---|
| H | 0.756 | – |
| O | 50.886 | 37.503 |
| [OH]:[Li] | Temperature, °C | Li, ppm | SO4, ppm | Conversion Efficiency, % | Li Recovery Rate, % |
|---|---|---|---|---|---|
| 1:0.1 | 60 | 1947.40 | 9.13 | 95.04 | 93.59 |
| 2:0.1 | 1874.92 | 11.27 | 93.91 | 89.57 | |
| 3:0.1 | 1832.0 | 12.66 | 93.29 | 85.83 | |
| 1:0.1 | 70 | 2886.13 | 3.14 | 98.78 | 98.86 |
| 2:0.1 | 2527.32 | 6.27 | 97.36 | 94.07 | |
| 3:0.1 | 2446.18 | 10.33 | 95.62 | 91.61 | |
| 1:0.1 | 80 | 2012.41 | 3.38 | 98.13 | 98.30 |
| 2:0.1 | 1976.17 | 7.47 | 95.96 | 94.55 | |
| 3:0.1 | 1968.45 | 12.17 | 93.51 | 92.83 |
| Temperature, °C | [OH]:[Li] mol Ratio | LiOH, g/L | Li2CO3, g/L | LiOH, % | Li2CO3, % |
|---|---|---|---|---|---|
| 60 | 1 | 3.875 | 0.9381 | 86.43 | 13.57 |
| 2 | 3.705 | 0.9038 | 86.34 | 13.66 | |
| 3 | 3.584 | 0.8136 | 87.17 | 12.83 | |
| 70 | 1 | 4.275 | 0.7097 | 90.29 | 9.71 |
| 2 | 4.025 | 0.7424 | 89.32 | 10.68 | |
| 3 | 3.942 | 0.6884 | 89.83 | 10.17 | |
| 80 | 1 | 4.248 | 0.7111 | 90.21 | 9.79 |
| 2 | 4.077 | 0.6981 | 90.01 | 9.99 | |
| 3 | 3.964 | 0.7438 | 89.16 | 10.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, S.; Wang, J.-P. Recovering Battery-Grade LiOH·H2O from Spent Lithium-Containing Sagger Crucible by Thermal Dehydration and BaSO4-Driven Double Decomposition. Metals 2025, 15, 1293. https://doi.org/10.3390/met15121293
Heo S, Wang J-P. Recovering Battery-Grade LiOH·H2O from Spent Lithium-Containing Sagger Crucible by Thermal Dehydration and BaSO4-Driven Double Decomposition. Metals. 2025; 15(12):1293. https://doi.org/10.3390/met15121293
Chicago/Turabian StyleHeo, Seongbong, and Jei-Pil Wang. 2025. "Recovering Battery-Grade LiOH·H2O from Spent Lithium-Containing Sagger Crucible by Thermal Dehydration and BaSO4-Driven Double Decomposition" Metals 15, no. 12: 1293. https://doi.org/10.3390/met15121293
APA StyleHeo, S., & Wang, J.-P. (2025). Recovering Battery-Grade LiOH·H2O from Spent Lithium-Containing Sagger Crucible by Thermal Dehydration and BaSO4-Driven Double Decomposition. Metals, 15(12), 1293. https://doi.org/10.3390/met15121293









