The Effects of Knife Milling and Ball Milling on Hydrogen Decrepitated Sm2TM17 Sintered Magnet Powder for Short-Loop Recycling
Abstract
1. Introduction
2. Methods and Materials
2.1. Starting Materials Characterisation
2.2. Hydrogen Decrepitation Trials
2.3. Oxygen/Nitrogen/Carbon Content Analysis
2.4. Milling of HD Powders
- Knife Milling—A total of 200–300 g of HD powder was loaded into a CGoldenWall electric grain grinder (CGoldenwall, Gongyi city, China), using 304 stainless-steel blades rotating at 20,000 RPM, and was milled for 1–4 min. After each minute of milling, the knife mill chamber was allowed to cool for 45 min to prevent cold welding of the sample to the chamber walls. All knife milling was completed inside a nitrogen-filled Mbraun glovebox to prevent sample oxidation.
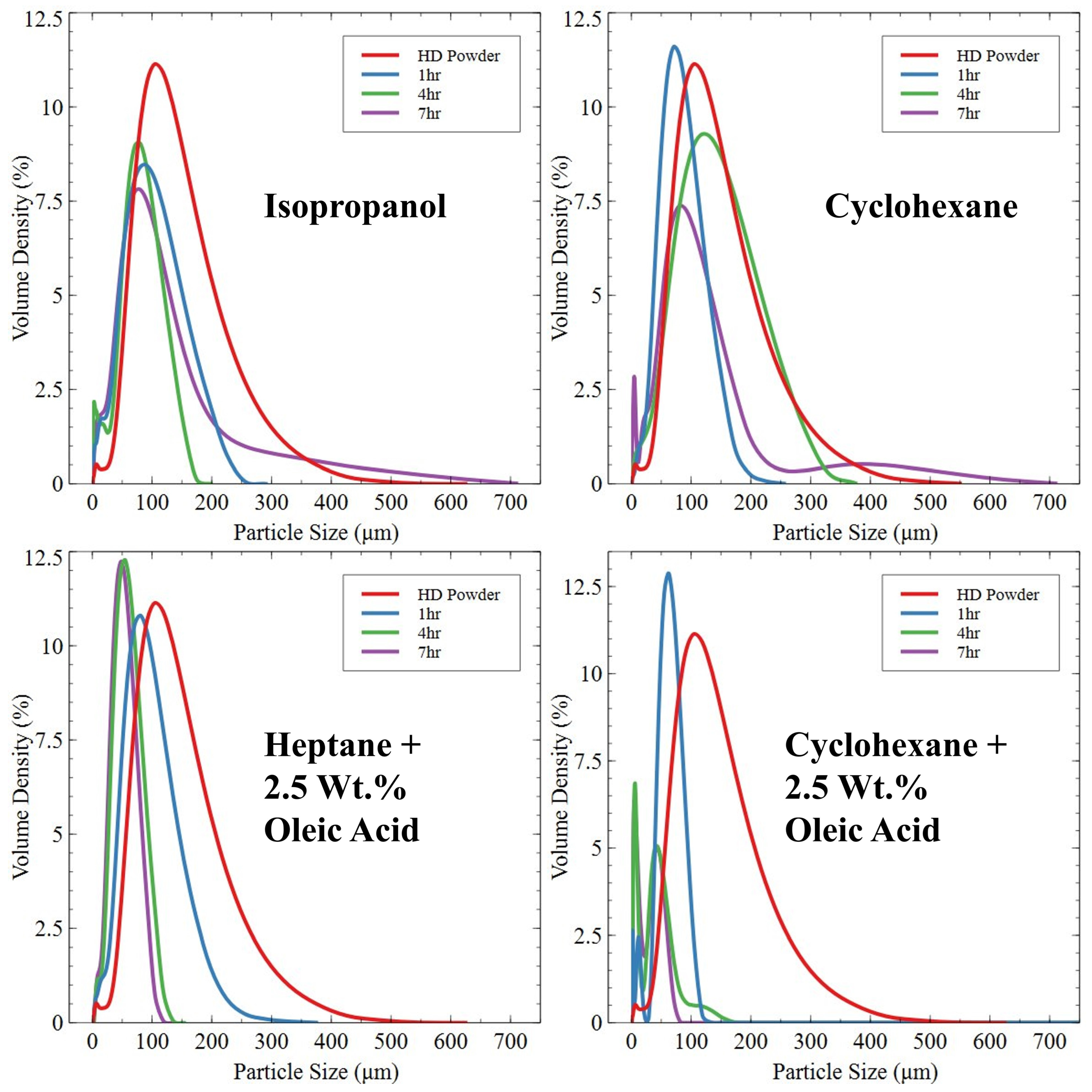
- Roller Ball Milling—A total of 35 g of HD powder or knife-milled powder was loaded into a stainless-steel milling pot alongside 250 g of stainless-steel milling balls (10 mm diameter) for a ball-to-powder ratio of approximately 7:1. Surfactants of isopropanol, cyclohexane and cyclohexane/heptane and oleic acid mixtures were added to the milling pot, with the former three being added as a 60 wt.% addition and the oleic acid being added at 2.5 wt.% of the HD powder mass. The milling pot was rotated at a speed of 100 RPM. Milled powders were dried in a vacuum chamber to remove volatile organic surfactants.
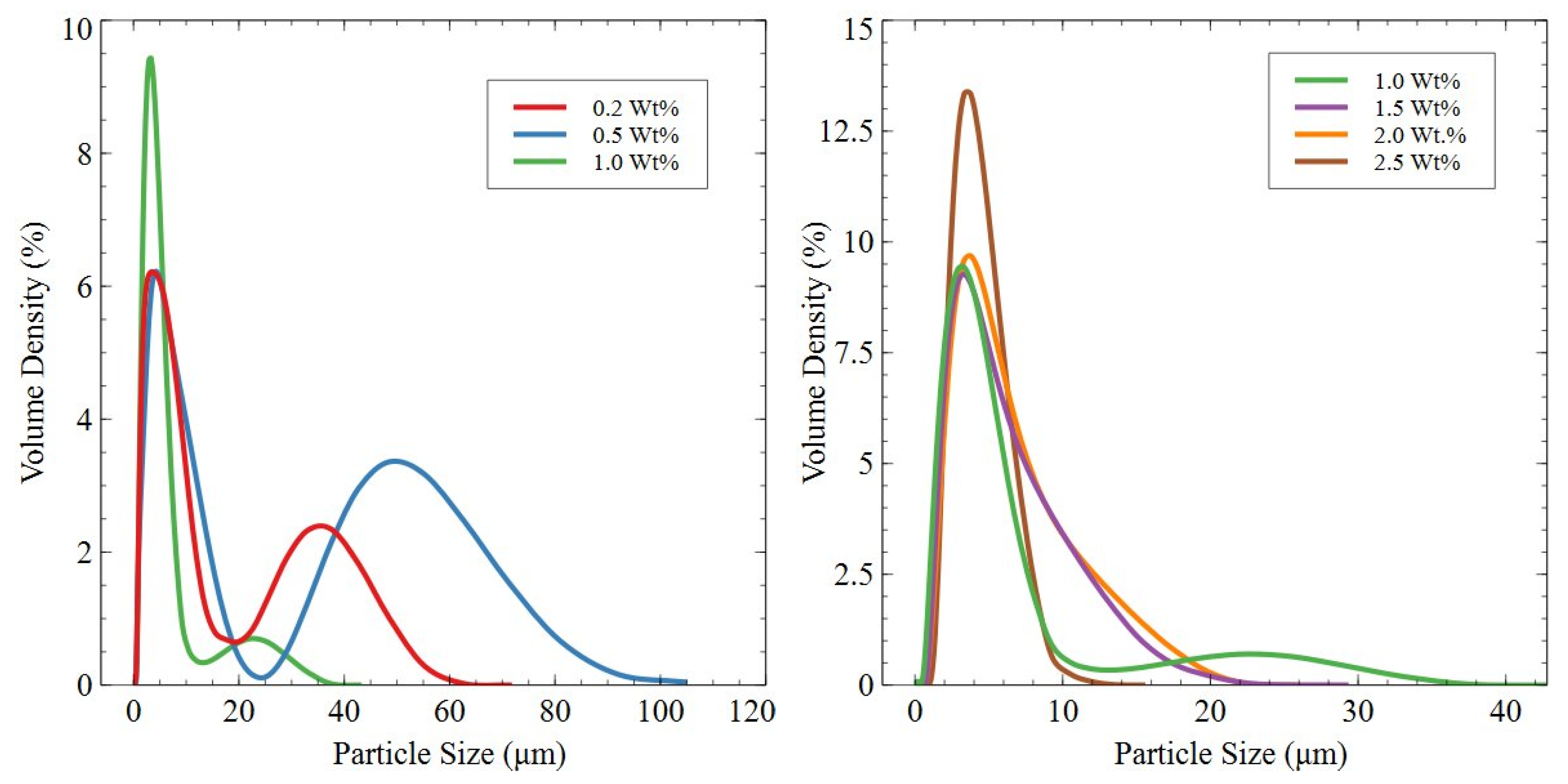
- Planetary Ball Milling—A total of 80 g of knife-milled powder was loaded into a tungsten carbide milling pot alongside 1200 g of tungsten carbide milling balls (5 mm diameter) for a ball-to-powder ratio of approximately 15:1. A surfactant mix of 60 wt.% cyclohexane and 0.5–2.5 wt.% oleic acid were added to the milling pot across the milling trials. The milling pot was rotated at a speed of 400 RPM for a total milling time of 10 min, with pauses in the cycle added to allow the pots to cool. Milled powders were dried in a vacuum chamber to remove volatile organic surfactants. For magnets manufactured with Sm-hydride additions, the hydride was crushed with a mortar and pestle and added to the planetary ball milling pot to be milled and mixed with HD powder.
2.5. Particle Size Analysis
2.6. Vacuum Degassing and Surfactant Removal for Milled Powders
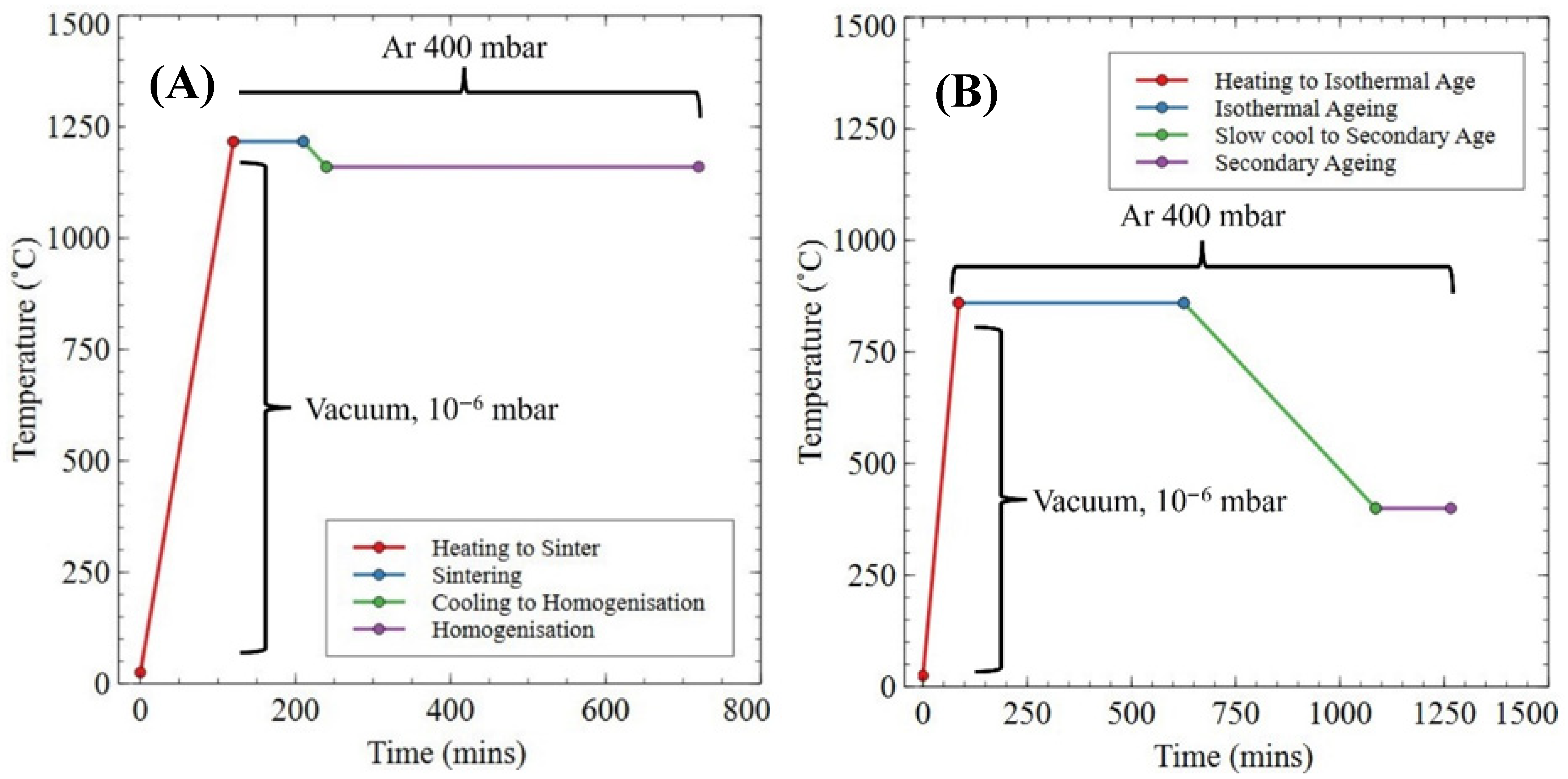
2.7. Recycled Magnet Manufacture
3. Results
3.1. As-Received Material Characterisation
3.2. Particle Size Analysis of HD-Processed and Milled Powders
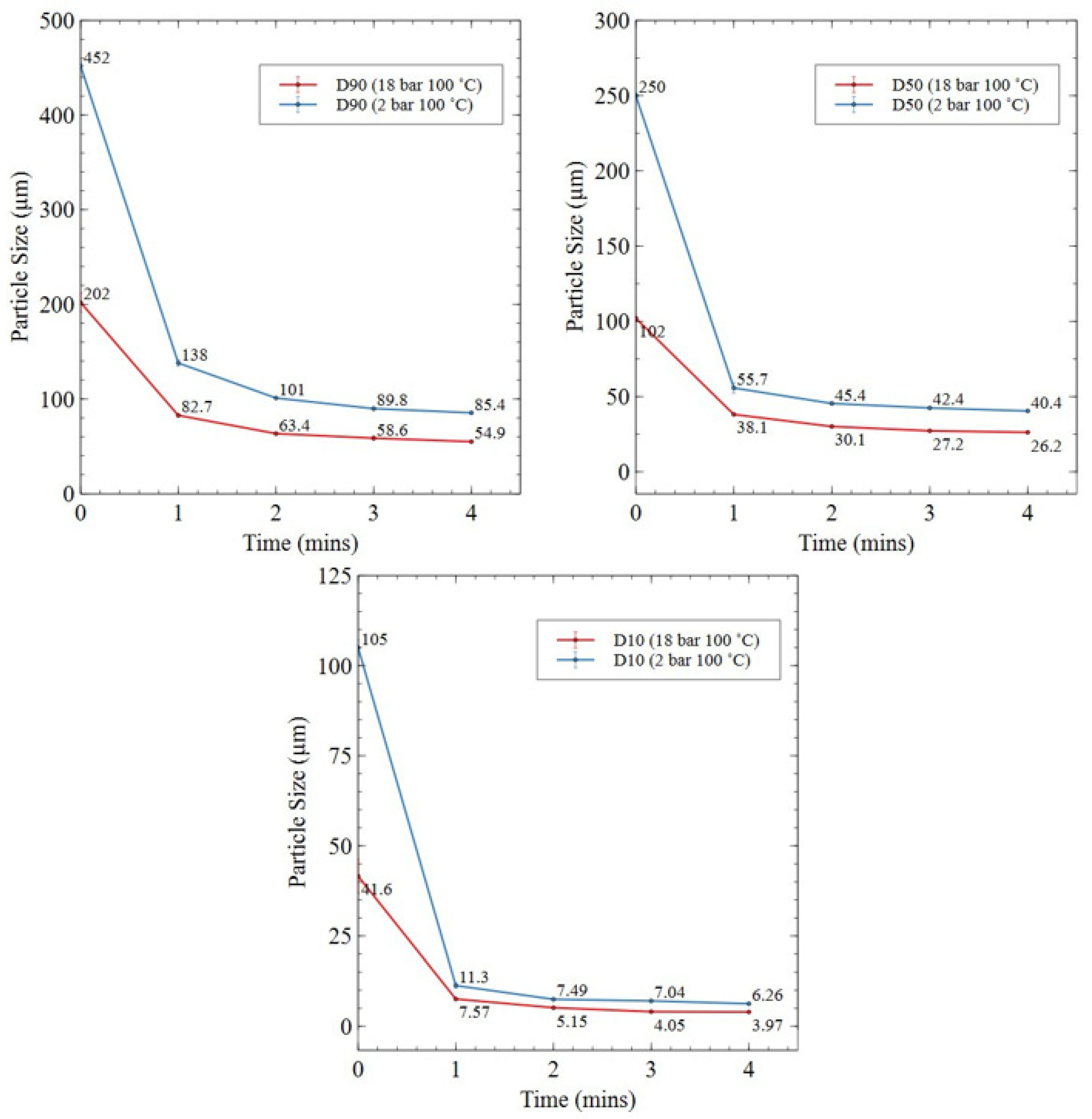
3.2.1. HD Powder
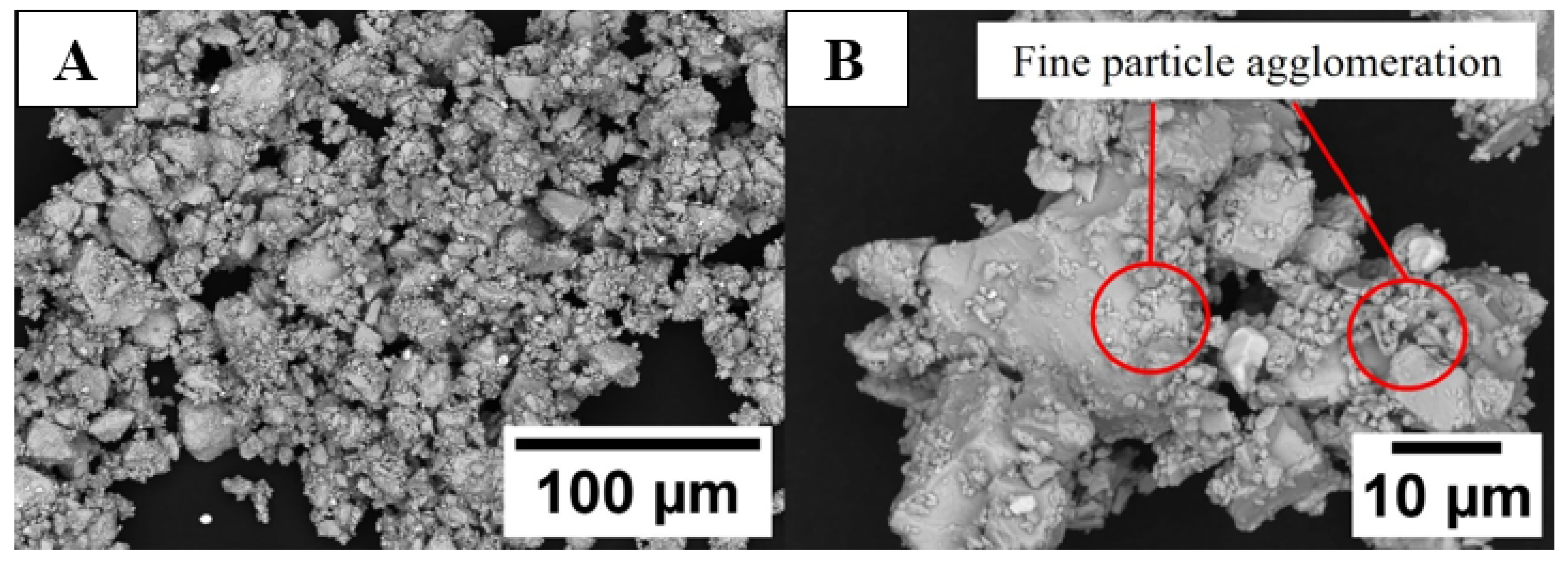
3.2.2. Knife-Milled Powder
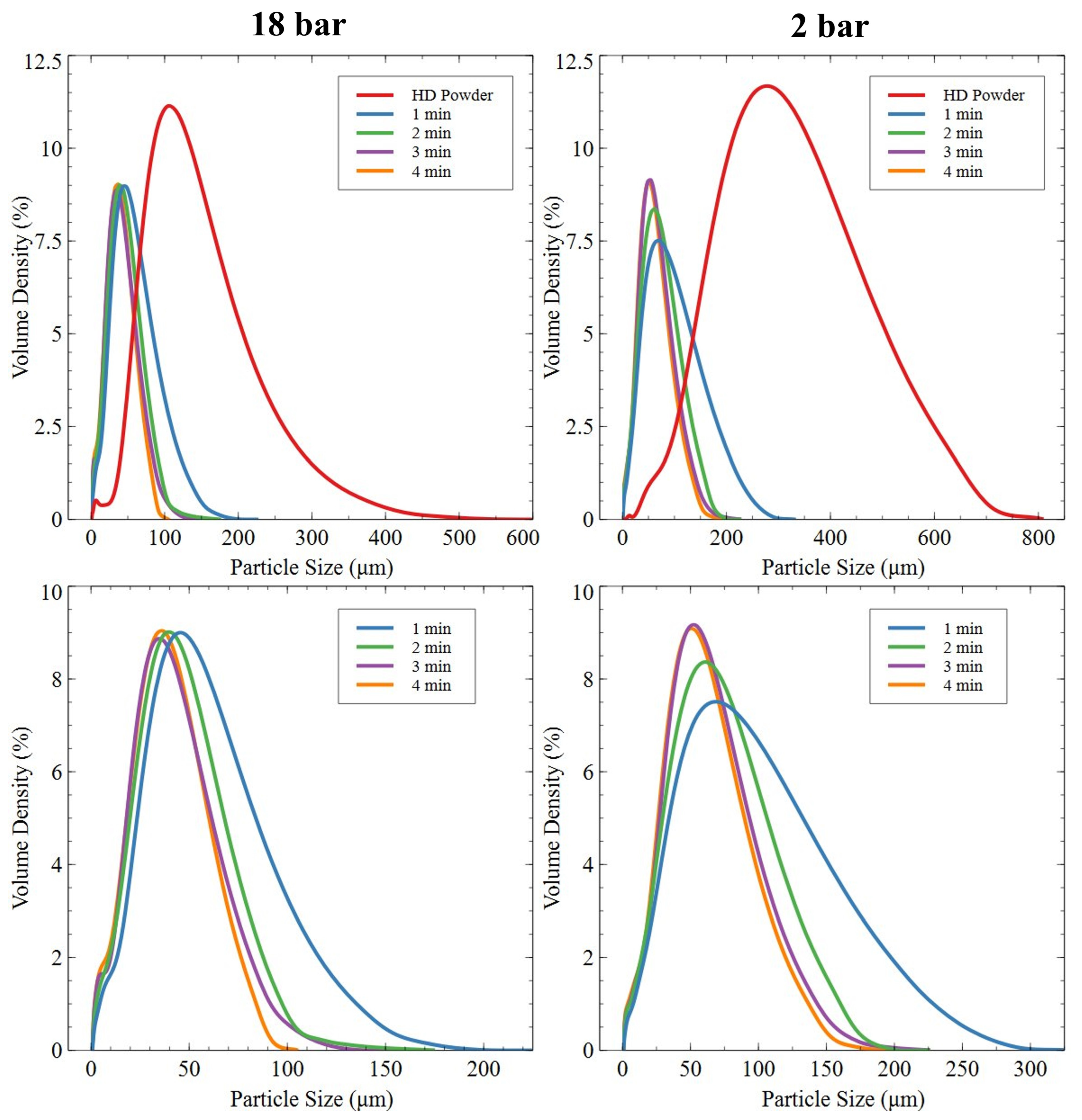
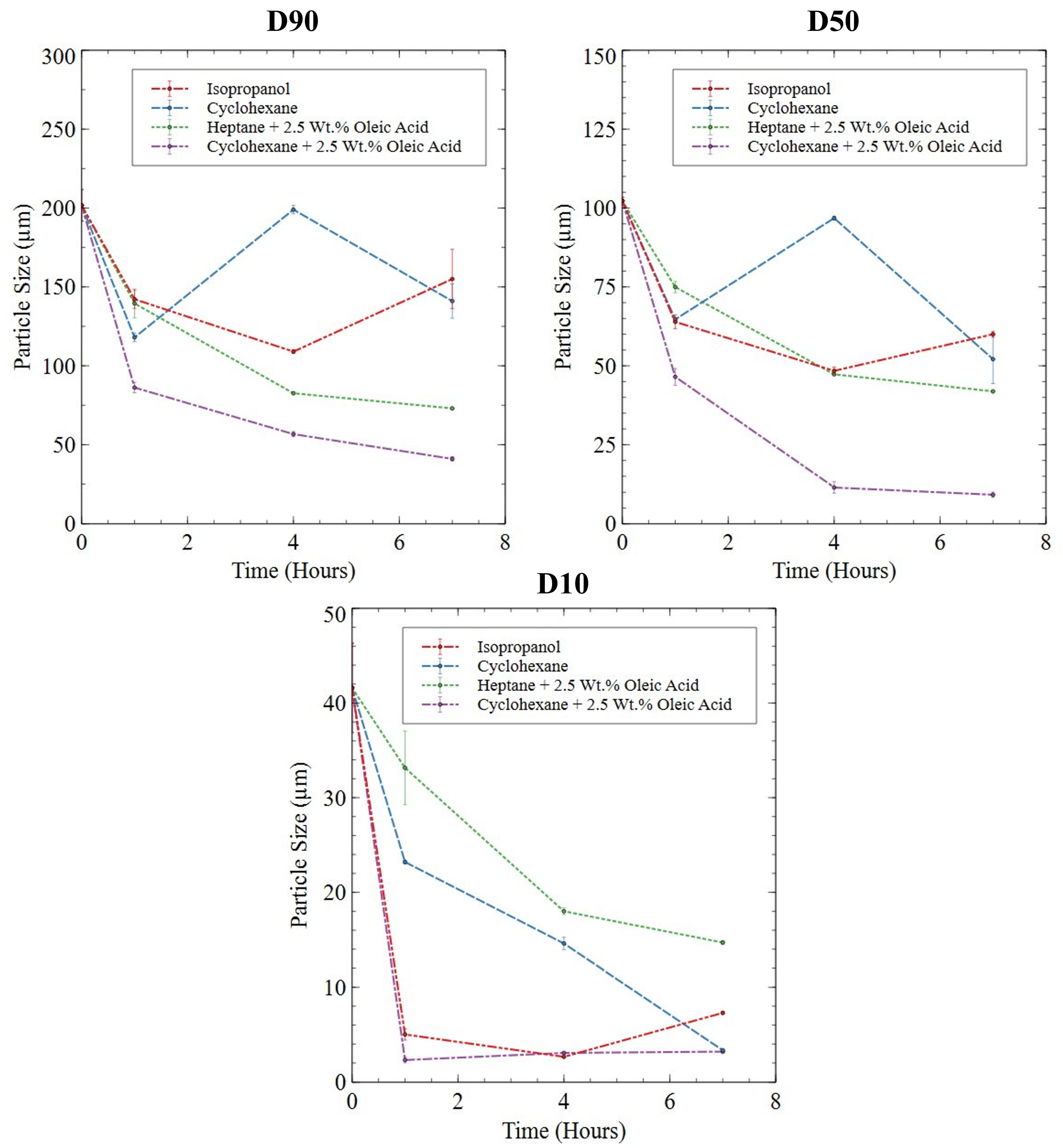
3.2.3. Roller Ball-Milled Powder
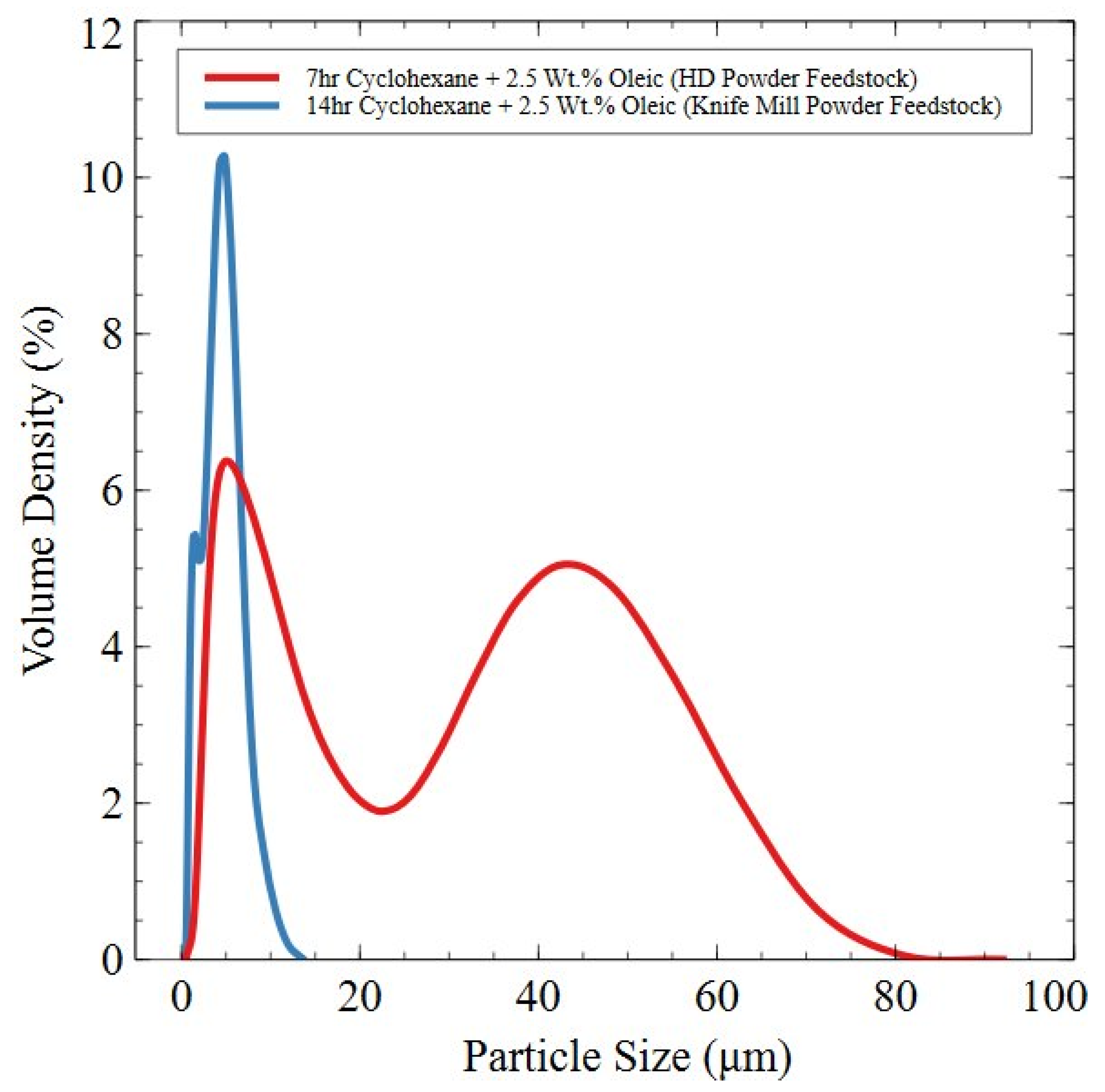
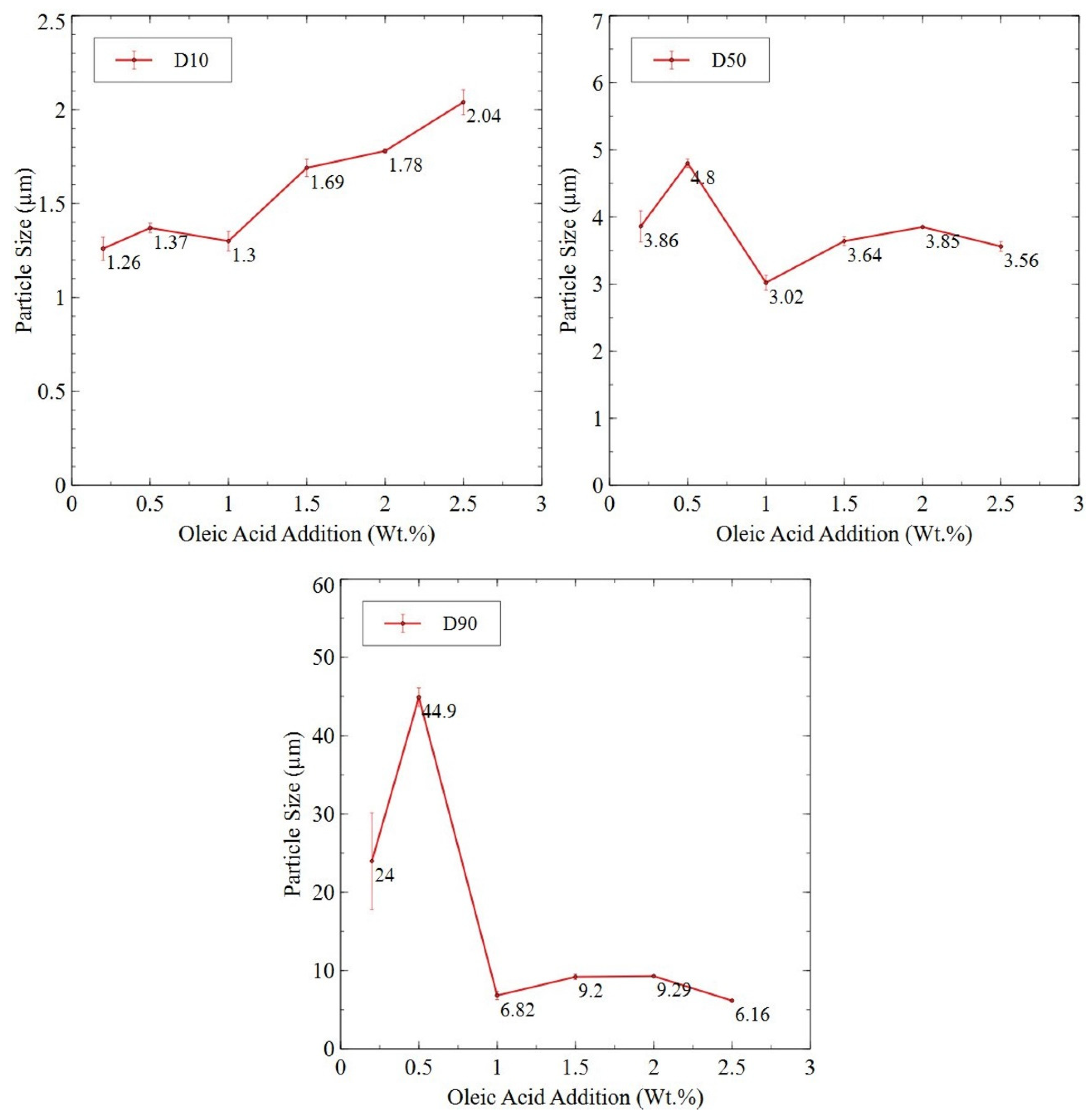
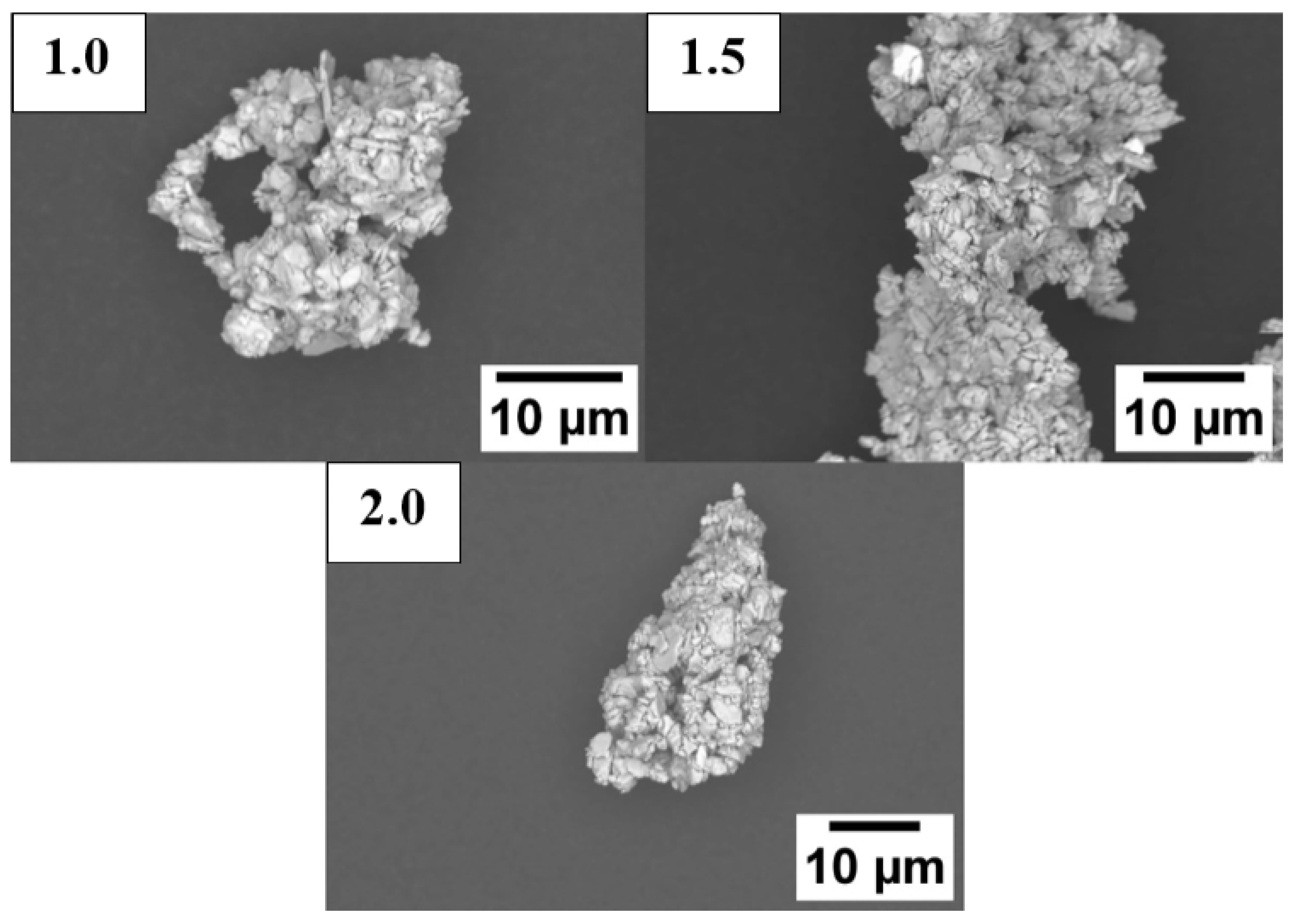
3.2.4. Planetary Ball-Milled Powder
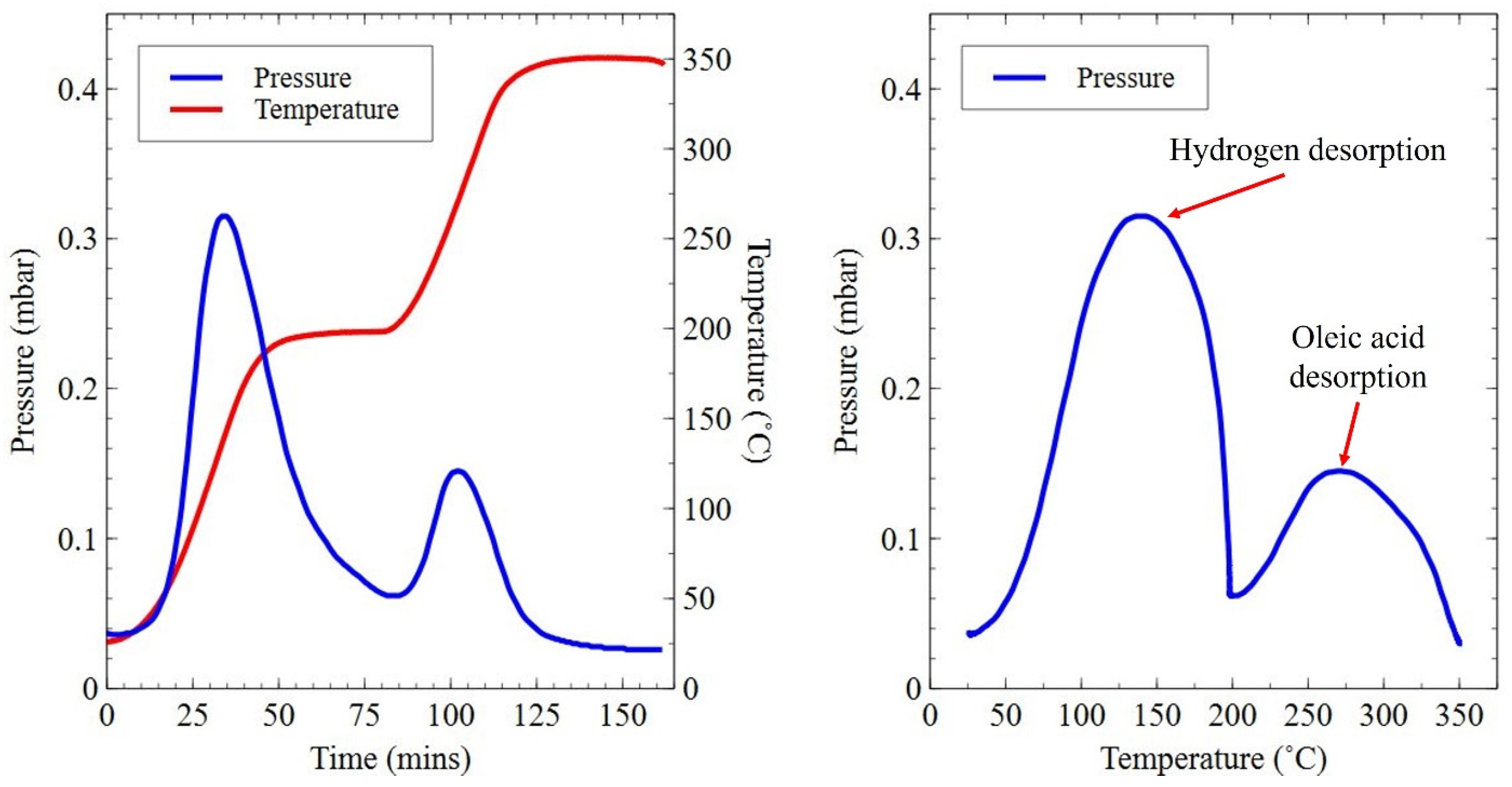
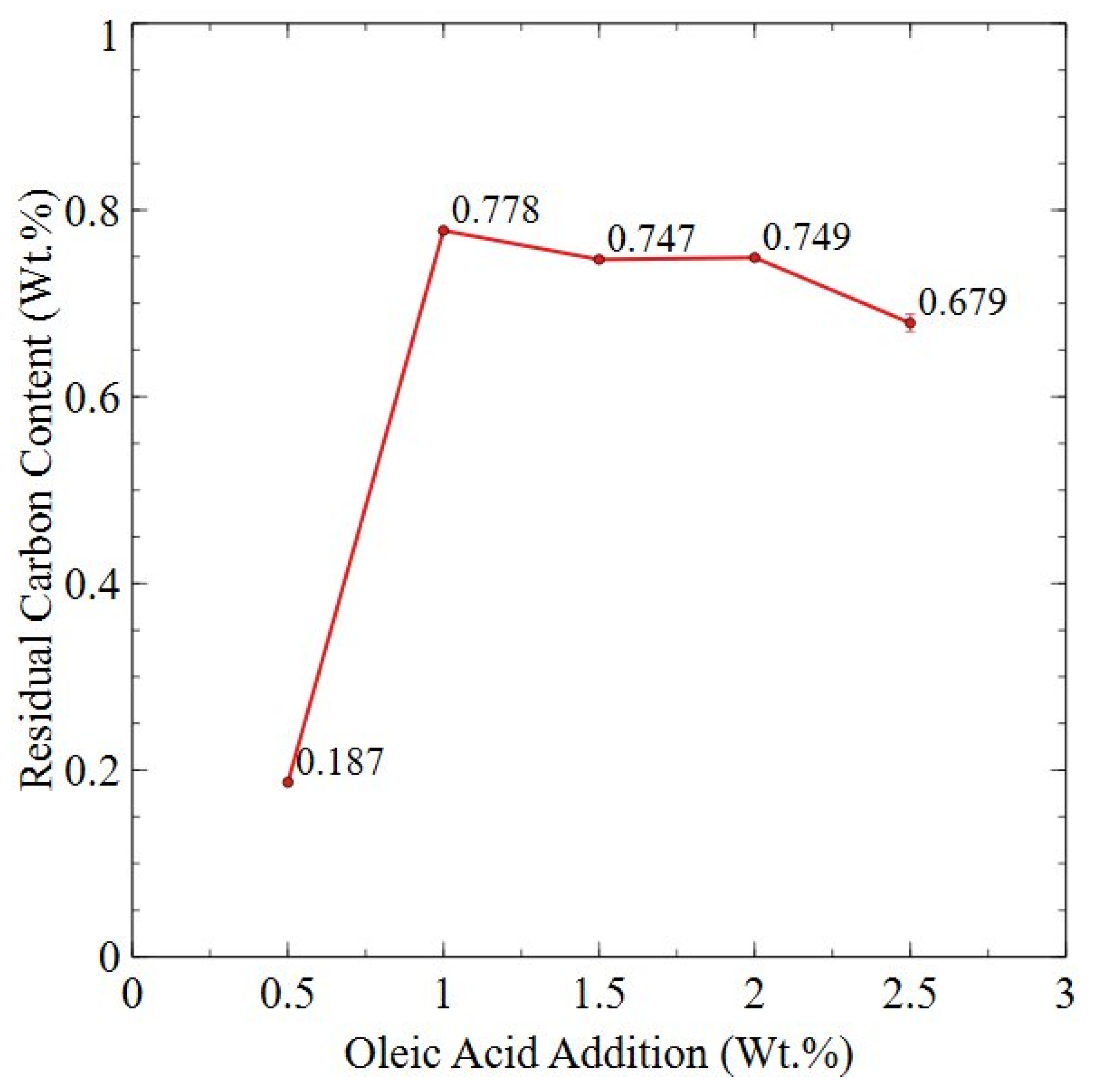
3.3. Degassing and Surfactant Removal from Planetary Ball-Milled Powder
3.4. Sintering Trials for Density (Planetary Ball Milling)
3.5. Sm Additions and Full Heat Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Strnat, K.J. The Hard-Magnetic Properties of Rare Earth-Transition Metal Alloys. IEEE Trans. Magn. 1972, 8, 511–516. [Google Scholar] [CrossRef]
- Ray, A.E.; Liu, S. Recent progress in 2:17-type permanent magnets. J. Mater. Eng. Perform. 1992, 1, 183–191. [Google Scholar] [CrossRef]
- Ojima, T.; Tomizawa, S.; Yoneyama, T.; Hori, T. New Type Rare Earth Cobalt Magnets with an Energy Product of 30 MGOe. Jpn. J. Appl. Phys. 1977, 16, 671–672. [Google Scholar] [CrossRef]
- Harris, I.R.; Jewell, G.W. Rare-earth magnets: Properties, processing and applications. In Functional Materials for Sustainable Energy Applications; Woodhead Publishing: Sawston, UK, 2012; pp. 600–639. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, P.; Lu, Y.; Cao, X.; Huang, Y.; Gill, V.; Li, Z. Unveiling the anomalous atomic stacking and formation mechanism of 1:3R Z-plates in Sm2Co17-type magnets. J. Alloys Compd. 2023, 934, 167890. [Google Scholar] [CrossRef]
- Duerrschnabel, M.; Yi, M.; Uestuener, K.; Liesegang, M.; Katter, M.; Kleebe, H.J.; Xu, B.; Gutfleisch, O.; Molina-Luna, L. Atomic structure and domain wall pinning in samarium-cobalt-based permanent magnets. Nat. Commun. 2017, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ding, Y.; Liu, L.; Sun, Y.; Wang, F.; Xu, D.; Hu, F.; Wang, F.; Liang, J.; Yan, A. Study on the grain growth and the evolution of defects around grain boundaries during heat treatment process in Sm2Co17 permanent magnets. J. Alloys Compd. 2023, 969, 172444. [Google Scholar] [CrossRef]
- Zhang, T.L.; Song, Q.; Wang, H.; Wang, J.M.; Liu, J.H.; Jiang, C.B. Effects of solution temperature and Cu content on the properties and microstructure of 2:17-type SmCo magnets. J. Alloys Compd. 2018, 735, 1971–1976. [Google Scholar] [CrossRef]
- Cataldo, L.; Lefèvre, A.; Ducret, F.; Cohen-Adad, M.T.; Allibert, C.; Valignat, N. Binary system Sm-Co: Revision of the phase diagram in the Co rich field. J. Alloys Compd. 1996, 241, 216–223. [Google Scholar] [CrossRef]
- Song, X.; Liu, Y.; Yuan, T.; Wang, F.; Fan, J.; Ma, T. Effect of post-solutionizing cooling rate on microstructure and magnetic properties of 2:17-type Sm-Co-Fe-Cu-Zr magnets. J. Alloys Compd. 2022, 896, 163080. [Google Scholar] [CrossRef]
- Xue, Z.Q.; Liu, L.; Liu, Z.; Li, M.; Lee, D.; Chen, R.J.; Guo, Y.Q.; Yan, A.R. Mechanism of phase transformation in 2:17 type SmCo magnets investigated by phase stabilization. Scr. Mater. 2016, 113, 226–230. [Google Scholar] [CrossRef]
- Cui, J.; Ormerod, J.; Parker, D.; Ott, R.; Palasyuk, A.; Mccall, S.; Paranthaman, M.P.; Kesler, M.S.; McGuire, M.A.; Nlebedim, I.C.; et al. Manufacturing Processes for Permanent Magnets: Part I—Sintering and Casting. JOM 2022, 74, 1279–1295. [Google Scholar] [CrossRef]
- Herrick, C.C. The vapor pressure and heat of sublimation of samarium. J. Less Common Met. 1964, 7, 618–620. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials for the EU. Technical Report. 2010. Available online: https://ec.europa.eu/commission/presscorner/detail/en/memo_10_263 (accessed on 17 January 2025).
- European Commission. Report on Critical Materials for the EU; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- European Commission. Study on the Review of the List of Critical Raw Materials: Executive Summary. 2017. Available online: https://op.europa.eu/en/publication-detail/-/publication/08fdab5f-9766-11e7-b92d-01aa75ed71a1/language-en (accessed on 17 January 2025).
- European Commission. Critical Raw Materials Resilience: Charting a Path Towards Greater Security and Sustainability; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Commission. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023; Available online: https://data.europa.eu/doi/10.2873/725585 (accessed on 17 January 2025).
- Department for Buisness Energy & Industrial Strategy. Policy Paper Resilience for the Future: The UK’s Critical Minerals Strategy. 2023. Available online: https://www.gov.uk/government/publications/uk-critical-mineral-strategy/4acf2ca4-70cf-4834-a081-cf16b7c66959 (accessed on 17 January 2025).
- Ren, X.; Wei, L.; Sun, A.; Xu, B.; Jiang, W.; Yang, B.; Wang, F. Recycling of Sm-Co permanent magnet waste: Perspectives and recent advances. J. Rare Earths 2025. [Google Scholar] [CrossRef]
- Harris, I.R. The potential of hydrogen in permanent magnet production. J. Less Common Met. 1987, 131, 245–262. [Google Scholar] [CrossRef]
- Walton, A.; Yi, H.; Rowson, N.A.; Speight, J.D.; Mann, V.S.J.; Sheridan, R.S.; Bradshaw, A.; Harris, I.R.; Williams, A.J. The use of hydrogen to separate and recycle neodymium-iron-boron-type magnets from electronic waste. J. Clean. Prod. 2015, 104, 236–241. [Google Scholar] [CrossRef]
- Eldosouky, A.; Škulj, I. Recycling of SmCo5 magnets by HD process. J. Magn. Magn. Mater. 2018, 454, 249–253. [Google Scholar] [CrossRef]
- Zheng, M.; Bi, Y.; Hou, Z.; Wang, C.; Wang, J.; Wang, L.; Duan, Z.; Zhang, B.; Fang, Y.; Zhu, M.; et al. Enhanced hydrogen adsorption capability of 2:17-type Sm-Co strips via microstructure modification. J. Alloys Compd. 2024, 987, 174182. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, D.; Zhang, H.; Shang, Z.; Wang, H.; Meng, C.; Liu, W.; Yue, M. Effect of hydrogen pressure on hydrogenation and pulverization behavior of Sm(CoFeCuZr)z ingot and strip casting flake. J. Alloys Compd. 2023, 930, 167427. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, D.; Zhang, H.; Li, Y.; Meng, C.; Teng, Y.; Liu, W.; Yue, M. Combination strategy for high-performance Sm(CoFeCuZr)z sintered permanent magnet: Synergistic improvement of the preparation process. Acta Mater. 2023, 251, 118901. [Google Scholar] [CrossRef]
- Eldosouky, A.; Škulj, I. Hydrogen Reaction with SmCo Compounds: Literature Review. J. Sustain. Metall. 2018, 4, 516–527. [Google Scholar] [CrossRef]
- Zakotnik, M.; Prosperi, D.; Williams, A.J. Kinetic studies of hydrogen desorption in SmCo 2/17-type sintered magnets. Thermochim. Acta 2009, 486, 41–45. [Google Scholar] [CrossRef]
- Zakotnik, M.; Williams, A.J.; Martinek, G.; Harris, I.R. Hydrogen decrepitation of a 2/17 sintered magnet at room temperature. J. Alloys Compd. 2008, 450, L1. [Google Scholar] [CrossRef]
- Griffiths, J.; Brooks, O.P.; Subramanian, G.; Kozak, V.; Brown, D.; Campbell, A.; Lambourne, A.; Sheridan, R.S. The effects of temperature and pressure on the hydrogen decrepitation and hydrogen desorption of Sm2TM17 sintered magnets. Intermetallics 2025, 184, 108831. [Google Scholar] [CrossRef]
- Schönfeldt, M.; Opelt, K.; Betz, S.; Metzmacher, C.; Yoon, S.; Gassmann, J. Chemical Stability and Hydrogen Reaction Behavior of SmCo 2:17-Type Permanent Magnets. Adv. Eng. Mater. 2023, 25, 2201934. [Google Scholar] [CrossRef]
- Chinnasamy, C.; Marinescu, M.; Liu, J. Large Scale Recycling of Industrial Scrap Sm (Co, Fe, Cu, Zr)z Magnets into Valuable Permanent Magnets. In Proceedings of the 23rd International Workshop Rare Earth and Future Permanet Magnets and Their Applications, Annapolis, MD, USA, 17–21 August 2015. [Google Scholar]
- Zhou, K.; Wang, A.; Zhang, D.; Zhang, X.; Yang, T. Sulfuric acid leaching of Sm–Co alloy waste and separation of samarium from cobalt. Hydrometallurgy 2017, 174, 66–70. [Google Scholar] [CrossRef]
- Kaya, E.E. An Integrated Hydrometallurgical Treatment and Combustion Process for Sustainable Production of Sm2O3 Nanoparticles from Waste SmCo Magnets. Min. Metall. Explor. 2024, 41, 2047–2056. [Google Scholar] [CrossRef]
- Liu, R.; Liu, X.; Li, J.; Yin, X.; Yang, Y. Bifunctional hydrophobic deep eutectic solvents for selective recovery of Sm and Co from waste SmCo permanent magnets. J. Ind. Eng. Chem. 2024, 130, 191–202. [Google Scholar] [CrossRef]
- Papakci, M.; Emil-kaya, E.; Stopic, S.; Gurmen, S.; Freidrich, B. Recovery of valuable metals from SmCo magnets through sulfation, selective oxidation, and water leaching. Sep. Sci. Technol. 2024, 59, 1241–1254. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Tang, Y.-C.; Shen, Y.-H. Leaching of Sm, Co, Fe, and Cu from Spent SmCo Magnets Using Organic Acid. Metals 2023, 13, 233. [Google Scholar] [CrossRef]
- Ormerod, J. The physical metallurgy and processing of sintered rare earth permanent magnets. J. Less Common Met. 1985, 111, 49–69. [Google Scholar] [CrossRef]
- Griffiths, J.; Brooks, O.; Kozak, V.; Xia, W.; Kitaguchi, H.; Brown, D.; Campbell, A.; Lambourne, A.; Sheridan, R.S. Hydrogen Decrepitation of Sm2TM17 Sintered Magnets from Scrap Rotor Assemblies. University of Birmingham: Birmingham, UK, 2025; submitted and under review, unpublished. [Google Scholar]
- Nayebossadri, S.; Awais, M.; Arnold, R.; Degri, M.; Mann, N.; Walton, A. Hydrogen assisted recycling of Nd-Fe-B magnets from the end-of-life audio products. J. Magn. Magn. Mater. 2024, 603, 172239. [Google Scholar] [CrossRef]
- Nouri, A.; Wen, C. Surfactants in mechanical alloying/milling: A catch-22 situation. Crit. Rev. Solid State Mater. Sci. 2014, 39, 81–108. [Google Scholar] [CrossRef]
- Sarwat, S.G. Contamination in wet-ball milling. Powder Metall. 2017, 60, 267–272. [Google Scholar] [CrossRef]
- Kwon, H.W.; Harris, I.R. Study of Sm(Co,Fe,Cu,Zr)7.1 magnets produced using a combination of hydrogen decrepitation and ball milling. J. Appl. Phys. 1991, 69, 5856–5858. [Google Scholar] [CrossRef]
- Degri, M.J.J. The Processing and Characterisation of Recycled NdFeB-Type Sintered Magnets. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2014. [Google Scholar]
- Pal, S.K.; Schultz, L.; Gutfleisch, O. Effect of milling parameters on SmCo5 nanoflakes prepared by surfactant-assisted high energy ball milling. J. Appl. Phys. 2013, 113, 013913. [Google Scholar] [CrossRef]
- Leontsev, S.; Lucas, M.; Shen, Y.; Sheets, A.; Horwath, J.; Karapetrova, E.; Crouse, C. Surfactant removal study for nano-scale SmCo5 powder prepared by high energy ball milling. IEEE Trans. Magn. 2013, 49, 3341–3344. [Google Scholar] [CrossRef]
- Jovic, V.; Lamovec, J.; Sojer, D.; Loncarevic, D.; Mladenovic, I.; Vasiljevic Radovic, D. Effect of high energy ball milling on the morphology and magnetic properties of powder prepared from HD Nd2Fe14B material. In Proceedings of the 2017 IEEE 30th International Conference on Microelectronics (MIEL), Nis, Serbia, 9–11 October 2017; pp. 131–134. [Google Scholar] [CrossRef]
- Yu, N.; Pan, M.; Zhang, P.; Ge, H.; Wu, Q. Effect of milling time on the morphology and magnetic properties of SmCo5 nanoflakes fabricated by surfactant-assisted high-energy ball milling. J. Magn. Magn. Mater. 2015, 378, 107–111. [Google Scholar] [CrossRef]
- Gabay, A.M.; Akdogan, N.G.; Marinescu, M.; Liu, J.F.; Hadjipanayis, G.C. Rare earth-cobalt hard magnetic nanoparticles and nanoflakes by high-energy milling. J. Phys. Condens. Matter 2010, 22, 164213. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, T.; Wang, H.; Jiang, C.; Liu, J. Effect of ball milling process on coercivity of nanocrystalline SmCo5 magnets. J. Magn. Magn. Mater. 2018, 446, 200–205. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, S.; Qu, X. Effects of oxygen and carbon on the magnetic properties and micro-structure of Sm2Co17 permanent magnets. Rare Met. 2007, 26, 299–304. [Google Scholar] [CrossRef]
- Andrew, D.; Ronald, L. Effect of molecular architecture of long chain fatty acids on the dispersion properties of titanium dioxide in non-aqueous liquids. Faraday Discuss. Chem. Soc. 1977, 65, 252–263. [Google Scholar]
- Tian, J.; Zhang, S.; Qu, X.; Akhtar, F.; Tao, S. Behavior of residual carbon in Sm(Co, Fe, Cu, Zr)z permanent magnets. J. Alloys Compd. 2007, 440, 89–93. [Google Scholar] [CrossRef]
- Christodoulou, C.N.; Takeshita, T. Reaction of samarium with hydrogen and nitrogen samarium oxides. J. Alloys Compd. 1992, 190, 99–106. [Google Scholar] [CrossRef]
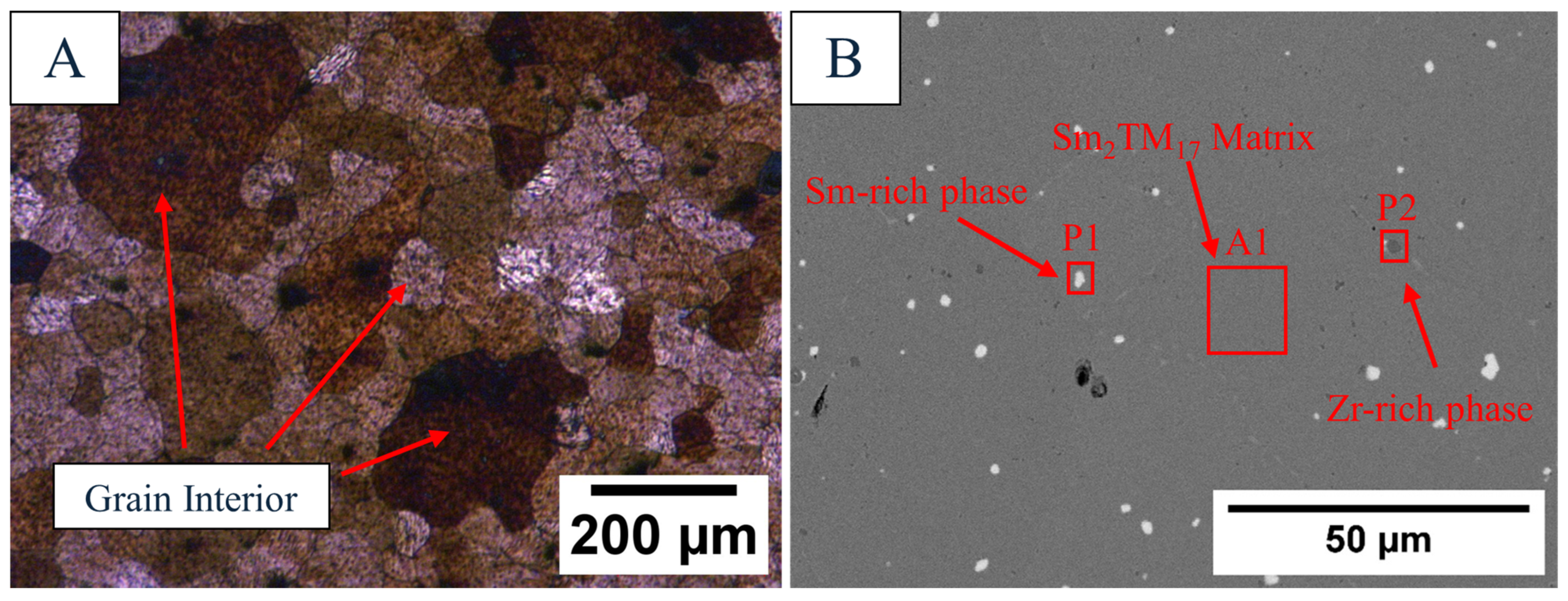
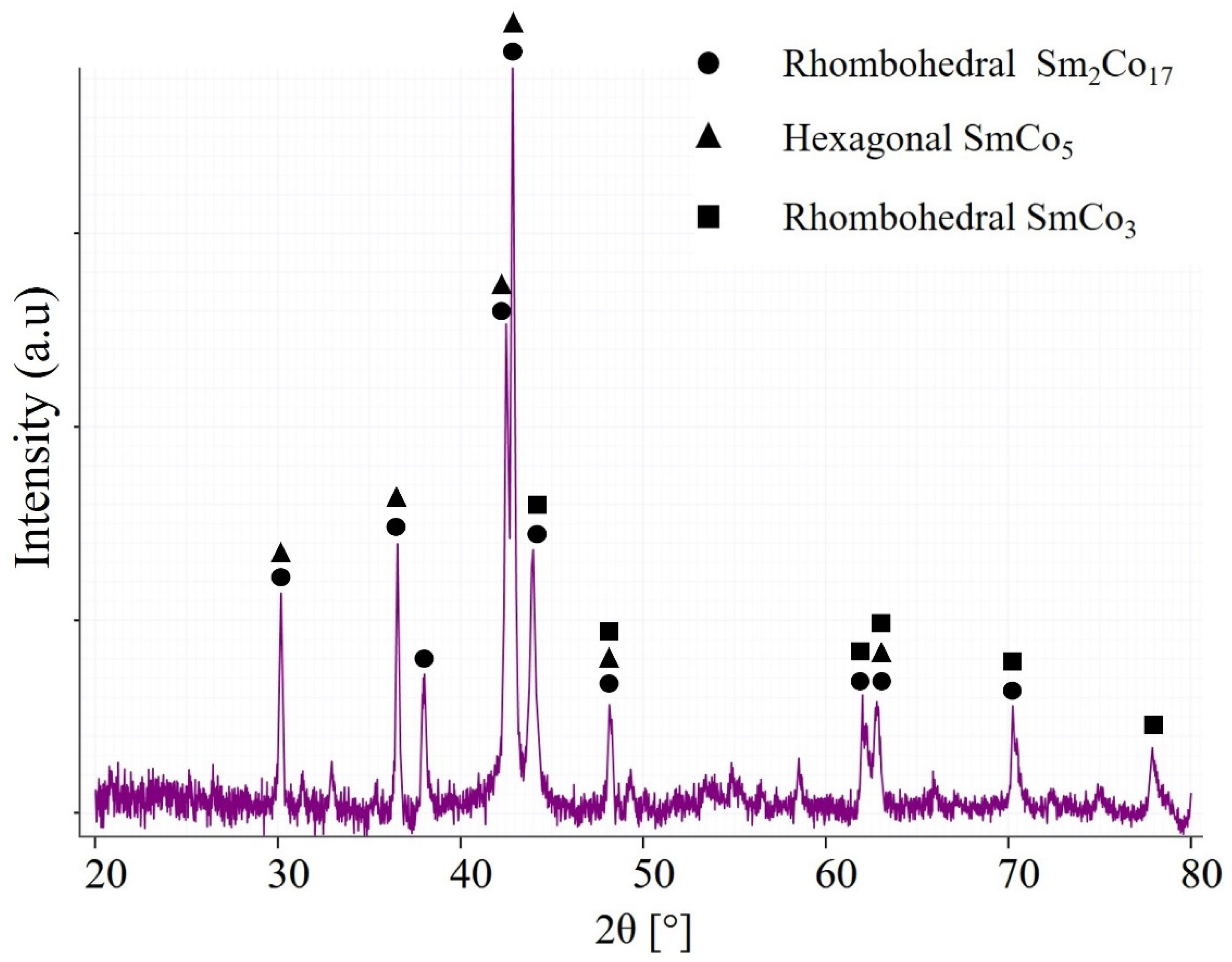
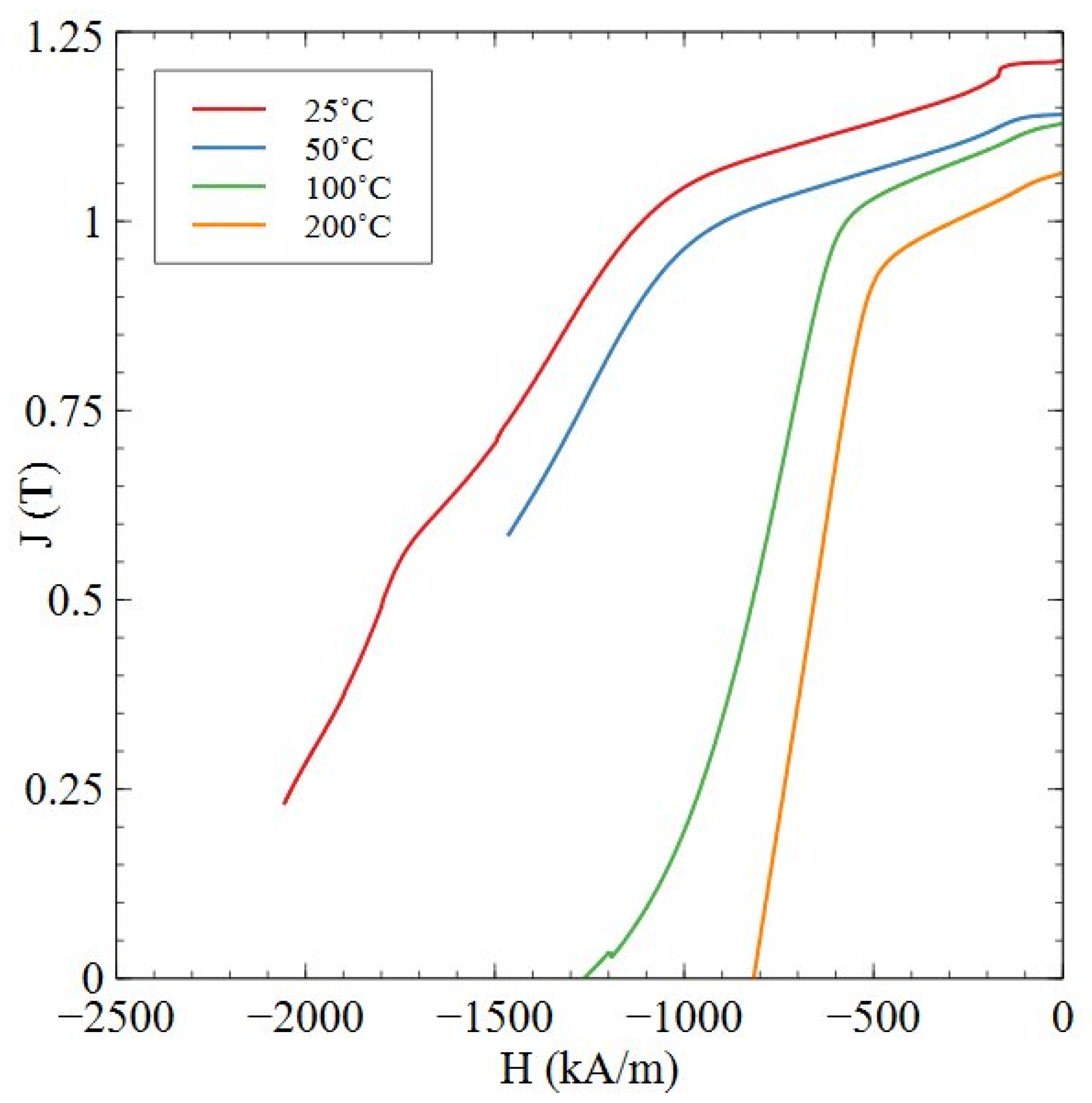

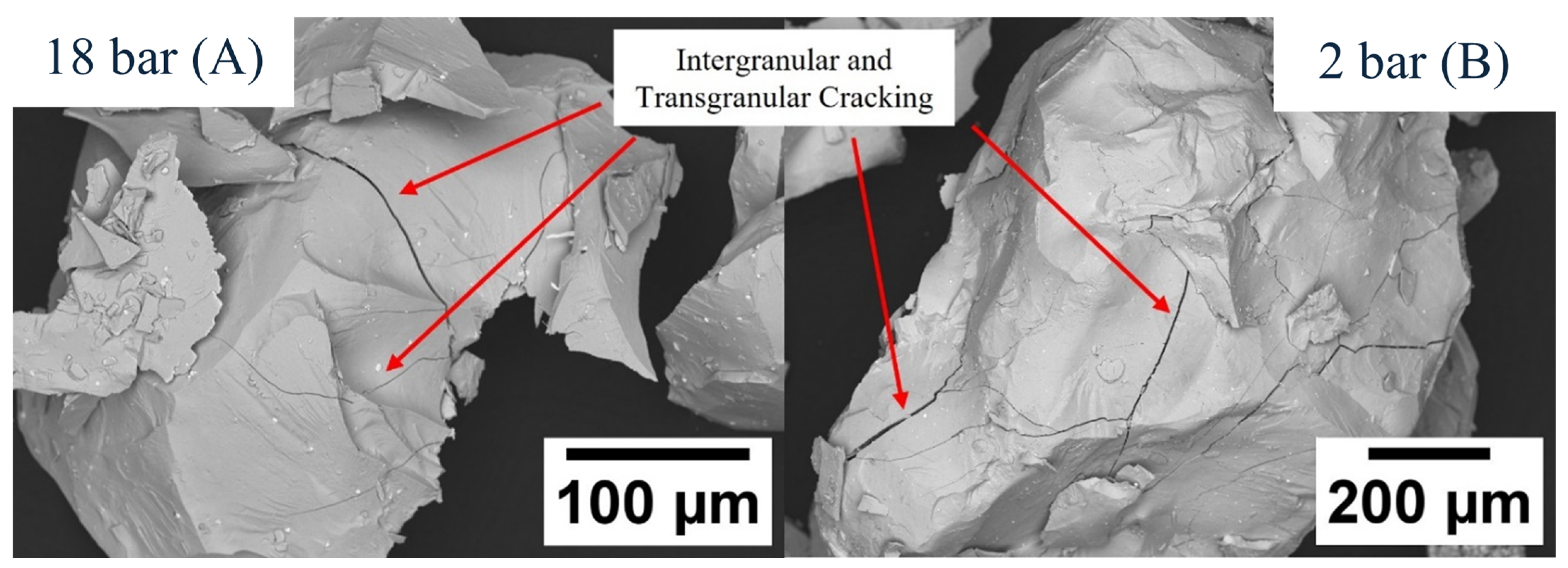


| Sample | Sm (wt.%) | Co (wt.%) | Fe (wt.%) | Cu (wt.%) | Zr (wt.%) | O (ppm) | N (ppm) | C (ppm) |
|---|---|---|---|---|---|---|---|---|
| As-received | 23.9 ± 0.16 | 49.3 ± 0.28 | 19.3 ± 0.10 | 4.8 ± 0.09 | 2.8 ± 0.12 | 1600 ± 86 | 1100 ± 84 | 850 ± 20 |
| EDS Site | Sm (wt.%) | Co (wt.%) | Fe (wt.%) | Cu (wt.%) | Zr (wt.%) |
|---|---|---|---|---|---|
| A1 | 24.9 ± 0.41 | 48.8 ± 0.54 | 18.8 ± 0.31 | 5.30 ± 0.78 | 2.21 ± 0.17 |
| P1 | 56.4 ± 0.58 | 28.8 ± 0.41 | 10.6 ± 0.30 | 2.82 ± 0.71 | 1.44 ± 0.19 |
| P2 | 8.86 ± 0.36 | 15.2 ± 0.29 | 6.09 ± 0.22 | 1.24 ± 0.52 | 68.6 ± 0.54 |
| Temperature (°C) | Remanence (T) | Coercivity (kA/m) | (BH)max (kJ/m3) |
|---|---|---|---|
| 25 | 1.21 | >2055 | >258 |
| 50 | 1.14 | >1464 | >232 |
| 100 | 1.13 | 1267 | 221 |
| 200 | 1.06 | 818 | 191 |
| Sample | Sm (wt.%) | Co (wt.%) | Fe (wt.%) | Cu (wt.%) | Zr (wt.%) | O (ppm) | N (ppm) | C (ppm) |
|---|---|---|---|---|---|---|---|---|
| As-received | 23.9 | 49.3 | 19.3 | 4.8 | 2.8 | 1600 | 1100 | 850 |
| 1.0 wt.% | 22.6 | 49.9 | 18.7 | 4.8 | 2.6 | 3400 | 2300 | 8200 |
| 1.5 wt.% | 19.4 | 50.2 | 19.4 | 4.4 | 3.0 | 4100 | 2700 | 7500 |
| 2.0 wt.% | 22.1 | 49.1 | 18.8 | 4.6 | 2.9 | 3500 | 2100 | 7200 |
| Sample | Density (g/cm3) |
|---|---|
| As-received | 8.40 ± 0.002 |
| 1.0 wt.% | 8.32 ± 0.006 |
| 1.5 wt.% | 8.30 ± 0.004 |
| 2.0 wt.% | 8.30 ± 0.01 |
| EDS Site | Sm (wt.%) | Co (wt.%) | Fe (wt.%) | Cu (wt.%) | Zr (wt.%) |
|---|---|---|---|---|---|
| 1 | 24.4 ± 0.40 | 50.0 ± 0.53 | 18.6 ± 0.31 | 7.1 ± 0.77 | - |
| 2 | 8.20 ± 0.30 | 59.0 ± 0.58 | 31.8 ± 0.38 | 1.1 ± 0.80 | - |
| 3 | 15.5 ± 0.54 | 46.4 ± 0.55 | 22.3 ± 0.43 | - | 15.8 ± 0.38 |
| 4 | 70.4 ± 0.35 | 29.6 ± 0.35 | - | - | - |
| 1.0 wt.% Oleic Acid | |||||
| 1 | 23.8 ± 0.40 | 49.1 ± 0.54 | 18.3 ± 0.31 | 7.5 ± 0.79 | 1.4 ± 0.16 |
| 2 | 2.11 ± 0.26 | 60.2 ± 0.65 | 34.6 ± 0.43 | 3.1 ± 0.91 | - |
| 3 | 15.6 ± 0.37 | 29.3 ± 0.37 | 9.7 ± 0.25 | 4.2 ± 0.64 | 41.3 ± 0.43 |
| 4 | 70.8 ± 0.35 | 29.2 ± 0.35 | - | - | - |
| 1.5 wt.% Oleic Acid | |||||
| 1 | 27.0 ± 0.45 | 47.2 ± 0.58 | 17.1 ± 0.32 | 6.3 ± 0.91 | 2.3 ± 0.17 |
| 2 | 12.2 ± 0.51 | 47.6 ± 0.49 | 23.5 ± 0.38 | - | 16.4 |
| 3 | 4.1 ± 0.37 | 4.8 ± 0.24 | 2.0 ± 0.20 | 0.2 ± 0.47 | 88.9 ± 0.61 |
| 4 | 68.1 ± 0.39 | 31.8 ± 0.37 | - | - | - |
| 2.0 wt.% Oleic Acid | |||||
| Sample | Sm (wt.%) | Co (wt.%) | Fe (wt.%) | Cu (wt.%) | Zr (wt.%) | C (ppm) |
|---|---|---|---|---|---|---|
| As-received | 23.9 ± 0.16 | 49.3 ± 0.28 | 19.3 ± 0.10 | 4.8 ± 0.09 | 2.8 ± 0.12 | 850 ± 20 |
| 2 wt.% Sm | 23.9 ± 0.11 | 48.7 ± 0.21 | 18.8 ± 0.14 | 4.5 ± 0.003 | 3.3 ± 0.008 | 8000 ± 19 |
| 3 wt.% Sm | 24.6 ± 0.02 | 48.5 ± 0.06 | 18.8 ± 0.009 | 4.5 ± 0.001 | 3.3 ± 0.02 | 7000 ± 130 |
| Sample | Density (g/cm3) |
|---|---|
| As-received | 8.40 ± 0.002 |
| 2.0 wt.% Sm | 8.23 ± 0.017 |
| 3.0 wt.% Sm | 8.37 ± 0.025 |
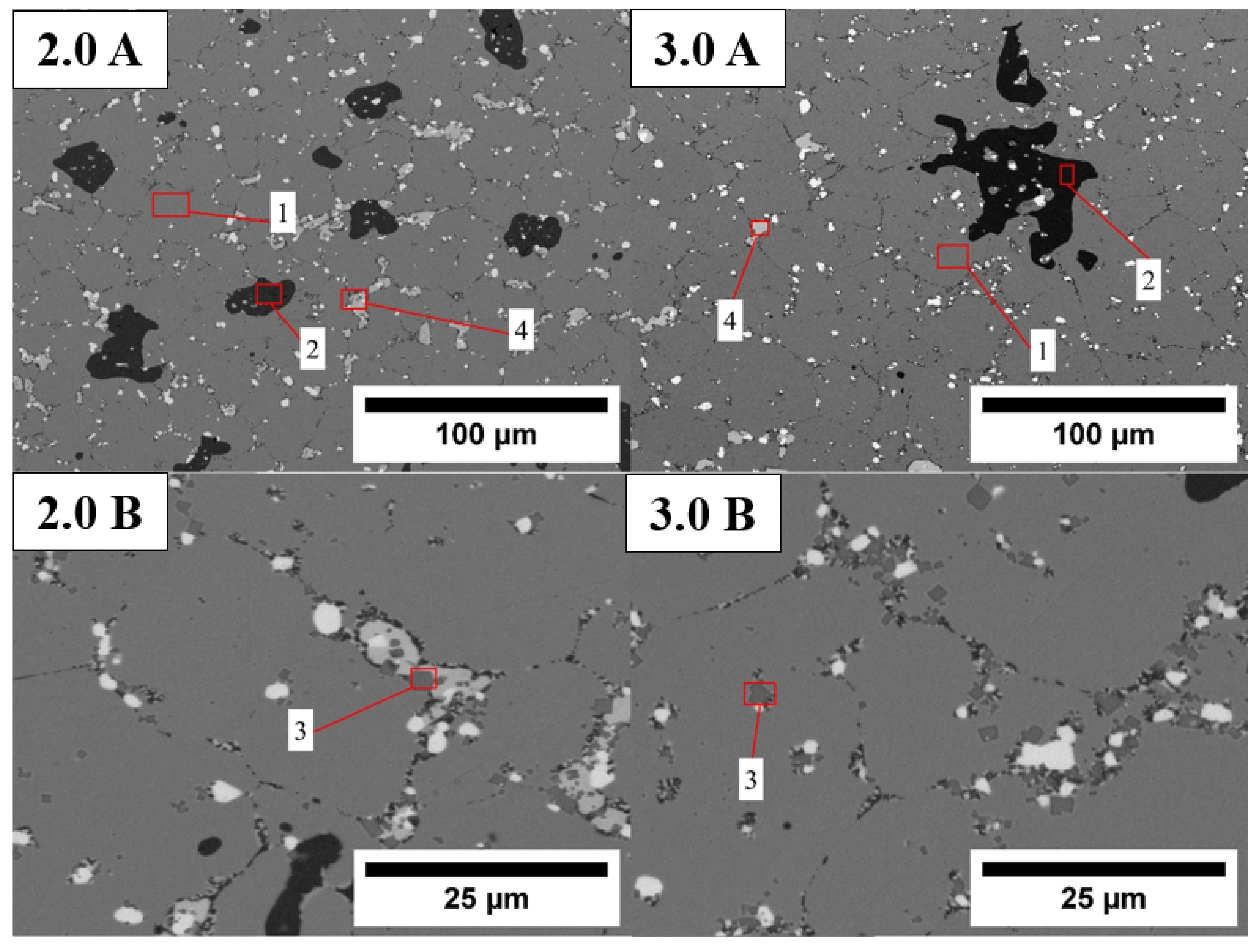
| EDS Site | Sm (wt.%) | Co (wt.%) | Fe (wt.%) | Cu (wt.%) | Zr (wt.%) |
|---|---|---|---|---|---|
| 1 | 24.7 ± 0.38 | 50.8 ± 0.51 | 18.1 ± 0.28 | 6.5 ± 0.74 | - |
| 2 | 1.51 ± 0.25 | 61.0 ± 0.31 | 37.5 ± 0.28 | - | - |
| 3 | 7.5 ± 0.31 | 5.1 ± 0.19 | 1.1 ± 0.16 | - | 86.3 ± 0.36 |
| 4 | 61.9 ± 0.55 | 32.2 ± 0.40 | - | 0.7 ± 0.65 | 5.2 ± 0.23 |
| 2.0 wt.% Sm | |||||
| 1 | 24.7 ± 0.39 | 49.5 ± 0.53 | 19.3 ± 0.30 | 6.5 ± 0.80 | - |
| 2 | - | 61.6 ± 0.27 | 38.4 ± 0.27 | - | - |
| 3 | 8.45 ± 0.32 | 14.8 ± 0.25 | 1.34 ± 0.5 | - | 69.3 ± 0.5 |
| 4 | 69.9 ± 0.36 | 27.5 ± 0.31 | 2.6 ± 0.26 | - | - |
| 3.0 wt.% Sm | |||||
| Sample | Remanence (T) | Coercivity (kA/m) | (BH)max (kJ/m3) |
|---|---|---|---|
| As-received | 1.21 | >2055 | >258 |
| 2 wt.% Sm | 0.26 | 9.1 | 0.7 |
| 3 wt.% Sm | 0.16 | 6.2 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffiths, J.T.; Brooks, O.P.; Kozak, V.; Lambourne, A.; Campbell, A.; Sheridan, R.S. The Effects of Knife Milling and Ball Milling on Hydrogen Decrepitated Sm2TM17 Sintered Magnet Powder for Short-Loop Recycling. Metals 2025, 15, 1258. https://doi.org/10.3390/met15111258
Griffiths JT, Brooks OP, Kozak V, Lambourne A, Campbell A, Sheridan RS. The Effects of Knife Milling and Ball Milling on Hydrogen Decrepitated Sm2TM17 Sintered Magnet Powder for Short-Loop Recycling. Metals. 2025; 15(11):1258. https://doi.org/10.3390/met15111258
Chicago/Turabian StyleGriffiths, James Thomas, Oliver Peter Brooks, Viktoria Kozak, Alexis Lambourne, Alexander Campbell, and Richard Stuart Sheridan. 2025. "The Effects of Knife Milling and Ball Milling on Hydrogen Decrepitated Sm2TM17 Sintered Magnet Powder for Short-Loop Recycling" Metals 15, no. 11: 1258. https://doi.org/10.3390/met15111258
APA StyleGriffiths, J. T., Brooks, O. P., Kozak, V., Lambourne, A., Campbell, A., & Sheridan, R. S. (2025). The Effects of Knife Milling and Ball Milling on Hydrogen Decrepitated Sm2TM17 Sintered Magnet Powder for Short-Loop Recycling. Metals, 15(11), 1258. https://doi.org/10.3390/met15111258





