3.1. Self-Defect Formation
We constructed a 3 × 3 × 2 supercell for investigating the formation and diffusion of defects. During the continuous reaction process of U-Mo alloy nuclear fuel, the constant consumption of uranium atoms inevitably generates many defects. The U
2Mo structure adopted in this study is a tetragonal crystal with the I4/mmm space group, which is widely used to describe experimentally observed phases in the U-Mo alloy system. Although this structure is not the thermodynamic ground state in first-principles calculations at 0 K, it exhibits good kinetic stability under high-temperature conditions. It frequently appears in experiments, making it widely used in theoretical studies. It should be noted that due to its metastable nature, the chemical potential construction process may not fully satisfy thermodynamic stability constraints. This study employs the standard states of elements as chemical potential references to calculate defect formation energies, as shown in
Figure 2. There are four basic point defects in U
2Mo: uranium vacancy (V
U), molybdenum vacancy (V
Mo), U substitutional defect on Mo site (U
Mo), and Mo substitutional defect on U site (Mo
U). In addition to vacancy and substitutional defects, we calculated the formation energies of molybdenum and uranium interstitial defects, denoted as I
Mo and I
U, respectively, as shown in
Figure 2. Since U
2Mo in this study is metastable, the following defect formation energies are analyzed based on its existence.
Figure 2 shows that among all considered point defect types, the two substitutional defects U
Mo (U atoms occupying Mo sites) and Mo
U (Mo atoms occupying U sites) exhibit the lowest formation energies, significantly lower than those of vacancies and interstitials. In U
2Mo structures, substitutional defects are the most readily formed point defect type. However, compared to substitutions and vacancies, interstitial-type defects display greater diversity, with their formation sites, lattice distortions, and energy distributions exhibiting more complex behaviors.
To comprehensively evaluate the evolution mechanisms of intrinsic defects, the subsequent analysis will investigate different configurations of interstitial defects and compare their thermodynamic feasibility with spatial distribution characteristics. This study primarily examines three possible interstitial occupation mechanisms for U and Mo: (a) U/Mo forming dumbbell-type interstitial defects; (b) U/Mo occupying octahedral interstitial sites; and (c) U/Mo occupying tetrahedral interstitial sites, as illustrated in
Figure 3. Based on the interstitial formation energies listed in
Figure 2 U/Mo tends to form dumbbell-type interstitial states in U
2Mo, making them one of the most probable defect types in the alloy. Among the intrinsic interstitial sites, the initially constructed defect was of the tet5 configuration. During geometric optimization, the interstitial atom spontaneously migrated and stabilized at a tet2 interstitial site, indicating that the tet5 site is not locally stable. In contrast, the tet2 site is thermodynamically more favorable in the current structure. Therefore, this study excludes tet5 interstitial sites from consideration in intrinsic interstitial defects. These results also show that for interstitial-type defects, the choice of initial configuration may significantly influence the final structure, necessitating special attention to their configurational reconstruction behavior.
As shown in
Figure 2, the formation energy of interstitial point defects is higher than that of other point defects, making interstitial defects more difficult to form. Therefore, we initially consider defect complexes that do not include interstitial defects. Isolated point defects may participate in atomic diffusion by aggregating to form defect complexes. Among defect complexes composed of two adjacent lattice-site point defects, the structures typically consist of five defect complexes [
38]. Thus, point defect aggregation in U
2Mo can form five defect complexes as diffusion media.
Table 1 presents the formation and binding energies of defect complexes calculated by Equations (4) and (5). Among these defect complexes, the V
U + U
Mo defect complex undergoes structural relaxation, after which the U atom moves from the substitutional position to the U vacancy, altering the original structure of the defect complex. Consequently, V
U + U
Mo cannot exist as a stable defect complex. The reason is that U atoms prefer to occupy U sites rather than Mo sites. Upon relaxation, the U atom substituting for Mo moves to the U site, fills the U vacancy, and reduces the system energy by eliminating the high formation energy of V
U.
As shown in
Table 1, the binding energies of all four defect complexes are positive values. These results show that point defects in U
2Mo exhibit attractive interactions and aggregate to form clusters. The binding energy of the V
Mo + Mo
U complex is higher than that of other defects, while its formation energy is lower than that of different defects. The results show that V
Mo + Mo
U is the predominant defect complex in U
2Mo, characterized by lower formation energy and higher binding energy, serving as potential mediators for the self-diffusion of Mo and U atoms in U
2Mo.
3.2. Self-Diffusion in U2Mo
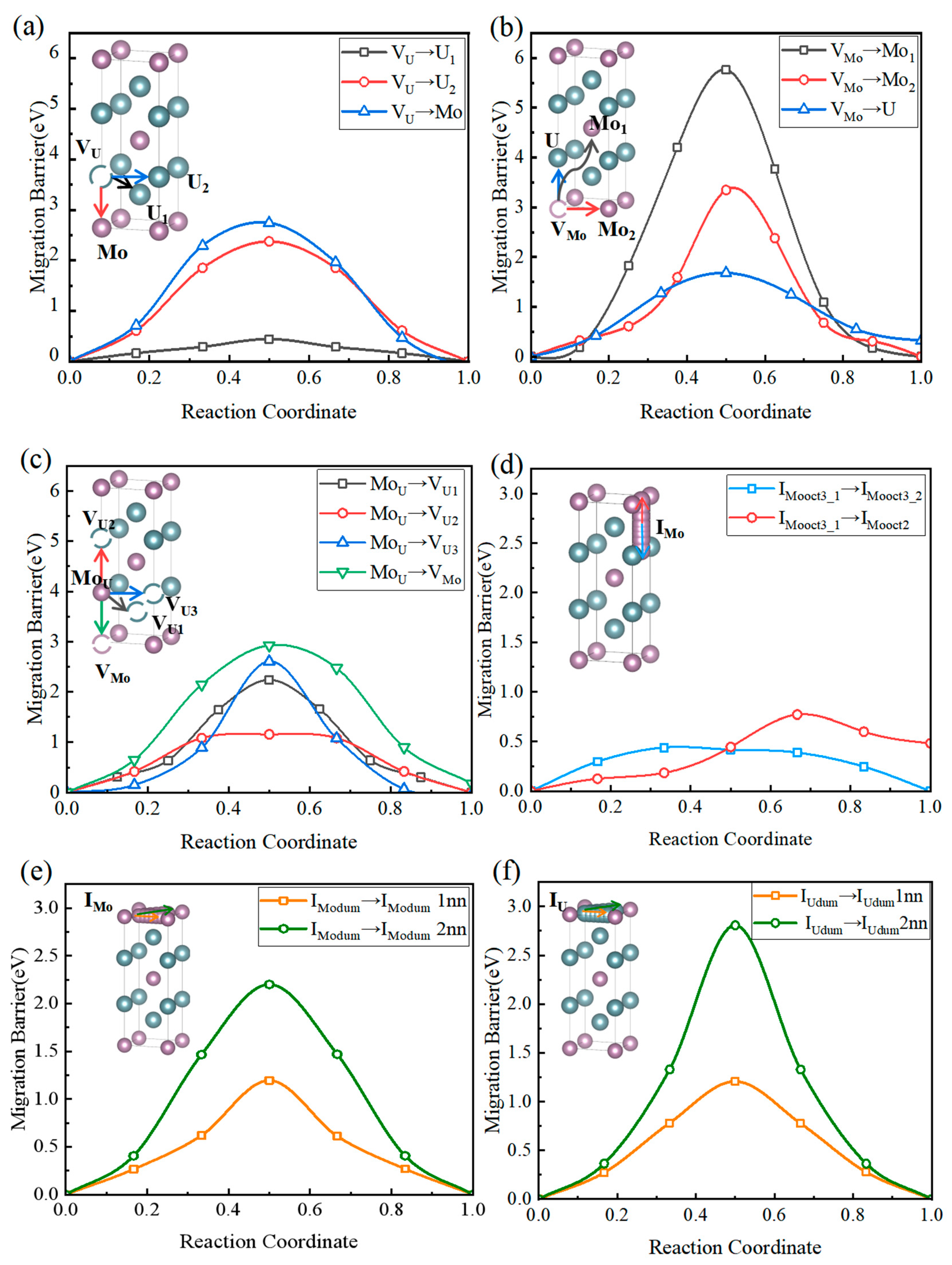
Due to the relatively low formation energies of vacancy and substitutional defects (as shown in
Figure 2), this paper first considers the migration of U and Mo atoms via vacancies (Mo or U vacancies) and substitutional defects through intrasublattice or intersublattice hopping.
Figure 4a illustrates the diffusion pathways and minimum energy paths (MEP) of molybdenum (Mo) and uranium (U) atoms via uranium vacancies within intrasublattice and intersublattice migrations. As shown, uranium atoms exhibit the lowest migration barrier (approximately 0.44 eV) when moving along the nearest-neighbor direction within the uranium sublattice (path: V
U → U
1), indicating strong intrinsic diffusivity. The barrier increases significantly with migration distance, reaching 2.38 eV for the second-nearest-neighbor (U
2) path, revealing pronounced spatial anisotropy and localized constraints in uranium-vacancy-mediated diffusion. In contrast, intersublattice migration of molybdenum atoms mediated by uranium vacancies (path: V
U → Mo) requires overcoming a higher barrier of ~2.74 eV. Intersublattice hopping of uranium vacancies (V
U) leads to their transition into the molybdenum sublattice, forming molybdenum substitutional defects (Mo
U) and molybdenum vacancies (V
Mo), thereby providing pathways for Mo diffusion. This shows that Mo atoms substituting U sites (Mo
U defects) are thermodynamically stable, but high migration barriers hinder their formation. Therefore, effective diffusion of substitutional defects is unlikely under irradiation or field-free conditions. Overall, U-vacancy-driven diffusion is confined to the U sublattice and governed by nearest-neighbor vacancy connectivity, whereas intersublattice substitutional defect migration remains kinetically inaccessible under conventional thermal conditions.
Figure 4b shows the migration pathways and MEPs for intra-sublattice and inter-sublattice jumps of Mo vacancies. Three migration pathways are identified: V
Mo → Mo
1, V
Mo → Mo
2, and V
Mo → U. Judging from the migration energy barriers, the Mo self-diffusion pathway (V
Mo → Mo
1) exhibits the highest energy barrier of approximately 5.76 eV, indicating significant diffusion resistance and thermodynamic unfavorability. Although V
Mo → Mo
2 represents second-nearest-neighbor diffusion, its energy barrier remains as high as 3.1 eV, demonstrating strong localization of Mo atoms in this system. In contrast, the diffusion pathway of Mo vacancies toward U lattice sites shows significantly lower migration barriers. The V
Mo → U pathway has an energy barrier of 1.68 eV, with a smooth and continuous trajectory devoid of additional local minima, suggesting favorable feasibility for this diffusion route. Notably, the V
Mo → U pathway possesses the lowest migration barrier, indicating that under realistic defect-containing or thermally perturbed conditions, Mo is more likely to preferentially undergo substitutional diffusion via the Mo → U mechanism rather than traditional Mo-Mo self-diffusion. These results show that inter-sublattice substitutional migration may emerge as the dominant diffusion channel in Mo-vacancy-dominated diffusion mechanisms, providing critical insights into understanding Mo behavior in alloy phases, coatings, or ceramics. (Intra-sublattice jumps of molybdenum vacancies (V
Mo) do not constitute the dominant mechanism governing Mo atomic self-diffusion. In U
2Mo, other more active migration mechanisms dominate the self-diffusion behavior of Mo atoms.)
This section further investigates the migration mechanism mediated by the defect complex (V
U + Mo
U) formed by uranium vacancies (V
U) and molybdenum substitutional defects (Mo
U). Since U atoms spontaneously migrate back to the U vacancy sites during structural optimization, making it impossible to maintain the substitutional defect configuration, this study did not select the U
Mo + V
U configuration as a stable defect for analysis. Our calculations show these two complexes exhibit the lowest formation energies among all defect configurations. Although researchers have identified such substitutional defect-assisted migration mechanisms in other alloys and intermetallic compounds [
39], these mechanisms remain unverified in U
2Mo.
Figure 4c illustrates the atomic migration mechanism of molybdenum substitutional defects (Mo
U) in γ′-U
2Mo by analyzing the minimum energy paths (MEPs) and migration barriers for Mo atoms occupying U sublattice sites and migrating toward adjacent U and Mo vacancies (V
U1, V
U2, V
U3, and V
Mo). Among these paths, the second-nearest-neighbor exchange Mo
U → V
U2 exhibits the lowest energy barrier of 1.16 eV, while diffusion toward a Mo vacancy (Mo
U → V
Mo) shows the highest barrier of 2.92 eV. Therefore, the substitution-assisted exchange (Mo
U + V
U) mechanism exhibits a diffusion-barrier range of 1.16–2.24 eV, and the Mo
U + V
Mo exchange path reaches up to 2.92 eV. These values are still markedly lower than the vacancy-mediated Mo migration barriers (3.34–5.76 eV,
Figure 4b). Overall, as shown in
Figure 4b,c, the substitution-assisted mechanism involves a lower energy cost and pronounced directional anisotropy, indicating that it dominates the Mo diffusion process in γ′-U
2Mo.
Although intrinsic metal atoms are generally considered not to diffuse via interstitial mechanisms in most metallic systems—primarily due to their large atomic radii and high interstitial formation energies, which render interstitial migration in bulk phases highly improbable—specific conditions such as high crystal defect density, loosely packed atomic structures near grain boundaries, or high-pressure/high-energy irradiation environments may enable Mo and U atoms to occupy interstitial sites locally, forming metastable states that induce interstitial diffusion behavior. To comprehensively evaluate potential diffusion pathways in this system, this study incorporates modeling of migration paths for Mo and U atoms in octahedral and tetrahedral interstitial sites and conventional substitutional diffusion mechanisms. The climbing image nudged elastic band (CI-NEB) method is employed to assess migration energy barriers and thermodynamic feasibility, providing a theoretical basis for understanding interstitial migration mechanisms that may be activated under non-equilibrium conditions.
To investigate the diffusion mechanisms of intrinsic interstitial atoms in the U-Mo system, initial configurations were constructed with U and Mo atoms occupying octahedral interstitial sites (oct3), followed by mapping their migration paths to the first-nearest-neighbor octahedral interstitial sites. This study performed CI-NEB calculations to determine the diffusion energy barriers. As shown in
Figure 4d, Mo atoms exhibit a well-defined diffusion barrier along the octahedral-octahedral migration path, with stable transition-state structures and energy barriers below 0.8 eV, consistent with typical metallic interstitial diffusion mechanisms.
In contrast, U atoms display markedly different diffusion behavior under the same structural conditions. One migration path features a distinct local minimum at the midpoint of the migration coordinate, forming an intermediate energy well and suggesting a stable intermediate configuration and a multi-step migration mechanism.
Another path exhibits a monotonically increasing energy profile without local transition states or energy minima, indicating thermodynamic instability of the final configuration of this pathway and kinetic inaccessibility.
Although U atoms have lower interstitial formation energies than Mo, their diffusion is strongly constrained by kinetics due to the absence of favorable low-energy migration pathways. In contrast, Mo atoms, despite their relatively higher formation energy, exhibit continuous and smooth migration potential energy surfaces with well-defined transition states, thereby possessing superior diffusion capabilities. In multi-component metallic systems, interstitial diffusion is determined not only by defect formation energies but also by the morphology of the migration path’s potential energy surface and its kinetic accessibility.
As shown in
Figure 4e,f, this study takes the dumbbell configuration, formed by U and Mo atoms occupying the 2a sites along the <110> direction, as the initial and final states. It constructs their migration paths to the first and second nearest neighbors. A diffusion energy barrier map was constructed for U and Mo, covering multiple octahedral and tetrahedral interstitial sites, to further elucidate the global migration pathways of interstitial atoms. We systematically calculated each path’s energy barriers and identified typical transition processes (O → T, T → O, T → T, etc.) and their barrier distributions. In
Figure 5a,b, interstitial atoms achieve long-range diffusion through multistep hopping. At the same time, the differences in energy barriers among various pathways show the connection probabilities between sites and the relative advantages of migration directions. As shown in
Figure 5a,b, the linear migration from tet2 → tet1 passes through a dumbbell interstitial site, where the formation energy of the dumbbell interstitial is lower than that of tetrahedral and octahedral interstitials, leading to a collapse of the energy barrier. Consequently, we insert an octahedral interstitial site midway.
By comparing the migration energy barriers of different diffusion paths, we find that the Mo atom most likely diffuses by jumping from one octahedral interstitial position to an adjacent equivalent octahedral interstitial position. In contrast, the most difficult diffusion occurs when jumping from one dumbbell interstitial position to the second nearest neighbor dumbbell interstitial position. The most difficult diffusion path for the U atom involves jumping from one dumbbell interstitial position to the second nearest neighbor dumbbell interstitial position.
3.3. Xe Diffusion in U2Mo
To gain an in-depth understanding of the diffusion behavior of xenon (Xe) atoms in U
2Mo, we systematically calculated and compared their formation energies at different lattice sites, including substitutional sites, tetrahedral interstitial sites, octahedral interstitial sites, and dumbbell configurations.
Table 2 lists the formation energies of various defect configurations.
In U
2Mo, the calculated formation energies for Xe atoms substituting U and Mo atoms are shown in
Table 2, with values of 6.59 eV and 7.13 eV, respectively. The formation energy of Xe substituting U atoms in the lattice is lower than that in α-U (6.69 eV [
40]). This shows that the U-atom sites in U
2Mo exhibit a more substantial capacity to accommodate oversized impurity Xe atoms, primarily due to the weaker lattice distortion response and higher geometric compatibility introduced by Xe substitution.
In U
2Mo, the formation of interstitial Xe defects is relatively favorable, with an average formation energy of 10.89 eV, lower than that in α-U (11.43 eV [
40]). Due to the complex atomic environment and diverse interstitial types in the U
2Mo crystal structure, it is challenging to precisely determine the most stable interstitial occupation site for Xe atoms.
This study focuses on analyzing three possible interstitial occupation mechanisms: (a) Xe forming dumbbell-type interstitial defects, (b) Xe occupying octahedral interstitial sites, and (c) Xe occupying tetrahedral interstitial sites. As an oversized impurity atom, Xe tends to form dumbbell-type interstitial states in U2Mo, making it one of the most likely defect types in the alloy.
Starting from the substitution defects, interstitial defects, and diffusion behavior of Xe atoms, this study comprehensively investigates the behavior of Xe in γ’-U2Mo. The substitution behavior of Xe atoms in the metal matrix reflects their ability to occupy vacancy defects. Since the energy required for direct hopping of Xe atoms through adjacent interstitial sites is relatively high, vacancies are introduced into the U2Mo system to examine their influence on the hopping of substitutional Xe atoms. During the hopping process, U vacancies easily capture Xe atoms, forming substitutional atoms. Due to the larger atomic radius of U compared to Mo, U occupies more space in the U2Mo system, making it more favorable to introduce Xe atoms.
Figure 6a,b, respectively, illustrate the migration of Xe atoms occupying U sites substitutionally (Xe
U) toward adjacent U vacancies (V
U) and the migration of Xe atoms occupying Mo sites substitutionally (Xe
Mo) toward adjacent Mo vacancies (V
Mo) in U
2Mo, both following a vacancy-assisted diffusion mechanism. A comparison between
Figure 6a,b show that the energy barrier for fission gas Xe occupying Mo fission gas Xe occupying U vacancies to hop to U vacancies. Relatively, the hopping of fission gas Xe occupying U vacancies to U vacancies occurs more readily. This is because, compared to Mo atoms, the size difference between U and Xe atoms is minor, resulting in weaker induced lattice distortion.
The Xe atom is an inert gas, exhibiting weak chemical adsorption capabilities in most metals and semiconductor materials, with a tendency toward physical adsorption or occupying interstitial sites. Comparative analysis of formation energies obtained through structural relaxation shows that the atom is more stable in the dumbbell interstitial site, with its formation energy being lower than that of both tetrahedral and octahedral interstitial sites.
The diffusion of interstitial Xe is representative of Xe migration behavior and is a primary focus in studying impurity element migration in nuclear fuels. Long-range diffusion of impurity atoms often relies on interstitial pathways, making the study of interstitial Xe diffusion significant for understanding Xe nucleation and growth. Due to its relatively large atomic radius, Xe diffusion typically requires assistance from other atoms, as observed in α-U [
40], which somewhat affects its diffusion rate.
In U2Mo, the migration pathways of Xe atoms can be categorized into three main types, based on the 2a-<110> dumbbell, octahedral interstitial, and tetrahedral interstitial sites: (1) diffusion within the (001) crystal plane, and (2) diffusion along the <001> direction from one (001) plane to another. Since the tetrahedral interstitial, octahedral interstitial, and 2a-<110> dumbbell sites all lie on the (001) plane and are close to each other, we anticipate that the diffusion energy barrier for Xe on this plane is relatively low. However, assistance from other atoms is often required when Xe migrates to another (001) plane.
By connecting various octahedral and tetrahedral interstitial sites, possible diffusion pathways for Xe are illustrated, as shown in
Figure 6e. Among the multiple migration paths tested, some exhibit distinct saddle-point structures in NEB simulations. In contrast, others flatten or revert after structural optimization, exhibit atomic rearrangements, or undergo local collapse or nonphysical distortion, thus failing to form practical migration barriers. Consequently, we calculated multiple diffusion paths for Xe atoms. As presented in
Table 3 and
Figure 6c–e, Path 6 incorporates an octahedral interstitial site because the direct migration from tet2 to tet1 passes through a dumbbell interstitial site, where the formation energy is lower than that of the tetrahedral interstitial site, leading to a collapse of the energy barrier.
Through comparison of the migration energy barriers along different diffusion pathways, it was found that the Xe atom has the lowest migration barrier along Path 6 (tet2 → oct2 → tet1), making it the most favorable diffusion direction; whereas Path 8 (dum → dum 2nn) exhibits the highest migration barrier, representing the most difficult diffusion pathway.