Comparison of the CO2 Balance in Electroslag Reduction of Cadmium with Pyrometallurgical and Hydrometallurgical Recovery Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Emissions Assessment Methodology
2.2. Comparable Cadmium Reduction Processes
2.2.1. Electroslag Reduction Method: 700 °C, KCl-NaCl Slag, Carbon, and No Cd Evaporation
- Mass of CdO: 128 g (1 mol);
- Mass of carbon (C): 500 g (41.7 mol);
- Reaction:
- Decomposition of cadmium hydroxide to obtain cadmium oxide.
- Reduction reaction of cadmium from cadmium oxide with carbon.
2.2.2. Pyrometallurgy (Distillation)
- Heat treatment of cadmium oxide in an open furnace, followed by condensation to produce cadmium oxide powder;
- Distillation in a closed furnace atmosphere, yielding metallic cadmium powder and an Fe–Ni alloy;
- Chlorination of batteries under a chlorine gas atmosphere or in hydrochloric acid at 960 °C to form cadmium chloride.
2.2.3. Hydrometallurgy
Hydrometallurgical Process Using the BATENUS Method
Hydrometallurgical Process Using the TNO Method
3. Results
4. Discussion
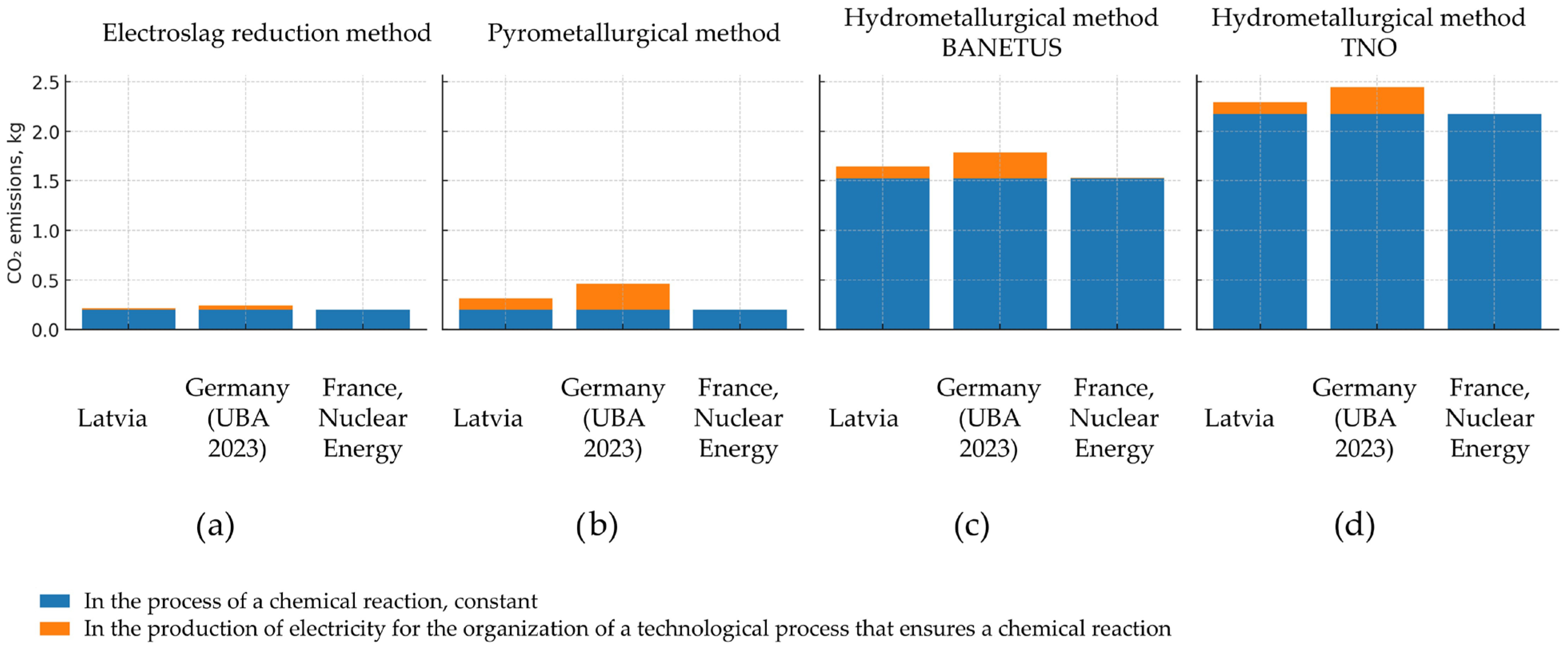
- Electroslag reduction demonstrates the lowest total CO2 emissions. This method benefits from low operating temperatures (≤700 °C) and a minimal flux role in emissions. Electroslag reduction technology for cadmium prevents cadmium evaporation, which also helps to reduce energy consumption and emissions.
- Pyrometallurgical recovery, though similarly reliant on carbon as a reductant, operates at higher temperatures (>850 °C) and requires additional energy for cadmium evaporation and condensation. While the chemical CO2 emissions remain identical to electroslag, the higher energy input increases total emissions.
- Hydrometallurgical recovery generates the highest CO2 emissions, not from energy usage but from reagent production. It is less environmentally favorable in terms of greenhouse gas emission.
5. Conclusions
- Electroslag reduction demonstrated the lowest total CO2 emissions per kilogram of recovered cadmium (0.196–0.241 kg CO2), primarily due to its moderate process temperature (700 °C), controlled environment preventing cadmium evaporation, and low energy demand. Its environmental performance is further enhanced when powered by low-carbon electricity sources.
- Pyrometallurgical methods exhibited slightly higher emissions (0.199–0.454 kg CO2/kg Cd), attributable to elevated operating temperatures (850–900 °C) and additional energy required for cadmium vaporization and condensation. Despite high recovery efficiency, the thermal intensity of this method presents a notable environmental drawback.
- Hydrometallurgical recovery showed significantly higher emissions (1.529–2.446 kg CO2/kg Cd), dominated by upstream emissions from the production of chemical reagents. Although advantageous for selective metal recovery and operation at lower temperatures, this route remains less favorable in terms of CO2 balance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission, Directorate-General for Internal Market, Industry, Entrepreneurship, and SMEs. Study on the Critical Raw Materials for the EU 2023: Final Report; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar] [CrossRef]
- Daosan, M.; Novoderezhkin, V.V.; Tomashevskij, F.F. Production of Electrolytic Accumulators (Прoизвoдствo Электрических Аккумулятoрoв in Russian); Visshaja Shkola: Moscow, Russia, 1977. [Google Scholar]
- Ismail, W.; El-Shaer, A.; Abdelfatah, M. Phase transition of Cd(OH)2 and physical properties of CdO microstructures prepared by precipitation method for optoelectronic applications. IOP Conf. Ser. Mater. Sci. Eng. 2020, 956, 012006. [Google Scholar] [CrossRef]
- Lyon, R.N.; Katz, D.L.V. Liquid-Metals Handbook; Navexos, P., Ed.; U.S. Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Volinskij, V.V. Methods for processing electrodes of nickel-cadmium batteries. Bull. Saratov State Tech. Univ. 2006, 3, 104–112. (In Russian) [Google Scholar]
- Blumbergs, E.; Maiorov, M.; Bogachov, A.; Platacis, E.; Ivanov, S.; Gavrilovs, P.; Pankratov, V. A Green Electroslag Technology for Cadmium Recovery from Spent Ni-CD Batteries Under Protective Flux with Electromagnetic Stirring by Electrovortex Flows. Metals 2025, 15, 959. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 10 October 2025).
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Mansur, M.B. Recycling batteries. In Waste Electrical and Electronic Equipment (WEEE) Handbook; Elsevier: Amsterdam, The Netherlands, 2019; pp. 371–391. [Google Scholar]
- Espinosa, D.C.R.; Tenório, J.A.S. Fundamental aspects of recycling of nickel–cadmium batteries through vacuum distillation. J. Power Sources 2004, 135, 320–326. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Tenório, J.A.S. Recycling of nickel–Cadmium batteries using coal as reducing agent. J. Power Sources 2006, 157, 600–604. [Google Scholar] [CrossRef]
- Huang, K.; Li, J.; Xu, Z. Characterization and recycling of cadmium from waste nickel–cadmium batteries. Waste Manag. 2010, 30, 2292–2298. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Bernardes, A.M.; Tenório, J.A.S. An overview on the current processes for the recycling of batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- Cox, A.; Fray, D.J. Recycling of cadmium from domestic, sealed NiCd battery waste by use of chlorination. Trans. Inst. Min. Metall. 1999, 108, C153–C158. [Google Scholar]
- Bernardes, A.M.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of batteries: A review of current processes and technologies. J. Power Sources 2004, 130, 291–298. [Google Scholar] [CrossRef]
- Anulf, T. SAB-NIFE recycling concept for nickel-cadmium batteries—An industrialised and environmentally safe process. In Proceedings of the Sixth International Cadmium Conference, Capri, Italy, 10–12 April 1990; pp. 161–163. [Google Scholar]
- Hanewald, R.H.; Schweyer, L.; Hoffman, M.D. High temperature recovery and reuse of specialty steel pickling materials and refractors at INMETCO. In Proceedings of the International Conference on Electric Furnace, San Diego, CA, USA, 5–7 November 1991; pp. 141–146. [Google Scholar]
- INMETCO. High Temperature Metal Recovery Process; INMETCO: Ellwood City, PA, USA, 1995. [Google Scholar]
- Liotta, J.J.; Onuska, J.C.; Hanewald, R.H. Nickel-Cadmium Battery Recycling through the INMETCO High Temperature Metals Recovery Process. In Proceedings of the 10th Annual Battery Conference on Applications and Advances, Long Beach, CA, USA, 10–13 January 1995; p. 333. [Google Scholar]
- Blumbergs, E.; Serga, V.; Platacis, E.; Maiorov, M.; Shishkin, A. Cadmium Recovery from Spent Ni-Cd Batteries: A Brief Review. Metals 2021, 11, 1714. [Google Scholar] [CrossRef]
- Schweers, M.E.; Onuska, J.C.; Hanewald, R.H. A pyrometallurgical process for recycling cadmium-containing batteries. In Proceedings of the HMC-South ’92 Exhibitor Conference and Exhibition, New Orleans, LA, USA, 26–28 February 1992; Hazardous Materials Control Research Institute: Washington, DC, USA, 1992; pp. 333–335. [Google Scholar]
- Nogueira, C.A.; Margarido, F. Recycling of spent Ni-Cd batteries by physical-chemical processing. In Proceedings of the 2006 TMS Fall Extraction and Processing Division: Sohn International Symposium, San Diego, CA, USA, 27–31 August 2006; Volume 5, pp. 305–312. [Google Scholar]
- Brückner, L.; Frank, J.; Elwert, T. Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 2020, 10, 1107. [Google Scholar] [CrossRef]
- Larouche, F.; Tedjar, F.; Amouzegar, K.; Houlachi, G.; Bouchard, P.; Demopoulos, G.P.; Zaghib, K. Progress and Status of Hydrometallurgical and Direct Recycling of Li-Ion Batteries and Beyond. Materials 2020, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Zulkapli, N.S.; Kamyab, H.; Taib, S.M.; Din, M.F.B.M.; Majid, Z.A.; Chaiprapat, S.; Kenzo, I.; Ichikawa, Y.; Nasrullah, M.; et al. Current technologies for recovery of metals from industrial wastes: An overview. Environ. Technol. Innov. 2021, 22, 101525. [Google Scholar] [CrossRef]
- Rudnik, E.; Nikiel, M. Hydrometallurgical recovery of cadmium and nickel from spent Ni–Cd batteries. Hydrometallurgy 2007, 89, 61–71. [Google Scholar] [CrossRef]
- Van Erkel, J. Recovery of Cd and Ni from Batteries. U.S. Patent 5,407,463, 18 April 1995. [Google Scholar]
- Randhawa, N.S.; Gharami, K.; Kumar, M. Leaching kinetics of spent nickel–cadmium battery in sulphuric acid. Hydrometallurgy 2016, 165, 191–198. [Google Scholar] [CrossRef]
- Lindermann, W.; Dombrowsky, C.H.; Sewing, D.; Muller, M.; Engel, S.; Joppien, R. The BATENUS process for recycling battery waste. In Proceedings of the International Symposium on Impurity Control and Disposal in Hydrometallurgical Processes, Toronto, ON, Canada, 21–24 August 1994; pp. 197–204. [Google Scholar]
- Tanong, K.; Coudert, L.; Mercier, G.; Blais, J.-F. Recovery of metals from a mixture of various spent batteries by a hydrometallurgical process. J. Environ. Manag. 2016, 181, 95–107. [Google Scholar] [CrossRef]
- Reza Khayati, G.; Dalvand, H.; Darezereshki, E.; Irannejad, A. A facile method to synthesis of CdO nanoparticles from spent Ni–Cd batteries. Mater. Lett. 2014, 115, 272–274. [Google Scholar] [CrossRef]
- Tanong, K.; Tran, L.-H.; Mercier, G.; Blais, J.-F. Recovery of Zn (II), Mn (II), Cd (II) and Ni (II) from the unsorted spent batteries using solvent extraction, electrodeposition and precipitation methods. J. Clean. Prod. 2017, 148, 233–244. [Google Scholar] [CrossRef]
- Reddy, B.R.; Priya, D.N.; Park, K.H. Separation and recovery of cadmium(II), cobalt(II) and nickel(II) from sulphate leach liquors of spent Ni–Cd batteries using phosphorus based extractants. Sep. Purif. Technol. 2006, 50, 161–166. [Google Scholar] [CrossRef]
- Nogueira, C.A.; Margarido, F. Nickel–Cadmium batteries: Effect of electrode phase composition on acid leaching process. Environ. Technol. 2012, 33, 359–366. [Google Scholar] [CrossRef]
- Fernandes, A.; Afonso, J.C.; Bourdot Dutra, A.J. Hydrometallurgical route to recover nickel, cobalt and cadmium from spent Ni–Cd batteries. J. Power Sources 2012, 220, 286–291. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Mayyas, M.; Sahajwalla, V. Recycling of Ni-Cd batteries by selective isolation and hydrothermal synthesis of porous NiO nanocuboid. J. Environ. Chem. Eng. 2018, 6, 4671–4675. [Google Scholar] [CrossRef]
- Singh, R.; Mahandra, H.; Gupta, B. Recovery of zinc and cadmium from spent batteries using Cyphos IL 102 via solvent extraction route and synthesis of Zn and Cd oxide nanoparticles. Waste Manag. 2017, 67, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.R.; Priya, D.N. Chloride leaching and solvent extraction of cadmium, cobalt and nickel from spent nickel–Cadmium, batteries using Cyanex 923 and 272. J. Power Sources 2006, 161, 1428–1434. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.H.; Thi, L.D.; Qureshi, T.I. Recycling of NiCd batteries by hydrometallurgical process on small scale. J. Chem. Soc. Pak. 2011, 33, 853–857. [Google Scholar]
- OpenCO2. Net Is a Carbon Footprint Platform Developed in Finland. Available online: https://www.openco2.net/en/ (accessed on 15 September 2025).
- Climatiq Emission Factor Database. Available online: https://www.climatiq.io/data/explorer?search=Hydrogen+peroxide&data_version=%5E26 (accessed on 15 September 2025).
- Nicholson, S.R.; Rorrer, N.A.; Uekert, T.; Avery, G.; Carpenter, A.C.; Beckham, G.T. Manufacturing Energy and Greenhouse Gas Emissions Associated With United States Consumption of Organic Petrochemicals. ACS Sustain. Chem. Eng. 2023, 11, 2198–2208. [Google Scholar] [CrossRef]
- Charbonneau, A.; Beyerle, M. Assessing the carbon footprint of industrial equipment—A case study in cane sugar processing. Sugar Ind. Int. 2025, 150, 265–272. [Google Scholar] [CrossRef]
- Edwards, R.; Padella, M.; Giuntoli, J.; Koeble, R.; O’ Connell, A.; Bulgheroni, C.; Marelli, L. Definition of Input Data to Assess GHG Default Emissions from Biofuels in EU Legislation; Version 1c, EUR 28349 EN, JRC104483; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Rincon, L.; Ruiz, C.A.; Contreras, R.R.; Almarza, J. Study of the NaOH(s)-CO2 (g) reaction creating value for industry: Green natrite production, energy, and its potential in different sustainable scenarios. Environ. Sci. Adv. 2023, 2, 957–966. [Google Scholar] [CrossRef]

| 2CdO | C | 2Cd | CO2 | |
|---|---|---|---|---|
| n, mol | 2 | 1 | 2 | 1 |
| , kJ/mol | −258.35 | 0 | 0 | 393.51 |
| Cd(OH)2 | CdO | Н2О | |
|---|---|---|---|
| n, mol | 1 | 1 | 1 |
| , kJ/mol | −563 | −258.35 | −241.83 |
| Electricity Source | Emission Factor (kg CO2/kWh) | Source |
|---|---|---|
| Latvia (Nowtricity) | 0.17 | https://www.nowtricity.com/country/latvia/ (accessed on 31 July 2025) Average 2024 year |
| Germany (Climatiq) | 0.33 | Climatiq Germany |
| Germany (UBA 2023) | 0.38 | UBA Germany |
| France (LCA) | 0.004 | https://www.sfen.org/rgn/les-emissions-carbone-du-nucleaire-francais-37g-de-co2-le-kwh/ (accessed on 31 July 2025) |
| Nuclear (LCA ADEME) | 0.006 | ADEME France |
| Solar (UNECE) EU28 | 0.011–0.037 | UNECE LCA 2021 |
| Natural gas, EU28 | 0.43 | UNECE LCA 2021 |
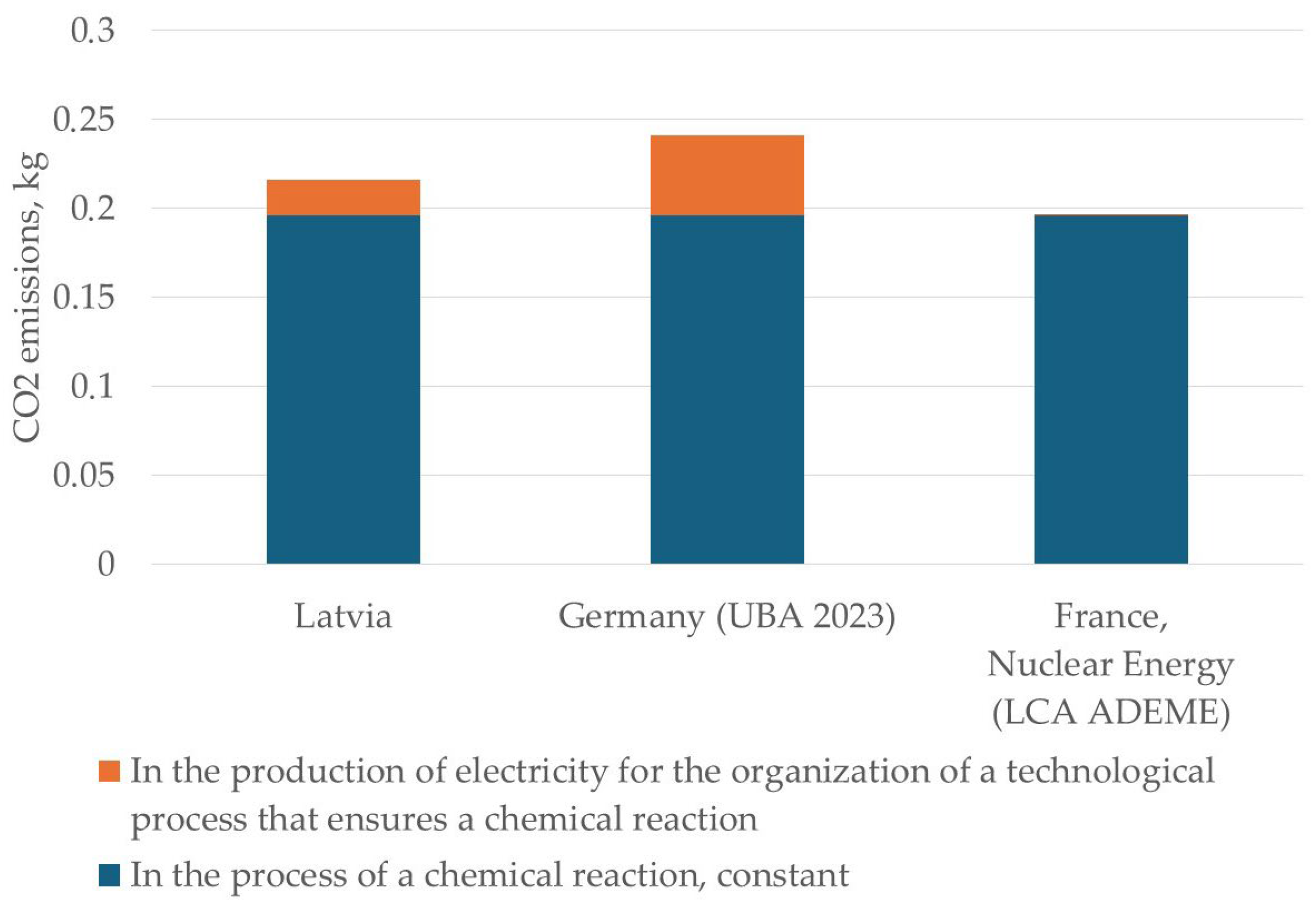
| Country | CO2 Emissions for the Reduction of 1 kg of Cadmium with Carbon Using the Electroslag Reduction Method, in kg | ||
|---|---|---|---|
| During a Chemical Reaction, the Value is Constant | The Costs of Organizing a Technical Process | Total | |
| Latvia | 0.1958 kg | 0.17 kg CO2/kWh × 0.11 kWh = 0.0187 kg | 0.2145 kg |
| Germany (UBA 2023) | 0.1958 kg | 0.38 kg CO2/kWh × 0.11 kWh = 0.0418 kg | 0.2376 kg |
| France, Nuclear Energy (LCA ADEME) | 0.1958 kg | 0.004 kg CO2/kWh × 0.11 kWh = 0.0004 kg | 0.1962 kg |
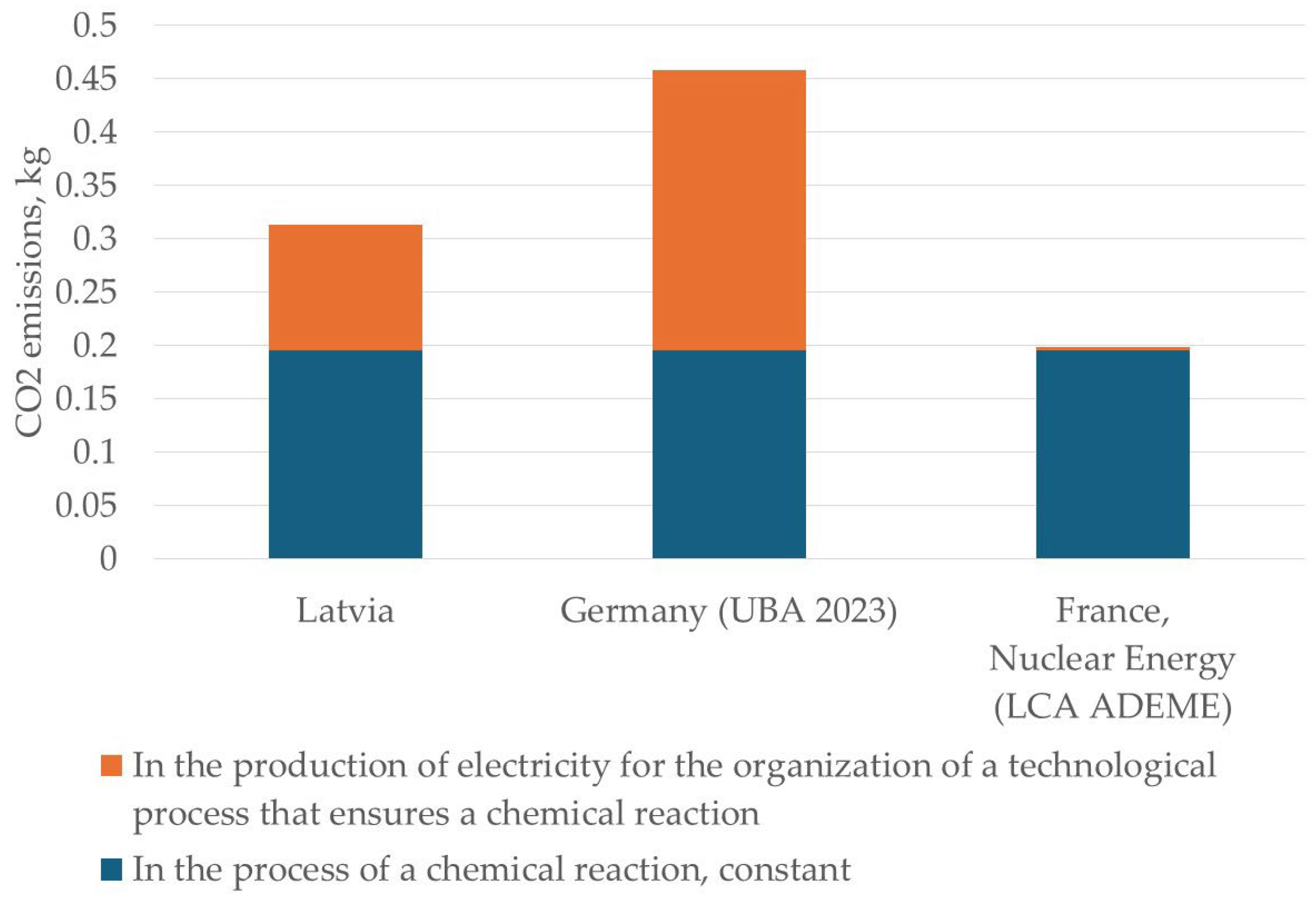
| Country | CO2 Emissions During Pyrometallurgical Reduction of 1 kg of Cadmium, in kg | ||
|---|---|---|---|
| During a Chemical Reaction, the Value Is Constant | The Costs of Organizing a Technical Process | Total | |
| Latvia | 0.1958 kg | 0.17 kg CO2/kWh × 0.68 kWh = 0.1156 kg | 0.3114 kg |
| Germany (UBA 2023) | 0.1958 kg | 0.38 kg CO2/kWh × 0.68 kWh = 0.2584 kg | 0.4542 kg |
| France, Nuclear Energy (LCA ADEME) | 0.1958 kg | 0.004 kg CO2/kWh × 0.68 kWh = 0.0027 kg | 0.1985 kg |
| Reagent | Mass (kg) | Specific Emissions, kg CO2/kg | CO2, kg |
|---|---|---|---|
| H2SO4 | 0.6058 | 0.14 | 0.0848 |
| H2O2 | 0.412 | 1.13 | 0.466 |
| TBP + ShellSol R | ~0.20 | 4.00 | 0.80 |
| Ion-exchange resin | ~0.05 | 3.50 | 0.175 |
| Total | 1.2678 | - | 1.5258 |
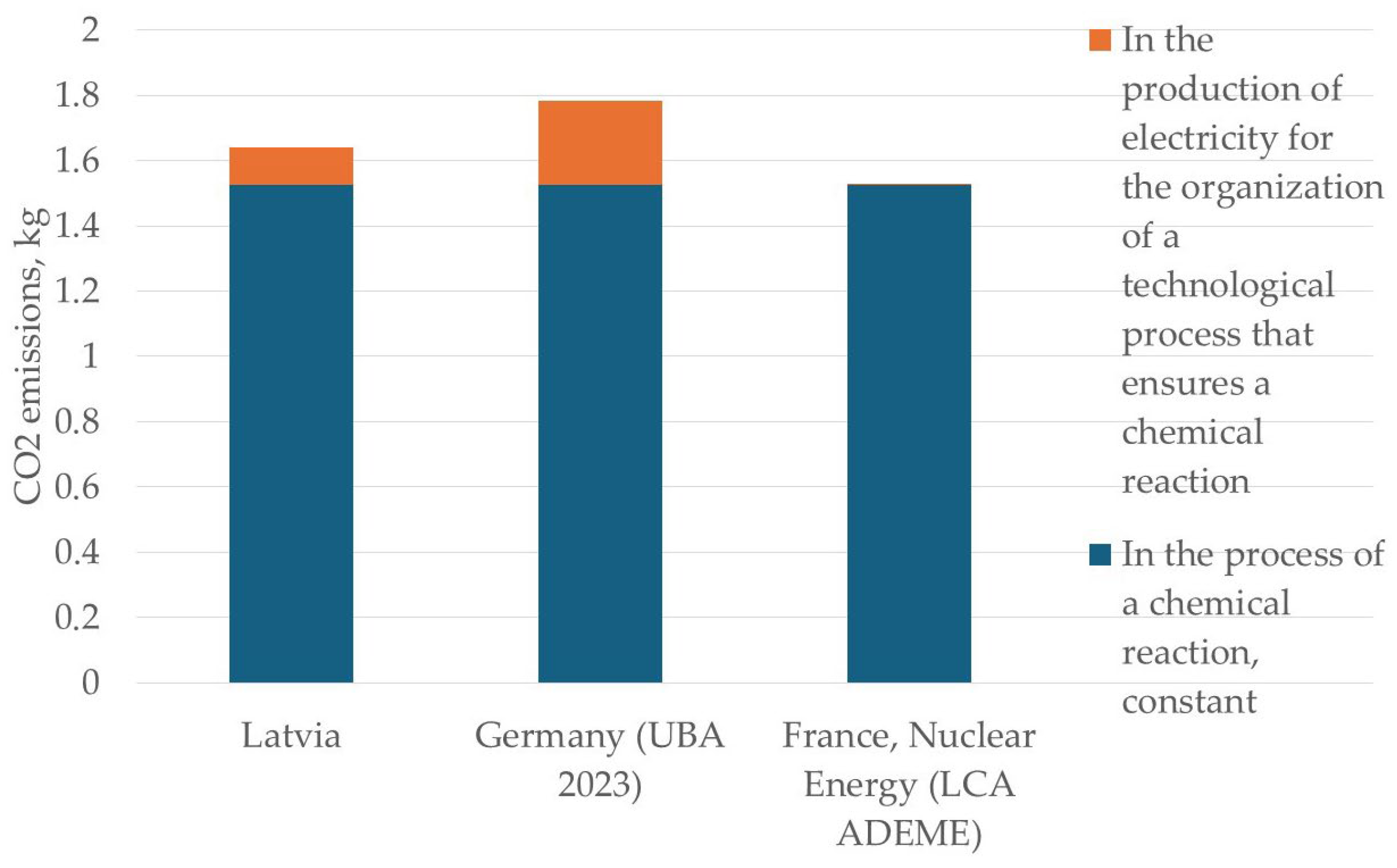
| Country | CO2 Emissions During Hydrometallurgical Reduction of 1 kg of Cadmium for the BATENUS Process, in kg | ||
|---|---|---|---|
| During a Chemical Reaction, the Value Is Constant | The Costs of Organizing a Technical Process | Total | |
| Latvia | 1.5258 kg | 0.17 kg CO2/kWh × 0.68 kWh = 0.1156 kg | 1.6414 kg |
| Germany (UBA 2023) | 1.5258 kg | 0.38 kg CO2/kWh × 0.68 kWh = 0.2584 kg | 1.7842 kg |
| France, Nuclear Energy (LCA ADEME) | 1.5258 kg | 0.004 kg CO2/kWh × 0.68 kWh = 0.0027 kg | 1.5285 kg |
| Reagent | Mass (kg) | Specific Emissions, kg CO2/kg | CO2, kg |
|---|---|---|---|
| HCl | 2 | 1.06 | 2.12 |
| TBP | 0.05 | No data | - |
| Na2CO3 | 0.1 | 0.52 | 0.052 |
| Total | 2.15 | - | 2.172 |
| Country | CO2 Emissions During Hydrometallurgical Reduction of 1 kg of Cadmium for the TNO Method, in kg | ||
|---|---|---|---|
| During a Chemical Reaction, the Value Is Constant | The Costs of Organizing a Technical Process | Total | |
| Latvia | 2.172 kg | 0.17 kg CO2/kWh × 0.72 kWh = 0.1224 kg | 2.2944 kg |
| Germany (UBA 2023) | 2.172 kg | 0.38 kg CO2/kWh × 0.72 kWh = 0.2736 kg | 2.4456 kg |
| France, Nuclear Energy (LCA ADEME) | 2.172 kg | 0.004 kg CO2/kWh × 0.72 kWh = 0.0029 kg | 2.1749 kg |
| Country | Latvia | Germany (UBA 2023) | France, Nuclear Energy (LCA ADEME) | |
|---|---|---|---|---|
| CO2 Emissions, kg | ||||
| Electroslag reduction method | In the process of a chemical reaction, constant | 0.1958 | 0.1958 | 0.1958 |
| The costs of organizing a technical process that ensures a chemical reaction occurs | 0.0187 | 0.0418 | 0.0004 | |
| Total | 0.2145 | 0.2376 | 0.1962 | |
| Pyrometallurgical method | In the process of a chemical reaction, constant | 0.1958 | 0.1958 | 0.1958 |
| The costs of organizing a technical process that ensures a chemical reaction occurs | 0.1156 | 0.2584 | 0.0027 | |
| Total | 0.3114 | 0.4542 | 0.1985 | |
| Hydrometallurgical method BANETUS | In the process of a chemical reaction, constant | 1.5258 | 1.5258 | 1.5258 |
| The costs of organizing a technical process that ensures a chemical reaction occurs | 0.116 | 0.258 | 0.0027 | |
| Total | 1.6418 | 1.7838 | 1.5285 | |
| Hydrometallurgical method TNO | In the process of a chemical reaction, constant | 2.172 | 2.172 | 2.172 |
| The costs of organizing a technical process that ensures a chemical reaction occurs | 0.1224 | 0.2736 | 0.0029 | |
| Total | 2.2944 | 2.4456 | 2.1749 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blumbergs, E.; Maiorov, M.; Brēķis, A.; Platacis, E.; Ivanov, S.; Nikitina, J.; Bogachov, A.; Pankratov, V. Comparison of the CO2 Balance in Electroslag Reduction of Cadmium with Pyrometallurgical and Hydrometallurgical Recovery Methods. Metals 2025, 15, 1197. https://doi.org/10.3390/met15111197
Blumbergs E, Maiorov M, Brēķis A, Platacis E, Ivanov S, Nikitina J, Bogachov A, Pankratov V. Comparison of the CO2 Balance in Electroslag Reduction of Cadmium with Pyrometallurgical and Hydrometallurgical Recovery Methods. Metals. 2025; 15(11):1197. https://doi.org/10.3390/met15111197
Chicago/Turabian StyleBlumbergs, Ervīns, Michail Maiorov, Artūrs Brēķis, Ernests Platacis, Sergei Ivanov, Jekaterina Nikitina, Artur Bogachov, and Vladimir Pankratov. 2025. "Comparison of the CO2 Balance in Electroslag Reduction of Cadmium with Pyrometallurgical and Hydrometallurgical Recovery Methods" Metals 15, no. 11: 1197. https://doi.org/10.3390/met15111197
APA StyleBlumbergs, E., Maiorov, M., Brēķis, A., Platacis, E., Ivanov, S., Nikitina, J., Bogachov, A., & Pankratov, V. (2025). Comparison of the CO2 Balance in Electroslag Reduction of Cadmium with Pyrometallurgical and Hydrometallurgical Recovery Methods. Metals, 15(11), 1197. https://doi.org/10.3390/met15111197






