Abstract
This study systematically investigates the effect of substituting Copper (Cu) with Nickel (Ni) on the glass-forming ability (GFA) and corrosion resistance of a Pt-based bulk metallic glass (BMG). We demonstrate that a minor substitution of 5 at.% Ni for Cu in the Pt40Pd20Cu20P20 base alloy significantly enhances both properties. The GFA is markedly improved, as evidenced by the supercooled liquid region (ΔTx) widening from 68 K to 91 K. The optimized Pt40Pd20Cu15Ni5P20 alloy exhibits a compressive fracture strength of 1.38 GPa. Electrochemical tests in a 3.5 wt.% NaCl solution reveal a substantial improvement in corrosion resistance. Compared to the Ni-free baseline alloy, the passive film resistance (Rf) and charge-transfer resistance (Rct) of the Ni-containing alloy are enhanced by factors of 2.75 and 2.60, respectively. This superior performance is attributed to a synergistic effect wherein Ni alloying both stabilizes the amorphous structure and promotes the formation of a more robust passive film. This work presents a viable strategy for designing cost-effective, high-performance multi-component BMGs for applications in aggressive chloride environments.
1. Introduction
Bulk metallic glasses (BMGs), characterized by their long-range disordered atomic structure, represent a paradigm shift from traditional crystalline alloys. The absence of microstructural defects such as grain boundaries, dislocations, and precipitates endows them with a unique combination of properties, including near-theoretical strength, high elastic strain limits, and exceptional chemical homogeneity [1,2,3,4,5]. This inherent structural uniformity makes BMGs particularly resistant to localized corrosion phenomena, such as pitting, which typically initiate at the chemically active sites prevalent in crystalline materials [6,7,8]. Consequently, BMGs are prime candidates for demanding applications where both mechanical robustness and long-term stability in aggressive environments are paramount.
Among the vast family of BMGs, systems incorporating noble metals like Platinum (Pt) and Palladium (Pd) are of particular interest [9,10,11]. These elements impart exceptional chemical inertness and catalytic activity, making Pt- and Pd-based BMGs highly attractive for biomedical devices [12], luxury goods [10], and electrocatalysis [13]. Prototypical systems such as Pt-Cu-P and the landmark Pd-Cu-Ni-P have demonstrated remarkable glass-forming ability (GFA), enabling the fabrication of bulk amorphous parts with thin dimensions [14,15,16]. However, the widespread application of these high-performance materials is often constrained by two key factors: the high cost of noble metals and the perpetual challenge of further enhancing their GFA to enable the manufacturing of larger and more complex components [17,18].
A promising strategy to address these challenges is through multi-component alloying, often termed the “confusion principle,” where the addition of new elements can frustrate crystallization and stabilize the amorphous phase [19,20,21]. Nickel (Ni), a cost-effective transition metal, presents a particularly compelling case as an alloying element. Ni is known to exhibit strong negative heat when mixing with metalloids like Phosphorus (P) and other constituent elements, which is a favorable thermodynamic indicator for glass formation [22,23]. Furthermore, Ni plays a crucial role in the passivation behavior of many corrosion-resistant alloys. It can significantly alter the chemistry and electronic properties of the surface passive layer (e.g., by forming stable NiO/Ni(OH)2 species), thereby offering a powerful lever to control pitting susceptibility and repassivation kinetics [24]. While the role of Ni in the celebrated Pd-Cu-Ni-P system is well-established, its specific effect as a substitute for a primary component like Cu in a Pt-Pd-Cu-P quinary system remains unexplored.
Inspired by this, in this study, by regulating the content of Ni, the influence of Ni on the Pd-Cu-Ni-P alloy was investigated. The addition of 5 at.% Ni was found to significantly enhance the glass-forming ability and thermal stability, increasing the supercooled liquid region width (ΔTx) from 68 K to 91 K. The optimized alloy exhibited a compressive fracture strength of 1.38 GPa. Impressively, this HE-MG not only retains excellent bending ductility after stress-relief annealing but also exhibits enhanced corrosion resistance in a 3.5% NaCl solution. The resistance of the passivation film and the charge transfer resistance were increased by 2.75 times and 2.60 times, respectively, implying the significant enhancement of corrosion resistance.
2. Materials and Methods
2.1. Alloy Fabrication
Amorphous alloy ribbons were fabricated in two sequential stages: first, to optimize the base composition of the quaternary Pt-Pd-Cu-P system, and second, to systematically investigate the effect of Ni addition on the optimized composition. For all alloys, master ingots were prepared from high-purity elements (Pt 99.9%, Pd 99.9%, Cu 99.99%, Ni 99.99%, P 99.5%) by arc-melting in a high-purity argon atmosphere. Each ingot was remelted four times to ensure chemical homogeneity. Amorphous ribbons were then produced from these ingots via single-roller melt-spinning. All subsequent characterization was performed on these as-spun ribbons.
Stage 1: Optimization of the Quaternary Base Alloy
In the initial stage, a broad range of quaternary alloys within the Pt-Pd-Cu-P system was fabricated to screen for the optimal glass-forming composition. This screening involved systematically varying the atomic ratios of Pt, Pd, and Cu. Based on a comprehensive analysis of their thermal properties, the composition Pt40Pd20Cu20P20 was identified as exhibiting a superior combination of glass-forming ability and thermal stability and was selected as the base alloy for the subsequent stage.
Stage 2: Investigation of Ni Alloying Effects
In the second stage, the effect of Ni was investigated by substituting Cu in the optimized base alloy, yielding a series of quinary compositions with the formula Pt40Pd20Cu(20−x)NixP20 (where x = 0, 2.5, 5, 5.5, 7.5, 10, and 15 at.%). For clarity, these samples are hereafter designated. Ni-0, Ni-2.5, Ni-5, etc., corresponding to their Ni content.
2.2. Experimental Procedure
The amorphous nature of the as-spun ribbons was verified by X-ray diffraction (XRD) using a Rigaku D/Max-2200/PC diffractometer (Tokyo, Japan) with Cu Kα radiation (λ = 1.5418 Å). Scans were performed over a 2θ range of 20–80° at a speed of 5°/min.
Thermal analysis was conducted to determine the characteristic temperatures of the alloys. The glass transition temperature (Tg) and the onset of crystallization (Tx) were measured using a Perkin-Elmer DSC-6 differential scanning calorimeter (DSC, Waltham, MA, USA). Samples of approximately 15 mg were heated at a constant rate of 40 K/min under a flowing pure argon atmosphere. The melting point (Tm) and liquidus temperature (Tl) were determined using a separate differential thermal analyzer (DTA) (Shimadzu Corporation, Kyoto, Japan) capable of reaching higher temperatures, under identical heating rates and atmospheric conditions. The supercooled liquid region is defined as ΔTx = Tx − Tg.
Uniaxial compression tests were performed on the Pt40Pd20Cu15Ni5P20 (Ni-5) alloy to evaluate its mechanical properties, including sample dimensions (cylindrical rods with a 2:1 aspect ratio) and strain rate (1 × 10−4 s−1). Tests were carried out on a Zwick/Roell Z100 servo-electric frame (Ulm, Germany) at room temperature. Cylindrical specimens with a diameter of 3 mm and length 6 mm (aspect ratio 2:1, in accordance with ASTM E9-19) were machined from the as-cast rods by slow-speed diamond cutting and subsequently ground with 2000-grit SiC paper to ensure parallel end faces (parallelism within ±5 µm). A constant engineering strain rate of 1 × 10−4 s−1 was imposed under displacement control. The fracture surfaces of the compressed samples were examined using scanning electron microscopy (SEM) (Zeiss, Oberkochen, Germany) to identify the deformation and fracture mechanisms.
Electrochemical measurements were conducted in a 3.5 wt.% NaCl aqueous solution at ambient temperature using a standard three-electrode cell configuration. The alloy ribbon served as the working electrode, a platinum mesh as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. Prior to each measurement, the working electrode was mechanically polished, cleaned, and then stabilized at its open-circuit potential (OCP) for 60 min.
Potentiodynamic polarization curves (PPC) were recorded by scanning the potential from −250 mV to +1200 mV versus OCP at a scan rate of 1 mV/s. Electrochemical impedance spectroscopy (EIS) was performed at the OCP over a frequency range from 100 kHz to 10 mHz with a 10 mV (rms) sinusoidal perturbation. The resulting EIS data were fitted to an equivalent electrical circuit (EEC) model to extract quantitative parameters. Mott–Schottky analysis was conducted by scanning the potential in the passive region at a frequency of 1 kHz to characterize the semiconductor properties of the passive film [25].
3. Experiment Result
3.1. Optimization of the Composition of Pt-Pd-Cu-P Amorphous Alloys
In order to understand the structure and characteristics of the samples. A systematic compositional screening was performed on the Pt-Pd-Cu-P quaternary system to optimize its GFA. The amorphous nature of the as-spun ribbons was confirmed via XRD. As presented in Figure 1a, the patterns for representative compositions, including Pt24Pd32Cu24P20, Pt32Pd16Cu32P20, and Pt48Pd16Cu16P20, each exhibit a single, broad diffraction halo centered at approximately 2θ ≈ 40°, with no discernible Bragg peaks corresponding to crystalline phases.
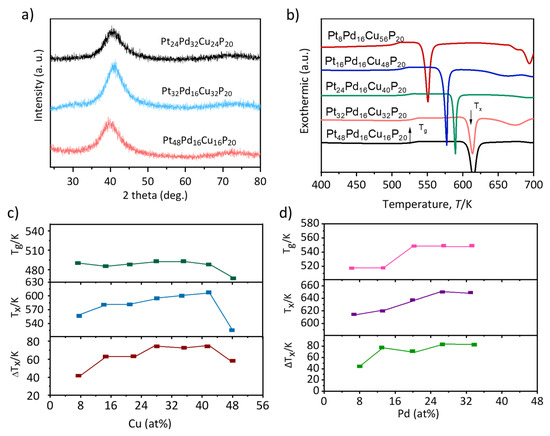
Figure 1.
(a) XRD patterns and (b) DSC curves of Pt80−x−yPdxCuyP20 (x = 8–56%, y = 8–56%) ribbons. (c) As the Cu content varies in Pt16Pd64−xCuxP20 (x = 8–56%), changes occur in the glass Tg, Tx, and ΔT. (d) The changes in the Pt content for Pt16Pd64−xCuxP20 (x = 8–56%).
The thermal behavior of the alloys was characterized by DSC, as shown in Figure 1b. The DSC traces universally display a distinct glass transition followed by a single exothermic crystallization event. The Tg and Tx were found to be in the ranges of 500–550 K and 550–600 K, respectively. This yields a supercooled liquid region (ΔTx = Tx − Tg) of approximately 50 K, indicating a favorable thermal stability window for potential thermoplastic forming.
To further refine the GFA, the influence of individual constituent elements was investigated. For the (Pt16Pd64−xCux)P20 series (Figure 1c), it was observed that while the Cu content had a minimal effect on Tg, it systematically elevated Tx. Consequently, the supercooled liquid region was widened, reaching a maximum for Cu concentrations between 32 and 48 at.%, which underscores the critical role of Cu in enhancing the thermal stability of the undercooled liquid. In contrast, for the (Pt64−xPdxCu16)P20 series (Figure 1d), the partial substitution of Pt with Pd resulted in a concurrent increase in both Tg and Tx. However, this led to a relatively stable ΔTx across the tested compositional range, suggesting that the Pt/Pd ratio has a less pronounced effect on the width of the supercooled liquid region in this specific series.
3.2. The Study of Structural Transformation of Amorphous Crystals
To contextualize the impact of Ni substitution, the GFA landscape of the parent Pt-Pd-Cu-P quaternary system was first surveyed. DSC traces (Figure 2a) reveal a pronounced compositional dependence of the super-cooled-liquid region width (ΔTx). A broad GFA domain is identified, with ΔTx attaining a maximum of 84 K at 16–48 at.% Pt, 16–32 at.% Pd and 16–32 at.% Cu. In Pd- or Cu-rich regimes, ΔTx declines markedly, signaling deteriorated GFA. The left panel of Figure 2b systematically maps the correlation between alloy composition and ΔTx, whereas the right panel denotes compositions that yield bulk-amorphous rods upon water quenching, corroborating the general rule that wider ΔTx implies superior GFA and thermal stability. Additionally, the DTA melting trace of the base alloy (Figure 2c) exhibits a single, sharp endothermic peak, evidencing a near-eutectic composition and intrinsically high glass-forming propensity.
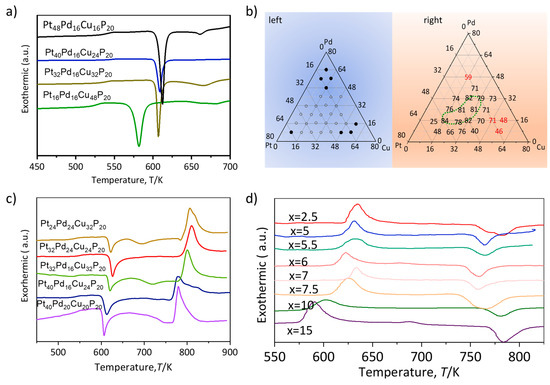
Figure 2.
(a) The DSC (Differential Scanning Calorimetry) curve of Pt-Pd-Cu-P bulk amorphous alloy. (b) The temperature of the supercooled region in the Pt-Pd-Cu alloy varies with composition changes (left) and the composition range for the formation of large amorphous phases of Pt-Pd-Cu alloy (right). (c) DSC curve of Pt80−x−yPdxCuyP20, (d) DTA curves of Pt-Pd-Cu-P-Ni alloy.
The thermal properties of the resulting PtPdCu(20−x)NixP (x = 0–15 at.%) alloy series are summarized in Table 1, with representative DTA curves shown in Figure 2d and Table 1. It reveals a pronounced enhancement in the thermal stability of the supercooled liquid upon Ni addition. The Ni-0 base alloy exhibits a supercooled liquid region width (ΔTx = Tx − Tg) of 68 K. Upon substitution with 5 at.% Ni (the Ni-5 alloy), ΔTx increases substantially to a maximum of 91 K, representing a 34% improvement. A wider ΔTx signifies a greater resistance to crystallization upon heating, which is a direct indicator of enhanced GFA.

Table 1.
Tg, Tx, Tm, Tl of Pt-Pd-Cu-P-Ni alloys and parameter values characterizing GFA.
To further quantify the GFA from a thermodynamic perspective, the reduced glass transition temperature (Trg = Tg/Tl) was calculated. This parameter, which normalizes the glass transition temperature against the liquidus temperature, is considered a more robust indicator of GFA. As detailed in Table 1, the Trg value also peaks at 0.69 for the Ni-5 composition, corroborating the trend observed with ΔTx and confirming that this composition possesses the highest GFA within the investigated series. Further evidence for the optimal nature of the 5 at.% Ni composition is found in the alloy’s melting behavior, as observed in the DTA traces (Figure 2d). For the Ni-5 alloy, the melting event is characterized by the sharpest endothermic peak, and the temperature interval between crystallization and melting is minimized. Such features are characteristic of a composition near a deep eutectic, which kinetically and thermodynamically favors the suppression of crystallization and promotes glass formation. The collective analysis of ΔTx, Trg, and DTA melting characteristics consistently indicates that a 5 at.% substitution of Cu with Ni is the optimal modification for maximizing the glass-forming ability of this alloy system. This enhancement can be partially attributed to the increase in the configurational entropy of mixing (ΔSmix) upon introducing a fifth element, which thermodynamically favors the formation of a disordered amorphous state over a competing crystalline phase.
Further XRD characterization (as shown in Figure 3) of Ni-5 revealed a pronounced broad diffuse halo centered at approximately 2θ ≈ 40°, and no sharp Bragg peaks corresponding to any known crystalline phases were observed across the entire scan range, indicating that the sample is amorphous (lacking long-range crystalline order).
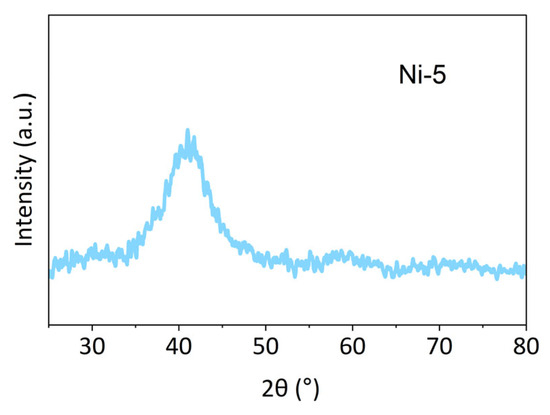
Figure 3.
XRD patterns of the Ni-5 sample.
4. The Study of the Properties of Pt-Pd-Cu-P-Ni Alloys
4.1. The Performance of Mechanical Property for Pt-Pd-Cu-Ni-P Alloys
The compressive stress–strain curve for the optimized Ni-5 alloy is shown in Figure 4. Compared to its initial as-cast state, a slight increase in compressive fracture strength is observed, accompanied by a modest reduction in fracture strain as the heat treatment temperature rises. This subtle change can be attributed to the minor adjustments in the alloy’s microstructure following heat treatment. The data clearly show that the as-cast high-entropy alloys exhibit a strength of 1.38 ± 0.2 GPa, although their plasticity is somewhat limited at 4.4%.
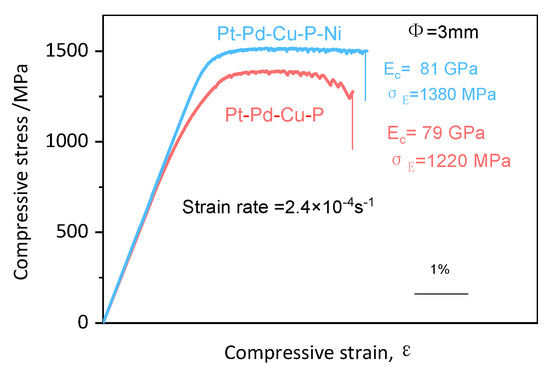
Figure 4.
The stress–strain curve of the Pt-Pd-Cu-P-Ni amorphous alloy.
The fracture surface morphology of the compressed Ni-5 sample was examined by SEM (Figure 5). The surface is dominated by a dense network of vein-like patterns (Figure 5a), which is a characteristic fracture feature of BMGs that have undergone plastic deformation. These patterns arise from the localized softening and viscous flow of material within the shear band just prior to catastrophic failure. Examination of the sample’s side surface (Figure 5b) reveals the presence of multiple, well-defined shear bands, which are the primary carriers of plastic deformation in amorphous alloys. The operation of these multiple shear bands before one becomes critical is consistent with the observed macroscopic plasticity of 4.4%.
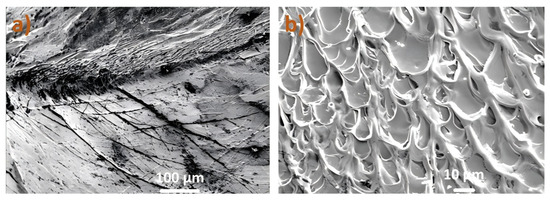
Figure 5.
SEM images of the Pt-Pd-Cu-P-Ni amorphous alloy’s surface at different scales from the compression test. (a) Sample surface morphology. (b) Sample side morphology.
4.2. Electrochemical Corrosion Performance
4.2.1. Potentiodynamic Polarization Curves
Based on its excellent structural properties, the corrosion performance of the alloys was evaluated primarily through electrochemical testing, encompassing both anodic and cathodic processes. Potentiodynamic polarization curves (PPC) were employed to characterize the electrochemical behavior of these alloys in a 3.5% NaCl solution. According to the data presented in Figure 6 and Table 2, as the Ni content increases, the corrosion potential of the alloys gradually rises, indicating a significant improvement in their stability in corrosive environments. Specifically, the corrosion potential of the Ni-0 alloy is 378 ± 21 mV, with a corrosion current density of 3.6 × 10−4 A/cm2; for the Ni-5 alloy, the corrosion potential is 485 ± 13 mV, with a corrosion current density of 3.1 × 10−4 A/cm2. The addition of Ni significantly enhances the corrosion potential of the alloy, thereby improving its corrosion stability. Overall, the increase in Ni content effectively enhances the corrosion resistance of the alloys, particularly by raising the corrosion potential, which leads to a marked improvement in their stability in corrosive environments.
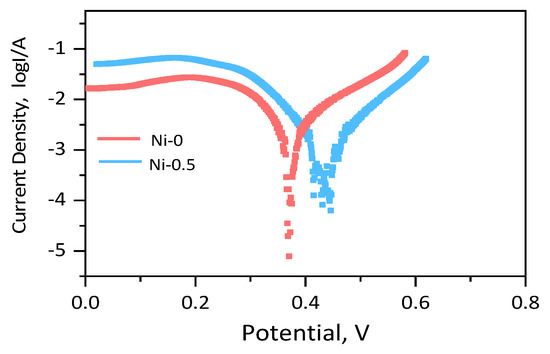
Figure 6.
PPCs of Pt-Pd-Cu-Ni-P and Pt-Pd-Cu-P immersion corrosion test.

Table 2.
Electrochemical parameters derived from polarization tests of cast HEAs in a 3.5 wt.% NaCl solution.
4.2.2. Electrochemical Impedance Spectroscopy (EIS)
To elucidate the intrinsic electrochemical corrosion mechanism of the alloys, we systematically investigated their steady-state response in a NaCl solution using electrochemical impedance spectroscopy (EIS). The EIS analysis (Figure 7) reveals that the Ni-5 alloy exhibits superior corrosion resistance compared to the Ni-0 baseline, a performance primarily attributed to the exceptional chemical stability and structural integrity of its surface passivation layer. This conclusion is directly substantiated by the impedance response. In the Nyquist plot (Figure 7a), the Ni-5 alloy displays a significantly enlarged capacitive arc, indicating a remarkably high polarization resistance. This is further corroborated in the Bode plot (Figure 7b), where its low-frequency impedance modulus (|Z|0.01Hz) exceeds that of the Ni-0 alloy by more than an order of magnitude to reach a level of 104 Ω·cm2. Concurrently, its broader phase angle peak approaching −90° signifies the formation of a dense and near-ideal capacitive passive interface.
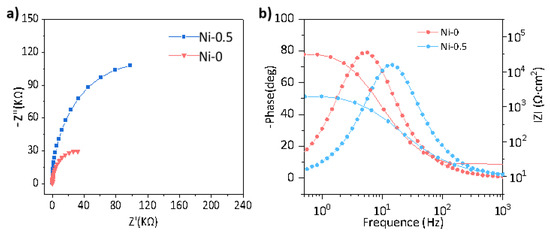
Figure 7.
(a) Nyquist and (b) Bode plots for Ni-0 and Ni-5.
To quantitatively deconvolute the contributions of the interfacial processes to the impedance, the data were fitted using a physically meaningful equivalent electrical circuit (EEC) model of Figure 8 (see Table 3 for fitting parameters). The results precisely unveil the origin of the performance disparity: the passive film resistance (Rf = 4.53 × 104 Ω·cm2) and charge-transfer resistance (Rct = 4.92 × 105 Ω·cm2) of the Ni-5 alloy are 2.75- and 2.60-fold higher, respectively, than those of the Ni-0 alloy. The marked increase in R f indicates a dense, low-defect passivation film acting as a formidable physical barrier that effectively suppresses the ingress of chloride ions. Meanwhile, the concurrent elevation in Rct implies that charge-transfer processes at the film/electrolyte interface, including anodic dissolution and cathodic reactions, are severely kinetically hindered.
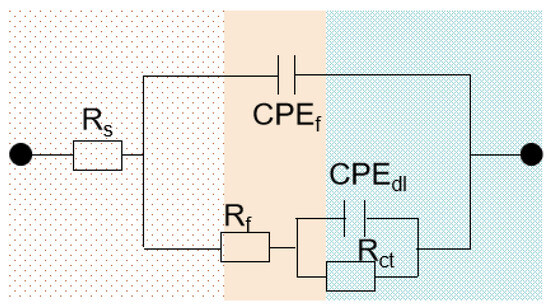
Figure 8.
An equivalent circuit model used to fit the EIS data of HEAs.

Table 3.
The parameters of the equivalent electrical circuit used for the electrochemical impedance spectroscopy analysis of the alloys.
The corrosion mechanism of the alloy is strongly linked with the semiconductor properties of the passive layer. As shown in Figure 9, the Mott–Schottky plots exhibit positive slopes, unequivocally confirming the n-type semiconducting character of the passive film. With increasing Ni additions, the absolute slope monotonically decreases [26].
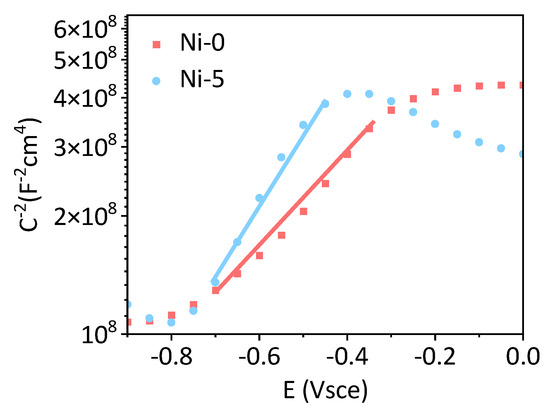
Figure 9.
Mott–Schottky plots of the passive layers of alloys.
Here, ε is the relative dielectric constant of the passive film (taken as 15.6), ε0 the vacuum permittivity (8.85 × 10−14 F cm−1), e the elementary charge (1.602 × 10−19 C), Efb the flat-band potential, K Boltzmann’s constant (1.38 × 10−23 J K−1), and T the absolute temperature [27]. According to the Mott–Schottky relation, the slope is inversely proportional to the donor density; consequently, the observed decrease in slope (Equation (1)) demonstrates that Ni incorporation increases the passive-film thickness.
The significant enhancement in corrosion resistance of the Ni-5 alloy compared to its Ni-0 counterpart in a NaCl solution can be attributed to the formation of a highly uniform amorphous structure. This structure, which features long-range atomic disorder and short-range chemical ordering, effectively eliminates conventional defects such as grain boundaries that typically serve as primary initiation sites for corrosion. As a result, the Ni-5 alloy exhibits significantly reduced corrosion, with the formation of a largely intact passive film (Figure 10b), while the Ni-0 alloy experiences severe corrosion and localized surface spalling (Figure 10a). Therefore, the role of Ni is twofold: it contributes thermodynamically to the formation of this uniform amorphous substrate and chemically enhances the stability and protective capacity of the overlying passive film. This synergy between an advanced structure and beneficial composition ultimately leads to superior corrosion resistance.
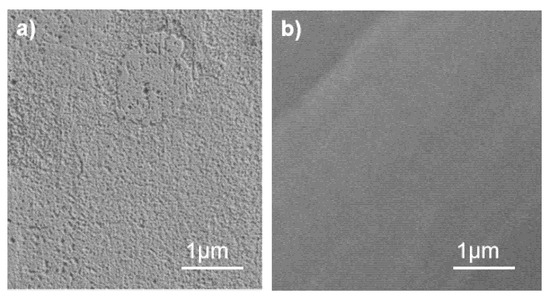
Figure 10.
SEM images of (a) Ni-0 and (b) Ni-5 inclusions for corrosion morphology after immersion.
5. Discussions
5.1. Mechanism of Enhanced Glass-Forming Ability
The substitution of 5 at.% Cu with Ni leads to a remarkable improvement in GFA, as evidenced by the significant increase in ΔTx and Trg. This can be understood through the established empirical rules for BMG formation, which emphasize the roles of atomic size mismatch, negative heats of mixing (ΔHmix), and multi-component “confusion”.
As shown in Table 4, Ni has an atomic radius (1.28 Å) similar to Cu (1.27 Å) but different from Pt (1.77 Å), Pd (1.69 Å), and P (0.98 Å). The introduction of Ni maintains the significant atomic size differences within the five-element system, which frustrates the efficient atomic packing required for crystallization. More importantly, Ni exhibits strong negative heats of mixing with Pt (−8 kJ/mol), Pd (−10 kJ/mol), and P (−5 kJ/mol). These strong, attractive interactions between dissimilar atom pairs increase the viscosity of the undercooled liquid and raise the energetic barrier for the atomic rearrangement necessary for crystal nucleation and growth, thereby stabilizing the disordered amorphous phase. The addition of a fifth component also increases the configurational entropy of the system, which thermodynamically favors the disordered glassy state over competing crystalline phases, an effect often termed the “confusion principle”. The peak GFA at 5 at.% Ni suggests that this composition achieves an optimal balance of these thermodynamic and kinetic factors, likely positioning it closer to a deep eutectic in the quinary phase space.

Table 4.
Properties of selected alloying elements (Adapted from Ref. [28]).
5.2. Unifying the Corrosion Mechanism
Defects as Facilitators of Repair: According to the Point Defect Model (PDM), point defects such as oxygen vacancies (the likely source of n-type behavior) are the primary charge carriers for ionic transport through the passive film. While a high defect density might imply a less-perfect insulator, it can also provide more pathways for ionic diffusion. A higher donor density (ND) can therefore lead to a higher ionic flux and, consequently, a faster film growth and repair rate [29,30]. Additionally, in an aggressive chloride environment, a passive film is not a static barrier. It is under constant attack, leading to localized breakdown events. The overall corrosion resistance of the material is determined less by the film’s initial perfection and more by its ability to rapidly repair these local breaches.
Thus, while Ni promotes interfacial charge transfer kinetics, its primary role is to stabilize the passive film electronically and structurally, shifting the corrosion mechanism toward a more controllable, passivation-dominated regime. This agrees with EIS and PDP data which corroborate this interpretation. The highest-Ni alloy displays ultimately degrading corrosion resistance.
6. Conclusions
The partial substitution of Cu with 5 at.% Ni in the Pt40Pd20Cu20P20 base alloy significantly improves its GFA. The supercooled liquid region (ΔTx) is widened from 68 K to 91 K, and the reduced glass transition temperature (Trg) increases from 0.65 to a peak of 0.69, indicating enhanced thermal stability of the undercooled liquid. The optimized Ni-5 alloy possesses robust mechanical properties, with a compressive fracture strength of 1.38 GPa and fracture morphology indicative of a ductile failure mode in a 3.5 wt.% NaCl solution compared to the Ni-free alloy. This is evidenced by a significant corrosion potential, which significantly increases in both passive film resistance (2.75-fold) and charge-transfer resistance (2.60-fold). A novel corrosion resistance mechanism is proposed to resolve the apparent contradiction between superior performance (EIS) and higher defect density (Mott–Schottky). We conclude that Ni incorporation endows passive films with enhanced self-healing capabilities, leading to greater dynamic stability in a chloride-rich environment.
Author Contributions
Conceptualization, P.A. and L.L. (Luliang Liao); Software, P.A., S.S., H.L. and L.L. (Lei Liu); Formal analysis, S.S. and L.L. (Lei Liu); Data curation, S.S., H.L. and L.L. (Luliang Liao); Writing—original draft, P.A.; Writing—review & editing, L.L. (Luliang Liao); Funding acquisition, P.A. and L.L. (Luliang Liao). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Program of Education Office of Jiangxi Province (grant numbers: GJJ212312 and GJJ202321).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, P.; Tan, J.; Tian, Y.; Yan, H.; Yu, Z. Research progress on selective laser melting (SLM) of bulk metallic glasses (BMGs): A review. Int. J. Adv. Manuf. Technol. 2022, 118, 2017–2057. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, X.G.; Chen, L.; Li, W.; Xu, X.; Pan, B.; Li, W.; Zhou, W.; Li, L.; Huang, W.; et al. Recent development in the application of bulk metallic glasses. J. Mater. Sci. Technol. 2022, 131, 115–121. [Google Scholar] [CrossRef]
- Sohrabi, N.; Jhabvala, J.; Logé, R.E. Additive manufacturing of bulk metallic glasses-process, challenges and properties: A review. Metals 2021, 11, 1279. [Google Scholar] [CrossRef]
- Li, B.; Yakubov, V.; Nomoto, K.; Ringer, S.P.; Gludovatz, B.; Li, X.; Kruzic, J.J. Superior mechanical properties of a Zr-based bulk metallic glass via laser powder bed fusion process control. Acta Mater. 2024, 266, 119685. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, D.; Yao, Y.; Zhang, G.; Wang, J.; Liu, L.; Li, F.; Fan, H.; Liu, X.; Wang, H.; et al. Substantially enhanced plasticity of bulk metallic glasses by densifying local atomic packing. Nat. Commun. 2021, 12, 6582. [Google Scholar] [CrossRef]
- Ding, F.; Feng, Y.; Xu, Y.; Shi, H.; Guo, X. Synergistic improvement of mechanical performance and corrosion resistance in Zr-based BMGs by Nb addition. J. Non-Cryst. Solids 2025, 665, 123619. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, S.; Du, P.; Yan, R.; Yuan, B.; Cai, Z.; Zhang, L.; Xie, G. Simultaneously improving the plasticity and corrosion resistance of biomedical Mg-based bulk metallic glass by introducing SnZn alloy solder. Intermetallics 2025, 182, 108791. [Google Scholar] [CrossRef]
- Liang, D.; Chen, Q.; Zhou, Y.; Liu, X.; Cai, Y.; Zhou, Q.; Huang, B.; Zhang, E.; Shen, J. Tailoring the structural heterogeneity and electrochemical behavior of Fe-based bulk metallic glasses by Ar+ irradiation. J. Alloys Compd. 2023, 936, 168332. [Google Scholar] [CrossRef]
- Sarac, B.; Sarac, A.S.; Eckert, J. Pd-based metallic glasses as promising materials for hydrogen energy applications. J. Electrochem. Soc. 2023, 170, 014503. [Google Scholar] [CrossRef]
- Houghton, O.S.; Greer, A.L. A conflict of fineness and stability: Platinum-and palladium-based bulk metallic glasses for jew-ellery: Part I: Introduction and properties of platinum-and palladium-based bulk metallic glasses. Johns. Matthey Technol. Rev. 2021, 65, 506–518. [Google Scholar] [CrossRef]
- Hughes, A.E.; Haque, N.; Northey, S.A.; Giddey, S. Platinum group metals: A review of resources, production and usage with a focus on catalysts. Resources 2021, 10, 93. [Google Scholar] [CrossRef]
- Lackington, W.A.; Wiestner, R.; Pradervand, E.; Schweizer, P.; Zuber, F.; Ren, Q.; Stoica, M.; Löffler, J.F.; Rottmar, M. Surface chemistry dictates the osteogenic and antimicrobial properties of palladium-, platinum-, and titanium-based bulk metallic glasses. Adv. Funct. Mater. 2023, 33, 2302069. [Google Scholar] [CrossRef]
- Mahajan, C.; Hasannaeimi, V.; Neuber, N.; Wang, X.; Busch, R.; Gallino, I.; Mukherjee, S. Model metallic glasses for superior electrocatalytic performance in a hydrogen oxidation reaction. ACS Appl. Mater. Interfaces 2023, 15, 6697–6707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Inoue, A. Bulk glassy alloys with low liquidus temperature in Pt-Cu-P system. Mater. Trans. 2003, 44, 1143–1146. [Google Scholar] [CrossRef]
- Wada, T.; Takenaka, K.; Nishiyama, N.; Inoue, A. Formation and mechanical properties of porous Pd-Pt-Cu-P bulk glassy alloys. Mater. Trans. 2005, 46, 2777–2780. [Google Scholar] [CrossRef]
- Na, J.H.; Corona, S.L.; Hoff, A.; Johnson, W.L. Observation of an apparent first-order glass transition in ultrafragile Pt–Cu–P bulk metallic glasses. Proc. Natl. Acad. Sci. USA 2020, 117, 2779–2787. [Google Scholar] [CrossRef]
- Raoof, J.B.; Ojani, R.; Esfeden, S.A.; Nadimi, S.R. Fabrication of bimetallic Cu/Pt nanoparticles modified glassy carbon electrode and its catalytic activity toward hydrogen evolution reaction. Int. J. Hydrogen Energy 2010, 35, 3937–3944. [Google Scholar] [CrossRef]
- Kugai, J.; Seino, S.; Nakagawa, T.; Yamamoto, T.A. Effect of phosphorus and copper additions on the structure of Pt and Pt–Cu nanoparticles in a radiation-induced reduction method. J. Nanopart. Res. 2014, 16, 2275. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C. More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Ma, H.; Shi, L.L.; Xu, J.; Li, Y.; Ma, E. Improving glass-forming ability of Mg−Cu−Y via substitutional alloying: Effects of Ag versus Ni. J. Mater. Res. 2006, 21, 2204–2214. [Google Scholar] [CrossRef]
- Calin, M.; Stoica, M.; Eckert, J.; Yavari, A.; Schultz, L. Glass formation and crystallization of Cu47Ti33Zr11Ni8X1 (X = Fe, Si, Sn, Pb) alloys. Mater. Sci. Eng. A 2005, 392, 169–178. [Google Scholar] [CrossRef]
- Johnson, W.L. Thermodynamic and kinetic aspects of the crystal to glass transformation in metallic materials. Prog. Mater. Sci. 1986, 30, 81–134. [Google Scholar] [CrossRef]
- Bajpai, A.; Bhatt, J.; Biswas, K.; Gurao, N.P. A new perspective to thermodynamical designing of high entropy bulk metallic glasses (HE-BMGs). Phys. B Condens. Matter 2020, 595, 412350. [Google Scholar] [CrossRef]
- Fu, K.; Yuan, D.; Yu, T.; Lei, C.; Kou, Z.; Huang, B.; Lyu, S.; Zhang, F.; Wan, T. Recent advances on two-dimensional nanomaterials supported single-atom for hydrogen evolution electrocatalysts. Molecules 2024, 29, 4304. [Google Scholar] [CrossRef] [PubMed]
- Feliu, S., Jr. Electrochemical impedance spectroscopy for the measurement of the corrosion rate of magnesium alloys: Brief review and challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Zhang, G.A.; Cheng, Y.F. Micro-electrochemical characterization and Mott–Schottky analysis of corrosion of welded X70 pipeline steel in carbonate/bicarbonate solution. Electrochim. Acta 2009, 55, 316–324. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Zhang, M.; Liu, F.; Wang, X.; Gong, P.; Tang, X. Influence of plastic deformation on the corrosion behavior of CrCoFeMnNi high entropy alloy. J. Alloys Compd. 2022, 891, 161822. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Sui, J.H.; Cai, W. Formation of diamond-like carbon (DLC) film on the NiTi alloys via plasma immersion ion implantation and deposition (PIIID) for improving corrosion resistance. Appl. Surf. Sci. 2006, 253, 2050–2055. [Google Scholar] [CrossRef]
- Fatimah, S.; Khoerunnisa, F.; Ko, Y.G. Decoration of an inorganic layer with nickel (hydr)oxide via green plasma electrolysis. RSC Adv. 2018, 8, 26804–26816. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).