3.2. Surface Characteristics After Nitriding
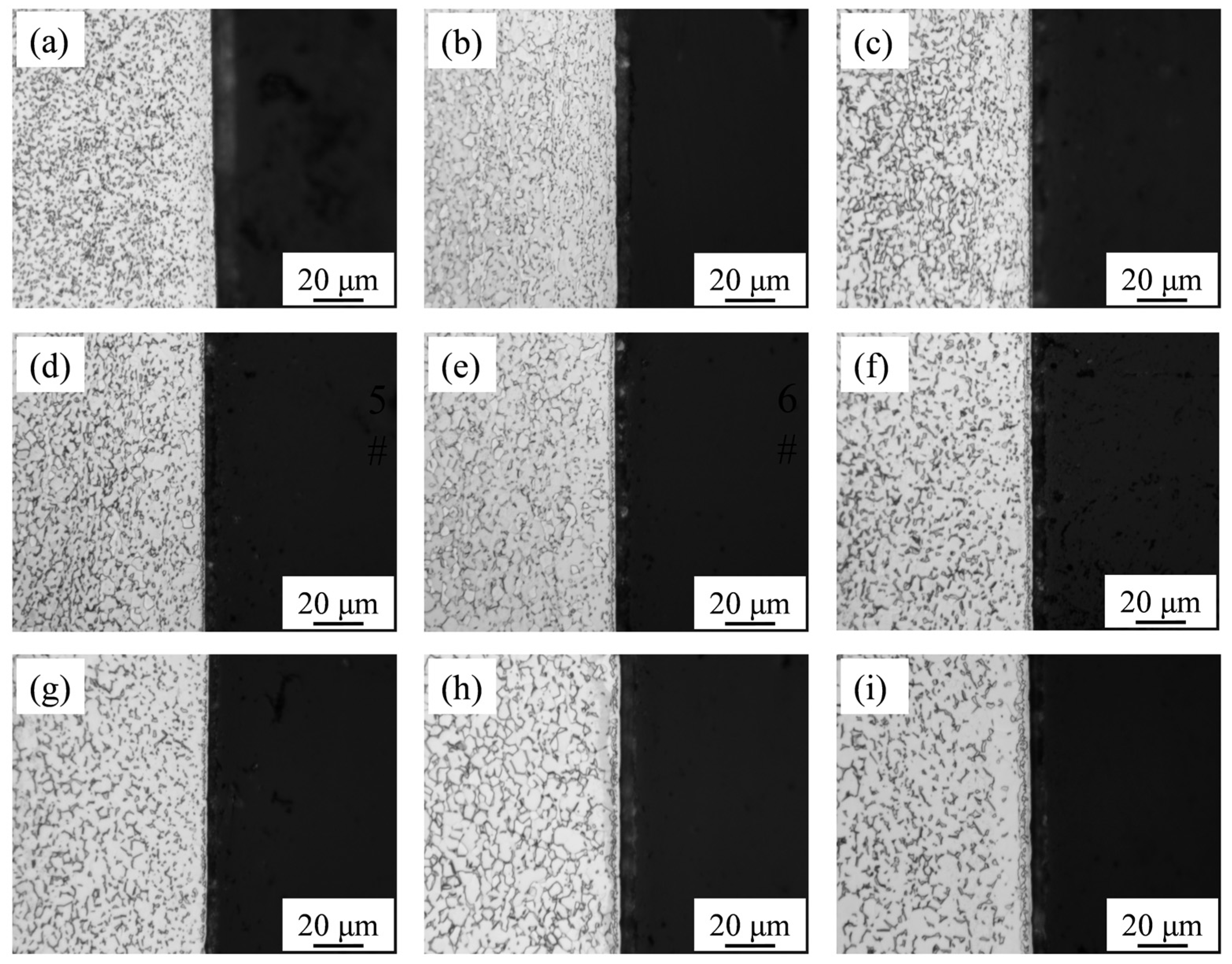
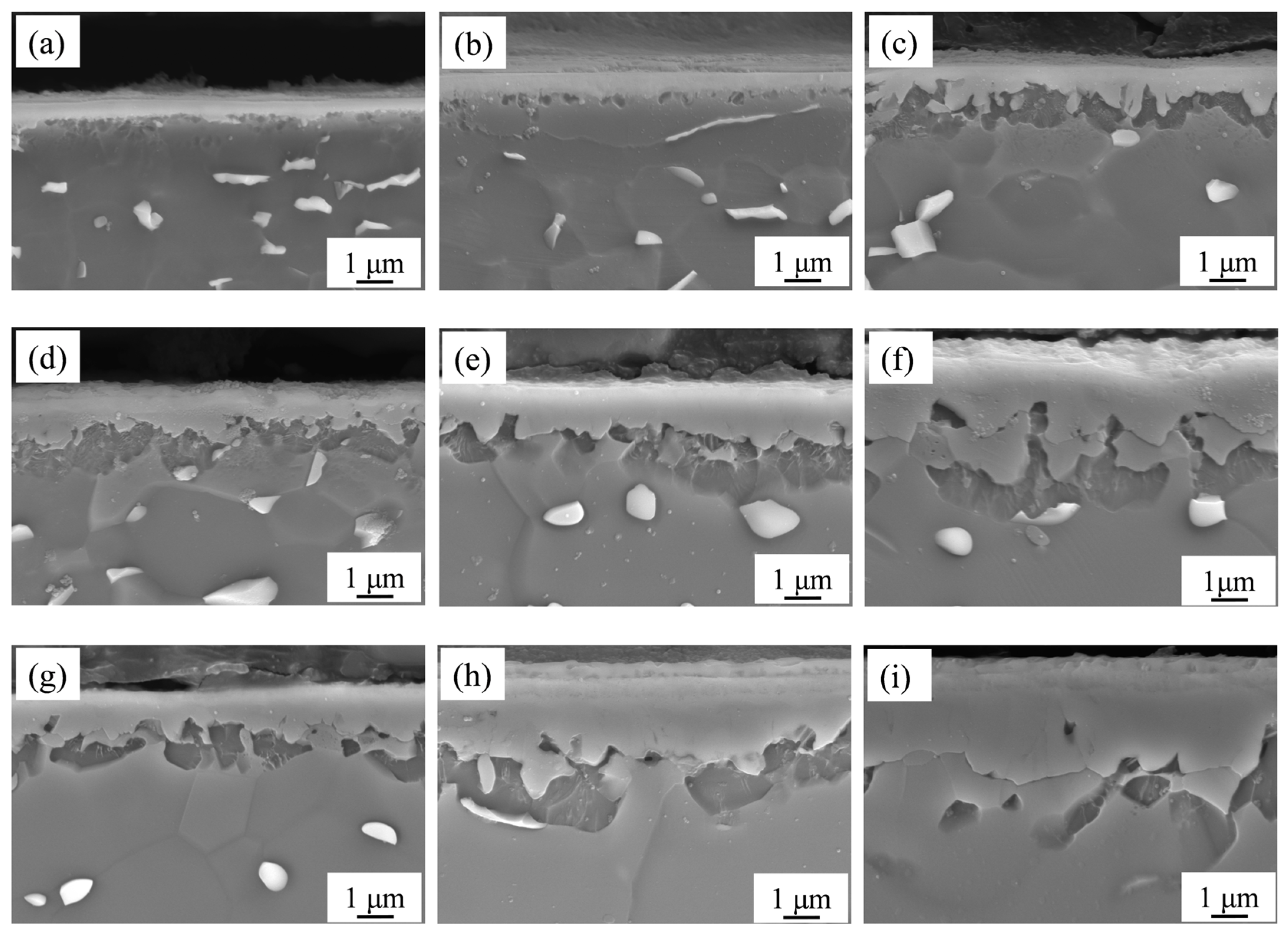
The cross-sections of the nitrided samples were also observed by SEM, as presented in
Figure 4. Irregular white blocks, typically regarded as the β phase [
2], were randomly precipitated in the α matrix. A series of light gray layers was observed on the sample surfaces. As the nitriding temperature increased and the nitriding duration was prolonged, the thickness of these layers increased.
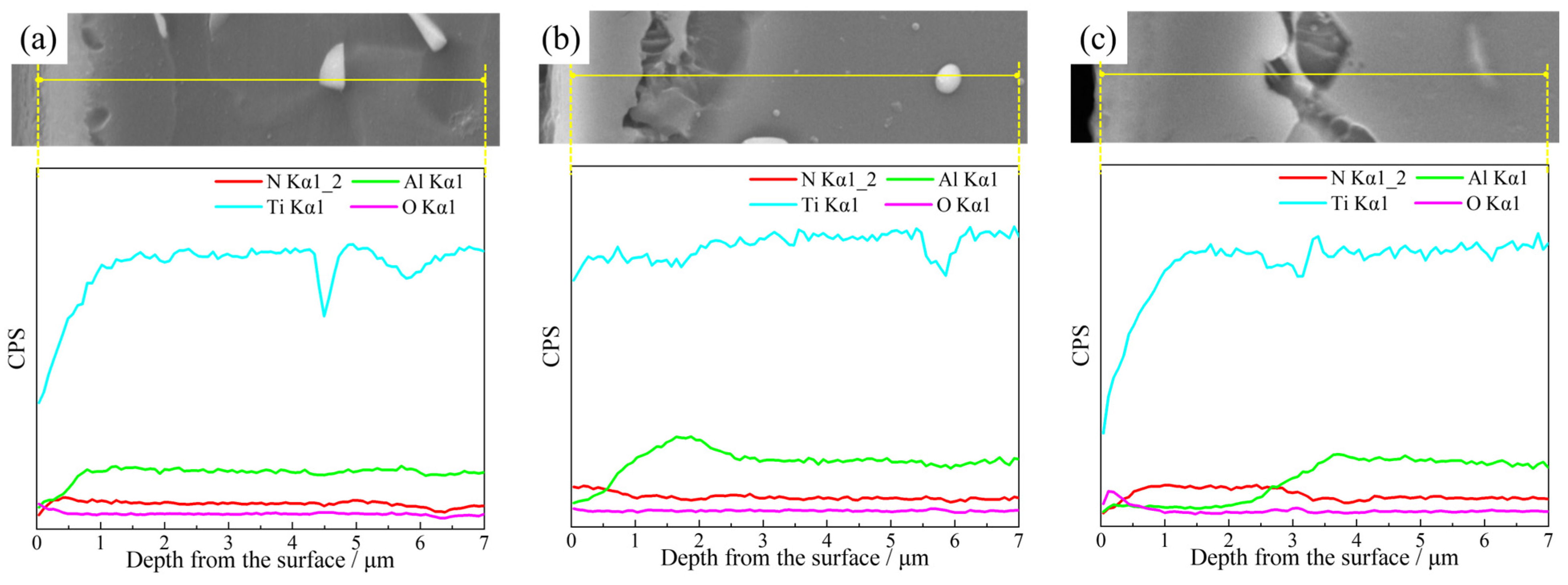
Due to the varying thicknesses of Samples 2#, 5#, and 9#, further analysis was conducted using EDS. The surface element distributions are presented in
Figure 5, and the cross-sectional line scanning results are presented in
Figure 6. For Sample 2#, which was nitrided at 850 °C for 2 h, the relatively low nitriding temperature and moderate duration limited the nitriding and diffusion of nitrogen. A slight enrichment of nitrogen was detected near the surface (
Figure 4a and
Figure 5a). For Sample 5#, nitrogen enrichment was more extensive (
Figure 5b and
Figure 6b), and this phenomenon became much more pronounced in Sample 9# (
Figure 5c and
Figure 6c).
These results indicate that increasing the nitriding temperature and duration enhances nitrogen diffusion and promotes the formation of the nitrided layer. The nitriding process involves three key steps: adsorption of nitrogen atoms on the surface, diffusion of nitrogen atoms into the subsurface, and formation of nitrides. Elevating the nitriding temperature increases the activity of both nitrogen atoms and substrate atoms, which facilitates these three processes. Additionally, prolonging the nitriding duration allows these processes to continue, enabling nitrogen atoms to diffuse deeper and the nitrided layer to grow thicker.
Additionally, oxygen was detected on the surfaces, indicating that oxidation was nearly unavoidable. Although the furnace was purged with nitrogen three times, a small amount of oxygen remained. Titanium has a strong affinity for oxygen, which results in the formation of oxides.
The concentration of aluminum was lower in the surface layer, particularly for Samples 5# and 9#, with a peak observed at the interface between the layer and the substrate—this suggests that aluminum gradually migrated from the surface and accumulated in the subsurface. Since nitrogen preferentially combines with titanium to form nitrides [
20], titanium tends to remain on or diffuse toward the surface; this, in turn, promotes the migration and enrichment of aluminum in the subsurface. Both nitrogen and aluminum are α stabilizers, capable of expanding the α phase region and reducing the volume fraction of the β phase in the alloy. As presented in
Figure 4, the number of β blocks near the surface decreased with increasing nitriding temperature and prolonged nitriding duration. This phenomenon is also attributed to the enrichment of nitrogen and migration of aluminum during nitriding.
Figure 7 presents the XRD patterns of the samples before and after nitriding. The as-received sample consisted of α and β phases, with the α phase being dominant. The primary nitrides formed in the nitrided samples were TiN, Ti
2N, and Ti
2AlN. Compared to the as-received sample, several TiN diffraction peaks were detected in Sample 1#, while Ti
2N and Ti
2AlN diffraction peaks were barely observable. In Sample 2#, the relative intensities of the Ti
2N diffraction peaks were extremely low, and the Ti
2AlN diffraction peaks remained barely detectable. Diffraction peaks corresponding to TiN, Ti
2N, and Ti
2AlN all appeared in Sample 3# as well as Samples 4#–9#.
Due to the sufficient nitrogen atoms on the sample surface, TiN preferentially formed during nitriding, according to
Subsequently, nitrogen atoms diffused inward, and Ti
2N formed according to
As nitridation progressed further, Al participated in the reaction, and Ti
2AlN was generated according to
Compared with the as-received sample, the diffraction peaks corresponding to the α phase in the nitrided samples shifted to the left, particularly the peak at approximately 38.40°. In the as-recieved sample, the lattice constants of α matrix were a = b = 2.9267 Å and c = 4.6752 Å, while in Sample 1#, they were a = b = 2.9268 Å and c = 4.6759 Å. With the increases in nitriding temperature and nitriding duration, the lattice further expanded. The lattice constants of α matrix were a = b = 2.9346 Å and c = 4.726 Å in Sample 2#, and they were a = b = 2.9327 Å and c = 4.7057 Å in Sample 4#. This phenomenon can be attributed to the solid solution of nitrogen atoms in the titanium lattice. Nitrogen atoms typically occupy interstitial sites in the titanium lattice, causing lattice expansion. The shift in the diffraction peaks indicates that, in addition to forming nitrides on the surface, some nitrogen atoms also diffused into the substrate, resulting in the formation of a nitrogen-diffusion layer.
Some diffraction peaks of TiO
2 were observed in the XRD patterns of the samples, indicating the formation of oxides during heating. The oxidation process is influenced by temperature, time, and nitrogen flow rate, with a competitive relationship existing between nitridation and oxidation. Among Samples 1#, 2#, and 3#, the TiO
2 diffraction peaks were weakest in Sample 3#, which was nitrided under the highest nitrogen flow rate (30 mL·min
−1) despite being heated for the longest duration. Samples 5# and 7#, which were also nitrided under a nitrogen flow rate of 30 mL·min
−1, exhibited barely detectable TiO
2 diffraction peaks. Moreover, as presented in
Figure 5 and
Figure 6, surface oxygen enrichment was noticeable in Samples 2# and 9# but weak in Sample 5#. These observations suggest that oxidation can be partially inhibited under high nitrogen flow rates.
3.3. Thickness and Surface Hardness
Table 3 presents the average thicknesses of the nitrided layers on the samples measured from SEM images, as well as the mean micro-Vickers hardness values obtained by applying the load directly to the sample surfaces. Range analysis and variance analysis were performed based on the data in
Table 3, with the results presented in
Table 4,
Table 5,
Table 6 and
Table 7.
From the range analysis results of the nitrided layer thickness (
Table 4), the range of nitriding temperature was the largest, whereas that of nitrogen flow rate was the smallest. This indicates that nitriding temperature exerts the greatest influence on the nitrided layer thickness, while nitrogen flow rate has the least impact. The variance analysis results were consistent with the range analysis results. The
-value for nitriding temperature was the smallest and less than 0.05 (
Table 5), indicating that its influence on thickness was significant. The influence of nitriding duration did not reach a significant level, though its
-value was close to 0.05. In contrast, the nitrogen flow rate exhibited the highest
-value, signifying the weakest influence. When comparing samples nitrided at different temperatures with the same nitriding duration, the thickness increased as the nitriding temperature rose. It was also observed that the thickness increased with the extension of nitriding duration at a constant nitriding temperature. According to the Arrhenius Law, the diffusion coefficient increases exponentially with a rise in temperature, as shown in the following formula:
where
is the frequency factor, which is related to the crystal structure and vibrational entropy;
is the diffusion activation energy;
is the gas constant;
is the absolute temperature. This means that the jump frequency of nitrogen atoms in the metal lattice increases significantly, thereby significantly accelerating the penetration rate and diffusion depth of nitrogen, which directly leads to an increase in the thickness of the penetration layer. While based on Fick’s Second Law, under constant temperature, the relationship between the penetration layer thickness
and time
is as shown in the following formula:
which means that extending the time does increase the penetration layer thickness, but the growth rate slows down gradually, and the influence of temperature on the thickness is much greater than that of time.
The surface hardness of the nitrided samples was higher than that of the as-received sample, confirming the hardening effect of nitriding. The results of range analysis and variance analysis on surface hardness revealed the distinct impacts of the three factors (
Table 6 and
Table 7). From the range analysis, nitrogen flow rate exerted the greatest impact on surface hardness, whereas nitriding temperature had the weakest effect. In contrast, the variance analysis indicated that the
-value of nitrogen flow rate was less than 0.05, which reached a statistically significant level. However, the
-values of nitriding temperature and nitriding duration were relatively close and did not meet the threshold for statistical significance.
At nitrogen flow rates of 10 mL·min−1 and 20 mL·min−1, the surface hardness was subject to a complex interplay between nitriding temperature and nitriding time. In contrast, when the nitrogen flow rate was increased to 30 mL·min−1, a consistent decrease in the hardness of all samples was observed. For samples treated at the same nitriding temperature, the hardness enhancement of those processed under a 30 mL·min−1 nitrogen flow was limited. For instance, Sample 3# exhibited lower hardness than Sample 2#, even though it was subjected to a longer nitriding duration. Analogously, Sample 5# underwent a longer nitriding duration than Sample 4# but also demonstrated a reduction in hardness. When comparing samples with identical nitriding durations (i.e., Samples 5# and 2#), it was found that the sample nitrided at 30 mL·min−1 (Sample 5#) had lower hardness than that nitrided at 20 mL·min−1 (Sample 2#)—despite the former being treated at a higher nitriding temperature. A similar phenomenon was also observed between Samples 7# and 4#.
The nitrogen flow rate directly regulates the supply rate and concentration of active nitrogen species, which in turn influences the adsorption and diffusion behaviors of nitrogen atoms on the material surface. Specifically, an excessively high nitrogen flow rate accelerates the escape of active nitrogen atoms, while an insufficiently low flow rate reduces the total amount of available active nitrogen atoms. Both scenarios are unfavorable for the progression of the nitriding reaction. In the present study, the
value corresponding to nitrogen flow rate in
Table 6 was the highest among
–
, indicating that a nitrogen flow rate of 20 mL·min
−1 was the most effective for enhancing the surface hardness of the samples.
3.4. Tensile Tests
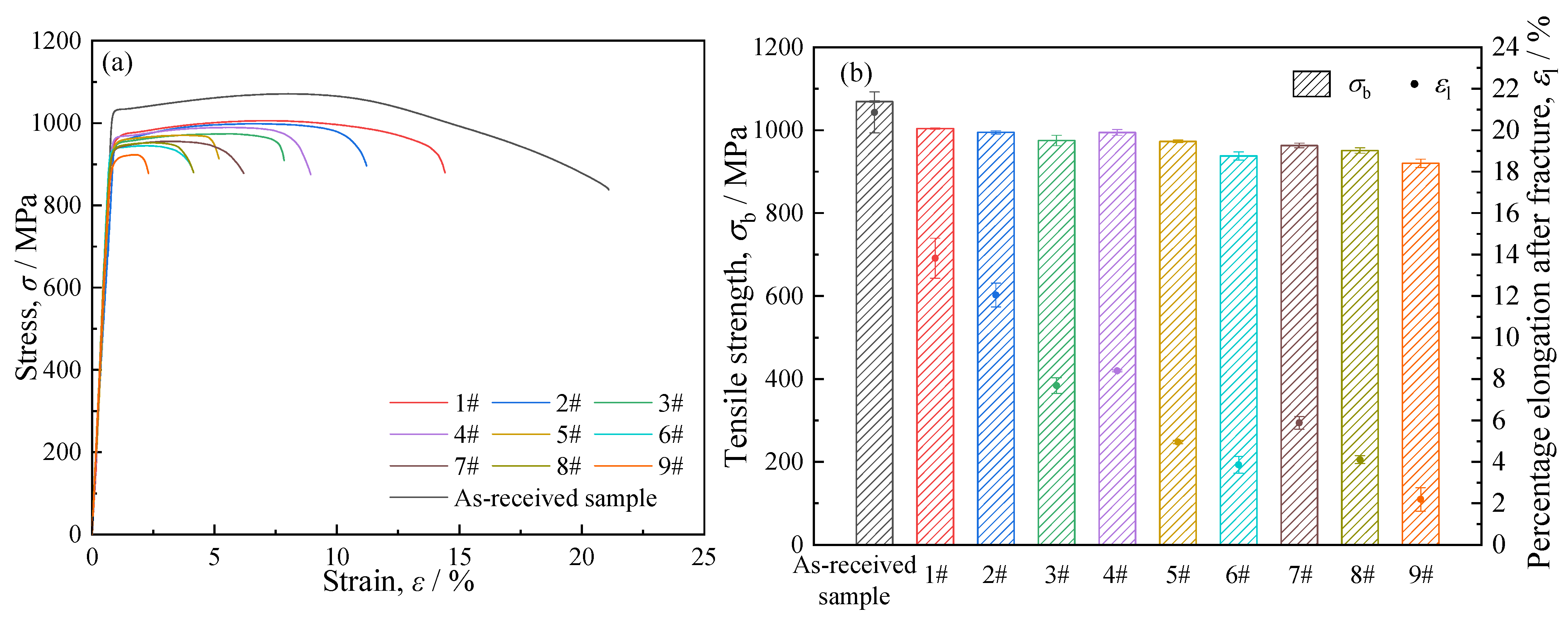
Figure 8 presents the tensile stress–strain curves of the samples, along with the tensile strengths and percentage elongations after fracture derived from the tensile tests. As illustrated in
Figure 8a, the stress–strain curves of all samples exhibited distinct elastic stages and plastic deformation stages. The elastic moduli of the nitrided samples varied from 122.52 to 131.23 GPa, which were slightly higher than that of the as-received sample (110.80 GPa). This phenomenon can be attributed to the formation of a hard nitrided layer on the sample surface. Nevertheless, the enhancement effect of the nitrided layer on the elastic modulus was limited, primarily due to the small thickness of these layers. Furthermore, the elastic modulus of a material is predominantly determined by the strength of interatomic bonds and its crystal structure, and variations in grain size exert negligible influence on the elastic modulus [
21], which explains the only slight differences observed in the elastic moduli between the nitrided and as-received samples.
The tensile strength and percentage elongation after fracture of each sample are presented in
Figure 8b. The as-received sample exhibited a tensile strength of ~1068.87 MPa and a percentage elongation after fracture of ~20.8%. After nitriding, both the tensile strength and percentage elongation after fracture of the samples decreased. Specifically, with the increase in nitriding temperature and the extension of nitriding duration, both properties exhibited a gradual downward trend. In this study, the nitrided layers were relatively thin, and all samples fractured during the plastic deformation stage rather than undergoing brittle fracture, indicating that the nitrided layers exerted only a slight influence on the tensile strength. As reflected in the aforementioned results, the nitriding temperature and duration induced microstructural evolution in the substrate, which in turn led to changes in the substrate’s hardness. Consequently, the variation in tensile strength was primarily attributed to the evolution of the substrate microstructure—a factor predominantly determined by the heating temperature and holding time during the nitriding process.
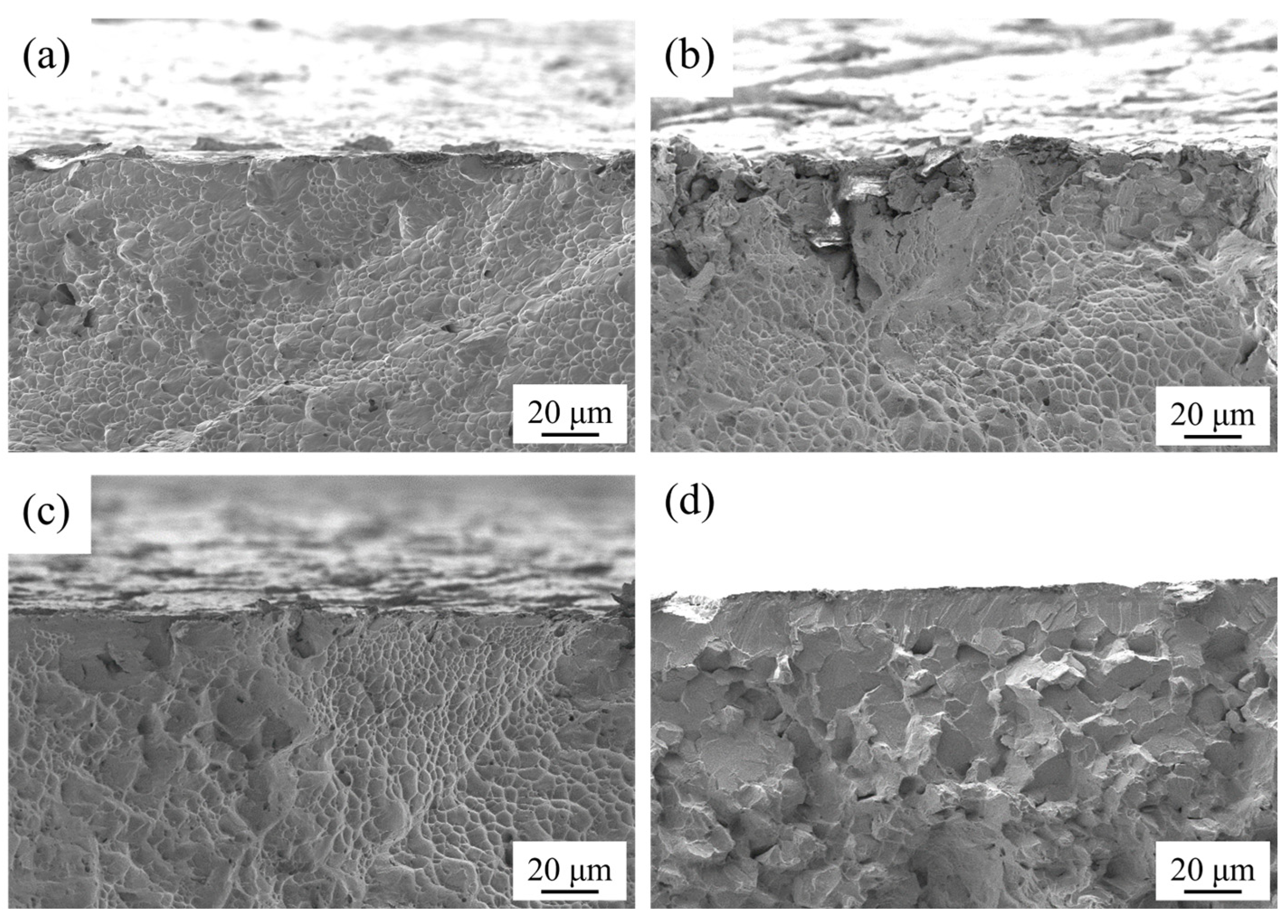
Additionally, the change in percentage elongation after fracture was found to exhibit a trend opposite to that of nitrided layer thickness. Since all nitrided samples retained a certain degree of plasticity, the percentage elongation after fracture was associated with the initiation and propagation of microcracks. The fracture cross-sections of the samples were observed by SEM, with representative images presented in
Figure 9. The as-received sample exhibited continuous dimples across its entire fracture cross-section–a hallmark of typical ductile fracture behavior. For Samples 2# and 5#, local cleavage features were observed in the near-surface region, while dimples dominated the remaining areas of the cross-sections. For Sample 9#, smooth cleavage planes were distributed along the surface and extended inward, resulting in a mixed fracture mode of transgranular and intergranular fracture; dimples were only locally present in the inner region of the cross-section. The fracture cross-sectional characteristics of Samples 2#, 5#, and 9# indicated that fracture initiation occurred at the surface—specifically, within the nitrided layer. Owing to the high hardness and brittleness of the nitrided layer, microcracks were more prone to initiation and propagation during tensile loading, which in turn led to a reduction in sample plasticity. As the nitriding temperature and duration increased, the thickness of the nitrided layer increased, thereby exacerbating the adverse effect on plasticity. Consequently, in the context of this study, a thicker nitrided layer was unfavorable for maintaining the mechanical properties of Ti-6Al-4V.
Based on the results presented in
Section 3.3 and
Section 3.4, the nitrided layer thickness, surface hardness, and tensile properties of the samples were found to be governed by distinct influencing factors. To achieve a thicker nitrided layer, higher nitriding temperatures and longer duration were required; however, this condition led to a reduction in both tensile strength and percentage elongation after fracture. Surface hardness was strongly influenced by the nitrogen flow rate, with 20 mL·min
−1 identified as an optimal value. For nitrided Ti-6Al-4V samples, the target performance requirement is to enhance wear resistance—this necessitates an increase in surface hardness relative to the as-received sample while preserving tensile properties as much as possible.
For Samples 4#–9#, nitriding resulted in a significant decrease in plasticity, indicating that 900 °C and 950 °C were unsuitable nitriding temperatures for this application. Additionally, the percentage elongation after fracture of Sample 3# was below 10%, suggesting that a 4 h nitriding duration was excessively long. In comparison to Sample 1#, Sample 2# exhibited a thicker nitrided layer and higher surface hardness, while its tensile properties remained comparable. Consequently, Sample 2#—which possessed a surface hardness of 661.31 HV0.3, a tensile strength of ~994.9 MPa, and a percentage elongation after fracture of ~12.1%—was deemed to have the most desirable comprehensive performance. This sample, along with the as-received sample, was therefore selected for subsequent accelerated corrosion and wear tests for comparative analysis.
3.5. Accelerated Corrosion Results
It is well established that nitrided layers formed on Ti alloys enhance corrosion resistance in commonly used electrolytes [
13,
15,
16,
17]. To evaluate the protective efficacy of the nitrided layer under harsh environmental conditions, accelerated corrosion tests were conducted: the as-received sample and Sample 2# were immersed in highly corrosive hydrofluoric acid (HF) solutions for comparative analysis. The cumulative weight losses of the two samples after immersion in HF solutions of varying concentrations are presented in
Figure 10.
For the as-received sample, the cumulative weight loss increased gradually with prolonged immersion time, although the rate of increase slowed over time. At the same immersion duration, the sample exhibited greater cumulative weight loss in higher-concentration HF solutions than in lower-concentration ones.
For Sample 2# immersed in a 1% HF solution, the cumulative weight loss remained nearly zero during the initial 20 min. Similarly, when Sample 2# was exposed to 3% and 5% HF solutions, its cumulative weight loss was also close to zero within the first 10 min of immersion. These observations demonstrate that the nitrided layer effectively protected the substrate from HF-induced etching during the initial corrosion stage. Once corrosion was initiated, the weight loss rate of Sample 2# in all three HF solutions first increased and then decreased—exhibiting a trend analogous to that of the as-received sample.
When immersed in a 1% HF solution, Sample 2# consistently exhibited lower cumulative weight loss than the as-received sample; however, the difference in cumulative weight loss between the two samples narrowed as immersion time increased. An analogous trend was observed when the two samples were exposed to a 3% HF solution. Furthermore, after 60 min of immersion in a 5% HF solution, the cumulative weight losses of the two samples became nearly identical. This phenomenon indicates that the protective efficacy of the nitrided layer was almost completely depleted following prolonged immersion.
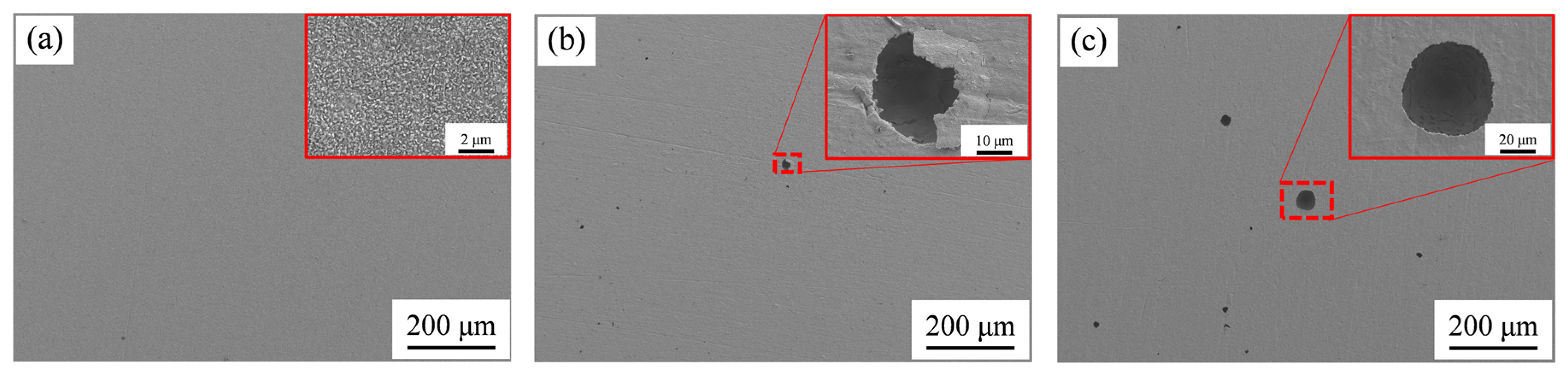
The surface morphologies of the samples after accelerated corrosion tests were observed via SEM, and the representative images are presented in
Figure 11. The surfaces of the as-received samples exhibited a uniform corrosion feature, as illustrated in
Figure 11a (a typical example). When examined under a higher magnification, some residual β-phase particles were clearly identified on the corroded surface, as presented in
Figure 11b.
For Sample 2# immersed in a 1% HF solution for 60 min (
Figure 11c,d), partial surface etching was observed, with a morphology similar to that of the as-received sample. Additionally, the nitrided layer remained locally residual on the surface. In contrast, when Sample 2# was immersed in 3% and 5% HF solutions for 60 min, residual nitrided layers were barely observed. Notably, both samples (immersed in 3% and 5% HF) exhibited morphologies analogous to that of the as-received sample.
Ti and TiO
2 are soluble in HF solutions, as described by the following reactions:
Therefore, the as-received samples underwent rapid etching when exposed to HF solutions.
TiN is also soluble in HF solutions, as described by the following reaction:
However, in a non-oxidizing environment, the reaction rate of Equation (6) was much slower than those of Equations (4) and (5). To gain insight into the corrosion process of Sample 2#, the surface morphologies of the sample after immersion in a 1% HF solution for 10, 20, and 30 min were observed via SEM, as presented in
Figure 12.
The change in the surface was rarely observed after 10 min of immersion (
Figure 12a). As the immersion time was extended to 20 min, pits began to emerge on the surface (
Figure 12b); a further extension of the immersion time to 30 min resulted in a noticeable increase in the size of these holes (
Figure 12c).
Galvanetto observed that the nitrided layer on Ti-6Al-4V peeled off following immersion in HCl solutions, and further pointed out that localized dissolution primarily occurred at defects in the nitrided layer [
22]. Fossati immersed glow-discharge nitrided Ti-6Al-4V in a 4 mol·L
−1 HNO
3 solution; nitrided layer peeling was also observed, and the Ti
2N layer exhibited a higher corrosion resistance than the TiN layer [
15]. In this study, the nitrided layer was composed of TiO
2 and nitrides. The localized dissolution of the nitrided layer at the initial stage could be attributed to the dissolution of TiO
2. Subsequently, the substrate was exposed to the solution and underwent continuous etching. As the immersion time increased, further localized dissolution occurred: the corrosion holes expanded, and more of the substrate was etched, which ultimately led to a gradual reduction in the protective efficacy of the nitrided layer. As the substrate preferentially reacted with HF, its dissolution led to the detachment of the nitrided layer from the substrate. This explains why corrosion accelerated after several minutes of immersion in HF solutions. Following immersion, black powder was observed in the solution; this powder is attributed to undissolved nitrides that had detached from the substrate.
Nevertheless, for the initial 10 min of immersion, the cumulative weight losses of Sample 2# in 1%, 3%, and 5% HF solutions were only 0.15%, 0.19%, and 1.50% of those of the as-received sample, respectively. This result clearly demonstrates the strong protective efficacy of the nitrided layer.
3.6. Wear Test Results
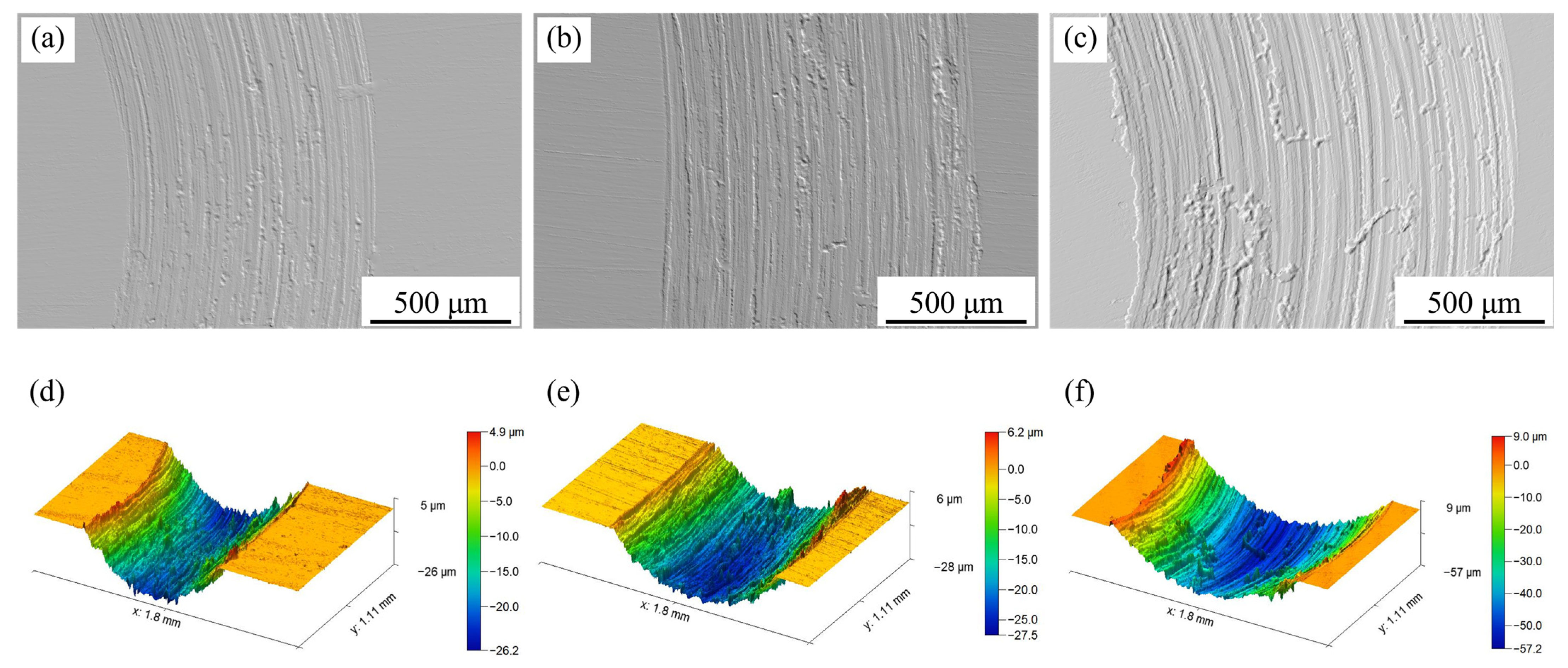
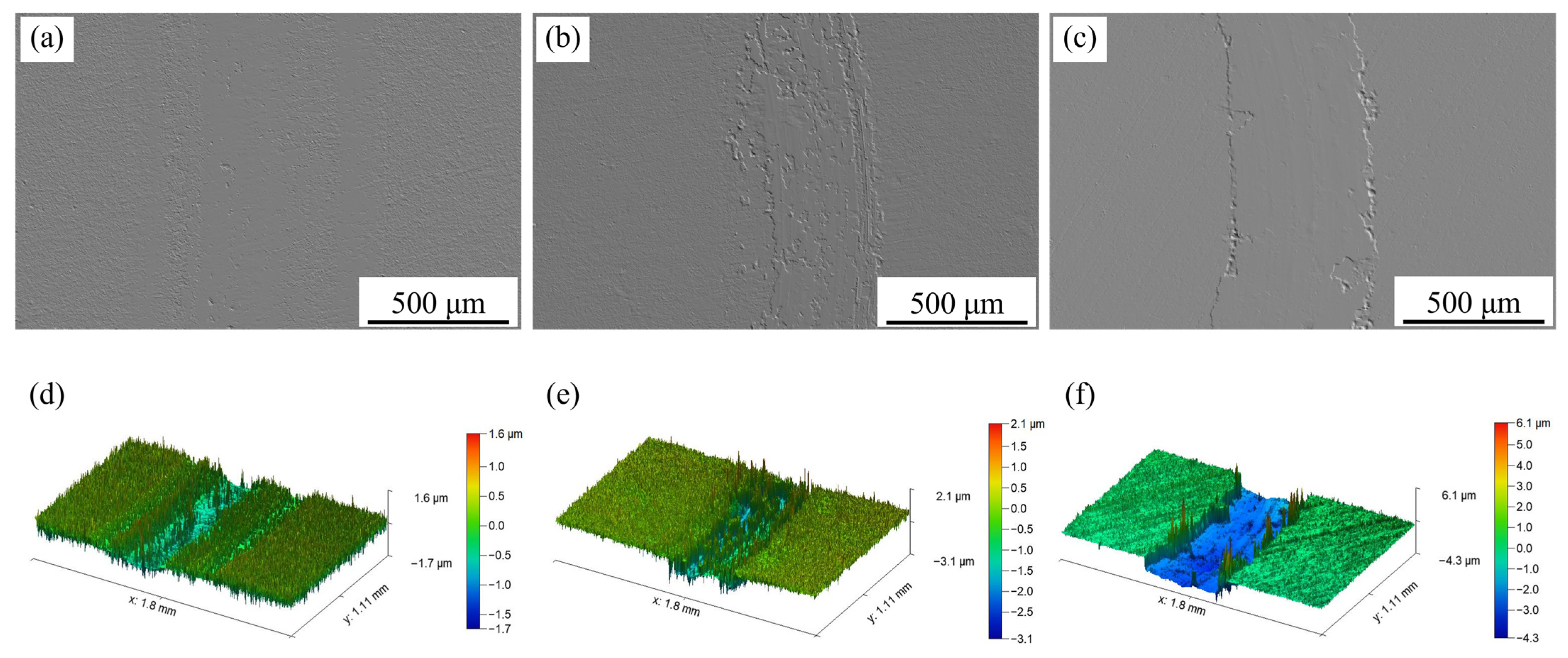
Figure 13 presents the variation in friction coefficient with time for the as-received sample and Sample 2#. The surface morphologies and three-dimensional morphologies of the worn sample surfaces are depicted in
Figure 14 and
Figure 15, respectively.
For the as-received sample tested under an applied load of 3 N, the friction coefficient increased slightly before stabilizing at approximately 0.5. When the applied load was increased to 5 N, the friction coefficient initially remained at approximately 0.3, followed by a gradual increase to around 0.5. Under an applied load of 10 N, the as-received sample exhibited an initial decrease in friction coefficient during the wear process, which was subsequently followed by an increase to approximately 0.5. When the applied load was small, a steady wear condition was achieved within a short time. As presented in
Figure 14a,d, furrows were the dominant morphological feature on the worn surface. With increasing applied load, small abrasive debris was generated, which in turn exerted a self-lubricating effect and reduced the friction coefficient. Subsequently, this abrasive debris either adhered to the substrate or the grinding ball, leading to the establishment of a relatively steady wear state. Additionally, localized scuffing was observed on the worn surfaces, as illustrated in
Figure 14b,e. When the applied load was further increased to 10 N, more small abrasive debris formed, resulting in a noticeable initial decrease in the friction coefficient. As a steady wear condition was gradually established, the friction coefficient increased progressively and eventually stabilized at approximately 0.5. Due to the high applied load of 10 N, plastic deformation and scuffing were prone to occur on the worn surface, as presented in
Figure 14c,f.
Under different applied loads, the initial friction coefficients of Sample 2# were nearly identical and consistently lower than those of the as-received sample (
Figure 13). Sample 2# featured a hard nitrided layer on its surface, which inhibited the occurrence of local plastic deformation.
As illustrated in
Figure 15, the nitrided layer exhibited lower surface smoothness compared to the as-received sample. This reduced smoothness led to a smaller initial contact area between the friction pairs, thereby minimizing frictional resistance and resulting in the consistently lower initial friction coefficients observed.
As noted in
Section 3.3, the nitrided layer of Sample 2# exhibited a thickness of approximately 0.73 μm. As presented in
Figure 15a,d, following the wear test under an applied load of 3 N, the nitrided layer remained largely intact, with almost no furrows detected on its worn surface. Additionally, the running-in period between the hard nitrided layer and the grinding ball was prolonged; a steady wear state was ultimately established between the two contact surfaces. Under applied loads of 5 and 10 N (
Figure 15b,c,e,f), the wear depths exceeded the thickness of the nitrided layer, indicating that the substrate was exposed and subjected to wear. Since the grinding ball came into contact with the relatively soft substrate within a short time, the running-in period was shortened. For Sample 2# tested under 5 N, the contact stress rises, leading to brittle fracture and spallation in local areas of the nitrided layer due to stress concentration. Meanwhile, the substrate with relatively low hardness comes into contact with the friction pair, and the detached hard wear debris from the surface layer acts as a third body at the friction interface. Consequently, during friction under this load condition, plastic deformation and wear on the substrate surface are aggravated, and plowing grooves are formed. When the applied load was increased to 10 N, the nitrided layer was more susceptible to wear. Abrasive debris from the nitrided layer partially filled the furrows on the substrate, thereby establishing a new steady wear state. Consequently, the worn surface of Sample 2# under 10 N appeared relatively smooth, with a clear boundary between the worn and unworn regions.
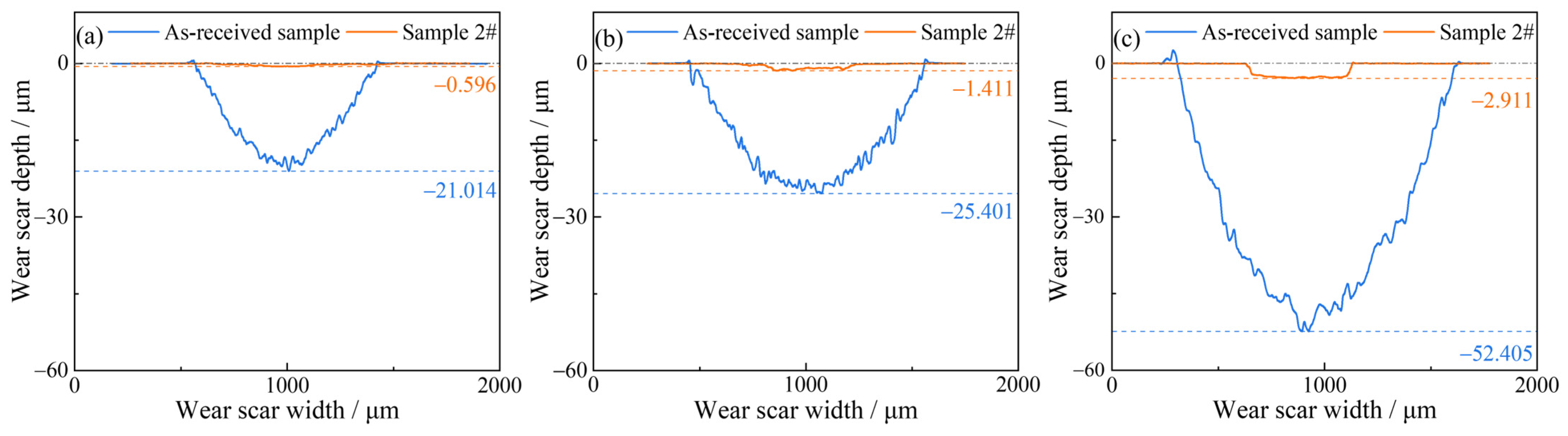
Figure 16 presents the fitted average cross-sectional profiles of the wear scars. Both the width and depth of the wear scar increased with increasing applied load. Compared to Sample 2#, the as-received sample exhibited wider and deeper wear scars; conversely, the wear scars of Sample 2# were narrower and shallower. The cross-sectional area (
A) of each wear scar was determined from the profiles in
Figure 16, and the wear rate (
w) was subsequently calculated using the following formula:
where
V is the wear volume of the sample,
F is the applied load, and
L is the sliding length of the grinding ball,
D is the diameter of the circular track used in the dry friction wear test,
n is the rotating speed (200 r·min
−1), and
t is the rotating time (30 min). By substituting the values of
n and
t into Equation (18), the wear rate
w was directly calculated using the following expression:
The calculated wear rates of the as-received sample and Sample 2# are presented in
Figure 17. Under an applied load of 3 N, the wear rate of Sample 2# was only 2.29% of that of the as-received sample. With increasing applied load, the wear rates of both samples increased; notably, Sample 2# exhibited a more pronounced upward trend, which was attributed to the gradual wear-through of its nitrided layer. Even under the highest applied load of 10 N, the nitrided layer retained most of its high wear resistance: the wear rate of Sample 2# remained as low as 2.89% of the as-received sample’s wear rate.
Farokhzadeh noted that a thick compound layer is prone to crack initiation, and the propagation of such cracks could be effectively suppressed in a timely manner—ultimately leading to reductions in both fatigue performance and wear resistance [
23]. Nolan proposed that a surface layer containing high-hardness nitrides required a lower-hardness nitrogen diffusion layer for structural support [
24]. Without this supportive diffusion layer, cracks were likely to initiate in the hard, brittle surface layer due to the plastic deformation of the substrate. These cracks could then lead to local spalling of the surface layer, which ultimately evolved into severe wear. Li removed the surface compound layer while retaining the diffusion layer after nitriding pure Ti and discovered that the diffusion layer could effectively inhibit the initiation and propagation of cracks, thereby enhancing the anti-cavitation ability [
25]. Therefore, it is concluded that nitrogen diffusion in the substrate exerts an important influence on the alloy’s wear resistance.
Additionally, Batory noted that thin nitrided layers formed via low-temperature, short-duration nitriding exhibited superior resistance to crack initiation and propagation compared to thicker nitrided layers produced through high-temperature, long-duration treatment [
26]. In this study, nitrogen atoms not only formed a nitrided layer on the sample surface but also diffused into the substrate interior. Thinner nitrided layers were obtained when the nitriding temperature was set to 850 °C. Even though the nitrided layer of Sample 2# was worn through under the higher applied loads, the underlying diffusion layer still exhibited noticeable wear resistance—indicating that the selected nitriding parameters were suitable.
Overall, Ti-6Al-4V samples were subjected to direct gas nitriding via a designed orthogonal experiment, with three process parameters—nitriding temperature, nitriding duration, and nitrogen flow rate—selected as the experimental factors. Given that the nitriding process requires heating the specimens at a relatively high temperature for a certain duration, the specimens undergo an equivalent heat treatment, which induces microstructural changes. Furthermore, despite the equipment’s desirable sealing performance and three rounds of gas purging, oxygen absorption and diffusion during the nitriding process remain unavoidable. The results indicate that the three process parameters exert contradictory effects. Increasing the nitriding temperature and extending the nitriding duration are beneficial for the growth of the nitrided layer but cause microstructural coarsening, which in turn reduces the material’s plasticity. Raising the nitrogen flow rate partially inhibits oxidation; however, it is unfavorable for achieving sufficient nitriding—an effect that limits improvements in hardness.
Among the nine designed experimental groups, the combination of a nitriding temperature of 850 °C, a nitriding duration of 2 h, and a nitrogen flow rate of 20 mL·min−1 was identified as the most suitable process parameter set. The protective effect of the nitrided layer was verified via HF etching and wear tests. Owing to the limited number of specimens prepared in this study, numerical simulations will be further conducted to model key processes—including nitrogen diffusion, nitride formation, stress evolution, and crack initiation within the nitrided layer—with the aim of further optimizing the nitriding process and thereby enhancing material properties. Additionally, the wear mechanisms could be investigated in greater depth through integrated testing and modeling under diverse conditions; this would help clarify the respective influences of the nitrided layer and diffusion layer, representing another valuable direction for future research.