Abstract
The influence of Cu addition on the age-hardening response, mechanical properties, and precipitation evolution of Si-rich Al–Mg–Si alloy was investigated by hardness test, room-temperature tensile test, and transmission electron microscopy analysis. The results indicate that the addition of Cu significantly enhances the aging–hardening response of the alloy, promotes the hardness and room-temperature tensile strength under the peak-aged state, and reduces the softening rate during over-aging. The peak-aged tensile strength of the Cu-added alloy (387 MPa) was approximately 9% higher than that of the Cu-free alloy (355 MPa), and the elongation to failure of the Cu-added alloy reached 19%, significantly exceeding the 15% exhibited by the Cu-free alloy. The Cu promotes the precipitation of under-aged and peak-aged β″ strengthening phases within the alloy grains, while also facilitating the formation of lath-shaped Q’ and L phases in peak-aged and over-aged microstructures. This enhances the room-temperature tensile properties of the alloy in the peak-aged state and reduces the attenuation of over-aged properties. Furthermore, Cu influences grain boundary precipitation behavior by promoting the formation of Cu-rich precipitates along grain boundaries and reducing the width of precipitation-free zones (PFZs).
1. Introduction
Al–Mg–Si–(Cu) alloys are age-hardenable aluminum alloys that exhibit excellent formability, high specific strength, and good corrosion resistance. These alloys are widely applied in rail transportation and the automotive industry. With the advancement of modern industrial requirements, there is a growing demand for improved mechanical properties in these alloys [1,2,3,4,5].
The aging precipitation sequence of the ternary Al–Mg–Si alloy system is generally recognized as follows [6,7,8,9,10]: supersaturated solid solution → clusters/GP zones → β″ → β′ (U1/U2/B’) → β, Si. The U1, U2, and B’ phases typically precipitate in the over-aged stage, particularly in alloys with relatively high Si content. Cu can significantly enhance the aging-induced hardness and mechanical properties of Al–Mg–Si alloys by modifying the precipitation sequence during artificial aging [5,11]. The precipitation sequence of the quaternary Al–Mg–Si–Cu alloy generally follows this progression [12,13,14]: supersaturated solid solution → clusters/GP zones → β″, L, C, QP, QC → β′, Q’ → Q, Si. Cu participates in the formation of solute clusters during the early stages of aging in Al–Mg–Si–Cu alloys [15], enhancing the thermal stability of these clusters and facilitating their evolution into the β″ phase via the GP zones [15,16], minimizing the adverse effects of natural aging on subsequent artificial aging processes for β″ precipitation [17]. The needle-like monoclinic β″ phase is commonly observed in peak-aged alloys and represents the most effective strengthening precipitate [5,18,19]. The Q and L phases, which contain Cu, contribute to additional strengthening and exhibit a relatively slow precipitation and coarsening rate during over-aging [5]. Under peak aging conditions, the 6061 alloy containing 0.08 at.% Cu and enriched in Si precipitates a lath-shape Q′ phase, whereas the conventional 6061 alloy with a relatively higher Mg content forms a lath-shape L phase. This difference in precipitation behavior may be attributed to the overall alloy composition [20]. However, Cu tends to segregate and precipitate at grain boundaries, thereby enhancing the susceptibility of the alloy to intergranular corrosion [5,11].
The primary alloying elements in 6XXX series aluminum alloys are magnesium and silicon, which combine in a stoichiometric ratio to form the Mg2Si phase [21]. An excess of silicon promotes the fine and uniform precipitation of the β″ phase, thereby enhancing the age-hardening response [21,22] and improving the mechanical properties of the alloy [23]. However, a higher silicon content also lowers the nucleation temperatures of the β′ and Q’ phases, thereby accelerating the over-aging kinetics of the alloy [23] and reducing the over-aging strength [21]. It should be noted that precipitate formation does not strictly follow the conventional precipitation sequence but is significantly influenced by the alloy composition and the aging treatment conditions.
Although numerous studies have examined the influence of Cu on the precipitation behavior and mechanical properties of Al–Mg–Si alloys, the majority of existing research has primarily focused on aging-hardening responses and precipitation characteristics at a single aging temperature. Furthermore, systematic investigations into the precipitation of lath-shape phases in Si-rich Al–Mg–Si–Cu alloys across different aging stages remain limited. This study systematically investigated the effect of adding 0.36 wt.% Cu on the age-hardening behavior of Si-rich Al–Mg–Si alloys across various aging temperatures, with particular emphasis on precipitation behavior and the associated mechanical properties during different stages of aging at the optimal temperature, as well as under heavily over-aged conditions at relatively high aging temperature.
2. Materials and Methods
In this study, high-purity elemental materials and master alloys, including aluminum with a purity of 99.99%, high-purity magnesium, Al-21%Si, Al-60%Cu, Al-10%Mn, Al-6%Er, and Al-10%Zr, were used as raw materials. Two alloy compositions were fabricated using the gravity casting method with metallic molds. The ingots each had a mass of 1.5 kg and approximate dimensions of 150 mm × 90 mm × 30 mm. The chemical compositions of both alloys were determined by means of inductively coupled plasma atomic emission spectrometry (ICP-AES) and are presented in Table 1. The as-cast ingots were subjected to a two-stage homogenization treatment, comprising an initial annealing step at 300 °C for 10 h, followed by a subsequent step at 535 °C for 12 h. Subsequently, the homogenized ingots were hot-rolled into sheets with a thickness of 4 mm, which were then air-cooled to room temperature. Specimens with dimensions of 12 mm (Rolling Direction, RD) × 10 mm (Transverse Direction, TD) × 4 mm (Normal Direction, ND) were machined from these sheets and subjected to solution treatment at 545 °C for 30 min, followed by immediate quenching with water to room temperature. Subsequently, the alloys were isothermally aged at 155 °C, 165 °C, 175 °C, and 185 °C, respectively.

Table 1.
Actual chemical compositions (wt.%) of the alloys prepared in this study.
All hardness tests and TEM samples were obtained from a cross-sectional area measuring 12 mm (RD) × 10 mm (TD). Microhardness was measured at room temperature using an HXD-1000TM/LCD Vickers hardness testing machine (Shanghai Taiming Optical Instrument Co., Ltd., Shanghai, China)with a load of 9.8 N and a dwell time of 10 s. The hardness value for each specimen was determined as the mean of six individual measurements. The tensile test was conducted using a universal testing machine at room temperature of 25 °C in accordance with GB/T 228.1-2010 [24], with a loading rate of 2 mm/min. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) observations were carried out using a JEM-2100F field-emission transmission electron microscope (JEOL, Tokyo, Japan) operated at 200 kV. TEM specimens were prepared as 3 mm-diameter thin disks and electropolished by RL-2 twin-jet equipment with a voltage of 18 V in a solution consisting of 70% methanol and 30% nitric acid maintained at approximately −25 °C. All microstructural observations were conducted along the <100>Al zone axis. Precipitate sizes were measured using Nano Measurer 1.2.5 software.
3. Results and Discussion
3.1. Age-Hardening Behavior
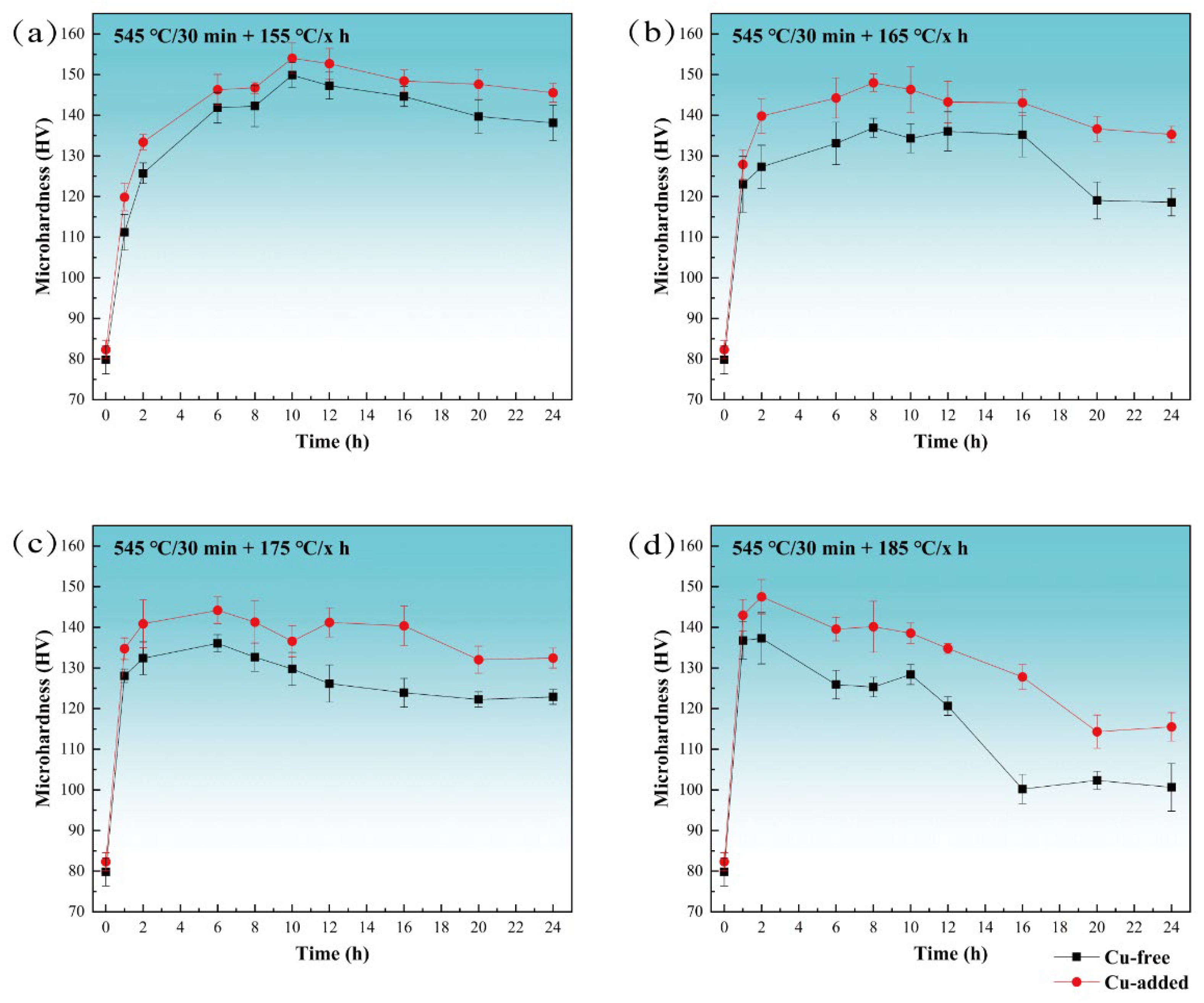
Figure 1 presents the hardness curves of Cu-free and Cu-added alloys after isothermal aging at temperatures of 155 °C, 165 °C, 175 °C, and 185 °C. The solid–solution hardness of both alloys is similar, approximately 80 HV. As shown in Figure 1a, the age-hardening behavior of the two alloys at 155 °C indicates a noticeable increase in hardness during the early stages of aging (1 h). The hardness values after 2 h of aging are 111 HV and 120 HV for the Cu-free and Cu-added alloys, respectively. At the peak-aged stage (10 h), the hardness of the Cu-free and Cu-added alloys reach 150 HV and 154 HV, respectively. Following over-aged stage (24 h), the hardness decreased to 138 HV and 146 HV, respectively.
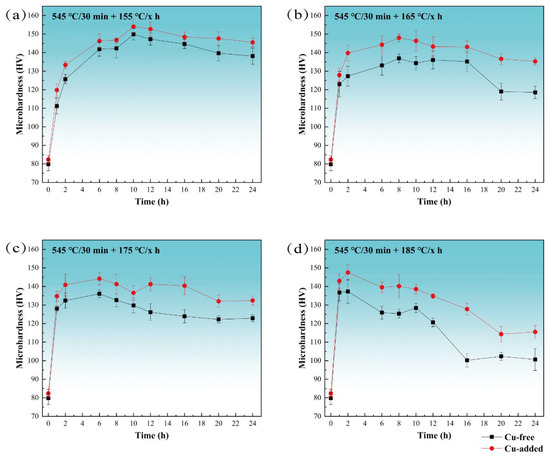
Figure 1.
The age-hardening curves of Cu-free and Cu-added alloys at various temperatures. (a) Aging at 155 °C, (b) aging at 165 °C, (c) aging at 175 °C, and (d) aging at 185 °C.
Figure 1b–d illustrate the age-hardening curves at 165 °C, 175 °C, and 185 °C, respectively. The general trend in hardness evolution is consistent across all tested temperatures. However, with the aging temperature increases, the time required to achieve peak hardness is shortened, and the rate of softening during over-aging accelerates. At 165 °C and after 8 h of aging, the maximum hardness values attained were 137 HV for the Cu-free alloy and 148 HV for the Cu-added alloy. At 175 °C (6 h), these values are 136 HV and 144 HV, and at 185 °C (2 h), they are 137 HV and 148 HV. The maximum hardness for both alloys is observed after peak aging at 155 °C. After heavily over-aging at 185 °C (24 h), the hardness decreased to 101 HV and 116 HV for the Cu-free and Cu-added alloys, respectively, representing reductions of 26.3% and 21.6% compared to their peak hardness at 185 °C (2 h). When compared to the peak hardness achieved at 155 °C (10 h), the reductions were 32.7% and 24.7%, respectively. The Cu-added alloy exhibits a significantly slower rate of hardness reduction during over-aging.
In summary, Figure 1 illustrates that under aging conditions, the Cu-added alloy exhibits a faster hardening response during the under-aged stage and a slower softening rate during over-aging, with its overall aging hardness consistently exceeding that of the Cu-free alloy.
3.2. Tensile Properties
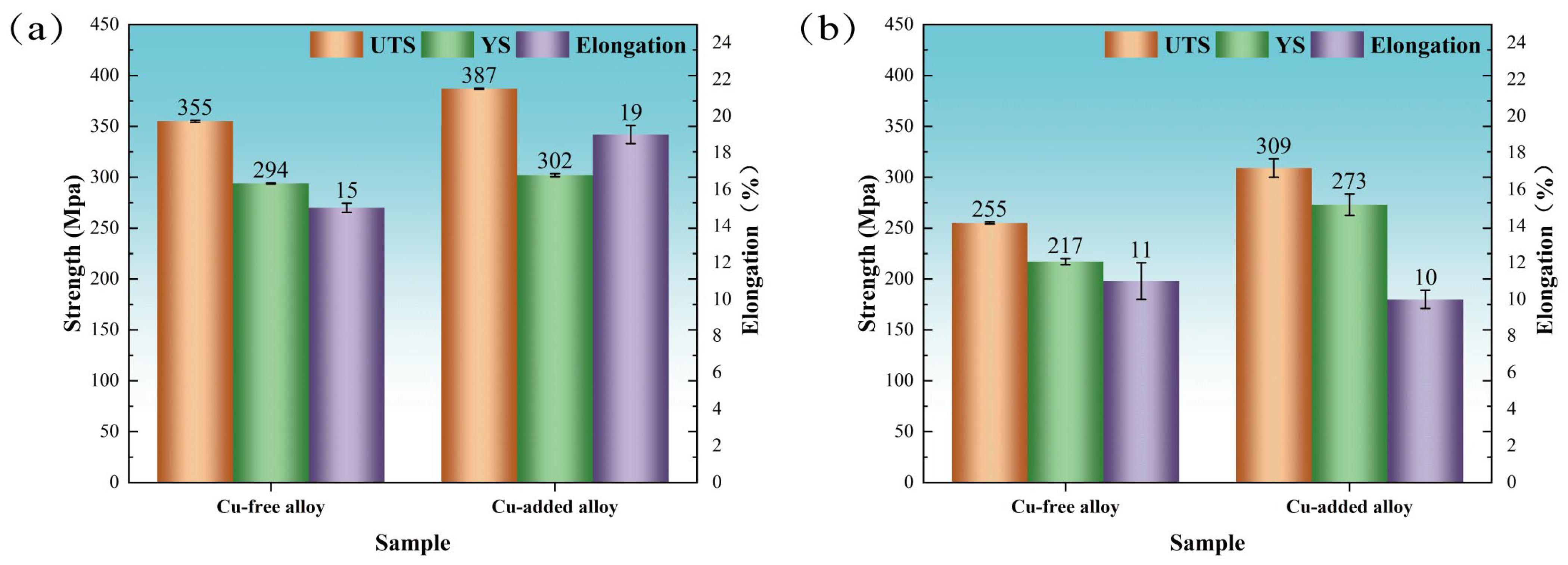
Figure 2a presents the room-temperature tensile mechanical properties of both alloys after peak aging at 155 °C for 10 h. The tensile strength of the Cu-added alloy (387 MPa) was approximately 9% higher than that of the Cu-free alloy (355 MPa), while the elongation at failure reached 19%, significantly exceeding the 15% recorded for the Cu-free counterpart. In addition, the Cu-added alloy exhibits a slightly higher yield strength (302 MPa) compared to the Cu-free alloy (294 MPa).
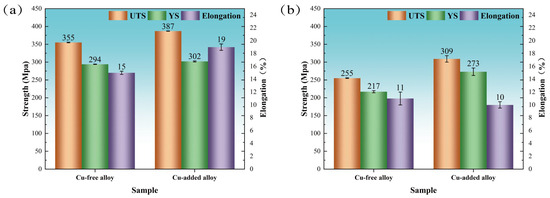
Figure 2.
Room-temperature tensile mechanical properties of Cu-free and Cu-containing alloys following various aging treatments. (a) Peak-aged condition (155 °C for 10 h); (b) heavily over-aged condition (185 °C for 24 h).
Figure 2b presents the room-temperature tensile mechanical properties of both alloys after being heavily over-aged at 185 °C for 24 h. The tensile strength (309 MPa) and yield strength (273 MPa) of the Cu-added alloy exhibited increases of 21.2% and 25.8%, compared to the Cu-free alloy. The elongation values of the two alloys are comparable. Under heavily over-aged conditions, the tensile strength and yield strength of Cu-free alloys decreased by 28.2% and 26.2%, respectively, relative to the peak-aged condition achieved at 155 °C for 10 h. The tensile strength and yield strength of the Cu-added alloy under heavily over-aged condition decreased by 20.2% and 9.6%, respectively, relative to the peak-aged condition achieved at 155 °C for 10 h. It is expected that increasing the aging temperature and prolonging the aging time will result in a reduction in mechanical performance. However, both the room-temperature tensile properties and aging hardness indicate that the Cu-added alloy exhibits a slower degradation in strength during over-aging and maintains higher strength compared to the Cu-free alloy. This behavior is closely associated with the precipitate state of the strengthening phase throughout the aging process.
3.3. TEM Bright-Field Images Aged at 155 °C
Based on the hardness data results presented in Figure 1, the samples aged at 155 °C for 2 h, 10 h and 24 h were selected to represent under-aged, peak-aged, and over-aged conditions, respectively. TEM bright-field imaging was conducted on both Cu-free and Cu-added alloys to investigate the influence of copper addition on the evolution of precipitates.
3.3.1. TEM Bright-Field Images of Under-Aged Alloys (Aged at 155 °C for 2 h)
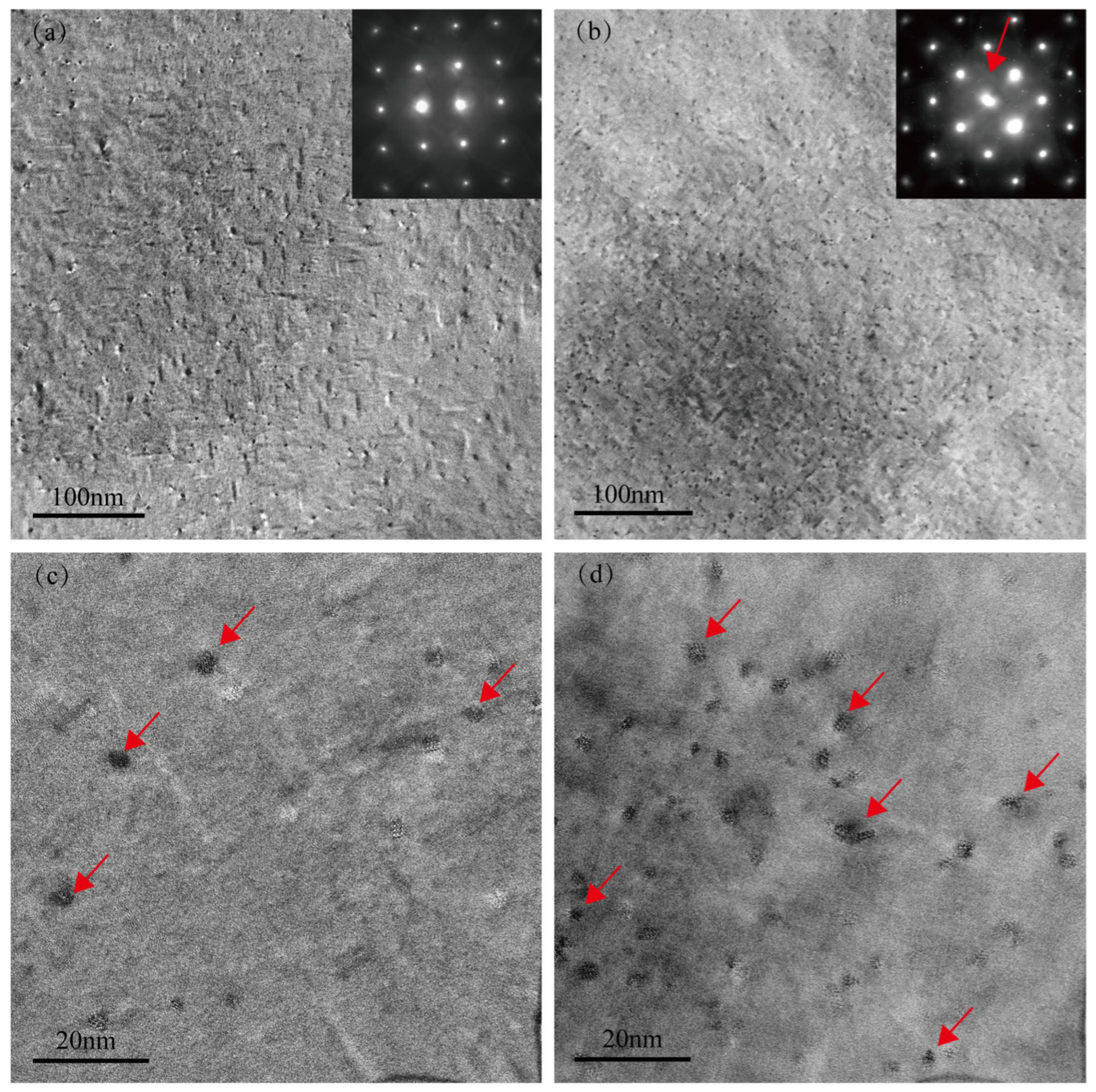
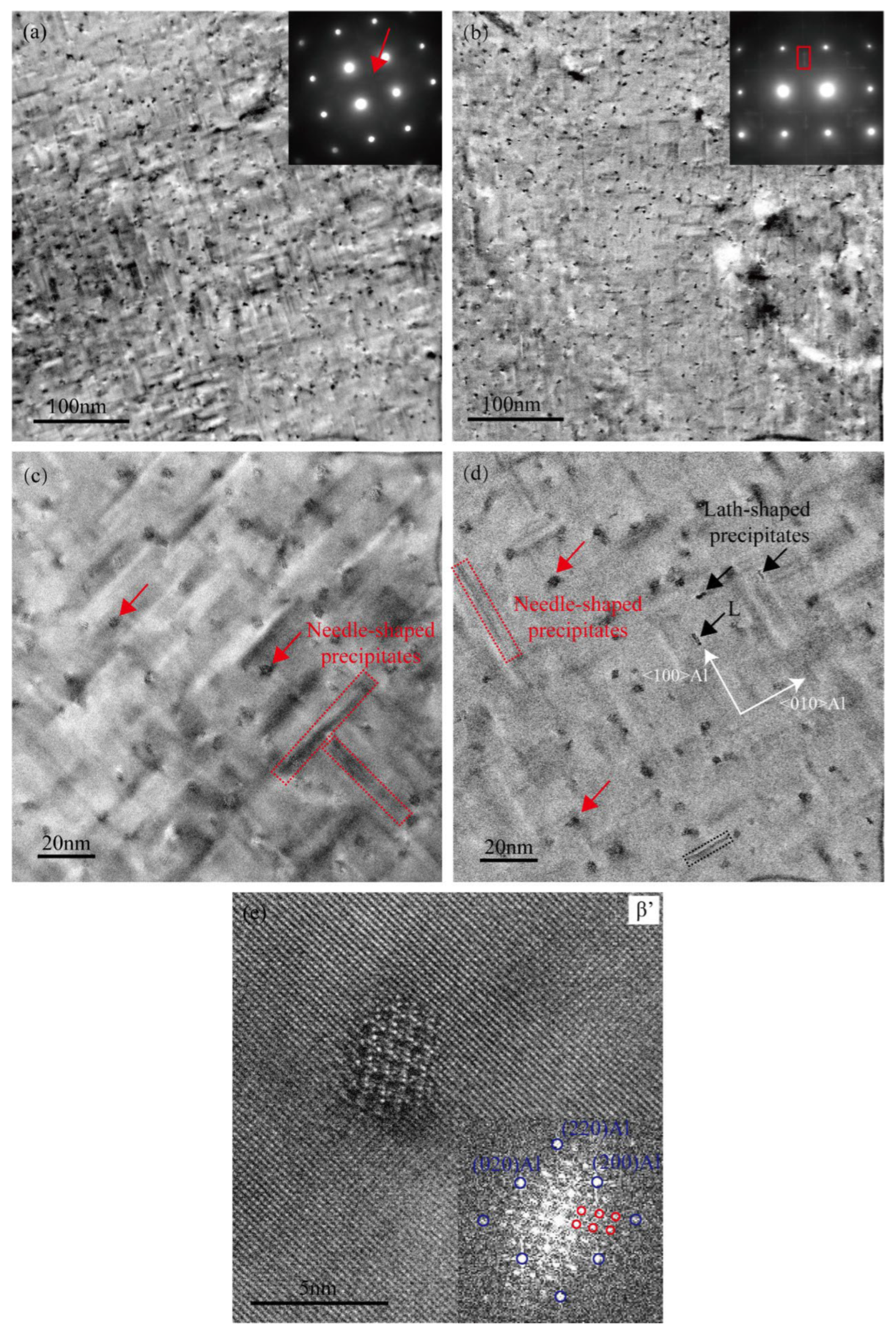
Figure 3a,b present the low-magnification TEM bright-field images and selected-area electron diffraction (SAED) patterns of the Cu-free and Cu-added alloys, respectively, after aging at 155 °C for 2 h. Very fine spherical precipitates exist in the Cu-free alloy matrix, as indicated by the red arrow in Figure 3c. However, apart from the diffraction spots corresponding to the aluminum matrix (inset in Figure 3a), no distinct additional diffraction signals are observed in the SAED pattern, suggesting that these precipitates are likely GP zones with no well-defined crystal structure [25,26]. In contrast, the Cu-added alloy exhibits a significantly higher number of spherical precipitates (as shown by the red arrow in Figure 3d), along with distinct diffraction streaks in the SAED pattern (inset in Figure 3b) [27,28]. This feature indicates that the precipitates in the Cu-added alloy possess a certain crystallinity and are structurally closer to the peak-aged β″ phase. This observation is consistent with the higher aging hardness exhibited by the Cu-added alloy in the under-aged condition, as shown in Figure 1. Previous studies have demonstrated that Cu can effectively enhance the age-hardening response of Al–Mg–Si alloy [5]. Furthermore, Weng et al. [15] utilized three-dimensional atom probe analysis (3DAP) to investigate the early-stage aging behavior in Al–Mg–Si–Cu alloys, demonstrating that Cu atoms participate in cluster formation and facilitate the transformation of clusters into the GP zones and β″ phases.
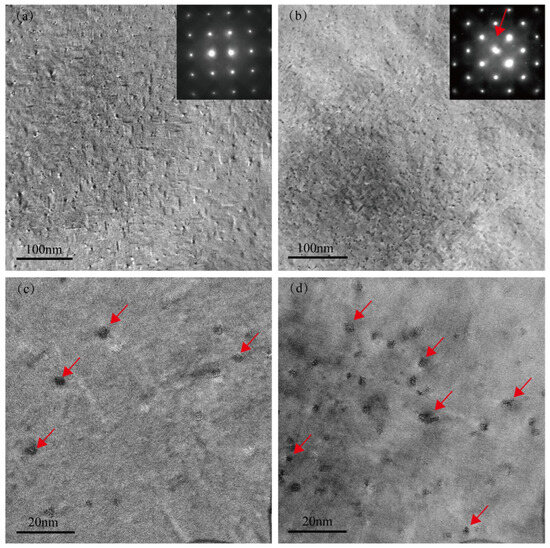
Figure 3.
TEM images of two alloys in the under-aged condition (155 °C for 2 h). (a,c) Cu-free alloy; (b,d) Cu-added alloy.
3.3.2. TEM Bright-Field Images of Peak-Aged Alloys (Aged at 155 °C for 10 h)
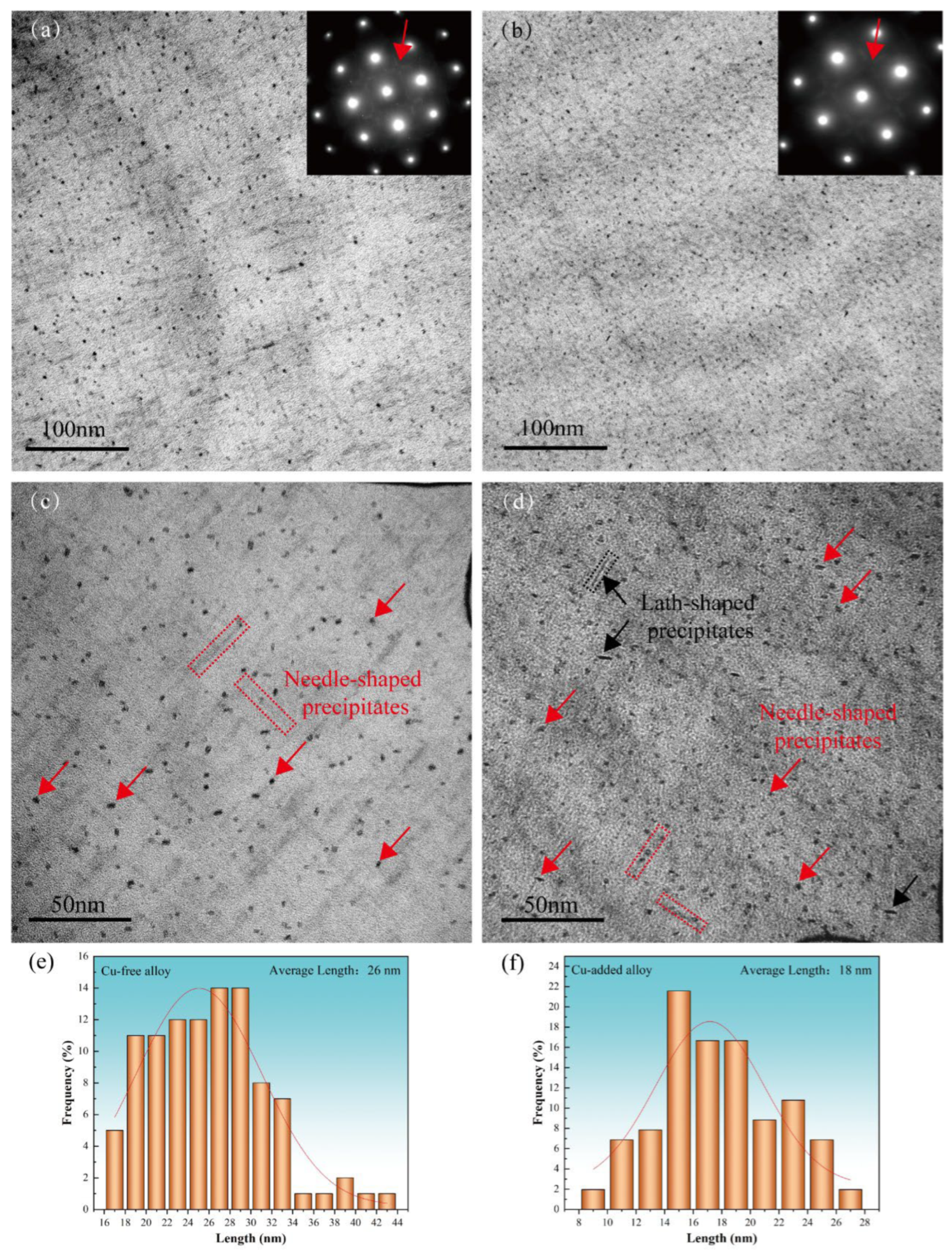
Figure 4a,b, respectively, present the low-magnification TEM bright-field images and SAED patterns of the Cu-free and Cu-added alloys in the peak-aged (aged at 155 °C for 10 h), respectively. Both alloys exhibit SAED patterns characteristic of the β″ phase, which appear as “cross shaped” streaks along the <100>Al direction [27,28]. The number density of precipitates in the peak-aged state is significantly higher than that observed in the under-aged stage. The β“ phase is short needle-shaped and precipitates along the <100>Al direction [28,29]. Therefore, under the electron beam B = [001] Al, two shapes can be observed: radial circular (as indicated by the red arrows in Figure 4c,d) and axial needle-shaped (as indicated by the red dotted rectangles in Figure 4c,d). The β″ phase adopts a monoclinic crystal structure, and under the [001] Al zone axis, a total of 12 different orientations of the β″ phase can be observed [28]. It is widely recognized as the most significant strengthening phase in peak-aged Al–Mg–Si–(Cu) alloys [5,19]. Additionally, a small number of lath-shaped precipitates, approximately 13 nm in length, were observed in the Cu-added alloy (as indicated by the black arrow and dotted rectangle in Figure 4d). These precipitates are likely associated with Cu-containing phases such as Q’ or L, and there is evidence suggesting their contribution to the overall hardening effect of the alloy [13,14].
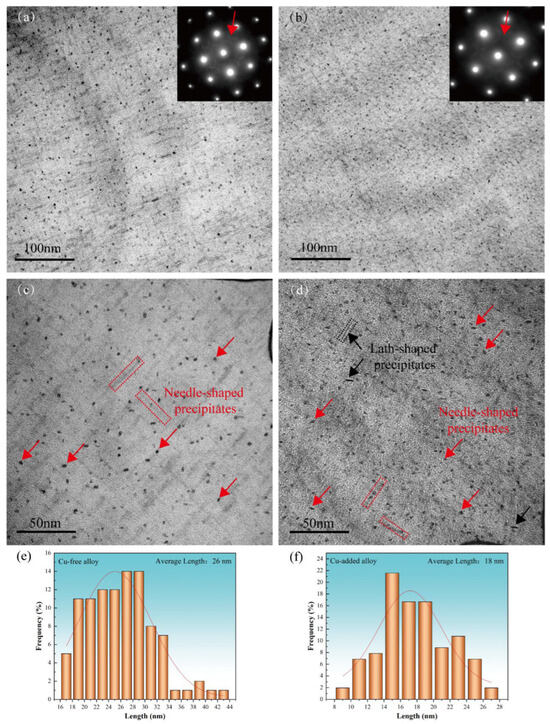
Figure 4.
TEM bright-field images (a–d) and the corresponding precipitate length distributions for two alloys (e–f) in the peak-aged condition (155 °C for 10 h). (a,c,e) Cu-free alloy; (b,d,f) Cu-added alloy.
The average axial lengths of the β″ precipitates in the peak-aged Cu-free and Cu-added alloys are 26 nm and 18 nm, respectively (as shown in Figure 4e,f), with radial average dimensions differing by approximately 2–3 nm. The β″ phase in the Cu-added alloy exhibits a more uniform and concentrated size distribution. The number of precipitates in a certain area of the TEM image was counted, and the planar number density is calculated by dividing the total number of precipitates by the area of the image [30]. The planar quantity densities of the β″ phase of the Cu-free alloy and the Cu-added alloy are estimated to be roughly 2.5 × 1016 m−2 and 4.3 × 1016 m−2, respectively. Therefore, the addition of Cu promotes the formation of the β″ phase, resulting in smaller precipitate sizes and a higher planar number density, which in turn enhances the peak-aged hardness and strength of the alloy. Previous studies [15,31] using 3DAP analysis and high-angle annular dark field (HAADF) imaging have revealed that the β″ precipitates in peak-aged Al–Mg–Si–Cu alloys are enriched with Mg, Si, and Cu. In these precipitates, Cu partially substitute for Mg and Si atoms, thereby enhancing the precipitate of the β″ phase.
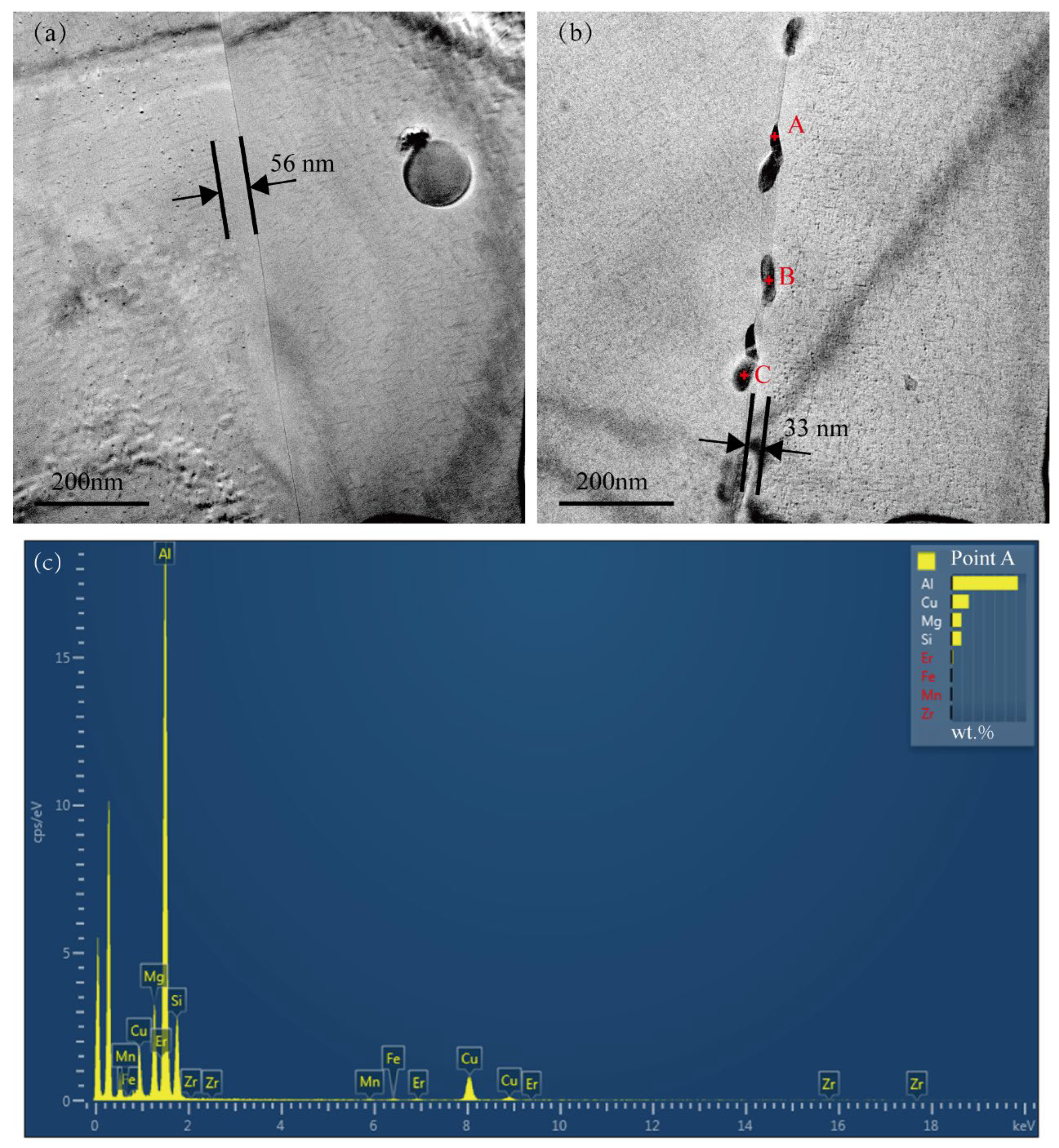
Figure 5a,b present typical TEM bright-field images of the grain boundary regions for the Cu-free alloy and the Cu-added alloy, respectively. Both alloys exhibit PFZs. The single-sided grain boundary PFZ width of the Cu-added alloy is approximately 33 nm, which is significantly narrower than that in the Cu-free alloy (56 nm). PFZs are commonly regarded as microstructural “weak zones” due to their lower hardness relative to the surrounding matrix, potentially contributing to a reduction in the alloy’s yield strength [32,33,34]. No precipitates were observed at the grain boundaries of the Cu-free alloy, as shown in Figure 5a. The Cu-added alloy displayed numerous precipitates along the grain boundaries, with an average length of approximately 80 nm and a width of 25 nm, as illustrated in Figure 5b. The EDX spectrum obtained from point A located along the grain boundary of the Cu-added alloy is displayed in Figure 5c. The EDX compositional analysis of precipitates at points A, B, and C is shown in Table 2. These grain boundary precipitates are predominantly composed of Al, Mg, Si, and Cu, and can be identified as the Q-phase [35,36]. In corrosive environments, the Cu-enriched Q-phase at the grain boundaries acts as cathodes, whereas the precipitate-free zones (PFZs) function as anodic regions and dissolution [26,37]. Consequently, the formation of Cu-enriched precipitates at grain boundaries tends to increase the alloy’s susceptibility to corrosion. Although no such precipitates were observed at the grain boundaries of the Cu-free alloy, previous studies [38] reported that only the Mg2Si phase is present in low-Cu alloys, and this phase does not promote intergranular corrosion (IGC).
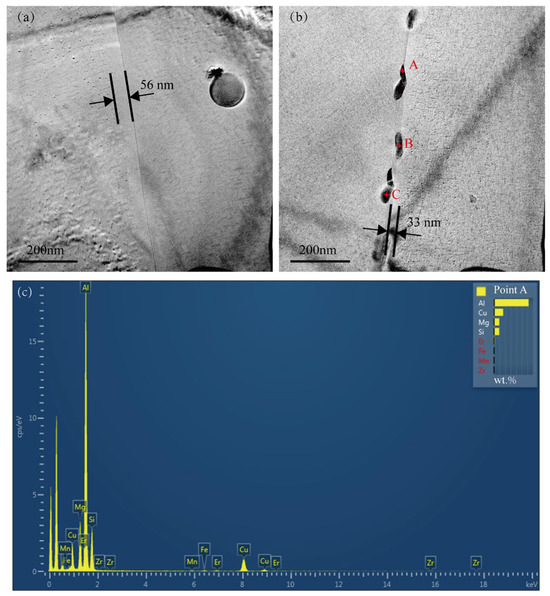
Figure 5.
TEM bright-field images and EDX compositional analysis of precipitates in the grain boundary regions of two alloys. (a) Cu-free alloy, (b) Cu-added alloy, and (c) EDX spectrum acquired from point A in (b).

Table 2.
EDX composition (wt.%) of grain boundary precipitates observed in the Cu-added alloy, as illustrated in Figure 5b.
3.3.3. TEM Bright-Field Images of Over-Aged Alloys (Aged at 155 °C for 24 h)
Figure 6a,c present TEM bright-field images of the Cu-free alloy in the over-aged condition (aged at 155 °C for 24 h). Compared to the peak-aged condition, a significant reduction in precipitate number density is observed, accompanied by noticeable coarsening of the axial dimensions of most needle-shaped precipitates, which have grown to approximately 30–40 nm, as indicated within the red dotted rectangles in Figure 6c. The presence of weak “cross-shaped” diffraction streaks in the SAED pattern suggests that a fraction of β″ phases remain retained within the alloy matrix. Figure 6e presents an HRTEM image of a representative precipitate in the Cu-free alloy. Fast Fourier transform (FFT) analysis verifies the presence of a hexagonal β′ phase [23,39], consistent with the microstructural evolution characteristic of the over-aged stage.
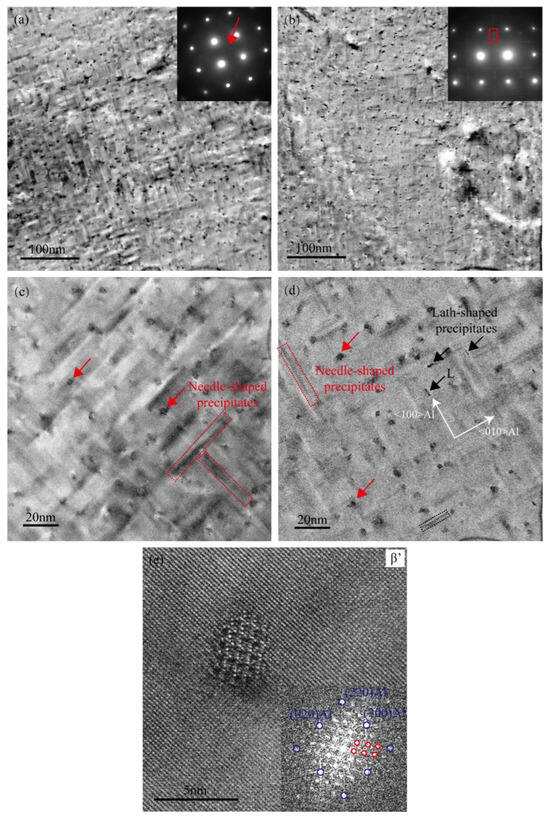
Figure 6.
TEM images of two alloys in the over-aged condition (155 °C for 24 h). (a,c,e) Cu-free alloy; (b,d) Cu-added alloy.
Under over-aged conditions (155 °C for 24 h), the Cu-added alloy exhibited a significantly higher density of lath-shaped precipitates compared to the peak-aged state. Two intensity maxima along the streak in the selected-area electron diffraction (SAED) pattern (indicated by the red rectangle in the inset of Figure 6b) closely match the simulated diffraction features corresponding to the (021)β′ and (211)Q’. This alignment may indicate concurrent precipitation of the β′ and Q’ phases [40]. The lath-shaped precipitate marked by the black arrow in Figure 6b, with its cross-section elongation along the <100>Al direction, was identified as the L phase [14,41]. Planar quantity densities of precipitates in the Cu-free and Cu-added alloys are estimated to be roughly 1.8 × 1016 m−2 and 2.2 × 1016 m−2, respectively. Additionally, the needle-shaped precipitates in the Cu-added alloy were relatively finer. Thus, the addition of Cu effectively retards the coarsening of precipitates during over-aging, preserving the alloy’s strength through the formation of lath-shaped precipitates and reducing the extent of softening associated with prolonged aging.
3.4. TEM Images of Heavily Over-Aged Alloys (Aged at 185 °C for 24 h)
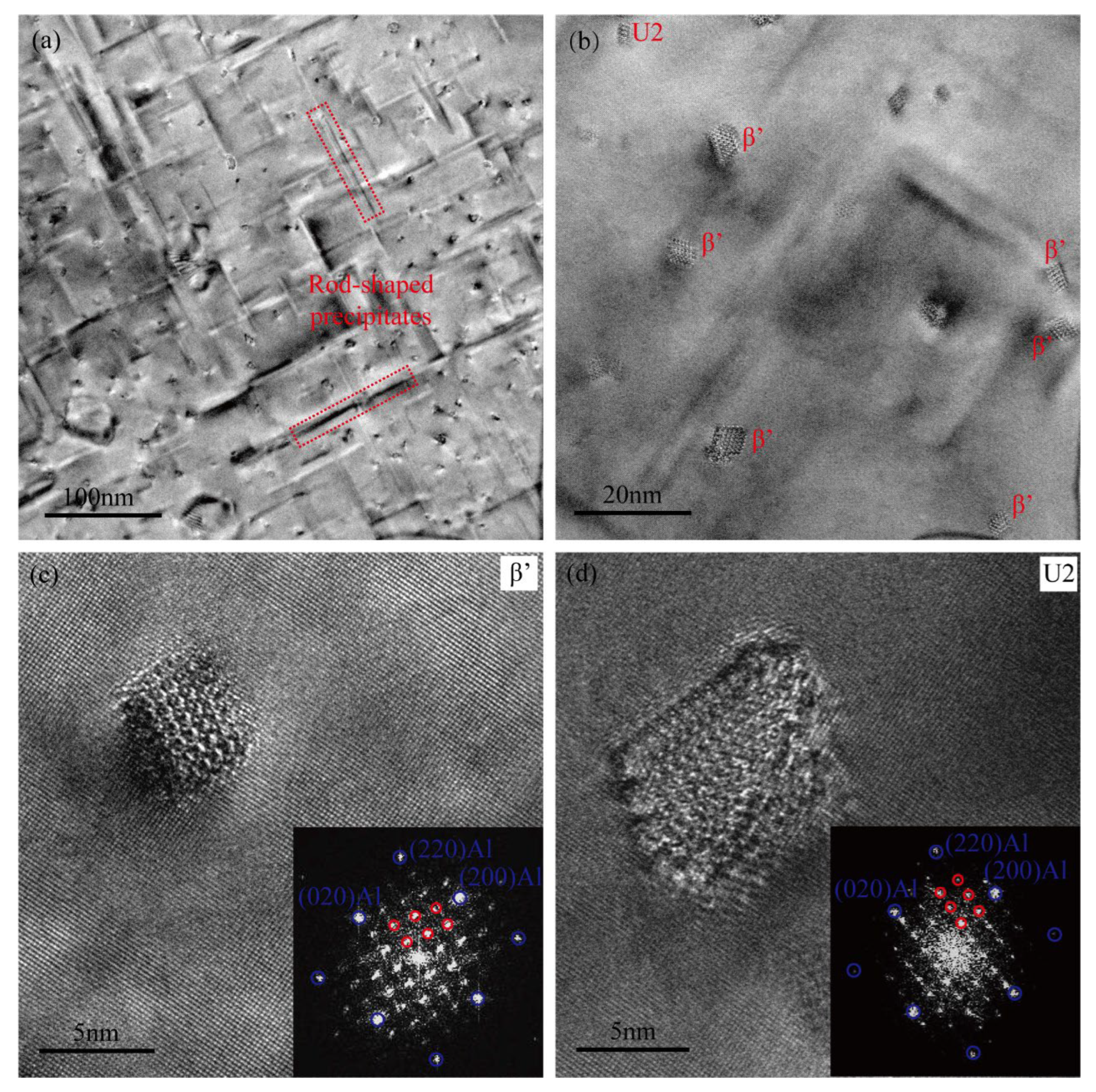
Figure 7a,b present the TEM micrographs of the heavily over-aged Cu-free alloy, aged at 185 °C for 24 h. Within the alloy matrix, the primary precipitates are coarse rod-shaped structures, with an average length of approximately 100 nm and a radial diameter of about 5 nm. The HRTEM images and associated FFT patterns of representative precipitates are presented in Figure 7c,d. These rod-shaped precipitates are predominantly hexagonal β′ phases, with only a small fraction exhibiting the orthorhombic U2 structure [9,10,42]. The spatial distribution of the two distinct types of precipitates is depicted in Figure 7b. The presence of the U2 phase is commonly observed during the over-aged stage condition in high-Si-content Al–Mg–Si alloys. The Cu-free alloy with a Mg/Si weight ratio of approximately 1.65 represents a typical Si-rich alloy system, and the observation of the U2 phase during the over-aged stage is in good agreement with findings reported in previous studies [9].
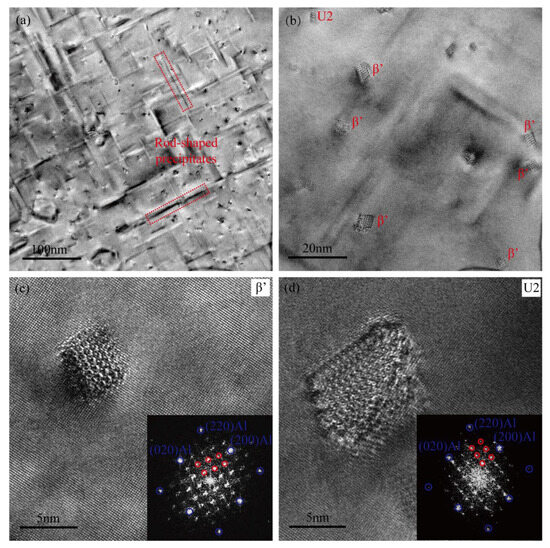
Figure 7.
TEM images of the Cu-free alloy following a heavily over-aged treatment at 185 °C for 24 h. (a,b) TEM bright-field images, (c,d) HRTEM images and the corresponding FFT patterns.
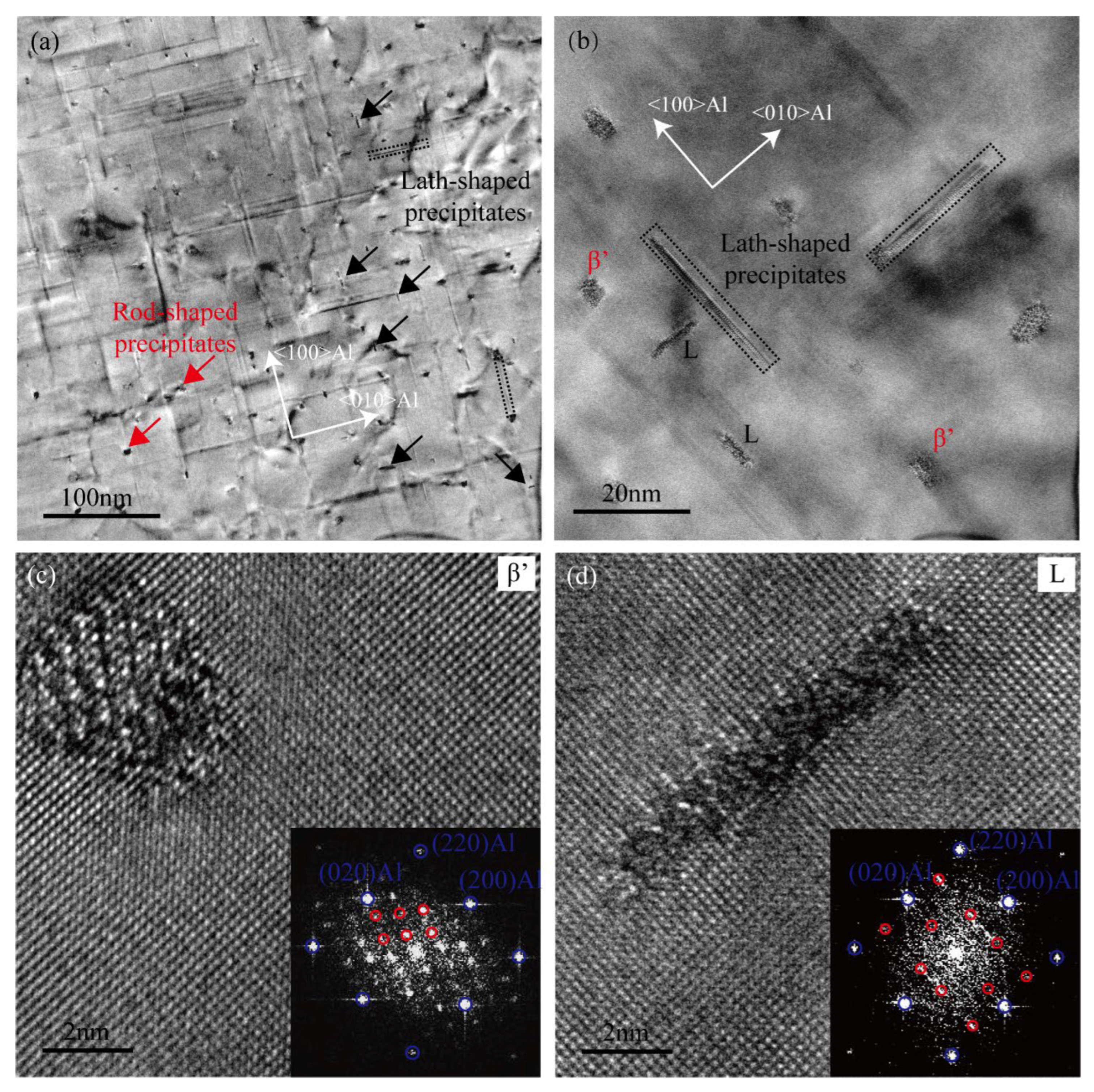
The TEM micrographs of the heavily over-aged Cu-added alloy are presented in Figure 8. A significant quantity of rod-like and lath-shaped precipitates can be observed in the microstructure. As shown in the HRTEM image (Figure 8c), the rod-shaped phase corresponds primarily to the β′ phase. Most of the lath-shaped precipitates are identified as L phases, which precipitate preferentially along the <100>Al direction [14,20,41]. Research indicates that the L phase is disordered and potentially belongs to the hexagonal crystal structure [14]. The red circles observed in the FFT pattern of Figure 8d indicate the presence of hexagonal sub-cells within the lath-shaped precipitate [20]. Lath-shaped Q’ phase, which typically form along the <510>Al direction, are rarely observed in the heavily over-aged Cu-added alloy. This scarcity may be attributed to the localized distribution of Mg and Cu. According to the literature, the Mg content in the L phase is lower than that in the Q’ phase [14]. In this study, the Cu-added alloy exhibits a higher Si content, which may facilitate the formation of the L phase. The extensive formation of stable lath-shaped L phase contributes to mitigating mechanical property degradation in the Cu-added alloy under the heavily over-aged condition. Therefore, the L phase plays a more significant role than the β′ phase in enhancing the mechanical strength of the alloy.
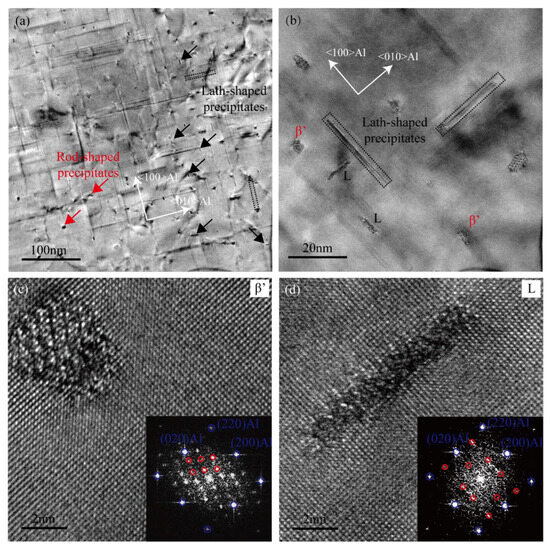
Figure 8.
TEM images of the Cu-added alloy in heavily over-aged condition (185 °C for 24 h). (a,b) TEM bright-field images, (c,d) HRTEM images and the corresponding FFT patterns.
4. Conclusions
The influence of Cu on the isothermal aged-hardening behavior of Si-rich Al–Mg–Si alloys and the associated precipitate evolution were systematically investigated. The main findings are summarized as follows:
- The Cu-added alloy exhibits higher hardness than the Cu-free alloy across various aging temperatures and demonstrates a more rapid age-hardening response during the under-aged stage. The time required for both alloys to reach peak hardness decreases with increasing aging temperature, with the most significant age-hardening effect observed at 155 °C.
- The relatively rapid age-hardening response observed in the under-aged condition of Cu-added alloys can be attributed to the accelerated clustering kinetics induced by Cu during the early stages of aging, which increases the number density of precipitate phases.
- Cu promotes the formation of the peak-aged β″ phase, refines the precipitate size, and induces the co-precipitation of a minor quantity of lath-shaped phases. It also promotes the precipitation of Cu-containing Q-phase at the grain boundary in peak-aged alloys and reduces the width of PFZs. Cu-added alloys exhibit superior mechanical properties in the peak-aged state, with a tensile strength of approximately 387 MPa and an elongation of ~19%, compared to 355 MPa and 15% for Cu-free alloy.
- Cu significantly reduces the softening rate of the alloy during the over-aging process. In the Cu-free alloy, the heavily over-aged microstructure is predominantly composed of rod-shaped β′ phases, with a minor presence of U2 phases. The Cu-added alloy exhibited a substantial amount of lath-shaped precipitates (predominantly the L phase) during the over-aging process. These lath-shaped precipitates exhibited significantly higher thermal stability compared to the β′ phase, thereby improving the mechanical properties of the alloy under heavily over-aged conditions.
Author Contributions
Conceptualization, Y.Z., W.W., H.H., Z.N. and S.W.; methodology, Y.Z., H.H. and S.W.; formal analysis, Y.Z., Y.L. and Z.R.; investigation, Y.L., Z.R.; resources, W.W. and H.H.; writing—original, Y.Z.; writing—review and editing, W.W., H.H. and S.W.; project administration, H.H., W.W. and Z.N.; funding acquisition, H.H., W.W. and Z.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science and Technology Major Project (2025ZD0611903).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zang, R.; Ding, L.; Ehlers, F.J.H.; Jia, Z.; Xu, S.; Li, Y.; Cao, L. The Influence of Cu Content and Mg/Si Ratio on the Strength and Formability in Al-Mg-Si-Cu Alloys. Mater. Charact. 2023, 205, 113355. [Google Scholar] [CrossRef]
- Du, J.; Guo, M.; Zhi, J.; Chen, X.; Zhuang, L.; Kestens, L.A.I. Coordinated Deformation Mechanisms of High Formability Al–Mg–Si–Cu–Zn–Fe Alloys via Coupling Control of Thermomechanical Processes. Mater. Chem. Phys. 2024, 320, 129471. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, W.; Zhao, Y.; Shi, W.; Zhou, X.; Rong, L.; Wen, S.; Wu, X.; Gao, K.; Huang, H.; et al. Effect of the Solid Solution and Aging Treatment on the Mechanical Properties and Microstructure of a Novel Al-Mg-Si Alloy. Materials 2023, 16, 7036. [Google Scholar] [CrossRef]
- Zhu, L.; Li, K.; Yang, X.; He, J.; Fang, J.; Zhang, Z.; Guo, M.; Zhang, J. Tailoring the Formability and Planar Anisotropy of Al-Mg-Si-Cu-Zn Alloys via Cross Hot Rolling and Two-Stage Cold Rolling. J. Alloys Compd. 2024, 985, 174089. [Google Scholar] [CrossRef]
- Hong, H.-B.; Hajra, R.N.; Shin, E.; Kim, J.-K.; Lee, J.-S.; Kim, J.H.; Kim, J.; Cho, H.-H. Effect of Heat Treatment and Cu Addition on the Mechanical, Electrochemical, and Precipitation Behavior of Al–Mg–Si Alloys. J. Mater. Res. Technol. 2025, 36, 1822–1834. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The Precipitation Sequence in Al–Mg–Si Alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Murayama, M.; Hono, K. Pre-Precipitate Clusters and Precipitation Processes in Al–Mg–Si Alloys. Acta Mater. 1999, 47, 1537–1548. [Google Scholar] [CrossRef]
- Frøseth, A.G.; Høier, R.; Derlet, P.M.; Andersen, S.J.; Marioara, C.D. Bonding in MgSi and Al-Mg-Si Compounds Relevant to Al-Mg-Si Alloys. Phys. Rev. B 2003, 67, 224106. [Google Scholar] [CrossRef]
- Andersen, S.J.; Marioara, C.D.; Frøseth, A.; Vissers, R.; Zandbergen, H.W. Crystal Structure of the Orthorhombic U2-Al4Mg4Si4 Precipitate in the Al–Mg–Si Alloy System and Its Relation to the Β′ and Β″ Phases. Mater. Sci. Eng. A 2005, 390, 127–138. [Google Scholar] [CrossRef]
- Andersen, S.J.; Marioara, C.D.; Vissers, R.; Frøseth, A.; Zandbergen, H.W. The Structural Relation between Precipitates in Al–Mg–Si Alloys, the Al-Matrix and Diamond Silicon, with Emphasis on the Trigonal Phase U1-MgAl2Si2. Mater. Sci. Eng. A 2007, 444, 157–169. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.; Wang, X.; Ma, P.-K.; Guan, Z.-P. Effect of Cu and Sn Additions on the Thermal Stability of Al–Mg–Si Alloy. Mater. Sci. Eng. A 2024, 894, 146158. [Google Scholar] [CrossRef]
- Saito, T.; Mørtsell, E.A.; Wenner, S.; Marioara, C.D.; Andersen, S.J.; Friis, J.; Matsuda, K.; Holmestad, R. Atomic Structures of Precipitates in Al–Mg–Si Alloys with Small Additions of Other Elements. Adv. Eng. Mater. 2018, 20, 1800125. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Stene, T.N.; Hasting, H.; Walmsley, J.; Van Helvoort, A.T.J.; Holmestad, R. The Effect of Cu on Precipitation in Al–Mg–Si Alloys. Philos. Mag. 2007, 87, 3385–3413. [Google Scholar] [CrossRef]
- Torsæter, M.; Lefebvre, W.; Marioara, C.D.; Andersen, S.J.; Walmsley, J.C.; Holmestad, R. Study of Intergrown L and Q′ Precipitates in Al–Mg–Si–Cu Alloys. Scr. Mater. 2011, 64, 817–820. [Google Scholar] [CrossRef]
- Weng, Y.; Ding, L.; Jia, Z.; Liu, Q. Effect of Combined Addition of Ag and Cu on the Precipitation Behavior for an Al-Mg-Si Alloy. Mater. Charact. 2021, 171, 110736. [Google Scholar] [CrossRef]
- Kawahara, Y.; Marioara, C.D.; Hell, C.M.; Thrane, E.S.; Mørtsell, E.A.; Røyset, J.; Holmestad, R.; Kaneko, K. Effect of Cu Addition on Atomic Clusters and Its Influence on the Dispersion of Precipitates in Si-Rich Al-Mg-Si Alloys. Mater. Sci. Eng. A 2025, 946, 149121. [Google Scholar] [CrossRef]
- Liu, T.; Scholtz, J.; Xue, F.; Yung, A.; Guiglionda, G.; Marquis, E.A. Effects of Cu and Pre-Aging on the Clustering Behavior in Al-Mg-Si Alloys. Materialia 2023, 32, 101917. [Google Scholar] [CrossRef]
- Zandbergen, H.W.; Andersen, S.J.; Jansen, J. Structure Determination of Mg5 Si6 Particles in Al by Dynamic Electron Diffraction Studies. Science 1997, 277, 1221–1225. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Y.X.; Chen, Z.Q.; Chen, S.L.; Gao, P.; Chen, J.H. Formation of β"-Related Composite Precipitates in Relation to Enhanced Thermal Stability of Sc-Alloyed Al-Mg-Si Alloys. J. Alloys Compd. 2021, 885, 160942. [Google Scholar] [CrossRef]
- Buchanan, K.; Colas, K.; Ribis, J.; Lopez, A.; Garnier, J. Analysis of the Metastable Precipitates in Peak-Hardness Aged Al-Mg-Si(-Cu) Alloys with Differing Si Contents. Acta Mater. 2017, 132, 209–221. [Google Scholar] [CrossRef]
- Gupta, A.K.; Lloyd, D.J.; Court, S.A. Precipitation Hardening in Al–Mg–Si Alloys with and without Excess Si. Mater. Sci. Eng. A 2001, 316, 11–17. [Google Scholar] [CrossRef]
- Zhong, H.; Rometsch, P.A.; Estrin, Y. The Influence of Si and Mg Content on the Microstructure, Tensile Ductility, and Stretch Formability of 6xxx Alloys. Metall. Mater. Trans. A 2013, 44, 3970–3983. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, M.; Zhang, J. Effect of Silicon Content on Nucleation and Growth of Multiscale Precipitates in Al–Mg–Si–Cu–Zn Alloys for Different Aging Paths. Mater. Sci. Eng. A 2022, 841, 143016. [Google Scholar] [CrossRef]
- GB/T 228.1-2010; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. Standards Press of China: Beijing, China, 2010.
- Buha, J.; Lumley, R.N.; Crosky, A.G.; Hono, K. Secondary Precipitation in an Al–Mg–Si–Cu Alloy. Acta Mater. 2007, 55, 3015–3024. [Google Scholar] [CrossRef]
- Li, H.; Zhao, P.; Wang, Z.; Mao, Q.; Fang, B.; Song, R.; Zheng, Z. The Intergranular Corrosion Susceptibility of a Heavily Overaged Al-Mg-Si-Cu Alloy. Corros. Sci. 2016, 107, 113–122. [Google Scholar] [CrossRef]
- Jacobs, M.H. The Structure of the Metastable Precipitates Formed during Ageing of an Al-Mg-Si Alloy. Philos. Mag. 1972, 26, 1–13. [Google Scholar] [CrossRef]
- Yang, W.; Wang, M.; Zhang, R.; Zhang, Q.; Sheng, X. The Diffraction Patterns from Β″ Precipitates in 12 Orientations in Al–Mg–Si Alloy. Scr. Mater. 2010, 62, 705–708. [Google Scholar] [CrossRef]
- Andersen, S.J.; Zandbergen, H.W.; Jansen, J.; Tr, C.; Tundal, U.; Reiso, O. The Crystal Structure of the β″ Phase in Al-Mg-Si Alloys. Acta Mater. 1998, 46, 3283–3298. [Google Scholar] [CrossRef]
- Esmaeili, S.; Wang, X.; Lloyd, D.J.; Poole, W.J. On the Precipitation-Hardening Behavior of the Al-Mg-Si-Cu Alloy AA6111. Metall. Mater. Trans. A 2003, 34, 751–763. [Google Scholar]
- Li, K.; Béché, A.; Song, M.; Sha, G.; Lu, X.; Zhang, K.; Du, Y.; Ringer, S.P.; Schryvers, D. Atomistic Structure of Cu-Containing Β″ Precipitates in an Al–Mg–Si–Cu Alloy. Scr. Mater. 2014, 75, 86–89. [Google Scholar] [CrossRef]
- Frodal, B.H.; Christiansen, E.; Myhr, O.R.; Hopperstad, O.S. The Role of Quench Rate on the Plastic Flow and Fracture of Three Aluminium Alloys with Different Grain Structure and Texture. Int. J. Eng. Sci. 2020, 150, 103257. [Google Scholar] [CrossRef]
- Poole, W.J.; Wang, X.; Embury, J.D.; Lloyd, D.J. The Effect of Manganese on the Microstructure and Tensile Response of an Al-Mg-Si Alloy. Mater. Sci. Eng. A 2019, 755, 307–317. [Google Scholar] [CrossRef]
- Arani, M.M.; Ramesh, N.S.; Wang, X.; Parson, N.; Li, M.; Poole, W.J. The Localization of Plastic Deformation in the Precipitate Free Zone of an Al-Mg-Si-Mn Alloy. Acta Mater. 2022, 231, 117872. [Google Scholar] [CrossRef]
- Bartawi, E.H.; Shaban, G.; Ambat, R. Role of Aging Time and Cu/Zn Additions on the Microstructure and Intergranular Corrosion Resistance of 6082 Al-Mg-Si Alloy. Corros. Sci. 2025, 254, 113027. [Google Scholar] [CrossRef]
- Svenningsen, G.; Larsen, M.H.; Walmsley, J.C.; Nordlien, J.H.; Nisancioglu, K. Effect of Artificial Aging on Intergranular Corrosion of Extruded AlMgSi Alloy with Small Cu Content. Corros. Sci. 2006, 48, 1528–1543. [Google Scholar] [CrossRef]
- Bartawi, E.H.; Mishin, O.V.; Shaban, G.; Nordlien, J.H.; Ambat, R. Electron Microscopy Analysis of Grain Boundaries and Intergranular Corrosion in Aged Al-Mg-Si Alloy Doped with 0.05 Wt% Cu. Corros. Sci. 2022, 209, 110758. [Google Scholar] [CrossRef]
- Svenningsen, G.; Lein, J.E.; Bjørgum, A.; Nordlien, J.H.; Yu, Y.; Nisancioglu, K. Effect of Low Copper Content and Heat Treatment on Intergranular Corrosion of Model AlMgSi Alloys. Corros. Sci. 2006, 48, 226–242. [Google Scholar] [CrossRef]
- Vissers, R.; Van Huis, M.A.; Jansen, J.; Zandbergen, H.W.; Marioara, C.D.; Andersen, S.J. The Crystal Structure of the β′ Phase in Al–Mg–Si Alloys. Acta Mater. 2007, 55, 3815–3823. [Google Scholar] [CrossRef]
- Miao, W.F.; Laughlin, D.E. Effects of Cu Content and Preaging on Precipitation Characteristics in Aluminum Alloy 6022. Metall. Mater. Trans. A 2000, 31, 361–371. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Røyset, J.; Reiso, O.; Gulbrandsen-Dahl, S.; Nicolaisen, T.-E.; Opheim, I.-E.; Helgaker, J.F.; Holmestad, R. Improving Thermal Stability in Cu-Containing Al-Mg-Si Alloys by Precipitate Optimization. Metall. Mater. Trans. A 2014, 45, 2938–2949. [Google Scholar] [CrossRef]
- Matsuda, K.; Sakaguchi, Y.; Miyata, Y.; Uetani, Y.; Sato, T.; Kamio, A.; Ikeno, S. Precipitation Sequence of Various Kinds of Metastable Phases in Al-1.0mass% Mg2Si-0.4mass% Si Alloy. J. Mater. Sci. 2000, 35, 179–189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).