Microstructure and Mechanical Property Regulation of As-Cast AlCoCrFeNi2.1Six (x = 0, 0.1, 0.2, 0.3) High-Entropy Alloys
Abstract
1. Introduction
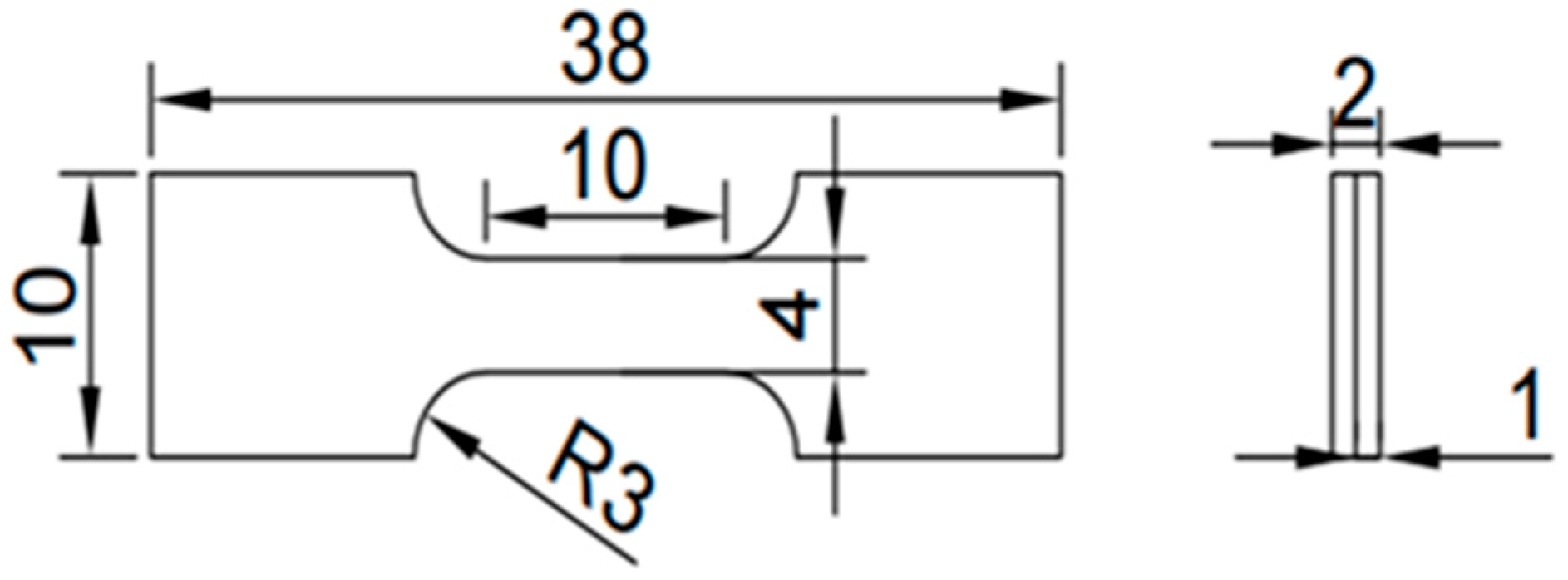
2. Materials and Methods
3. Results
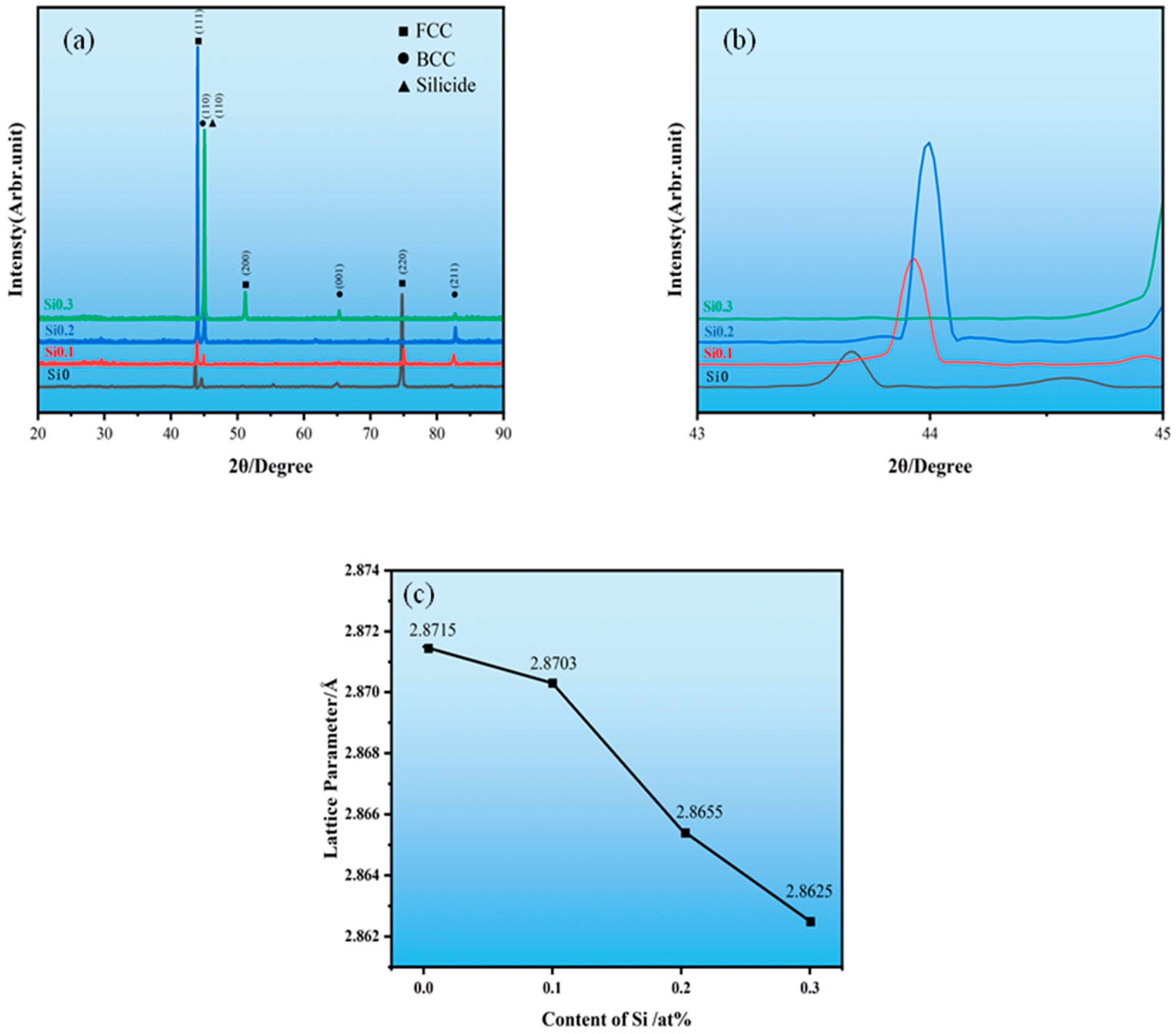
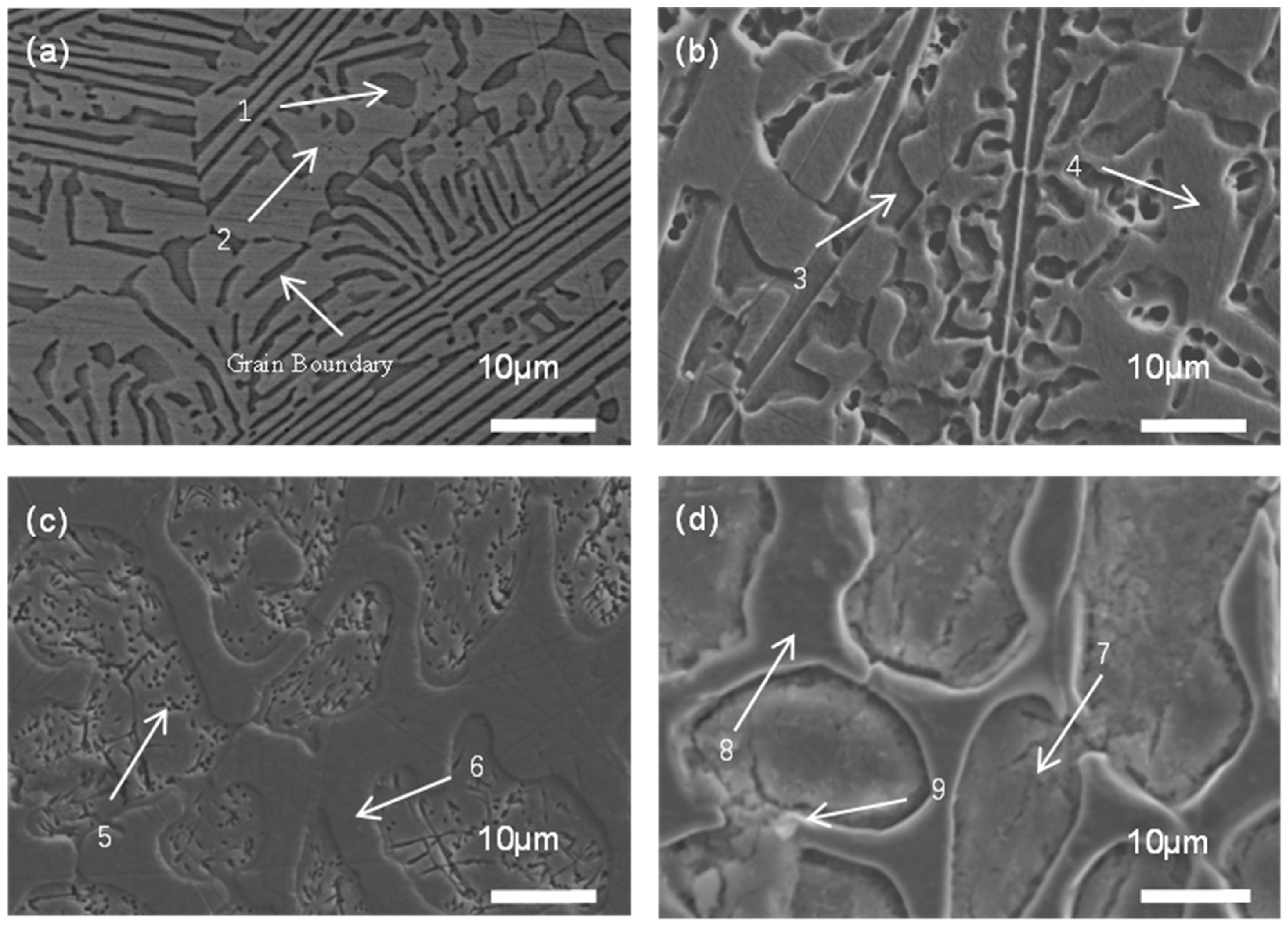
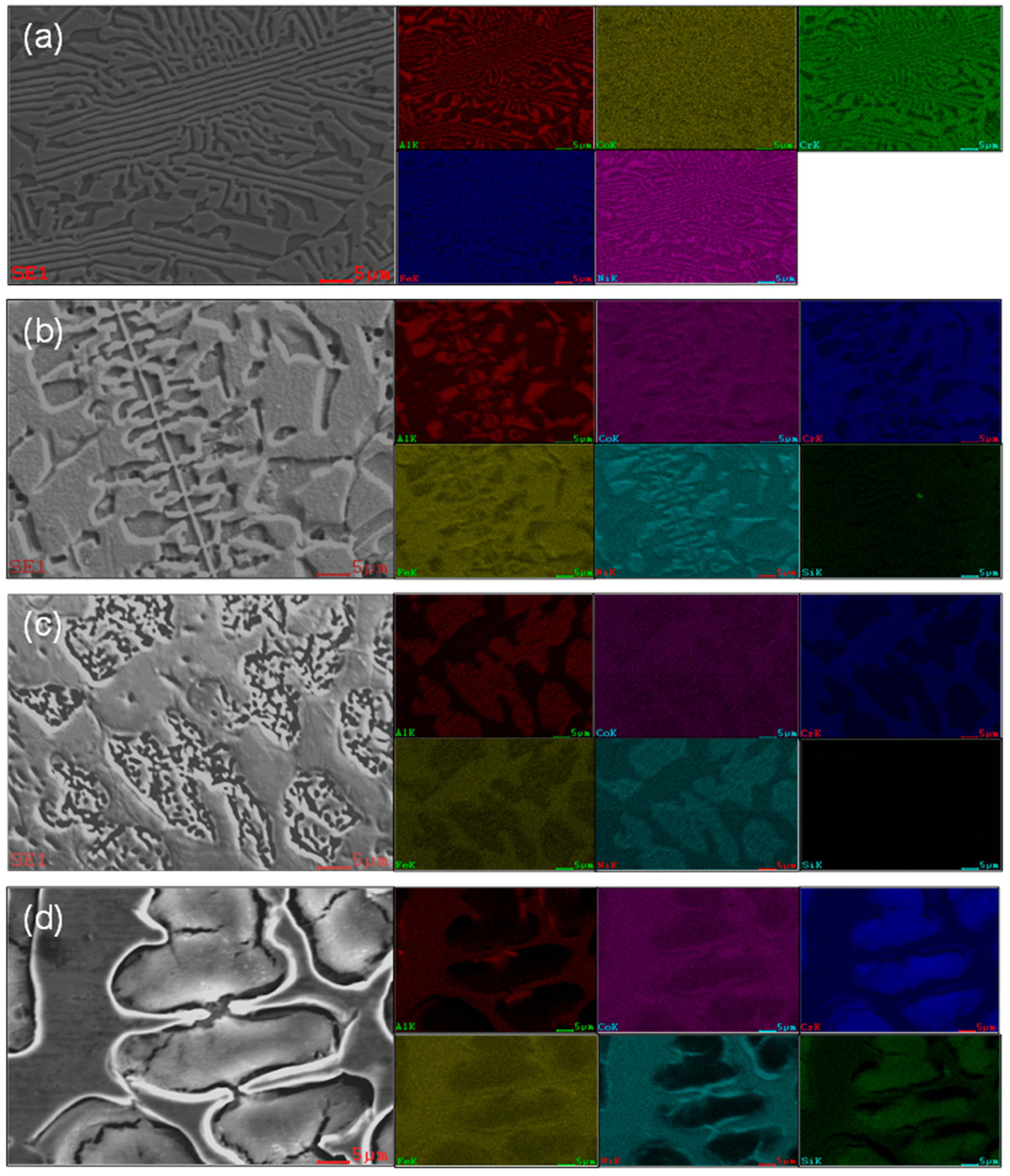
3.1. The Effect of Si Content on the Phase Structure and Microstructure of High-Entropy Alloys
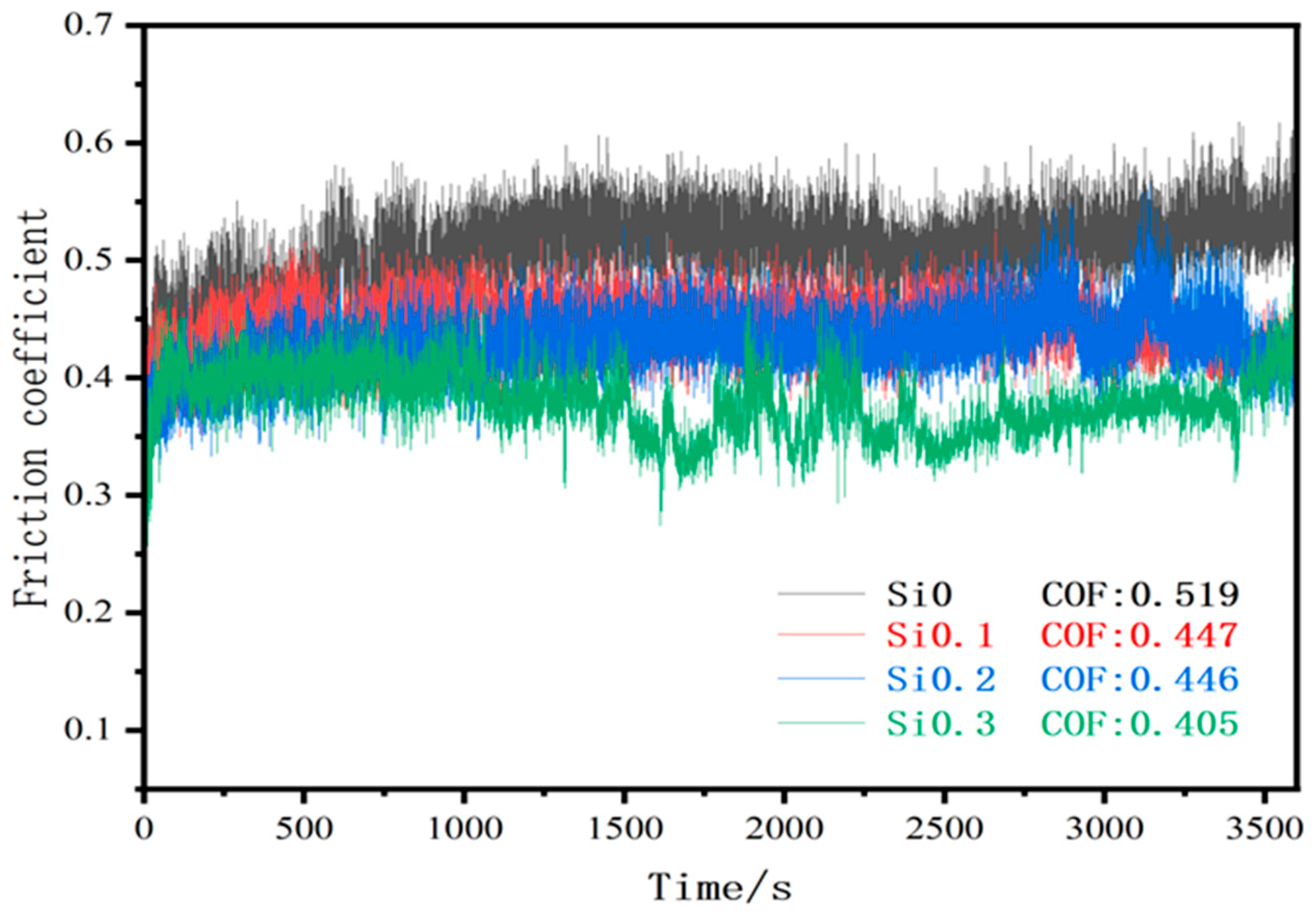
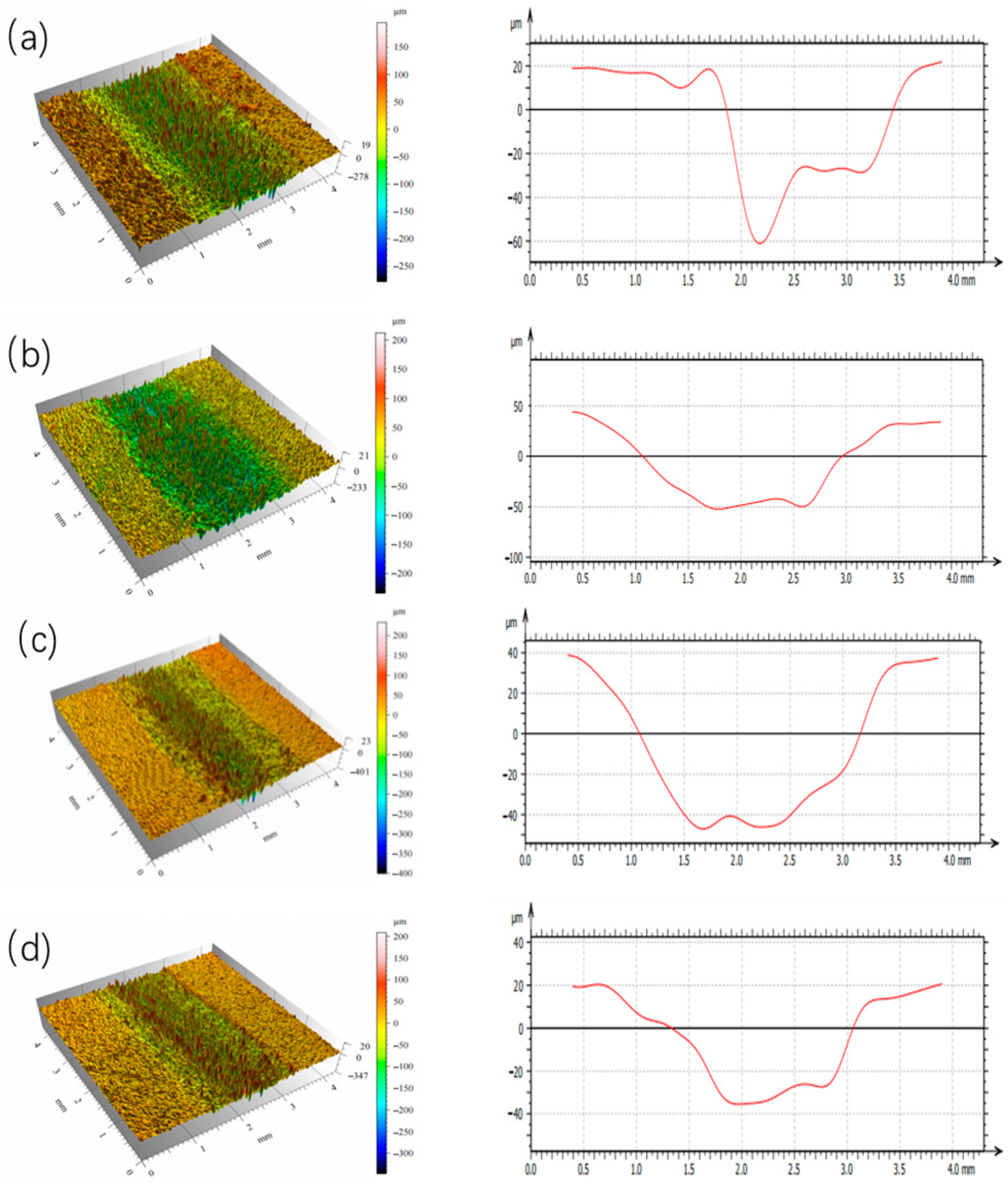
3.2. Influence of Si Content on the Characteristics of High-Entropy Alloys
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. 2004, 375–377, 213. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Luo, H.; Du, C.; Li, X. Recent advances on environmental corrosion behavior and mechanism of high-entropy alloys. J. Mater. Sci. Technol. 2021, 80, 217–233. [Google Scholar] [CrossRef]
- Li, W.; Liaw, P.K.; Gao, Y. Fracture resistance of high entropy alloys: A review. Intermetallics 2018, 99, 69–83. [Google Scholar] [CrossRef]
- Abid, T.; Akram, M.A.; Yaqub, T.B.; Karim, M.R.A.; Fernandes, F.; Zafar, M.F.; Yaqoob, K. Design and development of porous CoCrFeNiMn high entropy alloy (Cantor alloy) with outstanding electrochemical properties. J. Alloys Compd. 2024, 970, 172633. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.J.; Wang, T.M.; Wen, B.; Wang, Z.J.; Jie, J.C.; Cao, Z.Q.; et al. A promising new class of high-temperature alloys: Eutectic high-entropy alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Ma, P.; Yang, H.; Zhang, N.; Fang, Y.; Jia, Y.; Prashanth, K.G. Research Progress on Composition Design of Multicomponent Eutectic High Entropy Alloys. Trans. Indian Inst. Met. 2024, 77, 1455–1465. [Google Scholar] [CrossRef]
- Peng, P.; Feng, X.; Li, S.; Wei, B.; Zhang, M.; Xu, Y.; Zhang, X.; Ma, Z.; Wang, J. Effect of heat treatment on microstructure and mechanical properties of as-cast AlCoCrFeNi2.1 eutectic high entropy alloy. J. Alloys Compd. 2023, 939, 168843. [Google Scholar]
- Liang, N.N.; Xu, R.R.; Wu, G.Z.; Gao, X.Z.; Zhao, Y.H. High thermal stability of nanocrystalline FeNi2CoMo0.2V0.5 high-entropy alloy by twin boundary and sluggish diffusion. Mater. Sci. Eng. A 2022, 848, 143399. [Google Scholar] [CrossRef]
- Wan, D.; Guan, S.; Wang, D.; Lu, X.; Ma, J. Hydrogen embrittlement of additively manufactured AlCoCrFeNi2.1 eutectic high-entropy alloy. Corros. Sci. 2022, 195, 110007. [Google Scholar] [CrossRef]
- Cao, L.; Lin, X.; Zhang, Z.; Bai, M.; Wu, X. Effect of hot deformation and heat treatment on the microstructure and properties of spray-formed Al-Zn-Mg-Cu alloys. Metals 2024, 14, 451. [Google Scholar] [CrossRef]
- Peng, Z.; Guo, Q.; Sun, J.; Li, K.; Luan, H.; Gong, P. Effect of Mo Addition on Tribological Properties of Al19Fe20−xCo20−xNi41Mo2x Eutectic High-Entropy Alloys. Rare Met. Mater. Eng. 2024, 53, 17–22. [Google Scholar]
- Dong, Y.; Lu, Y. Effects of tungsten addition on the microstructure and mechanical properties of near-eutectic AlCoCrFeNi2 high-entropy alloy. J. Mater. Eng. Perform. 2018, 27, 109–115. [Google Scholar] [CrossRef]
- Yang, H.; Liu, X.; Li, A.; Li, R.; Xu, S.; Zhang, M.; Yu, P.; Yu, S.; Jiang, M.; Huo, C.; et al. Effect of silicon addition on the corrosion resistance of Al0.2CoCrFe1.5Ni high-entropy alloy in saline solution. J. Alloys Compd. 2023, 964, 171226. [Google Scholar] [CrossRef]
- Chen, Q.S.; Lu, Y.P.; Dong, Y.; Wang, T.M.; Li, T.J. Effect of minor B addition on microstructure and properties of AlCoCrFeNi multi-compenent alloy. Rare Met. Mater. Eng. 2017, 46, 651–656. [Google Scholar] [CrossRef]
- Feng, J.J.; Gao, S.; Han, K.; Miao, Y.D.; Qi, J.Q.; Wei, F.X.; Ren, Y.J.; Zhan, Z.Z.; Sui, Y.W.; Sun, Z.; et al. Effects of minor B addition on microstructure and properties of Al19Co20Fe20Ni41 eutectic high-entropy alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 1049–1058. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Ma, Z.L.; Tan, Y.; Wang, M.; Zhao, Y.; Cheng, X.W. Effects of Si additions on microstructures and mechanical properties of VNbTiTaSix refractory high-entropy alloys. J. Alloys Compd. 2022, 900, 163517. [Google Scholar] [CrossRef]
- Babilas, R.; Łoński, W.; Boryło, P.; Kądziołka-Gaweł, M.; Gębara, P.; Radoń, A. The influence of cooling rate, chromium and silicon addition on the structure and properties of AlCoCrFeNiSi high entropy alloys. J. Magn. Magn. Mater. 2020, 502, 166492. [Google Scholar] [CrossRef]
- Pan, Y.; Pu, D.L.; Yu, E.D. Structural, electronic, mechanical and thermodynamic properties of Cr–Si binary silicides from first-principles investigations. Vacuum 2021, 185, 110024. [Google Scholar] [CrossRef]
- Gu, X.; Zhuang, Y.; Jia, P. Evolution of the microstructure and mechanical properties of as-cast Al0.3CoCrFeNi high entropy alloys by adding Si content. Mater. Sci. Eng. A 2022, 840, 142983. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.J.; Mueller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb–20Mo–20Cr–20Ti–20Al with and without Si addition. J. Alloys Compd. 2016, 688, 468–477. [Google Scholar]
- Chang, H.; Zhang, T.W.; Ma, S.G.; Zhao, D.; Xiong, R.L.; Wang, T.; Li, Z.Q.; Wang, Z.H. Novel Si-added CrCoNi medium entropy alloys achieving the breakthrough of strength-ductility trade-off. Mater. Des. 2021, 197, 109202. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A 2012, 532, 480–486. [Google Scholar]
- Yakaboylu, G.A.; Sabolsky, K.; Sabolsky, E.M. Phase stability, microstructure and high-temperature properties of NbSi2-and TaSi2-oxide conducting ceramic composites. J. Mater. Sci. 2018, 53, 9958–9977. [Google Scholar]
- Kuznetsova, T.; Lapitskaya, V.; Solovjov, J.; Chizhik, S.; Pilipenko, V.; Aizikovich, S. Properties of CrSi2 layers obtained by rapid heat treatment of Cr film on silicon. Nanomaterials 2021, 11, 1734. [Google Scholar] [CrossRef]
- Huang, L.; Wang, X.; Jia, F.; Zhao, X.; Huang, B.; Ma, J.; Wang, C. Effect of Si element on phase transformation and mechanical properties for FeCoCrNiSix high entropy alloys. Mater. Lett. 2021, 282, 128809. [Google Scholar] [CrossRef]
- Mehrotra, R.C. Synthesis and properties of alkoxy-and acyloxysilanes. Pure Appl. Chem. 1966, 13, 111–132. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.Y.; Cotton, J.D.; Zhang, Y. Phase stability of low-density, multiprincipal component alloys containing aluminum, magnesium, and lithium. JOM 2014, 66, 2009–2020. [Google Scholar] [CrossRef]
- Zheng, S.; Zhou, F.; Kuang, S.; Liu, W.; Liu, Q. Microstructure and mechanical properties of multi-phase reinforced MoFeCrTiWNb2.5 (Al2O3)x high-entropy alloy laser cladding coatings. J. Therm. Spray Technol. 2022, 31, 1662–1672. [Google Scholar] [CrossRef]
- Wei, D.; Gong, W.; Tsuru, T.; Lobzenko, I.; Li, X.; Harjo, S.; Kawasaki, T.; Do, H.-S.; Bae, J.W.; Wagner, C.; et al. Si-addition contributes to overcoming the strength-ductility trade-off in high-entropy alloys. Int. J. Plast. 2022, 159, 103443. [Google Scholar]
- Shuai, L.; Zou, X.; Rao, Y.; Lu, X.; Yan, H. Synergistic effects of La and Y on the microstructure and mechanical properties of cast Al-Si-Cu alloys. Materials 2022, 15, 7283. [Google Scholar] [PubMed]
- Kalhapure, M.G.; Dighe, P.M. Impact of silicon content on mechanical properties of aluminum alloys. Int. J. Sci. Res. 2015, 4, 38–40. [Google Scholar]
- Su, Y.; Tian, S.; Yu, H.; Yu, L. Effect of pre-compressive treatment on creep behavior of a <0 1 1>-oriented single-crystal Ni-based superalloy. Scr. Mater. 2014, 93, 24–27. [Google Scholar]
- Yue, T.; Huang, K. Laser cladding of Cu0.5NiAlCoCrFeSi high entropy alloy on AZ91D magnesium substrates for improving wear and corrosion resistance. World J. Eng. 2012, 9, 119–124. [Google Scholar]
- Liu, Y.; Liskiewicz, T.W.; Beake, B.D. Dynamic changes of mechanical properties induced by friction in the Archard wear model. Wear 2019, 428, 366–375. [Google Scholar] [CrossRef]
- Khan, A.A.; Kaiser, M.S. Wear properties analysis on Al-based automotive alloy with varied levels of Si in dry, 3.5% NaCl and seawater corrosive environments. Arch. Metall. Mater. 2024, 69, 881–890. [Google Scholar]
- Saber, D.; Abdel-Karim, R.; Kandel, A.A.; El-Aziz, K.A. Corrosive wear of alumina particles reinforced Al–Si alloy composites. Phys. Met. Metallogr. 2020, 121, 188–194. [Google Scholar]
- Kang, N.; Coddet, P.; Chen, C.; Wang, Y.; Liao, H.; Coddet, C. Microstructure and wear behavior of in-situ hypereutectic Al–high Si alloys produced by selective laser melting. Mater. Des. 2016, 99, 120–126. [Google Scholar] [CrossRef]
- Udoye, N.E.; Fayomi, O.S.I.; Inegbenebor, A.O. Assessment of wear resistance of aluminium alloy in manufacturing industry—A review. Procedia Manuf. 2019, 35, 1383–1386. [Google Scholar]
- Xu, D.; Wang, M.; Li, T.; Wei, X.; Lu, Y. A critical review of the mechanical properties of CoCrNi-based medium-entropy alloys. Microstructures 2022, 2, 2022001. [Google Scholar]
- Hsu, S.M.; Munro, R.; Shen, M.C. Wear in boundary lubrication. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2002, 216, 427–441. [Google Scholar] [CrossRef]
- Otto, F.; Dlouhý, A.; Somsen, C.; Bei, H.; Eggeler, G.; George, E.P. The influences of temperature and microstructure on the tensile properties of a CoCrFeMnNi high-entropy alloy. Acta Mater. 2013, 61, 5743–5755. [Google Scholar]
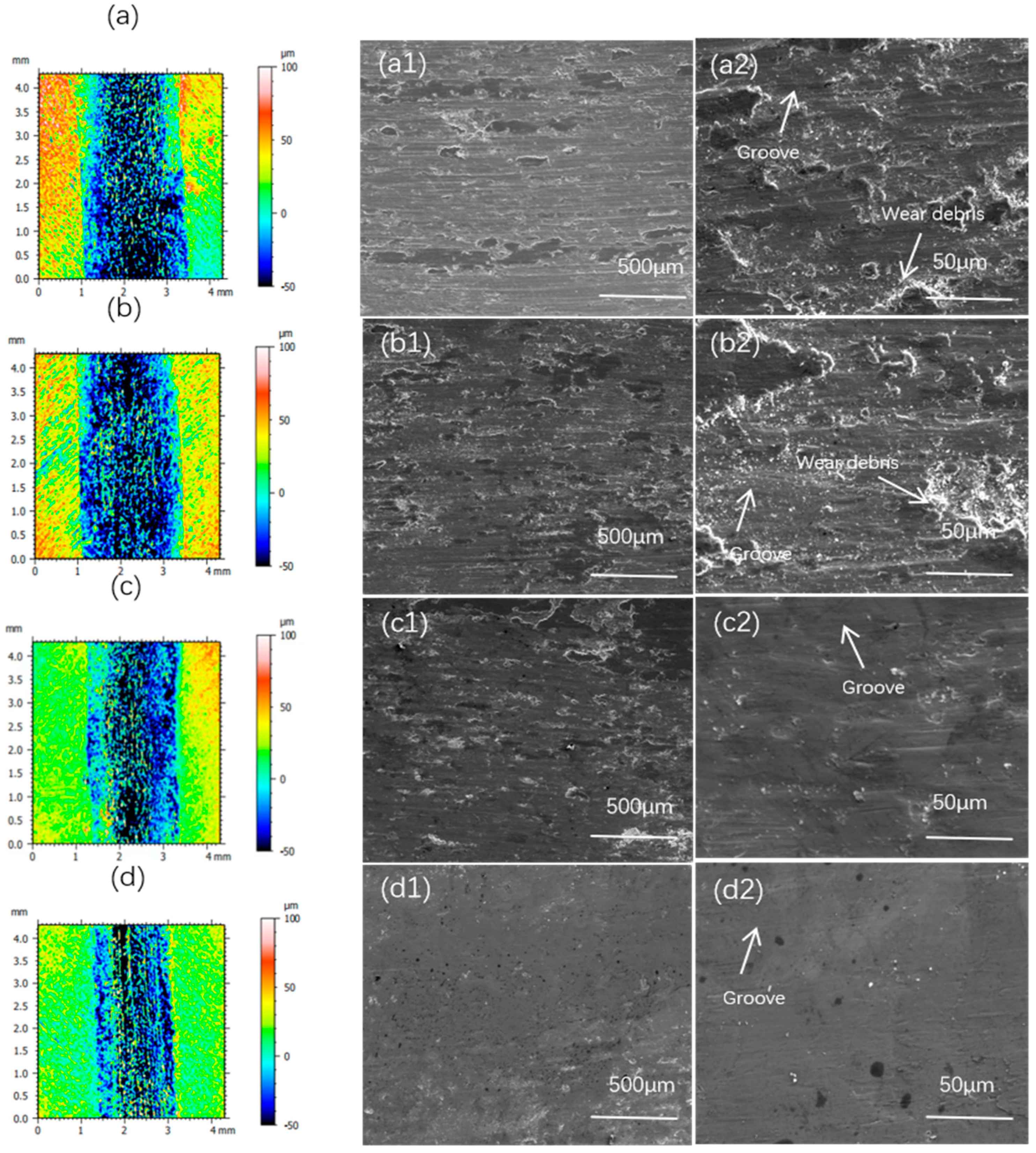
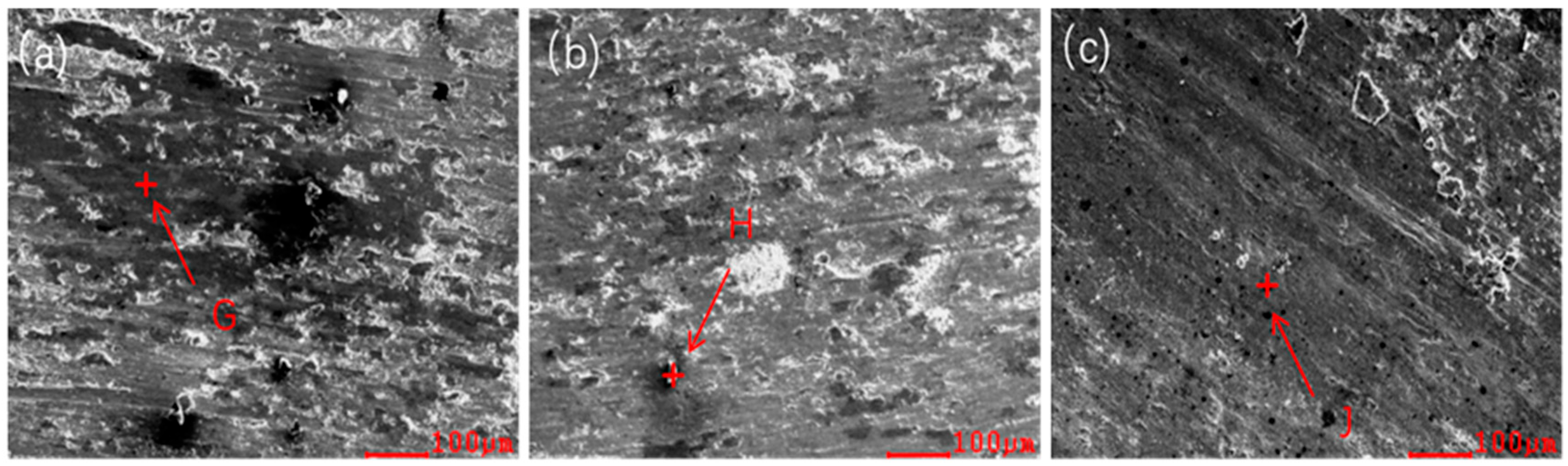
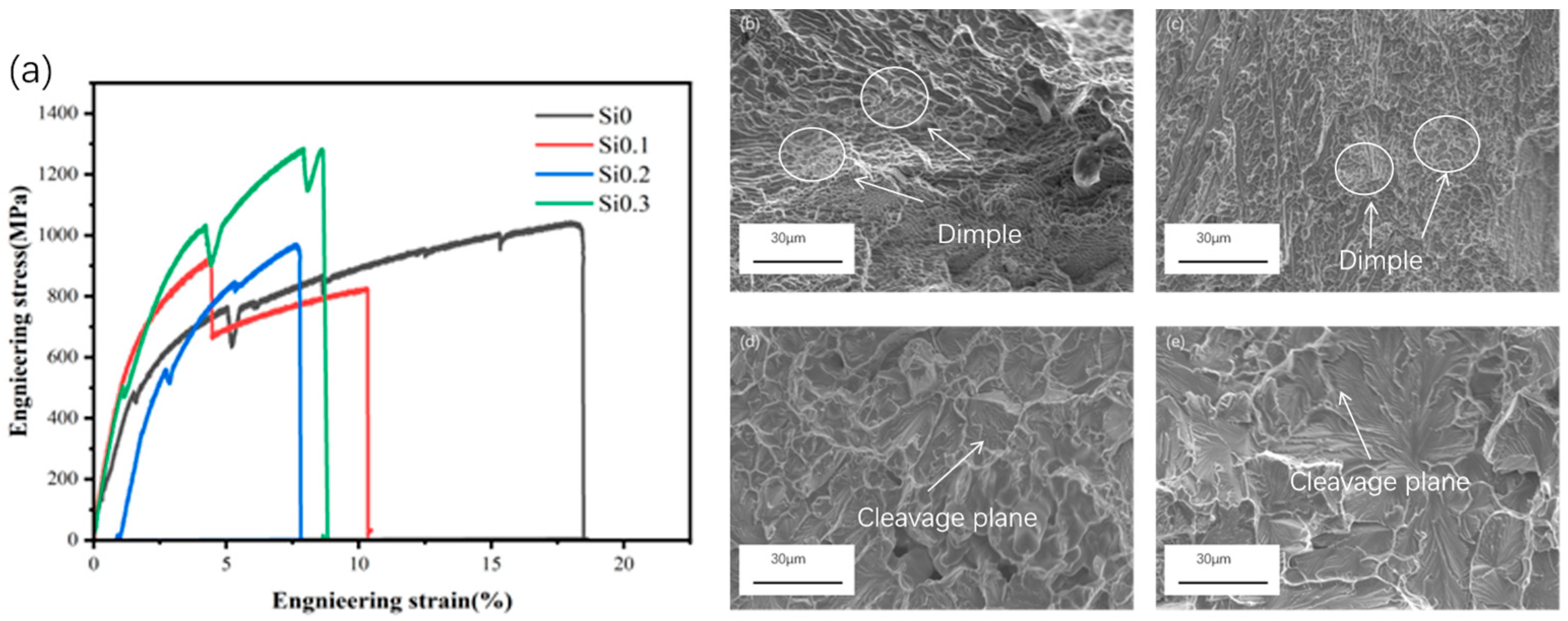
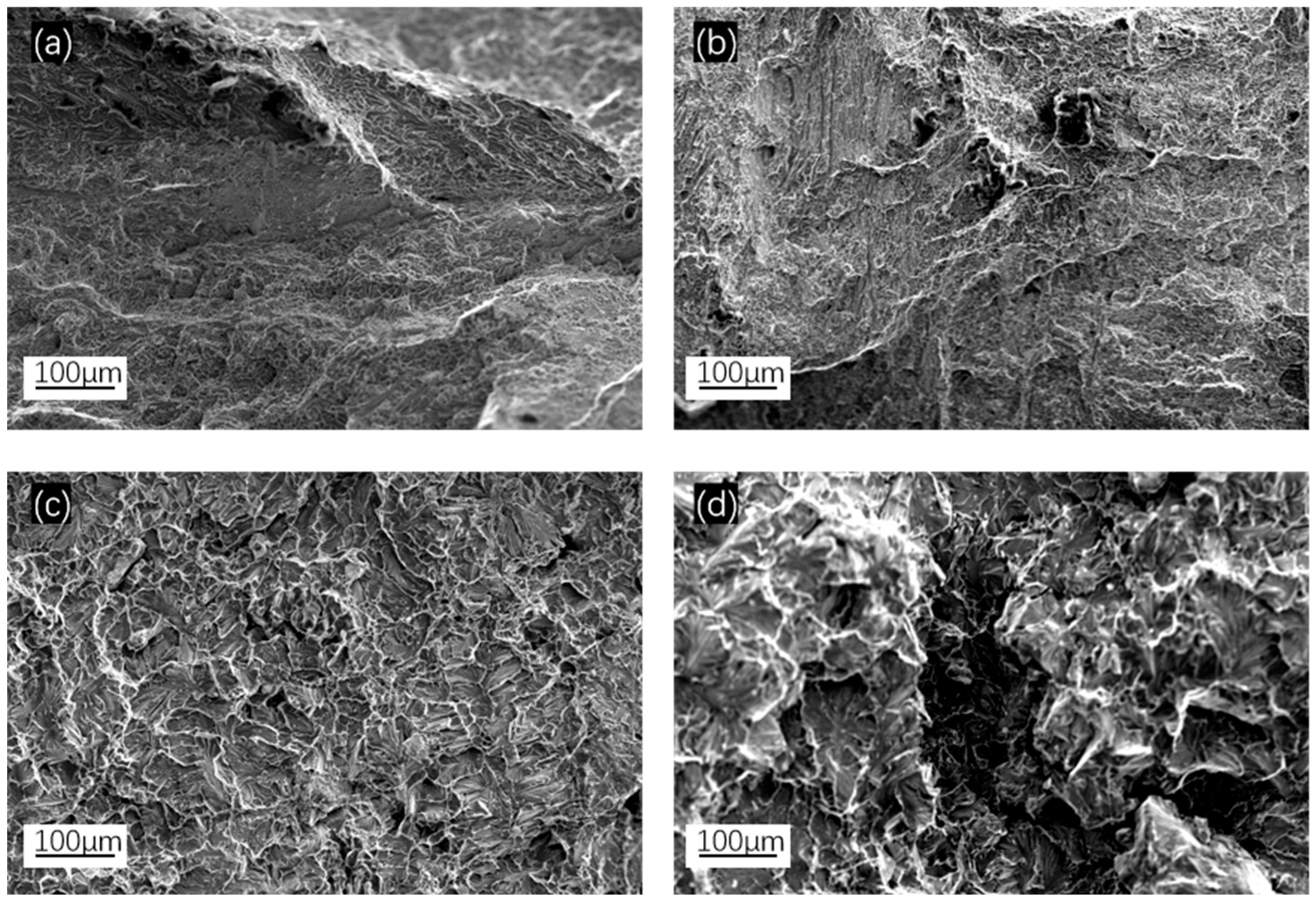
| x | Al | Co | Cr | Fe | Ni | Si |
|---|---|---|---|---|---|---|
| 0 | 8.51 | 18.59 | 16.40 | 17.61 | 38.88 | — |
| 0.1 | 8.43 | 18.42 | 16.25 | 17.46 | 38.53 | 0.87 |
| 0.2 | 8.36 | 18.27 | 16.12 | 17.31 | 38.20 | 1.74 |
| 0.3 | 8.29 | 18.11 | 15.97 | 17.16 | 37.89 | 2.58 |
| No. | Elements | Phase Structure | ||||||
|---|---|---|---|---|---|---|---|---|
| Al | Co | Cr | Fe | Ni | Si | |||
| X = 0 | 1 (BCC) | 30.11 | 12.98 | 8.37 | 12.19 | 36.35 | — | FCC + BCC |
| 2 (FCC) | 10.87 | 16.19 | 21.34 | 18.86 | 32.74 | — | ||
| X = 0.1 | 3 (BCC) | 25.87 | 13.42 | 7.53 | 12.03 | 39.57 | 1.58 | FCC + BCC |
| 4 (FCC) | 13.31 | 16.97 | 18.82 | 18.64 | 30.65 | 1.61 | ||
| X = 0.2 | 5 (BCC) | 17.92 | 15.59 | 13.42 | 12.70 | 37.39 | 2.98 | FCC + BCC |
| 6 (FCC) | 12.12 | 18.67 | 19.55 | 17.36 | 29.26 | 3.04 | ||
| 7 (BCC) | 11.93 | 12.37 | 28.51 | 10.15 | 32.25 | 4.79 | FCC + BCC + CrSi2 | |
| X = 0.3 | 8 (FCC) | 16.46 | 14.71 | 15.99 | 13.29 | 36.23 | 3.32 | |
| 9 (CrSi2) | 10.70 | 14.65 | 23.91 | 16.61 | 14.49 | 19.64 | ||
| Elements | Si | Ni | Al | Fe | Cr | Co |
|---|---|---|---|---|---|---|
| Atomic Radius/pm | 115 | 124 | 143 | 126 | 128 | 125 |
| Si | Si | −22.9 | −5.4 | −17.7 | −20.4 | −21.2 |
| Ni | Ni | −22 | −1.6 | −6.7 | −0.2 | |
| Al | Al | −11 | −10 | −19 | ||
| Fe | Fe | −0.5 | −1.6 | |||
| Cr | Cr | −4.5 | ||||
| Co | Co |
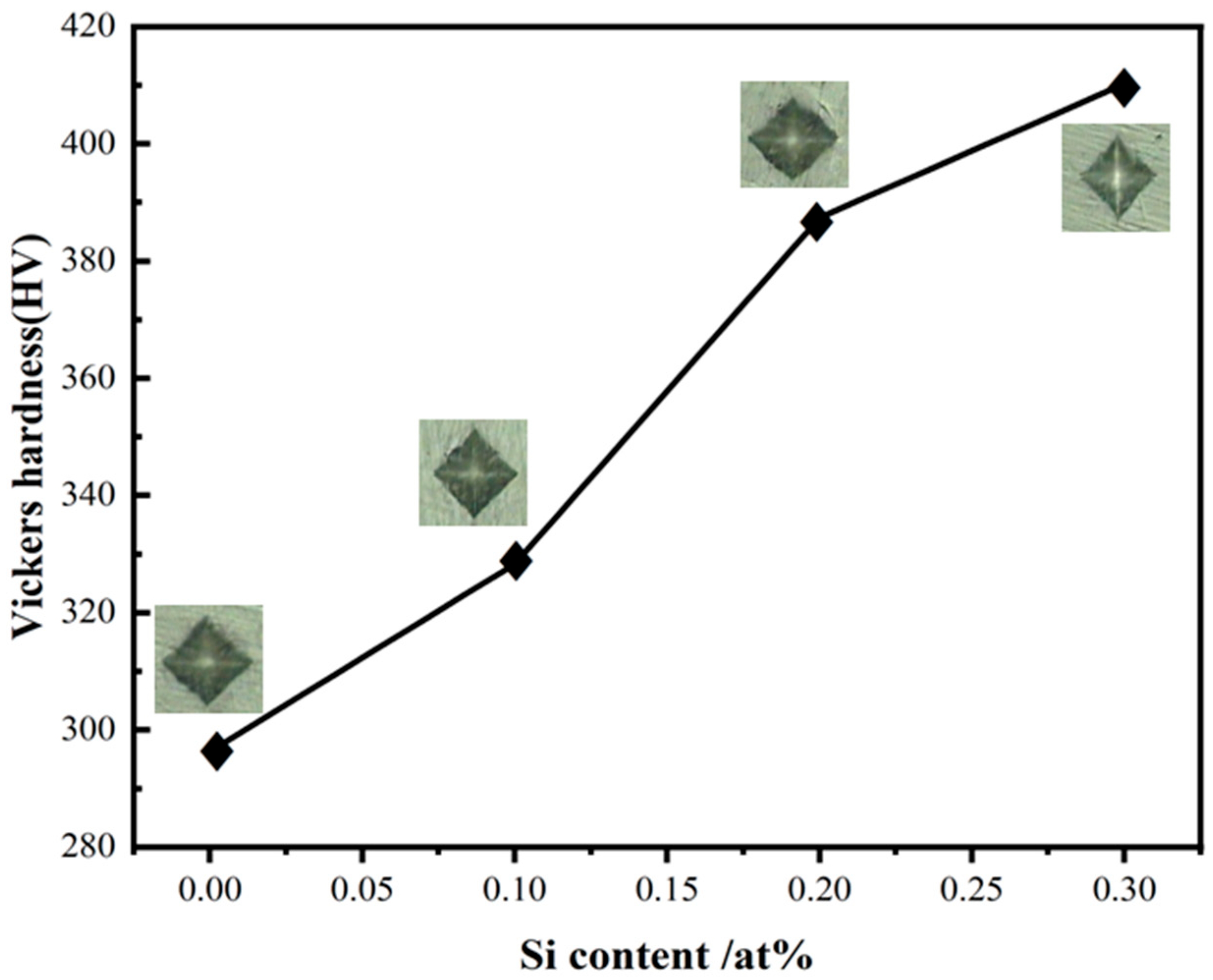
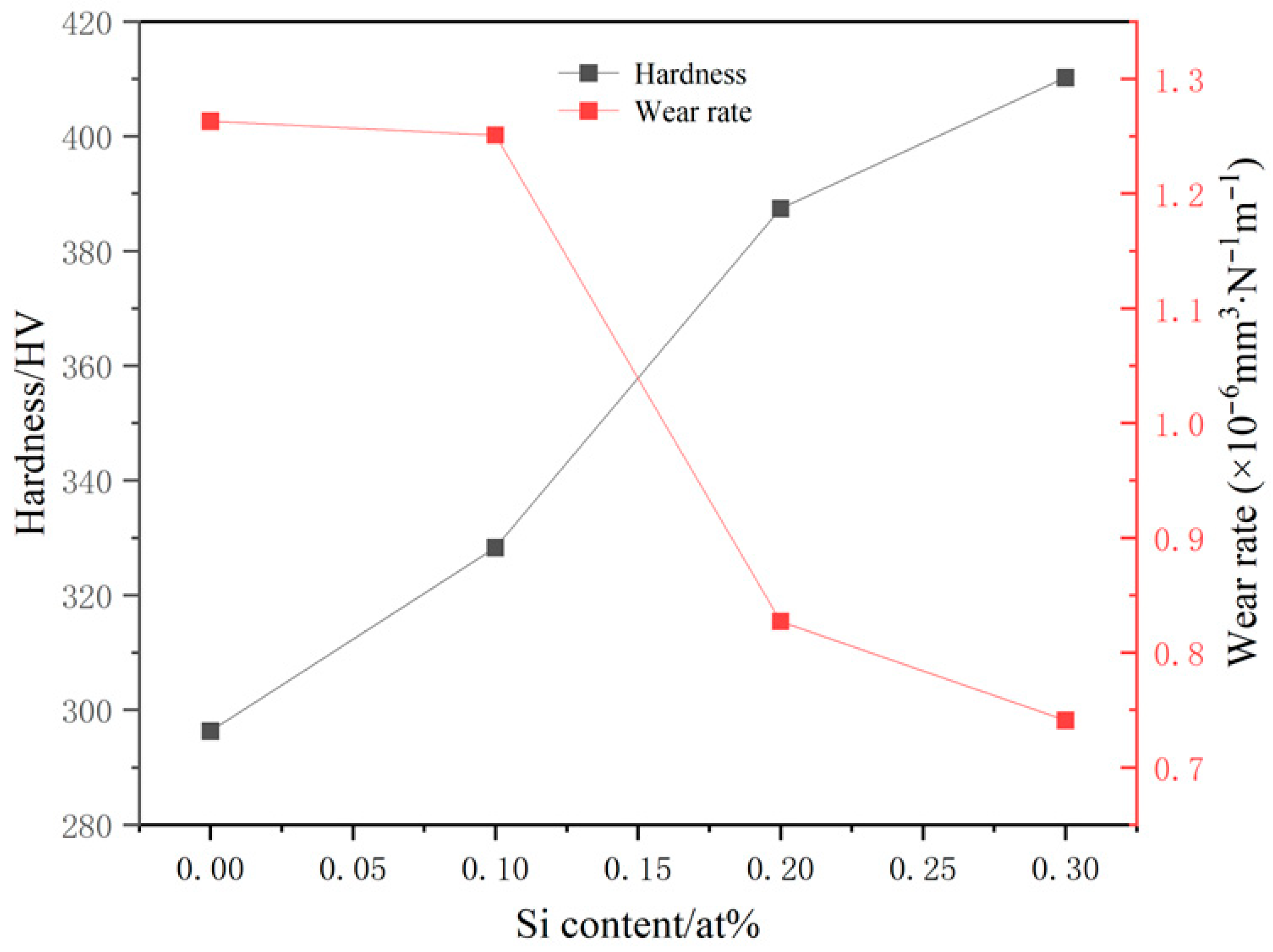
| x | Hardness (HV) | Average Value | Relative Error (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| 0 | 276.7 | 291.7 | 295.6 | 295.6 | 293.6 | 312.1 | 314.3 | 299.6 | 284.0 | 299.6 | 296.3 | 12.68 |
| 0.1 | 325.4 | 327.8 | 325.4 | 323.2 | 332.4 | 320.9 | 327.8 | 342.1 | 330.1 | 327.8 | 328.3 | 6.46 |
| 0.2 | 413.3 | 368.2 | 330.1 | 379.5 | 403.7 | 420.0 | 382.4 | 385.3 | 403.7 | 388.3 | 387.4 | 23.1 |
| 0.3 | 385.3 | 437.3 | 426.8 | 373.8 | 371.0 | 368.2 | 440.9 | 455.7 | 423.4 | 420.0 | 410.2 | 21.3 |
| X | Regions | Al | Co | Cr | Fe | Ni | Si | O |
|---|---|---|---|---|---|---|---|---|
| 0.1 | G | 14.58 | 12.85 | 12.88 | 12.70 | 27.86 | 1.62 | 17.52 |
| 0.2 | H | 11.87 | 12.84 | 13.88 | 12.74 | 25.91 | 3.87 | 18.89 |
| 0.3 | J | 13.71 | 13.01 | 10.97 | 10.72 | 22.23 | 5.43 | 23.93 |
| Alloys | Yiels Strength (Mpa) | Uitimate Tensile Strength (Mpa) | Elongation (%) |
|---|---|---|---|
| Si0 | 516.8 | 1043 | 18.50 |
| Si0.1 | 544.6 | 916 | 10.34 |
| Si0.2 | 585.6 | 972 | 7.80 |
| Si0.3 | 686.6 | 1284 | 8.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Li, S.; Zhang, J.; Tian, J. Microstructure and Mechanical Property Regulation of As-Cast AlCoCrFeNi2.1Six (x = 0, 0.1, 0.2, 0.3) High-Entropy Alloys. Metals 2025, 15, 1146. https://doi.org/10.3390/met15101146
Li R, Li S, Zhang J, Tian J. Microstructure and Mechanical Property Regulation of As-Cast AlCoCrFeNi2.1Six (x = 0, 0.1, 0.2, 0.3) High-Entropy Alloys. Metals. 2025; 15(10):1146. https://doi.org/10.3390/met15101146
Chicago/Turabian StyleLi, Rongbin, Saiya Li, Jiahao Zhang, and Jiaming Tian. 2025. "Microstructure and Mechanical Property Regulation of As-Cast AlCoCrFeNi2.1Six (x = 0, 0.1, 0.2, 0.3) High-Entropy Alloys" Metals 15, no. 10: 1146. https://doi.org/10.3390/met15101146
APA StyleLi, R., Li, S., Zhang, J., & Tian, J. (2025). Microstructure and Mechanical Property Regulation of As-Cast AlCoCrFeNi2.1Six (x = 0, 0.1, 0.2, 0.3) High-Entropy Alloys. Metals, 15(10), 1146. https://doi.org/10.3390/met15101146





