Application of Ultrasonic-Enhanced Leaching for the Recovery of Metal Elements from Mineral Raw Materials and Secondary Resources
Abstract
1. Introduction
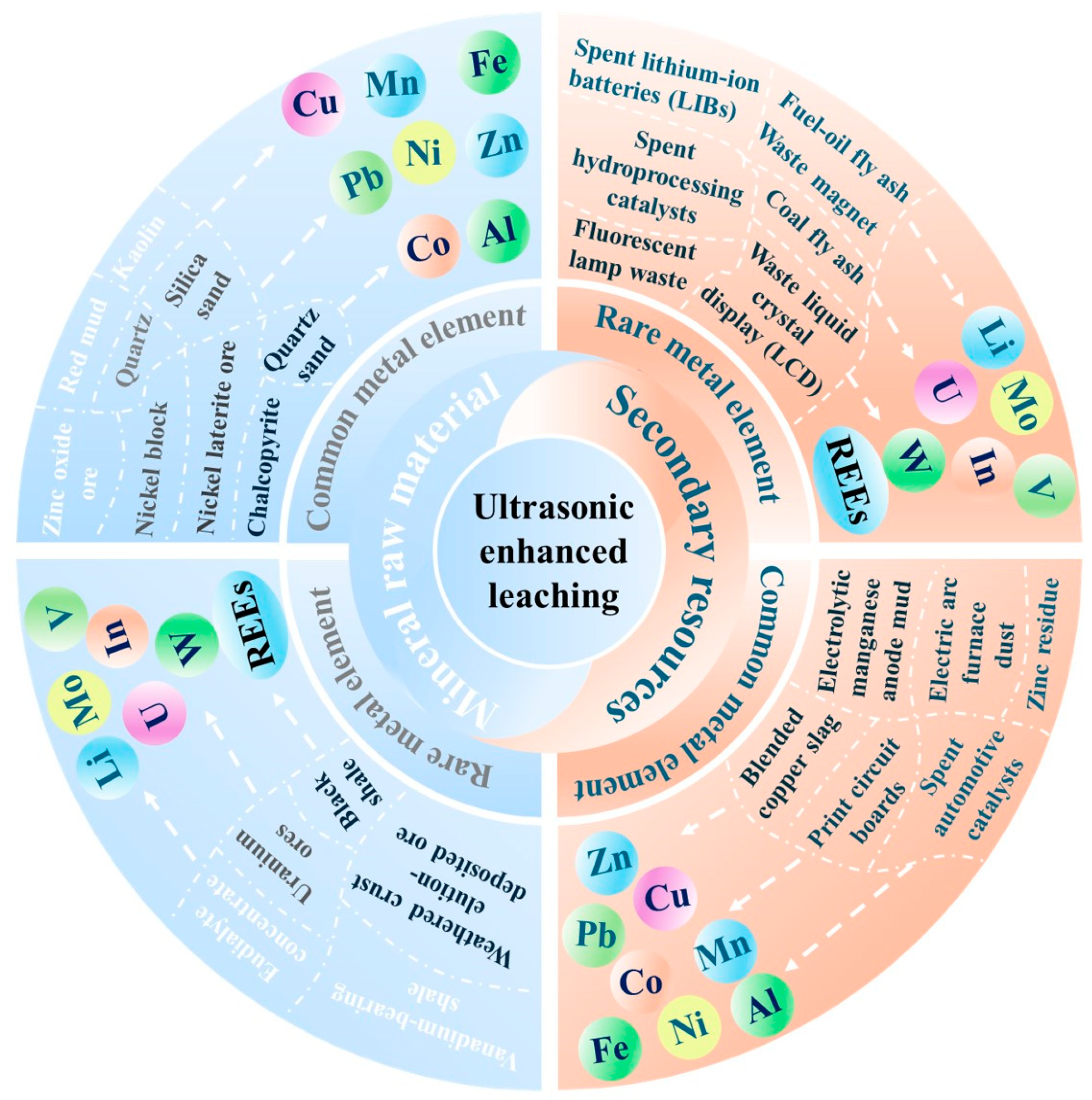
2. Recovery of Metal Elements from Mineral Raw Material
2.1. Recovery of Common Metal Elements
2.2. Recovery of Rare Metal Elements
3. Recovery of Metal Elements from Secondary Sources
3.1. Recovery of Common Metal Elements
3.2. Recovery of Rare Metal Elements
4. Conclusions
- (1)
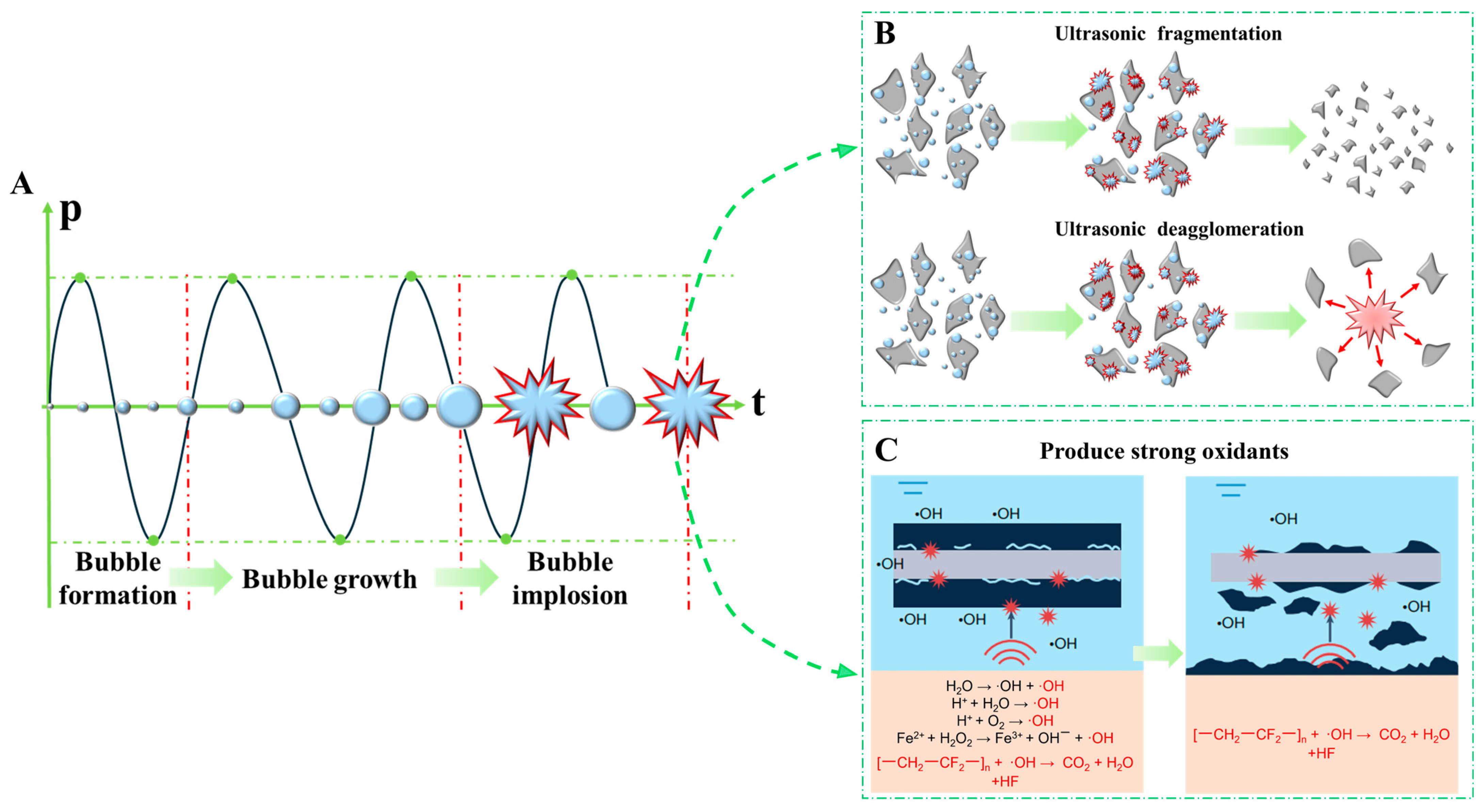
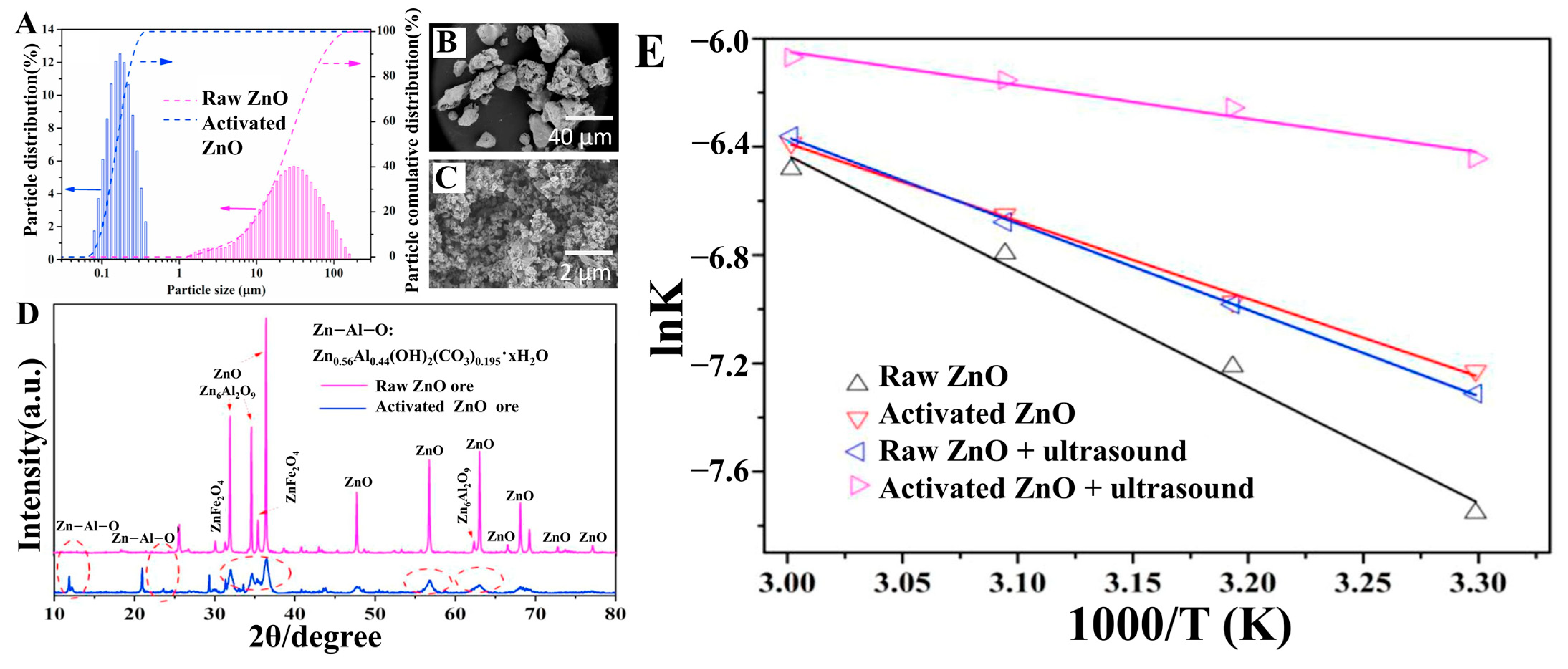
- By comparing the ultrasonic-enhanced leaching method with the traditional leaching method, the physicochemical properties and reaction mechanisms of ultrasonic-enhanced leaching are summarized, and three advantages of ultrasonic-enhanced leaching are summarized: (i) The use of the ultrasonic cavitation physical effect on the raw material particles of the crushing changes the morphology of the particles (reduces the size of the particles, destroys the surface morphology to increase the specific surface area, and removes the surface of the raw material of the passivation layer), which is conducive to the mass transfer and diffusion of chemical agents on the particle surface and in the solution and the increase in the collision frequency between the leaching agent molecules and the raw material. (ii) The ultrasonic-enhanced leaching method can effectively reduce the activation energy of the leaching reaction, accelerate the leaching reaction efficiency, and realize the efficient leaching and recovery of metal elements. (iii) By using the chemical effect of ultrasonic cavitation to generate a strong oxidizing reagent (·OH or H2O2), the strong oxidizing reagent generated by the ultrasound replaces the chemical reagent, which improves the leaching efficiency and reduces the consumption of chemical reagents, realizing the cost savings and efficiency improvement of the leaching reaction process.
- (2)
- The combined use of ultrasonic-enhanced leaching with new green enhanced methods (applied electric field, microwave heating) and reagents (microorganisms, organic acids, ionic liquids) can be adopted for the efficient recycling of low-grade minerals and secondary resources. The huge potential in the recovery of metal elements through ultrasound, in combination with active agents or other processes, requires further research. In addition, numerical simulations can be performed using software such as ANSYS Fluent (ANSYS Fluent, 2025 R2, ANSYS Inc., Canonsburg, PA, USA) [91] and MATLAB (MATLAB, R2025a, The MathWorks, Inc., Natick, MA, USA) [92], which can optimize the experimental parameters and reveal the strengthening mechanism [93]. The traditional method of high energy consumption and serious secondary pollution is transformed into a green and sustainable recycling method. However, based on the ultrasonic-enhanced leaching process, most of the research is in the laboratory stage; the small processing sample size, the limited ultrasonic cavitation area, and the poor stability of long-time operation are the main constraints for the industrial application of ultrasonic-enhanced leaching technology. In order to further promote the industrial application of ultrasonic leaching, systematic research should be conducted according to the types of samples to be treated and the ultrasonic leaching parameters to achieve better results.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Anderson, A.; Rezaie, B. Geothermal technology: Trends and potential role in a sustainable future. Appl. Energy 2019, 248, 18–34. [Google Scholar] [CrossRef]
- Amigues, J.-P.; Le Kama, A.A.; Moreaux, M. Equilibrium transitions from non-renewable energy to renewable energy under capacity constraints. J. Econ. Dyn. Control 2015, 55, 89–112. [Google Scholar] [CrossRef]
- Jurasz, J.; Canales, F.A.; Kies, A.; Guezgouz, M.; Beluco, A. A review on the complementarity of renewable energy sources: Concept, metrics, application and future research directions. Sol. Energy 2020, 195, 703–724. [Google Scholar] [CrossRef]
- Qazi, A.; Hussain, F.; Rahim, N.A.; Hardaker, G.; Alghazzawi, D.; Shaban, K.; Haruna, K. Towards Sustainable Energy: A Systematic Review of Renewable Energy Sources, Technologies, and Public Opinions. IEEE Access 2019, 7, 63837–63851. [Google Scholar] [CrossRef]
- Bao, S.; Tang, Y.; Zhang, Y.; Liang, L. Recovery and Separation of Metal Ions from Aqueous Solutions by Solvent-Impregnated Resins. Chem. Eng. Technol. 2016, 39, 1377–1392. [Google Scholar] [CrossRef]
- Abkhoshk, E.; Jorjani, E.; Al-Harahsheh, M.; Rashchi, F.; Naazeri, M. Review of the hydrometallurgical processing of non-sulfide zinc ores. Hydrometallurgy 2014, 149, 153–167. [Google Scholar] [CrossRef]
- Brunelli, K.; Dabalà, M. Ultrasound effects on zinc recovery from EAF dust by sulfuric acid leaching. Int. J. Miner. Met. Mater. 2015, 22, 353–362. [Google Scholar] [CrossRef]
- Ding, W.; Bao, S.; Zhang, Y.; Xiao, J. Efficient Selective Extraction of Scandium from Red Mud. Miner. Process. Extr. Met. Rev. 2023, 44, 304–312. [Google Scholar] [CrossRef]
- Yin, X.; Opara, A.; Du, H.; Miller, J.D. Molecular dynamics simulations of metal–cyanide complexes: Fundamental considerations in gold hydrometallurgy. Hydrometallurgy 2010, 106, 64–70. [Google Scholar] [CrossRef]
- Xie, Y.; Xie, S.; Chen, X.; Gui, W.; Yang, C.; Caccetta, L. An integrated predictive model with an on-line updating strategy for iron precipitation in zinc hydrometallurgy. Hydrometallurgy 2015, 151, 62–72. [Google Scholar] [CrossRef]
- Park, J.; Jung, Y.; Kusumah, P.; Lee, J.; Kwon, K.; Lee, C. Application of Ionic Liquids in Hydrometallurgy. Int. J. Mol. Sci. 2014, 15, 15320–15343. [Google Scholar] [CrossRef]
- Lai, Y.; Li, J.; Zhu, S.; Liu, K.; Xia, Q.; Huang, M.; Hu, G.; Zhang, H.; Qi, T. Recovery of rare earths, lithium, and fluorine from rare earth molten salt electrolytic slag by mineral phase reconstruction combined with vacuum distillation. Sep. Purif. Technol. 2023, 310, 123105. [Google Scholar] [CrossRef]
- Yang, D.; Yu, M.; Mubula, Y.; Yuan, W.; Huang, Z.; Lin, B.; Mei, G.; Qiu, T. Recovering rare earths, lithium and fluorine from rare earth molten salt electrolytic slag using sub-molten salt method. J. Rare Earths 2023, 42, 1774–1781. [Google Scholar] [CrossRef]
- Tong, Z.; Hu, X.; Wen, H. Effect of roasting activation of rare earth molten salt slag on extraction of rare earth, lithium and fluorine. J. Rare Earths 2023, 41, 300–308. [Google Scholar] [CrossRef]
- Tian, L.; Chen, L.; Gong, A.; Wu, X.; Cao, C.; Xu, Z. Recovery of rare earths, lithium and fluorine from rare earth molten salt electrolytic slag via fluoride sulfate conversion and mineral phase reconstruction. Miner. Eng. 2021, 170, 106965. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H. Selective extraction of rare earths and lithium from rare earth fluoride molten-salt electrolytic slag by sulfation. Miner. Eng. 2021, 160, 106711. [Google Scholar] [CrossRef]
- Mahfouz, M.G.; Galhoum, A.A.; Gomaa, N.A.; Abdel-Rehem, S.S.; Atia, A.A.; Vincent, T.; Guibal, E. Uranium extraction using magnetic nano-based particles of diethylenetriamine-functionalized chitosan: Equilibrium and kinetic studies. Chem. Eng. J. 2015, 262, 198–209. [Google Scholar] [CrossRef]
- Larrabure, G.; Chero-Osorio, S.; Silva-Quiñones, D.; Benndorf, C.; Williams, M.; Gao, F.; Gamarra, C.; Alarcón, A.; Segura, C.; Teplyakov, A.; et al. Surface processes at a polymetallic (Mn-Fe-Pb) sulfide subject to cyanide leaching under sonication conditions and with an alkaline pretreatment: Understanding differences in silver extraction with X-ray photoelectron spectroscopy (XPS). Hydrometallurgy 2021, 200, 105544. [Google Scholar] [CrossRef]
- Mubula, Y.; Yu, M.; Yang, D.; Niu, H.; Qiu, T.; Mei, G. Recovery of Rare Earths from Rare-Earth Melt Electrolysis Slag by Mineral Phase Reconstruction. JOM 2024, 76, 4732–4748. [Google Scholar] [CrossRef]
- Cai, G.; Fung, K.; Ng, K.; Wibowo, C. Process development for the recycle of spent lithium ion batteries by chemical Precipitation. Ind. Eng. Chem. Res. 2014, 53, 18245–18259. [Google Scholar] [CrossRef]
- Schipper, F.; Aurbach, D. A brief review: Past, present and future of lithium ion batteries. Russ. J. Electrochem. 2016, 52, 1095–1121. [Google Scholar] [CrossRef]
- Luo, F.; Wei, C.; Zhang, C.; Gao, H.; Niu, J.; Ma, W.; Peng, Z.; Bai, Y.; Zhang, Z. Operando X-ray diffraction analysis of the degradation mechanisms of a spinel LiMn2O4 cathode in different voltage windows. J. Energy Chem. 2020, 44, 138–146. [Google Scholar] [CrossRef]
- Chawla, N.; Bharti, N.; Singh, S. Recent Advances in Non-Flammable Electrolytes for Safer Lithium-Ion Batteries. Batteries 2019, 5, 19. [Google Scholar] [CrossRef]
- Xia, S.; Huang, W.; Shen, X.; Liu, J.; Cheng, F.; Liu, J.-J.; Yang, X.; Guo, H. Rearrangement on surface structures by boride to enhanced cycle stability for LiNi0.80Co0.15Al0.05O2 cathode in lithium ion batteries. J. Energy Chem. 2020, 45, 110–118. [Google Scholar] [CrossRef]
- Mubula, Y.; Yu, M.; Gu, H.; Wang, L.; Chen, M.; Qiu, T.; Mei, G. Recovery of rare earths from rare earth molten salt electrolytic slag using alkali phase reconstruction method with the aid of external electric field. J. Rare Earths 2024, 43, 2285–2294. [Google Scholar] [CrossRef]
- Mubula, Y.; Yu, M.; Yang, D.; Niu, H.; Gu, H.; Qiu, T.; Mei, G. Microwave-assisted atmospheric alkaline leaching process and leaching kinetics of rare earth melt electrolysis slag. Heliyon 2024, 10, e32278. [Google Scholar] [CrossRef]
- Kumari, A.; Dipali; Randhawa, N.S.; Sahu, S.K. Electrochemical treatment of spent NdFeB magnet in organic acid for recovery of rare earths and other metal values. J. Clean. Prod. 2021, 309, 127393. [Google Scholar] [CrossRef]
- Çetintaş, S.; Bingöl, D. Performance evaluation of leaching processes with and without ultrasound effect combined with reagent-assisted mechanochemical process for nickel recovery from Laterite: Process optimization and kinetic evaluation. Miner. Eng. 2020, 157, 106562. [Google Scholar] [CrossRef]
- Nalesso, S.; Bussemaker, M.J.; Sear, R.P.; Hodnett, M.; Lee, J. A review on possible mechanisms of sonocrystallisation in solution. Ultrason. Sonochemistry 2019, 57, 125–138. [Google Scholar] [CrossRef]
- Ozonek, J. Application of Hydrodynamic Cavitation in Environmental Engineering; Reference and Research Book News; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Chen, Y.; Truong, V.N.; Bu, X.; Xie, G. A review of effects and applications of ultrasound in mineral flotation. Ultrason. Sonochemistry 2020, 60, 104739. [Google Scholar] [CrossRef]
- Altay, R.; Sadaghiani, A.K.; Sevgen, M.I.; Şişman, A.; Koşar, A. Numerical and Experimental Studies on the Effect of Surface Roughness and Ultrasonic Frequency on Bubble Dynamics in Acoustic Cavitation. Energies 2020, 13, 1126. [Google Scholar] [CrossRef]
- Yasui, K. Unsolved Problems in Acoustic Cavitation. In Handbook of Ultrasonics and Sonochemistry; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1. [Google Scholar]
- Ezzatneshan, E.; Vaseghnia, H. Dynamics of an acoustically driven cavitation bubble cluster in the vicinity of a solid surface. Phys. Fluids 2021, 33, 123311. [Google Scholar] [CrossRef]
- Nazari-Mahroo, H.; Pasandideh, K.; Navid, H.; Sadighi-Bonabi, R. Influence of liquid density variation on the bubble and gas dynamics of a single acoustic cavitation bubble. Ultrasonics 2020, 102, 106034. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, J.; Zhu, L.; Yao, Q.; Sun, Y.; Guo, C.; Chen, H.; Yang, B. Efficient recovery of valuable metals from waste printed circuit boards via ultrasound-enhanced flotation. Process. Saf. Environ. Prot. 2022, 169, 869–878. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Wang, Y.; Jiang, Y.; Tan, X.; Han, W.; Wang, S. Recycling of LiFePO4 cathode materials from spent lithium-ion batteries through ultrasound-assisted Fenton reaction and lithium compensation. Waste Manag. 2021, 136, 67–75. [Google Scholar] [CrossRef]
- Xin, C.; Xia, H.; Jiang, G.; Zhang, Q.; Zhang, L.; Xu, Y.; Cai, W. Mechanism and kinetics study on ultrasonic combined with oxygen enhanced leaching of zinc and germanium from germanium-containing slag dust. Sep. Purif. Technol. 2022, 302, 122167. [Google Scholar] [CrossRef]
- Chen, B.; Bao, S.; Zhang, Y.; Li, S. A high-efficiency and sustainable leaching process of vanadium from shale in sulfuric acid systems enhanced by ultrasound. Sep. Purif. Technol. 2020, 240, 116624. [Google Scholar] [CrossRef]
- Xue, J.; Lu, X.; DU, Y.; Mao, W.; Wang, Y.; Li, J. Ultrasonic-assisted Oxidation Leaching of Nickel Sulfide Concentrate. Chin. J. Chem. Eng. 2010, 18, 948–953. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Yin, S. Ultrasound augmented leaching of nickel sulfate in sulfuric acid and hydrogen peroxide media. Ultrason. Sonochemistry 2018, 40, 1021–1030. [Google Scholar] [CrossRef]
- He, H.; Cao, J.; Duan, N. Synergistic effect between ultrasound and fierce mechanical activation towards mineral extraction: A case study of ZnO ore. Ultrason. Sonochemistry 2018, 48, 163–170. [Google Scholar] [CrossRef]
- Mohammadnejad, S.; Provis, J.L.; van Deventer, J.S. Effects of grinding on the preg-robbing potential of quartz in an acidic chloride medium. Miner. Eng. 2013, 52, 31–37. [Google Scholar] [CrossRef]
- Kaupp, G. Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm 2009, 11, 388–403. [Google Scholar] [CrossRef]
- Wang, J.; Faraji, F.; Ghahreman, A. Effect of Ultrasound on the Oxidative Copper Leaching from Chalcopyrite in Acidic Ferric Sulfate Media. Minerals 2020, 10, 633. [Google Scholar] [CrossRef]
- Li, S.; Chen, W.; Yin, S.; Ma, A.; Yang, K.; Xie, F.; Zhang, L.; Peng, J. Impacts of ultrasound on leaching recovery of zinc from low grade zinc oxide ore. Green Process Synth. 2015, 4, 323–328. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Peng, J.; Srinivasakannan, C.; Li, S.; Yin, S. Microwave and ultrasound augmented leaching of complicated zinc oxide ores in ammonia and ammonium citrate solutions. Metals 2017, 7, 216. [Google Scholar] [CrossRef]
- Knaislová, A.; Vu, H.N.; Dvořák, P. Microwave and Ultrasound Effect on Ammoniacal Leaching of Deep-Sea Nodules. Minerals 2018, 8, 351. [Google Scholar] [CrossRef]
- Li, X.; Li, T.; Gao, J.; Huang, H.; Li, L.; Li, J. A novel “green” solvent to deeply purify quartz sand with high yields: A case study. J. Ind. Eng. Chem. 2016, 35, 383–387. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, X.; Huang, H.; Zhou, L.; Xiong, T. High efficiency iron removal from quartz sand using phosphoric acid. Int. J. Miner. Process 2012, 114–117, 30–34. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Li, X.; Zhang, Z. Improvement of iron removal from silica sand using ultrasound-assisted oxalic acid. Ultrason. Sonochemistry 2011, 18, 389–393. [Google Scholar] [CrossRef]
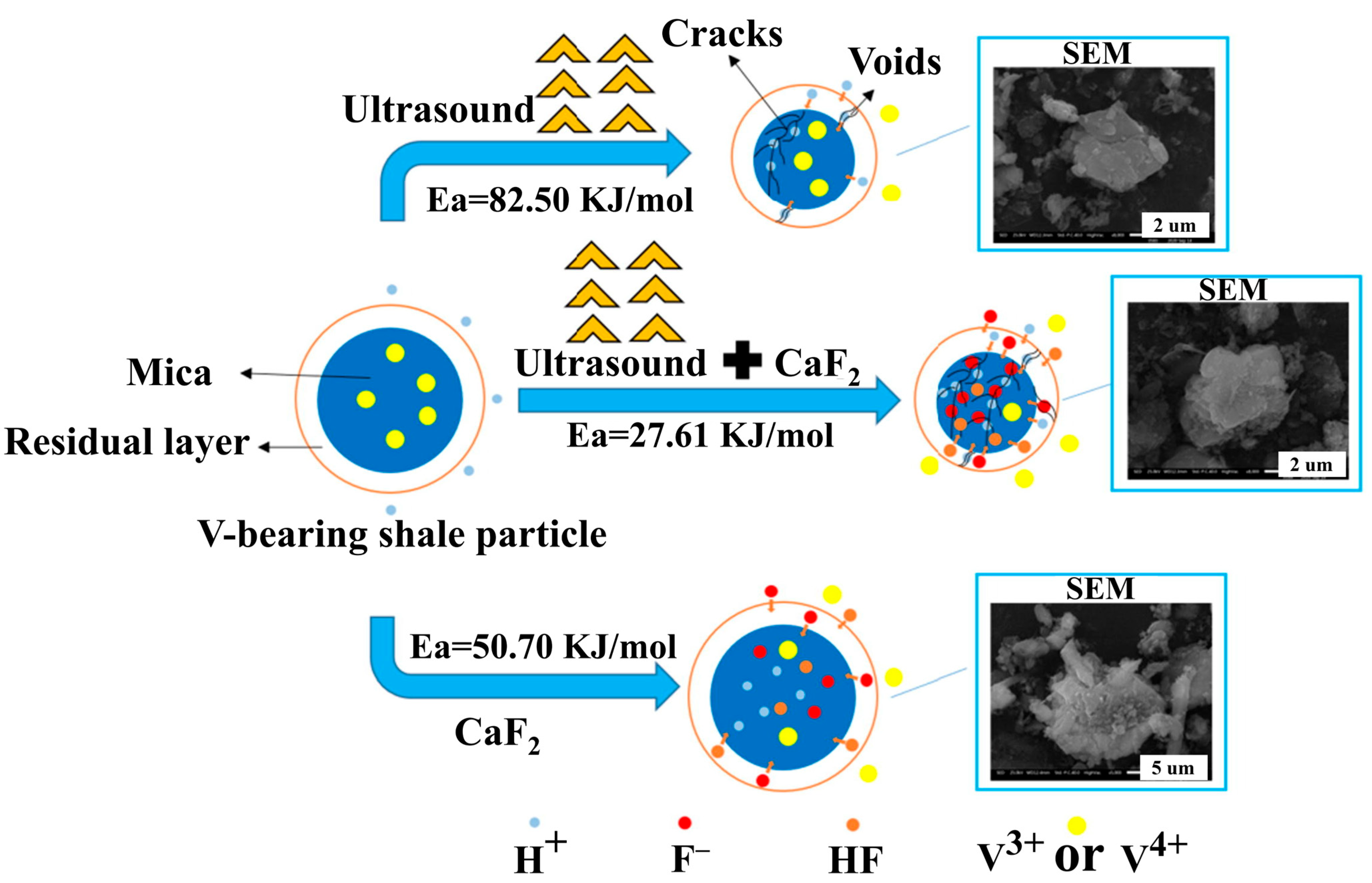
- Chen, B.; Bao, S.; Zhang, Y. Synergetic strengthening mechanism of ultrasound combined with calcium fluoride towards vanadium extraction from low-grade vanadium-bearing shale. Int. J. Min. Sci. Technol. 2021, 31, 1095–1106. [Google Scholar] [CrossRef]
- Yang, J.-H.; He, L.-H.; Liu, X.-H.; Ding, W.-T.; Song, Y.-F.; Zhao, Z.-W. Comparative kinetic analysis of conventional and ultrasound-assisted leaching of scheelite by sodium carbonate. Trans. Nonferrous Met. Soc. China 2018, 28, 775–782. [Google Scholar] [CrossRef]
- Johansson, Ö.; Pamidi, T.; Shankar, V. Extraction of tungsten from scheelite using hydrodynamic and acoustic cavitation. Ultrason. Sonochemistry 2021, 71, 105408. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, L.; Wang, S.; Cui, W.; Peng, J. Synergistic extraction of gold from the refractory gold ore via ultrasound and chlorination-oxidation. Ultrason. Sonochemistry 2017, 37, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.; Hu, Y.; Wang, S.; Zhang, L. Mechanism of synergistic pretreatment with ultrasound and ozone to improve gold and silver leaching percentage. Appl. Surf. Sci. 2022, 576, 151726. [Google Scholar] [CrossRef]
- Cilek, E.C.; Ciftci, H.; Karagoz, S.G.; Tuzci, G. Extraction of silver from a refractory silver ore by sono-cyanidation. Ultrason. Sonochemistry 2020, 63, 104965. [Google Scholar] [CrossRef]
- Zhao, Z.; Ding, W.; Liu, X.; Liang, Y. Effect of ultrasound on kinetics of scheelite leaching in sodium hydroxide. Can. Met. Quart. 2013, 52, 138–145. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, X.-J.; Li, K.-F.; Qian, G.-R.; Zhou, M. Kinetics of leaching refractory gold ores by ultrasonic-assisted electro-chlorination. Int. J. Miner. Met. Mater. 2012, 19, 473–477. [Google Scholar] [CrossRef]
- Yin, S.; Pei, J.; Jiang, F.; Li, S.; Peng, J.; Zhang, L.; Ju, S.; Srinivasakannan, C. Ultrasound-assisted leaching of rare earths from the weathered crust elution-deposited ore using magnesium sulfate without ammonia-nitrogen pollution. Ultrason. Sonochemistry 2018, 41, 156–162. [Google Scholar] [CrossRef]
- Chanturiya, V.; Minenko, V.; Samusev, A.; Chanturia, E.; Koporulina, E.; Bunin, I.; Ryazantseva, M. The Effect of Energy Impacts on the Acid Leaching of Eudialyte Concentrate. Miner. Process. Extr. Met. Rev. 2021, 42, 484–495. [Google Scholar] [CrossRef]
- Mubula, Y.; Yu, M.; Yang, D.; Lin, B.; Guo, Y.; Qiu, T. Recovery of valuable elements from solid waste with the aid of external electric field: A review. J. Environ. Chem. Eng. 2023, 11, 111237. [Google Scholar] [CrossRef]
- Esmaeili, M.; Rastegar, S.; Beigzadeh, R.; Gu, T. Ultrasound-assisted leaching of spent lithium ion batteries by natural organic acids and H2O2. Chemosphere 2020, 254, 126670. [Google Scholar] [CrossRef]
- Xiao, X.; Hoogendoorn, B.W.; Ma, Y.; Sahadevan, S.A.; Gardner, J.M.; Forsberg, K.; Olsson, R.T. Ultrasound-assisted extraction of metals from Lithium-ion batteries using natural organic acids. Green Chem. 2021, 23, 8519–8532. [Google Scholar] [CrossRef]
- Ding, W.; Bao, S.; Zhang, Y.; Xiao, J. Mechanism and kinetics study on ultrasound assisted leaching of gallium and zinc from corundum flue dust. Miner. Eng. 2022, 183, 107624. [Google Scholar] [CrossRef]
- Hosseinzadeh, F.; Rastegar, S.O.; Ashengroph, M.; Gu, T. Ultrasound-assisted Fenton-like reagent to leach precious metals from spent automotive catalysts: Process optimization and kinetic modeling. Int. J. Environ. Sci. Technol. 2021, 18, 3449–3458. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, S.; Chen, Y.; Xiang, D.; Zhang, L.; Liu, J.; Ye, J. Ultrasound enhanced in-situ chemical oxidation for leaching Ag from zinc leaching residue and response surface optimization. Chem. Eng. J. 2024, 489, 151243. [Google Scholar] [CrossRef]
- Kong, J.; Xing, P.; Wei, D.; Jin, X.; Zhuang, Y. Ultrasound-Assisted Leaching of Iron from Silicon Diamond-Wire Saw Cutting Waste. JOM 2021, 73, 791–800. [Google Scholar] [CrossRef]
- Xie, H.; Li, S.; Guo, Z.; Xu, Z. Extraction of lead from electrolytic manganese anode mud by microwave coupled ultrasound technology. J. Hazard. Mater. 2021, 407, 124622. [Google Scholar] [CrossRef]
- Oza, R.; Shah, N.; Patel, S. Recovery of nickel from spent catalysts using ultrasonication-assisted leaching. J. Chem. Technol. Biotechnol. 2011, 86, 1276–1281. [Google Scholar] [CrossRef]
- Wang, X.; Yang, D.-J.; Srinivasakannan, C.; Peng, J.-H.; Duan, X.-H.; Ju, S.-H. A Comparison of the Conventional and Ultrasound-Augmented Leaching of Zinc Residue Using Sulphuric Acid. Arab. J. Sci. Eng. 2014, 39, 163–173. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Zhang, Y.; Zhang, L.; Kong, D. Synergistic mechanism and decopperization kinetics for copper anode slime via an integrated ultrasound-sodium persulfate process. Appl. Surf. Sci. 2022, 589, 153032. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, F.; Ma, Y. Ultrasonic recovery of copper and iron through the simultaneous utilization of Printed Circuit Boards (PCB) spent acid etching solution and PCB waste sludge. J. Hazard. Mater. 2011, 185, 155–161. [Google Scholar] [CrossRef]
- Wang, S.; Cui, W.; Zhang, G.; Zhang, L.; Peng, J. Ultra fast ultrasound-assisted decopperization from copper anode slime. Ultrason. Sonochemistry 2017, 36, 20–26. [Google Scholar] [CrossRef]
- Turan, M.D.; Sari, Z.A.; Demiraslan, A. Ultrasound-assisted leaching and kinetic study of blended copper slag. Met. Mater. Trans. B 2019, 50, 1949–1956. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Lee, M.S. Improvement of Alumina Dissolution from the Mechanically Activated Dross Using Ultrasound-Assisted Leaching. Korean J. Met. Mater. 2019, 57, 154–161. [Google Scholar] [CrossRef]
- Li, X.; Xing, P.; Du, X.; Gao, S.; Chen, C. Influencing factors and kinetics analysis on the leaching of iron from boron carbide waste-scrap with ultrasound-assisted method. Ultrason. Sonochemistry 2017, 38, 84–91. [Google Scholar] [CrossRef]
- Masoum, H.G.; Rastegar, S.O.; Khamforoush, M. Ultrasound-Assisted Leaching of Vanadium and Yttrium from Coal Ash: Optimization, Kinetic and Thermodynamic Study. Chem. Eng. Technol. 2021, 44, 2249–2256. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.-P. Effect of ultrasound on bioleaching of hydrodesulphurization spent catalyst. Environ. Technol. Innov. 2019, 14, 100310. [Google Scholar] [CrossRef]
- Sadeghi, S.M.; Jesus, J.; Pinto, E.; Almeida, A.A.; Soares, H.M. A simple, efficient and selective process for recycling La (and Al) from fluid cracking catalysts using an environmentally friendly strategy. Miner. Eng. 2020, 156, 106375. [Google Scholar] [CrossRef]
- Behera, S.; Panda, S.K.; Mandal, D.; Parhi, P. Ultrasound and Microwave assisted leaching of neodymium from waste magnet using organic solvent. Hydrometallurgy 2019, 185, 61–70. [Google Scholar] [CrossRef]
- Jiang, F.; Chen, Y.; Ju, S.; Zhu, Q.; Zhang, L.; Peng, J.; Wang, X.; Miller, J.D. Ultrasound-assisted leaching of cobalt and lithium from spent lithium-ion batteries. Ultrason. Sonochemistry 2018, 48, 88–95. [Google Scholar] [CrossRef]
- Ning, P.; Meng, Q.; Dong, P.; Duan, J.; Xu, M.; Lin, Y.; Zhang, Y. Recycling of cathode material from spent lithium ion batteries using an ultrasound-assisted DL-malic acid leaching system. Waste Manag. 2020, 103, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Jiang, T.; Gao, H.; Liu, Y.; Zheng, X.; Xue, X. Comparison of Ultrasound-Assisted and Regular Leaching of Vanadium and Chromium from Roasted High Chromium Vanadium Slag. JOM 2018, 70, 155–160. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Waste Catalyst Utilization: Extraction of Valuable Metals from Spent Hydroprocessing Catalysts by Ultrasonic-Assisted Leaching with Acids. Ind. Eng. Chem. Res. 2011, 50, 9495–9501. [Google Scholar] [CrossRef]
- Pinto, I.S.; Soares, H.M. Selective leaching of molybdenum from spent hydrodesulphurisation catalysts using ultrasound and microwave methods. Hydrometallurgy 2012, 129–130, 19–25. [Google Scholar] [CrossRef]
- Wang, L.; Chao, L.; Qu, W.; Xu, S.; Zhang, L.; Peng, J.; Ye, X. Ultrasound-assisted oil removal of γ-Al2O3-based spent hydrodesulfurization catalyst and microwave roasting recovery of metal Mo. Ultrason. Sonochemistry 2018, 49, 24–32. [Google Scholar] [CrossRef]
- Toache-Pérez, A.D.; Bolarín-Miró, A.M.; Jesús, F.S.-D.; Lapidus, G.T. Facile method for the selective recovery of Gd and Pr from LCD screen wastes using ultrasound-assisted leaching. Sustain. Environ. Res. 2020, 30, 20. [Google Scholar] [CrossRef]
- Tunsu, C.; Ekberg, C.; Retegan, T. Characterization and leaching of real fluorescent lamp waste for the recovery of rare earth metals and mercury. Hydrometallurgy 2014, 144–145, 91–98. [Google Scholar] [CrossRef]
- Zhang, L.; Song, Q.; Xu, X.; Xu, Z. Process simulation of Ohno continuous casting for single crystal copper prepared from scrap copper in waste printed circuit boards. Waste Manag. 2021, 124, 94–101. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Y.; Xu, Z. Eddy current separation technology for recycling printed circuit boards from crushed cell phones. J. Clean. Prod. 2017, 141, 1316–1323. [Google Scholar] [CrossRef]
- Niu, B.; E, S.; Song, Q.; Xu, Z.; Han, B.; Qin, Y. Physicochemical reactions in e-waste recycling. Nat. Rev. Chem. 2024, 8, 569–586. [Google Scholar] [CrossRef]


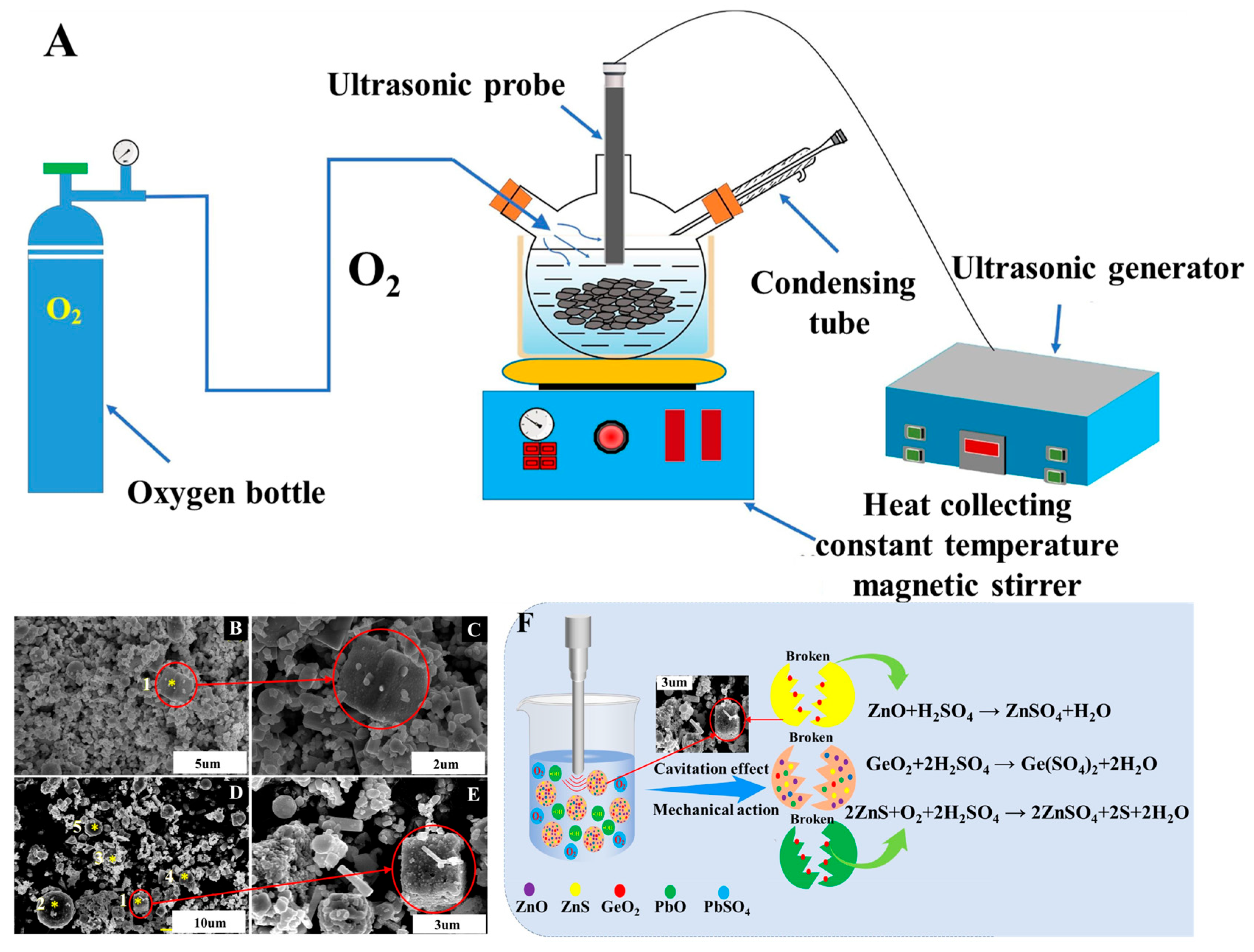
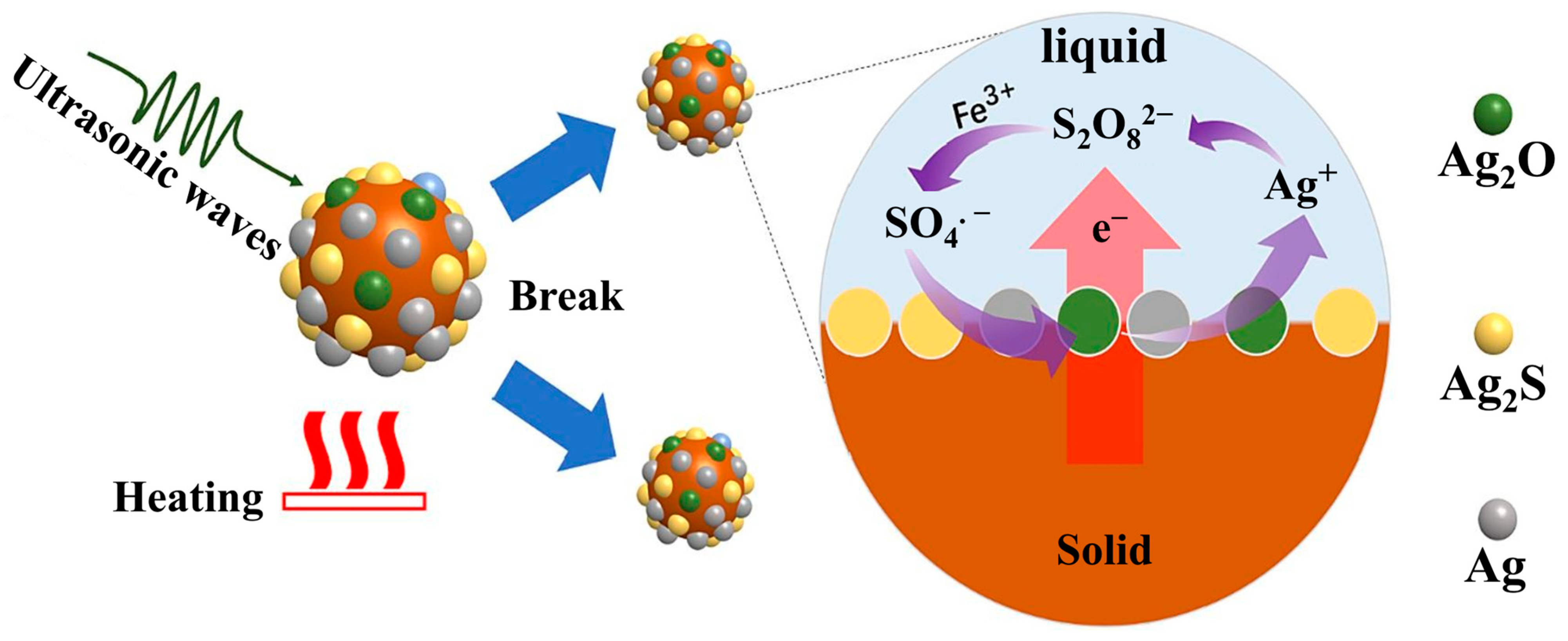
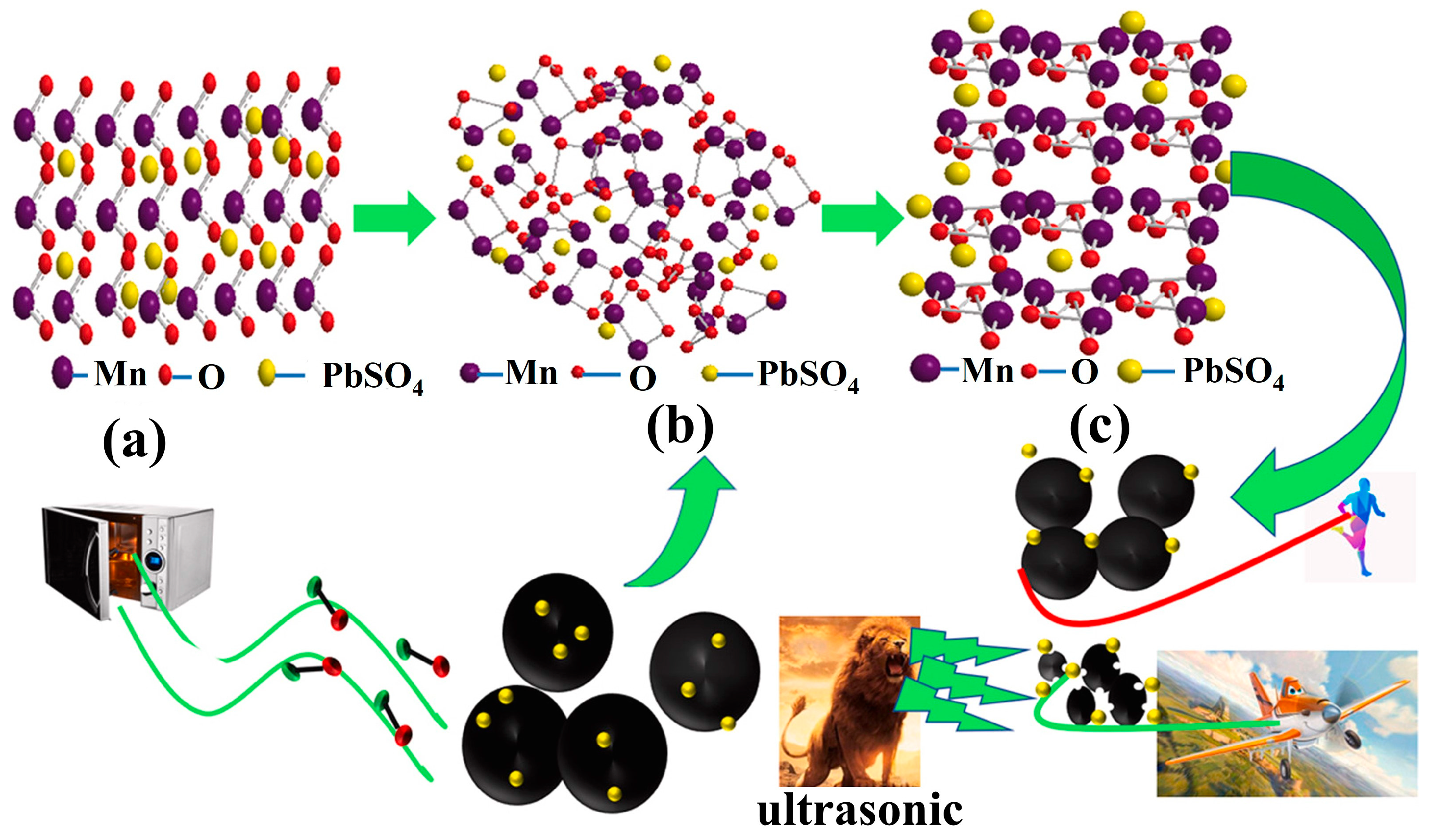

| Target Elements | Raw Materials | Chemical Reagents | Temperature | Time | Ultrasonic Power/Frequency | Leaching Rate | Ref. |
|---|---|---|---|---|---|---|---|
| Ni | Nickel sulfide ore | Na2S2O8-AgNO3 | 70 °C | 120 min | 220 W | 82.95% | [41] |
| Nickel block | H2SO4-H2O2 | 60 °C | 240 min | 200 W | 60.41% | [42] | |
| Nickel laterite ore | H2SO4 | 95 °C | 10 min | 720 W | 96.18% | [29] | |
| Zn | Zinc oxide ore | NH3–(NH4)2SO4 | 30 °C | 60 min | 600 W | 83.33% | [47] |
| Zinc oxide ore | NH3-C6H5O7(NH4)3 | 25 °C | 120 min | 600 W | 88.57% | [48] | |
| Zinc oxide ore | H2SO4 | 60 °C | 90 min | 150 W | 75% | [43] | |
| Cu | Chalcopyrite | Fe2(SO4)3-H2SO4 | 80 °C | 300 min | 80 W | 57.5% | [46] |
| Deep-sea manganese nodules | (NH4)2S2O3 | 85 °C | 90 min | 100 W | 93% | [49] | |
| Al | Quartz | Na2CO3 | 80 °C | 25 min | 150 W | 42.3% | [50] |
| Fe | Quartz sand | H3PO4 | 80 °C | 240 min | 150 W | 77.1% | [51] |
| Silica sand | H2C2O4 | 95 °C | 30 min | 150 W | 75.4% | [52] |
| Target Elements | Raw Materials | Chemical Reagents | Temperature | Time | Ultrasonic Power/Frequency | Leaching Rate | Ref. |
|---|---|---|---|---|---|---|---|
| V | Vanadium-bearing shale | H2SO4-CaF2 | 95 °C | 30 min | 900 W | 92.93% | [40] |
| W | Scheelite | Na3CO3 | 90 °C | 450 min | 1000 W | 22.5% | [54] |
| Scheelite | HNO3 | 80 °C | 360 min | 131 kWh/kg | 71.5% | [55] | |
| Scheelite | NaOH | 90 °C | 146 min | 176 W | 90% | [59] | |
| Au | Refractory gold ores | NaClO-NaOH | 30 °C | 120 min | 200 W | 68.55% | [56] |
| Refractory gold ores | HCl-Cl2 | 50 °C | 120 min | 300 W | 50% | [60] | |
| Refractory gold ore | Na3(CN)3C3H3N6O3 | 80 °C | 240 min | 480 W | 93.52% | [57] | |
| Ag | Refractory gold ore | Na3(CN)3C3H3N6O3 | 80 °C | 240 min | 480 W | 61.25% | [57] |
| Refractory silver ores | NaCN | 30 °C | 2880 min | 100 W | 90% | [58] | |
| REEs | Weathered crust elution- deposited ore | MgSO4 | 25 °C | 30 min | 700 W | 99% | [61] |
| Eudialyte concentrate | HNO3 | 80 °C | 140 min | 22 KHz | 94.5% | [62] |
| Target Elements | Raw Materials | Chemical Reagents | Temperature | Time | Ultrasonic Power/Frequency | Leaching Rate | Ref. |
|---|---|---|---|---|---|---|---|
| Ni | Spent catalysts | HNO3 | 90 °C | 50 min | 30 KHz | 95% | [71] |
| Spent lithium-ion batteries (LIBs) | lemon juice–H2O2 | 40 °C | 35 min | 37 KHz | 100% | [64] | |
| Spent LIBs | citric or acetic | 50 °C | 1440 min | 110 W | 99% | [65] | |
| Zn | Electric arc furnace dust | H2SO4 | 80 °C | 30 min | 60 W | 90% | [8] |
| Corundum flue dust | H2SO4 | 90 °C | 50 min | 900 W | 99.57% | [66] | |
| Zinc residue | H2SO4 | 65 °C | 180 min | 160 W | 80% | [72] | |
| Cu | Copper anode slime | H2SO4- Na2S2O8 | 50 °C | 50 min | 400 W | 98.11% | [73] |
| Print circuit boards | spent etching solution | 25 °C | 30 min | 300 W | 93.76% | [74] | |
| Copper anode slime | Ultrasound Lixiviant: H2SO4 | Room temperature | 600 min | 800 W | 85.18% | [75] | |
| Blended copper slag | Lixiviant: H2O2- CH3COOH | 65 °C | 60 min | 10% ultrasound power | 93% | [76] | |
| Al | Spent automotive catalysts | FeSO4-H2O2 | 70 °C | 40 min | 37 KHz | 81.7% | [67] |
| Aluminum dross | NaOH | 50 °C | 240 min | 100 W | 60% | [77] | |
| Fe | Silicon diamond wire saw cutting waste | H2SO4 | 60 °C | 50 min | 270 W | 95.24% | [69] |
| Boron carbide waste- scrap | H2SO4 | 50 °C | 50 min | 210 W | 94.5% | [78] |
| Target Elements | Raw Materials | Chemical Reagents | Temperature | Time | Ultrasonic Power/Frequency | Leaching Rate | Ref. |
|---|---|---|---|---|---|---|---|
| Li | Spent LIBs | H2SO4-H2O2 | 30 °C | 30 min | 360 W | 98.62% | [83] |
| Spent LIBs | DL-malic acid-H2O2 | 80 °C | 30 min | 90 W | 98% | [84] | |
| V | Chromium-vanadium slag | H2SO4 | 60 °C | 60 min | 800 W | 90.89% | [85] |
| Coal fly ash | H2SO4-H2O2 | 50 °C | 60 min | 60 KHz | 100% | [79] | |
| Mo | Spent hydroprocessing catalysts | Critic acid | 60 °C | 360 min | 320 W | 95% | [86] |
| Spent hydrodesulphurization catalysts | NaOH | 80 °C | 10 min | 200 W | 66% | [87] | |
| Spent hydrodesulphurization catalysts | Na2CO3 | 55 °C | 120 min | 600 W | 94.3% | [88] | |
| REEs | Spent fluid cracking catalysts | HCl | 60 °C | 60 min | 200 W | 97.1% | [81] |
| LCD screen wastes | P2O74- | 30 °C | 60 min | 120 W | 85% | [89] | |
| Fluorescent lamp waste | HNO3 | 20 °C | 1440 min | 120 W | 95% | [90] | |
| Waste magnet | CH3COOH | 30 °C | 120 min | 90 W | 99.99% | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubula, Y.; Yu, M.; Niu, H.; Zhu, Z.; Xu, K. Application of Ultrasonic-Enhanced Leaching for the Recovery of Metal Elements from Mineral Raw Materials and Secondary Resources. Metals 2025, 15, 1115. https://doi.org/10.3390/met15101115
Mubula Y, Yu M, Niu H, Zhu Z, Xu K. Application of Ultrasonic-Enhanced Leaching for the Recovery of Metal Elements from Mineral Raw Materials and Secondary Resources. Metals. 2025; 15(10):1115. https://doi.org/10.3390/met15101115
Chicago/Turabian StyleMubula, Yusufujiang, Mingming Yu, Heyue Niu, Zhehan Zhu, and Kun Xu. 2025. "Application of Ultrasonic-Enhanced Leaching for the Recovery of Metal Elements from Mineral Raw Materials and Secondary Resources" Metals 15, no. 10: 1115. https://doi.org/10.3390/met15101115
APA StyleMubula, Y., Yu, M., Niu, H., Zhu, Z., & Xu, K. (2025). Application of Ultrasonic-Enhanced Leaching for the Recovery of Metal Elements from Mineral Raw Materials and Secondary Resources. Metals, 15(10), 1115. https://doi.org/10.3390/met15101115






