Vacuum Diffusion Bonding Process Optimization for the Lap Shear Strength of 7B04 Aluminum Alloy Joints with a 7075 Aluminum Alloy Powder Interlayer Using the Response Surface Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Treatment
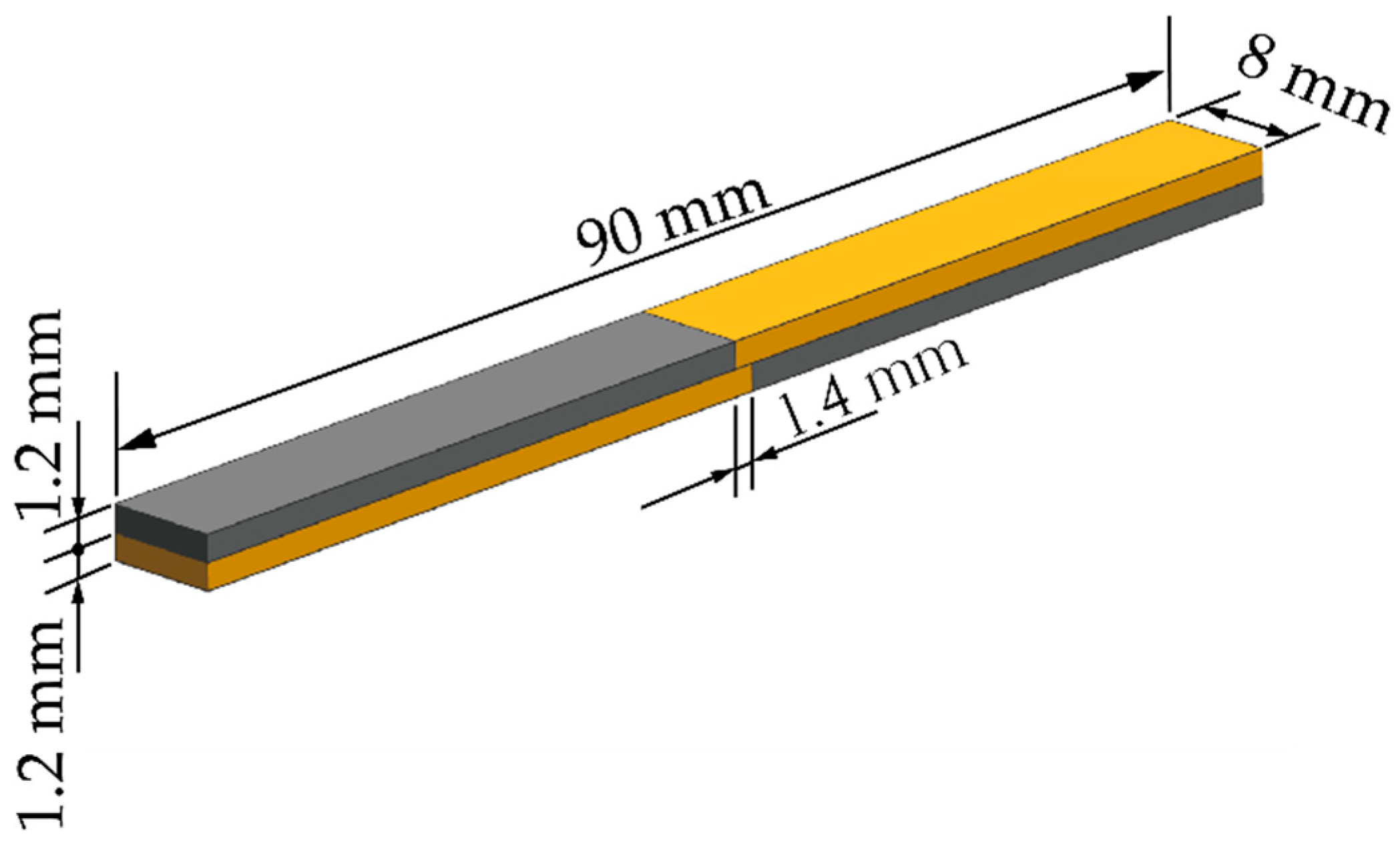
2.3. Diffusion Bonding Experiment
2.4. Microstructure Characterization
2.5. Mechanical Test
3. Results and Discussion
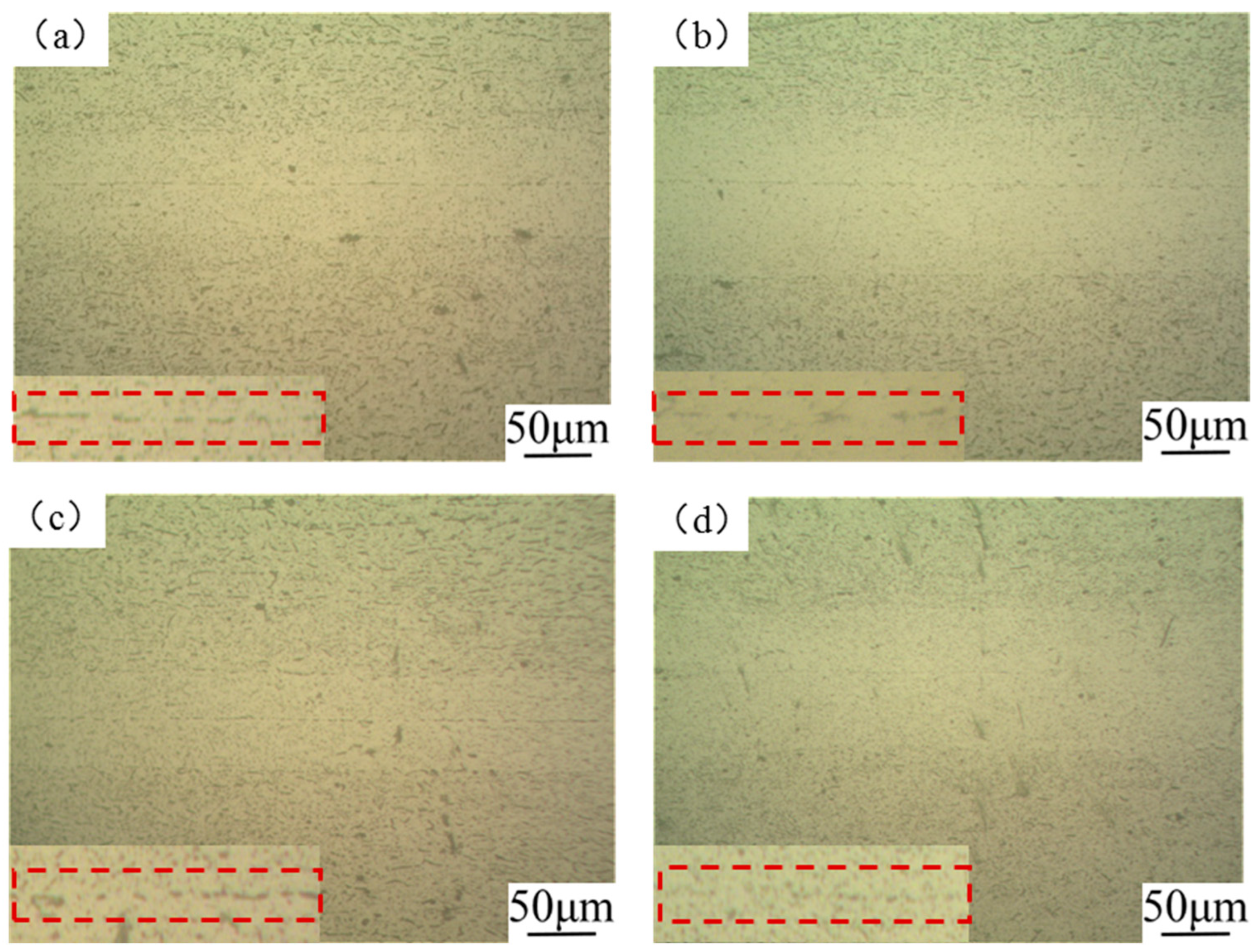
3.1. Effect of Surface Treatment on DB Joint Performance
3.1.1. Physics/Chemistry Cleaning
3.1.2. Plasma Treatment
3.2. DB Process Parmeter Optimization with RSM
3.2.1. Establishment of LSS Prediction Model
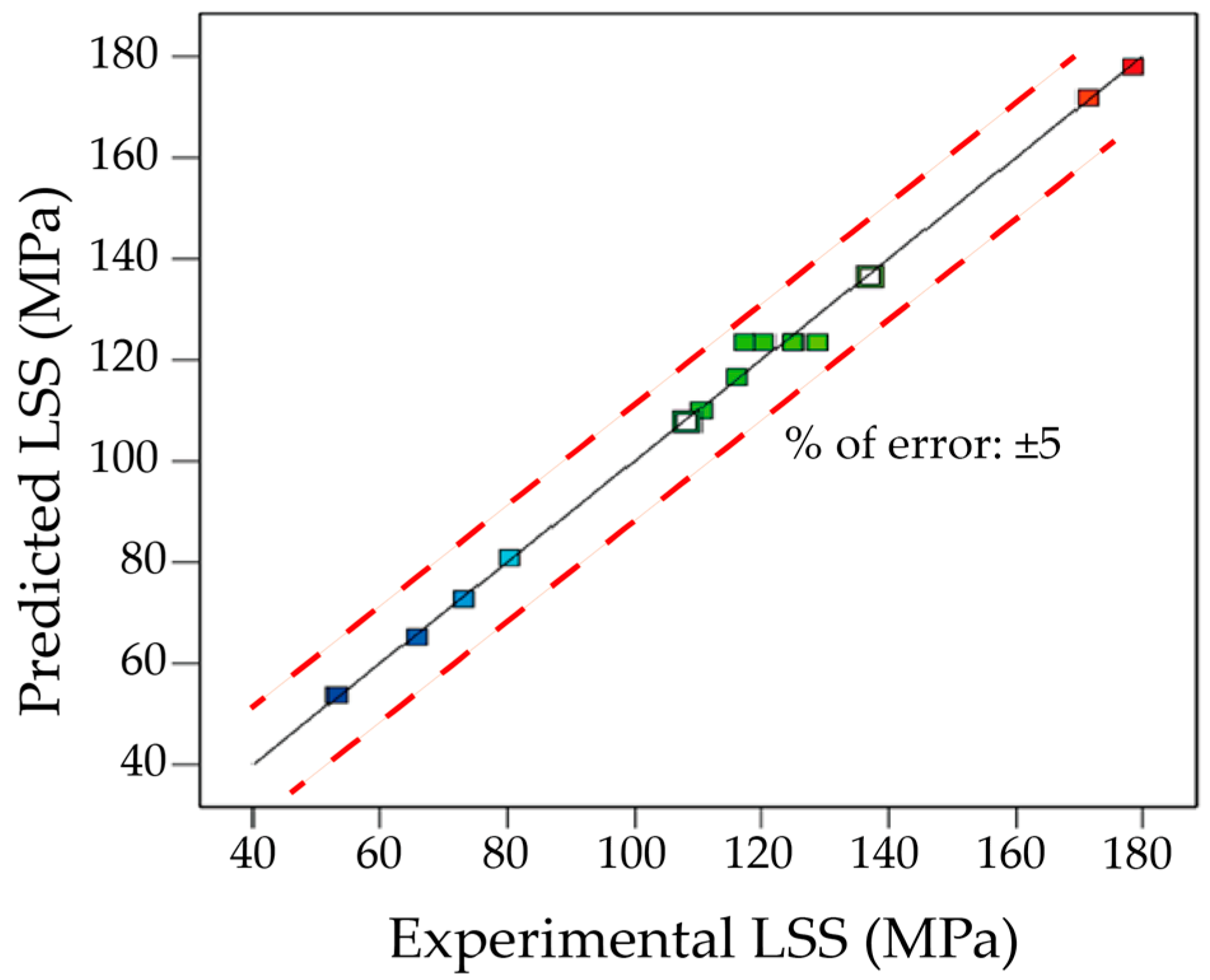
3.2.2. Reliability Assessment of the LSS Prediction Model
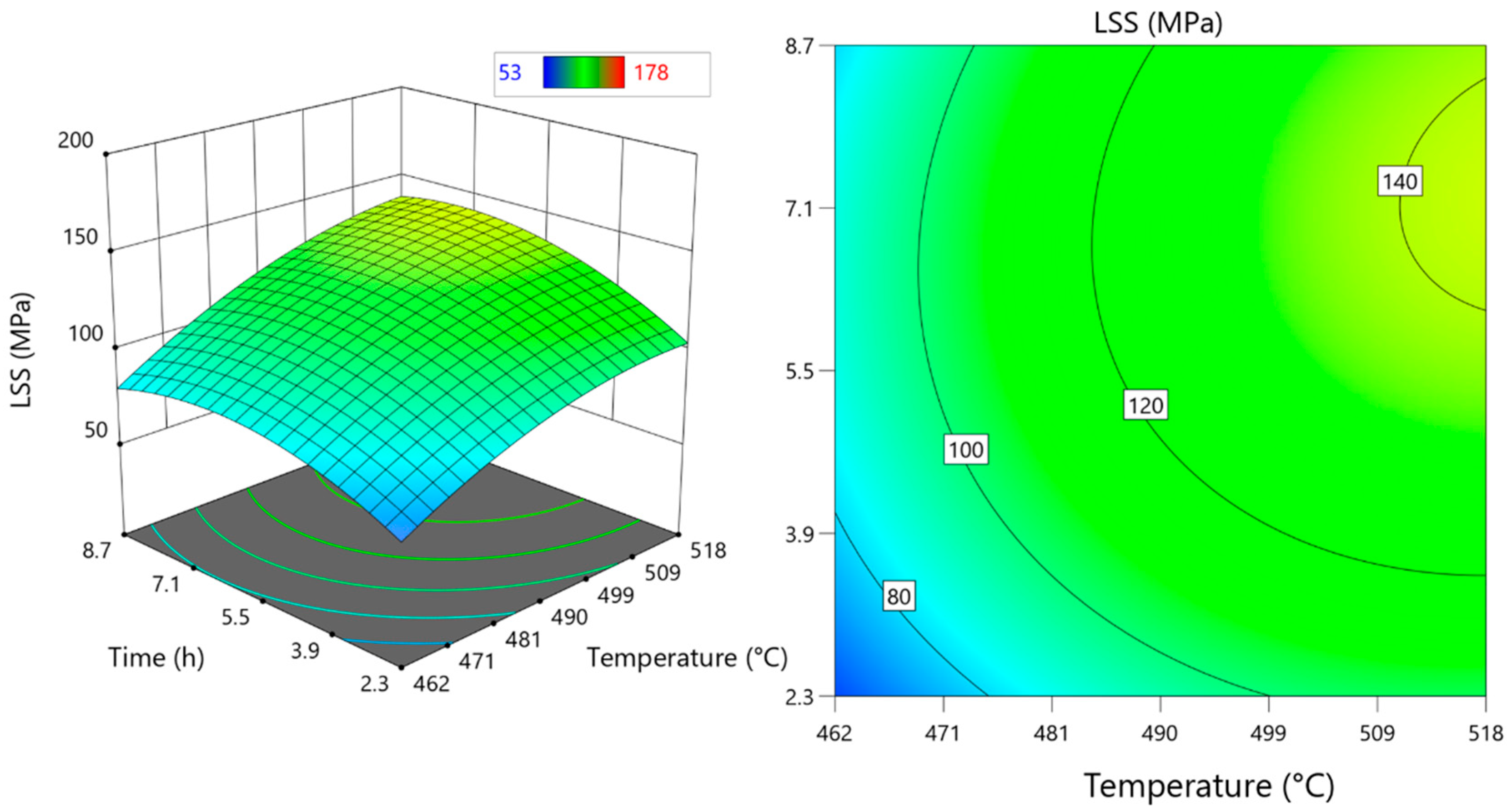
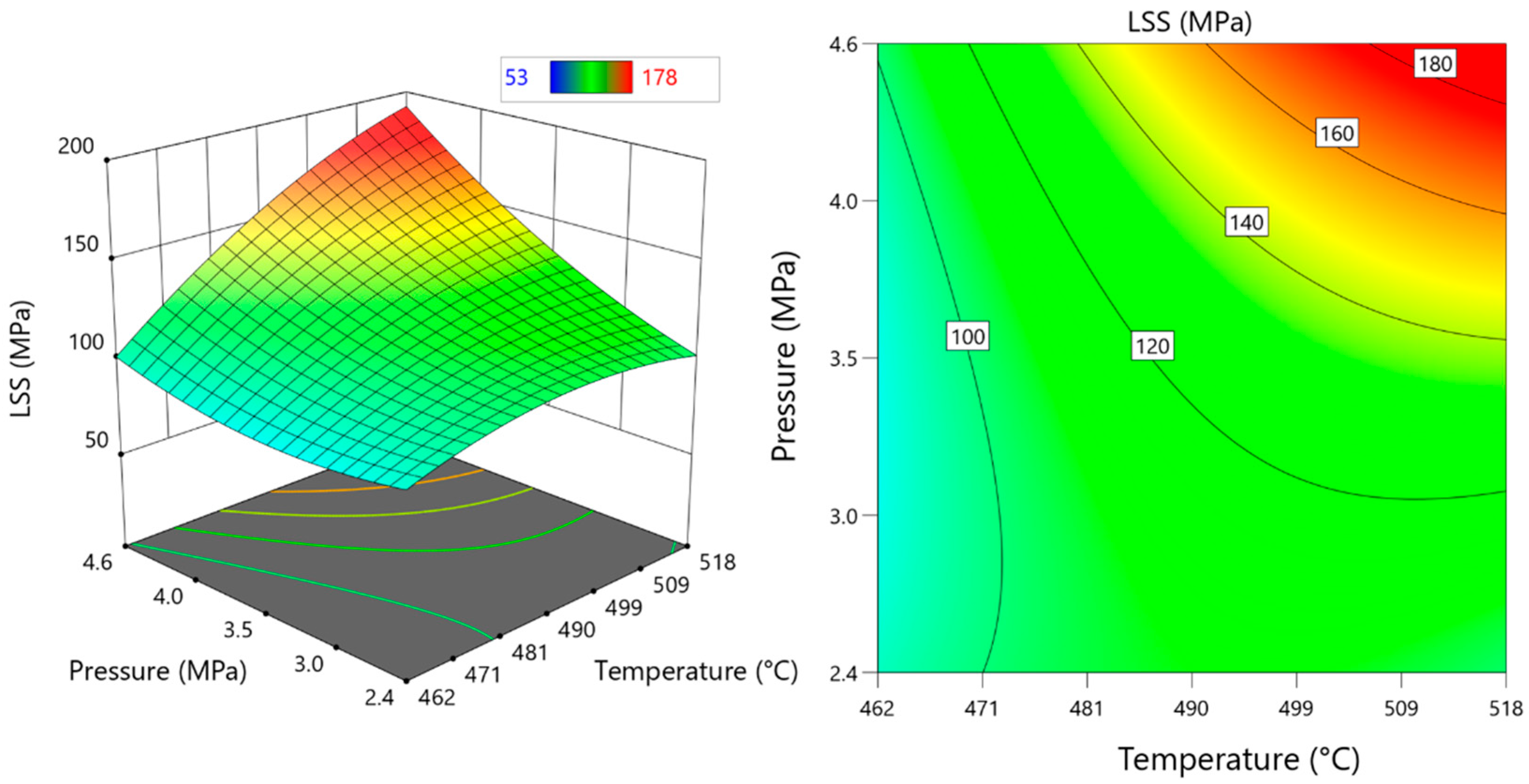
3.2.3. Effects of DB Parameters on LSS
- Temperature effect on LSS
- Effect of time on LSS
- The effect of pressure on LSS
3.2.4. Optimization and Verification of the DB Process Parameter
Di = ((Yi − Li)/(Hi − Li))Wti Li ≤ Yi ≤ Hi
1 Yi > Hi
Di = ((Hi − Yi)/(Hi − Li))Wti Li ≤ Yi ≤ Hi
1 Yi < Li
Di = ((Yi − Hi)/(Ti − Hi))Wti2 Ti ≤ Yi ≤ Hi
0 other
Di = 0 other
4. Conclusions
- A comparative analysis of the effects of the surface treatment process on the performance of the diffusion joint shows no significant difference in the welding microstructure of the base material with different surface roughness levels, ranging from 400 # to 1500 # sandpaper polish. The plasma treatment enhanced diffusion bonding. Polishing with 1500 # sandpaper combined with plasma treatment results in the best overall joint performance.
- Based on experiments designed by CCD with RSM, a quadratic regression model was established relating process parameters—temperature, pressure, and time—to the response parameter LSS, with a prediction accuracy of 99.52%. The model demonstrates high reliability and can be used to predict LSS for the DB joint of 7B04 aluminum alloy.
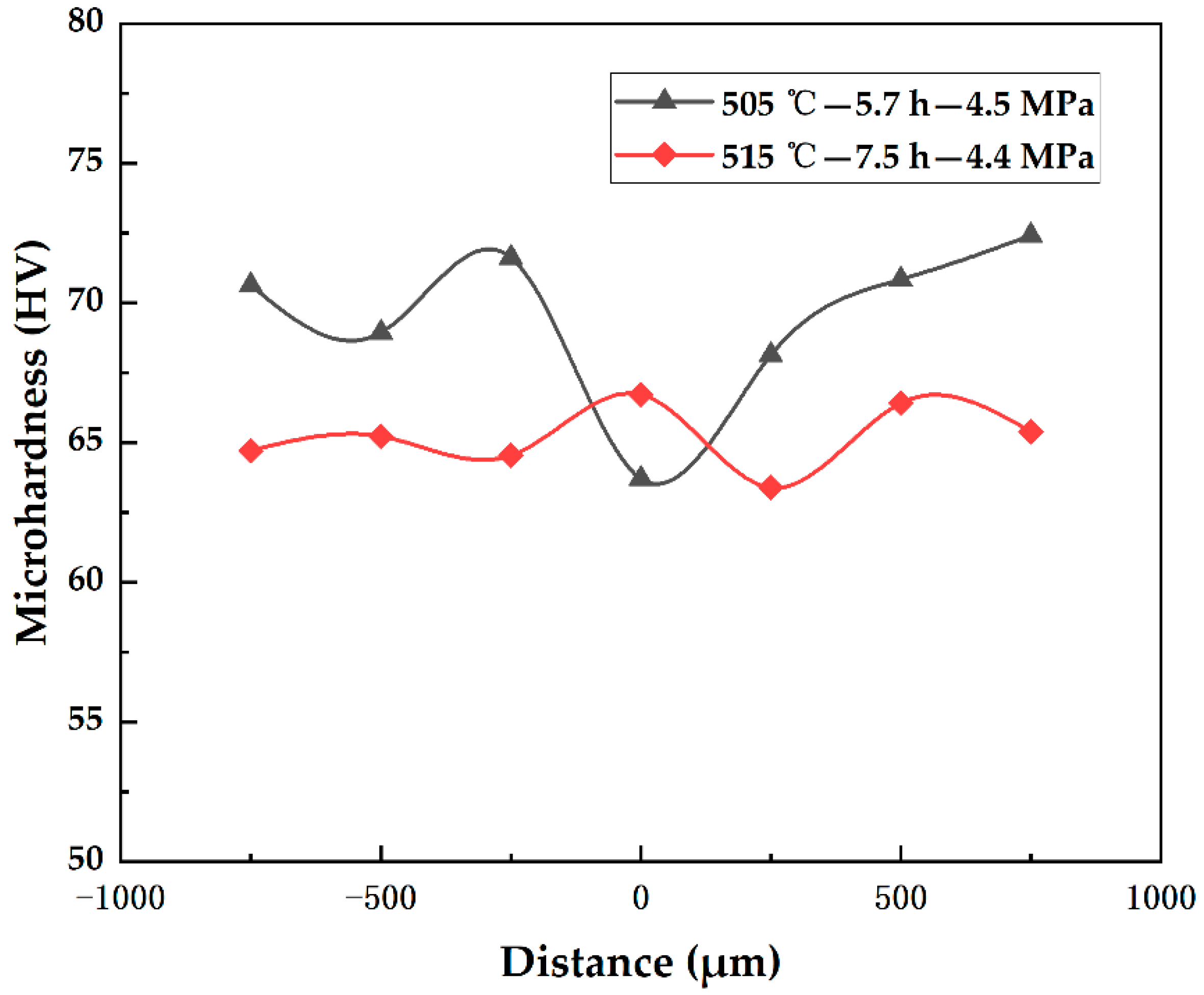
- The satisfaction function optimization method was used to optimize the process parameters. The results were then experimentally verified, showing good agreement with the actual measured values, with an error of approximately 5%. The optimal process parameters for diffusion bonding 7B04 aluminum alloy were found to be 515 °C-7.5 h-4.4 MPa.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DB | Diffusion bonding |
| LSS | Lap shear strength |
| RSM | Response surface method |
| IMC | Intermetallic compounds |
| SPS | Spark plasma sintering |
| GA | Genetic algorithm |
| CCD | Central composite design |
| ANOVA | Analysis of variance |
| CV | Coefficient of variation |
References
- Wang, Y.; Chai, P.; Guo, X.; Qi, B. Effect of Connection Processes on Mechanical Properties of 7B04 Aluminum Alloy Structures. China Weld. 2021, 30, 50–57. [Google Scholar] [CrossRef]
- Du, J.; Wang, L.; Li, J.; Cui, L.; Jiang, X. The Influence of Pre-Strain Levels on the Microstructure and Performance of Aircraft 7b04 Aluminum Alloy Skin. J. Phys. Conf. Ser. 2024, 2691, 012059. [Google Scholar] [CrossRef]
- Lao, S.; Zhan, L.; Qian, W.; Xu, Y.; Ma, B.; Liu, C.; Huang, M.; Yang, Y.; Chen, K.; Peng, N.; et al. Creep Aging Behavior of a Thermo-Mechanical Treated 7B04 Aluminum Alloy. Metals 2023, 13, 182. [Google Scholar] [CrossRef]
- Ramaswamy, A.; Malarvizhi, S.; Balasubramanian, V. Effect of Variants of Gas Metal Arc Welding Process on Tensile Properties of AA6061-T6 Aluminium Alloy Joints. Int. J. Adv. Manuf. Technol. 2020, 108, 2967–2983. [Google Scholar] [CrossRef]
- John, V.C.; Abdel-Hamid, I.M.; Muhammad, M.S.; Shivamurthy, B. Review of Recent Trends in Friction Stir Welding Process of Aluminum Alloys and Aluminum Metal Matrix Composites. Trans. Nonferrous Met. Soc. China 2021, 31, 3281–3309. [Google Scholar] [CrossRef]
- Ardika, R.D.; Triyono, T.; Muhayat, N.; Triyono. A Review Porosity in Aluminum Welding. Procedia Struct. Integr. 2021, 33, 171–180. [Google Scholar] [CrossRef]
- Lathashankar, B.; Tejaswini, G.C.; Suresh, R.; Swamy, N.H.S. Advancements in Diffusion Bonding of Aluminium and Its Alloys: A Comprehensive Review of Similar and Dissimilar Joints. Adv. Mater. Process. Technol. 2022, 8, 4659–4677. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Bai, Y.; Liu, Y.; Zhu, Z.; Chen, H. The Influence of Hydrogen Content on the Zr-4 Alloy Diffusion Bonding Joint: From Experiments to Molecular Dynamics Simulations. Mater. Today Commun. 2024, 40, 109568. [Google Scholar] [CrossRef]
- Li, Q.; Liu, M.; Hou, B.; Qin, H.; Dai, Z. Diffusion Welding Process and Welding Properties of 6061 Aluminum Alloy Water-cooled Plate. Mater. Res. Appl. 2018, 12, 303–307. [Google Scholar]
- Aravinda, T.; Niranjan, B.H.; Babu, S.B.; Ravi, U.M. Solid State Diffusion Bonding Process-A Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1013, 012011. [Google Scholar] [CrossRef]
- Chang, R.; Guo, Q.; Ma, Z.; Ding, R.; Liu, C.; Liu, Y. Kinetics of Voids Evolution during Diffusion Bonding of Dissimilar Metals on Consideration of the Realistic Surface Morphology: Modeling and Experiments. Acta Mater. 2024, 276, 120121. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, S.; Jia, L.; Li, Q.; Ma, M. Study on the Influence of Surface Roughness and Temperature on the Interface Void Closure and Microstructure Evolution of Stainless Steel Diffusion Bonding Joints. Metals 2024, 14, 812. [Google Scholar] [CrossRef]
- Li, H.; Li, M.Q.; Kang, P.J. Void Shrinking Process and Mechanisms of the Diffusion Bonded Ti–6Al–4V Alloy with Different Surface Roughness. Appl. Phys. A 2016, 122, 18. [Google Scholar] [CrossRef]
- Aravinda, T.; Niranjan, H.B.; Boppana, S.B.; Dayanand, R.S. Solid State Diffusion Bonding of Al2024 Sheets. J. Mines Met. Fuels 2021, 69, 455–460. [Google Scholar]
- Zhang, J.; Zhou, Y.; Liu, X.; Niu, J. Effects of Surface Treatment on the Diffusion Bonding of LF6 Alloy. Mater. Sci. Technol. 1995, 3, 108–111. [Google Scholar]
- Kenevisi, M.S.; Mousavi Khoie, S.M.; Alaei, M. Microstructural Evaluation and Mechanical Properties of the Diffusion Bonded Al/Ti Alloys Joint. Mech. Mater. 2013, 64, 69–75. [Google Scholar] [CrossRef]
- Kurgan, N. Investigation of the Effect of Diffusion Bonding Parameters on Microstructure and Mechanical Properties of 7075 Aluminium Alloy. Int. J. Adv. Manuf. Technol. 2014, 71, 2115–2124. [Google Scholar] [CrossRef]
- Liu, D.; Xu, J.; Li, X.; Wei, P.; Liang, Y.; Qin, J.; Sun, H.; Ding, T.; Ding, Z.; Zhong, S.; et al. Influence of Al Foil Interlayer on Performance of Vacuum Diffusion Bonding Joint of 6061 Aluminium Alloy. J. Iron Steel Res. Int. 2024, 31, 2404–2412. [Google Scholar] [CrossRef]
- Song, K.; Lv, L.; Zhu, S.; Liu, F.; Luo, J.; Qing, Z.; Zhong, Z.; Zhu, Z.; Wu, Y. Microstructure and Mechanical Properties of Spark Plasma Diffusion-Bonded 5A06Al Joints with Al–20Cu–5Si–2Ni Interlayer. Int. J. Adv. Manuf. Technol. 2021, 114, 3627–3643. [Google Scholar] [CrossRef]
- Hosseinabadi, N.; Sarraf-Mamoory, R.; Mohammad Hadian, A. Diffusion Bonding of Alumina Using Interlayer of Mixed Hydride Nano Powders. Ceram. Int. 2014, 40, 3011–3021. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, W.; Zhang, B.; Mao, C.; Wang, X.; Pan, W.; Du, J.; Xiao, H. Enhanced Interfacial Properties and Deformation Behavior of Ti/Al Composite Plates through Pure Al Interlayer-Assisted Diffusion Bonding. Mater. Sci. Eng. A 2025, 944, 148947. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, T.; Zhao, Y.; Zhang, Z.; Shao, W.; Huang, J.; Zhang, X.; Ye, Z.; Wang, W.; Yang, J. Microstructure and Mechanical Properties of Copper/al Interlayer/Graphite Joint Prepared Utilizing “Two-Stage Method” Vacuum Diffusion Bonding. Vacuum 2024, 227, 113414. [Google Scholar] [CrossRef]
- Yin, F.; Liu, C.; Zhang, Y.; Qin, Y.; Liu, N. Effect of Ni Interlayer on Characteristics of Diffusion Bonded Mg/Al Joints. Mater. Sci. Technol. 2018, 34, 1104–1111. [Google Scholar] [CrossRef]
- Silva, M.; Ramos, A.S.; Simões, S. Joining Ti6Al4V to Alumina by Diffusion Bonding Using Titanium Interlayers. Metals 2021, 11, 1728. [Google Scholar] [CrossRef]
- Alhazaa, A.; Albrithen, H.; Hezam, M.; Shar, M.A.; Alhwaimel, I.; Alharbi, Y.; Estournes, C. Diffusion Bonding of Al7075 to Ti-6Al-4V by Spark Plasma Sintering and Using a Copper Interlayer. Crystals 2022, 12, 1293. [Google Scholar] [CrossRef]
- Rajakumar, S.; Balasubramanian, V. Diffusion Bonding of Titanium and AA 7075 Aluminum Alloy Dissimilar Joints—Process Modeling and Optimization Using Desirability Approach. Int. J. Adv. Manuf. Technol. 2016, 86, 1095–1112. [Google Scholar] [CrossRef]
- Fernandus, M.J.; Senthilkumar, T.; Balasubramanian, V.; Rajakumar, S. Optimizing Diffusion Bonding Parameters to Maximize the Strength of AA6061 Aluminum and AZ61A Magnesium Alloy Joints. Exp. Tech. 2014, 38, 21–36. [Google Scholar] [CrossRef]
- Elangovan, S.; Venkateshwaran, S.; Prakasan, K. Experimental investigations on optimization of ultrasonic welding parameters for copper to brass joints using response surface method and genetic algorithm. Int. J. Adv. Eng. Res. Stud. 2012, 1, 1–6. [Google Scholar]
- Fernandus, M.J.; Senthilkumar, T.; Balasubramanian, V.; Rajakumar, S. Optimizing Diffusion Bonding Parameters in AA6061-T6 Aluminum and AZ80 Magnesium Alloy Dissimilar Joints. J. Mater. Eng. Perform. 2012, 21, 2303–2315. [Google Scholar] [CrossRef]
- Negemiya, A.; Rajakumar, S.; Sonar, T.; Ivanov, M. Mathematical Modeling and Optimization of Vacuum Diffusion Bonding Parameters for Predicting and Enhancing the Strength of Dissimilar IN-718/MSS-410 Joints Using RSM for Power Generation Applications. J. Adv. Join. Process. 2024, 9, 100214. [Google Scholar] [CrossRef]





| Element | Zn | Mg | Cu | Cr | Fe | Mn | Si | Al |
|---|---|---|---|---|---|---|---|---|
| 7B04 | 5.0–6.5 | 1.8–2.8 | 1.4–2.0 | 0.1–0.25 | 0.05–0.25 | 0.2–0.6 | 0.1 | Bal. |
| 7075 | 5.1–6.1 | 2.1–2.9 | 1.2–2.0 | 0.16–0.28 | 0.5 | 0.3 | 0.4 | Bal. |
| Parameter | Unit | Notation | Levels | ||||
|---|---|---|---|---|---|---|---|
| −21/2 | −1 | 0 | 1 | 21/2 | |||
| Temperature | °C | T | 450 | 462 | 490 | 518 | 530 |
| Time | h | t | 1 | 2.3 | 5.5 | 8.7 | 10 |
| Pressure | MPa | P | 2 | 2.4 | 3.5 | 4.6 | 5 |
| Sr. No. | Coded Value | Actual Value | LSS (MPa) | ||||
|---|---|---|---|---|---|---|---|
| T | t | P | Temperature (°C) | Time (h) | Pressure (MPa) | ||
| 1 | 1 | 1 | −1 | 518 | 8.7 | 2.4 | 116 |
| 2 | 1 | −1 | 1 | 518 | 2.3 | 4.6 | 171 |
| 3 | −1 | 1 | 1 | 462 | 8.7 | 4.6 | 80 |
| 4 | −1 | −1 | −1 | 462 | 2.3 | 2.4 | 53 |
| 5 | 1.414 | 0 | 0 | 530 | 5.5 | 3.5 | 137 |
| 6 | −1.414 | 0 | 0 | 450 | 5.5 | 3.5 | 66 |
| 7 | 0 | 1.414 | 0 | 490 | 10 | 3.5 | 111 |
| 8 | 0 | −1.414 | 0 | 490 | 1 | 3.5 | 73 |
| 9 | 0 | 0 | 1.414 | 490 | 5.5 | 5 | 178 |
| 10 | 0 | 0 | −1.414 | 490 | 5.5 | 2 | 108 |
| 11 | 0 | 0 | 0 | 490 | 5.5 | 3.5 | 117 |
| 12 | 0 | 0 | 0 | 490 | 5.5 | 3.5 | 120 |
| 13 | 0 | 0 | 0 | 490 | 5.5 | 3.5 | 125 |
| 14 | 0 | 0 | 0 | 490 | 5.5 | 3.5 | 125 |
| 15 | 0 | 0 | 0 | 490 | 5.5 | 3.5 | 129 |
| Source | LSS | |||||
|---|---|---|---|---|---|---|
| Sum of Squares | DF | Mean Square | F-Value | P1-Value | ||
| Model | 17,262.16 | 9 | 1918.02 | 115.76 | <0.0001 | significant |
| A—Temperature | 2537.57 | 1 | 2537.57 | 153.16 | <0.0001 | |
| B—Time | 694.90 | 1 | 694.90 | 41.94 | 0.0013 | |
| C—Pressure | 2465.42 | 1 | 2465.42 | 148.80 | <0.0001 | |
| AB | 36.07 | 1 | 36.07 | 2.18 | 0.2001 | |
| AC | 816.52 | 1 | 816.52 | 49.28 | 0.0009 | |
| BC | 352.07 | 1 | 352.07 | 21.25 | 0.0058 | |
| A2 | 990.57 | 1 | 990.57 | 59.79 | 0.0006 | |
| B2 | 1990.11 | 1 | 1990.11 | 120.11 | 0.0001 | |
| C2 | 723.35 | 1 | 723.35 | 43.66 | 0.0012 | |
| Residual | 82.84 | 5 | 16.57 | |||
| Lack of Fit | 3.11 | 1 | 3.11 | 0.1560 | 0.7130 | not significant |
| Pure Error | 79.73 | 4 | 19.93 | |||
| Cor Total | 17,345.00 | 14 | ||||
| Fit Statistics | Std.Dev | 4.07 | R2 | 0.9952 | ||
| Mean | 114.02 | Adjusted R2 | 0.9866 | |||
| C.V.% | 3.57 | Predicted R2 | 0.9737 | |||
| Sr. No. | Parameter | Value | Joint | Defects |
|---|---|---|---|---|
| 1 | Temperature | 530 °C |  | Excessive plastic deformation |
| 2 | Temperature | 450 °C |  | Unbonded |
| 3 | Pressure | 5 MPa |  | Excessive plastic deformation |
| 4 | Pressure | 2 MPa |  | Unbonded |
| 5 | Time | 10 h |  | Excessive plastic deformation |
| 6 | Time | 1 h |  | Unbonded |
| Parameter | Limit | Importance | Guidelines | |
|---|---|---|---|---|
| Upper Limit | Lower Limit | |||
| Temperature (°C) | 462 | 518 | 3 | In range |
| Time (h) | 2.3 | 8.7 | 3 | In range |
| Pressure (MPa) | 2.4 | 4.6 | 3 | In range |
| LSS (MPa) | 53.2 | 178 | 3 | Maximize |
| Temperature (°C) | Time (h) | Pressure (MPa) | LSS (MPa) | Satisfaction Values | |
|---|---|---|---|---|---|
| 1 | 505 | 5.7 | 4.5 | 179 | 1.000 |
| 2 | 515 | 7.5 | 4.5 | 182 | 1.000 |
| Sr. No. | LSS (MPa) | λ | Deformation Rate (%) |
|---|---|---|---|
| 1 | 169 | 1.19 | 3.9 |
| 2 | 174 | 1.34 | 4.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Xie, L.; Chen, M. Vacuum Diffusion Bonding Process Optimization for the Lap Shear Strength of 7B04 Aluminum Alloy Joints with a 7075 Aluminum Alloy Powder Interlayer Using the Response Surface Method. Metals 2025, 15, 1109. https://doi.org/10.3390/met15101109
Wang N, Xie L, Chen M. Vacuum Diffusion Bonding Process Optimization for the Lap Shear Strength of 7B04 Aluminum Alloy Joints with a 7075 Aluminum Alloy Powder Interlayer Using the Response Surface Method. Metals. 2025; 15(10):1109. https://doi.org/10.3390/met15101109
Chicago/Turabian StyleWang, Ning, Lansheng Xie, and Minghe Chen. 2025. "Vacuum Diffusion Bonding Process Optimization for the Lap Shear Strength of 7B04 Aluminum Alloy Joints with a 7075 Aluminum Alloy Powder Interlayer Using the Response Surface Method" Metals 15, no. 10: 1109. https://doi.org/10.3390/met15101109
APA StyleWang, N., Xie, L., & Chen, M. (2025). Vacuum Diffusion Bonding Process Optimization for the Lap Shear Strength of 7B04 Aluminum Alloy Joints with a 7075 Aluminum Alloy Powder Interlayer Using the Response Surface Method. Metals, 15(10), 1109. https://doi.org/10.3390/met15101109







