Application of Thermodynamic Calculations in the Study of Slag Melting Characteristics and Aluminum Loss Control
Abstract
1. Introduction
2. Methods
3. Results and Discussion
3.1. The Influence of Slag Composition on the Melting Characteristics of Slag
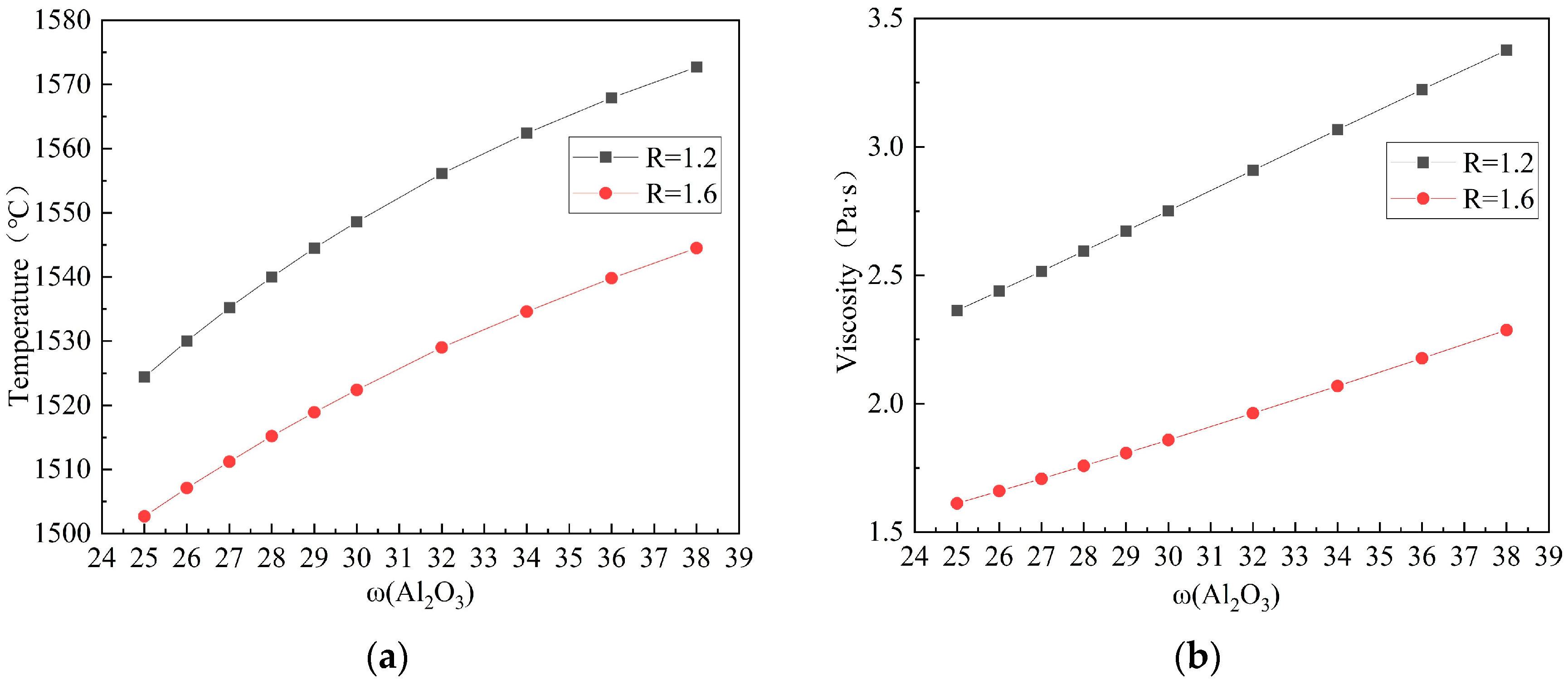
3.1.1. Effect of Al2O3 on the Melting Temperature and Viscosity of Slag
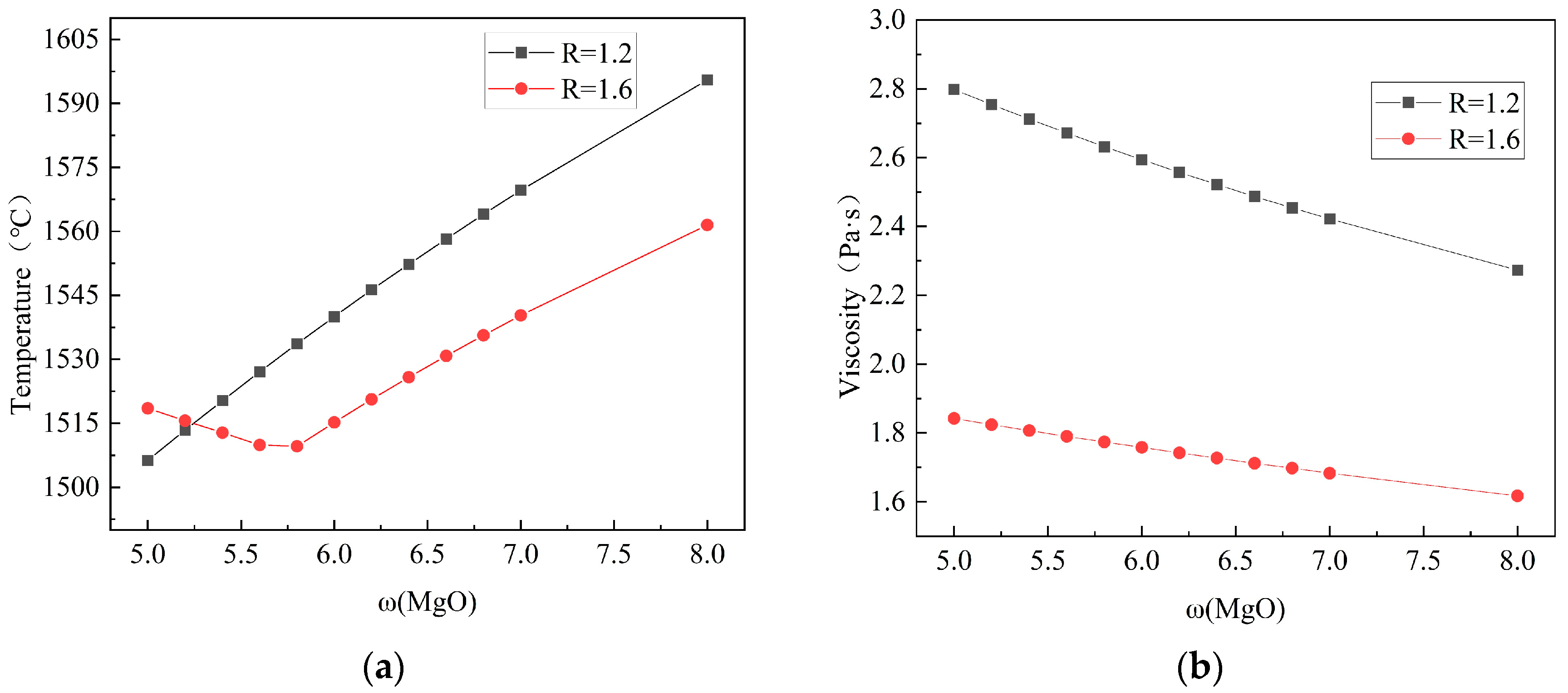
3.1.2. Effect of MgO on the Melting Temperature and Viscosity of Slag
3.1.3. Effect of Alkalinity on the Melting Temperature and Viscosity of Slag
3.1.4. Effect of FeO on the Melting Temperature and Viscosity of Slag
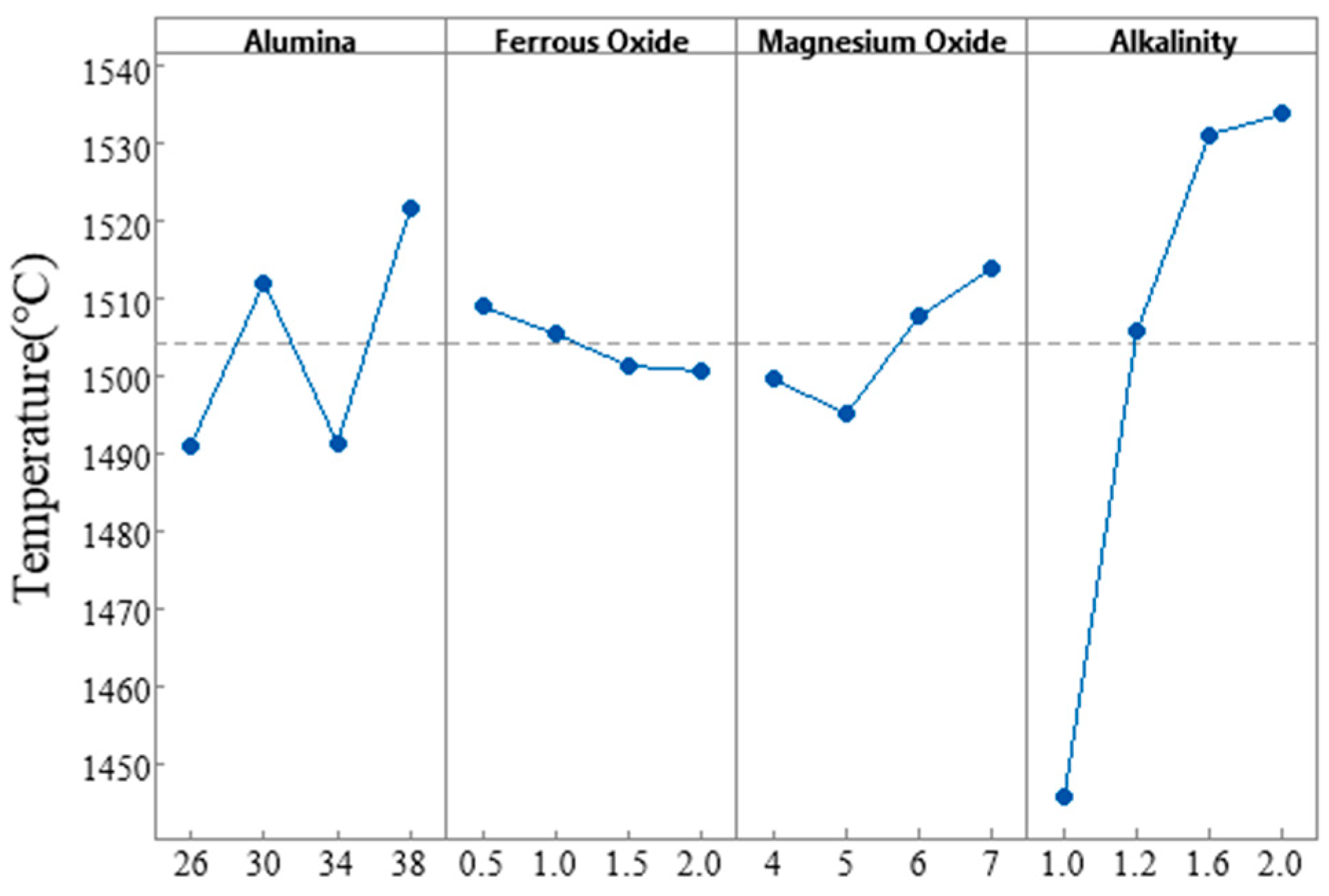
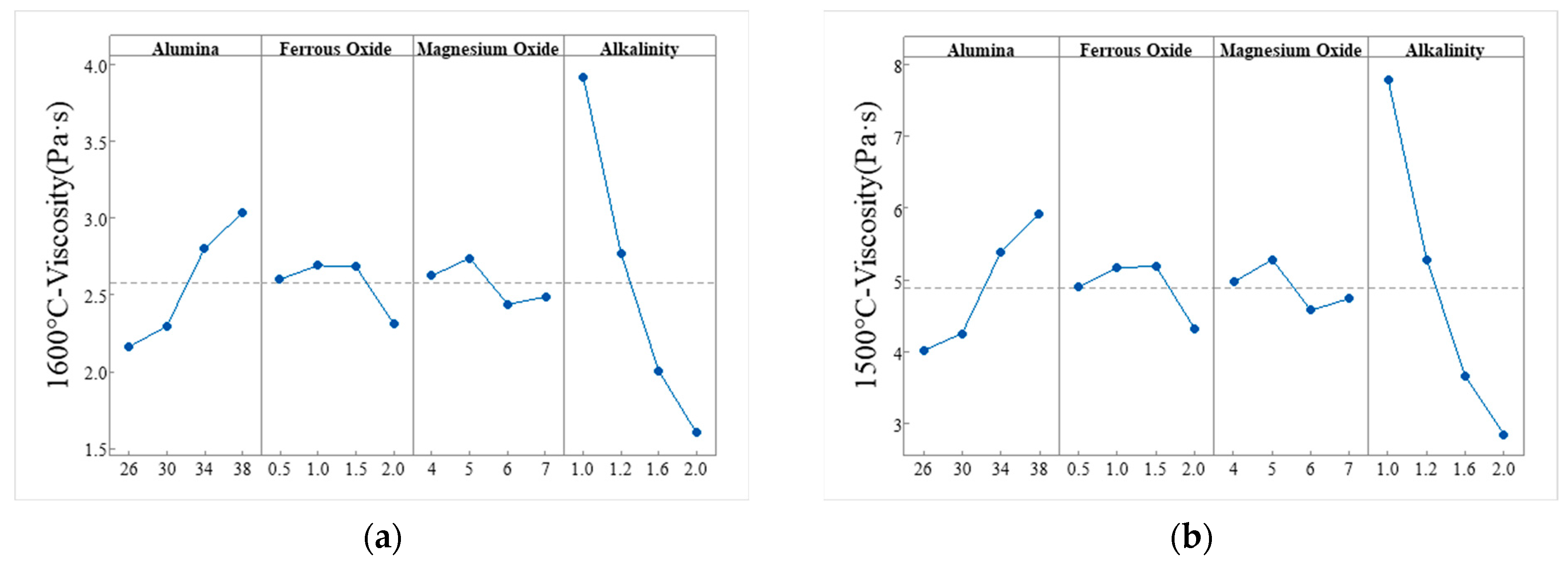
3.1.5. Orthogonal Experimental Study on the Melting Characteristics of Slag
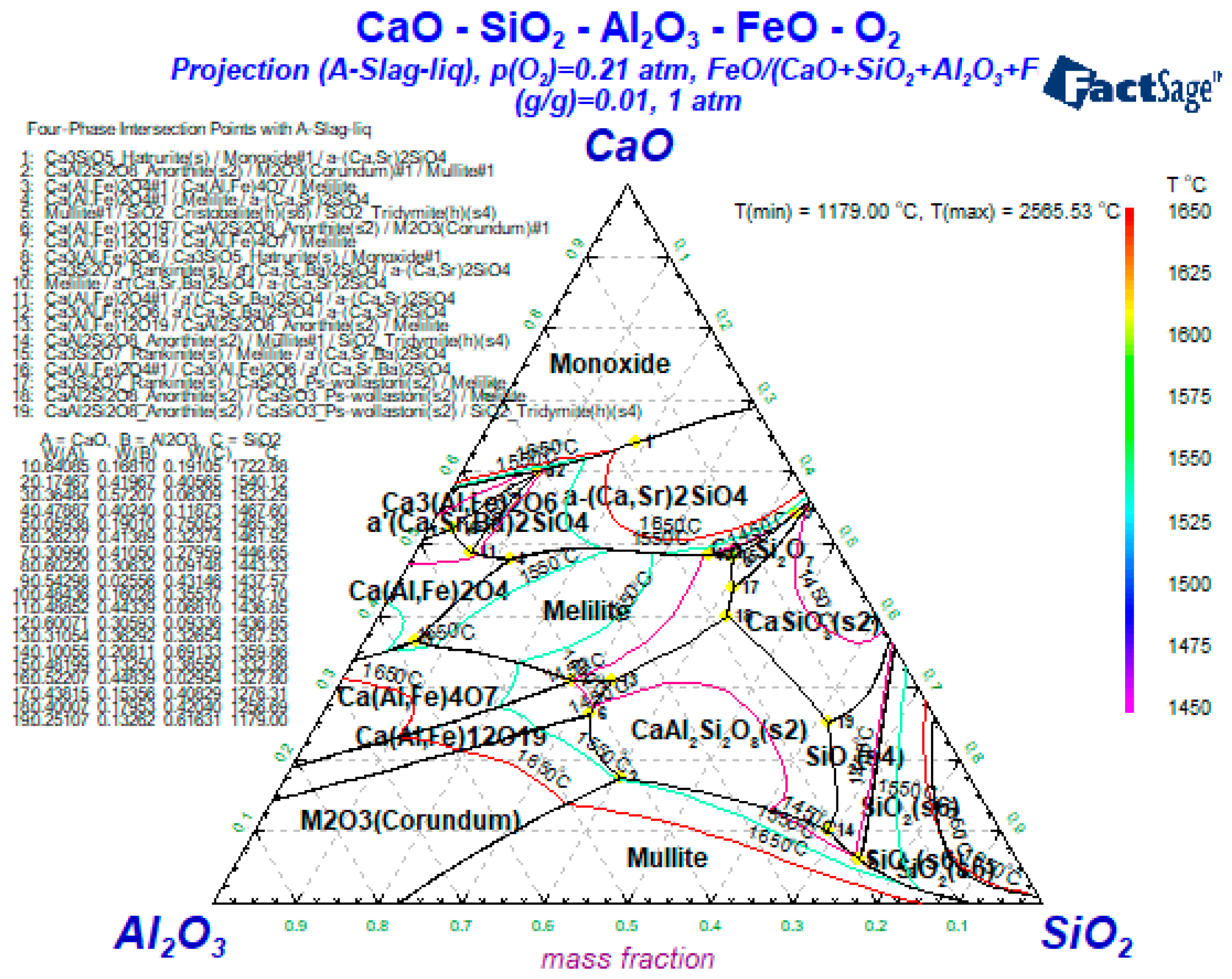
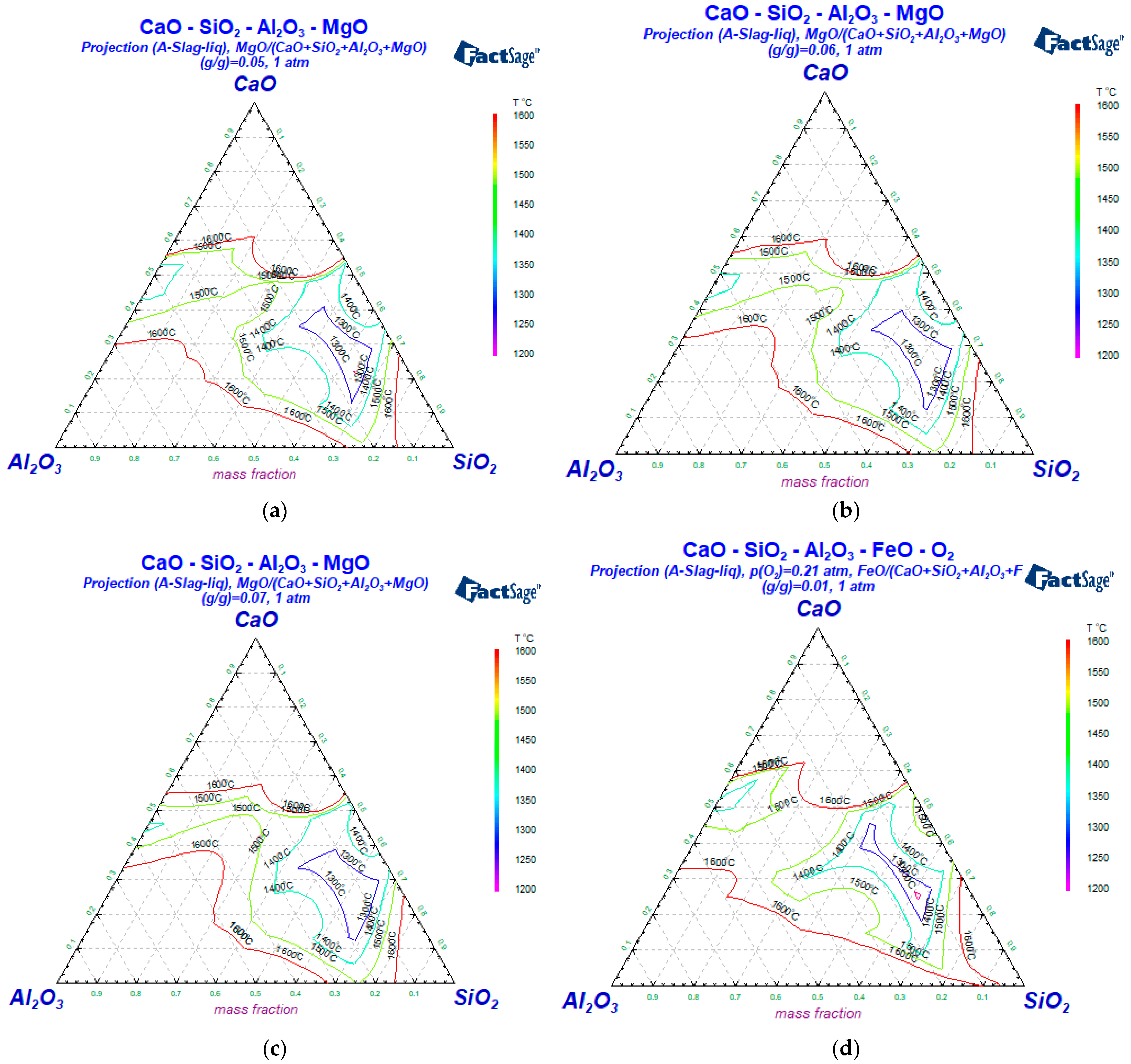
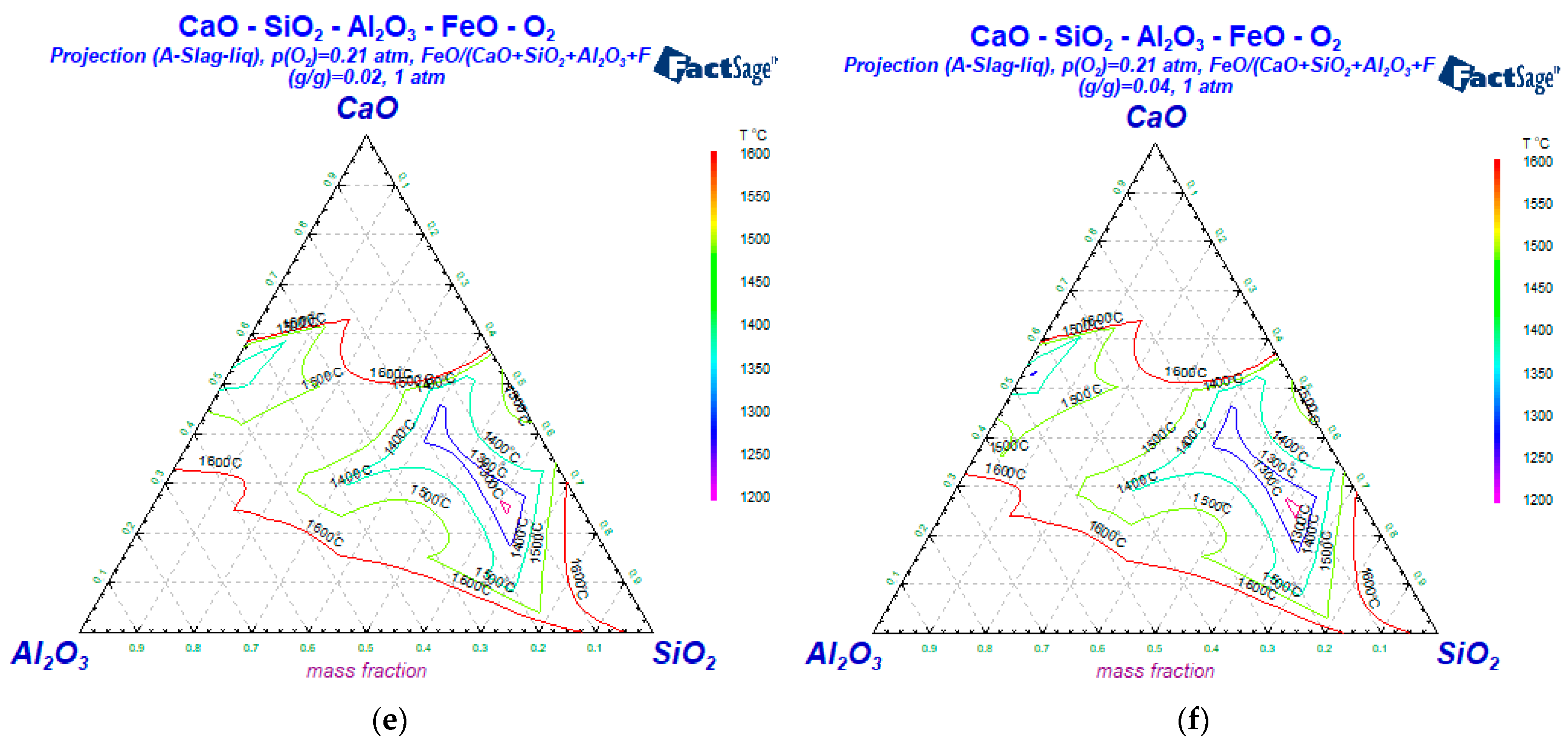
3.2. The Influence of the Slag Composition on the Liquid Phase Zone
3.3. The Influence of Slag Oxidation on Aluminum Loss
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RH | RH Vacuum Degassing Process |
References
- Song, T.L.; Wang, Z.L.; Bao, Y.P.; Gu, C. Research progress on key production technologies of high-quality oriented silicon steel. Electr. Steel 2023, 5, 5–12. [Google Scholar]
- Ning, X.; Wang, Y.L.; Liang, Y.F.; Lin, J.P. Preparation process and development trend of oriented silicon steel. Precis. Form. Eng. 2022, 14, 1–10. [Google Scholar]
- Huo, H.X.; Li, Y.X.; Sun, Z.D.; Liu, P.C.; Huang, F. Development of new production processes for oriented silicon steel. Baosteel Technol. 2020, 46, 30–32. [Google Scholar] [CrossRef]
- Shi, P.Z.; Li, J.B.; Qiao, J.L.; Guo, F.H.; Qiu, S.T. High temperature mechanical properties and hot working properties of high magnetic induction oriented silicon steel. Steel Vanadium Titan. 2023, 44, 135–141. [Google Scholar] [CrossRef]
- Li, L.; Jia, Z.W.; Song, Q.Y.; Luo, L.; Zhang, J.; Pang, S.F. The effect of acid soluble aluminum content on the precipitation of normalizing inhibitors in oriented silicon steel. Electr. Steel 2021, 3, 12. [Google Scholar]
- Su, D.X.; Ma, J.C.; Zhang, Z.P. Comparison of cleanliness of low carbon aluminum killed steel with different RH refining process. Steelmaking 2015, 31, 36–39. [Google Scholar]
- Zhu, C.Y.; Zheng, J.X.; Li, G.Q.; Chen, X.H.; Zhang, F. The effect of acid soluble aluminum on the composition and morphology of second phase particles in cast oriented silicon steel. Spec. Steel 2014, 35, 60–64. [Google Scholar]
- Yang, J.H. The smelting method of alloying oriented silicon steel in RH furnace. Introd. Metall. Inf. 2022, 59, 24–26. [Google Scholar]
- Zhao, J.Q.; Cai, X.F.; Zou, C.D. Development and Application of Desulfurization Process for 180 t RH Vacuum Refining Furnace. Iron Steel 2018, 53, 41–46. [Google Scholar] [CrossRef]
- Spencer, P.J. The origins, growth and current industrial impact of Calphad. Calphad 2022, 79, 102489. [Google Scholar] [CrossRef]
- Nicholas, U.; Richard, O.; Vilupanur, R. Generalized method of sensitivity analysis for uncertainty quantification in Calphad calculations. Calphad 2022, 79, 102504. [Google Scholar] [CrossRef]
- Pang, S.F.; You, Q.L.; Jiang, Q.W.; Jia, Z.W.; Luo, L. Calculation and analysis of thermodynamic equilibrium precipitates in oriented silicon steel. Electr. Steel 2023, 5, 7–13. [Google Scholar]
- Zhang, Y.; Ren, Y.; Zhang, L. Kinetic study on compositional variations of inclusions, steel and slag during refining process. Metall. Res. Technol. 2018, 115, 415. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Sun, G.M.; Li, Z.W.; Peng, Y.S.; Liu, W.S.; Zhu, S.L. Optimization and industrial testing of LF refining slag system for 65Mn steel. Spec. Steel 2023, 44, 65–69. [Google Scholar]
- Wang, B.C. Research on RH refining slag control process. Tianjin Metall. 2022, 1, 24–26, 34. [Google Scholar]
- Zhang, Y.H.; Zhou, Y.J.; Hu, C.X. Control of oxide inclusions during refining process. Zhejiang Metall. 2022, 4, 4–7. [Google Scholar]
- Bai, S.X.; Zeng, Y.N.; Ning, X.; Tang, G.Z. The influence of refining slag components on the composition of bearing steel liquid. J. North China Univ. Technol. (Nat. Sci. Ed.) 2023, 45, 114–120. [Google Scholar]
- Liu, W.B.; Song, B.; Cai, C.K.; Mao, J.H. A study on the viscosity of CaO-Al2O3-MgO-SiO2-CeO2 pentagonal refining slag system. Rare Earth 2021, 42, 13–20. [Google Scholar] [CrossRef]
- Dai, Z.D.; Chen, B.X.; Deng, Q.Y.; Hu, M.L. Theoretical analysis of the influence of Al2O3 on the physicochemical properties of blast furnace slag. J. Chongqing Univ. 2022, 45, 100–110. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, G.G.; Zhang, T.; Zhang, Y.Q.; Long, H.; Guo, J.Y. Plasticity control of CaO-SiO2-Al2O3-MgO inclusions in 45 steel. Steelmaking 2023, 39, 82–89, 96. [Google Scholar]
- Tian, M.; Wan, X.B.; Chen, M.; Taskinen, P.; Tiljander, M.; Jokilaakso, A. Phase equilibria of FeOx-SiO2-Al2O3 slag system at 1200 °C and PO2 of 10−8.6 atm. Calphad 2022, 79, 102502. [Google Scholar] [CrossRef]
- Xie, S.; Liao, C.F.; Zhao, B.J. Phase Equilibrium studies in the system “FeO”-SiO2-Al2O3-CaO-MgO at 1200 °C and PO2 10−8 atm. Calphad 2023, 82, 102588. [Google Scholar] [CrossRef]
- Fredriksson, P.; Seetharaman, S. Thermodynamic studies of slags containing FeO and their impact on ladle refining process. Ironmak. Steelmak. 2013, 32, 47–53. [Google Scholar] [CrossRef]
- Alves, C.P.; Rocha, D.C.V.; Pereira, M.A.J.; Bielefeldt, W.V.; Vilela, A.C.F. Review and planning of experiments with steel and slag in laboratory furnace. J. Mater. Res. Technol. 2018, 7, 387–394. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, F.Y.; Deng, T.F.; Liu, Y.L.; Yang, L.F.; Guo, C.S.; Tan, J.; Ma, T.Y.; Chen, W.; Du, Y. Phase equilibria in the FeO-Fe2O3-SiO2 system: Experimental measurement and thermodynamic modeling. Calphad 2022, 79, 102459. [Google Scholar] [CrossRef]
- Hu, W.; Xu, B.; Huang, J.Q.; Zhu, W.R.; Li, J.; Li, R.C.; Liu, J.S.; Chen, R.J. Optimization production practice of DC06 top slag modifier for 260t converter RH process IF steel. Spec. Steel 2021, 42, 27–30. [Google Scholar] [CrossRef]
- Shan, W.; Zhao, J.W.; An, C.; Wang, C. Research on controlling inclusion composition based on RH slag composition adjustment. Shanxi Metall. 2023, 46, 46–49. [Google Scholar]
- Shi, C.B.; Wang, H.; Li, J. Effects of Reoxidation of Liquid Steel and Slag Composition on the Chemistry Evolution of Inclusions During Electroslag Remelting. Metall. Mater. Trans. 2018, 49, 1675–1689. [Google Scholar] [CrossRef]
- Deng, A.J.; Fan, D.D.; Wang, H.C.; Li, C.H. Simulation research on oxygen mass transfer between steel and slag in IF steel refining process. J. Iron Steel Res. Int. 2020, 27, 409–419. [Google Scholar] [CrossRef]
- Yang, F.; Luo, H.M.; Wei, X.D. Inclusion Control in Oriented Silicon Steel. Contin. Cast. 2020, 39, 49–53. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Yuan, Y.; Wang, J.; Chen, T.; Wu, L.; Chen, X.H.; Jiang, B.; Tang, A.T.; Pan, F.S. Effect of alloying elements on the dissolution behavior of iron in magnesium melt. Calphad 2022, 79, 102503. [Google Scholar] [CrossRef]




| Al2O3 | FeO | MgO | CaO | SiO2 | S | TiO2 | P2O5 | R |
| 32 | 1 | 5.5 | 36 | 24 | ≤0.03 | ≤0.4 | ≤0.05 | 1.5 |
| ω(Al2O3) | ω(FeO) | ω(MgO) | Alkalinity |
|---|---|---|---|
| 26 | 0.5 | 4 | 1 |
| 30 | 1 | 5 | 1.2 |
| 34 | 1.5 | 6 | 1.6 |
| 38 | 2 | 7 | 2 |
| Group | ω(Al2O3) | ω(FeO) | ω(MgO) | Alkalinity | Melt Temperature | 1600 °C Viscosity | 1500 °C Viscosity |
|---|---|---|---|---|---|---|---|
| 1 | 26 | 0.5 | 4 | 1 | 1434.1 | 3.564 | 6.916 |
| 2 | 30 | 0.5 | 5 | 1.2 | 1498 | 2.864 | 5.437 |
| 3 | 34 | 0.5 | 6 | 1.6 | 1534.6 | 2.12 | 3.884 |
| 4 | 38 | 0.5 | 7 | 2 | 1569.5 | 1.862 | 3.364 |
| 5 | 26 | 1 | 5 | 1.6 | 1513.9 | 1.735 | 3.093 |
| 6 | 30 | 1 | 7 | 2 | 1546.4 | 1.49 | 2.614 |
| 7 | 34 | 1 | 4 | 1 | 1432.5 | 4.354 | 8.803 |
| 8 | 38 | 1 | 6 | 1.2 | 1529.4 | 3.192 | 6.19 |
| 9 | 26 | 1.5 | 6 | 2 | 1510.1 | 1.352 | 2.349 |
| 10 | 30 | 1.5 | 7 | 1.6 | 1547.3 | 1.737 | 3.089 |
| 11 | 34 | 1.5 | 5 | 1.2 | 1489 | 3.014 | 5.785 |
| 12 | 38 | 1.5 | 4 | 1 | 1459.4 | 4.647 | 9.547 |
| 13 | 26 | 2 | 7 | 1.2 | 1506.9 | 2.006 | 3.725 |
| 14 | 30 | 2 | 6 | 1 | 1457.3 | 3.099 | 5.901 |
| 15 | 34 | 2 | 5 | 2 | 1509.7 | 1.718 | 3.076 |
| 16 | 38 | 2 | 4 | 1.6 | 1529.4 | 2.434 | 4.576 |
| Factor | Al2O3 | FeO | MgO | Alkalinity |
|---|---|---|---|---|
| Mean 1 | 1491 | 1509 | 1500 | 1446 |
| Mean 2 | 1512 | 1506 | 1495 | 1506 |
| Mean 3 | 1491 | 1501 | 1508 | 1531 |
| Mean 4 | 1522 | 1501 | 1514 | 1534 |
| Range (R) | 31 | 8 | 19 | 88 |
| Rank | 2 | 4 | 3 | 1 |
| Factor | 1600 °C Viscosity | 1500 °C Viscosity | ||||||
|---|---|---|---|---|---|---|---|---|
| Al2O3 | FeO | MgO | Alkalinity | Al2O3 | FeO | MgO | Alkalinity | |
| Mean 1 | 2.164 | 2.603 | 2.625 | 3.916 | 4.021 | 4.9 | 4.973 | 7.792 |
| Mean 2 | 2.298 | 2.693 | 2.741 | 2.769 | 4.26 | 5.175 | 5.288 | 5.284 |
| Mean 3 | 2.801 | 2.688 | 2.441 | 2.006 | 5.387 | 5.193 | 4.581 | 3.66 |
| Mean 4 | 3.034 | 2.314 | 2.49 | 1.606 | 5.919 | 4.319 | 4.745 | 2.851 |
| Range (R) | 0.87 | 0.378 | 0.3 | 2.31 | 1.898 | 0.873 | 0.707 | 4.941 |
| Rank | 2 | 2 | 4 | 1 | 2 | 3 | 4 | 1 |
| Group | Al2O3 | Alkalinity | MgO | FeO | ∆FeO | |
|---|---|---|---|---|---|---|
| 1 | before | 27.54 | 1.29 | 5.3 | 1.24 | −0.23 |
| after | 28.49 | 1.27 | 6.7 | 1.01 | ||
| 2 | before | 29.91 | 1.81 | 6.17 | 0.62 | −0.14 |
| after | 30.01 | 1.81 | 6.65 | 0.48 | ||
| 3 | before | 29.78 | 1.73 | 6.23 | 0.89 | 0.01 |
| after | 28.37 | 1.07 | 5.71 | 0.90 | ||
| 4 | before | 29.85 | 1.72 | 6.1 | 0.99 | −0.07 |
| after | 27.51 | 0.92 | 5.48 | 0.92 | ||
| 5 | before | 25.562 | 1.14 | 5.64 | 2.18 | −0.86 |
| after | 25.984 | 0.95 | 5.94 | 1.32 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Zhang, Q.; Zheng, S.; Dai, F. Application of Thermodynamic Calculations in the Study of Slag Melting Characteristics and Aluminum Loss Control. Metals 2025, 15, 1099. https://doi.org/10.3390/met15101099
Liu T, Zhang Q, Zheng S, Dai F. Application of Thermodynamic Calculations in the Study of Slag Melting Characteristics and Aluminum Loss Control. Metals. 2025; 15(10):1099. https://doi.org/10.3390/met15101099
Chicago/Turabian StyleLiu, Ting, Qingxia Zhang, Shenglan Zheng, and Fangqin Dai. 2025. "Application of Thermodynamic Calculations in the Study of Slag Melting Characteristics and Aluminum Loss Control" Metals 15, no. 10: 1099. https://doi.org/10.3390/met15101099
APA StyleLiu, T., Zhang, Q., Zheng, S., & Dai, F. (2025). Application of Thermodynamic Calculations in the Study of Slag Melting Characteristics and Aluminum Loss Control. Metals, 15(10), 1099. https://doi.org/10.3390/met15101099





