Abstract
A sheet-type sinter-bonding material was developed to form thermally stable and highly heat-conductive joints suitable for wide-bandgap (WBG) semiconductor dies and high-heat-flux devices, and its bonding characteristics were investigated. To enhance the cost-competitiveness of the bonding material, Ag-coated Cu (Cu@Ag) particles were employed as fillers instead of conventional Ag particles. To facilitate accelerated sintering, a bimodal particle size distribution comprising several micron- and submicron-sized particles was adopted by synthesizing and mixing both size ranges. For sheet fabrication, a decomposable resin was used as the essential binder component, which could be removed during the bonding process via thermal decomposition. This approach enabled the formation of a sintered bond line composed entirely of Cu@Ag particles. Thermogravimetric and differential thermal analyses revealed that the decomposition of the resin in the sheet occurred within the temperature range of 290–340 °C. Consequently, sinter-bonding conducted at 350 °C and 370 °C exhibited significantly superior bondability compared to bonding at 330 °C. In particular, sinter-bonding at 350 °C for just 60 s resulted in a highly densified joint microstructure with a low porosity of 7.6% and high shear strength exceeding 25 MPa. The formation of the bond line was initiated by sintering between the outer Ag shells of the adjacent particles. However, with increasing bonding time or temperature, sintering driven by Cu diffusion from the particle cores to the outer Ag shells, particularly in the submicron-sized particles, was progressively enhanced. These results obtained from the fabricated sheet-type materials demonstrate that, even with the use of resin, rapid solid-state sintering between filler particles combined with the removal of resin through decomposition enables the formation of a metallic bond line with excellent thermal conductivity.
1. Introduction
Power semiconductor materials are gradually being replaced by wide-band-gap (WBG) semiconductors such as SiC owing to their superior high-speed switching characteristics, reduced switching losses, and improved energy conversion efficiency compared to Si. This has resulted in the conventional soldering-based die-attach process increasingly becoming replaced by sinter-bonding techniques employing Ag particles [1,2,3]. Unlike conventional solder joints, the Ag bond line formed through sinter bonding exhibits a high melting point and excellent thermal conductivity, thereby ensuring the reliable performance of and efficient heat dissipation in SiC devices, even under high-temperature operating conditions [4,5]. Consequently, sinter bonding using Ag particles is expected to be continuously adopted not only for power semiconductor dies but also for thermally demanding devices such as light-emitting diodes (LEDs). One of the most notable advantages of sinter bonding is the significantly higher melting point of the resulting bond line compared to the bonding process temperature, which ensures the thermal stability and reliability of the joint in high-temperature environments.
The materials used for forming Ag bond lines via sinter bonding are broadly categorized into paste- and sheet-type formats [6]. Owing to their relatively simple fabrication process, Ag pastes have been extensively studied and numerous research outcomes have been reported to date. However, the conventional process involving the patterning of Ag paste followed by die placement and thermocompression often introduces process complexity and may result in defects such as bleed-out and kerf creep [7,8,9,10]. By contrast, although sheet-type materials are more challenging to fabricate, they offer the advantages of simplified processing and inherently lower material flow during thermocompression, thereby minimizing the occurrence of such defects. Consequently, sheet-type sinter-bonding materials are often preferred over pastes in industrial settings. These Ag-particle-based sheets have already been commercialized and are currently used in semiconductor manufacturing processes worldwide [11]. Moreover, the die-attach process using sheet-type materials can be implemented at the wafer level by laminating the sheet onto the wafer followed by dicing, which offers significant potential for process streamlining and productivity enhancement [12]. Despite their technological significance, the fabrication methods for Ag-particle-based sheet materials remain proprietary, and the literature to date has primarily focused on reporting applications or device-level results using such materials, rather than detailing their synthesis routes [13,14,15].
In this study, a novel sheet-type sinter-bonding material was developed based on a fabrication concept distinct from that of commercially available Ag-particle-based sheet materials. Specifically, when a resin is incorporated during the sheet preparation process, the material is designed to thermally decompose the resin during sinter bonding, thereby minimizing the resin residue in the final bond line and achieving the formation of a predominantly metallic Ag bond line. This approach aims to produce a flexible sheet-type bonding material suitable for high-reliability applications. The bonding performance of the developed sheet was subsequently evaluated using copper finishes. To further enhance the cost-competitiveness compared with conventional Ag-based sheets, Ag particles were replaced with Ag-coated Cu (Cu@Ag) particles. In addition, to promote rapid densification during sintering, a bimodal particle-size distribution was achieved by mixing micro- and submicron-sized Cu@Ag particles.
2. Materials and Methods
2.1. Fabrication of Bimodal Cu@Ag Particles
Submicron-sized Cu particles were synthesized in-house using a proprietary wet-chemical method to prepare small fractions of bimodal Cu@Ag particles [16]. To prepare the Cu slurry, 5 g of the synthesized Cu particles were dispersed in 100 mL of deionized water. Separately, an Ag-coating solution was prepared by sequentially dissolving 5 M NaOH (98%, Samchun Chemicals, Seoul, Republic of Korea), 1.25 M ethylenediaminetetraacetic acid (EDTA; C10H16N2O8, 98.5%, Yakuri Pure Chemicals, Kyoto-fu, Japan), and 0.16 M AgNO3 (99.99%, Hojeonable Inc., Daejeon, Republic of Korea) in 60 mL of deionized water. EDTA acts as a chelating agent by forming complexes with Ag+ ions, thereby lowering their reduction potential and minimizing the potential difference with Cu. This facilitates a more uniform and stable electroless Ag deposition reaction [17,18]. The Ag-coating solution was then added dropwise to the Cu slurry at a rate of 5 mL/min to initiate the coating process. The mixed solution was stirred at 300 rpm for 30 min to promote galvanic displacement, thereby forming submicron Cu@Ag core–shell particles. After the reaction, the resulting particles were subjected to four centrifugation cycles at 7000 rpm and washed with deionized water. A final washing step was performed with ethanol, followed by drying under vacuum at room temperature for 6 h. A schematic of the fabrication process for the submicron Cu@Ag particles is shown in Figure 1.
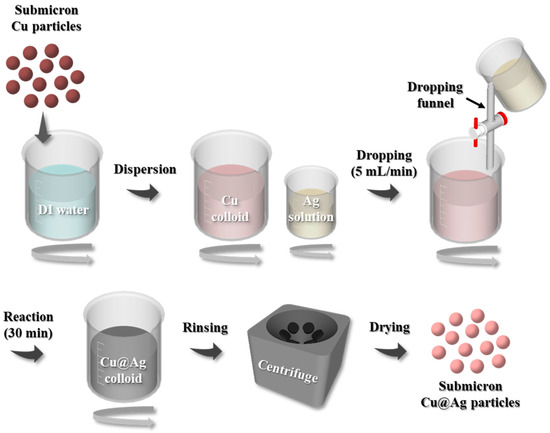
Figure 1.
Schematic of the fabrication process for submicron-sized Cu@Ag particles.
To prepare the bimodal Cu@Ag particles, micron-sized Cu@Ag powders supplied by JoinM (Nonsan-si, Republic of Korea) were mixed with the previously synthesized submicron Cu@Ag particles at a weight ratio of 6:4 in ethanol to achieve close packing. The mixture was then subjected to ultrasonic dispersion and magnetic stirring to ensure homogeneous mixing. The resulting slurry was dried at room temperature in a vacuum chamber for 6 h to yield bimodal Cu@Ag particles.
2.2. Fabrication of the Bonding Sheet
The bonding sheet fabrication process is shown in Figure 2. To prepare the resin formulation, an acrylic resin was dissolved in a solvent at room temperature for approximately 3 days. The prepared resin solution was mixed with a reductant (EW-10; Epsilon Epowder, Seongnam-si, Republic of Korea) and the bimodal Cu@Ag particles to form a homogeneous paste. The resulting paste was printed onto a glass substrate in a square shape of 20 × 20 × 0.1 mm3, followed by drying on a hot plate at 100 °C for approximately 2 min to evaporate the residual solvent. Thus, a bonding material in the form of a bimodal Cu@Ag sheet was successfully fabricated, as shown in Figure 3.
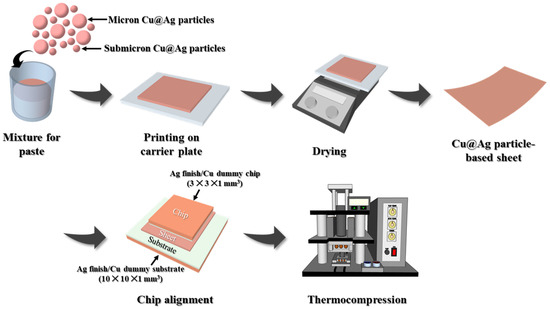
Figure 2.
Schematic of the fabrication process and sinter bonding of a bimodal Cu@Ag particle-based sheet.
Figure 3.
Photograph of a sheet containing bimodal Cu@Ag particles.
2.3. Thermal Properties of the Bonding Sheet and Compression Sinter-Bonding Process
The thermal behavior of the fabricated bonding sheet containing bimodal Cu@Ag particles and the developed resin formulation was evaluated using thermogravimetric and differential thermal analysis (TG-DTA; DTG-60H, Shimadzu Corporation, Kyoto, Japan). The analysis was conducted under an air atmosphere using a dynamic heating mode up to 500 °C at a heating rate of 20 °C/min.
To assess the bonding performance of the developed sheet material, compression sinter bonding was performed in air, as illustrated in the latter part of Figure 2, using dummy substrates and chips coated with a 500 nm thick Ag finish. A square sheet (3 × 3 mm2) was cut and aligned on a substrate (10 × 10 × 1 mm3), followed by the placement of a chip (3 × 3 × 1 mm3) on top to form a sandwich structure. Bonding was achieved using an in-house fabricated thermocompression bonder at bonding temperatures of 330, 350, and 370 °C, for bonding times of 30, 60, and 90 s, respectively, under a constant compression of 5 MPa.
The shear strengths of the bonded joints formed under the different sintering conditions were evaluated using shear tests. The tests were conducted using a bond tester (HAWK-8200S, Kovis Technology, Seoul, Republic of Korea), where the shear tip was positioned 200 µm above the substrate surface and moved laterally at a speed of 200 µm/s to apply a shear force to the chip.
The morphology, particle size, and dispersion state of the bimodal Cu@Ag particles were examined using high-resolution field-emission scanning electron microscopy (HR-FE-SEM; SU8010, Hitachi High-Technologies Corporation, Mito, Japan). To evaluate the quality of the Ag coating, the morphology of the coated layers, cross-sectional microstructure of the bonded joints, and fracture surfaces after sintering were observed in the back-scattered electron (BSE) mode. The acquired cross-sectional SEM images were further analyzed using an image analysis software (ImageJ, US National Institutes of Health, Bethesda, MD, USA) to quantitatively evaluate the porosity of the formed bond line.
3. Results and Discussion
3.1. Characteristics of the Bimodal Cu@Ag Particles
The BSE images of the cross-sectional microstructures of the micron- and submicron-sized Cu@Ag particles and a secondary electron image of the mixed bimodal Cu@Ag particles are presented in Figure 4. In the BSE micrographs shown in Figure 4a,b, some voids were observed at the Cu/Ag interfaces, which likely resulted from the galvanic displacement reaction used for the Ag coating [19]. Nevertheless, a well-defined core–shell structure was clearly confirmed, with Ag shells uniformly encapsulating the Cu cores, thereby providing effective protection against the oxidation of the Cu particles. In Figure 4c, slight aggregation among the submicron Cu@Ag particles is observed; however, the submicron particles are distributed within the interstitial spaces among the larger micron-sized Cu@Ag particles, indicating that the mixing of large and small particles was achieved. This particle distribution was expected to become more uniform during the subsequent paste preparation process through additional mixing. Based on image-based particle size analysis, the average size of the micron Cu@Ag particles was approximately 2 µm, whereas that of the submicron Cu@Ag particles was approximately 350 nm.
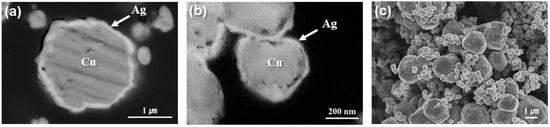
Figure 4.
Cross-sectional BSE images of (a) micron and (b) submicron Cu@Ag particles and (c) SEM image of bimodal Cu@Ag particles after mixing.
3.2. Thermal Properties of the Bonding Sheet
Figure 5 shows the TG-DTA results for the resin formulation (Figure 5a) and the bonding sheet containing bimodal Cu@Ag particles (Figure 5b) under dynamic heating in air. In Figure 5a, the initial weight loss observed below approximately 150 °C is attributed to the evaporation of residual solvent and reductant. For the resin formulation, thermal decomposition initiated at around 300 °C, accompanied by an endothermic reaction near 376 °C, indicating the characteristic decomposition behavior of the resin. By contrast, the bimodal Cu@Ag sheet shown in Figure 5b exhibited complete resin decomposition at 334 °C, followed by a distinct exothermic peak at 342 °C. This exothermic behavior is interpreted as the result of rapid physical contact between the Cu@Ag particles upon resin burnout, which in turn triggers particle sintering and the associated heat release. Moreover, the decomposition temperature of the resin was reduced by approximately 41 °C upon the incorporation of Cu@Ag particles. This behavior is attributed to the outward diffusion of Cu through the Ag shell at elevated temperatures above 200 °C, followed by oxidation of the diffused Cu. The resulting Cu oxide acts as a catalytic agent that promotes thermal decomposition of the resin [20,21]. Specifically, Cu oxide can be reduced by the residual reductant remaining in the sheet, thereby generating active oxygen species. These oxygen species shift the thermal decomposition pathway of the resin toward a combustion-like mechanism, which accelerates the decomposition process and leads to a further reduction in the decomposition temperature [22,23].
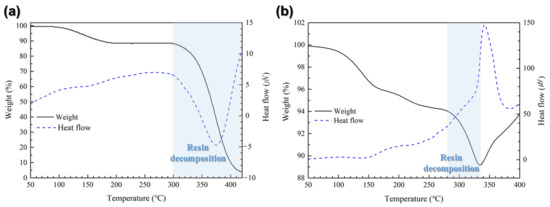
Figure 5.
TG-DTA results of (a) resin formulation and (b) the sheet containing bimodal Cu@Ag particles.
3.3. Sinter-Bonding Properties of the Bonding Sheet
Figure 6 presents the average shear strength values of joints sinter-bonded using the bimodal Cu@Ag sheet under a compression of 5 MPa in air at bonding temperatures of 330, 350, and 370 °C and bonding times of 30, 60, and 90 s. The shear strength increased with increasing bonding temperature and time, indicating that the extent of sintering increased in proportion to these parameters. At 330 °C, relatively low shear strengths of 13 and 15.7 MPa were obtained for bonding times of 30 and 60 s, respectively. However, the strength increased to 21.5 MPa at 90 s, exceeding the threshold of 20 MPa. Considering the thermal behavior shown in Figure 5b, this result suggests that insufficient resin decomposition occurred at 330 °C, leading to residual resin that hindered effective particle–particle contact and limited the sintering process. By contrast, at 350 °C, significantly higher and satisfactory shear strengths of 23.4, 25.5, and 27.5 MPa were obtained for bonding times of 30, 60, and 90 s, respectively. These values imply that the elevated temperature enabled more complete resin removal, allowing improved interparticle contact and enhanced sintering, thereby resulting in substantial strength improvement. At 370 °C, where resin decomposition was essentially complete, further enhancement in the sintering process led to additional strength gains. Shear strengths of 24.2, 27.0, and 33.7 MPa were recorded at bonding times of 30, 60, and 90 s, respectively, demonstrating a progressive increase in joint integrity under more aggressive bonding conditions.
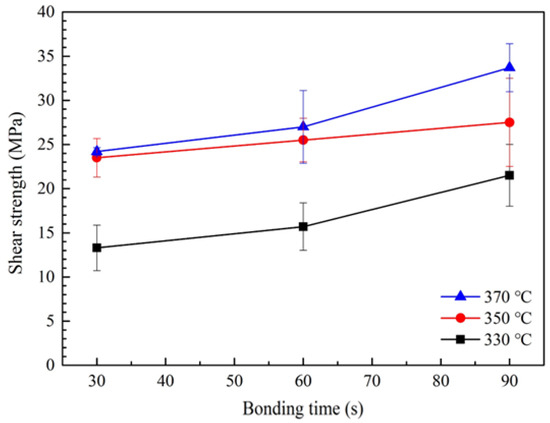
Figure 6.
Shear strengths of bond lines formed under 5 MPa with different bonding temperatures and bonding times using the sheet containing bimodal Cu@Ag particles in air.
Figure 7 displays the cross-sectional microstructures of bond lines formed by sinter bonding using the bimodal Cu@Ag particle-containing sheet under ambient conditions at bonding temperatures of 330–370 °C and bonding times of 60 and 90 s. At 330 °C, a porous microstructure containing numerous voids was observed, which is attributed to the insufficient sintering caused by the large amount of residual resin remaining between the particles. This microstructural feature correlates well with the relatively low shear strength values (15.7–21.5 MPa) reported in Figure 6. By contrast, at 350 °C, enhanced thermal decomposition of the resin facilitated improved particle sintering, resulting in a marked reduction in void formation and an increase in the overall density of the joint. At 370 °C, resin removal was nearly complete, allowing extensive sintering among particles and yielding the densest joint structure among the tested conditions. Additionally, the interfaces between the Cu@Ag sheet and the top and bottom Ag finishes became increasingly dense and well bonded with increasing bonding temperature and time. This observation suggests that variations in the bonding conditions may influence the fracture mode observed in subsequent shear testing by altering the interfacial bonding quality.
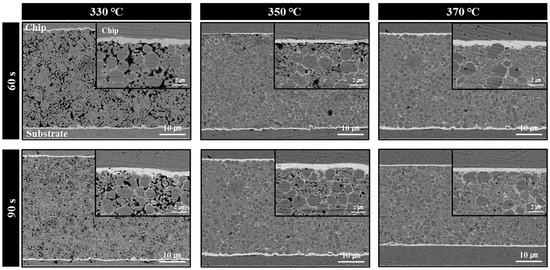
Figure 7.
Cross-sectional low- and high-magnification (inset) BSE images of bond lines formed under 5 MPa for different times at different temperatures using the sheet containing bimodal Cu@Ag particles in air.
Figure 8 shows the porosities of the formed bond lines calculated based on the cross-sectional microstructures using image analysis software. At a bonding time of 60 s, the porosities (red-colored areas) measured at bonding temperatures of 330, 350, and 370 °C were 18.7%, 7.6%, and 3.0%, respectively. At a bonding time of 90 s, the porosity decreased further to 16.2%, 5.9%, and 2.5% under the same respective temperature conditions. Notably, porosity levels below 8% are rarely observed after 60–90 s of sinter bonding under a relatively low compression pressure of 5 MPa in Cu-based particle systems [24,25,26]. These results clearly demonstrate that increasing the bonding temperature and extending the bonding time significantly reduce the porosity of the bond line, which directly accounts for the corresponding improvements in the shear strength observed in Figure 6.
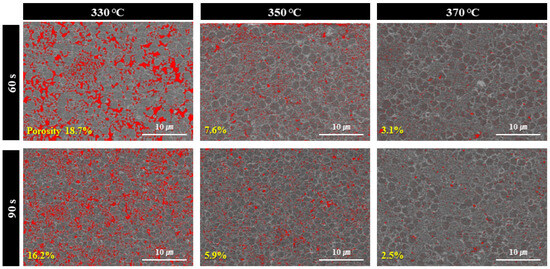
Figure 8.
Cross-sectional porosity images of bond-lines sinter-bonded under 5 MPa for different time at different temperatures in air using the sheet containing bimodal Cu@Ag particles.
Figure 9 shows the fracture surfaces observed after the shear testing of joints that were sinter-bonded using the bimodal Cu@Ag particle-containing sheet at 330–370 °C for 90 s under ambient conditions. At 330 °C, where a considerable amount of resin remained, the original morphology of the added particles was largely preserved, indicating minimal sintering between particles. In this case, fractures predominantly occurred along the chip/substrate interface, corresponding to an interfacial failure mode. This suggests that the resin decomposition was insufficient at this temperature, leading to weak interfacial bonding and poor sintering across the joint interface. At 350 °C, partial decomposition of the resin led to a transition in fracture mode from interfacial to cohesive failure. Localized sintering was confirmed by necking between particles, and elongated fracture features aligned with the shear direction were occasionally observed. At 370 °C, nearly complete removal of the resin facilitated extensive sintering between particles. Consequently, an elongated and dense fracture surface microstructure developed throughout most of the joint area, with the fractures propagating across the interior of the bond line, which is a typical feature of cohesive failure. This indicates that higher bonding temperatures significantly enhance particle–particle sintering and shift the failure mode from interfacial to cohesive. In conclusion, an increase in bonding temperature strongly correlates with improved sinterability and a transition in fracture behavior. The overall trend of the fracture mode evolution is schematically shown in Figure 10.

Figure 9.
(a–c) Low- and (d–f) high-magnification SEM images of the fracture surfaces of the bond lines formed under 5 MPa for 90 s at (a,d) 330 °C, (b,e) 350 °C, and (c,f) 370 °C using the sheet containing bimodal Cu@Ag particles in air.
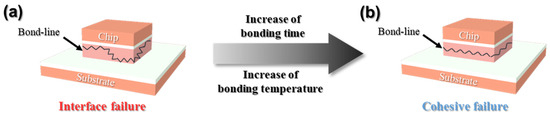
Figure 10.
Schematic of the fracture modes in the bond line: (a) interfacial and (b) cohesive fracture.
4. Conclusions
In this study, a novel bonding material, in the form of a sheet containing bimodal Cu@Ag particles, was developed considering potential applications in high-power semiconductor devices and high-heat-flux LEDs. To evaluate its rapid sinter-bonding behavior under ambient conditions, bonding tests were conducted between the Ag-finished dummy Cu chips and substrates. The proposed bonding sheet was designed to decompose and can be removed during sintering, ultimately forming a thermally stable and highly conductive metallic bond line. Submicron-sized Cu@Ag particles were synthesized using an in-house aqueous reduction method, and a bimodal particle system was achieved by mixing micron- and submicron-sized Cu@Ag particles in a weight ratio of 6:4 to facilitate fast sintering. The prepared particles were uniformly mixed with a resin formulation to produce a paste that was printed and thermally dried to fabricate a bonding sheet.
The fabricated bonding sheet exhibited a significant reduction in the resin decomposition temperature by approximately 41 °C compared to the resin formulation alone, which is attributed to the catalytic effect of Cu oxide generated during heating. When sinter bonding was performed in air using this sheet, it was observed that both particle sintering and the average shear strength improved with increasing bonding temperature and time. For example, at 350 °C, a sufficient shear strength of 23.4 MPa was achieved with only 30 s of bonding, while under the most aggressive conditions of 370 °C for 90 s, the porosity was remarkably reduced to 2.5%, and a high shear strength of 33.7 MPa was obtained. Furthermore, the fracture mode shifted from interfacial to cohesive failure as the bonding conditions became more severe. The improvements in bond line strength and the transition in fracture behavior were closely correlated with the reduction in residual resin and the associated enhancement of sinterability. These findings confirm that the bimodal Cu@Ag particle-based sheet is a promising alternative to conventional Ag-based bonding sheets. This system not only provides excellent cost competitiveness but also enables rapid sinter bonding in air, making it a viable candidate as a next-generation bonding material for high-power semiconductor and high-heat-flux LED applications.
Author Contributions
Conceptualization, J.-H.L.; methodology, H.-M.L. and J.-H.L.; validation, H.-M.L.; formal analysis, H.-M.L. and J.-H.L.; investigation, H.-M.L.; resources, J.-H.L.; data curation, H.-M.L.; writing—original draft preparation, H.-M.L. and J.-H.L.; writing—review and editing, J.-H.L.; visualization, H.-M.L.; supervision, J.-H.L.; project administration, J.-H.L.; funding acquisition, J.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No: 2021R1A2C1007400).
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Knoerr, M.; Kraft, S.; Schletz, A. Reliability assessment of sintered nano-silver die attachment for power semiconductors. In Proceedings of the 12th Electronics Packaging Technology Conference, Singapore, 8–10 December 2010; pp. 56–61. [Google Scholar]
- Jakubowska, M.; Jarosz, M.; Kiełbasinski, K.; Młożniak, A. New conductive thick-film paste based on silver nanopowder for high power and high temperature applications. Microelectron. Reliab. 2011, 51, 1235–1240. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.; Suetake, A.; Hsieh, M.-C.; Suganuma, K. Reliability of Ag sinter-joining die attach under harsh thermal cycling and power cycling tests. J. Electron. Mater. 2021, 50, 6597–6606. [Google Scholar] [CrossRef]
- Tobita, M.; Yasuda, Y.; Ide, E.; Ushio, J.; Morita, T. Optimal design of coating material for nanoparticles and its application for low-temperature interconnection. J. Nanopart. Res. 2010, 12, 2135–2144. [Google Scholar] [CrossRef]
- Bai, J.G.; Lu, G.-Q. Thermomechanical reliability of low-temperature sintered silver die-attachment. In Proceedings of the IEEE Transactions on Device and Materials Reliability, Chicago, IL, USA, 26–28 September 2006. [Google Scholar]
- Siow, K.S.; Lin, Y.T. Identifying the development state of sintered silver (Ag) as a bonding material in the microelectronic packaging via a patent landscape study. J. Electron. Packag. 2016, 138, 020804. [Google Scholar] [CrossRef]
- Wan, M.F.; Chiew, Y.H.; Tey, Y.Y. Detailed analysis of conductive die attach film in miniature package. In Proceedings of the 2022 IEEE 39th International Electronics Manufacturing Technology Conference (IEMT), Kuala Lumpur, Putrajaya, Malaysia, 19–21 October 2022. [Google Scholar]
- Seng, A.L.W.; Yu, L.; Shyan, P.H.; Murali, S.; Kumar, B.S.; Kang, S.S. Non-pressure Ag sinter paste without residual bleed-out: Studied on different Au based substrates. In Proceedings of the 2022 IEEE 24th Electronics Packaging Technology Conference (EPTC), Singapore, 7–9 December 2022. [Google Scholar]
- Wang, X.; Liu, L.; Liu, J.; Zhang, J.; Du, X.; Zhang, Z. Optical measurement for the alarming height of silver paste overflow surrounding APD chip in optical component packaging. IEEE Trans. Instrum. Meas. 2025, 74, 5012310. [Google Scholar] [CrossRef]
- Yan, H.; Mei, Y.; Li, X.; Li, H.; Lu, G.-Q. Effect of as-printed bondline thickness on assembling high power laser diodes by sintering of nanosilver paste. In Proceedings of the 17th International Conference on Electronic Packaging Technology (ICEPT), Wuhan, China, 16–19 August 2016. [Google Scholar]
- Dai, J.; Li, J.; Agyakwa, P.; Corfield, M.; Johnson, C.M. Shear strength of die attachments prepared using dry nanosilver film by a time-reduced sintering process. Microelectron. Reliab. 2020, 111, 113740. [Google Scholar] [CrossRef]
- Yamagishi, M.; Ishii, Y.; Kirihata, T.; Miyawaki, M. A novel ultra-thin dicing die attach film for various dicing processes. In Proceedings of the 2024 IEEE 10th Electronics System-Integration Technology Conference (ESTC), Berlin, Germany, 11–13 September 2024. [Google Scholar]
- Le Henaff, F.; Greca, G.; Salerno, P.; Mathieu, O.; Reger, M.; Khaselev, O. Reliability of double side silver sintered devices with various substrate metallization. In Proceedings of the PCIM Europe; International Exhibition and Conference for Power Electronics, Intelligent Motion, Renewable Energy and Energy Management, Nuremberg, Germany, 10–12 May 2016. [Google Scholar]
- Khazaka, R.; Thollin, B.; Mendizabal, L.; Henry, D.; Khazaka, R.; Hanna, R. Characterization of nanosilver dry films for high-temperature applications. IEEE Trans. Device Mater. Reliab. 2015, 15, 149–155. [Google Scholar] [CrossRef]
- Greca, G.; Salerno, P.; Durham, J.; Le Henaff, F.; Harel, J.C.; Hamelink, J. Double side sintered IGBT 650 V/200 A in a TO-247 package for extreme performance and reliability. In Proceedings of the 2016 IEEE 18th Electronics Packaging Technology Conference (EPTC), Singapore, 30 November–3 December 2016. [Google Scholar]
- Choi, E.B.; Lee, J.-H. Submicron Ag-coated Cu particles and characterization methods to evaluate their quality. J. Alloys Compd. 2016, 689, 952–958. [Google Scholar] [CrossRef]
- De Oliveira, G.M.; Barbosa, L.L.; Broggi, R.L.; Carlos, I.A. Voltammetric study of the influence of EDTA on the silver electrodeposition and morphological and structural characterization of silver films. J. Electroanal. Chem. 2005, 578, 151–158. [Google Scholar] [CrossRef]
- Kim, Y.J.; Eom, Y.-S.; Choi, K.-S.; Lee, J.-H. Suppression of Ag dewetting and sinterability improvement of submicron Ag-coated Cu particles as fillers in sintering paste by surface modification with stearic acid. Trans. Nonferrous Met. Soc. China 2025, 35, 2008–2020. [Google Scholar] [CrossRef]
- Kamat, G.A.; Yan, C.; Osowiecki, W.T.; Moreno-Hernandez, I.A.; Ledendecker, M.; Alivisatos, A.P. Self-limiting shell formation in Cu@Ag core–shell nanocrystals during galvanic replacement. J. Phys. Chem. Lett. 2020, 11, 5318–5323. [Google Scholar] [CrossRef] [PubMed]
- Kociołek-Balawejder, E.; Stanisławska, E.; Jacukowicz-Sobala, I.; Mucha, I. Copper rich composite materials based on carboxylic cation exchangers and their thermal transformation. Polymers 2021, 13, 3199. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-G.; Yeh, C.S. The effects of copper oxides on the thermal degradation of bismaleimide triazine prepreg. Polym. Degrad. Stab. 2004, 83, 529–537. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, H.-J.; Kim, J.-H.; Kim, J.-H.; Kang, S.-H.; Ryu, J.-H.; Park, N.-K.; Yun, D.-S.; Bae, J.-W. Noble-metal-based catalytic oxidation technology trends for volatile organic compound (VOC) removal. Catalysts 2022, 12, 63. [Google Scholar] [CrossRef]
- Liu, R.; Wu, H.; Shi, J.; Xu, X.; Zhao, D.; Ng, Y.H.; Zhang, M.; Liu, S.; Ding, H. Recent progress on catalysts for catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2022, 12, 6945–6991. [Google Scholar] [CrossRef]
- Nakako, H.; Natori, M.; Ishikawa, D.; Kazuhiko, M.; Matsushima, S. Relationship between the porosity in Cu sintered bonding and thermal cycle reliability. In Proceedings of the PCIM Europe; International Exhibition and Conference for Power Electronics, Intelligent Motion, Renewable Energy and Energy Management, Nürnberg, Germany, 11–13 June 2024. [Google Scholar]
- Wu, Y.; Zou, G.; Wang, S.; Guo, W.; Zhang, H.; Peng, P.; Feng, B.; Liu, L. Rapid and low temperature sintering bonding using Cu nanoparticle film for power electronic packaging. Appl. Surf. Sci. 2022, 603, 154422. [Google Scholar] [CrossRef]
- Nakako, H.; Natori, M.; Ishikawa, D.; Tanaka, T.; Ejiri, Y. Copper sintering pastes for die bonding. In Proceedings of the PCIM Europe Digital Days 2021, International Exhibition and Conference for Power Electronics, Intelligent Motion, Renewable Energy and Energy Management, Online, 3–7 May 2021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).