Microstructure Evolution of a TRIP Fe–1.4Si–2.6Mn–0.17C Steel After Intercritical Treating and Its Effect on Mechanical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Numerical Methodology
2.2. Experimental Methodology
3. Results and Discussion
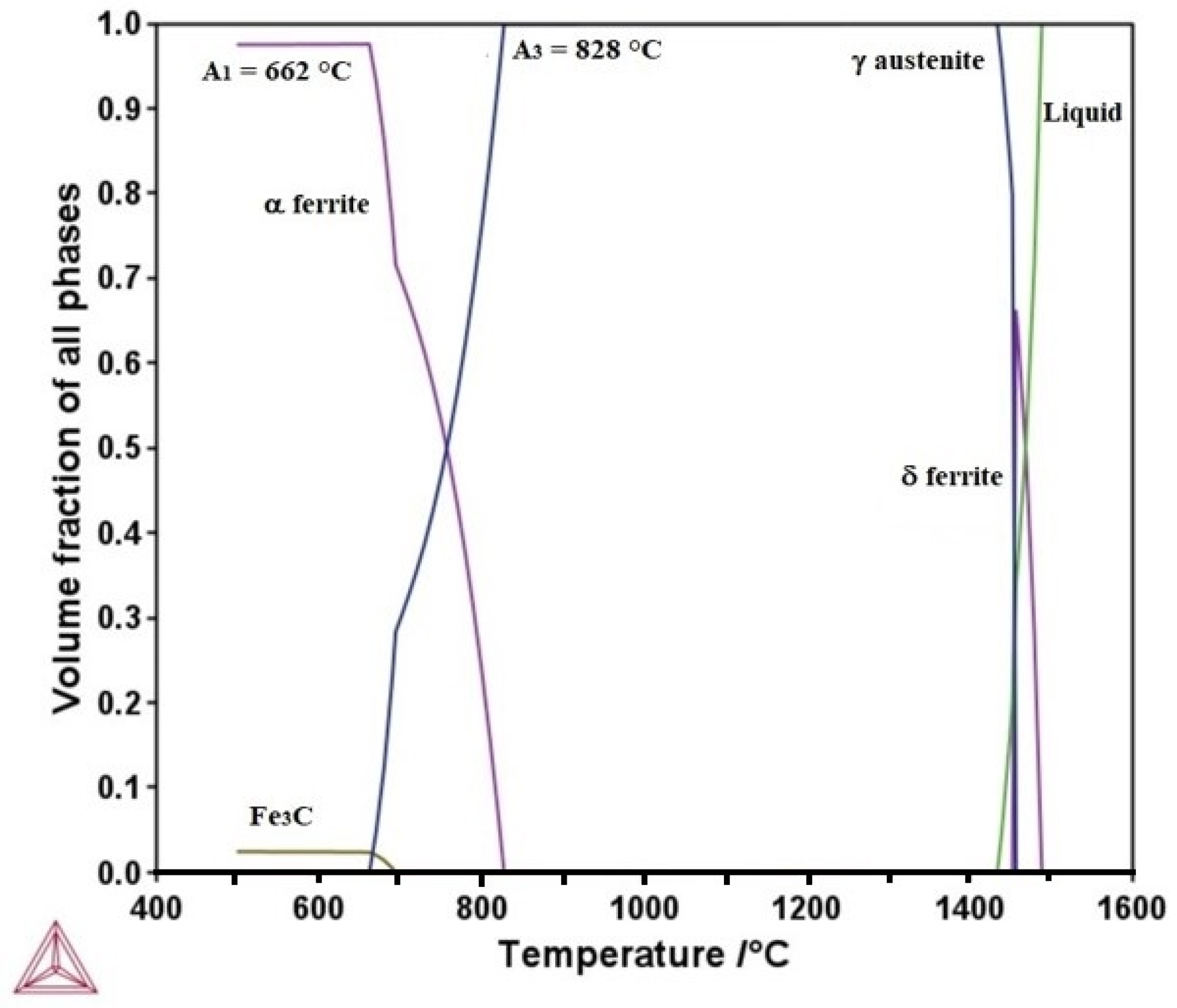
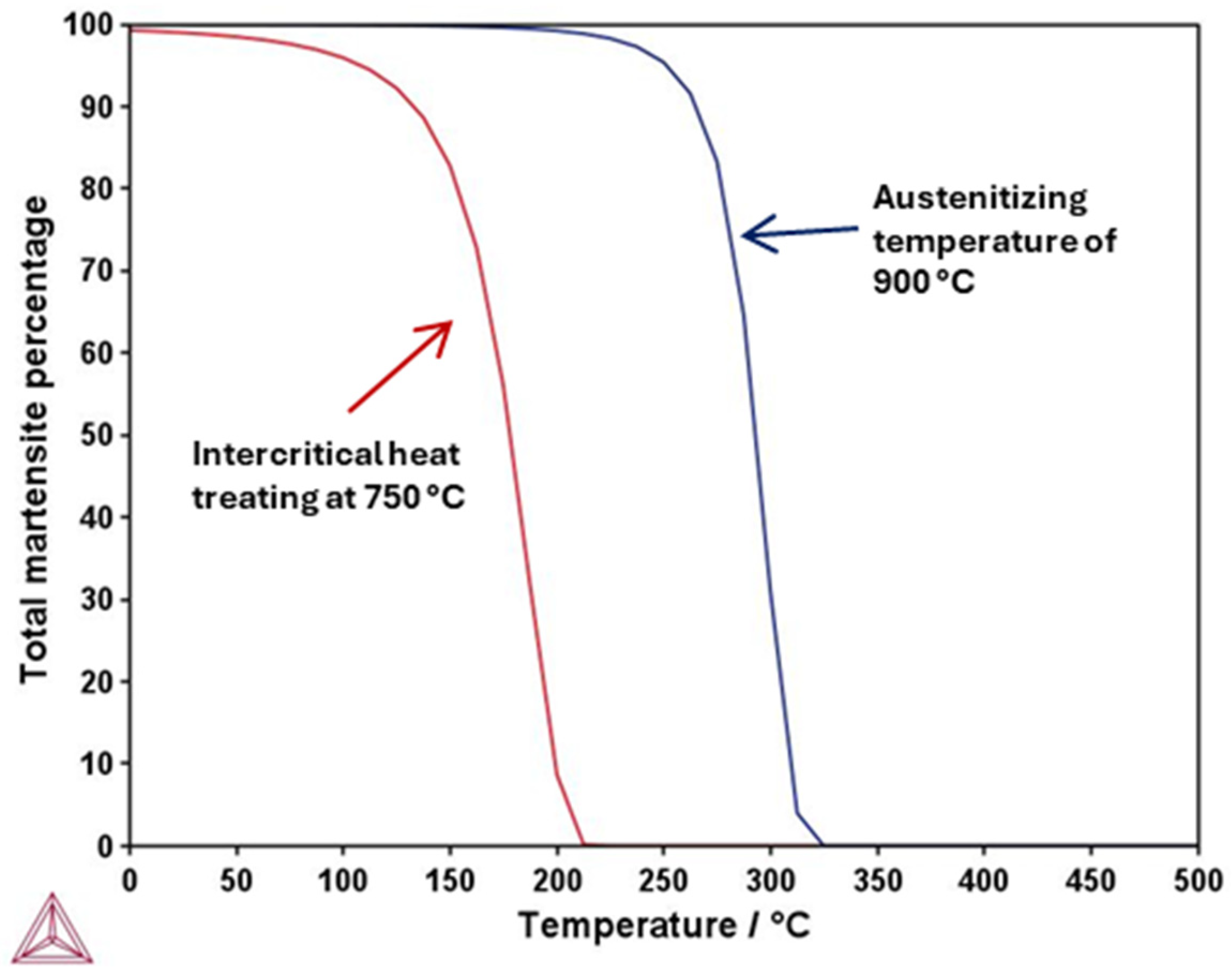
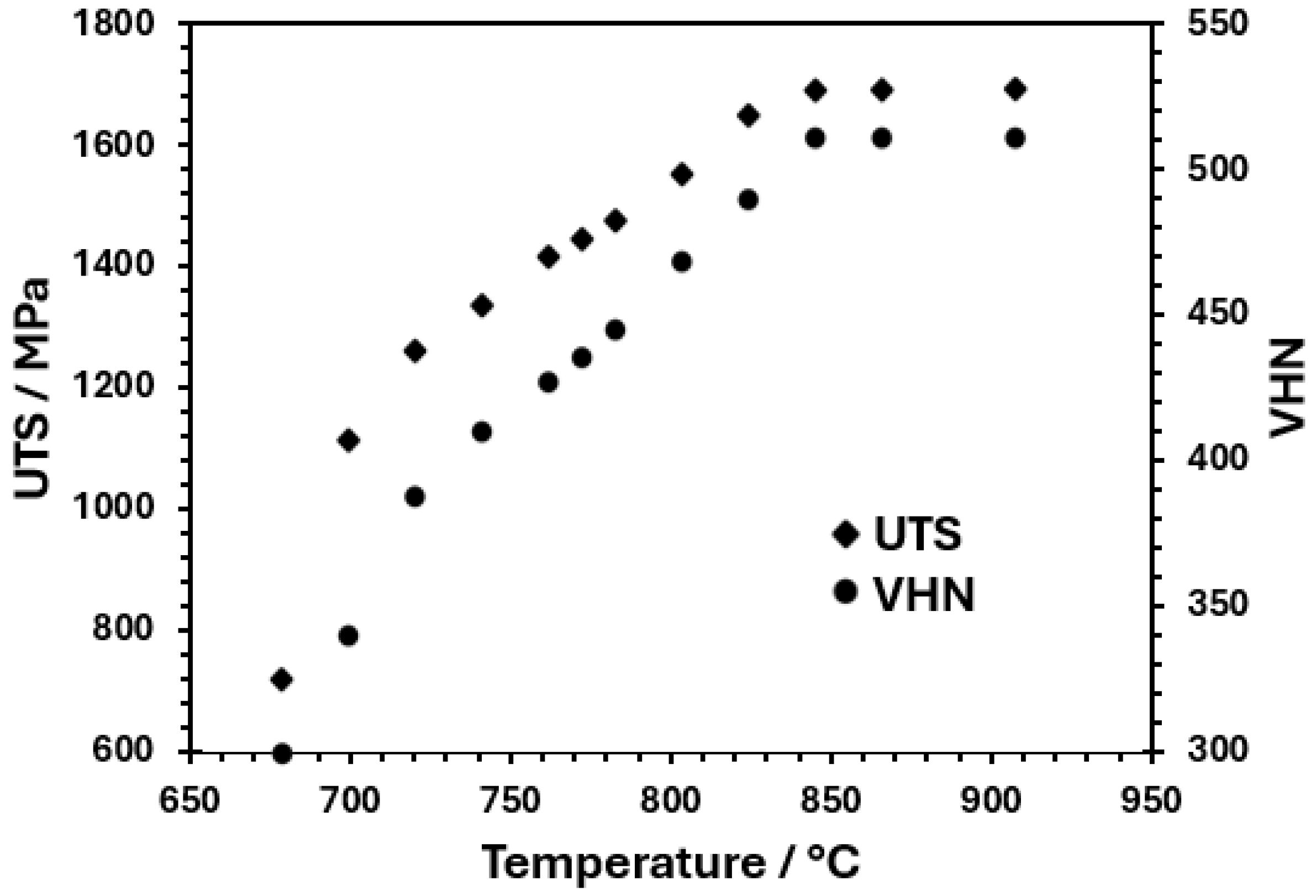
3.1. Thermo-Calc Analysis of Equilibrium and Nonequilibrium Phases
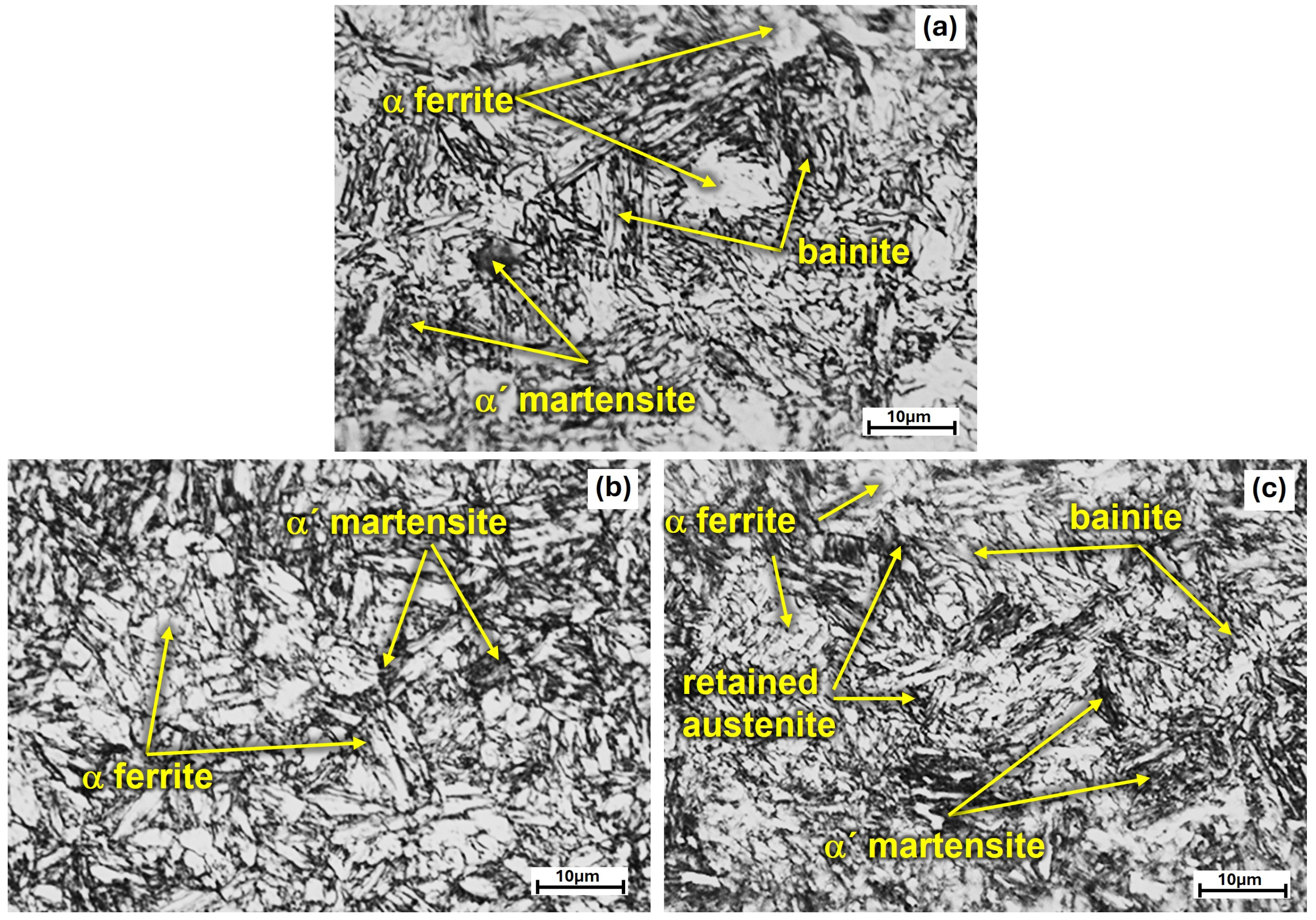
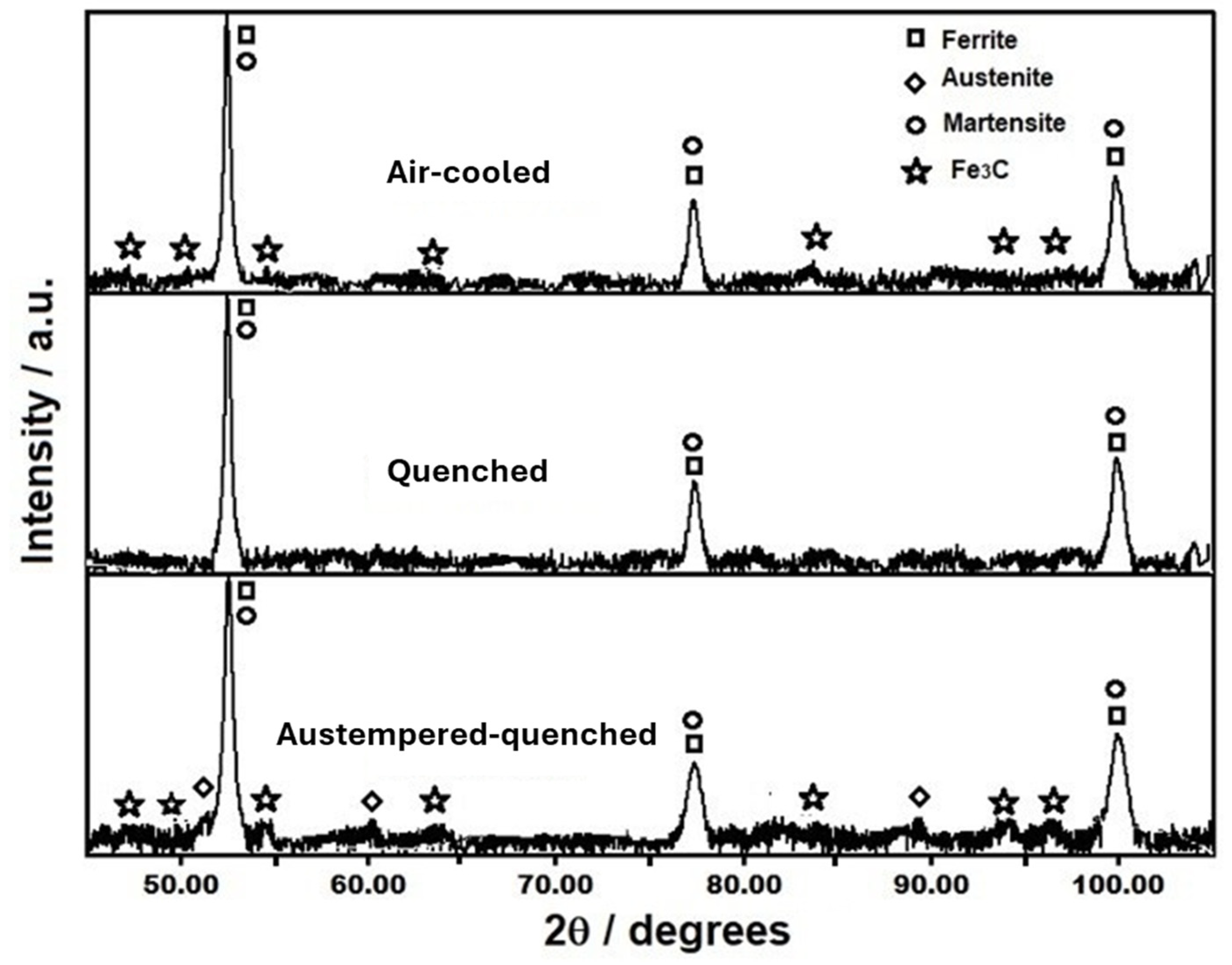
3.2. Microstructural Characterization of Heat Treated Steel
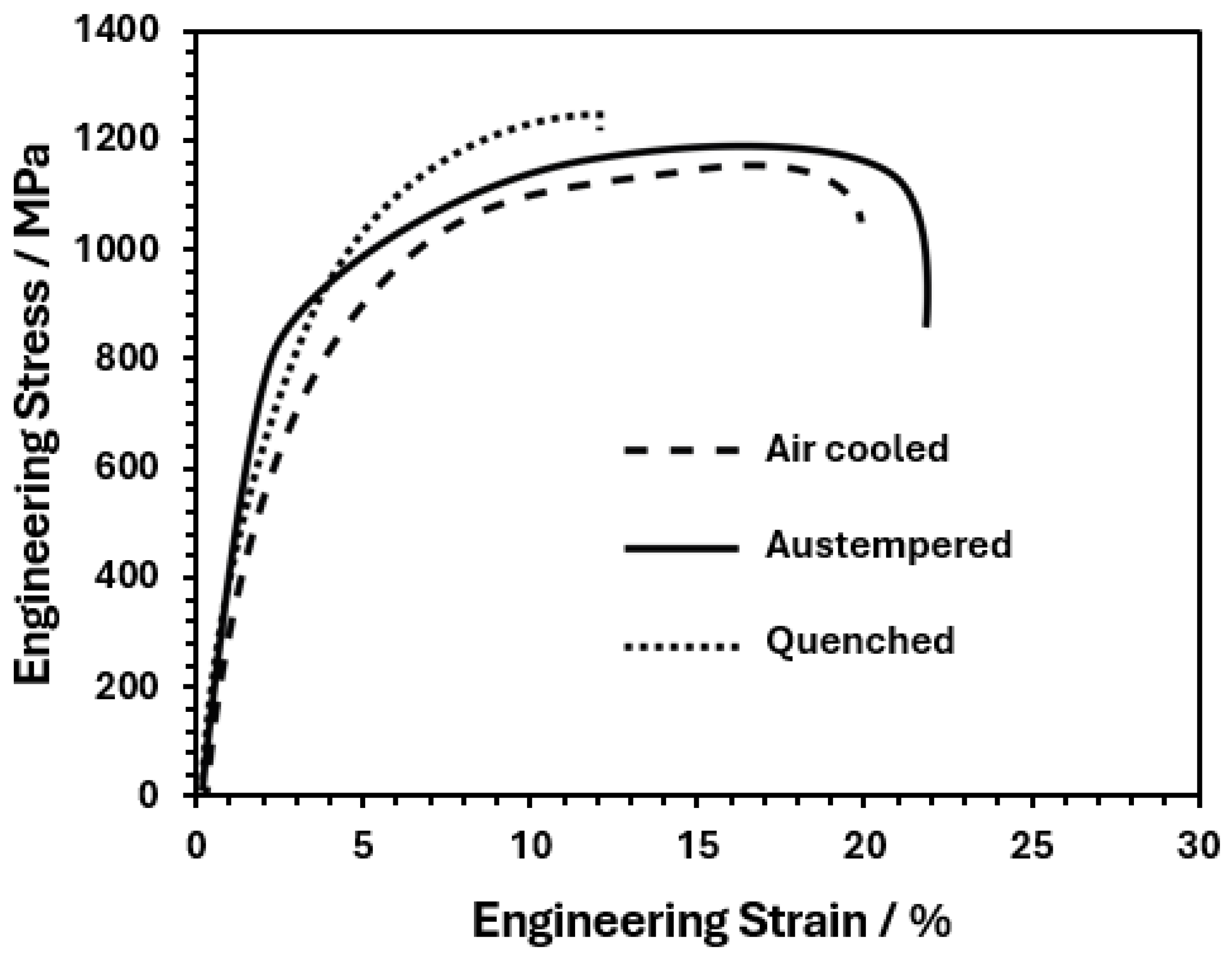
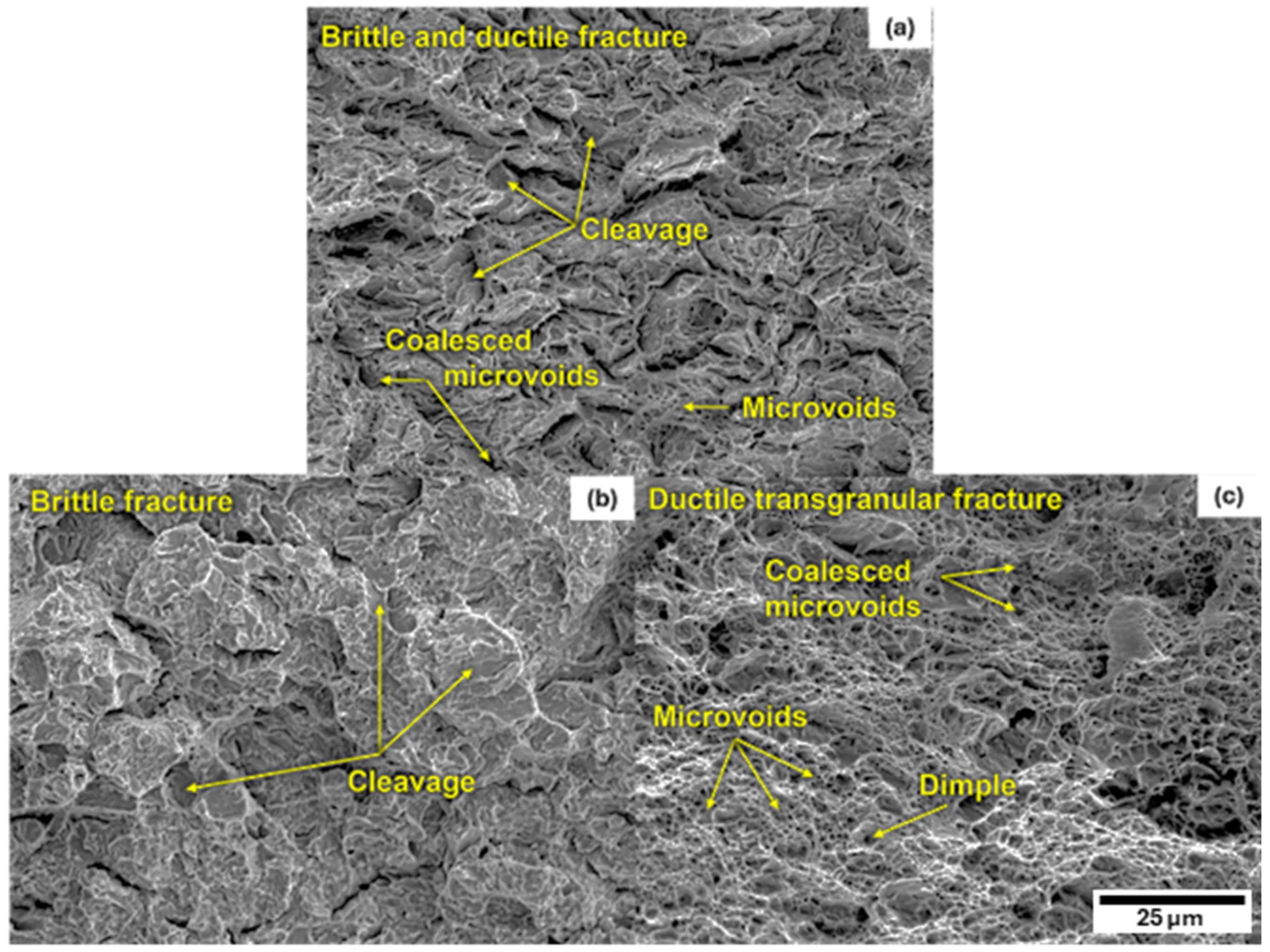
3.3. Mechanical Characterization of Heat-Treated Steel
3.4. Relation of Microstructure to Mechanical Properties
4. Conclusions
- TC-calculated microstructure evolution and mechanical properties for the heat- treated steel specimens agree well with the experimental ones. Additionally, Thermo-Calc permits the determination of TTT diagrams at intercritical temperatures, which can be used to design heat treatments and to predict the phases and microconstituents after heat treatment.
- The quenching treatment of this TRIP steel, from intercritical temperatures, is a viable alternative for producing hardened martensite with VHN value up to 430 and UTS of 1255 MPa. However, ductility decreases as the intercritical treatment temperature increases due to the presence of a higher volume fraction of martensite.
- The austempered-quenched steel, after intercritical treatment, presented a tensile strength similar to that of the quenched steel but with more ductility, which is attributable to the stabilization of retained γ austenite associated with the high Si and Mn content.
- The air-cooling treatment of this TRIP steel is also a good possibility, since it provides a high VHN value of 406 and UTS of 1190 MPa, along with good ductility, due to the bainite formation and a small fraction of martensite during air cooling, which is attributed to its high hardenability, as shown in the TC-calculated TTT diagram at 750 °C.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rana, R.; Singh, S.B. (Eds.) Automotive Steels: Design, Metallurgy, Processing and Applications; Woodhead Publishing Series in Metals and Surface Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 217–249. [Google Scholar]
- Fonstein, N. Advanced High Strength Sheet Steels: Physical Metallurgy, Design, Processing, and Properties; Springer International Publishing: Cham, Switzerland, 2015; pp. 17–60. [Google Scholar]
- Avila, D.; van Bohemen, S.M.C.; Huizenga, R.M.; Offerman, S.E.; Santofimia, M.J. Shortening the heat treatment of third-generation AHSS by forming carbide-free bainite in the presence of martensite. Mater. Sci. Eng. A 2025, 931, 148241. [Google Scholar] [CrossRef]
- Morales-Rivas, L. Viewpoints on technological aspects of advanced high-strength bainitic steels. Metals 2022, 12, 195. [Google Scholar] [CrossRef]
- Bai, X.; Yu, Y.; Hu, B.; Wang, H.; Wang, W.; Liu, S.; Liu, W. Insights into the role of retained austenite stability in TRIP-aided steel: Ductilizing and toughening. J. Mater. Res. Technol. 2024, 33, 7698–7708. [Google Scholar] [CrossRef]
- Yang, G.; Zhuang, C.; Li, C.; Lan, F.; Yao, H. Study on high-temperature mechanical properties of Fe–Mn–C–Al TWIP/TRIP steel. Metals 2021, 11, 821. [Google Scholar] [CrossRef]
- Jia, G.; Li, Y.; Ding, W. Alloy composition and process design based on thermodynamic and kinetic simulation: The case of medium Mn steel. Calphad 2023, 82, 102601. [Google Scholar] [CrossRef]
- Mehrabi, A.; Zurob, H.S.; McDermid, J.R. Process maps for predicting austenite fraction (vol %) in medium-Mn third-generation AHSS. Materials 2024, 17, 993. [Google Scholar] [CrossRef]
- Lu, J.; Wang, S.; Yu, H.; Wu, G.; Gao, J.; Wu, H.; Zhao, H.; Zhang, C.; Mao, X. Structure-property relationship in vanadium micro-alloyed TRIP steel subjected to the isothermal bainite transformation process. Mater. Sci. Eng. A 2023, 878, 145208. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, C.G.; Choi, I.; Lee, S. Effects of heat treatment and alloying elements on the microstructures and mechanical properties of 0.15 wt % C TRIP-aided cold-rolled steel sheets. Metall. Mater. Trans. A 2001, 32A, 505–514. [Google Scholar] [CrossRef]
- Pantilimon, M.C.; Berbecaru, A.C.; Gherghescu, I.A.; Coman, G.; Ciucă, S.; Grecu, A.; Sohaciu, M.G.; Dumitrescu, R.E.; Predescu, C. Comparative evaluation of the TRIP effect in steels with different contents of Mn and Al. Metals 2022, 12, 443. [Google Scholar] [CrossRef]
- Morawiec, A.; Skowronek, A.; Kozłowska, A.; Garcia-Mateo, C.; Grajcar, A. Effect of prior martensite formation on the bainite transformation in kinetics in high-strength 3% Mn multiphase steel. J. Therm. Anal. Calorim. 2023, 148, 1365–1371. [Google Scholar] [CrossRef]
- Soleimani, M.; Kalhor, A.; Mirzadeh, H. Transformation-induced plasticity (TRIP) in advanced steels: A review. Mater. Sci. Eng. A 2020, 795, 140023. [Google Scholar] [CrossRef]
- Thermo-Calc Software. Solutions for ICME. Available online: https://thermocalc.com/solutions/solutions-for-icme/ (accessed on 1 July 2025).
- Kumpati, J.; Hasan, S.M.; Bonvalet Rolland, M.; Borgenstam, A. Retained austenite stability: A Comparative Study of Two-Phase and Bulk Microstructures. Metall. Mater. Trans. A 2024, 55, 466–476. [Google Scholar] [CrossRef]
- Mehrabi, A.; Zurob, H.S.; Benrabah, I.-E.; McDermid, J.R. Austenite formation in a medium-Mn steel during intercritical annealing via in situ high-energy X-ray diffraction. J. Mater. Res. Technol. 2024, 30, 2158–2167. [Google Scholar] [CrossRef]
- Lamari, M.; Allain, S.Y.P.; Geandier, G.; Ponçot, M.; Perlade, A.; Zhu, K.O. Behaviour of TRIP-aided medium Mn steels investigated by in situ synchrotron X-ray diffraction experiments and microstructure-based micromechanical modelling. Int. J. Plast. 2024, 173, 103866. [Google Scholar] [CrossRef]
- Kozłowska, A. Dilatometric study on phase transformations in non-deformed and plastically deformed medium-Mn multiphase steels with increased Al and Si additions. Materials 2024, 17, 1051–1058. [Google Scholar] [CrossRef]
- Thermo-Calc Software. Steel Model Library. Available online: https://thermocalc.com/products/add-on-modules/steel-model-library/ (accessed on 2 July 2025).
- Yan, J.; Ågren, J.; Jeppsson, J. Pearlite in multicomponent steels: Phenomenological steady-state modeling. Metall. Mater. Trans. A 2020, 51, 1978–2001. [Google Scholar] [CrossRef]
- Leach, L.; Kolmskog, P.; Höglund, L.; Hillert, M.; Borgenstam, A. Critical driving forces for formation of bainite. Metall. Mater. Trans. A 2018, 49, 4509–4520. [Google Scholar] [CrossRef]
- Leach, L.; Ågren, J.; Höglund, L.; Borgenstam, A. Diffusion-controlled lengthening rates of bainitic ferrite a part of the Steel Genome. Metall. Mater. Trans. A 2019, 50, 2613–2618. [Google Scholar] [CrossRef]
- Huyan, F.; Hedström, P.; Höglund, L.; Borgenstam, A. A thermodynamic-based model to predict the fraction of martensite in steels. Metall. Mater. Trans. A 2016, 47, 4404–4410. [Google Scholar] [CrossRef]
- Stormvinter, A.; Borgenstam, A.; Ågren, J. Thermodynamically based prediction of the martensite start temperature for commercial steels. Metall. Mater. Trans. A 2012, 43, 3870–3879. [Google Scholar] [CrossRef]
- Park, S.J.; Heogh, W.; Yang, J.; Kang, S.; Jeong, W.; Lee, H.; Jang, T.S.; Jung, H.D.; Jahazi, M.; Han, S.C.; et al. Meta-structure of amorphous-inspired 65.1Co28.2Cr5.3Mo lattices augmented by artificial intelligence. Adv. Compos. Hybrid Mater. 2024, 7, 224. [Google Scholar] [CrossRef]
- Thermo-Calc Software. Steel and Fe-Alloys Databases, TCFE Databases; Thermo-Calc Software: Solna, Sweden, 2021; Available online: https://thermocalc.com/products/databases/steel-and-fe-alloys/ (accessed on 1 July 2025).
- ASTM E8/E8M-22; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM E384-22; Standard Test Method for Microindentation Hardness of Materials. ASTM International: West Conshohocken, PA, USA, 2022.
- Callister, W.D., Jr.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 10th ed.; Wiley: Hoboken, NJ, USA, 2022; Chapter 9; p. 337. [Google Scholar]
- Bhadeshia, H.K.D.H.; Honeycombe, R.W.K. Steels: Microstructure and Properties, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2017; p. 61. [Google Scholar]
- Erişir, E.; Bilir, O.G. Effect of intercritical annealing temperature on phase transformations in medium carbon dual phase steels. J. Mater. Eng. Perform. 2014, 23, 1055–1061. [Google Scholar] [CrossRef]
- Hofer, C.; Leitner, H.; Winkelhofer, F.; Clemens, H.; Primig, S. Structural characterization of ‘carbide-free’ bainite in a Fe–0.2C–1.5Si–2.5Mn steel. Mater. Charact. 2015, 102, 85–91. [Google Scholar] [CrossRef]
- Gulbay, O.; Gramlich, A.; Krupp, U. Effects of silicon and aluminum alloying on phase transformation and microstructure evolution in Fe–0.2C–2.5Mn steel: Insights from continuous-cooling-transformation and time-temperature-transformation diagrams. Steel Res. Int. 2024, 95, 2400159. [Google Scholar] [CrossRef]
- Vander Voort, G.F. (Ed.) Metallography and Microstructures. In ASM Handbook; ASM International: Materials Park, OH, USA, 2004; Volume 9, pp. 103–462. [Google Scholar]
- Li, F.; Shang, X.; Zhao, J.; Wang, X.; Xu, X.; Wu, Z. Effects of annealing temperature on microstructure and mechanical properties of a low density δ-TRIP steel plate via intercritical quenching-annealing process. Mater. Sci. Eng. A 2024, 900, 146503. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, W.; Hu, F.; Wu, K.; Yershov, S. Influence of intercritical annealing temperature on TRIP/TWIP effect of retained austenite and tensile deformation mechanism of IQ&P steel. J. Mater. Res. Technol. 2024, 33, 3619–3634. [Google Scholar]
- Bhadhon, K.M.H.; Wang, X.; McNally, E.A.; McDermid, J.R. Effect of intercritical annealing parameters and starting microstructure on the microstructural evolution and mechanical properties of a medium-Mn third generation advanced high strength steel. Metals 2022, 12, 356. [Google Scholar] [CrossRef]
- Rana, R.; Cordova-Tapia, E.; Morales-Rivas, L.; Jimenez, J.A.; Garcia-Mateo, C. Mechanical behaviour and microstructural characteristics of high-silicon ultra-strong bainitic steels for hot rolling practice. Mater. Sci. Eng. A 2025, 931, 148236. [Google Scholar] [CrossRef]
- Hu, Z.; Fu, H. Effect of Si Content on Microstructure and Properties of Low-Carbon Medium-Manganese Steel after Intercritical Heat Treatment. Metals 2024, 14, 675. [Google Scholar] [CrossRef]
- Xiao, H.; Zhao, G.; Xu, D.; Cheng, Y.; Bao, S. Effect of Microstructure Morphology of Q&P Steel on Carbon and Manganese Partitioning and Stability of Retained Austenite. Metals 2022, 12, 1613. [Google Scholar] [CrossRef]
- Long, X.; Liu, W.; Zhu, R.; Zhang, Y.; Zhang, F.; Yang, Z.; Li, Y. Effect of the Cooling Rate in the Medium Temperature Zone on the Phase Transformation and Microstructure of Carbide-Free Bainitic Steel. J. Mater. Res. Technol. 2024, 29, 59. [Google Scholar] [CrossRef]



| Element | C | Mn | Si | Al | Ti |
| wt. % | 0.17 | 2.60 | 1.40 | 0.023 | 0.024 |
| Element | V | Cu | Nb | P | S |
| wt. % | 0.01 | 0.012 | 0.01 | 0.007 | 0.001 |
| Specimen | VHN | YS (MPa) | UTS (MPa) | Elongation % |
|---|---|---|---|---|
| Air-cooled | 406 | 690 | 1190 | 15 |
| Quenched | 430 | 665 | 1255 | 9 |
| Austempered-quenched | 420 | 820 | 1200 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda-Lopez, V.; Beltrán-Zúñiga, M.A.; Lopez-Hirata, V.M.; Dorantes-Rosales, H.J.; Saucedo-Muñoz, M.L. Microstructure Evolution of a TRIP Fe–1.4Si–2.6Mn–0.17C Steel After Intercritical Treating and Its Effect on Mechanical Properties. Metals 2025, 15, 1096. https://doi.org/10.3390/met15101096
Miranda-Lopez V, Beltrán-Zúñiga MA, Lopez-Hirata VM, Dorantes-Rosales HJ, Saucedo-Muñoz ML. Microstructure Evolution of a TRIP Fe–1.4Si–2.6Mn–0.17C Steel After Intercritical Treating and Its Effect on Mechanical Properties. Metals. 2025; 15(10):1096. https://doi.org/10.3390/met15101096
Chicago/Turabian StyleMiranda-Lopez, Valeria, Manuel Alejandro Beltrán-Zúñiga, Victor M. Lopez-Hirata, Hector J. Dorantes-Rosales, and Maribel L. Saucedo-Muñoz. 2025. "Microstructure Evolution of a TRIP Fe–1.4Si–2.6Mn–0.17C Steel After Intercritical Treating and Its Effect on Mechanical Properties" Metals 15, no. 10: 1096. https://doi.org/10.3390/met15101096
APA StyleMiranda-Lopez, V., Beltrán-Zúñiga, M. A., Lopez-Hirata, V. M., Dorantes-Rosales, H. J., & Saucedo-Muñoz, M. L. (2025). Microstructure Evolution of a TRIP Fe–1.4Si–2.6Mn–0.17C Steel After Intercritical Treating and Its Effect on Mechanical Properties. Metals, 15(10), 1096. https://doi.org/10.3390/met15101096







