Effect of Mechanical Activation on Electrochemical Properties of Chalcopyrite in Iron-Containing Sulfuric Acid Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chalcopyrite Samples
2.2. Mechanical Activation Experiments
2.3. Electrochemical Experiments
3. Results and Discussion
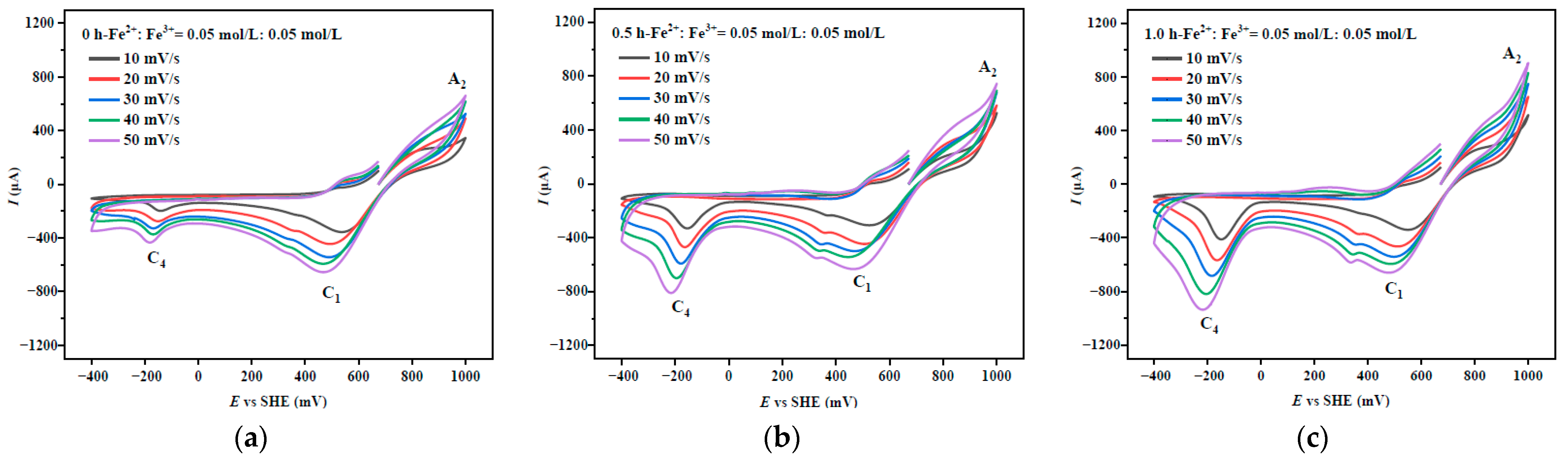
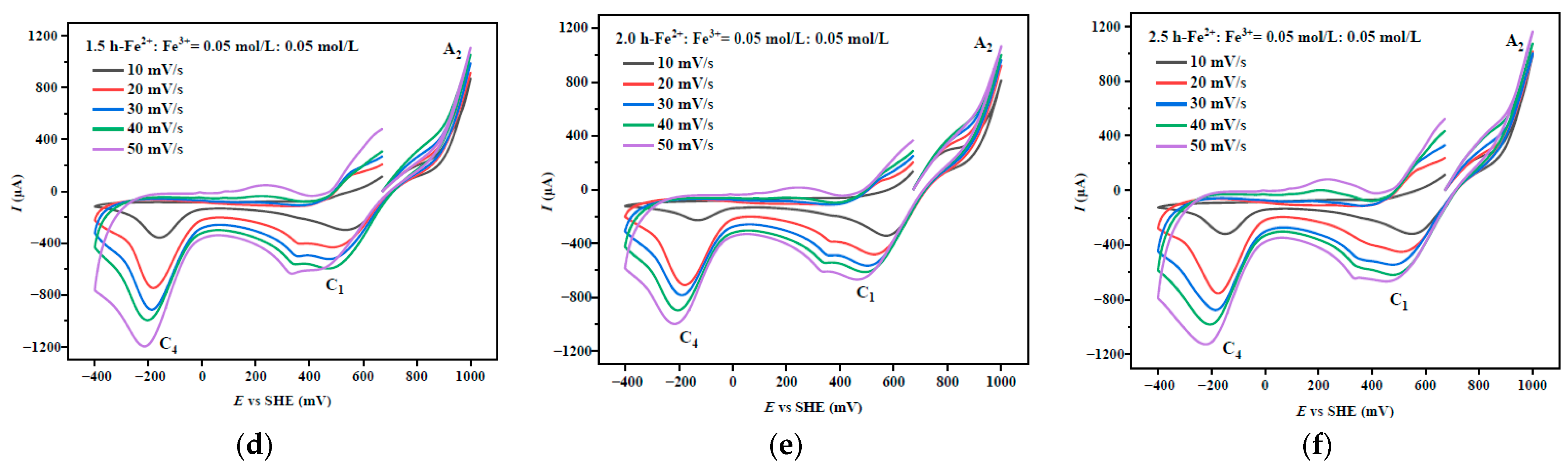
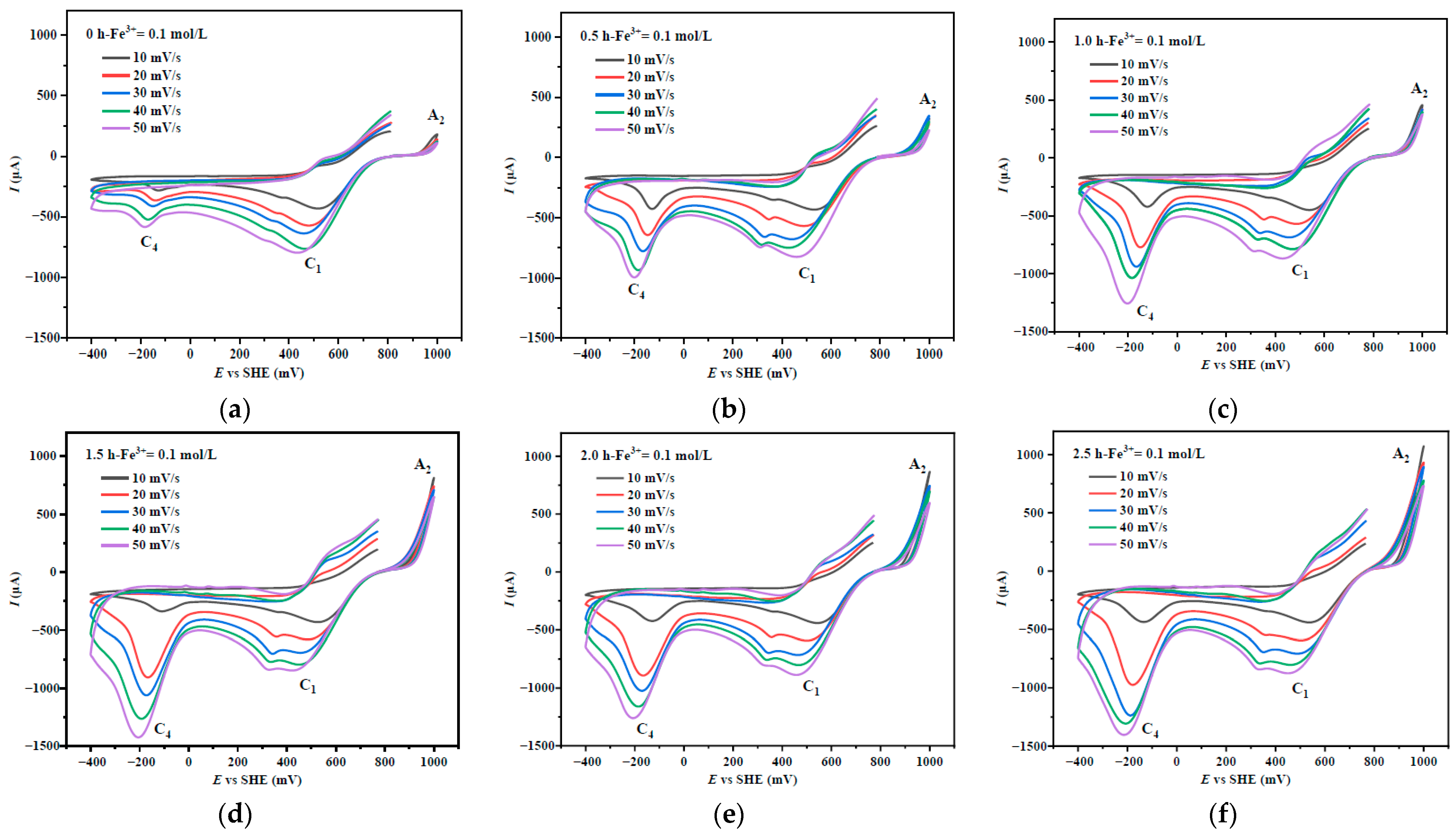
3.1. Cyclic Voltammetry of Chalcopyrite After Mechanical Activation
3.2. Effect of Mechanical Activation on the Semiconductor Properties of Chalcopyrite
3.3. Tafel Curve of Chalcopyrite After Mechanical Activation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kartal, M.; Xia, F.; Ralph, D.; Rickard, W.D.A.; Renard, F.; Li, W. Enhancing chalcopyrite leaching by tetrachloroethylene-assisted removal of sulphur passivation and the mechanism of jarosite formation. Hydrometallurgy 2020, 191, 105192. [Google Scholar] [CrossRef]
- Khoshkhoo, M.; Dopson, M.; Engström, F.; Sandström, Å. New insights into the influence of redox potential on chalcopyrite leaching behaviour. Miner. Eng. 2017, 100, 9–16. [Google Scholar] [CrossRef]
- Chen, B.W.; Wen, J.K. Feasibility study on heap bioleaching of chalcopyrite. Rare Met. 2013, 32, 524–531. [Google Scholar] [CrossRef]
- Ríos, D.; Bellenberg, S.; Christel, S.; Lindblom, P.; Giroux, T.; Dopson, M. Potential of single and designed mixed cultures to enhance the bioleaching of chalcopyrite by oxidation-reduction potential control. Hydrometallurgy 2024, 224, 106245. [Google Scholar] [CrossRef]
- Barton, I.F.; Hiskey, J.B. Chalcopyrite leaching in novel lixiviants. Hydrometallurgy 2022, 207, 105775. [Google Scholar] [CrossRef]
- Ahn, J.; Wu, J.J.; Lee, J. A Comparative Kinetic Study of Chalcopyrite Leaching Using Alternative Oxidants in Methanesulfonic Acid System. Mineral Process. Extr. Metall. Rev. 2022, 43, 390–401. [Google Scholar] [CrossRef]
- O’Connor, G.M.; Lepkova, K.; Eksteen, J.J.; Oraby, E.A. Electrochemical behaviour and surface analysis of chalcopyrite in alkaline glycine solutions. Hydrometallurgy 2018, 182, 32–43. [Google Scholar] [CrossRef]
- Jiang, L.S.; Leng, H.G.; Han, B.S. Dissolution and Passivation Mechanism of Chalcopyrite during Pressurized Water Leaching. Minerals 2023, 13, 996. [Google Scholar] [CrossRef]
- Zhou, S.; Gan, M.; Zhu, J.Y.; Li, Q.; Jie, S.Q.; Yang, B.J.; Liu, X.D. Catalytic effect of light illumination on bioleaching of chalcopyrite. Bioresour. Technol. 2015, 182, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Crundwell, F.K. The semiconductor mechanism of dissolution and the pseudo-passivation of chalcopyrite. Can. Metall. Q. 2015, 54, 279–288. [Google Scholar] [CrossRef]
- Zhao, H.B.; Huang, X.T.; Wang, J.; Li, Y.N.; Liao, R.; Wang, X.X.; Qiu, X.; Xiong, Y.M.; Qin, W.Q.; Qiu, G.Z. Comparison of bioleaching and dissolution process of p-type and n-type chalcopyrite. Miner. Eng. 2017, 109, 153–161. [Google Scholar] [CrossRef]
- Zhao, C.X.; Yang, B.J.; Liao, R.; Hong, M.X.; Yu, S.C.; Liu, S.T.; Wang, J.; Qiu, G.Z. Combined effect and mechanism of visible light and Ag+ on chalcopyrite bioleaching. Miner. Eng. 2022, 175, 107283. [Google Scholar] [CrossRef]
- Li, Y.B.; Wang, B.; Xiao, Q.; Lartey, C.; Zhang, Q.W. The mechanisms of improved chalcopyrite leaching due to mechanical activation. Hydrometallurgy 2017, 173, 149–155. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, W.; Zhao, S.; Lu, D.; Xie, F.; Dreisinger, D. Effect of Mechanical Activation on Leaching Behavior and Mechanism of Chalcopyrite. Miner. Process. Extr. Metall. Rev. 2022, 43, 440–452. [Google Scholar] [CrossRef]
- Xiao, Q.; Qiu, G.Z.; Hu, Y.H. Computational simulation to mechanical activation of pyrite (I)—Relation of structural strain to chemistry reaction activity. Chin. J. Nonferrous Met. 2001, 11, 900–905. [Google Scholar] [CrossRef]
- Wei, Z.L.; Yang, X.; Li, W.Q.; Ma, Q.; Wu, X.Y.; Li, Y.B. An Improved Understanding of Chalcopyrite Leaching Mechanisms: The Influence of Anisotropic Crystal Planes. Minerals 2023, 13, 1461. [Google Scholar] [CrossRef]
- Baláž, P. Mechanical activation in hydrometallurgy. Int. J. Miner. Process. 2003, 72, 341–354. [Google Scholar] [CrossRef]
- Granata, G.; Takahashi, K.; Kato, T.; Tokoro, C. Mechanochemical activation of chalcopyrite: Relationship between activation mechanism and leaching enhancement. Miner. Eng. 2019, 131, 280–285. [Google Scholar] [CrossRef]
- Cao, S.T.; Zheng, X.F.; Nie, Z.Y.; Zhou, Y.H.; Liu, H.C.; Chen, J.H.; Yang, H.Y.; Xia, J.L. Mechanical Activation on Bioleaching of Chalcopyrite: A New Insight. Minerals 2020, 10, 788. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhao, S.X.; Wang, G.R.; Zhang, Q.; Jin, Z.N.; Tong, L.L.; Chen, G.B.; Qiu, X.M. Mechanical activation modes of chalcopyrite concentrate and relationship between microstructure and leaching efficiency. Hydrometallurgy 2022, 207, 105778. [Google Scholar] [CrossRef]
- Yang, C.R.; Jiao, F.; Qin, W.Q. Leaching of chalcopyrite: An emphasis on effect of copper and iron ions. J. Cent. South Univ. 2018, 25, 2380–2386. [Google Scholar] [CrossRef]
- Vilcáez, J.; Yamada, R.; Inoue, C. Effect of pH reduction and ferric ion addition on the leaching of chalcopyrite at thermophilic temperatures. Hydrometallurgy 2009, 96, 62–71. [Google Scholar] [CrossRef]
- Vilcáez, J.; Inoue, C. Mathematical modeling of thermophilic bioleaching of chalcopyrite. Miner. Eng. 2009, 22, 951–960. [Google Scholar] [CrossRef]
- Kaplun, K.; Li, J.; Kawashima, N.; Gerson, A.R. Cu and Fe chalcopyrite leach activation energies and the effect of added Fe3+. Geochim. Cosmochim. Acta 2011, 75, 5865–5878. [Google Scholar] [CrossRef]
- Córdoba, E.M.; Muñoz, J.A.; Blázquez, M.L.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Santos, A.L.A.; Arena, F.A.; Benedetti, A.V.; Bevilaqua, D. Effect of redox potential on chalcopyrite dissolution imposed by addition of ferrous ions. Eclética Quím. J. 2017, 42, 40. [Google Scholar] [CrossRef]
- Yang, C.R.; Li, Y.X.; Tian, Z.Y.; Qin, W.Q.; Liu, X.D.; Wang, X. Enhanced chalcopyrite leaching by mechanical activation: New insights from microstructure. Miner. Eng. 2024, 212, 108719. [Google Scholar] [CrossRef]
- Li, Y.X.; Tian, Z.Y.; Wang, X.; Wen, J.; Mao, Q.M.; Yang, C.R. Enhanced chalcopyrite bioleaching with mechanical activation and redox potential regulation. Miner. Eng. 2025, 227, 109292. [Google Scholar] [CrossRef]
- Xie, X.; Gan, T.; Sun, D.; Wu, K. Application of Multi-walled Carbon Nanotubes/Nafion Composite Film in Electrochemical Determination of Pb2+. Fuller. Nanotub. Carbon Nanostruct. 2008, 16, 103–113. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Chen, M.; Qian, D.J. Electrochemistry of Single-Walled Carbon Nanotubes-Viologen Composites on the Glass Carbon Electrodes. J. Nanosci. Nanotechnol. 2009, 9, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Nazari, G.; Dixon, D.G.; Dreisinger, D.B. The Mechanism of Chalcopyrite Leaching in the Presence of Silver-Enhanced Pyrite in the Galvanox™ Process. Hydrometallurgy 2012, 113–114, 122–130. [Google Scholar] [CrossRef]
- Liang, C.L.; Xia, J.L.; Yang, Y.; Nie, Z.Y.; Zhao, X.J.; Zheng, L.; Ma, C.Y.; Zhao, Y.D. Characterization of the thermo-reduction process of chalcopyrite at 65 °C by cyclic voltammetry and XANES spectroscopy. Hydrometallurgy 2011, 107, 13–21. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Tomashevich, Y.V.; Asanov, I.P.; Okotrub, A.V.; Varnek, V.A.; Vyalikh, D.V. Spectroscopic and electrochemical characterization of the surface layers of chalcopyrite (CuFeS2) reacted in acidic solutions. Appl. Surf. Sci. 2004, 225, 395–409. [Google Scholar] [CrossRef]
- Elsherief, A.E. The influence of cathodic reduction, Fe2+ and Cu2+ ions on the electrochemical dissolution of chalcopyrite in acidic solution. Miner. Eng. 2002, 15, 215–223. [Google Scholar] [CrossRef]
- Zeng, W.M.; Qiu, G.Z.; Zhou, H.B.; Chen, M. Electrochemical behaviour of massive chalcopyrite electrodes bioleached by moderately thermophilic microorganisms at 48 °C. Hydrometallurgy 2011, 105, 259–263. [Google Scholar] [CrossRef]
- Sauber, M.; Dixon, D.G. Electrochemical study of leached chalcopyrite using solid paraffin-based carbon paste electrodes. Hydrometallurgy 2011, 110, 1–12. [Google Scholar] [CrossRef]
- Price, D.W.; Warren, G.W. The influence of silver ion on the electrochemical response of chalcopyrite and other mineral sulfide electrodes in sulfuric acid. Hydrometallurgy 1986, 15, 303–324. [Google Scholar] [CrossRef]
- López-Juárez, A.; Gutiérrez-Arenas, N.; Rivera-Santillán, R.E. Electrochemical behavior of massive chalcopyrite bioleached electrodes in presence of silver at 35 °C. Hydrometallurgy 2006, 83, 63–68. [Google Scholar] [CrossRef]
- Gómez, C.; Figueroa, M.; Muñoz, J.; Blázquez, M.L.; Ballester, A. Electrochemistry of chalcopyrite. Hydrometallurgy 1996, 43, 331–344. [Google Scholar] [CrossRef]
- Gu, G.H.; Hu, K.T.; Zhang, X.; Xiong, X.X.; Yang, H.S. The stepwise dissolution of chalcopyrite bioleached by Leptospirillum ferriphilum. Electrochim. Acta 2013, 103, 50–57. [Google Scholar] [CrossRef]
- Lee, M.S.; Nicol, M.J.; Basson, P. Cathodic processes in the leaching and electrochemistry of covellite in mixed sulfate–chloride media. J. Appl. Electrochem. 2008, 38, 363–369. [Google Scholar] [CrossRef]
- Nava, D.; González, I.; Leinen, D.; Ramos-Barrado, J.R. Surface characterization by X-ray photoelectron spectroscopy and cyclic voltammetry of products formed during the potentiostatic reduction of chalcopyrite. Electrochim. Acta 2008, 53, 4889–4899. [Google Scholar] [CrossRef]
- Nava, D.; González, I. Electrochemical characterization of chemical species formed during the electrochemical treatment of chalcopyrite in sulfuric acid. Electrochim. Acta 2006, 51, 5295–5303. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.F.; Luo, J.L. Electronic Structure and Pitting Susceptibility of Passive Film on Carbon Steel. Electrochim. Acta 1999, 44, 2947–2957. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; Gotor, F.J.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571. [Google Scholar] [CrossRef] [PubMed]
- Flitt, H.J.; Schweinsberg, D.P. Evaluation of corrosion rate from polarisation curves not exhibiting a Tafel region. Corros. Sci. 2005, 47, 3034–3052. [Google Scholar] [CrossRef]
- Rybalka, K.V.; Beketaeva, L.A.; Bukhan’ko, N.G.; Davydov, A.D. Electrochemical behavior and the rate of general corrosion of NiAl intermetallic compound in the unbuffered sodium chloride solutions. Corros. Sci. 2011, 53, 630–636. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS Analysis of Chalcopyrite (CuFeS2) Dissolution in Sulfuric Acid Solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]


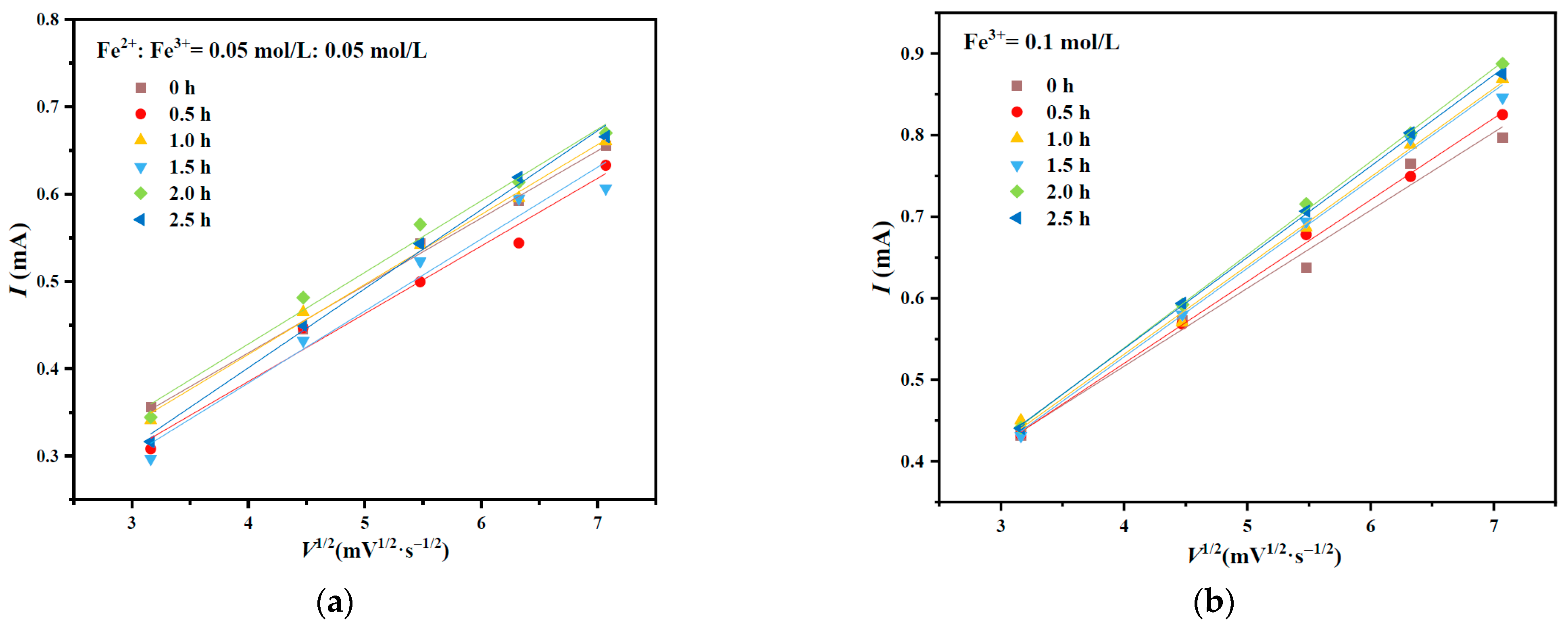

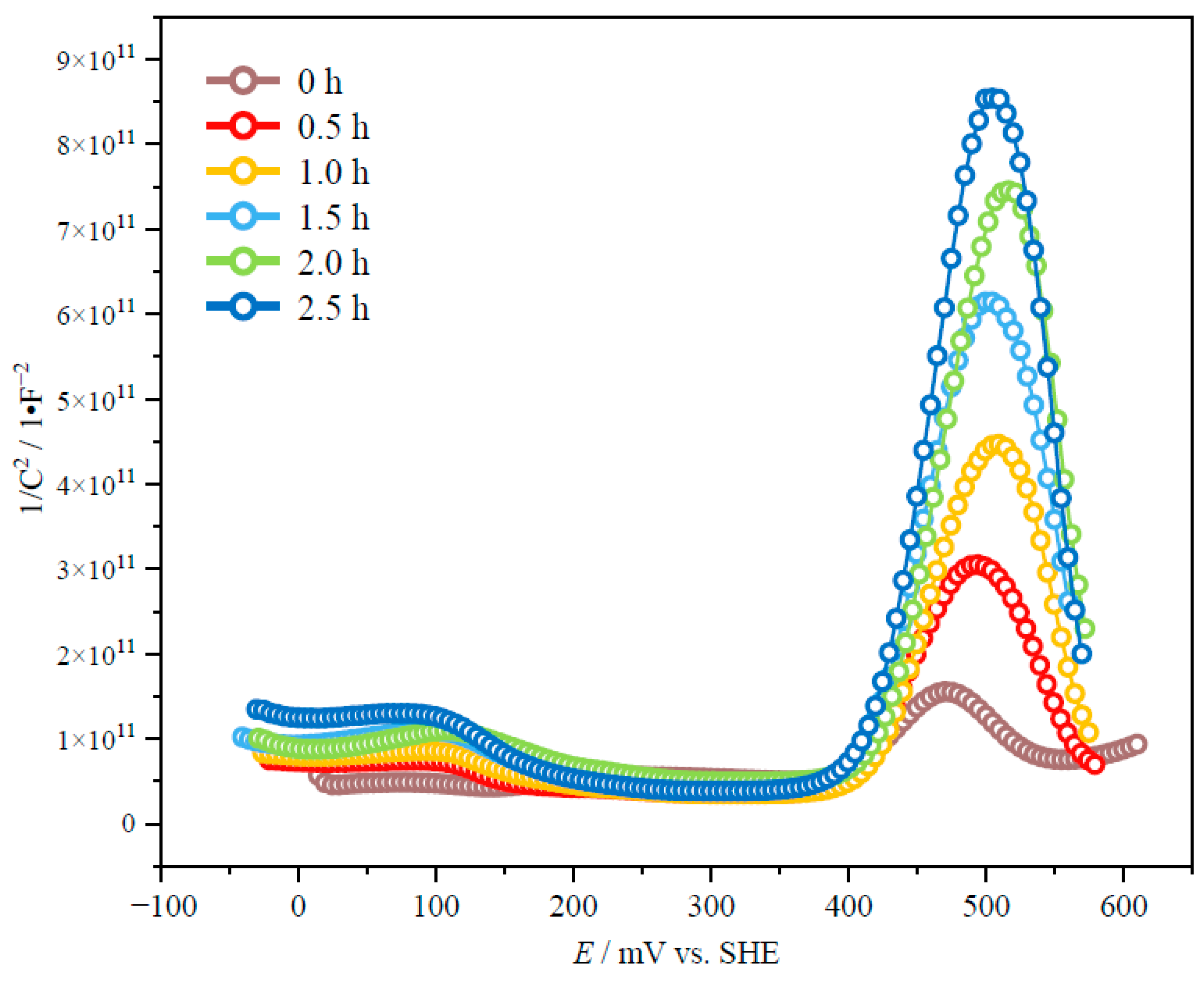
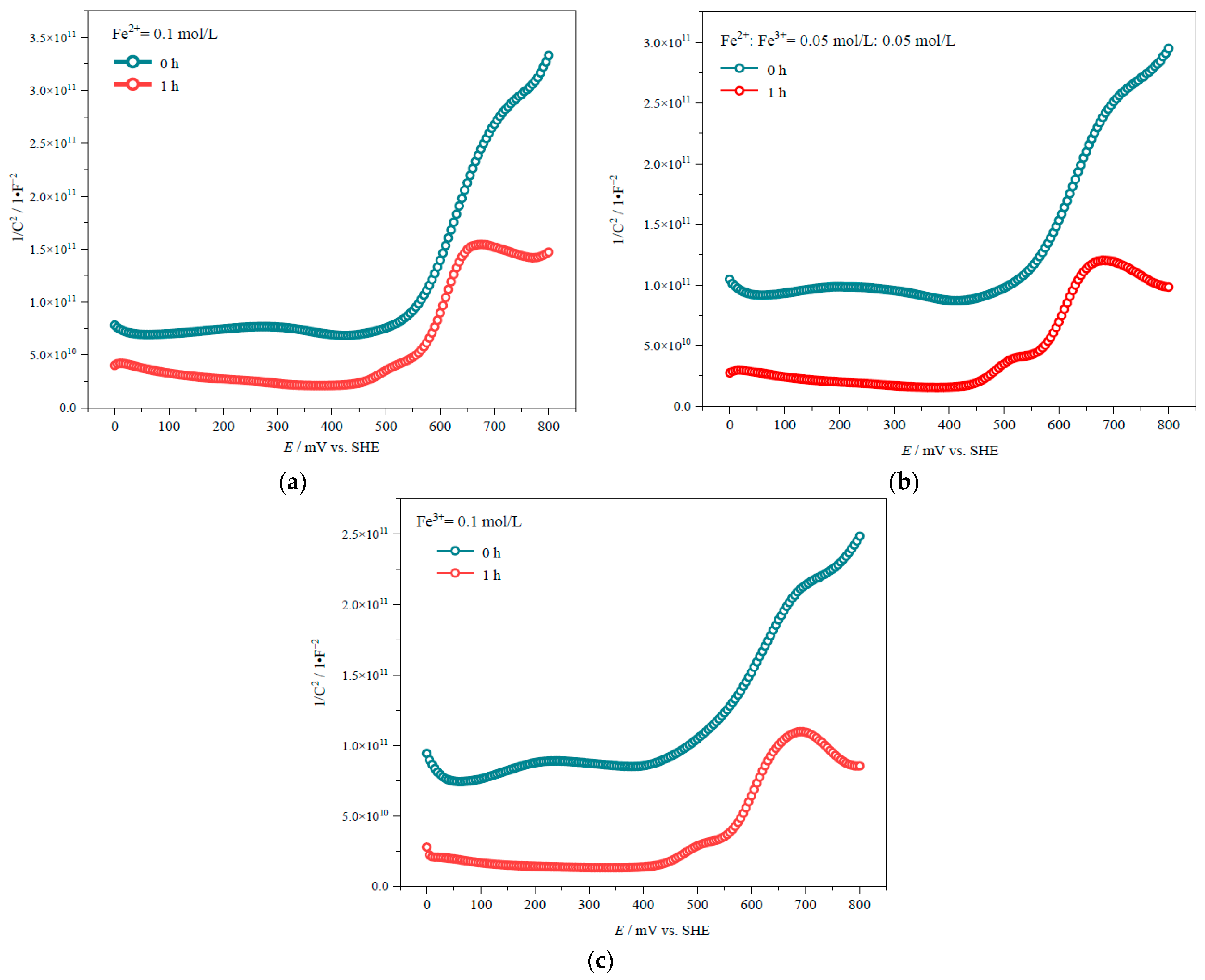
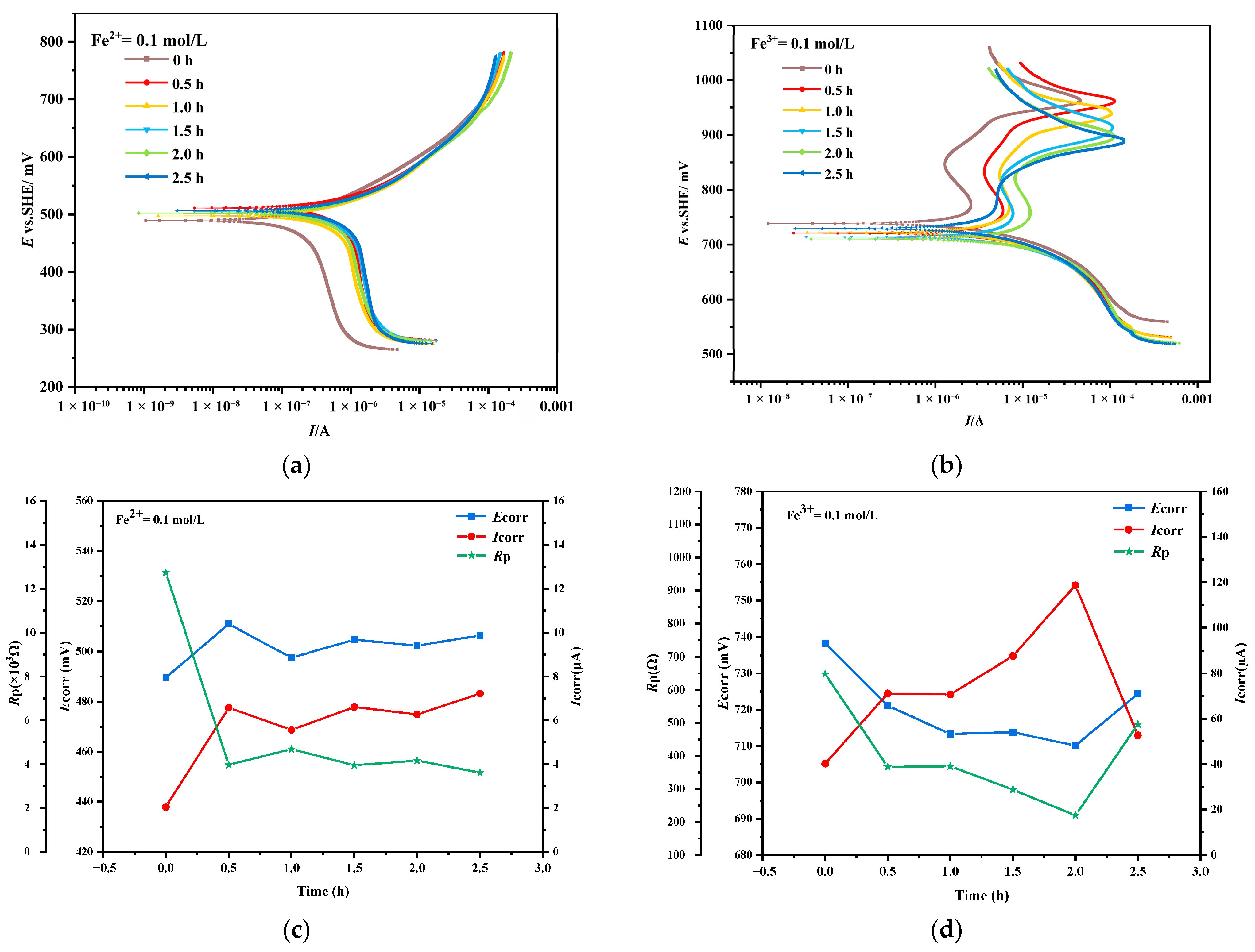
| Time/h | Fe2+:Fe3+ = 0.05 mol/L:0.05 mol/L | Fe3+ = 0.1 mol/L | ||
|---|---|---|---|---|
| K | R2 | K | R2 | |
| 0 | 0.077 | 0.994 | 0.096 | 0.979 |
| 0.5 | 0.078 | 0.970 | 0.100 | 0.998 |
| 1.0 | 0.080 | 0.994 | 0.109 | 0.996 |
| 1.5 | 0.082 | 0.959 | 0.109 | 0.994 |
| 2.0 | 0.082 | 0.983 | 0.114 | 0.999 |
| 2.5 | 0.090 | 0.993 | 0.112 | 0.999 |
| Time/h | Fe2+ = 0.1 mol/L | Fe2+:Fe3+ =0.05 mol/L:0.05 mol/L | Fe3+ = 0.1 mol/L | |||
|---|---|---|---|---|---|---|
| K | R2 | K | R2 | K | R2 | |
| 0 | 0.039 | 0.973 | 0.059 | 0.993 | 0.077 | 0.959 |
| 0.5 | 0.081 | 0.961 | 0.124 | 0.992 | 0.148 | 0.989 |
| 1.0 | 0.123 | 0.952 | 0.133 | 0.992 | 0.201 | 0.974 |
| 1.5 | 0.159 | 0.967 | 0.202 | 0.959 | 0.266 | 0.949 |
| 2.0 | 0.133 | 0.914 | 0.185 | 0.873 | 0.206 | 0.923 |
| 2.5 | 0.149 | 0.950 | 0.195 | 0.932 | 0.242 | 0.892 |
| Time/h | Flat Band Potential (EFB)/mV | Semiconductor Physics Type |
|---|---|---|
| 0 | 289.2 | n |
| 0.5 | 259.6 | n |
| 1.0 | 244.1 | n |
| 1.5 | 247.0 | n |
| 2.0 | 237.1 | n |
| 2.5 | 242.0 | n |
| Leaching System | Time/h | Flat Band Potential (EFB)/mV | Semiconductor Type |
|---|---|---|---|
| Fe2+ = 0.1 mol/L | 0 | 149.2 | n |
| 1.0 | 118.4 | n | |
| Fe2+: Fe3+ = 0.05 mol/L:0.05 mol/L | 0 | 192.5 | n |
| 1.0 | 123.8 | n | |
| Fe3+ = 0.1 mol/L | 0 | 261.7 | n |
| 1.0 | 131.2 | n |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tian, Z.; Wang, X.; Yang, C. Effect of Mechanical Activation on Electrochemical Properties of Chalcopyrite in Iron-Containing Sulfuric Acid Solutions. Metals 2025, 15, 1075. https://doi.org/10.3390/met15101075
Li Y, Tian Z, Wang X, Yang C. Effect of Mechanical Activation on Electrochemical Properties of Chalcopyrite in Iron-Containing Sulfuric Acid Solutions. Metals. 2025; 15(10):1075. https://doi.org/10.3390/met15101075
Chicago/Turabian StyleLi, Yuxin, Zuyuan Tian, Xu Wang, and Congren Yang. 2025. "Effect of Mechanical Activation on Electrochemical Properties of Chalcopyrite in Iron-Containing Sulfuric Acid Solutions" Metals 15, no. 10: 1075. https://doi.org/10.3390/met15101075
APA StyleLi, Y., Tian, Z., Wang, X., & Yang, C. (2025). Effect of Mechanical Activation on Electrochemical Properties of Chalcopyrite in Iron-Containing Sulfuric Acid Solutions. Metals, 15(10), 1075. https://doi.org/10.3390/met15101075







