High-Performance Porous Aluminum Alloys from Recycled A356 Scrap: Synergistic Foaming Approach Using TiH2 and Na2B4O7·10H2O
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Microstructure Characterization
2.3. Density and Porosity Measurement
2.4. Evaluation of Mechanical and Corrosion Properties
3. Results and Discussion
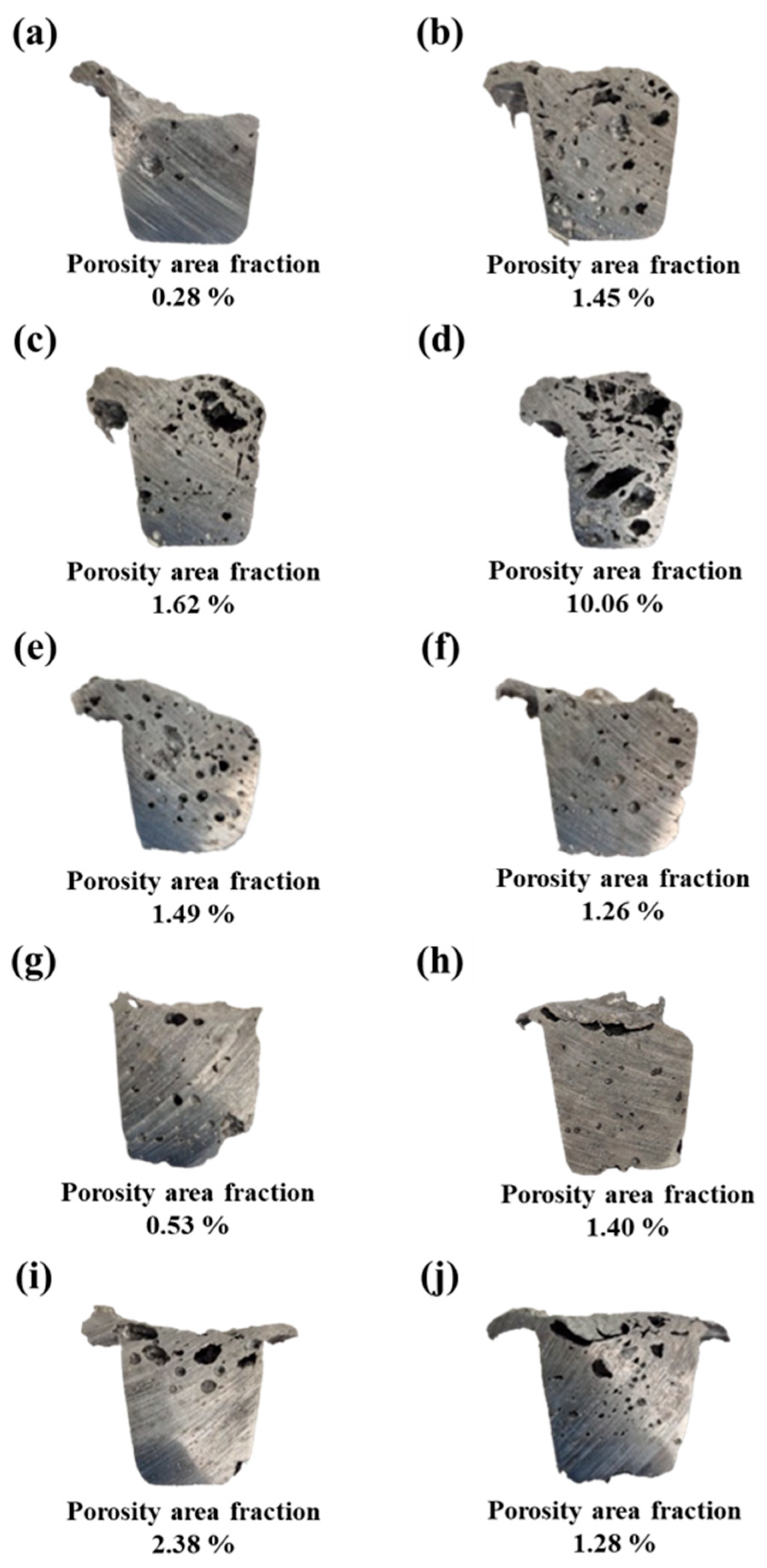
3.1. Porosity Analysis
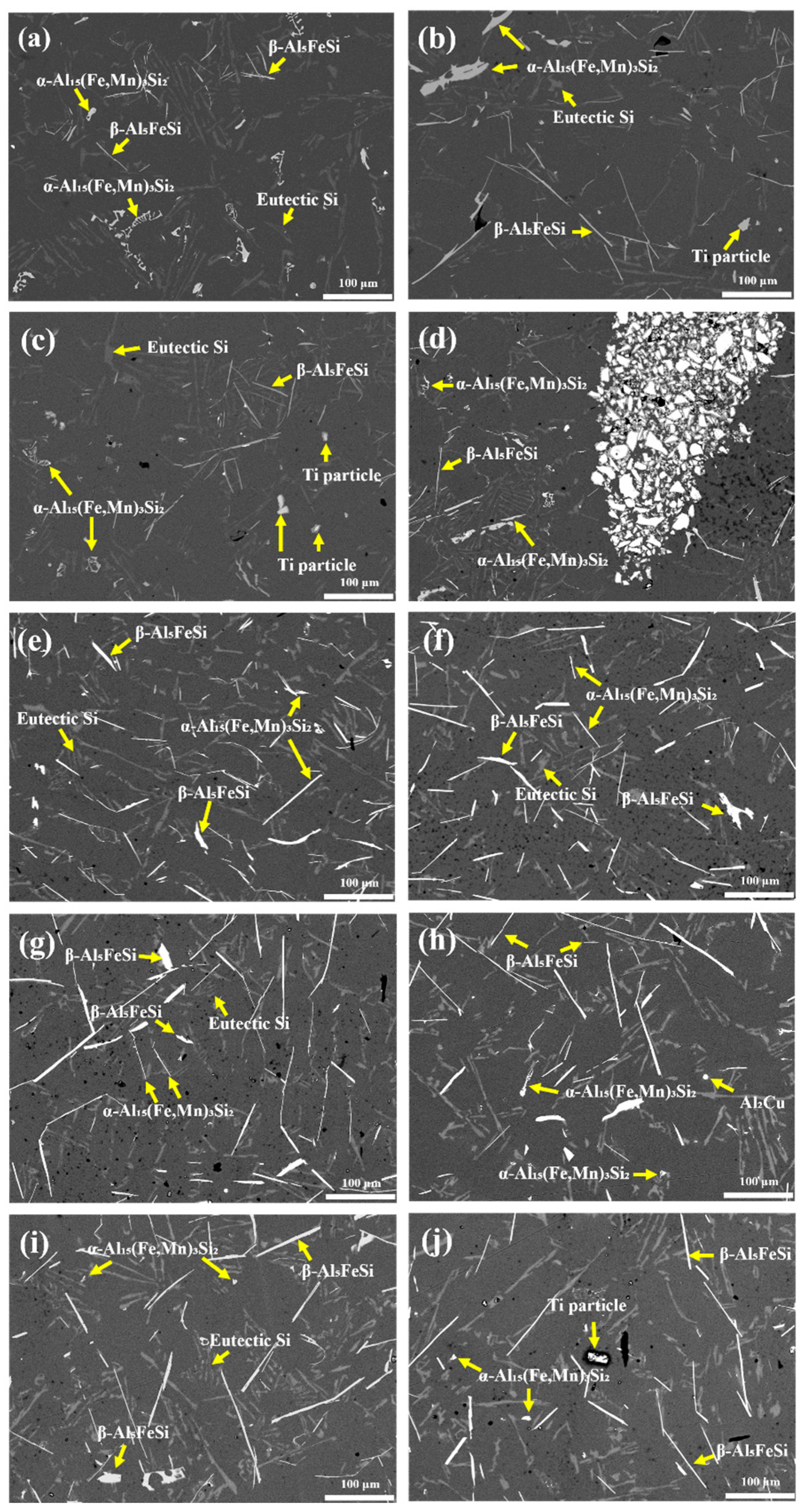
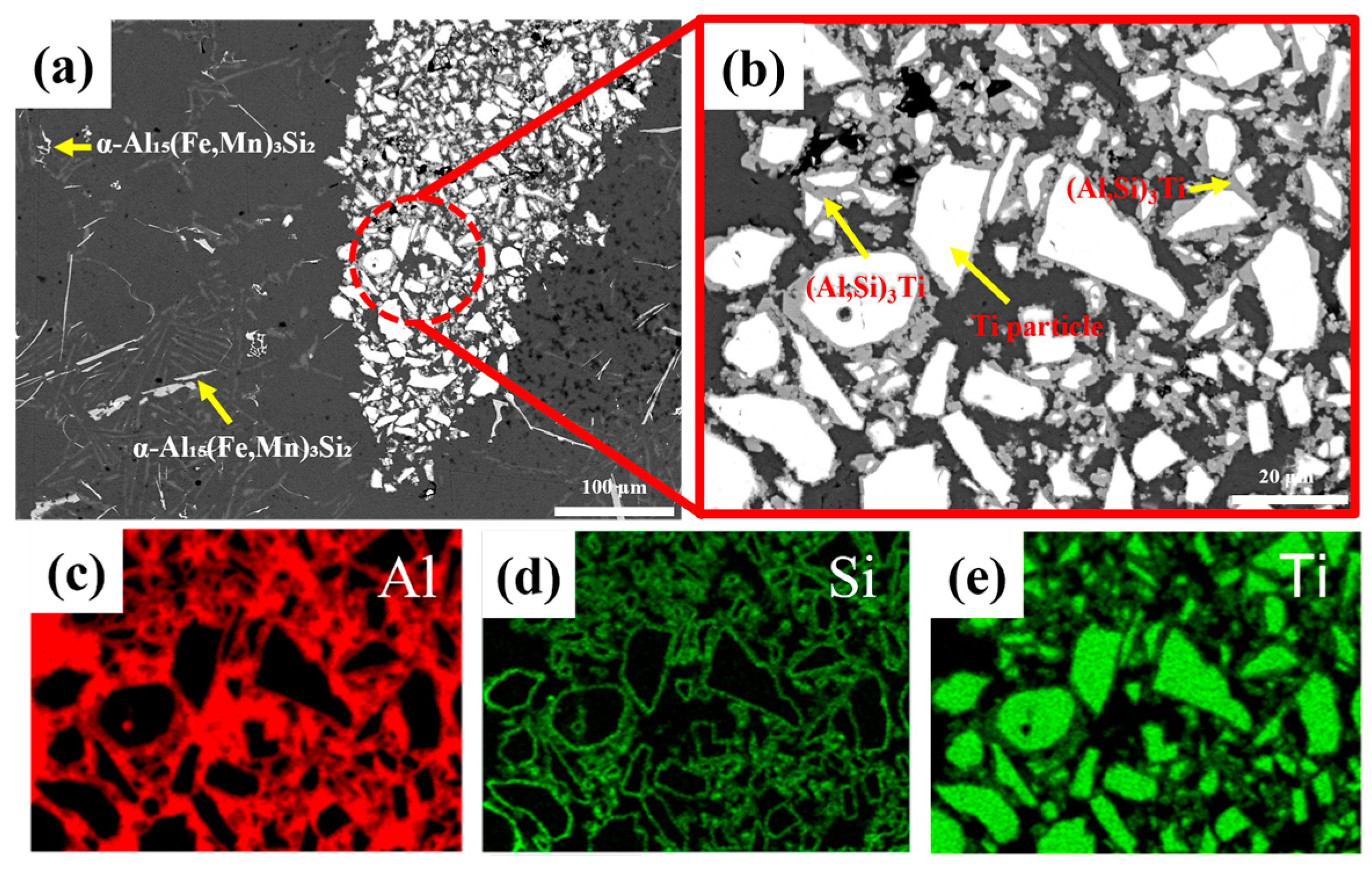
3.2. Microstructure
3.3. SDAS
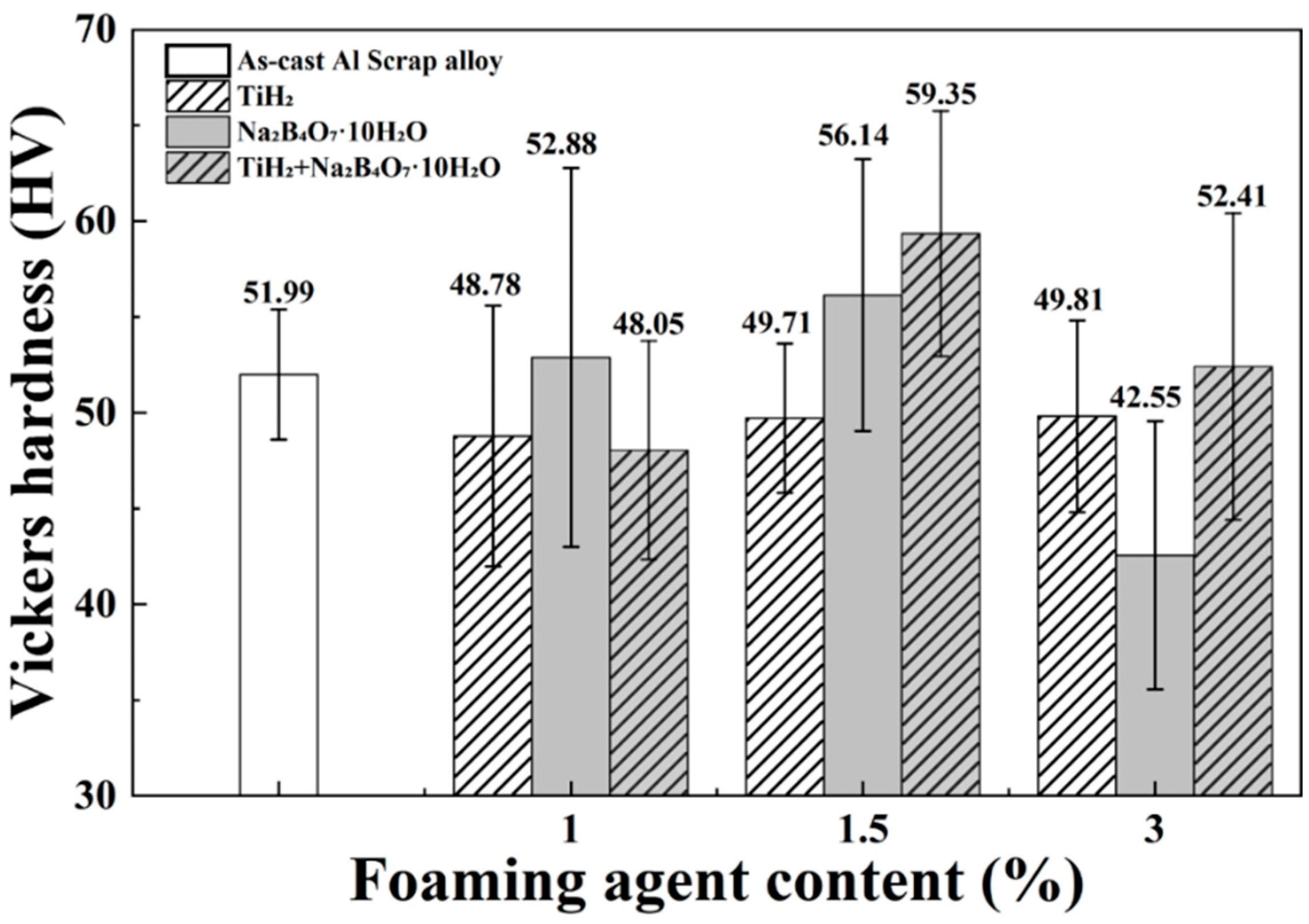
3.4. Vickers Hardness
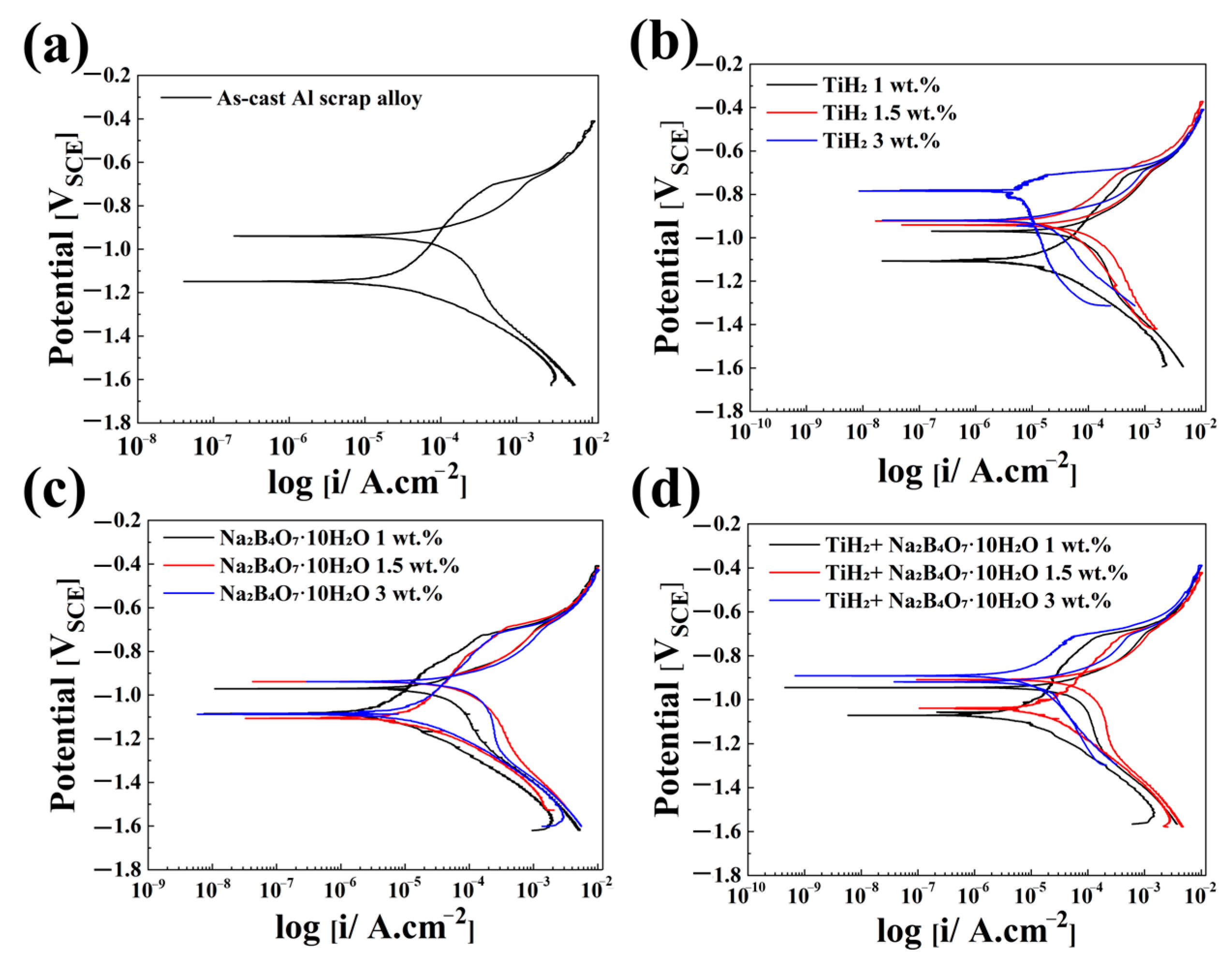
3.5. Electrochemical Measurement
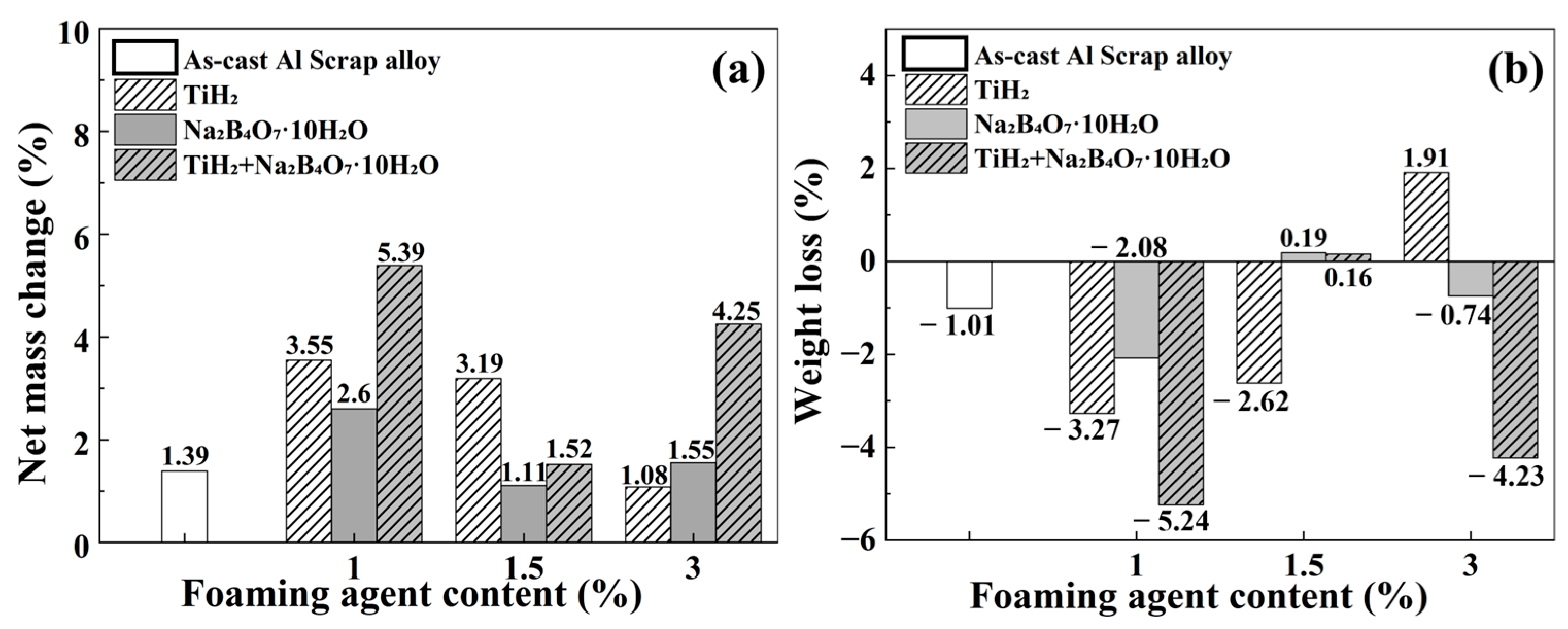
3.6. Weight Loss and Corrosion Rate
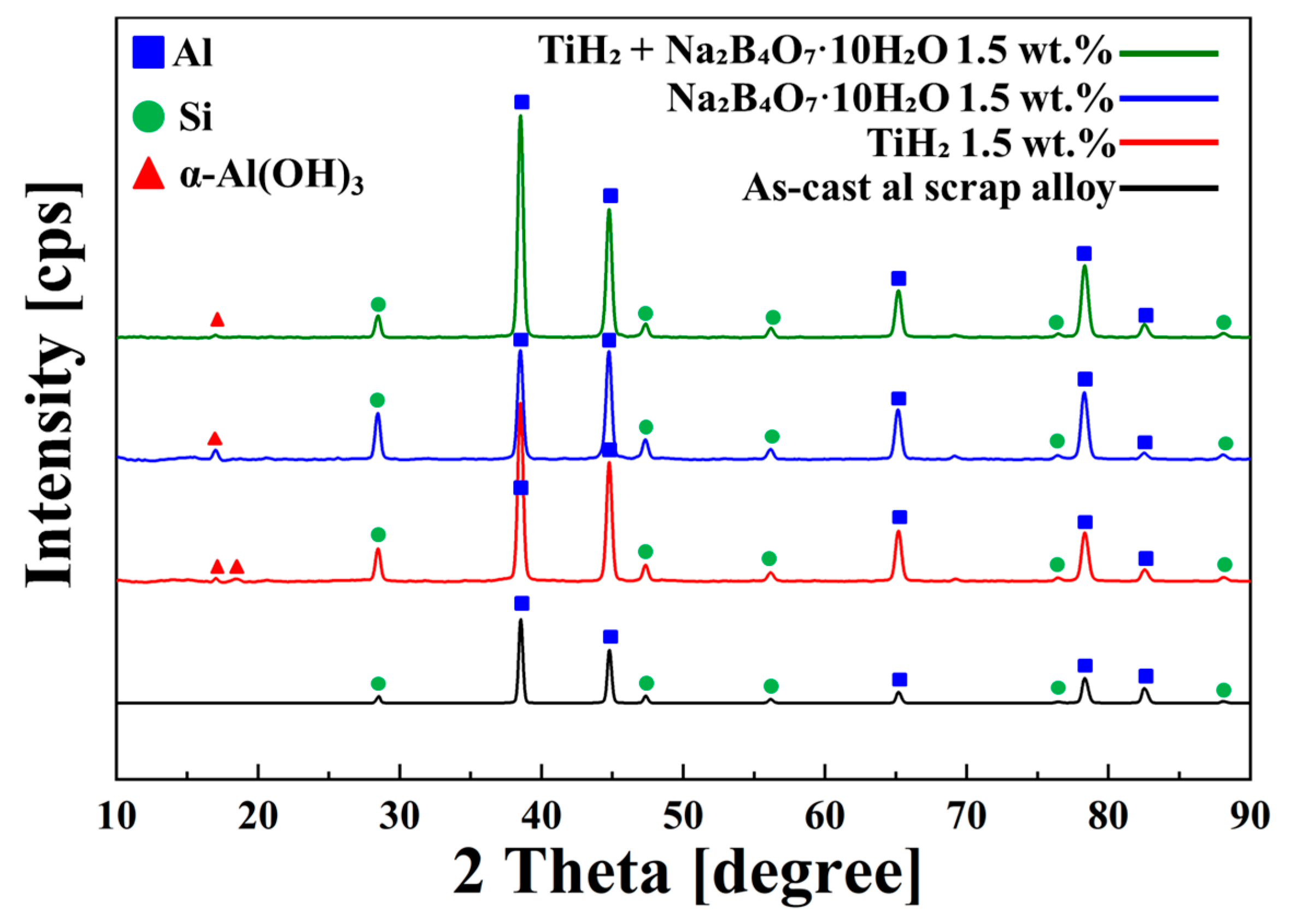
3.7. XRD
4. Conclusions
- (1)
- With the addition of TiH2 alone, increasing its content led to a higher porosity due to increased hydrogen evolution and induced grain refinement. Moreover, a stable corrosion potential and pitting potential were maintained, resulting in excellent corrosion resistance. However, at 3 wt.%, non-uniform pore distribution and metal loss were observed, indicating a potential degradation in corrosion resistance.
- (2)
- With the addition of Na2B4O7·10H2O alone, increasing content generally led to reduced corrosion resistance. In particular, the 3 wt.% condition exhibited grain coarsening, a negative shift in pitting potential, and an increase in corrosion current density. In contrast, the 1 and 1.5 wt.% conditions showed relatively stable pore formation and improved hardness, demonstrating positive effects in terms of mechanical performance.
- (3)
- In the combined addition conditions, an overall balance was achieved across porosity, microstructure, mechanical properties, and corrosion resistance. Particularly, the 1.5 wt.% combined condition exhibited the best overall performance, including high pore area fraction (2.38%), porosity (27.0%), SDAS (48.1 ± 4.8 µm), hardness (59.35 ± 6.4 HV), corrosion potential (−1.039 V), pitting potential (−0.709 V), and corrosion current density (4.956 μA/cm2). The metal loss after corrosion product removal (+1.30 mpy) was also relatively minor.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Yang, Y.; Zhuang, Y.; Wang, H.; Chen, C. Corrosion Test and Corrosion Fatigue Numerical Simulation Research on Marine Structures. Ocean. Eng. 2025, 316, 119931. [Google Scholar] [CrossRef]
- Adedipe, O.; Brennan, F.; Kolios, A. Review of Corrosion Fatigue in Offshore Structures: Present Status and Challenges in the Offshore Wind Sector. Renew. Sustain. Energy Rev. 2016, 61, 141–154. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, C.; Zhuang, Y.; Suo, Z. Reviewing the Progress of Corrosion Fatigue Research on Marine Structures. Front. Mater. 2024, 11, 1399292. [Google Scholar] [CrossRef]
- Thomas, D.J. A Life at Sea and the Corrosion Fatigue Lives of Offshore Structures. J. Fail. Anal. Prev. 2021, 21, 707–710. [Google Scholar] [CrossRef]
- Abbas, M.; Shafiee, M. An Overview of Maintenance Management Strategies for Corroded Steel Structures in Extreme Marine Environments. Mar. Struct. 2020, 71, 102718. [Google Scholar] [CrossRef]
- Shifler, D.A. Understanding Material Interactions in Marine Environments to Promote Extended Structural Life. Corros. Sci. 2005, 47, 2335–2352. [Google Scholar] [CrossRef]
- Yeh, C.L.; Sun, W.E. Use of TiH2 as a Reactant in Combustion Synthesis of Porous Ti5Si3 and Ti5Si3/TiAl Intermetallics. J. Alloys Compd. 2016, 669, 66–71. [Google Scholar] [CrossRef]
- Gölbaşı, Z.; Öztürk, B.; Beköz Üllen, N. The Structural and Mechanical Properties of Open-Cell Aluminum Foams: Dependency on Porosity, Pore Size, and Ceramic Particle Addition. J. Alloys Compd. 2024, 1009, 176921. [Google Scholar] [CrossRef]
- Parveez, B.; Jamal, N.A.; Maleque, A.; Yusof, F.; Jamadon, N.H.; Adzila, S. Review on Advances in Porous Al Composites and the Possible Way Forward. J. Mater. Res. Technol. 2021, 14, 2017–2038. [Google Scholar] [CrossRef]
- Gaur, S.K.; Sahoo, R.R.; Sarkar, J. Performance Optimization of Triple Tube Heat Exchanger Using Aluminum Metal Foam: A Comprehensive Numerical Investigation. Int. Commun. Heat Mass Transf. 2024, 158, 107953. [Google Scholar] [CrossRef]
- Antohe, B.V.; Lage, J.L.; Price, D.C.; Weber, R.M. Numerical Characterization of Micro Heat Exchangers Using Experimentally Tested Porous Aluminum Layers. Int. J. Heat Fluid Flow 1996, 17, 594–603. [Google Scholar] [CrossRef]
- Barragán De Los Rios, G.A.; Salazar Martínez, S.A.; Mendoza Fandiño, E.; Fernández-Morales, P. Numerical Simulation of Aluminum Foams by Space Holder Infiltration. Int. J. Met. 2024, 18, 3506–3522. [Google Scholar] [CrossRef]
- Duarte, I.; Banhart, J. A Study of Aluminium Foam Formation—Kinetics and Microstructure. Acta Mater. 2000, 48, 2349–2362. [Google Scholar] [CrossRef]
- Wang, T.; Zuo, X.; Yi, J.; Zhou, Y.; Luo, X.; Guo, S.; Song, W. Enhancing Melt Foam Stability, Form-Filling Process, and Pore Structure Evolution of Shaped Aluminum Foam. J. Mater. Res. Technol. 2024, 33, 5708–5719. [Google Scholar] [CrossRef]
- Thulasikanth, V.; Padmanabhan, R. Fabrication of Sustainable Closed-Cell Aluminium Foams Using Recycled Fly Ash and Eggshell Powder. Mater. Today Commun. 2023, 37, 107302. [Google Scholar] [CrossRef]
- Brugnolo, F.; Costanza, G.; Tata, M.E. Manufacturing and Characterization of AlSi Foams as Core Materials. Procedia Eng. 2015, 109, 219–227. [Google Scholar] [CrossRef]
- Ji, C.; Huang, H.; Wang, T.; Huang, Q. Recent Advances and Future Trends in Processing Methods and Characterization Technologies of Aluminum Foam Composite Structures: A Review. J. Manuf. Process. 2023, 93, 116–152. [Google Scholar] [CrossRef]
- Bang, J.; Lee, E. Enhancing Wear Resistance of A390 Aluminum Alloy: A Comprehensive Evaluation of Thermal Sprayed WC, CrC, and Al2O3 Coatings. Coatings 2024, 14, 853. [Google Scholar] [CrossRef]
- Han, J.; Dong, G.; Li, S.; Zheng, J.; Wang, J.; Li, H.; Starostenkov, M.D.; Bi, J. Uneven Distribution of Cooling Rate, Microstructure and Mechanical Properties for A356-T6 Wheels Fabricated by Low Pressure Die Casting. J. Manuf. Process. 2024, 127, 196–210. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Cao, Y.; Peng, J.; Yang, D.; Song, D.; Okonkwo, B.O.; Wang, J.; Han, E. Revealing the Effect of Iron Content on the Microstructure Evolution, Mechanical Properties and Corrosion Resistance of the A356-T6 Aluminum Alloys. J. Mater. Res. Technol. 2024, 33, 2263–2274. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, Characterisation and Application of Cellular Metals and Metal Foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Sun, B.; Lan, X.; Wang, Z.; Sun, N.; Guo, Z. Grade-Preserving Recycling of Highly Polluted Al-Mg-Si Alloys Scrap: Continuous Filtration under Supergravity-Induced. Sustain. Mater. Technol. 2024, 40, e00918. [Google Scholar] [CrossRef]
- Gottmyers Melwyn, J.; Chandragandhi, B.; Sathiyaseelan, G.; Srinath, P. Aluminium Scrap Recycling in a Production Furnace: Minimizing Dross Formation for Sustainable and Efficient Recovery. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Korban, P.; Leszczyńska-Madej, B. Effect of Barbotage Refining Time and Recycled Scrap Content on the Microstructure of EN AC 44200 Alloy. Mater. Today Commun. 2023, 37, 107038. [Google Scholar] [CrossRef]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminum Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Chen, X.; Saada, M.B.; Lavisse, B.; Ammar, A. Recent Advances in the Remelting Process for Recycling Aluminium Alloy Chips: A Critical Review. Int. J. Mater. Form. 2025, 18, 42. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Ren, Y.; Ma, W. Removal of Fe Impurities from Al Alloy Scraps by Electromagnetic Directional Solidification Combined with Si Addition. J. Mater. Res. Technol. 2023, 26, 8738–8747. [Google Scholar] [CrossRef]
- Wan, B.; Li, W.; Liu, F.; Lu, T.; Jin, S.; Wang, K.; Yi, A.; Tian, J.; Chen, W. Determination of Fluoride Component in the Multifunctional Refining Flux Used for Recycling Aluminum Scrap. J. Mater. Res. Technol. 2020, 9, 3447–3459. [Google Scholar] [CrossRef]
- ASTM G31; Standard Guide for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2021.
- Samuel, A.M.; Samuel, E.; Songmene, V.; Samuel, F.H. A Review on Porosity Formation in Aluminum-Based Alloys. Materials 2023, 16, 2047. [Google Scholar] [CrossRef]
- Gao, J.W.; Shu, D.; Wang, J.; Sun, B.D. Study on Iron Purification from Aluminium Melt by Na2B4O7 Flux. Mater. Sci. Technol. 2009, 25, 619–624. [Google Scholar] [CrossRef]
- Kuchariková, L.; Medvecká, D.; Tillová, E.; Belan, J.; Kritikos, M.; Chalupová, M.; Uhríčik, M. The Effect of the β-Al5FeSi Phases on Microstructure, Mechanical and Fatigue Properties in A356.0 Cast Alloys with Higher Fe Content without Additional Alloying of Mn. Materials 2021, 14, 1943. [Google Scholar] [CrossRef]
- Liu, G.; Ren, Y.; Ma, W.; Morita, K.; Lei, Y.; Zhan, S.; Lv, G.; Li, S.; Zeng, Y.; Li, R. Development Process and Future Trends of Chemical Refining Agents’ Influence on Grain Refinement in Aluminum Alloys. J. Mater. Res. Technol. 2024, 29, 242–257. [Google Scholar] [CrossRef]
- Bang, J.; Byon, E.; Lee, E. Effects of Na2B4O7·10H2O on Microstructure and Mechanical Properties of AlSi7Mg0.3 and AlSi10MnMg Alloys. J. Adv. Mar. Eng. Technol. 2021, 45, 363–370. [Google Scholar] [CrossRef]
- Abo Nama, H.A.H.; Esen, İ.; Karakurt, V.; Ahlatci, H. Effect of the Ti Addition on the Corrosion Behavior of Newly Developed AA7075-Ti Alloys. J. Alloys Compd. 2023, 969, 172349. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, W.; Wang, X.; Jiang, B.; Wang, Y.; Zhu, D.; Hu, M. Effect of Ti on Microstructure and Mechanical Properties of Al0.8Nb0.5TixV2Zr0.5 Refractory Complex Concentrated Alloys. Int. J. Refract. Met. Hard Mater. 2023, 117, 106383. [Google Scholar] [CrossRef]
- Dias, V.; Maciel, H.; Fraga, M.; Lobo, A.O.; Pessoa, R.; Marciano, F.R. Atomic Layer Deposited TiO2 and Al2O3 Thin Films as Coatings for Aluminum Food Packaging Application. Materials 2019, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Staszuk, M.; Pakuła, D.; Reimann, Ł.; Musztyfaga-Staszuk, M.; Socha, R.; Tański, T. Investigation of Ti/Al2O3 + TiO2 and Ti + TiO2/Al2O3 + TiO2 Hybrid Coatings as Protection of Ultra-Light Mg–(Li)–Al–RE Alloys against Corrosion. Sci. Rep. 2022, 12, 19363. [Google Scholar] [CrossRef]

| Foaming Agent Type | Element | Al (%) | Si (%) | Mg (%) | Fe (%) | Ti (%) | Cu (%) | Mn (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Content | |||||||||
| As-cast Al Scrap | No foaming agent added | 87.1 | 10.07 | 1.74 | 0.59 | 0.08 | 0.07 | 0.07 | |
| TiH2 | 1 wt.% | 88.74 | 10.18 | 1.54 | 0.72 | 0.08 | 0.08 | 0.06 | |
| 1.5 wt.% | 88.14 | 8.81 | 1.73 | 0.77 | 0.12 | 0.05 | 0.07 | ||
| 3 wt.% | 88.11 | 8.52 | 1.96 | 0.72 | 0.14 | 0.06 | 0.06 | ||
| Na2B4O7·10H2O | 1 wt.% | 88.40 | 8.64 | 1.42 | 1.04 | 0.09 | 0.06 | 0.07 | |
| 1.5 wt.% | 86.41 | 10.23 | 1.91 | 0.87 | 0.07 | 0.10 | 0.07 | ||
| 3 wt.% | 88.42 | 9.45 | 1.19 | 0.94 | 0.05 | 0.34 | 0.03 | ||
| TiH2 + Na2B4O7·10H2O | 1 wt.% | 86.91 | 10.48 | 1.42 | 0.75 | 0.09 | 0.09 | 0.05 | |
| 1.5 wt.% | 85.80 | 11.86 | 0.89 | 1.05 | 0.09 | 0.07 | 0.06 | ||
| 3 wt.% | 88.95 | 8.16 | 1.00 | 1.48 | 0.08 | 0.03 | 0.09 | ||
| Foaming Agent Type | Content | Density (g/cm3) | Porosity (%) |
|---|---|---|---|
| As-cast Al Scrap | No foaming agent added | 2.170 | 19.0 |
| TiH2 | 1 wt.% | 2.028 | 24.3 |
| 1.5 wt.% | 1.969 | 26.5 | |
| 3 wt.% | 1.190 | 55.6 | |
| Na2B4O7·10H2O | 1 wt.% | 1.973 | 26.3 |
| 1.5 wt.% | 2.110 | 21.3 | |
| 3 wt.% | 2.170 | 19.0 | |
| TiH2 + Na2B4O7·10H2O | 1 wt.% | 2.127 | 20.6 |
| 1.5 wt.% | 1.956 | 27.0 | |
| 3 wt.% | 2.062 | 23.1 |
| Foaming Agent Type | Content | Intermetallic Phase Length (μm) | Area Fraction of the Fe-Rich Phase (%) | ||
|---|---|---|---|---|---|
| α-Al15(Fe,Mn)3Si2 | β-Al5FeSi | α-Al15(Fe,Mn)3Si2 | β-Al5FeSi | ||
| As-cast Al Scrap | No foaming agent added | 26.35 ± 2.01 | 30.95 ± 5.37 | 1.61 ± 0.24 | 0.35 ±0.27 |
| TiH2 | 1 wt.% | 38.03 ± 13.17 | 142.35 ± 19.83 | 1.78 ± 0.17 | 1.85 ± 0.32 |
| 1.5 wt.% | 22.13 ± 6.71 | 138.73 ± 19.47 | 1.67 ± 0.14 | 1.63 ± 0.28 | |
| 3 wt.% | 36.98 ± 13.17 | 122.89 ± 13.17 | 2.50 ± 0.69 | 1.29 ± 0.42 | |
| Na2B4O7·10H2O | 1 wt.% | 97.02 ± 7.36 | 25.27 ± 8.13 | 2.16 ± 0.26 | 2.07 ± 0.62 |
| 1.5 wt.% | 71.61 ± 7.34 | 22.63 ± 6.71 | 1.97 ± 0.18 | 1.50 ± 0.30 | |
| 3 wt.% | 110.53 ± 14.91 | 31.26 ± 7.44 | 1.90 ± 0.01 | 1.25 ± 0.26 | |
| TiH2 + Na2B4O7·10H2O | 1 wt.% | 10.07 ± 1.87 | 107.94 ± 8.79 | 0.48 ± 0.44 | 1.42 ± 0.16 |
| 1.5 wt.% | 10.54 ± 2.16 | 127.14 ± 21.67 | 1.52 ± 0.28 | 2.93 ± 0.99 | |
| 3 wt.% | 7.37 ± 2.05 | 107.62 ± 9.39 | 0.35 ± 0.03 | 1.56 ± 0.47 | |
| Foaming Agent Type | Content | Corrosion Potential (Ecorr, V) | Corrosion Current Density (Icorr, μA/cm2) | Pitting Potential (Epit, V) |
|---|---|---|---|---|
| As-cast Al Scrap | No foaming agent added | −1.149 | 14.740 | −0.704 |
| TiH2 | 1 wt.% | −1.107 | 6.201 | −0.722 |
| 1.5 wt.% | −0.922 | 1.782 | −0.704 | |
| 3 wt.% | −0.784 | 2.008 | −0.708 | |
| Na2B4O7·10H2O | 1 wt.% | −1.103 | 1.102 | −0.717 |
| 1.5 wt.% | −1.074 | 3.479 | −0.719 | |
| 3 wt.% | −1.088 | 6.410 | −0.728 | |
| TiH2 + Na2B4O7·10H2O | 1 wt.% | −1.071 | 1.970 | −0.715 |
| 1.5 wt.% | −1.039 | 4.956 | −0.709 | |
| 3 wt.% | −1.008 | 2.748 | −0.714 |
| Foaming Agent Type | Content | Corrosion Rate (mpy) |
|---|---|---|
| As-cast Al Scrap | No foaming agent added | −8.85 |
| TiH2 | 1 wt.% | −19.04 |
| 1.5 wt.% | −12.69 | |
| 3 wt.% | +9.90 | |
| Na2B4O7·10H2O | 1 wt.% | −15.06 |
| 1.5 wt.% | +1.74 | |
| 3 wt.% | −5.63 | |
| TiH2 + Na2B4O7·10H2O | 1 wt.% | −45.41 |
| 1.5 wt.% | +1.30 | |
| 3 wt.% | −24.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.; Lee, H.; Bang, J.; Lee, E. High-Performance Porous Aluminum Alloys from Recycled A356 Scrap: Synergistic Foaming Approach Using TiH2 and Na2B4O7·10H2O. Metals 2025, 15, 1068. https://doi.org/10.3390/met15101068
Baek J, Lee H, Bang J, Lee E. High-Performance Porous Aluminum Alloys from Recycled A356 Scrap: Synergistic Foaming Approach Using TiH2 and Na2B4O7·10H2O. Metals. 2025; 15(10):1068. https://doi.org/10.3390/met15101068
Chicago/Turabian StyleBaek, Jinwoo, Hyuncheul Lee, Jaehui Bang, and Eunkyung Lee. 2025. "High-Performance Porous Aluminum Alloys from Recycled A356 Scrap: Synergistic Foaming Approach Using TiH2 and Na2B4O7·10H2O" Metals 15, no. 10: 1068. https://doi.org/10.3390/met15101068
APA StyleBaek, J., Lee, H., Bang, J., & Lee, E. (2025). High-Performance Porous Aluminum Alloys from Recycled A356 Scrap: Synergistic Foaming Approach Using TiH2 and Na2B4O7·10H2O. Metals, 15(10), 1068. https://doi.org/10.3390/met15101068






