Heat Treatment Effect on the Corrosion Resistance of 316L Stainless Steel Produced by Laser Powder Bed Fusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electrochemical Corrosion Testing
2.3. Testing for Evaluation of Microstructural Features
3. Results and Discussion
3.1. Results
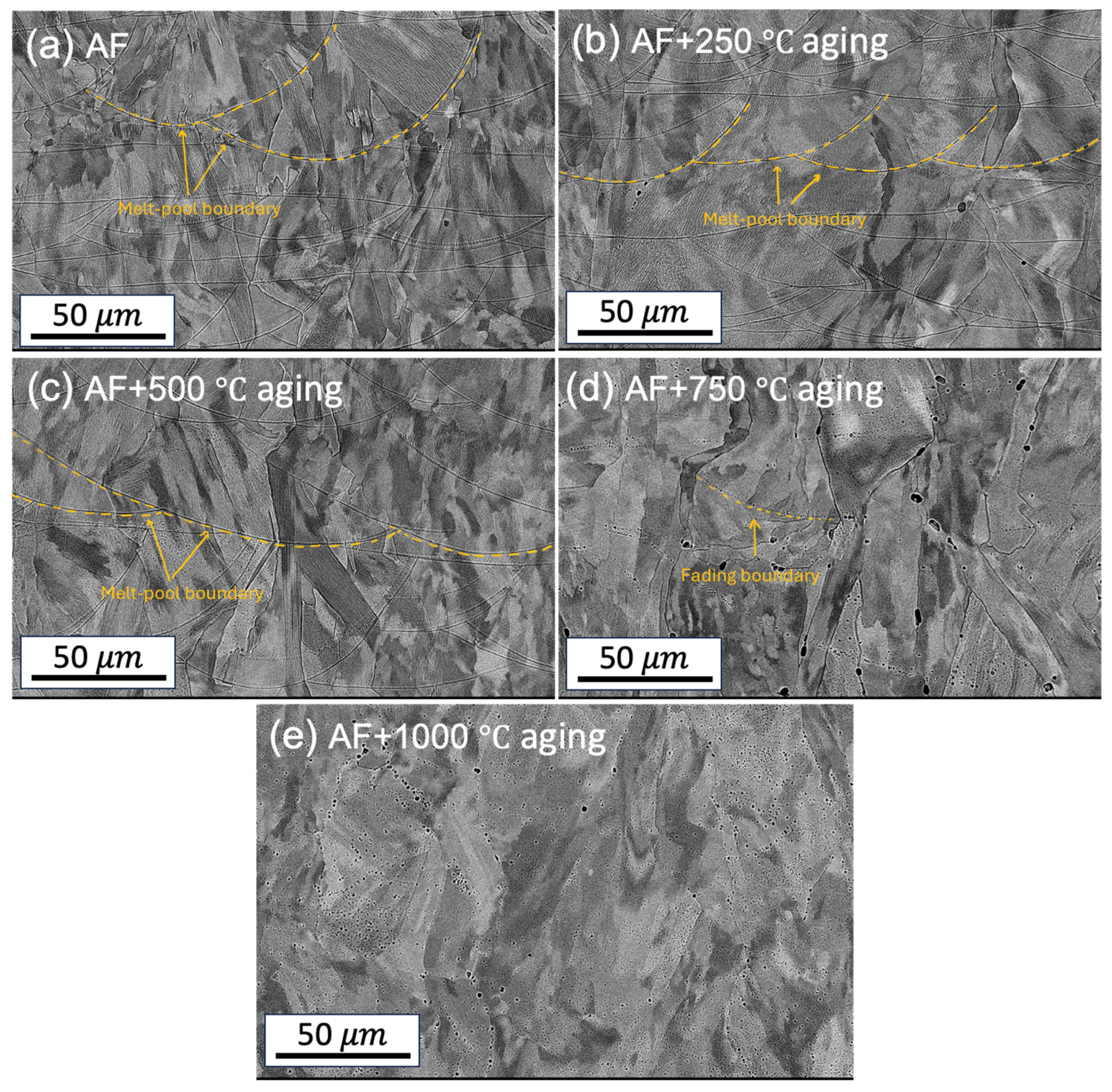
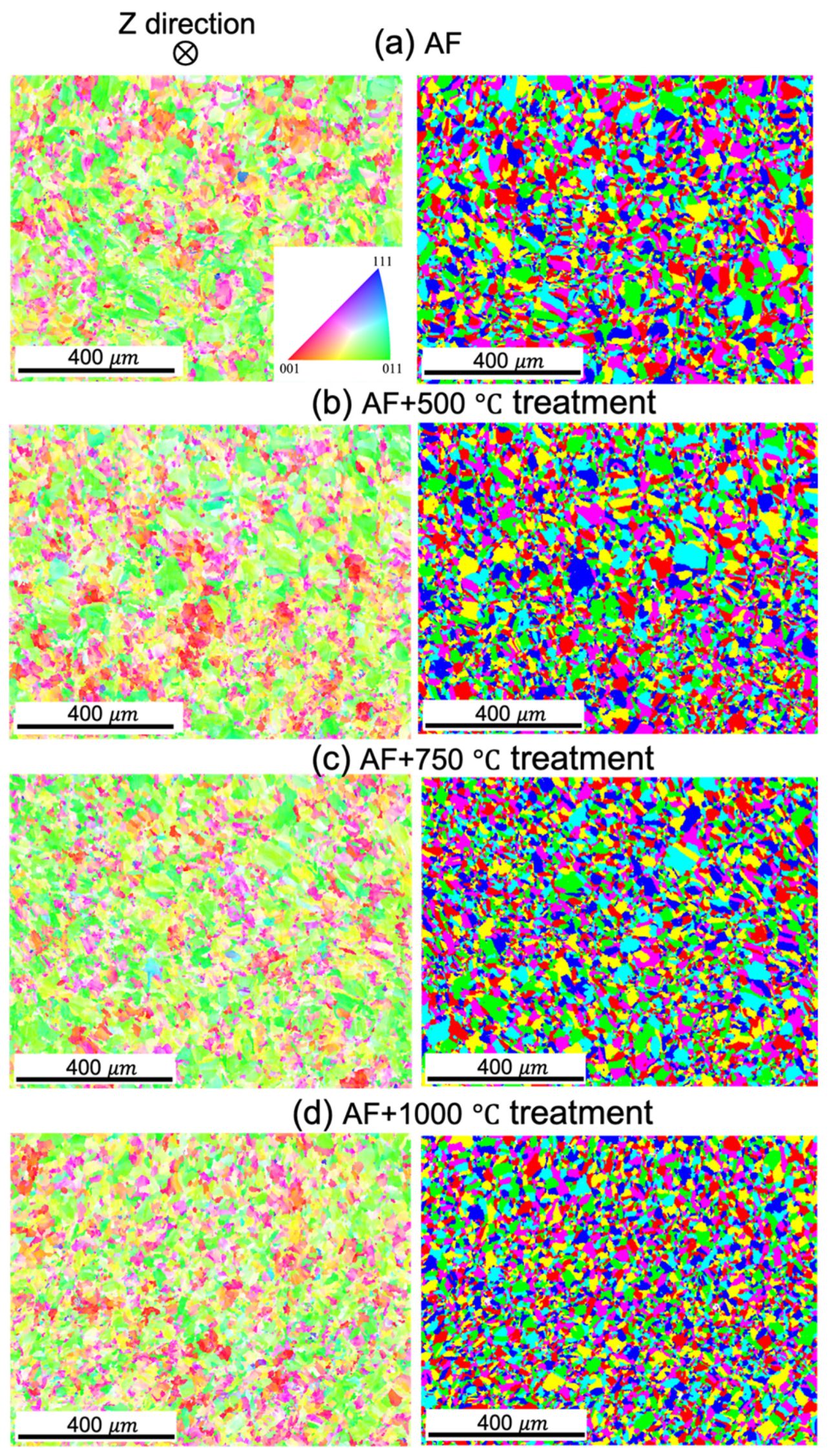
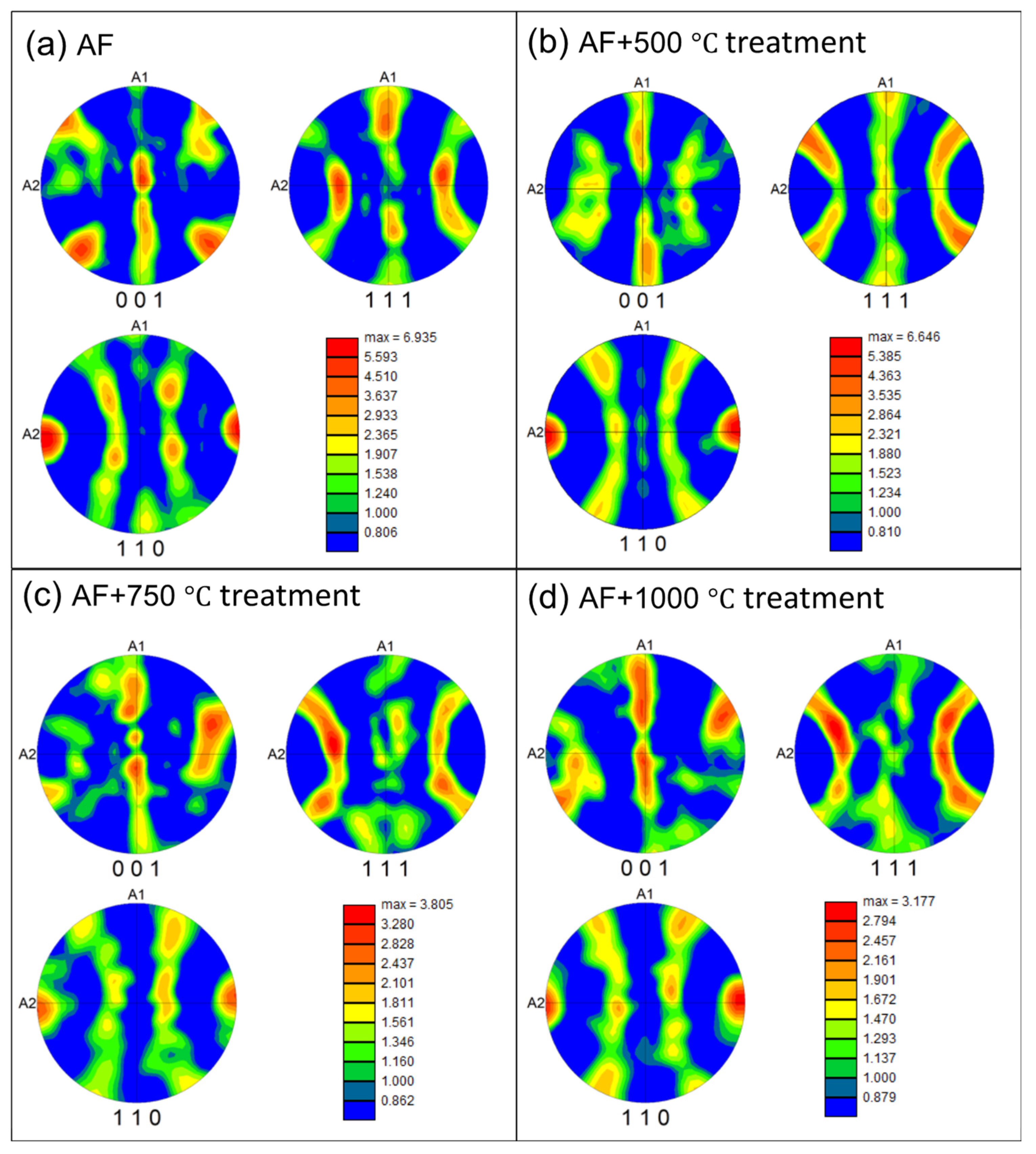
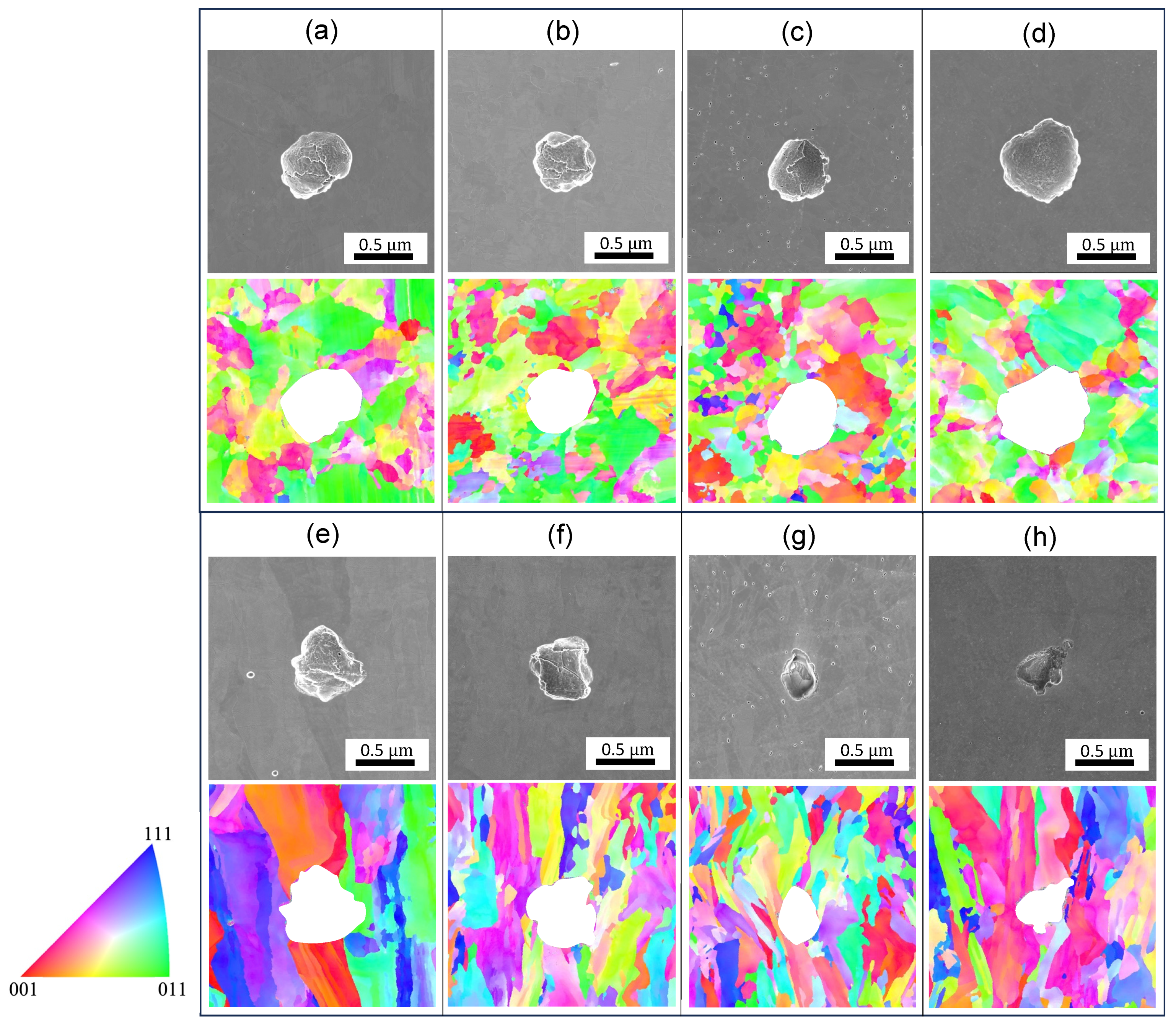
3.1.1. Microstructural Characterization
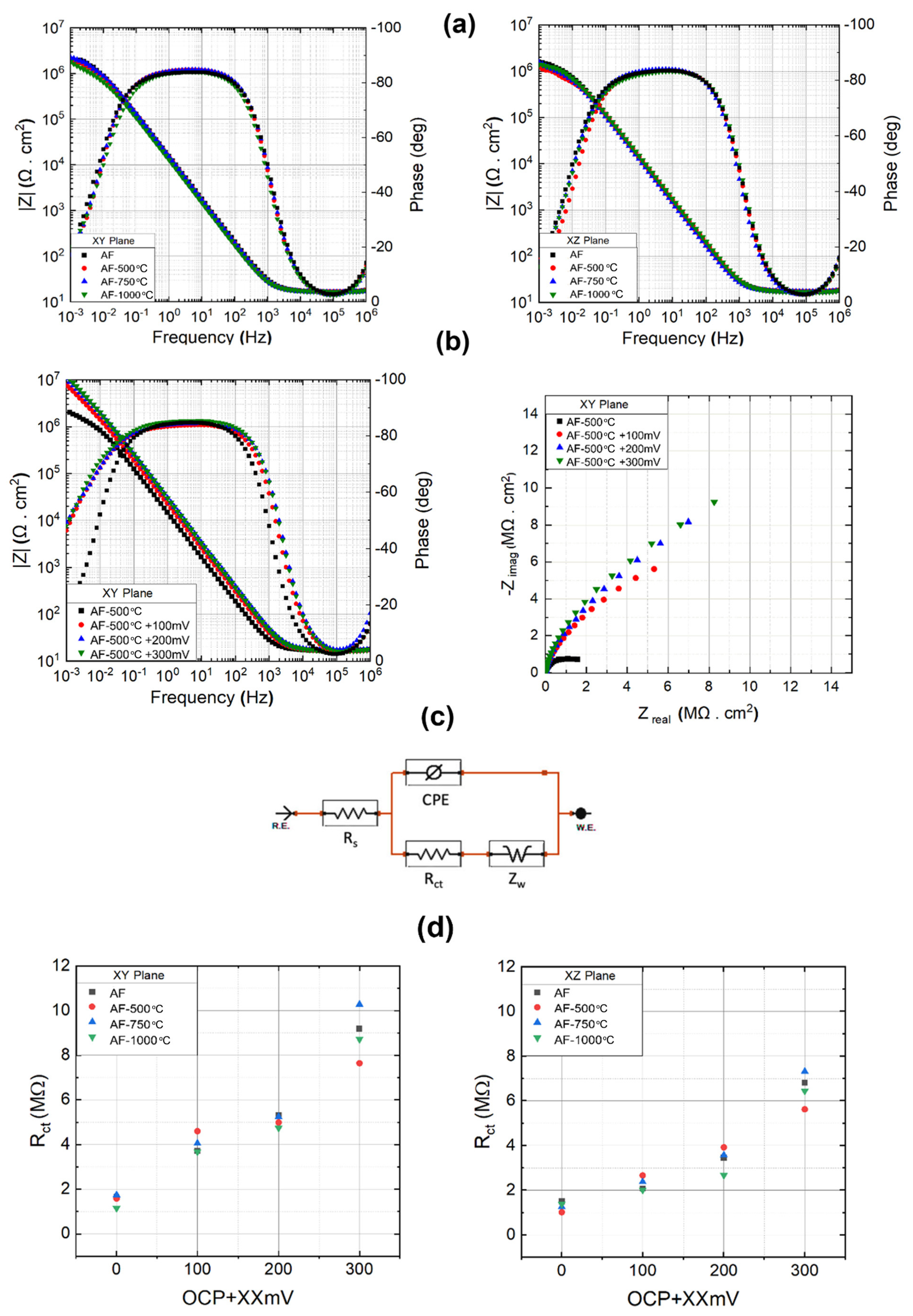
3.1.2. Electrochemical Analysis
3.2. Discussion
4. Conclusions
- The microstructures are significantly altered during the heat treatment. The changes include diminished melt pool boundaries and dislocation of cellular structures, along with a reduced grain size, with increasing treatment temperatures. The grains remained elongated and columnar-shaped during the process.
- A deterioration in corrosion resistance with an increased annealing temperature was evidenced by reduced pitting and repassivation potentials.
- Anisotropic corrosion resistance was also observed. The vertical plane (parallel to the build direction) exhibited a higher pitting and repassivation potential, yet a higher current density was observed post-repassivation. The degree of anisotropy gradually diminished with increasing treatment temperature.
- The increased grain boundaries and reduced dislocation cell densities are attributed to the different corrosion performances after heat treatment.
5. Practical Implementation
- Industries producing components for applications demanding corrosion resistance, such as marine industries, should prioritize low-temperature heat treatments (500 °C). This temperature regime achieves microstructural homogenization and stress relief without introducing adverse grain growth and comprises pitting and repassivation behaviors.
- Anisotropic corrosion behavior suggests that designers should strategically orient critical components during fabrication. Vertical orientations, with their demonstrated susceptibility to higher corrosion currents, may require additional surface treatments or coatings to ensure long-term durability in aggressive environments.
- The main findings of this research contribute to a deeper understanding of the post-fabrication processing–structure–property relationships in 316L stainless steel fabricated via L-PBF, pointing towards the importance of optimizing manufacturing and heat treatment parameters to enhance material performance in corrosive environments.
6. Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sumita, M.; Hanawa, T.; Teoh, S. Development of nitrogen-containing nickel-free austenitic stainless steels for metallic biomaterials—Review. Mater. Sci. Eng. C 2004, 24, 753–760. [Google Scholar] [CrossRef]
- Imbaby, M.; Jiang, K.; Chang, I. Net shape fabrication of stainless-steel micro machine components from metallic powder. J. Micromech. Microeng. 2008, 18, 115018. [Google Scholar] [CrossRef]
- Choi, J.-P.; Shin, G.-H.; Brochu, M.; Kim, Y.-J.; Yang, S.-S.; Kim, K.-T.; Yang, D.-Y.; Lee, C.-W.; Yu, J.-H. Densification behavior of 316L stainless steel parts fabricated by selective laser melting by variation in laser energy density. Mater. Trans. 2016, 57, 1952–1959. [Google Scholar] [CrossRef]
- Salman, O.O.; Funk, A.; Waske, A.; Eckert, J.; Scudino, S. Additive manufacturing of a 316L steel matrix composite reinforced with CeO2 particles: Process optimization by adjusting the laser scanning speed. Technologies 2018, 6, 25. [Google Scholar] [CrossRef]
- Kurgan, N.; Varol, R. Mechanical properties of P/M 316L stainless steel materials. Powder Technol. 2010, 201, 242–247. [Google Scholar] [CrossRef]
- Gnanasekaran, B.; Song, J.; Vasudevan, V.; Fu, Y. Corrosion fatigue characteristics of 316L stainless steel fabricated by laser powder bed fusion. Metals 2021, 11, 1046. [Google Scholar] [CrossRef]
- Pragana, J.P.; Pombinha, P.; Duarte, V.R.; A Rodrigues, T.; Oliveira, J.P.; Bragança, I.M.; Santos, T.G.; Miranda, R.M.; Coutinho, L.; Silva, C.M. Influence of processing parameters on the density of 316L stainless steel parts manufactured through laser powder bed fusion. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2020, 234, 1246–1257. [Google Scholar] [CrossRef]
- Ziri, S.; Hor, A.; Mabru, C. Combined effect of powder properties and process parameters on the density of 316L stainless steel obtained by laser powder bed fusion. Int. J. Adv. Manuf. Technol. 2022, 120, 6187–6204. [Google Scholar] [CrossRef]
- Huang, G.; Wei, K.; Deng, J.; Liu, M.; Zeng, X. High-power laser powder bed fusion of 316L stainless steel: Defects, microstructure, and mechanical properties. J. Manuf. Process. 2022, 83, 235–245. [Google Scholar] [CrossRef]
- Piazza, S.; Merrigan, B.; Dowling, D.P.; Celikin, M. The effects of geometry and laser power on the porosity and melt pool formation in additively manufactured 316L stainless steel. Int. J. Adv. Manuf. Technol. 2020, 111, 1457–1470. [Google Scholar] [CrossRef]
- Sander, G.; Thomas, S.; Cruz, V.; Jurg, M.; Birbilis, N.; Gao, X.; Brameld, M.; Hutchinson, C.R. On the corrosion and metastable pitting characteristics of 316L stainless steel produced by selective laser melting. J. Electrochem. Soc. 2017, 164, C250. [Google Scholar] [CrossRef]
- Sun, Y.; Moroz, A.; Alrbaey, K. Sliding wear characteristics and corrosion behaviour of selective laser melted 316L stainless steel. J. Mater. Eng. Perform. 2014, 23, 518–526. [Google Scholar] [CrossRef]
- Schaller, R.F.; Taylor, J.M.; Rodelas, J.; Schindelholz, E.J. Corrosion properties of powder bed fusion additively manufactured 17-4 PH stainless steel. Corrosion 2017, 73, 796–807. [Google Scholar] [CrossRef]
- Geenen, K.; Röttger, A.; Theisen, W. Corrosion behavior of 316L austenitic steel processed by selective laser melting, hot-isostatic pressing, and casting. Mater. Corros. 2017, 68, 764–775. [Google Scholar] [CrossRef]
- Song, J.; Sangoi, K.; Nadimi, M.; Fu, Y. Remarkably enhanced corrosion performance of 316 L stainless steel via laser powder bed fusion thin-layer deposition. Mater. Today Commun. 2024, 38, 107690. [Google Scholar] [CrossRef]
- Laleh, M.; Hughes, A.E.; Xu, W.; Cizek, P.; Tan, M.Y. Unanticipated drastic decline in pitting corrosion resistance of additively manufactured 316L stainless steel after high-temperature post-processing. Corros. Sci. 2020, 165, 108412. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.D.; De, A.; Zhang, W. Additive manufacturing of metallic components–process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Sprouster, D.J.; Cunningham, W.S.; Halada, G.P.; Yan, H.; Pattammattel, A.; Huang, X.; Olds, D.; Tilton, M.; Chu, Y.S.; Dooryhee, E.; et al. Dislocation microstructure and its influence on corrosion behavior in laser additively manufactured 316L stainless steel. Addit. Manuf. 2021, 47, 102263. [Google Scholar] [CrossRef]
- Trelewicz, J.R.; Halada, G.P.; Donaldson, O.K.; Manogharan, G. Microstructure and corrosion resistance of laser additively manufactured 316L stainless steel. JOM 2016, 68, 850–859. [Google Scholar] [CrossRef]
- Ziętala, M.; Durejko, T.; Polański, M.; Kunce, I.; Płociński, T.; Zieliński, W.; Łazińska, M.; Stępniowski, W.; Czujko, T.; Kurzydłowski, K.J.; et al. The microstructure, mechanical properties and corrosion resistance of 316L stainless steel fabricated using laser engineered net shaping. Mater. Sci. Eng. A 2016, 677, 1–10. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Zhang, L.; Man, C.; Yao, J.; Xiao, K.; Li, X. Heat treatment effect on the microstructure and corrosion behavior of 316L stainless steel fabricated by selective laser melting for proton exchange membrane fuel cells. Electrochim. Acta 2018, 276, 293–303. [Google Scholar] [CrossRef]
- Wang, K.; Chao, Q.; Annasamy, M.; Hodgson, P.D.; Thomas, S.; Birbilis, N.; Fabijanic, D. On the pitting behaviour of laser powder bed fusion prepared 316L stainless steel upon post-processing heat treatments. Corros. Sci. 2022, 197, 110060. [Google Scholar] [CrossRef]
- Bedmar, J.; García-Rodríguez, S.; Roldán, M.; Torres, B.; Rams, J. Effects of the heat treatment on the microstructure and corrosion behavior of 316 L stainless steel manufactured by Laser Powder Bed Fusion. Corros. Sci. 2022, 209, 110777. [Google Scholar] [CrossRef]
- Ronneberg, T.; Davies, C.M.; Hooper, P.A. Revealing relationships between porosity, microstructure and mechanical properties of laser powder bed fusion 316L stainless steel through heat treatment. Mater. Des. 2020, 189, 108481. [Google Scholar] [CrossRef]
- Dong, P.; Vecchiato, F.; Yang, Z.; Hooper, P.; Wenman, M. The effect of build direction and heat treatment on atmospheric stress corrosion cracking of laser powder bed fusion 316L austenitic stainless steel. Addit. Manuf. 2021, 40, 101902. [Google Scholar] [CrossRef]
- Kluczyński, J.; Śnieżek, L.; Grzelak, K.; Oziębło, A.; Perkowski, K.; Torzewski, J.; Szachogłuchowicz, I.; Gocman, K.; Wachowski, M.; Kania, B. Comparison of different heat treatment processes of selective laser melted 316L steel based on analysis of mechanical properties. Materials 2020, 13, 3805. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Xiao, R.; Li, H.; Ruan, Y. Effects of phase selection and microsegregation on corrosion behaviors of Ti-Al-Mo alloys. Corros. Sci. 2022, 200, 110232. [Google Scholar] [CrossRef]
- Li, M.; Chen, W.-Y.; Zhang, X. Effect of heat treatment on creep behavior of 316 L stainless steel manufactured by laser powder bed fusion. J. Nucl. Mater. 2022, 559, 153469. [Google Scholar] [CrossRef]
- Jinhui, L.; Ruidi, L.; Wenxian, Z.; Liding, F.; Huashan, Y. Study on formation of surface and microstructure of stainless steel part produced by selective laser melting. Mater. Sci. Technol. 2010, 26, 1259–1264. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, S.; Shi, Q.; Tao, H.; Song, Y.; Zheng, J.; Xu, P.; Zhang, L. Improvement of corrosion resistance of SS316L manufactured by selective laser melting through subcritical annealing. Corros. Sci. 2019, 164, 108353. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Wang, D. A study on the residual stress during selective laser melting (SLM) of metallic powder. Int. J. Adv. Manuf. Technol. 2016, 87, 647–656. [Google Scholar] [CrossRef]
- Feaugas, X.; Haddou, H. Effects of grain size on dislocation organization and internal stresses developed under tensile loading in fcc metals. Philos. Mag. 2007, 87, 989–1018. [Google Scholar] [CrossRef]
- Bertsch, K.; de Bellefon, G.M.; Kuehl, B.; Thoma, D. Origin of dislocation structures in an additively manufactured austenitic stainless steel 316L. Acta Mater. 2020, 199, 19–33. [Google Scholar] [CrossRef]
- Chao, Q.; Cruz, V.; Thomas, S.; Birbilis, N.; Collins, P.; Taylor, A.; Hodgson, P.D.; Fabijanic, D. On the enhanced corrosion resistance of a selective laser melted austenitic stainless steel. Scr. Mater. 2017, 141, 94–98. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting Corrosion of Metals: A Review of the Critical Factors. J. Electrochem. Soc. 1998, 145, 2186–2198. [Google Scholar] [CrossRef]
- Khamaj, J.A. Cyclic polarization analysis of corrosion behavior of ceramic coating on 6061 Al/SiCp composite for marine applications. Prot. Met. Phys. Chem. Surfaces 2016, 52, 886–893. [Google Scholar] [CrossRef]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Pang, J.; Briceno, A.; Chander, S. A Study of Pyrite/Solution Interface by Impedance Spectroscopy. J. Electrochem. Soc. 1990, 137, 3447–3455. [Google Scholar] [CrossRef]
- Salman, O.; Gammer, C.; Chaubey, A.; Eckert, J.; Scudino, S. Effect of heat treatment on microstructure and mechanical properties of 316L steel synthesized by selective laser melting. Mater. Sci. Eng. A 2019, 748, 205–212. [Google Scholar] [CrossRef]
- Terada, M.; Saiki, M.; Costa, I.; Padilha, A.F. Microstructure and intergranular corrosion of the austenitic stainless steel 1.4970. J. Nucl. Mater. 2006, 358, 40–46. [Google Scholar] [CrossRef]
- Terada, M.; Escriba, D.M.; Costa, I.; Materna-Morris, E.; Padilha, A.F. Investigation on the intergranular corrosion resistance of the AISI 316L(N) stainless steel after long time creep testing at 600 °C. Mater. Charact. 2008, 59, 663–668. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, J.; Hu, S.; Tao, H.; Fang, B.; Li, L.; Zheng, J.; Zhang, L. Enhanced corrosion resistance of additively manufactured 316L stainless steel after heat treatment. J. Electrochem. Soc. 2020, 167, 141504. [Google Scholar] [CrossRef]
- Montero-Sistiaga, M.L.; Godino-Martinez, M.; Boschmans, K.; Kruth, J.-P.; Van Humbeeck, J.; Vanmeensel, K. Microstructure evolution of 316L produced by HP-SLM (high power selective laser melting). Addit. Manuf. 2018, 23, 402–410. [Google Scholar] [CrossRef]
- Jeon, J.M.; Park, J.M.; Yu, J.-H.; Kim, J.G.; Seong, Y.; Park, S.H.; Kim, H.S. Effects of microstructure and internal defects on mechanical anisotropy and asymmetry of selective laser-melted 316L austenitic stainless steel. Mater. Sci. Eng. A 2019, 763, 138152. [Google Scholar] [CrossRef]
- Ni, X.; Kong, D.; Wu, W.; Zhang, L.; Dong, C.; He, B.; Lu, L.; Wu, K.; Zhu, D. Corrosion behavior of 316L stainless steel fabricated by selective laser melting under different scanning speeds. J. Mater. Eng. Perform. 2018, 27, 3667–3677. [Google Scholar] [CrossRef]
- Gorsse, S.; Hutchinson, C.; Gouné, M.; Banerjee, R. Additive manufacturing of metals: A brief review of the characteristic microstructures and properties of steels, Ti-6Al-4V and high-entropy alloys. Sci. Technol. Adv. Mater. 2017, 18, 584–610. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, K.; Gao, X.; Zhong, Y.; Shen, Z. Hardened austenite steel with columnar sub-grain structure formed by laser melting. Mater. Sci. Eng. A 2015, 625, 221–229. [Google Scholar] [CrossRef]
- Schaller, R.F.; Mishra, A.; Rodelas, J.M.; Taylor, J.M.; Schindelholz, E.J. The role of microstructure and surface finish on the corrosion of selective laser melted 304L. J. Electrochem. Soc. 2018, 165, C234. [Google Scholar] [CrossRef]
- Lodhi, M.; Iams, A.; Sikora, E.; Palmer, T. Microstructural features contributing to macroscopic corrosion: The role of oxide inclusions on the corrosion properties of additively manufactured 316L stainless steel. Corros. Sci. 2022, 203, 110354. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangoi, K.; Nadimi, M.; Song, J.; Fu, Y. Heat Treatment Effect on the Corrosion Resistance of 316L Stainless Steel Produced by Laser Powder Bed Fusion. Metals 2025, 15, 41. https://doi.org/10.3390/met15010041
Sangoi K, Nadimi M, Song J, Fu Y. Heat Treatment Effect on the Corrosion Resistance of 316L Stainless Steel Produced by Laser Powder Bed Fusion. Metals. 2025; 15(1):41. https://doi.org/10.3390/met15010041
Chicago/Turabian StyleSangoi, Kevin, Mahdi Nadimi, Jie Song, and Yao Fu. 2025. "Heat Treatment Effect on the Corrosion Resistance of 316L Stainless Steel Produced by Laser Powder Bed Fusion" Metals 15, no. 1: 41. https://doi.org/10.3390/met15010041
APA StyleSangoi, K., Nadimi, M., Song, J., & Fu, Y. (2025). Heat Treatment Effect on the Corrosion Resistance of 316L Stainless Steel Produced by Laser Powder Bed Fusion. Metals, 15(1), 41. https://doi.org/10.3390/met15010041






