Preparation of Two Novel Stable Silica-Based Adsorbents for Selective Separation of Sr from Concentrated Nitric Acid Solution
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
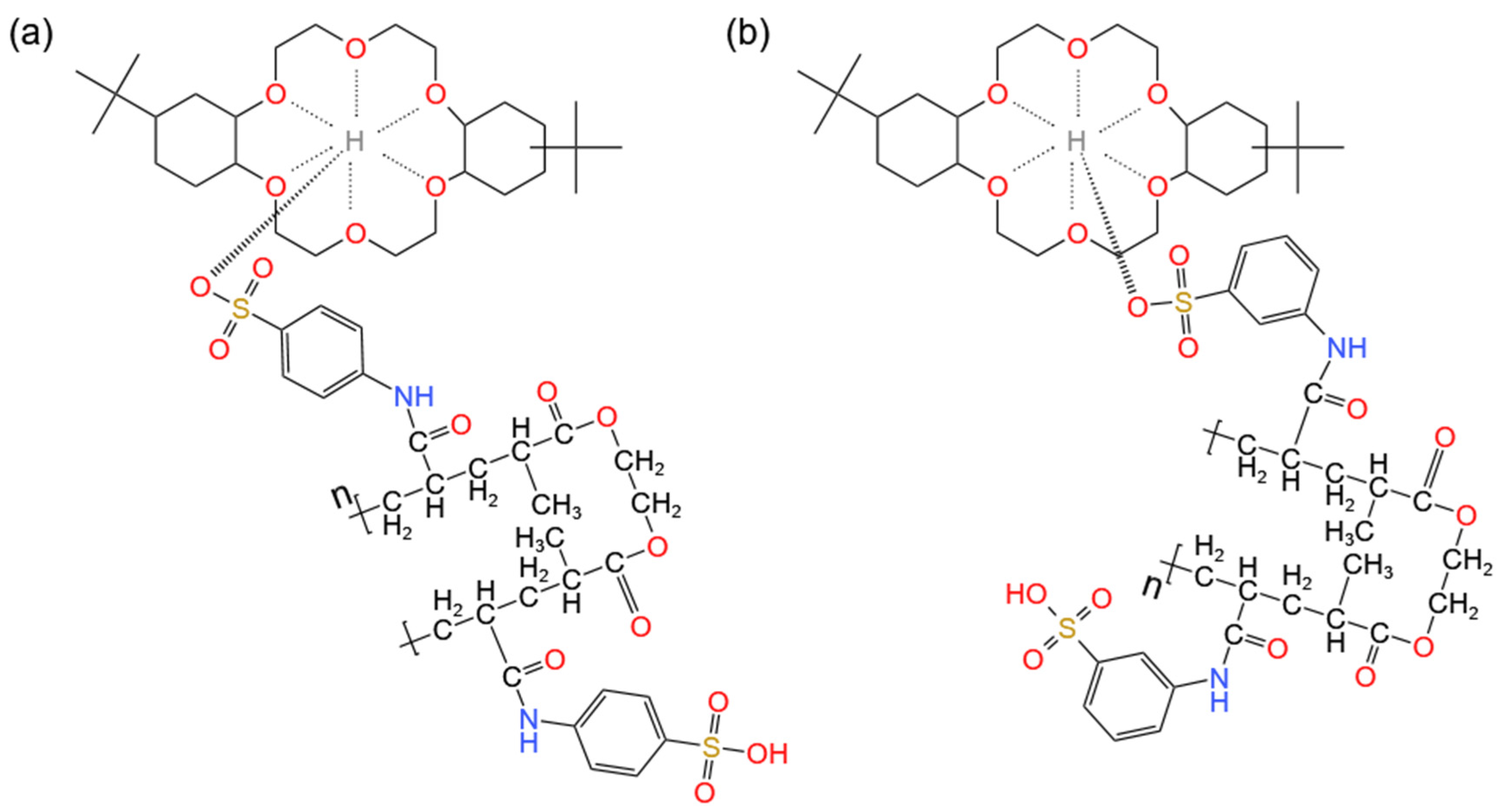
2.2. Synthesis
2.3. Characterization
2.4. Batch Experiments
2.5. Column Experiments
3. Results and Discussion
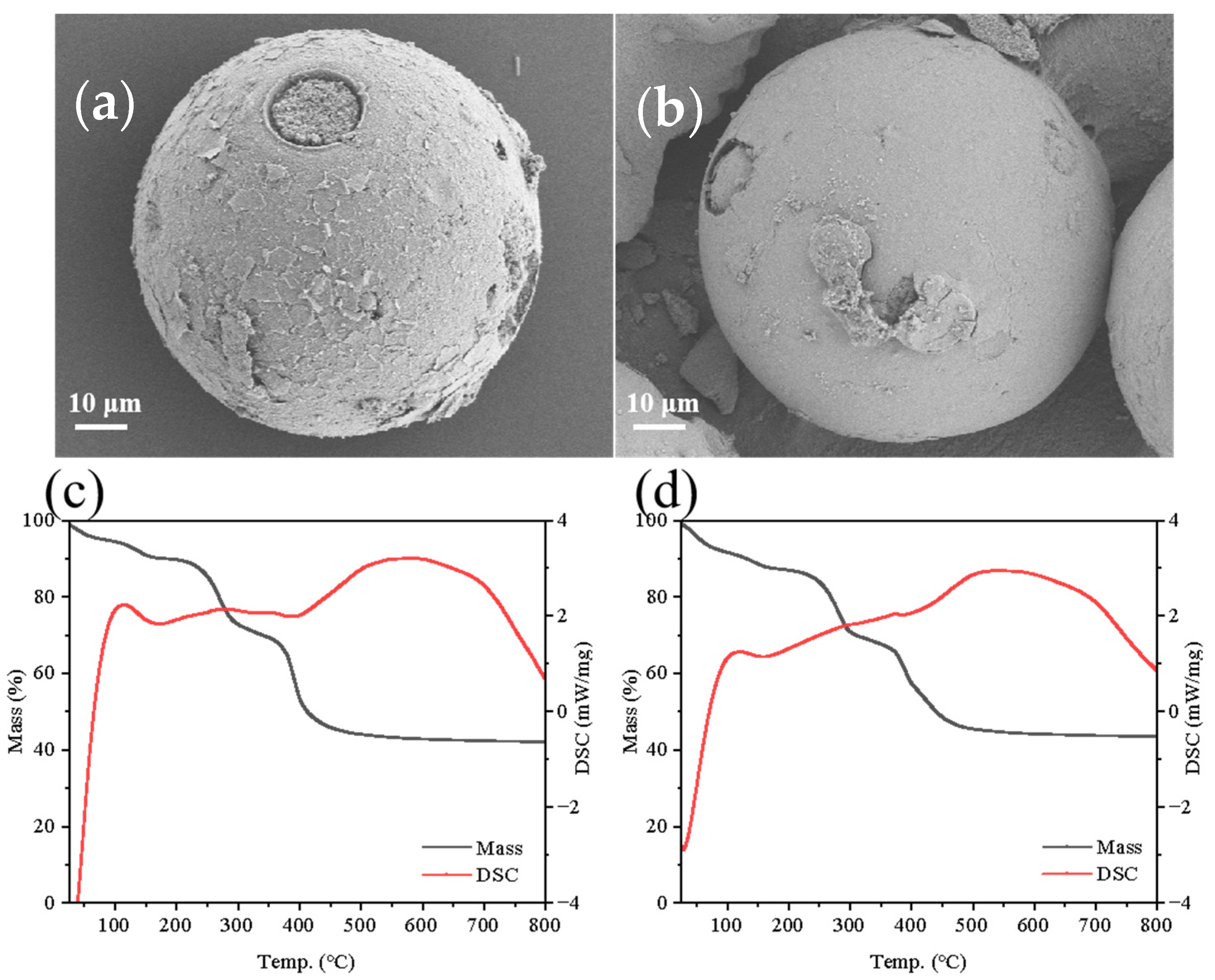
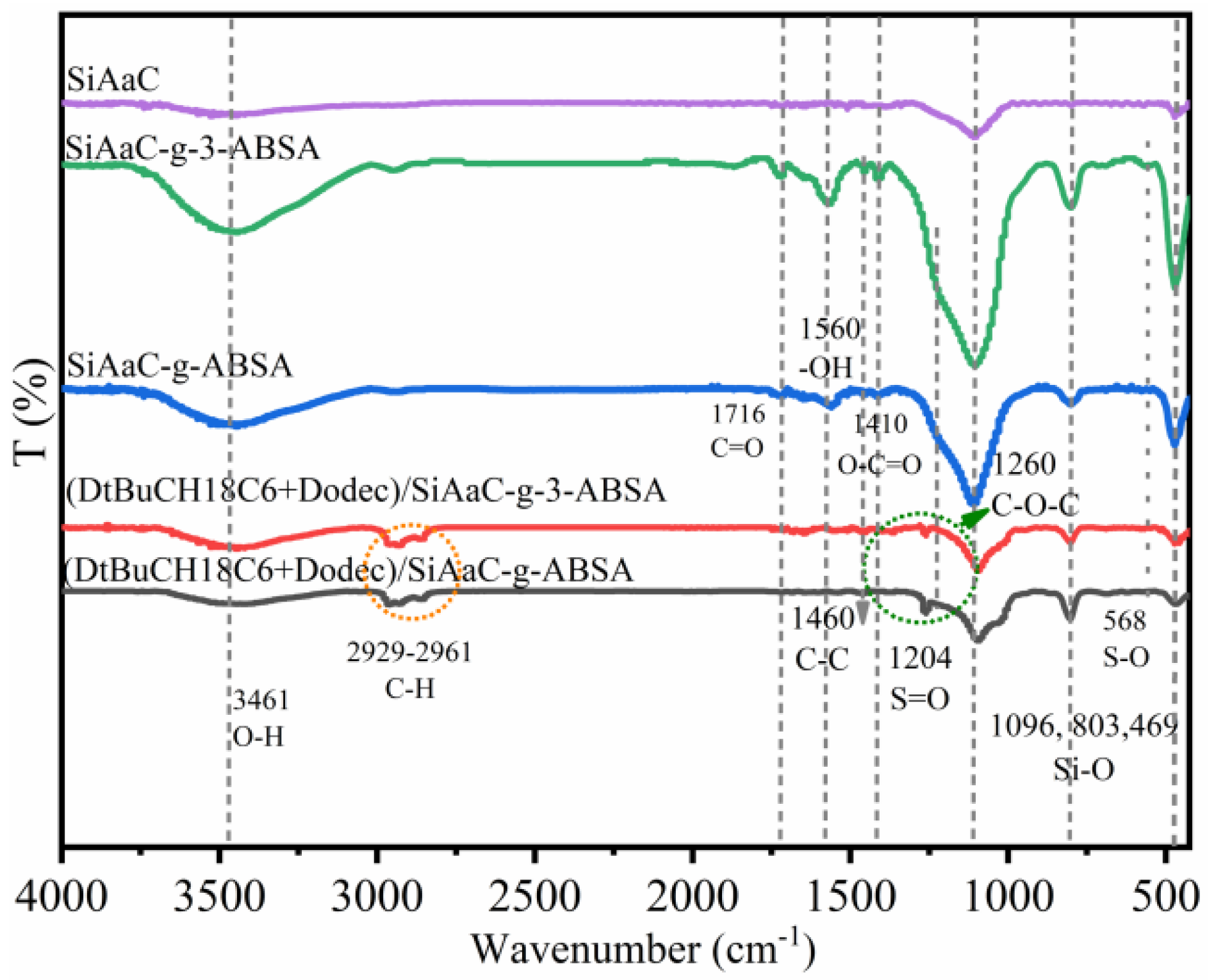
3.1. Characterization of the Materials
3.2. Batch Adsorption Experiments
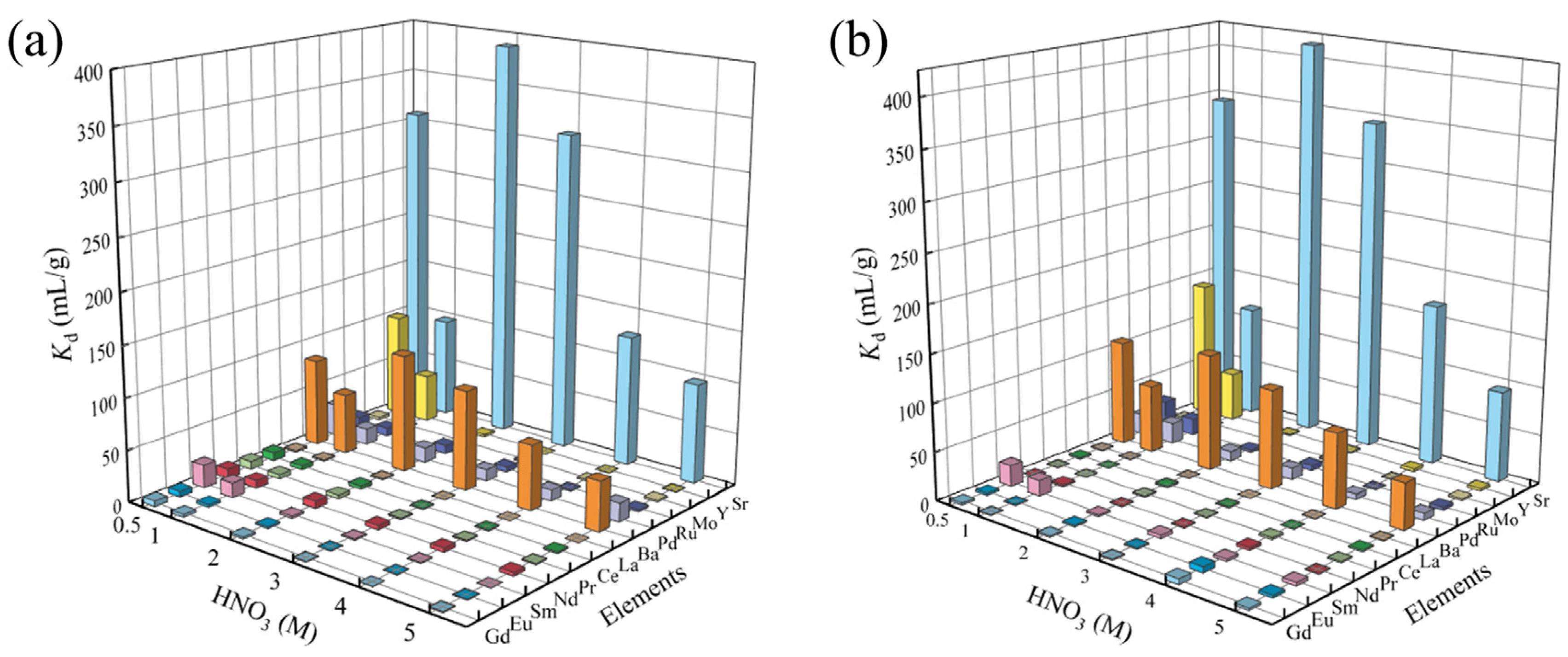
3.2.1. Adsorption Selectivity
3.2.2. Kinetics
3.2.3. Isotherms
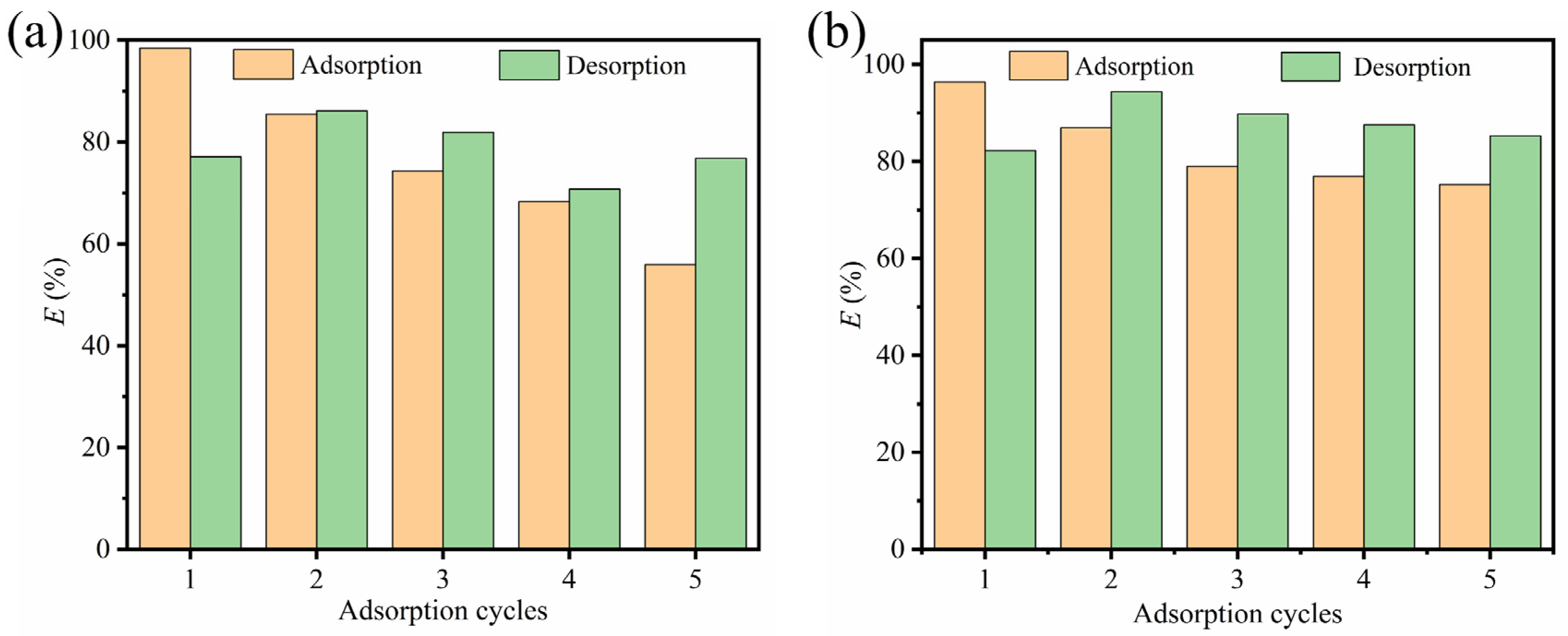
3.3. Reusability and Stability
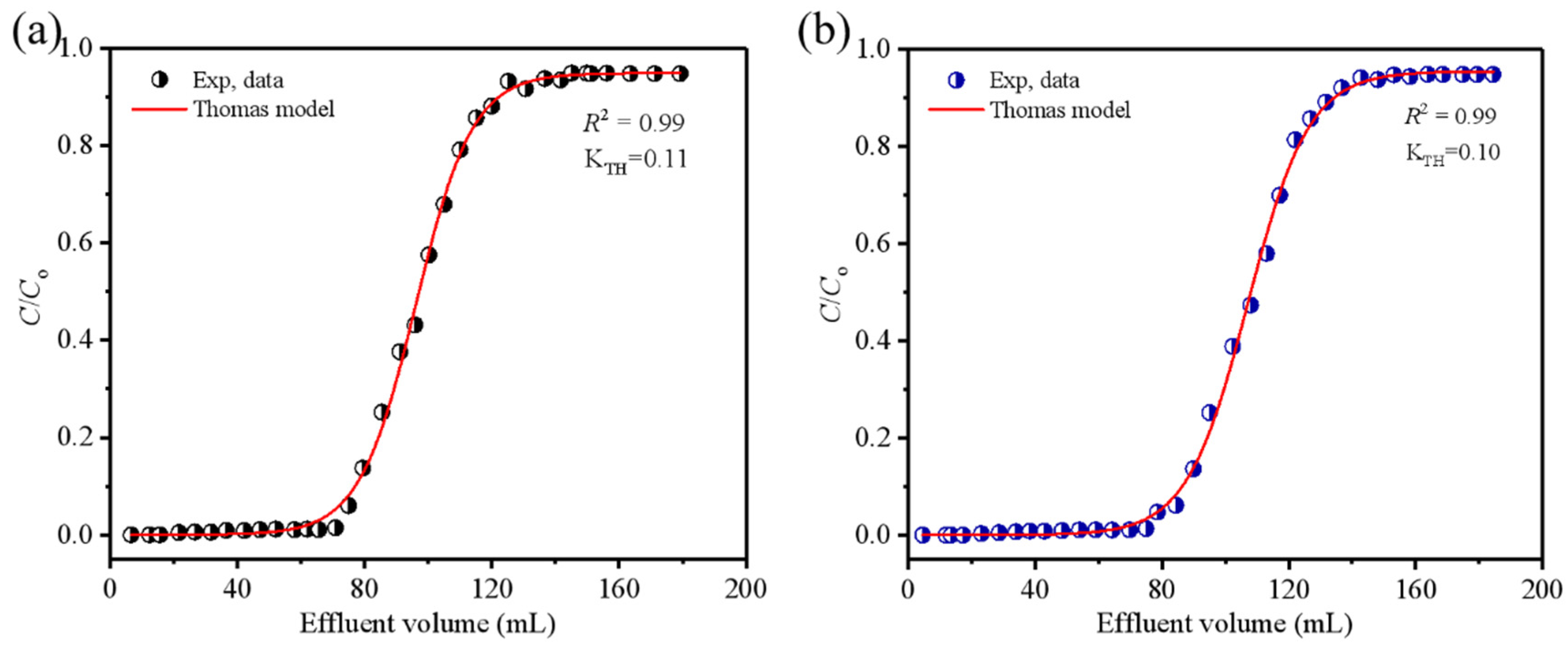
3.4. Column Experiments
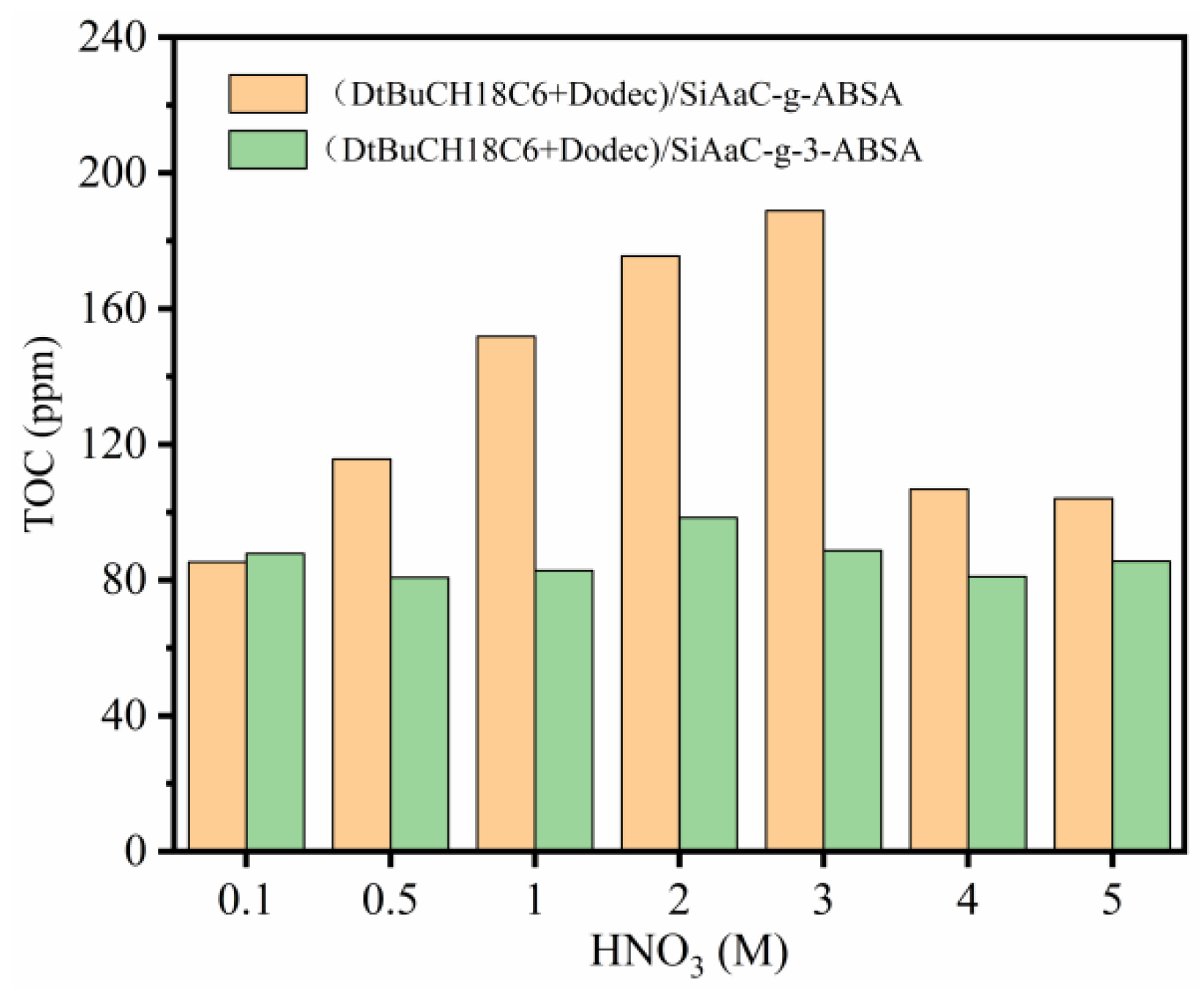
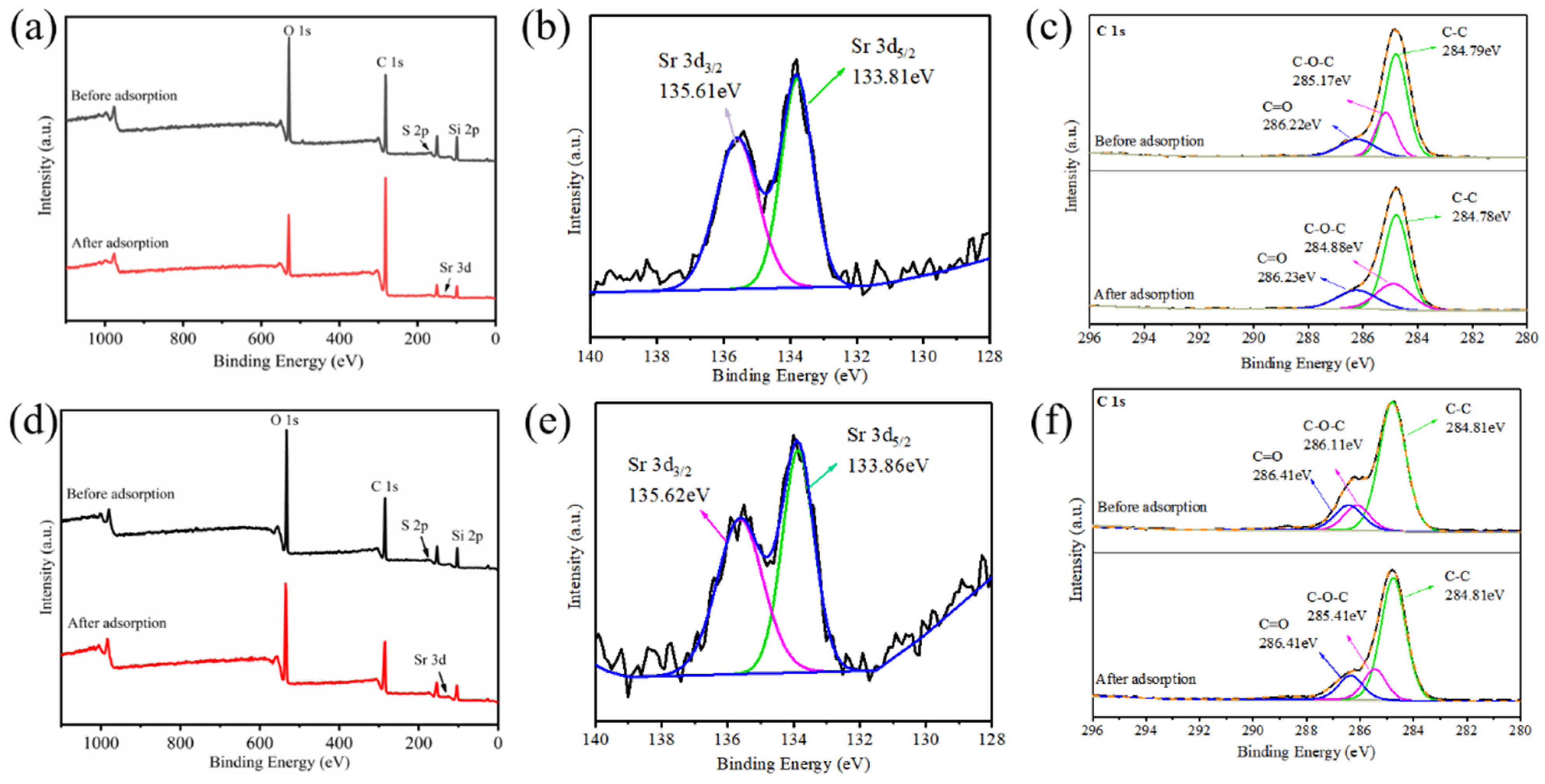
3.5. Mechanism Study
3.6. Comparison of (DtBuCH18C6 + Dodec)/SiAaC-g-ABSA and (DtBuCH18C6 + Dodec)/SiAaC-g-3-ABSA with Other Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Kim, S.Y.; Ito, T.; Nakazawa, K.; Funaki, Y.; Tada, T.; Hitomi, K.; Ishii, K. Adsorption and separation behavior of yttrium and strontium in nitric acid solution by extraction chromatography using a macroporous silica-based adsorbent. J. Chromatogr. A 2012, 1263, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, K.A.; Selvan, B.R.; Antony, M.P.; Srinivasan, T.G.; Rao, P.R.V. Extraction of palladium from nitric acid medium by commercial resins with phosphinic acid, methylene thiol and isothiouronium moieties attached to polystyrene-divinylbenzene. J. Radioanal. Nucl. Chem. 2005, 266, 431–440. [Google Scholar] [CrossRef]
- Khan, P.N.; Bhattacharyya, A.; Banerjee, D.; Sugilal, G.; Kaushik, C.P. Partitioning of heat generating fission product (137Cs & 90Sr) from acidic medium by 1,3-dioctyloxy-calix[4]arenecrown-6 (CC6) & Octabenzyloxyoctakis[[[(N,N-diethylamino)carbonyl)]methyl]oxy]calix[8]arene (BOC8A) in nitro octane diluent: Batch scale study & process parameter optimization. Sep. Purif. Technol. 2021, 274, 119102. [Google Scholar] [CrossRef]
- Xiao, C.L.; Wang, C.Z.; Yuan, L.Y.; Li, B.; He, H.; Wang, S.; Zhao, Y.L.; Chai, Z.F.; Shi, W.Q. Excellent selectivity for actinides with a tetradentate 2,9-diamide-1,10-phenanthroline ligand in highly acidic solution: A hard–soft donor combined strategy. Inorg. Chem. 2014, 53, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, A.; Pu, N.; Xu, C.; Chen, J. Development of Two novel silica based symmetric triazine-ring opening N-donor ligands functional adsorbents for highly efficient separation of palladium from HNO3 solution. J. Hazard. Mater. 2019, 376, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.J.; Oleynikov, P.; Moon, W.K.; Ma, Y.; Mayoral, A.; Kim, H.; Dejoie, C.; Song, M.K.; Terasaki, O.; Yoon, K.B. Removal of 90Sr from highly Na+-rich liquid nuclear waste with a layered vanadosilicate. Energy Environ. Sci. 2019, 12, 1857–1865. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, P.; Yan, S.; Pan, S.; Dong, L.; Zhang, G. A novel nanomaterial and its new application for efficient radioactive strontium removal from tap water: KZTS-NS metal sulfide adsorbent versus CTA-F-MF process. Chem. Eng. J. 2020, 391, 123486. [Google Scholar] [CrossRef]
- Song, Y.; Du, Y.; Lv, D.; Ye, G.; Wang, J. Macrocyclic receptors immobilized to monodisperse porous polymer particles by chemical grafting and physical impregnation for strontium capture: A comparative study. J. Hazard. Mater. 2014, 274, 221–228. [Google Scholar] [CrossRef]
- Saha, D.; Vithya, J.; Kumar, R.; Joseph, M. Studies on purification of 89Sr from irradiated yttria target by multi-column extraction chromatography using DtBuCH18-C-6/XAD-7 resin. Radiochim. Acta 2019, 107, 479–487. [Google Scholar] [CrossRef]
- Yi, R.; Xu, C.; Sun, T.; Wang, Y.; Ye, G.; Wang, S.; Chen, J. Improvement of the extraction ability of bis(2-propyloxy)calix[4]arene-crown-6 toward cesium cation by introducing an intramolecular triple cooperative effect. Sep. Purif. Technol. 2018, 199, 97–104. [Google Scholar] [CrossRef]
- Wu, Y.; Kim, S.-Y.; Tozawa, D.; Ito, T.; Tada, T.; Hitomi, K.; Kuraoka, E.; Yamazaki, H.; Ishii, K. Equilibrium and kinetic studies of selective adsorption and separation for strontium using DtBuCH18C6 loaded resin. J. Nucl. Sci. Technol. 2012, 49, 320–327. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, C.; Chai, Z. SPEC Process II. Adsorption of strontium and some typical co-existent elements contained in high level liquid waste onto a macroporous silica-based crown ether impregnated functional composite. J. Radioanal. Nucl. Chem. 2009, 280, 181–191. [Google Scholar] [CrossRef]
- Guo, C.; Yuan, M.; He, L.; Cheng, L.; Wang, X.; Shen, N.; Ma, F.; Huang, G.; Wang, S. Efficient capture of Sr2+from acidic aqueous solution by an 18-crown-6-etherbased metal organic framework. CrystEngComm 2021, 23, 3349–3355. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Wei, Y. Adsorption characteristics and radiation stability of a silica-based DtBuCH18C6 adsorbent for Sr (II) separation in HNO3 medium. J. Radioanal. Nucl. Chem. 2014, 299, 485–491. [Google Scholar] [CrossRef]
- Sharma, J.N.; Khan, P.N.; Dhami, P.S.; Jagasia, P.; Tessy, V.; Kaushik, C.P. Separation of strontium-90 from a highly saline high level liquid waste solution using 4,4′(5′)-[di-tert-butyldicyclohexano]-18-crown-6 + isodecyl alcohol/n-dodecane solvent. Sep. Purif. Technol. 2019, 229, 115502. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, C.; Kuraoka, E.; Kumagai, M. Preparation of a novel silica-based DtBuCH18C6 impregnated polymeric composite modified by tri-n-butyl phosphate and its application in chromatographic partitioning of strontium from high level liquid waste. Ind. Eng. Chem. Res. 2007, 46, 2164–2171. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Y.; Mao, C.; Sang, H.; Wu, Y.; Li, H.; Wei, Y. Development of chromatographic process for the dynamic separation of 90Sr from high level liquid waste through breakthrough curve simulation and thermal analysis. Sep. Purif. Technol. 2022, 282, 120103. [Google Scholar] [CrossRef]
- Liu, H.; Ning, S.; Zhang, S.; Wang, X.; Chen, L.; Fujita, T.; Wei, Y. Preparation of a mesoporous ion-exchange resin for efficient separation of palladium from simulated electroplating wastewater. J. Environ. Chem. Eng. 2022, 10, 106966. [Google Scholar] [CrossRef]
- Ma, F.Y.; Li, Z.; Zhou, W.; Li, Q.N.; Zhang, L. Application of polyantimonic acid-polyacrylonitrile for removal of strontium (II) from simulated high-level liquid waste. J. Radioanal. Nucl. Chem. 2017, 311, 2007–2013. [Google Scholar] [CrossRef]
- Ning, S.Y.; Wang, X.P.; Zou, Q.; Shi, W.Q.; Tang, F.D.; He, L.F.; Wei, Y.Z. Direct separation of minor actinides from high level liquid waste by Me2-CA-BTP/SiO2-P adsorbent. Sci. Rep. 2017, 7, 14679. [Google Scholar] [CrossRef]
- Liu, J.Q.; Liu, Y.J.; Talay, D.K.; Calverley, E.; Brayden, M.; Martinez, M. A new carbon molecular sieve for propylene/propane separations. Carbon 2015, 85, 201–211. [Google Scholar] [CrossRef]
- Chang, K.C.; Lo, H.-M.; Lin, K.-L.; Liu, M.-H.; Chiu, H.-Y.; Lo, F.-C.; Chang, J.H. Cu adsorption in fixed bed column with three different influent concentration. E3S Web Conf. 2019, 120, 03003. [Google Scholar] [CrossRef]
- Unuabonah, E.I.; Omorogie, M.O.; Oladoja, N.A. Modeling in adsorption: Fundamentals and applications, Compos. Nanoadsorbents 2018, 85–118. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Li, J.S.; Liu, F.X.; Wang, X.; Chen, H.; Ni, H.Z.; Wang, Z. Composite membranes based on polybenzimidazole and ionic liquid functional Si-O-Si network for HT-PEMFC applications. Int. J. Hydrogen Energy 2017, 42, 21913–21921. [Google Scholar] [CrossRef]
- Kozubal, J.; Heck, T.; Metz, R.B. Vibrational Spectroscopy of Intermediates and C-H Activation Products of Sequential Zr+ Reactions with CH4. J. Phys. Chem. A 2020, 124, 8235–8245. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, Y.; Zhang, A.Y. Preparation and characterization of a mesoporous polycrown impregnated silica and its adsorption for palladium from highly acid medium. J. Porous Mater. 2017, 24, 1037–1045. [Google Scholar] [CrossRef]
- Tang, J.; Liao, L.; He, X.; Lv, L.; Yin, X.; Li, W.; Wei, Y.; Ning, S.; Chen, L. Efficient separation of radium from natural thorium using a mesoporous silica-supported composite resin with sulfonic acid groups for the acquisition of targeted α-nuclides 212Pb. Chem. Eng. J. 2024, 485, 150022. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Wang, W.H.; Chai, Z.F.; Kumagai, M. Separation of strontium ions from a simulated highly active liquid waste using a composite of silica-crown ether in a polymer. J. Sep. Sci. 2008, 31, 3148–3155. [Google Scholar] [CrossRef]
- Lu, L.; Na, C.Z. Gibbsian interpretation of Langmuir, Freundlich and Temkin isotherms for adsorption in solution. Philos. Mag. Lett. 2022, 102, 239–253. [Google Scholar] [CrossRef]
- Ezzati, R.; Pseudo-First-Order, D.O. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Martín, F.S.; Kracht, W.; Vargas, T. Attachment of Acidithiobacillus ferrooxidans to pyrite in fresh and saline water and fitting to Langmuir and Freundlich isotherms. Biotechnol. Lett. 2020, 42, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Zhang, S.; Zhang, W.; Zhou, J.; Wang, S.; Wang, X.; Wei, Y. Separation and recovery of Rh, Ru and Pd from nitrate solution with a silica-based isoBu-BTP/SiO2-P adsorbent. Hydrometallurgy 2019, 191, 105207. [Google Scholar] [CrossRef]
- Baseri, H.; Tizro, S. Treatment of nickel ions from contaminated water by magnetit based nanocomposite adsorbents: Effects of thermodynamic and kinetic parameters and modeling with Langmuir and Freundlich isotherms. Process Saf. Environ. Prot. 2017, 109, 465–477. [Google Scholar] [CrossRef]
- Su, Z.; Ning, S.; Li, Z.; Zhang, S. High-efficiency separation of palladium from nitric acid solution using a silica-polymer-based adsorbent isoPentyl-BTBP/SiO2-P. J. Environ. Chem. Eng. 2022, 10, 107928. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.; Chen, L.; Li, Z.; Wu, K.; Zhang, Y.; Su, Z.; Yin, X.; Hamza, M.F.; Wei, Y.; et al. Efficient separation of palladium from nitric acid solution by a novel silica-based ion exchanger with ultrahigh adsorption selectivity. Sep. Purif. Technol. 2023, 322, 124326. [Google Scholar] [CrossRef]
- Adimule, V.; Nandi, S.S.; Yallur, B.C.; Bhowmik, D.; Jagadeesha, A.H. Optical, Structural and Photoluminescence Properties of Gdx SrO: CdO Nanostructures Synthesized by Co Precipitation Method. J. Fluoresc. 2021, 31, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gu, P.; Zhang, M.; Yan, S.; Dong, L.; Zhang, G. Synthesis of a robust layered metal sulfide for rapid and effective removal of Sr2+ from aqueous solutions. Chem. Eng. J. 2019, 372, 1205–1215. [Google Scholar] [CrossRef]
- Gupta, K.; Yuan, B.; Chen, C.; Varnakavi, N.; Fu, M.-L. K2xMnxSn3−xS6 (x = 0.5–0.95) (KMS-1) immobilized on the reduced graphene oxide as KMS-1/r-GO aerogel to effectively remove Cs+ and Sr2+ from aqueous solution. Chem. Eng. J. 2019, 369, 803–812. [Google Scholar] [CrossRef]
- Yin, L.; Kong, X.; Shao, X.; Ji, Y. Synthesis of DtBuCH18C6-coated magnetic metal–organic framework Fe3O4@UiO-66-NH2 for strontium adsorption. J. Environ. Chem. Eng. 2019, 7, 103073. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Y.; Ouyang, J.; Wu, X.; Luo, J.; Liu, S.; Gong, X. A facile preparation of dicyclohexano-18-crown-6 ether impregnated titanate nanotubes for strontium removal from acidic solution. Solid State Sci. 2019, 90, 49–55. [Google Scholar] [CrossRef]
- Kudo, T.; Ito, T.; Kim, S.-Y. Adsorption behavior of Sr (II) from high-level liquid waste using crown ether with ionic liquid impregnated silica adsorbent. Energy Procedia 2017, 131, 189–194. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, C.; Liu, Y.; Hu, Q.; Chen, C.; Kuraoka, E. Preparation of macroporous silica-based crown ether materials for strontium separation. J. Porous Mater. 2010, 17, 153–161. [Google Scholar] [CrossRef]

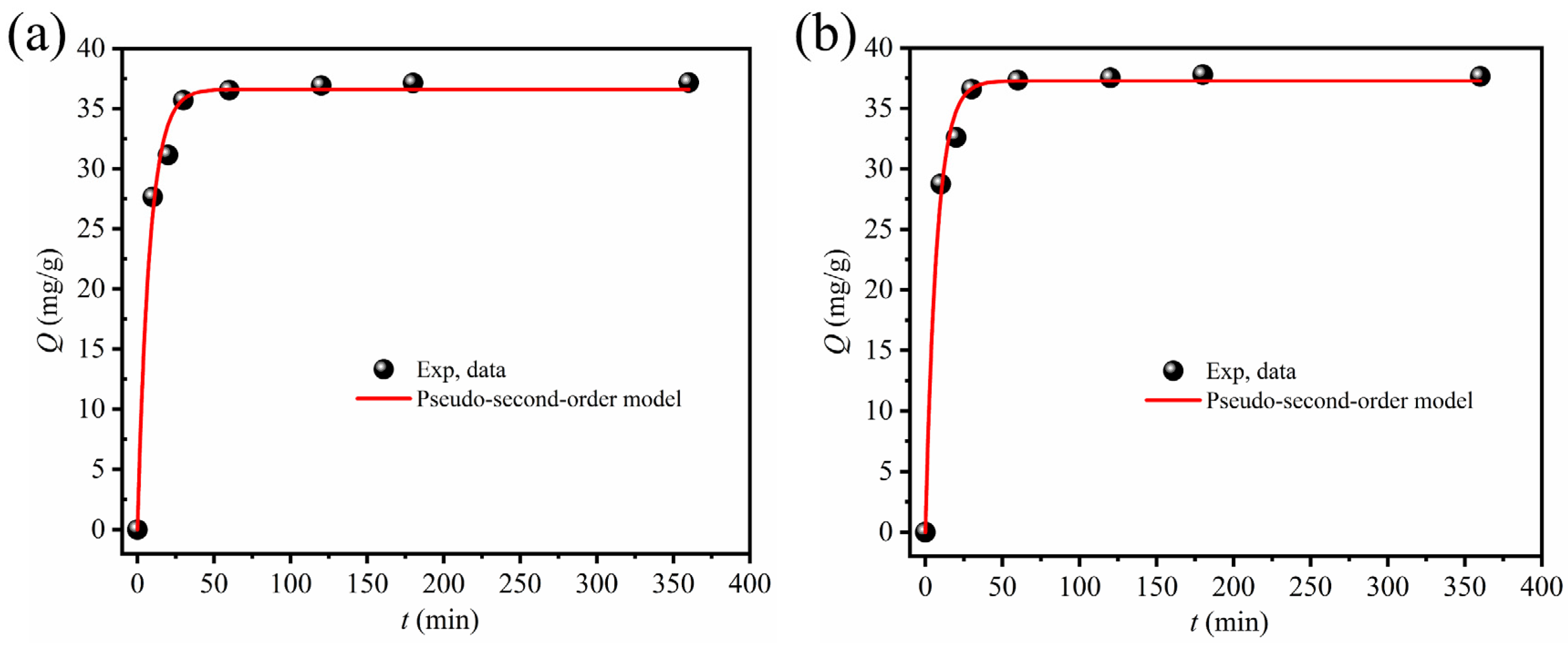

| T (K) | Adsorbents | Pseudo-Second-Order Model | ||
|---|---|---|---|---|
| K2 (g·h/mg) | Qe (mg/g) | R2 | ||
| 298 | (DtBuCH18C6 + Dodec)/ SiAaC-g-ABSA | 0.13 | 36.6 | 0.99 |
| (DtBuCH18C6 + Dodec)/ SiAaC-g-3-ABSA | 0.14 | 37.4 | 0.99 | |
| Adsorbents | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL (L/mg) | qm (mg/g) | R2 | n | KF (mg1−n·Ln/g) | R2 | |
| (DtBuCH18C6 + Dodec)/SiAaC-3-ABSA | 0.50 | 47.4 | 0.99 | 0.50 | 16.6 | 0.94 |
| (DtBuCH18C6 + Dodec)/SiAaC-g-3-ABSA | 0.51 | 48.2 | 0.99 | 0.4 | 17.1 | 0.93 |
| Adsorbents | Other Species | C(HNO3) | Kd (cm3/g) | Reusability | Ref. |
|---|---|---|---|---|---|
| (DtBuCH18C6 + [C2mim] [NTf2])/SiO2-P | Sr, Ba, Na, Ca, La, Nd, Sm, Gd, Ru, Pd, Zr, Mo | 3 M | 30 | – | [42] |
| (DtBuCH18C6 + Oct)/SiO2-P | Ru, Pd, Ba, Mo, La, Y, Cs, Na, K | 2 M | <200 | – | [43] |
| (DtBuCH18C6 + Dodec)/SiO2-P | Cs, Ru, Pd, La, Nd, Sm, Gd, Zr, Mo | 3 M | 182.0 | – | [11] |
| (DtBuCH18C6 + Dodec + DBS)/SiO2-P | – | 3 M | 260.3 | – | [17] |
| (DtBuCH18C6 + Dodec)/SiAaC-g-ABSA | Gd, Eu, Sm, Nd, Pr, Ce, La, Ba, Pd, Ru, Mo, Y | 2 M | 389.68 | 4 | This study |
| (DtBuCH18C6 + Dodec)/SiAaC-g-3-ABSA | 2 M | 416.68 | ≥5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhang, S.; Wang, X.; Chen, L.; Yin, X.; Hamza, M.F.; Wei, Y.; Ning, S. Preparation of Two Novel Stable Silica-Based Adsorbents for Selective Separation of Sr from Concentrated Nitric Acid Solution. Metals 2024, 14, 627. https://doi.org/10.3390/met14060627
Liu C, Zhang S, Wang X, Chen L, Yin X, Hamza MF, Wei Y, Ning S. Preparation of Two Novel Stable Silica-Based Adsorbents for Selective Separation of Sr from Concentrated Nitric Acid Solution. Metals. 2024; 14(6):627. https://doi.org/10.3390/met14060627
Chicago/Turabian StyleLiu, Chang, Shichang Zhang, Xinpeng Wang, Lifeng Chen, Xiangbiao Yin, Mohammed F. Hamza, Yuezhou Wei, and Shunyan Ning. 2024. "Preparation of Two Novel Stable Silica-Based Adsorbents for Selective Separation of Sr from Concentrated Nitric Acid Solution" Metals 14, no. 6: 627. https://doi.org/10.3390/met14060627
APA StyleLiu, C., Zhang, S., Wang, X., Chen, L., Yin, X., Hamza, M. F., Wei, Y., & Ning, S. (2024). Preparation of Two Novel Stable Silica-Based Adsorbents for Selective Separation of Sr from Concentrated Nitric Acid Solution. Metals, 14(6), 627. https://doi.org/10.3390/met14060627












