Abstract
The corrosion resistance of low-carbon steel and two new low-alloy, corrosion-resistant steels containing Cu-Sb and Cu was studied in a simulated seawater environment. The effects of Cu and Sb on corrosion resistance were analyzed by an electrochemical test and accelerated corrosion test. The results show that Cu and Sb reduce the corrosion current density by increasing the corrosion potential and increasing the polarization resistance. Sb can promote the formation of Cu-containing compounds with a strong corrosion inhibition performance, and it can enhance the overall corrosion resistance of steel. In addition, Sb can also promote the conversion of Fe2+ ions into a corrosion-resistant compound, α-FeOOH, and it also further improves the corrosion resistance of steel.
1. Introduction
Offshore environmental conditions, when coupled with the high concentration of salt and chloride ions in seawater, is a corrosively adverse environment for metallic materials. The corrosion caused by the chloride erosion of steel directly contributes to structural failure. Enhancing the service life in such harsh environments can significantly reduce maintenance costs. A range of anti-corrosion measures and techniques have been extensively studied, including electrochemical protection [1,2], protective coatings [3,4] and enhancing the inherent corrosion resistance of steel reinforcement [5]. The enhancement of corrosion resistance in traditional carbon steel to stainless steel primarily involves significant additions of alloying elements such as Cr, Ni, Mo, etc. The production cost of stainless steels is typically 4–6 times higher than that of ordinary carbon steels, and it is limited to a selective use in key parts of construction [6]. One of the earliest known structures using stainless steels as the primary material is located in Progreso Marina, North America, in the Gulf of Mexico. Even now, after sixty years, the rebar inside the concrete structure has shown no signs of corrosion. The Hong Kong–Zhuhai–Macao Bridge (HZMB), which commenced construction in 2009, utilizes 2304 stainless steel (UNS S32304) reinforcement components. This type of stainless steel is 60 times more resistant to pitting corrosion compared to ordinary carbon steels, and it is expected to have a service life of up to 120 years [7].
In recent years, low-alloy steels have been intensive studied, due to their ability to offer good corrosion resistance by forming a dense rust layer on the surface. The layer acts as an insulating barrier against corrosive media, while maintaining superior mechanical properties. Nishimura et al. [8] successfully developed low-alloy steels with Ni and Co (with mass fractions ranging from 1 to 3%), whereby an excellent corrosion resistance was demonstrated in both wet and dry cycling tests. Similarly, Zhou et al. [9] worked on low-alloy steels containing Cr (wt 0.25–0.5%), Ni (wt 0.4–0.5%) and Cu (wt 0.2%) to enhance the corrosion resistance of the steels. By improving the structure of the rust layer in a Cl− environment and promoting the formation of a dense internal rust layer, the aforementioned steels showed an improved performance against corrosion. Current corrosion-resistant steels have reduced the amount of Cr and Ni added through low-alloying approaches; however, they still face limitations in engineering applications due to the price. Therefore, the development of new, cost-effective low-alloyed steels holds significant market potential in addressing these challenges.
In China, HRB400 is the most commonly used carbon steel bar in ordinary concrete structures. It has excellent mechanical properties and is widely applied in construction projects. In the present paper, two different compositions of chloride ion corrosion-resistant steels were developed, using HRB400 rebar as the base material. Instead of using high-cost elements like Cr and Ni, alloying elements such as Cu, P and Sb were added to the steel. The compositions were adjusted using a vacuum induction furnace. The research involved simulating the electrochemical properties of the corrosion-resistant steel in a marine environment through electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization in the fully immersed zone. Additionally, this study simulated marine atmospheric conditions by conducting cyclic immersion tests and salt spray tests to analyze and evaluate the corrosion resistance of the steel. The physical phase and structure of the corrosion products were further analyzed using an X-ray diffractometer (XRD), a Zeiss scanning electron microscope (SEM) and an X-ray photoelectron spectrometer (XPS). These analyses provided valuable insights into the corrosion behavior and effectiveness of the developed corrosion-resistant steels in harsh marine environments.
2. Materials and Methods
2.1. Materials
The corrosion-resistant steel was melted in a 50 kg vacuum induction furnace using high-purity Ar gas as the protective gas, and ingots were then produced after casting. These ingots were then heated, forged and continuously rolled at 1150 °C to achieve a thickness of 20 mm, before being cooled to room temperature naturally. In comparison, the HRB400 steel was sourced from a domestic steel mill. Table 1 lists the chemical compositions of the three steel samples used in the experiment.

Table 1.
The chemical compositions (mass fraction, wt%) of the steel samples used in the experiment.
2.2. Electrochemical Measurements
The specimens were wire-cut into small squares with a side length of 10 mm × 10 mm. The specimens were polished to 2000# using different grits of sandpaper. In addition, they were polished with diamond spray polish with a grain size of 1 μm until the surface was free of scratches. The samples were soldered to wires and encapsulated with acrylic resin in order to study their electrochemical properties. A three-electrode system was used to test the electrochemical properties of the specimens, with the encapsulated specimen as the working electrode, the metal Pt electrode as the counter electrode and the saturated calomel electrode as the reference electrode. Before the test, the whole system was placed in the test solution, and the workstation was connected to test the open-circuit potential (OCP) after 600 s of static operation. After the OCP stabilized, the potential value at a stable potential of −0.6 V was set as the initial potential of the potentiodynamic polarization, with a scanning range of 1.2 V and a scanning speed of 0.001 V/s. Then, the value of the stable potential was set as the EIS starting potential in the range of 105 Hz to 10−2 Hz, with an amplitude of 0.01 V [10]. Electrochemical measurements were performed on a CHI-760E workstation, and the results were analyzed using ZSimpWin 3.60 software.
2.3. Accelerated Corrosion Test
The specimens used for the cycle immersion experiments and salt spray experiments were 50 mm × 25 mm × 5 mm in size. The specimens were ultrasonically cleaned using acetone and anhydrous ethanol, after being polished up to 800#. Cyclic immersion experiments were carried out at room temperature, and the corrosive solution used had a pH of 6.5–7.2, included a 3.5 wt (mass fraction) NaCl solution and was put through the experiment in a 24 h cycle. Each cycle consisted of 12 h of immersion and 12 h of drying. Salt spray experiments were carried out on a 2 K-60 A neutral salt spray tester, and the corrosion solution had a pH of 6.5–7.2 and included a 5 wt (mass fraction) NaCl solution. The corrosion time was set to 96 h, and the ambient temperature of the salt spray chamber was set at (45 ± 2) °C. NaCl solution was supplementally added once every 24 h.
2.4. Corrosion Rate Measurements
At the end of the accelerated corrosion test, the specimens, after being gently scraped from the rust layer, were vertically immersed into the cleaning solution (500 mL of HCl + 500 mL of H2O + 3.5 g of C6H12N4). After ten minutes of cleaning, the specimens were washed, sonicated and blow-dried using deionized water, acetone and anhydrous ethanol, respectively. Following this, the specimens were weighed. Each experiment was repeated three times, and the average value was taken as the result. The weight loss method was used to measure the corrosion rate, and the following equation was used:
where V is the corrosion rate, g/(m2·h); m0 − m1 is the mass loss, g; S is the total surface area, m2; and t is the exposure time, h.
2.5. Corrosion Product Analysis
X-ray diffraction spectroscopy (XRD, PANalytical Empyre, Panico, Almelo, Netherlands) with a cobalt (Co) target was utilized to analyze the phase composition of the corrosion products. The XRD analysis was performed at a scanning rate of 5°/min over a range of 20° to 80° (in 2θ). The forms of the Cu and Sb in the rust layer were investigated using X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo, Waltham, MA, USA). The binding energy of the C 1s peak at a 284.8 eV was employed as a calibration reference for the test results. The morphology of the corrosion products was examined through scanning electron microscopy (SEM, GeminiSEM300, Carl Zeiss, Oberkochen, Germany), and the elemental compositions on the surface of the corrosion products were analyzed using energy-dispersive spectroscopy (EDS).
3. Results
3.1. Microstructure
Figure 1(a-1,a-2) show the microstructure of HRB400 steel, which is primarily composed of pearlite and ferrite. Figure 1(a-3) shows the EDS results of the grain boundaries in HRB400 steel, which also indicate the enrichment of alloy elements such as Mn, Cr and Si at the grain boundaries. Figure 1(b-1,b-2) show the microstructure of 1#, where the addition of Cu and Sb facilitated grain growth. They also resulted in significantly larger grain sizes of ferrite and pearlite compared to the HRB400 samples that are shown in Figure 1(a-1). In Figure 1(b-2), the formation of the new phases that were observed at the grain boundaries is shown. Figure 1(b-3) presents the EDS results of the new phase. Figure 1(b-2) shows the enrichment of Cu and Sb at the grain boundaries of 1#. Figure 1(c-1,c-2) show the microstructure of 2#, which is mainly composed of pearlite and ferrite. Figure 1(c-3) presents the EDS results of the material shown in Figure 1(c-2), which, in turn, shows the enrichment of Cu at the grain boundaries, where the differences in phases are also compared to other grain boundaries. Based on these findings, it was suspected that Cu might have formed new intermetallic compounds with the Cr and Mn elements and precipitated at the grain boundaries.
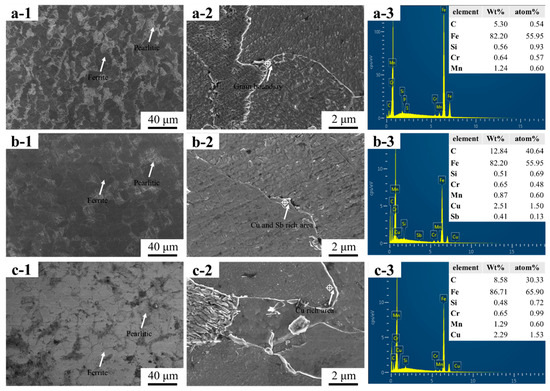
Figure 1.
The microstructures of the three groups of steels: ((a-1)–(a-3)) HRB400; ((b-1)–(b-3)) 1#; and ((c-1)–(c-3)) 2#.
3.2. EIS Results
Figure 2 shows the Nyquist and Bode plots that were obtained from the electrochemical impedance testing of the HRB400, 1# and 2# specimens in a 3.5% (mass fraction) NaCl solution. According to the characteristics of the Bode plot, the maximum phase angle occurred at a middle frequency, which indicates a capacitive response. Furthermore, the maximum phase angle was less than 90°. This was attributed to the presence of a certain roughness to the corrosion products on the surface of the electrode, which can lead to a signal offset; thus, the constant–phase–element (CPE) angle was usually used instead of the double-layer capacitor for the equivalent circuit fittings [11]. The value of the impedance was found to be related to the angular frequency of the signal source via the following relation:
where Y0 denotes the conductance modulus and n is the dimensionless dispersion coefficient. When n = 0, the CPE behaved as a pure resistor; when n = 1, the CPE behaved as a pure capacitor, where j is the imaginary unit and w is the angular frequency.
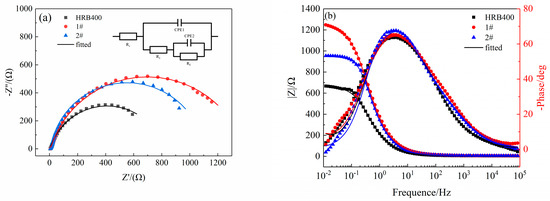
Figure 2.
The EIS results of the three groups of steels. (a) Nyquist plots. (b) Bode plots.
The values of the CPE can be calculated according to the following formula:
where “x” represents the number for each RC loop in the EEC (x = 1,2).
The characteristics of the Bode plot were used to fit an equivalent circuit with the circuit code Rs(CPE1(R1(CPE2Rf))). The solution resistance was denoted by Rs. The resistance of the surface corrosion product and the constant–phase–element angle of the double-layer capacitance were denoted by R1 and CPE1, respectively. The charge transfer resistance was represented by Rf, while the constant–phase–element angle of the corrosion product and the substrate surface that formed the double-layer capacitance were denoted by CPE2. The fitted values of each element of the equivalent circuit are listed in Table 2, and the chi-square (χ2) test values were all in the range of 10−3. This indicates that the selected equivalent circuit was effective in fitting the elements. The size of the impedance arc represents the ease of the charge transfer, with a larger diameter indicating a higher Rf value (suggesting that the corrosion reaction is less likely to occur), which thus results in the steel having a better corrosion resistance [12,13,14]. It can be seen from Table 2 that the 1# specimen with a Cu-Sb composite added to it had the lowest capacitance value and the highest Rf value, thus indicating that its resistance to electrochemical corrosion increased after low alloying [15]. Moreover, the 1# specimen with a Cu-Sb composite added to it had the best corrosion resistance performance.

Table 2.
The EIS equivalent circuit fitting results for the three groups of steels.
3.3. Potentiodynamic Polarization Results
Figure 3 displays the potentiodynamic polarization curves in a 3.5 wt% (mass fraction) NaCl solution. Table 3 presents the fitted values of the parameters, including the linear polarization resistance Rp, the anodic slope (βa) and cathodic slope (βc) of the Tafel line, which were directly obtained from the workstation. To determine the corrosion current density, the tangent lines of the cathodic and anodic polarization curves were extended to the intersection point at the origin. The current density that corresponded to this intersection point is represented by the corrosion current density Icorr. From Table 3, it is evident that the corrosion current densities of the low-alloyed test steels 1# and 2# were greatly reduced, with 1# exhibiting better corrosion resistance. This is consistent with the AC impedance results. The addition of the Cu and Sb elements gave rise to an increase in self-corrosion potential from −0.5 V to approximately −0.4 V, which led to a substantial increase in the polarization resistance. A study by Wu et al. [16] suggested that a specific ratio of Cu and Sb can alter the structure of the rust layer, which facilitates the formation of a dense and thin corrosion product film. This film is ion-selective and hinders the penetration of Cl− ions into the substrate surface, thereby explaining the increase in the EIS fitting circuit R1.
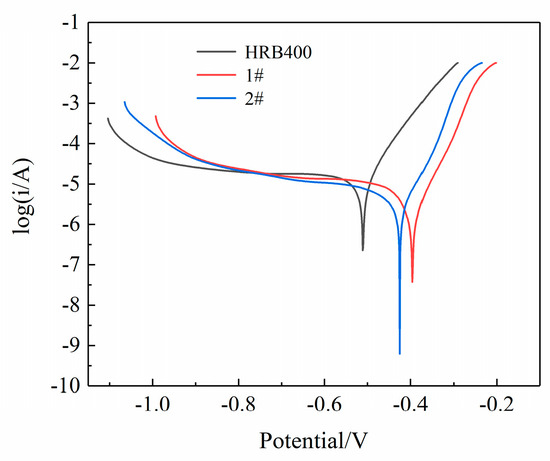
Figure 3.
The potentiodynamic polarization curve results of the three groups of steels.

Table 3.
The fitting results for the three groups of steels.
3.4. Cyclic Immersion Test
Figure 4 presents the XRD patterns of the corrosion products and the relative content of rust layer structures for the different cycles. The results were obtained using the relative intensity ratio (RIR) method, which was used in conjunction with cycle immersion tests in a 3.5 wt% (mass fraction) NaCl solution for 15, 30 and 45 days. Figure 5 illustrates the average corrosion rates of the three groups of steels. The rust layers of HRB400 and the test steels were found to consist of spinel-type iron oxides (Fe3O4 and Fe2O3) and hydroxyl iron oxides (α-FeOOH and β-FeOOH), which were collectively represented as Fe3O4 in this study, due to the similar structure of Fe3O4 and Fe2O3 (this also made them difficult to distinguish using XRD) [17]. Kamimura T et al. [18] proposed a protective ability index (PAI) of the rust layer, which is the ratio of the mass fraction of crystalline α-FeOOH to the sum of the mass fractions of γ-FeOOH and Fe3O4 (denoted as α/γ*), where a higher value indicates a better protective performance from the rust layer. In the early stages of steel corrosion, the alloying elements at the steel matrix interface promoted the charge transfer process, thereby accelerating the generation of corrosion products. Consequently, the corrosion rates of 1# and 2# were initially significantly higher than that of HRB400. However, as the corrosion time progressed, a complete protective rust layer gradually formed on the steel surface, leading to a rapid reduction in the corrosion rate.
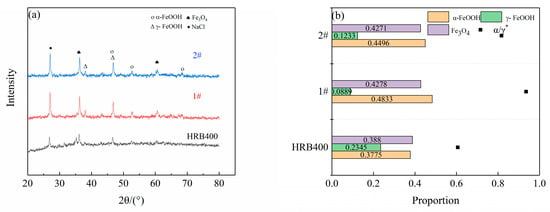
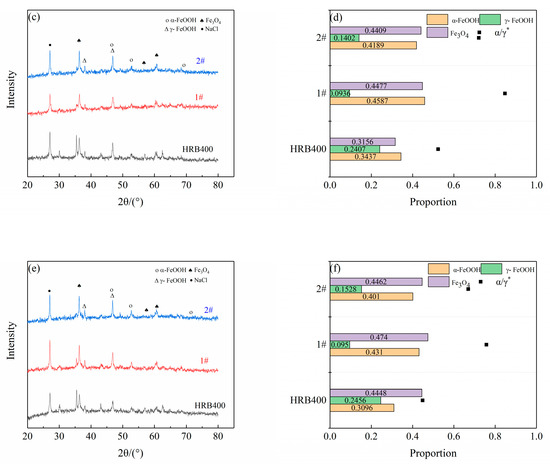
Figure 4.
The XRD patterns of the corrosion products, and the proportion of the various phases formed on the three groups of steels after cyclic corrosion tests. (a,b) 15 day, (c,d) 30 day and (e,f) 45 day.
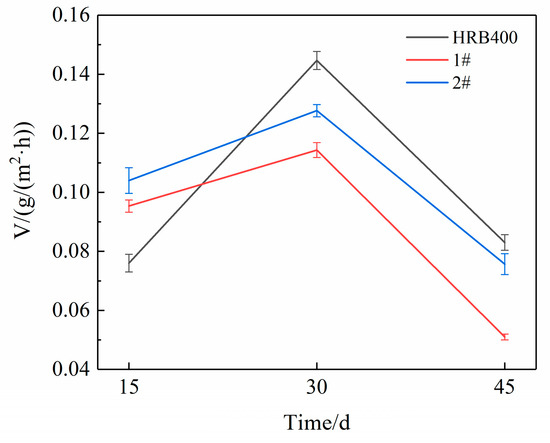
Figure 5.
Average corrosion rates of the three groups of steels after cyclic corrosion tests.
3.5. Salt Spray Test
Figure 6 shows the microscopic morphology and EDS test results of the outer rust layer of the steel specimens after 96 h of a neutral salt spray test. Figure 7 shows the microscopic morphologies that were observed after removing the surface corrosion products. Figure 6(a-1) shows the salt spray corrosion morphology of HRB400 steel. Corrosion was observed on the sample surface, mainly in the ferrite region. The pitting and rupture of the rust layer at the grain boundaries were also observed. This indicated that the corrosion behavior of HRB400 steel was mainly intra-granular- and inter-granular-type corrosion. As shown in Figure 6(a-2), a significant number of cubic particles that were adsorbed on the rusted surface of the ferrite were observed, and these were confirmed to be NaCl crystals by the EDS results. Figure 6(a-3) reveals that these NaCl particles were adsorbed onto a lamellar structure. Figure 6(a-4) indicates the microscopic morphology of γ-FeOOH, which exhibited a distinct lamellar structure, thereby suggesting the NaCl particles shown in Figure 6(a-3) were adsorbed into γ-FeOOH. Due to its porous structure, γ-FeOOH facilitated further Cl− penetration into the uncorroded grains and grain boundaries after corrosion [19]. The EDS results, which are shown in Figure 6(a-5), confirmed that NaCl adsorbed into γ-FeOOH. This phenomenon explains the occurrence of the pitting corrosion. When inter-granular corrosion intensifies, ferrite or pearlite grains will detach from the corroded surface, leading to material loss. On this note, the HRB400 steel exhibited the highest corrosion loss among all of the samples.
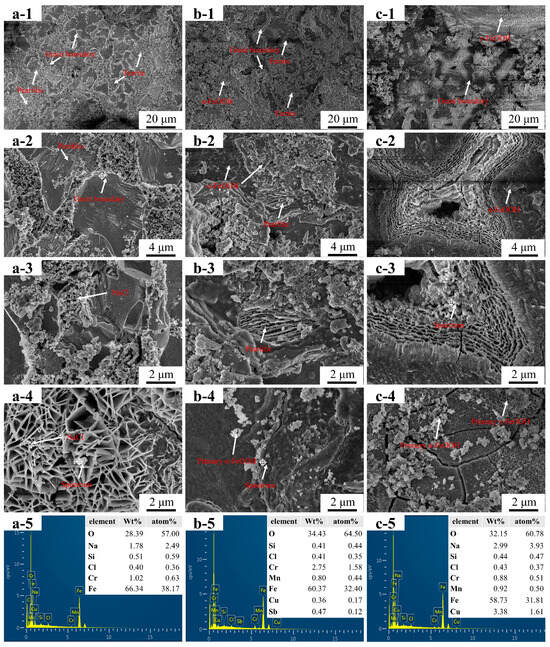
Figure 6.
The microscopic morphology and EDS test results of the three groups of steels’ rust layers after a 96 h salt spray test. ((a-1)–(a-5)) HRB400, ((b-1)–(b-5)) 1# and 2#, ((c-1)–(c-5)) 2#.

Figure 7.
The microscopic morphology of the three groups of steels after removal of the corrosion products. (a) HRB400, (b) 1# and (c) 2#.
Figure 6(b-1) shows the corrosion morphology of 1#, where no NaCl particles were adsorbed in the rust layer and no enrichment of corrosion products was found at the grain boundaries. Corrosion mainly occurred within the grains. Research has suggested that intra-granular corrosion rates are much lower than inter-granular corrosion rates [20]. Therefore, the reduction in the corrosion product enrichment at the grain boundaries most likely inhibited the inter-granular corrosion. This might have also been the cause for the lowest corrosion loss in Sample 1. However, corroded pearlite was also observed, as shown in Figure 6(b-2), but this could be attributed to the higher potential of the Fe3C in the pearlite (even with no enrichment of the Cu and Sb elements) than in the α-Fe. The potential difference formed a galvanic cell within the pearlite, thereby leading to a complete corrosion of α-Fe that thus left behind Fe3C. This effect formed a lamellar structure, as shown in Figure 6(b-2,b-3). The EDS results, as shown in Figure 6(b-3), confirmed that Cu and Sb were mainly enriched at the grain boundaries. Research has indicated that Cu and Sb increase the potential at the grain boundaries [21,22]. During galvanic cell corrosion, the α-Fe within the grains was preferentially corroded over the grain boundaries, thus reducing the inter-granular corrosion. Figure 6(b-4) illustrates the corrosion product morphology that was observed at the grain boundaries, as shown in Figure 6(b-2). In addition, the spherical α-FeOOH corrosion products were found to be identical to those observed by other researchers [23], thus indicating that a change in the corrosion product type at the grain boundaries alters the rust layer morphology. As α-FeOOH is denser than γ-FeOOH, it avoided Cl− adsorption. The EDS results in the grain boundary region confirmed an α-FeOOH formation in the inner rust layer, which is where the Cu and Sb were enriched. Research has suggested that Cu promotes the transformation of amorphous corrosion products to crystalline iron oxides, and it participates in the early stage of rust layer formation [24], thus leading to corrosion product accumulation and a densification of the rust layer. Therefore, the transformation of γ-FeOOH to α-FeOOH, as well as the formation of more corrosion-resistant Cu-containing compounds under the combined action of Cu and Sb, explain the lowest corrosion loss that was found in the samples containing Cu and Sb.
Figure 6(c-1) shows the rust layer morphology of 2#, which had partially cracked rust layers and lamellar γ-FeOOH. The pitting corrosion pits, which occurred in regions enriched with γ-FeOOH, are shown in Figure 6(c-2). Parts of the rust layer had a laminar structure, which led to Cl− adsorption and an enlargement of the pitting corrosion pits. Figure 6(c-5) depicts the EDS results of Figure 6(c-3), where Cu signals were detected near the pitting corrosion pits, thus indicating the presence of Cu in the corrosion products. However, since Sb further promoted the production of α-FeOOH, the 1# sample exhibited the best corrosion resistance.
Figure 8 shows the XRD patterns of the corrosion products of the rust layer, and Figure 9 presents the average corrosion rate after a 96 h salt spray test. All of the three groups of steels formed a stable rust layer [25], where their structures were dominated by Fe3O4 and α-FeOOH accompanied by some γ-FeOOH. They produced similar corrosion products as were obtained by the cycle immersion test. The content of the Fe3O4 in the two test steels was not overly diverse. The content of the α-FeOOH in 1# was higher, which meant that the rust layer of 1# was more protective and that Sb can promote the formation of α-FeOOH. Fe3O4 and α-FeOOH are thermodynamically stable with good densification [26], and they attach to the substrate surface as corrosion proceeds, thus hindering the migration of Cl− and reducing the corrosion rate. The morphology of Cu and Sb in the rust layer of Samples #1 and #2 was investigated by XPS, as shown in Figure 10. The Cu presented as Cu+ and Cu2+ in both steels, and the formation of a dense oxide film protected the steel substrate and inhibited the anodic reaction, thereby reducing the corrosion rate. Due to the low content of Sb, the signal-to-noise ratio was low. Its peaks were not obvious, and the binding energy was close to that of O, thus meaning it can be easily confused with peaks containing O, such as FeOOH and Fe3O4. The Sb in the 1# steel existed in the form of Sb2O3. The peak intensity of the Cu in the 1# steel with added Sb was significantly higher than that in the 2# specimen without Sb addition, thus indicating that Sb can promote the formation of Cu-containing compounds.
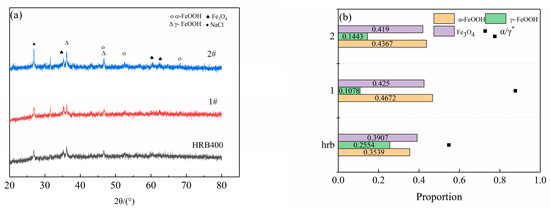
Figure 8.
The three groups of steels after cyclic corrosion tests. (a) The XRD patterns of the corrosion products and (b) the proportion of the various phases.
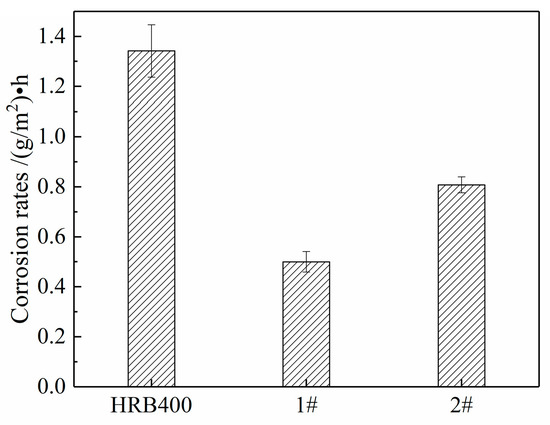
Figure 9.
The average corrosion rates of the three groups of steels after a 96 h salt spray test.
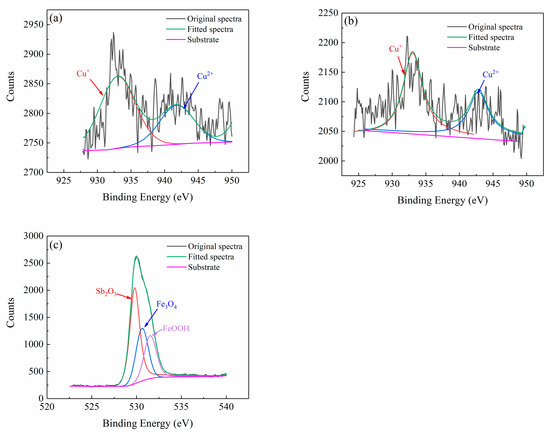
Figure 10.
XPS analysis of the surface rust layer. (a) 1# Cu 2p, (b) 2# Cu 2p and (c) 1#Sb 3d.
4. Discussion
The results of the electrochemical, cyclic immersion and salt spray tests show that micro-alloying affects the corrosion behavior of steel in a Cl− environment. The addition of Cu and Sb increased self-corrosion potential by reducing the corrosion current density per unit area and suppressing the generation of corrosion pits. They also changed the corrosion form from pitting corrosion to homogeneous corrosion. The XRD results prove that the alloying elements can promote the transformation of generating γ-FeOOH into α-FeOOH in the presence of Cl−, and the results also showed how they can improve the structure of the rust layer. At the early stages of corrosion, the corrosion medium can easily diffuse through the loose rust to the surface of the steel substrate, thus accelerating the corrosion process. The following reactions then occur at the anode [9]:
Fe→Fe2+ + 2e−;
Fe2+ + 2Cl− + 4H2O → FeCl2·4H2O;
FeCl2·4H2O → Fe(OH)2 + 2Cl− + 2H+ + 2H2O;
4Fe(OH)+ + 4OH− + O2 → 4FeOOH + 2H2O.
The following reactions then occur at the cathode:
O2 + 2H2O + 4e− → 4OH−;
Fe2+ + 8FeOOH + 2e− → 3Fe3O4 + 4H2O.
With the extension of the corrosion time, a part of the γ-FeOOH was reduced to Fe3O4, and the corrosion resistance was directly related to the content of α-FeOOH and Fe3O4. After cycle immersion tests of a varying duration, the Cu-Sb composite-added test steel possessed less γ-FeOOH but a higher α-FeOOH and Fe3O4 when compared to the HRB400 sample with only Cu and no alloying. This suggests that Sb promotes the conversion of Fe2+ to α-FeOOH and Fe3O4, thereby reducing the content of the more electrochemically active γ-FeOOH. The oxides of Cu and Sb provide nucleation sites for crystallization in the rust, as well as refining the structure of the corrosion product film layer, and their PAI index proves to be higher than that of the remaining two steels, thus implying that the rust layer has better protective properties. The PAI indices of the three groups of steels in the salt spray test also proved that the composite addition had the best corrosion resistance.
According to the XPS results, Sb exists as Sb2O3 in the rust layer, whereas Sb2O5 needs to be formed under strong oxidizing conditions in a strongly acidic solution. Therefore, it was not present in the XPS spectra. According to Yang et al. [27], Sb can consume H+ to generate SbH3 in order to alleviate local acidification. This then reacts with O2 to generate a stable Sb2O3, which is deposited in the micropores and microcracks of the rust layer to increase the pH of the steel surface in the corrosive environment. This is conducive to the transformation of γ-FeOOH [28]. Therefore, the rust layer of Sb-containing steel is more stable than the remaining two steels, and the formation process of Sb2O3 is as follows:
Sb + 3H+ + 3e− → SbH3;
2SbH3 + 3O2 → Sb2O3 + 3H2O.
5. Conclusions
In this paper, the effects of adding the alloying elements Cu and Sb on the electrochemical properties and rust layer protection of HRB400 in a simulated marine environment were investigated. The main conclusions derived are as follows:
(1) As confirmed through the analysis of the kinetic potential polarization curves and AC impedance spectra, micro-alloying using Cu-Sb can increase the self-corrosion potential of the test steel shift significantly, thus increasing polarization resistance and reducing corrosion current density through the formation of a dense rust layer on the surface. The specimen that underwent the simultaneous addition of Cu and Sb had the highest corrosion potential (−0.392 V) and the lowest corrosion current density (2.086 × 10−6 A·cm−2), which, in turn, significantly slowed down electrochemical corrosion.
(2) After different cycles of immersion tests, the main components of the rust layer were Fe3O4 and α-FeOOH, which were accompanied by some γ-FeOOH. The steel that underwent the simultaneous addition of Cu and Sb had the highest PAI index and the lowest corrosion rate.
(3) The salt spray test shows that HRB400 mainly has pitting corrosion, forming a loose rust layer. The tested steel underwent a mainly uniform corrosion, i.e., it formed a uniform and dense rust layer. The corrosion rate was less than half that of HRB400, with good corrosion resistance. The addition of Sb to steel promotes the generation of more copper-containing corrosion inhibitors, where the rust layer is more uniform and dense, without obvious cracks; as such, it can more effectively prevent corrosion ions from entering the matrix.
Author Contributions
Conceptualization, Y.C. and Z.M.; methodology, Y.C. and Z.M.; software, Y.L.; validation, Y.C. and Y.L.; formal analysis, Y.C.; investigation, Y.C.; data curation, Y.C.; writing—original draft preparation, Y.C.; writing—review and editing, Z.M. and J.S.; visualization, Y.C.; supervision, Z.M.; funding acquisition, Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Guangxi Major Science and Technology Project, China (AA22068073) and the National Natural Science Foundation of China (52204340).
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jeong, J.; Ha, J. Improvement of throwing power of cathodic protection in reinforced concrete structure with conductive mortar. J. Adv. Mar. Eng. Technol. 2021, 45, 21–25. [Google Scholar] [CrossRef]
- Chen, J.; Ji, L.; Song, J. Study of Crevice Corrosion Behavior and Cathodic Protection of Carbon Steel Reinforcement in Concrete. Int. J. Electrochem. Sci. 2022, 17, 220140. [Google Scholar] [CrossRef]
- Fürbeth, W. Special Issue: Advanced Coatings for Corrosion Protection. Materials 2020, 13, 3401. [Google Scholar] [CrossRef] [PubMed]
- Cuong, P.; Thao, D. Effect of surface roughness and plasma current to adhesion of Cr3C2-NiCr coating fabricated by plasma spray technique on 16Mn steel. Int. J. Mod. Phys. B 2021, 35, 2140037. [Google Scholar] [CrossRef]
- Wei, X.; Fu, D.; Chen, M.; Wu, W.; Wu, D.; Liu, C. Data mining to effect of key alloying elements on corrosion resistance of low alloy steels in Sanya seawater environmentAlloying Elements. J. Mater. Sci. Technol. 2021, 64, 222. [Google Scholar] [CrossRef]
- Wu, Y. Brief Analysis of Stainless Steel, from the Type, Performance, Cost and the Application. South. Met. 2018, 3, 43–47. [Google Scholar]
- Jing, Q.; Fang, X.; Ni, J.; Tang, B. Use of 2304 Stainless Steel Reinforcement in Hong Kong-Zhuhai-Macau Bridge—Corrosion Behaviors of 2304 Stainless Steel Reinforcement. J. Highw. Transp. Res. Dev. 2017, 34, 51–56. [Google Scholar]
- Nishimura, T.; Katayama, H.; Noda, K.; Kodama, T. Effect of Co and Ni on the corrosion behavior of low alloy steels in wet/dry environments. Corros. Sci. 2000, 42, 1611–1621. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Xu, Y.; Liu, Z. Effects of Cr, Ni and Cu on the Corrosion Behavior of Low Carbon Microalloying Steel in a CF− Containing Environment. J. Mater. Sci. Technol. 2013, 29, 168–174. [Google Scholar] [CrossRef]
- Yin, X.; Meng, Z.; Hu, H. Research on Corrosion Resistance of Low Cost Al-Ti-Cr High Strength Seawater Corrosion Resistant Steel. Mater. Rev. 2021, 35, 14084–14088. [Google Scholar]
- Holm, S.; Holm, T.; Martinsen, O.G. Simple circuit equivalents for the constant phase element. PLoS ONE 2021, 16, 0248786. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Liu, C. Microstructure, Mechanical Properties, and Corrosion Behavior of Ultra-Low Carbon Bainite Steel with Different Niobium Content. Materials 2021, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, G.; Ye, H.; Zeng, Q.; Zhang, Z.; Tian, Z.; Jin, X.; Jin, N.; Chen, Z.; Wang, J. Corrosion of steel rebar in concrete induced by chloride ions under natural environments. Constr. Build. Mater. 2023, 369, 130504. [Google Scholar] [CrossRef]
- Feng, G.; Jin, Z.; Xiong, C.; Fan, J. Corrosion Behavior of Corrosion-resistant Steel in Cracked Concrete Exposed to Marine Tidal Area for 700 Days. Mater. Rev. 2020, 34, 8064–8070. [Google Scholar]
- Moshtaghi, M.; Eskinja, M.; Mori, G.; Griesser, T.; Safyari, M.; Cole, I. The effect of HPAM polymer for enhanced oil recovery on corrosion behaviour of a carbon steel and interaction with the inhibitor under simulated brine conditions. Corros. Sci. 2023, 217, 111118. [Google Scholar] [CrossRef]
- Wu, W.; Dai, Z.; Liu, Z.; Liu, C.; Li, X. Synergy of Cu and Sb to enhance the resistance of 3%Ni weathering steel to marine atmospheric corrosion. Corros. Sci. 2021, 183, 109353. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Q.; Yang, L.; Liu, Z.; Li, X.; Li, Y. Corrosion and SCC initiation behavior of low-alloy high-strength steels microalloyed with Nb and Sb in a simulated polluted marine atmosphere. J. Mater. Res. Technol. 2020, 9, 12976–12995. [Google Scholar] [CrossRef]
- Kamimura, T.; Hara, S.; Miyuki, H.; Yamashita, M.; Uchida, H. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corros. Sci. 2006, 48, 2799–2812. [Google Scholar] [CrossRef]
- Hao, W.; Liu, Z.; Wu, W.; Li, X.; Du, C.; Zhang, D. Electrochemical characterization and stress corrosion cracking of E690 high strength steel in wet-dry cyclic marine environments. Mater. Sci. Eng. A 2018, 710, 318–328. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, Y.; Liu, Z.; Hu, W.; Wang, Q.; Hu, C. The Role of Grain Boundaries in the Corrosion Process of Fe Surface: Insights from ReaxFF Molecular Dynamic Simulations. Metals 2022, 12, 876. [Google Scholar] [CrossRef]
- Pan, M.; Liu, X.; Jin, W.; Fu, S.; Yang, E.; Liu, X.; Zhao, L.; Zhang, X.; Yan, M. Effect of Cu grain boundary modification on microstructure and corrosion resistance in recycled Nd-Fe-B sintered magnets. J. Magn. Magn. Mater. 2022, 550, 169109. [Google Scholar] [CrossRef]
- Du, H.; An, N.; Wang, X.; Li, Y.; Liu, Z.; Jin, A.; Yang, R.; Pan, Y.; Li, X. Enhancing the SCC Resistance of the Anchor Steel with Microalloying in a Simulated Mine Environment. Materials 2023, 16, 5965. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Q.; Li, X.; Xu, J.; Xu, Y.; Shi, W.; Wang, S. Microscopic Analysis of Steel Corrosion Products in Seawater and Sea-Sand Concrete. Materials 2019, 12, 3330. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Dong, J.; Wei, J.; Etim, I.; Ke, W. Effect of Cu on corrosion behavior of low alloy steel under the simulated bottom plate environment of cargo oil tank. Corros. Sci. 2017, 121, 84–93. [Google Scholar] [CrossRef]
- Ratnani, S.; Kumari, A.; Verma, C. Corrosion protection effect of rust and scales: A “metal protects metal” perception by considering the examples of hematite and magnetite. Asia-Pac. J. Chem. Sci. 2024, 19, 3003. [Google Scholar] [CrossRef]
- Daniel, E.; Li, C.; Wang, C.; Dong, J.; Udoh, I.; Okafor, P.; Zhang, D.; Zhong, W.; Zhong, S. Insights into the characteristics of corrosion products formed on the contact and exposed regions of C1045 steel bolt and nut fasteners exposed to aqueous chloride environments. J. Mater. Sci. Technol. 2022, 135, 250–264. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, X.; Zhao, J.; Fan, Y.; Li, X. A study of rust layer of low alloy structural steel containing 0.1 % Sb in atmospheric environment of the Yellow Sea in China. Corros. Sci. 2021, 188, 109549. [Google Scholar] [CrossRef]
- Ahn, S.; Park, K.; Oh, K.; Hwang, S.; Park, B.; Kwon, H.; Shon, M. Effects of Sn and Sb on the Corrosion Resistance of AH 32 Steel in a Cargo Oil Tank Environment. Met. Mater. Int. 2015, 21, 865–873. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).