Abstract
The effects of different MgO contents (0.3 wt.%, 0.5 wt.%, 0.7 wt.% and 1.0 wt.%) on the microstructure and properties of Mg-1Zn-0.5Ca alloy (ZX) were systematically investigated to promote the clinical application of Mg alloys. The results showed that a MgO addition promoted the precipitates of Ca2Mg6Zn3 and Mg2Ca after hot extrusion. Meanwhile, the average grain size of the ZX alloy decreased abruptly from 17.73 μm to 5.54 μm after the addition of 0.3 wt.% MgO and then reduced slowly as further increasing the MgO contents to 1.0 wt.%. The microhardness and yield strength (YS) increased gradually from 59.43 HV and 102.0 MPa in ZX to 69.81 HV and 209.5 MPa in ZX1.0, respectively. However, the elongation to failure (EL) decreased from 26.7% in ZX to 21.2% in ZX1.0 due to the increase of volume fraction of the second phase and decrease of grain size as increasing the MgO. The corrosion result showed that ZX alloy exhibited local corrosion while ZX composites (ZX0.3, ZX0.5 and ZX0.7) displayed relatively uniform corrosion owing to the fine grain size, dispersed fine second and the protective effect of corrosion product after MgO hydrolyzation. However, excessive MgO (ZX1.0) easily caused the aggregation of itself and the precipitates and deteriorated the corrosion resistance of the material.
1. Introduction
In recent years, magnesium (Mg) and its alloys have attracted much attention as a potential bone repair material due to their excellent biocompatibility, biodegradability and mechanical compatibility, such as the close density and Young’s modulus to natural bone [1,2,3,4,5,6]. However, the wide application of Mg alloys is still limited due to their poor corrosion resistance and low strength [7,8,9]. Many methods have been taken to solve these problems; therefore, alloying with the essential element for the human body was considered to be one of the most effective methods. Zheng et al. [10] reported that the weight of metallic elements larger than 1 g in the human body are Ca, K, Na, Mg, Fe and Zn. Considering the high activity of K and Na elements and the harmful element of Fe for the corrosion resistance of Mg, they are excluded.
Zn is a daily essential element that plays a key role in human health [11], and studies have demonstrated that Zn has good biocompatibility for the human body. Zn has antiproliferative effects and strong anti-atherosclerotic properties that reduce neoplastic endothelial hyperplasia, regulate inflammatory cytokines, and prevent restenosis after stenting [12]. Cai et al. [13] demonstrated that the addition of the Zn element refined the grain size of the Mg alloy significantly and increased the volume fraction of the second phase. The tensile properties and corrosion resistance of Mg-Zn alloys are closely related to the volume fraction and the form of existence of the second phase. An appropriate increase in the volume fraction of the second phase is conducive to the improvement of corrosion resistance, but excessive Zn will generate harmful MgZn intermetallic compounds, leading to accelerated micro-coupled corrosion. This was mainly attributed to the high potential difference between the MgZn phase and the α-Mg matrix [14,15].
Ca is involved in a large number of biochemical and physiological reactions in the human body, which is necessary to maintain normal metabolism and physiological functions [16,17]. Ca additions in Mg-Zn alloys significantly refine the grain size [18] and improve formability [19]. The addition of appropriate Ca contents to Mg alloys can improve the corrosion resistance [20,21,22]. Ca solubility in Mg is about 1.34 wt.% under equilibrium conditions, and it plays a microstructure refining role, enhancing strength and creep properties due to the formation of intermetallic phases. However, these intermetallic compounds are brittle, which could initiate cracking, and electrochemically active, which may lead to galvanic phenomena [23]. Therefore, the moderate alloying element is helpful to maintain the balance between the mechanical property and the corrosion rate of Mg alloys, but this cannot meet the required mechanical properties of the material for fracture fixation, and it is necessary to introduce extra strengthening mode. Previous work revealed that bioactive oxide particles such as hydroxyapatite (HA), tricalcium sphere (TCP), and magnesium oxide (MgO) [24] had excellent strengthening effects and promoted bone formation ability. Our work proved that the incorporation of MgO NPs effectively promoted the formation of fine microstructure by promoting the dynamic recrystallization and hindering the migration of grain boundaries during the hot extrusion process, and this led to the improvement of the strength of Mg-Zn-Ca alloy [25].
Meanwhile, MgO could promote the formation of Mg(OH)2, making the corrosion product layer denser and more durable. However, the optimal MgO content needs to be clarified so as to obtain a balanced mechanical property and corrosion resistance of Mg-Zn-Ca alloy. Table 1 summarizes the literature related to the research work mentioned above.

Table 1.
A brief overview of the literature.
Based on this, different MgO contents were introduced into Mg-1Zn-0.5Ca alloy to explore its influence on the microstructure, mechanical properties, and corrosion behavior of the low-alloyed Mg-Zn-Ca alloy.
2. Experimental
2.1. Materials Preparation
MgO nanoparticles were prepared using MgCl2-6H2O, CTAB solution with a concentration of 1%, and H2C2O4 in a weight ratio of 6.6:0.04:1. The three substances were then added sequentially in a water bath at 43 °C, with each drug being stirred for 20 min at intervals. Then, the solution was filtered 4–5 times using a vacuum pump and filtration flask. The fabricated magnesium oxalate solid was then dried in a drying oven at 80 °C for 14 h. Subsequently, the solid should be heated to 630 °C and maintained at that temperature for 5 h. Finally, nano-sized MgO (50 ± 3.75 nm) particles were obtained after grinding and sieving.
ZX composites with different MgO ( = 0.3 wt.%, 0.5 wt.%, 0.7 wt.% or 1.0 wt.%) were prepared from pure Mg (99.99 wt.%), pure Zn (99.99 wt.%), Mg-Ca master alloy (Mg-25Ca wt.%) and self-prepared nano-sized MgO particles as shown in Figure 1a, whose average particle size is 50 nm. The XRD(D/Max/2500PC) (Rigaku Corporation, Tokyo, Japan) result only showed a single sharp characteristic peak of MgO. Pure Mg ingots were placed in the resistance furnace (MTC-5EA), and the furnace temperature was raised to 973 K using N2 and SF6 as protective gases. After the Mg ingot was completely melted, other raw materials were placed into the melt and stirred by a high-shear device. After quiescence for 20 min, the melt was poured into the preheated steel mold. To ensure the consistency of the experiments, the Mg-1Zn-0.5Ca alloy was smelted in the same way.
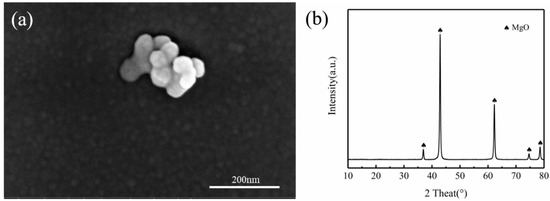
Figure 1.
(a) SEM morphology and (b) XRD patterns of MgO particles.
The ZX composites with different MgO contents were solid solution-treated at 420 °C for 12 h. Before extrusion, the molds and ingots were preheated in a muffle furnace at 350 °C for 1 h and then were extruded at 350 °C with an extrusion speed of 0.2 mm/s, and extrusion ratio of 81:1 (Extruder model: YJQ-300).
2.2. Microstructural Characterization
The samples for observation by an optical microscope (OM) were cut from the extrusion rods parallel to the extrusion direction (ED). They were ground with 800#, 1500#, 3000#, and 5000# sandpaper, polished with 0.5 μm diamond gesso until the surface was smooth and then chemically etched (2.75 g of picric acid, 2.5 mL of acetic acid, 5 mL of deionized water, and 45 mL of anhydrous ethanol). The second phase distribution in the polished specimens was observed using an ultra-high resolution field emission scanning electron microscope (FE-SEM) and Focused Ion Beam Scanning Electron Microscopy (FIB-SEM) (Thermo Fisher Scientific, Waltham, MA, USA) coupled with an energy dispersive spectrometer (EDS) (SEM model: Verios 460L) system. The average grain size and particle size of the specimens were determined using Nano Measurer 1.2 software.
2.3. XRD Phase Analysis
The extruded materials were cut into φ8 × 3 mm discs using an autobiographic cutter and then sandpapered according to the order of 800#, 1500#, and 3000# to ensure that the oxidized film on the surface of the samples was removed. Subsequently, the specimen was ultrasonically cleaned for ten minutes, and after blow-drying the sample, the sample was quickly tested using an X-ray diffractometer (Rigaku Corporation, Tokyo, Japan). A Cu target Kα line X-ray diffractometer was used to test the specimens with an accelerating constant voltage of 40 kV, a constant current of 100 mA, a scanning angle of 20°~80°, and a scanning speed of 5°/min.
2.4. Mechanical Performance Test
2.4.1. Hardness Test
For the microhardness test, the specimens were cut into φ8×3 mm pieces and sanded with 800#, 1500#, and 3000# sandpaper until the surface was flat. Then, the specimens were polished with a W1 polishing solution and a matching polishing disk until the surface was free of scratches. The microhardness was tested using an HMV-2T microhardness tester (Shimadzu, Kyoto, Japan) with a loading load of 490.3 mN and a loading time of 10 s. For each material, twenty points were examined, and the average hardness was calculated.
2.4.2. Tensile Test
Tensile tests were conducted using a universal testing machine (DDL10) at room temperature with a tensile speed of 0.5 mm/min. The specimens with a cross-section size of 25 mm and a diameter of 5 mm were prepared in accordance with GB/T 24176-2009 [26]. Three specimens for each material were tested to ensure repeatability.
2.5. Corrosion Test
Electrochemical tests were carried out using an electrochemical workstation (Zahner-Zennium) with a three-electrode working system, which includes the working electrode of the test specimen, the counter electrode of a graphite electrode and the reference electrode of a saturated calomel electrode (SCE).
A cylindrical sample with a diameter of 8 mm and a thickness of 3 mm was ground for the electrochemical testing. Three electrodes were placed into the beaker and subjected to open circuit potential (OCP) test, electrochemical impedance spectroscopy (EIS) test and kinetic potential polarization test sequentially in the simulated body fluid (SBF) at 37 °C. Before the A.C. impedance and polarization tests started, the samples were subjected to OCP tests for approximately 30 min to obtain a stable OCP. The testing frequency range of A.C. impedance was 105 Hz~10−1 Hz, and the polarization was tested with a scanning rate of 1 mV/s from −500 mV to 500 mV. Nyquist spectra were fitted in ZView 2 software, and the equivalent circuit was drawn according to the fitting results. The corrosion current density (Icorr) was calculated using the Tafel method. Three specimens of each material were performed to ensure the reliability of the experimental results. In vitro immersion tests (ASTM-G31-72) (ASTM, West Conshohocken, PA, USA) were all carried out using a cylindrical sample with a diameter of 8 mm and a thickness of 3 mm. The test plane was perpendicular to ED. All the samples were ground by sandpaper to remove the surface oxide layer and obtain a flat surface. During the immersion process, the solution was changed every 2 days to keep it fresh, and the pH of the solution was measured. After immersion for various times (1, 3, 5, 7, and 14 days), the samples were taken out and washed with ultra-pure water. Then, they were dried and sealed for preservation. The corrosion products were removed by chromic acid (200 g/L, CrO3) and ultrasonic cleaning for 5 min. The degradation rate of the sample can be calculated according to Equation (1) [27]:
where (mm/year) is the annual corrosion rate of degradable metal, is a constant of 8.76 × 104, (g/cm3) is the density of the material, (g) is the mass loss.
3. Results
3.1. Microstructural Characterization
Figure 2 shows the OM images of the extruded ZX composites. The study found that all five materials underwent complete dynamic recrystallization during extrusion, with no non-recrystallized zones detected. The average grain size of ZX alloy reaches 17.73 μm. With the addition of MgO, the microstructures of the composites are refined gradually. After the addition of 0.3 wt.% MgO, the average grain size of the ZX0.3 composite is reduced to 5.54 μm, which decreases by 68.6% compared to ZX alloy. Further increasing MgO contents, the refinement effect decreases, and the average grain sizes of ZX0.5, ZX0.7, and ZX1.0 composites are 4.96 μm, 4.37 μm, and 3.95 μm, respectively (Figure 2f). This indicates that incorporating MgO has a refinement efficiency for Mg alloys. This is similar to the results studied by Fan et al. [28], who believe that the diffusely distributed MgO facilitates non-homogeneous nucleation and promotes grain refinement. However, the effect of MgO contents on microstructure refinement is not significant (Figure 2b–e). Meanwhile, some suspected small-sized MgO agglomerates are found in the ZX1.0 composite and marked by the red circle, as shown in Figure 2e.
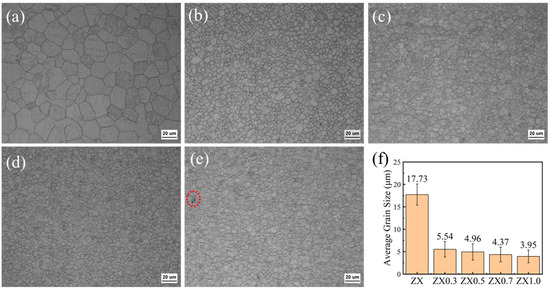
Figure 2.
Shows the OM microstructure of extruded ZX composites, (a) ZX, (b) ZX0.3, (c) ZX0.5, (d) ZX0.7, (e) ZX1.0, and (f) ZX1.0 partial enlargement.
3.2. XRD Physical Phase Analysis of Extruded Composites
Figure 3 shows the XRD diffraction spectra of the extruded ZX composites, along with localized magnification of the diffraction peaks of the MgO (Figure 3b) and Mg2Ca (Figure 3c). As can be seen in Figure 3a, except for the diffraction peaks of α-Mg, diffraction peaks of the Mg2Ca phase, Ca2Mg6Zn3 phase, and MgO are also observed in these materials. The diffraction peak intensity of MgO is enhanced by increasing the MgO contents, as shown in Figure 1b. It shows the same variation for the intensity of Ca2Mg6Zn3 as well as the Mg2Ca phase (Figure 3c). It is noted that the diffraction peaks of the Mg2Ca phase and MgO are not found in the ZX alloy.
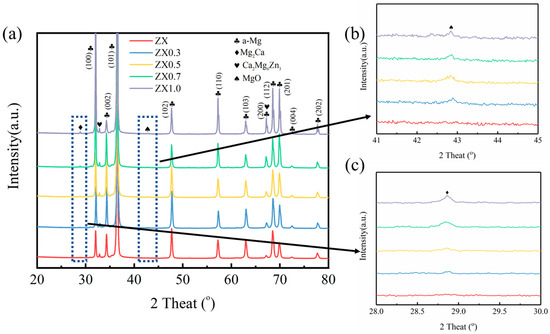
Figure 3.
XRD diffraction pattern (a) of extruded ZX composite and local magnification of (b) MgO and (c) Mg2Ca diffraction peaks.
Figure 4 shows the SEM images of extruded ZX alloy and ZX composites. The size of the second phase in the extruded composites is small, with an average size of Mg2Ca and Ca2Mg6Zn3 between 186–229 nm and 107–128 nm, as shown in Table 2. It can be seen that the size of the second phase of the extruded composite is very small, and with the increase of MgO contents, the number of the second phase increases gradually.
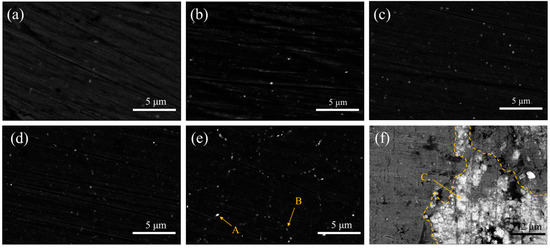
Figure 4.
SEM images of extruded materials (a) ZX; (b); ZX0.3 (c) ZX0.5; (d) ZX0.7; (e) ZX1.0 and (f) shows the agglomeration of the MgO particles in the ZX1.0 composite. The MgO nanoparticles are gathered in the range of 6 μm and thus become the potential defects.

Table 2.
Grain size and phase size statistics.
This was consistent with the study by Shen [29] et al., who found that MgO particles can be used as a more effective heterogeneous nucleation substrate of Mg alloys, promoting the precipitation of the second phase.
Combining the XRD results and EDS in Table 3, the bright white phase at point A in the ZX1.0 composite is considered to be Mg2Ca, and the grayish white phase at point B is regarded as Ca2Mg6Zn3, while point C is the agglomerated MgO. The area fraction of the second phases in the five materials is given in Figure 5. The results indicate that both Ca2Mg6Zn3 and Mg2Ca increase as the MgO content increases. The total area fraction of the second phase increases from 0.61% in the ZX alloy to 1.5% in the ZX1.0 composite. It is noted that the Ca2Mg6Zn3 is the main second phase in all the materials.

Table 3.
EDS results corresponding to regions in Figure 4.
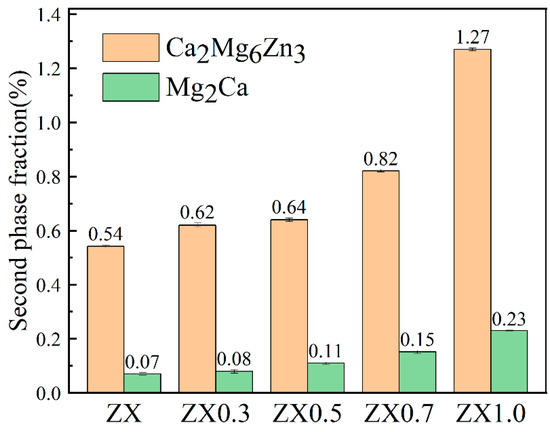
Figure 5.
Second phase content of extruded ZX composites.
Owing to the small size and the similar color of MgO to the Mg matrix, it is difficult to observe a large number of MgO particles from TEM images. Therefore, only several MgO particles were provided in our previous work [25,30,31]. Based on this, a Focused Ion Beam-Scanning Electron Microscope (FIB-SEM) was employed to analyze the distribution of MgO nanoparticles in ZX0.7 composites and the results are displayed in the following Figure 6. MgO NPs are indicated by yellow circles in the figure. The center distance between these MgO NPs (protruding points) was approximately 1.5 µm, which is close to the theoretical center distance (1.7 µm) calculated by the following Equation (2). Therefore, Figure 6a can represent the distribution of MgO NPs in the matrix.
where is the mean center-to-center spacing between particles, is the volume fraction of second particles, and is the mean diameter of the particles, = 500 is a computational constant.
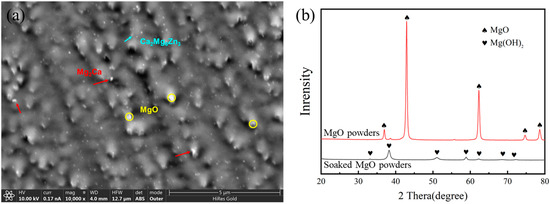
Figure 6.
(a) FIB-SEM investigation of MgO nanoparticle distribution in the ZX0.7 composites and (b) XRD of MgO powders and soaked MgO powders.
In addition, calculations based on Equation (3) demonstrate that the volume of MgO particles increases to approximately 2.2 times compared with their original size. Considering the fact that nano-MgO hydrolyzed easily and formed Mg(OH)2 when they were contacted with water during the sample preparation for SEM and TEM observation, the expansion of volume can be reasonably explained. Additionally, the EDS results indicated higher oxygen content in the larger particles. Therefore, we believe that a part of MgO may be transformed to Mg(OH)2 during preparation.
where is the volume, is the molar mass, and is density.
Furthermore, the agglomeration of the MgO particles in the ZX1.0 composite is shown in Figure 4f.
3.3. Mechanical Properties
Figure 7 displays the Vickers hardness of extruded ZX composites. The microhardnesses of the extruded composites gradually increased with the increase of MgO contents, and they are 59.43 HV, 63.38 HV, 65.23 HV, 66.46 HV, and 69.81 HV for ZX, ZX0.3, ZX0.5, ZX0.7, and ZX1.0, respectively. The higher Vickers hardness values are mainly attributed to the reduced grain size and increased nanometer-sized second phase [32].
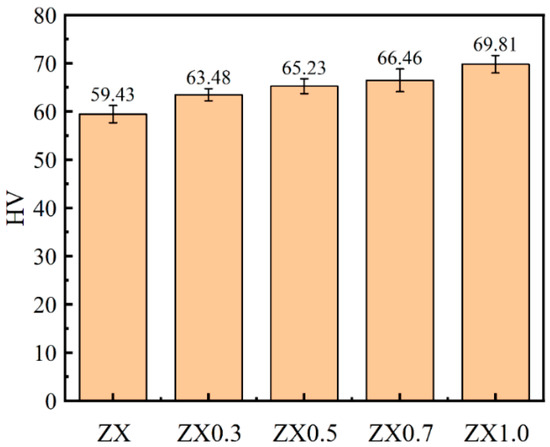
Figure 7.
Microhardness of extruded ZX alloy and ZX composites in extruded state.
Figure 8 shows the tensile properties of the extruded materials. Both yield strength (YS) and ultimate tensile strength (UTS) increase after adding the MgO, especially for the YS of the materials. The YS of the ZX1.0 composite is about 209 MPa, which is twice of the ZX alloy. This indicates that the MgO addition plays an important role in strengthening Mg alloys. Though the increment of UTS is not significant, it also increases from 213 MPa in the ZX alloy to 247 MPa in the ZX1.0 composite. While for the ductility of the materials, it decreased gradually with increasing the MgO contents. This was mainly attributed to the growing volume fraction of the second phase, which impeded the dislocation slip during tension. However, considering their small size and uniform distribution, their ductility decreased slightly.
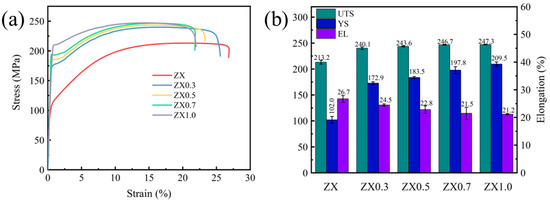
Figure 8.
(a) Typical tensile stress-strain curves and (b) tensile properties of the extruded ZX composites.
3.4. Corrosion Performance
Figure 9 shows the electrochemical test results of the extruded ZXx composites. The kinetic potential polarization curves are shown in Figure 9a. The results show that the polarization voltage tends to increase along the positive direction as the MgO content increases, indicating that the corrosion product layer has a positive effect on the surface potential of the material. All MgO-containing composites showed passivation in the anodic region, while ZX alloy did not. This indicates that the addition of MgO enhances the protective effect of the corrosion product layer. The electrochemical analysis results were obtained from the polarization curves in Figure 9. In this case, although the polarization voltage of ZX1.0 is higher than that of ZX0.7, the polarization current is much higher than that of ZX0.7, and it is corrosion current density that affects the corrosion more significantly, therefore the corrosion resistance of ZX0.7 is better than that of ZX1.0.
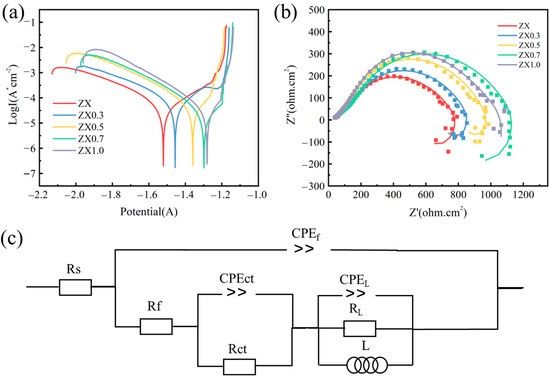
Figure 9.
(a) Potentiodynamic polarization curve (b) impedance diagram, and (c) equivalent circuit diagram of extruded ZX composites.
The Nyquist curve shown in Figure 9b shows similar shapes of all materials, including capacitive loops at high and medium frequencies and inductive loops at low frequencies. The diameters of the high-frequency capacitance loops are close in all materials, indicating that they have similar corrosion resistance during the initial immersion phase. At medium frequencies, the diameter difference increases significantly and becomes progressively larger with increasing MgO content. Among them, ZX1.0 shows a decreased value, indicating that the diameter of the inductive circuit becomes smaller when the MgO content is too much and the corrosion resistance is relatively lower. Usually, a large diameter represents high corrosion resistance, therefore ZX0.7 has the best corrosion performance based on this.
Figure 9c shows the equivalent circuit diagram obtained by fitting with ZSimpWin 3.3 software, where Rs denotes the resistance of the electrolyte, CPEf and Rf denote the capacity and resistance of the corrosion product film formed in the SBF solution, respectively. CPEct and Rct are the double-layer capacitance and the charge-transfer resistance, respectively. L is the circuit formed by the alloy due to pitting in the corrosion process, CPEL denotes the induction circuit capacity, and RL is its corresponding induction resistance. The electrochemical test results (Table 4) show that the value of Rct increases gradually with the increase of MgO content, but it decreases with the ZX1.0 composite, indicating a decrease in the hindering effect on the charge. This indicates that the addition of MgO enhances the charge transfer hindering effect on the surface of the specimen, which in turn inhibits the corrosion expansion. However, too much MgO may lead to adverse effects.

Table 4.
Electrochemical tests data of extruded ZX composites.
In order to further investigate the corrosion performance of the extruded materials, an in vitro immersion test in SBF is carried out for 14 days. Figure 10a shows the pH change curves of the extruded materials, and it shows an approximately linear growth trend for all the materials. In the initial immersion stage, within 2 days, ZX alloy exhibits a higher pH than other composites, whose pH is subequal. With increasing immersion times, the pH differences among the materials are enlarged. Therefore, the pH of ZX alloy is the highest during the whole immersion process, while for the composites, the pH increment is decreased gradually with the increase of MgO contents except for the ZX1.0 composite. The pH of ZX0.7 is the lowest after 4 days of immersion, and this is kept at a minimum until the end of the test. It is noted that the pH value of the ZX1.0 composite shows a rapid growth compared with ZX0.7 after immersion of 4 days. Figure 10b shows the average annual corrosion rate of extruded materials for different immersion times through the weight loss method. It can be seen from the figure that the average annual corrosion rate of the extruded materials gradually decreases with the increase in immersion time. This may be attributed to the protection of the gradually increased corrosion product layer. ZX0.7 composite exhibits the lowest corrosion rate throughout the whole immersion test and the average annual corrosion rate of the five materials is in descending order of ZX (0.99 g/m2/h) < ZX0.3 (0.84 g/m2/h) < ZX1.0 (0.82 g/m2/h) < ZX0.5 (0.8 g/m2/h) < ZX0.7 (0.77 g/m2/h) after immersion 14 days, which is consistent with the changing trend of the pH values.
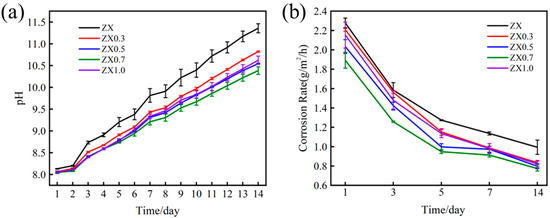
Figure 10.
(a) pH curve and (b) average annual corrosion rate and ZX composites soaked in SBF for different times.
Figure 11 shows the corrosion cross-section of the extruded materials after 14 days of immersion. Figure 11a illustrates the non-uniform thickness of the corrosion layer between the dotted red lines in the ZX alloy and the large undulations in the corrosion interface. The addition of MgO gradually flattens the corrosion interface of the extruded composites, and there are no large corrosion pits in the matrix, indicating a relatively uniform corrosion. This reveals that the addition of MgO particles inhibits the local corrosion and improves the corrosion homogeneity of extruded ZX alloy. The average thickness of the corrosion product layer in the ZX0.3, ZX0.5 and ZX0.7 is similar, but then an increase is observed in the ZX1.0 composite. Moreover, local corrosion is reemerged in the ZX1.0 composite.
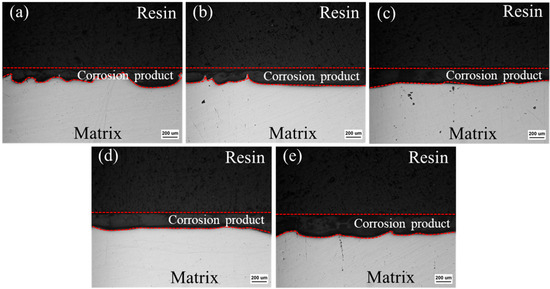
Figure 11.
Corrosion section of extruded ZX composite after 14 days immersion in SBF (a) ZX; (b) ZX0.3 (c) ZX0.5; (d) ZX0.7 and (e) ZX1.0.
As shown in Figure 12, when the samples were immersed for 14 days, the surface of the ZX alloy was corroded violently, a corrosion phenomenon. The corrosion resistance of the ZX1.0 composite is lower than that of the ZX alloy, but it is more susceptible to edge peeling. In the case of ZX0.7, the surface of the specimen is relatively flat, and almost half of the surface is smooth. Meanwhile, most of the shallow pitting is mutually independent and does not form large corrosion pits. Moreover, the edge of the sample is still relatively intact, and there is only local shedding, indicating that the corrosion in the ZX0.7 composite is slow and uniform.
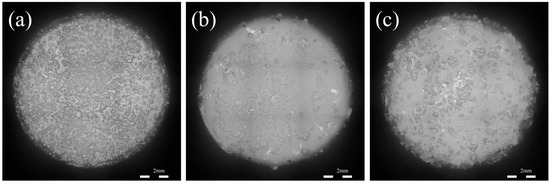
Figure 12.
Surface of extruded ZX alloy (a) ZX0.7 (b) and ZX1.0 composite (c) without corrosion products after soaking in SBF for 14 days.
Figure 13 shows the SEM images of corroded surfaces of ZX alloy, ZX0.7 and ZX1.0 composites after 14 days of immersion. The surfaces of all specimens are covered by corrosion product layers, and the corrosion product layer on the surface of ZX alloy is divided into large blocks by wide and deep cracks. A large number of pitting can be observed on the surface of the corrosion product layer (Figure 12a). The magnified image shown in Figure 13d shows that the number of craters is significantly higher, and the surface is rougher compared to the other components. In addition, many areas are covered by bright white loose corrosion products and visible cracks. From the EDS results in Table 5, it can be seen that the elemental distributions in the dense region (point A, C and E) and loose corrosion product layers(square B and F, point D) are similar. In other words, the corrosion surface is still dominated by Ca-P deposits and Mg(OH)2. However, the protective effect of the loose corrosion layer on the substrate is greatly reduced. No corrosion product layer detachment is found on the surface of the ZX0.7 composite, and the corrosion layer is split into small blocks with narrow corrosion cracks running through them, as shown in Figure 13b. The surface of the composite is still relatively flat. Figure 13e shows the magnification microstructure of the corrosion product layer, and there is no loose corrosion product. Although there are a small number of microcracks on the surface, there is no shedding phenomenon. For ZX1.0 composites, the surface of the corrosion products became inhomogeneous with large fluctuations. It is divided into large blocks, and corrosion pits are also found, but the number of pits is small. Sparse areas can also be observed in the high-magnification image (Figure 13f) due to the detachment of the dense layer.
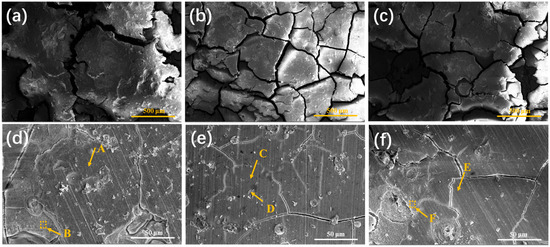
Figure 13.
Surface morphology of extruded specimens after 14 days of immersion in SBF for ZX alloy (a,d), ZX0.7 alloy (b,e) and ZX1.0 alloy (c,f).

Table 5.
EDS spectrum data at different positions as shown in Figure 13.
Figure 14 shows the SEM images and corresponding EDS results of ZX alloy and ZX0.7 and ZX1.0 composites after immersion in SBF for 14 days. As the main components of the corrosion product layer, the distribution of Ca, P and O elements can be used to determine the thickness of different corrosion product layers. Firstly, the EDS results showed that the P, Ca and O elements were uniformly distributed in the corrosion product layer between the dotted red lines after 14 days of immersion. The corrosion interface of the ZX matrix displays a zigzag shape from the low magnification SEM image (Figure 14), while a straight interface was obtained in the ZX0.7 composite, but it becomes uneven again in the ZX1.0 composite. Though the interface is not straight from the macro level in the ZX1.0 composite, it is still flat in the local area, which is better than that of ZX alloy. Lines 1, 2 and 3 are surface line scan elemental analyses of ZX alloy, ZX0.7 and ZX1.0, respectively. The calcium and phosphorus contents in the ZX alloy are lower than those in the ZX0.7 and ZX1.0 composites. This indicates that the density of the Ca-P layer is lower in ZX, so the protective effect on this material is weak. In addition, the distribution of the O element in the corrosion product layer is similar to that of the Ca-P layer, indicating that the density of Mg(OH)2 in ZX is also less than that of ZX0.7 and ZX1.0. Therefore, it cannot form a good protection effect on the substrate.
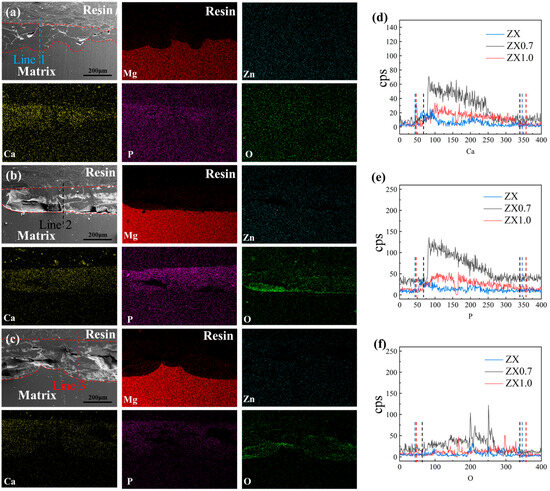
Figure 14.
Cross-sectional SEM and EDS micrographs after immersion 14 days. (a–c) ZX, ZX0.7, ZX1.0 (d–f) Line scans result of the elements Ca, P and O.
4. Discussion
4.1. Strengthening Mechanism of MgO on Mg-Zn-Ca Alloy
The microhardness and tensile properties shown in Figure 7 and Figure 8 demonstrate that the addition of MgO has played an important role in improving the mechanical properties of ZX alloy, and with the increase of MgO content, the strengthening effect is enhanced gradually. Based on the change of the microstructures shown in Figure 2 and Figure 4, this improvement of strength is mainly attributed to grain size refinement, Orowan strengthening due to the incorporation of MgO, the increase of the second phase, and thermal expansion mismatch strengthening. The increase in yield strength due to fine grain strengthening can be expressed by the Hall–Petch Equation (4) [33]:
where is a constant with the value of , and and are the average grain sizes of the composites in the solid solution as well as in the extruded state, respectively.
The increment due to Orowan strengthening induced by the incorporation of MgO and the second phase can be calculated from Equation (5) [34]:
where is the Taylor factor with a value of 6.5, is the Brønested vector of the Mg matrix composite = 0.321 nm, is the shear modulus of the Mg matrix with a value of 16.5 GPa, = 0.35 is the Poisson’s ratio of Mg, and and are the volume fraction of the ministerial with MgO particles and the average dynamic recrystallization grain size, respectively.
Due to the coefficient of thermal expansion (CTE) mismatch between the MgO and the Mg matrix, incompatible plastic deformation may occur in the composite, and residual thermal stresses and dislocations may be generated, leading to an increase in the strength of the material. This strengthening increase can be calculated using Equation (6) [35]:
where and are the Taylor factor and shear modulus of Mg, and their values are the same as in Equation (5). is the average diameter of the MgO, is the volume fraction of the MgO, is the Bergstrom vector with the value of 0.321 nm, is the difference in the thermal expansion coefficient between the MgO (11.2 × 10−6 K−1) and Mg matrix (30.7 × 10−6 K−1), and is the temperature difference between the temperature of mechanical testing and the extrusion deformation temperature.
For the extruded ZX alloy and composites with 0.3%, 0.5%, 0.7%, and 1.0% MgO, the theoretically calculated values of the three strengthening mechanisms for yield strength enhancement are shown in Table 6. The table displays the calculated theoretical and experimental strengths of the yield strength enhancement for the three mechanisms of enhancement across five cases. The yield strength of the ZX alloy was assumed to be equal to its theoretical strength. It is found that the experimental yield strength is higher than that of the theoretical yield strength for all the ZX composites. With increasing the MgO contents, the differences between experimental strength and theoretical strength are enlarged. This difference is likely caused by the texture strengthening [36].

Table 6.
Theoretical calculation values of yield strength enhancement by three strengthening mechanisms.
From the table, it can be seen that the yield strength increment caused by grain refinement is larger than that caused by Orowan strengthening and CTE mismatch when the MgO addition is small, indicating that the fine grain reinforcement has the greatest effect on the tensile properties. With the increase of MgO mass fraction, the strength enhancement induced by fine grain strengthening, Orowan strengthening and CTE mismatch gradually increased. It is noted that the strength enhancement caused by CTE mismatch is very weak, and it can be neglected.
4.2. Corrosion Mechanism of ZXx Materials
The corrosion results show that the addition of MgO reduced the corrosion rate, and its value depended on the MgO content. The changes in the microstructure between ZX and ZX composites are mainly on the grain size, the volume fraction of the second phases (Ca2Mg6Zn3 and Mg2Ca) and MgO contents in the matrix.
Grain refinement improves not only mechanical properties but also corrosion resistance. The material’s corrosion resistance significantly improved due to the rapid formation of a corrosion product layer [37,38,39,40,41,42], which acted as a barrier against destructive ions. The addition of MgO results in a larger average grain size reduction, but the MgO contents had a weak effect on the grain size (Figure 2). The protective effect provided by grain refinement can be reflected directly by the passivation platform in Figure 9 and the reduced pH and corrosion rate in the initial immersion stage (Figure 10). In SBF solution, the Mg(OH)2 film of ZX composites is denser than that of ZX due to fine grains. Therefore, more dense corrosion layers are formed in the process of corrosion, and these corrosion layers have a protective effect on the substrate and the fine grain size is favorable for the uniformity of corrosion, which avoids the occurrence of pitting corrosion to a certain extent.
The mechanical properties of Mg alloy are affected not only by the volume and size of the second phase particles but also by the type and distribution of the second phase [43,44]. Patrick et al. [45] found that the Mg2Ca phase acts as an anodic phase, resulting in slow and uniform degradation when present in moderate phase fractions. The preferentially corroded Mg2Ca phase has a protective effect on the substrate. Xu et al. [46] discovered that the Mg2Ca phase corrodes preferentially, indicating that the Mg2Ca and α-Mg phases act as anode and cathode, respectively. The work function of Mg2Ca was found to be smaller than that of α-Mg through a combination of first-principles calculations and SKPFM potential measurements, suggesting that Mg2Ca is more susceptible to electron loss and, therefore, more susceptible to corrosion.
Furthermore, Cha et al. [47] concluded that the higher corrosion resistance of Mg-Zn-Ca alloys, compared to binary Mg-Ca, is mainly attributed to the Zn-rich Mg2Ca phase, rather than the corrosion barrier of Ca2Mg6Zn3. The effect of Mg2Ca is small due to the lower content. Additionally, Ca2Mg6Zn3 generally acts as the cathode, which is not favorable for corrosion performance. However, the protective effect of MgO is still present.
Several studies [48,49,50] have shown that the addition of MgO can reduce grain size and improve mechanical properties. Fan et al. [48] found that MgO particles are effective nucleation sites for the primary α-Mg phase, and the diffusely distributed MgO has a refining effect. The study of Ma et al. [49] showed that the grain size of Mg-3%Al alloys at casting was about 535 ± 31 μm0.5%, and with the increase of MgO additions up to 0.5% and 1%, the average grain sizes were further refined to 267 ± 18 μm and 146 ± 6 μm, respectively. Lin et al. [50] showed that the grain size, YS and EL values of Mg-3Zn-0.2Ca and Mg-3Zn-0.2Ca-0.5MgO were 2 μm, 257.23 MPa, 16.6% and 1.5 μm, 300.00 MPa, 14.1%, respectively. MgO acts as a nucleation substrate for the second phase, which can promote the precipitation of the second phase. An appropriate amount of MgO can effectively improve the mechanical and corrosion properties of the material, but an excessive amount of MgO can lead to excessive precipitation or even agglomeration of the second phase.
Figure 15 shows a schematic of the corrosion principle of the ZX alloy and ZX composites based on the above discussion. Firstly, the composites have high grain boundary density and uniform grain size, and the fine grains can promote the formation of a dense corrosion product layer, leading to the high corrosion resistance of the ZX composites. Secondly, with the addition of MgO, more precipitates are generated, and the moderate amount of diffusely distributed second phase is conducive to slowing down the corrosion. However, when the content of the second phase is too high, for example, in ZX1.0, the total volume fraction of the second is about 1.5%, which is 1.5 times higher than that of ZX0.7, these abundant second phases accelerate the overall corrosion rate of ZX1.0. Thirdly, the Mg(OH)2 particles formed by the reaction between MgO and the SBF solution sealed the cracks of the corrosion product layer, effectively preventing the penetration of the corrosion medium into the Mg matrix along the cracks. Ultimately, the reduction in the corrosion rate means a reduction in the rate of hydrogen release, and this effectively reduces the accumulation of hydrogen in the cracks of the corrosion product layer and the destructive tendency of hydrogen bubbles to the corrosion product layer. This ensures that the corrosion product layer can cover the surface of the specimen for a long time and protect the matrix, whereas, for the ZX1.0 composite, the local agglomeration of MgO particles becomes a defect and is easily eroded by the destructive ion.
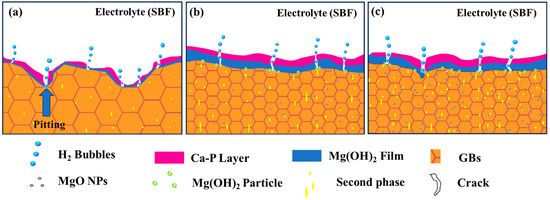
Figure 15.
Schematic of corrosion of (a) ZX alloy, (b) ZX0.7, and (c) ZX1.0 composite.
Therefore, the ZX0.7 composite exhibited a slower and uniform corrosion than that of the ZX alloy and ZX1.0 composite.
5. Conclusions
The microstructure, mechanical properties and corrosion resistance of the ZX composites with different MgO contents were studied. The following conclusions were drawn:
- The incorporation of MgO particles significantly inhibited the grain boundary migration through the Zener pinning force, and the average grain size of the ZX, ZX0.3, ZX0.5, ZX0.7, and ZX1.0 materials were 17.73 μm, 5.54 μm, 4.96 μm, 4.37 μm, and 3.95 μm was obtained in the ZX1.0 composite. However, it did not change the type of second phases (Ca2Mg6Zn3 and Mg2Ca) and only increased the volume fraction of them by increasing the MgO contents.
- The mechanical properties of the composites were enhanced due to the fine grain strengthening effect brought by the grain size refinement, as well as the Orowan strengthening caused by the incorporation of MgO particles and the thermal expansion mismatch strengthening. The yield strength and microhardness increased from 102.0 MPa and 59.43 HV in the ZX alloy to 209.5 MPa and 69.81 HV in the ZX1.0 composite. However, the corresponding fracture elongation decreased from 26.7% to 21.2%.
- The differences in corrosion resistance of the ZX composites are influenced by grain size as well as the type and distribution of the second phase. The ZX alloy corrodes rapidly due to the large grain size and the low adhesion of the corrosion product film. The ZX0.7 composite (ZX0.3, ZX0.5 and ZX) exhibited the best corrosion performance with the corrosion rate of 0.77 g/m2/h owing to the fine grain size, evenly distributed fine second phase as well as the enhanced corrosion product layer by the hydrolysis of MgO. However, excessive MgO (1.0 wt.%) caused the self-agglomeration and increasing second phases, which accelerated the speed of galvanic corrosion. Therefore, ZX1.0 had a faster corrosion rate of 0.82 g/m2/h than the ZX0.7 composite.
Author Contributions
Conceptualization, Q.Z., S.L. and G.Z.; Methodology, Q.Z., S.L. and G.Z.; Validation, M.C. and S.L.; Investigation, Q.Z., S.L. and G.Z.; Data curation, Q.Z. and G.Z. Writing—original draft, Q.Z., S.L. and G.Z.; Writing—review and editing, Q.Z., S.L. and G.Z.; Resources, M.C.; Funding acquisition, M.C. and S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (Nos. 52201301 and 52171241); Tianjin Natural Science Foundation: 22JCQNJC00750.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (privacy).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Verma, A.; Ogata, S. Magnesium based alloys for reinforcing biopolymer composites and coatings: A critical overview on biomedical materials. Adv. Ind. Eng. Polym. Res. 2023, 6, 341–355. [Google Scholar] [CrossRef]
- Meenachi, P.; Subashini, R.; Lakshminarayanan, A.K.; Gupta, M. Comparative study of the biocompatibility and corrosion behaviour of pure Mg, Mg Ni/Ti, and Mg 0.4Ce/ZnO2 nanocomposites for orthopaedic implant applications. Mater. Res. Express. 2023, 10, 056503. [Google Scholar]
- Yang, Y.; Xiong, X.M.; Chen, J.; Peng, X.D.; Chen, D.L.; Pan, F.S. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloy. 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, P.; Qiu, Y.; Jao, C.; Blawert, C.; Lamaka, S.; Bouali, A.; Lu, X.; Zheludkevich, M.L.; Li, W. Experimental and quantum chemical studies of carboxylates as corrosion inhibitors for AM50 alloy in pH neutral NaCl solution. J. Magnes. Alloy. 2022, 10, 555–568. [Google Scholar] [CrossRef]
- Du, P.; Mei, D.; Furushima, T.; Zhu, S.; Wang, L.; Zhou, Y.; Guan, S. In vitro corrosion properties of HTHEed Mg-Zn-Y-Nd alloy microtubes for stent applications: Influence of second phase particles and crystal orientation. J. Magnes. Alloy. 2022, 10, 1286–1295. [Google Scholar] [CrossRef]
- Gao, M.; Yang, K.; Tan, L.; Ma, Z. Role of bimodal-grained structure with random texture on mechanical and corrosion properties of a Mg-Zn-Nd alloy. J. Magnes. Alloy. 2022, 10, 2147–2157. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Egorkin, V.S.; Ustinov, A.Y.; Sergienko, V.; Gnedenkov, S. The detailed corrosion performance of bioresorbable Mg-0.8Ca alloy in physiological solutions. J. Magnes. Alloy. 2022, 10, 1326–1350. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Qi, Y.; Peng, Z.; Ye, Y.; Liang, J. Corrosion and tribocorrosion resistance of MAO-based composite coating on AZ31 magnesium alloy. J. Magnes. Alloy. 2022, 10, 3406–3417. [Google Scholar] [CrossRef]
- Feng, B.; Liu, G.; Yang, P.; Huang, S.; Qi, D.; Chen, P.; Wang, C.; Du, J.; Zhang, S.; Liu, J. Different role of second phase in the micro-galvanic corrosion of WE43 Mg alloy in NaCl and Na2SO4 solution. J. Magnes. Alloy. 2022, 10, 1598–1608. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.F.; Chen, X.H.; Yang, J.A.; Pan, H.B.; Chen, D.F.; Wang, L.N.; Zhang, J.L.; Zhu, D.H.; Wu, S.L.; et al. Fundamental Theory of Biodegradable Metals-Definition, Criteria, and Design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Hassan, N.; Krieg, T.; Zinser, M.; Schröder, K.; Kröger, N. An Overview of Scaffolds and Biomaterials for Skin Expansion and Soft Tissue Regeneration: Insights on Zinc and Magnesium as New Potential Key Elements. Polymers 2023, 15, 3854. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. C-Mater. Biol. Appl. 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Hurley, M.F.; Efaw, C.M.; Davis, P.H.; Croteau, J.R.; Graugnard, E.; Birbilis, N. Volta Potentials Measured by Scanning Kelvin Probe Force Microscopy as Relevant to Corrosion of Magnesium Alloys. Corrosion 2014, 71, 160–170. [Google Scholar] [CrossRef]
- Suh, J.S.; Ha, H.Y.; Suh, B.C.; Kang, J.W. Determination of Optimum Zn Content for Mg-xZn-0.5Mn-0.5Sr Alloy in Terms of Mechanical Properties and In Vitro Corrosion Resistance. Met. Mater. Int. 2023, 29, 1841–1852. [Google Scholar] [CrossRef]
- Fang, H.; Wang, C.; Zhou, S.; Zheng, Z.; Lu, T.; Li, G.; Tian, Y.; Suga, T. Enhanced adhesion and anticorrosion of silk fibroin coated biodegradable Mg-Zn-Ca alloy via a two-step plasma activation. Corros. Sci. 2020, 168, 108466. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Hong, C.H.; Kim, J.C.; Lee, S.; Baek, S.; Jeong, H.Y.; Park, S.S. Factors affecting the grain refinement of extruded Mg–6Zn–0.5Zr alloy by Ca addition. Scr. Mater. 2020, 187, 24–29. [Google Scholar] [CrossRef]
- Liu, R.X.; Wang, J.; Wang, L.Y.; Zeng, X.Q.; Jin, Z.H. Cluster Hardening Effects on Twinning in Mg-Zn-Ca Alloys. Metals 2022, 12, 693. [Google Scholar] [CrossRef]
- Jin, Y.M.; Blawert, C.; Feyerabend, F.; Bohlen, J.; Campos, M.S.; Gavras, S.; Wiese, B.; Mei, D.; Deng, M.; Yang, H.; et al. Time-sequential corrosion behaviour observation of micro-alloyed Mg-0.5Zn-0.2Ca alloy via a quasi-in situ approach. Corros. Sci. 2019, 158, 108096. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, C.; Huang, J.; Zhao, Y.C.; Han, T.Z.; Wang, G.G.; Wu, L.; Huang, G.S. Effect of Ca Element on Microstructure and Corrosion Behavior of Single-Phase Mg-Sc Alloy. Metals 2022, 12, 93. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.H.; Huang, Q.Y.; Li, Y.J.; Yang, Y.S. Effect of the microstructure parameters on the corrosion characteristics of Mg-Zn-Ca alloy with columnar structure. J. Magnes. Alloy. 2023, 11, 1709–1720. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloy. 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Venkatesh, R.; Kanagasabapath, H. Analysing the characteristics of magnesium based composites for biomedical applications. Mater. Today. Proc. 2023. [Google Scholar] [CrossRef]
- Tang, C.K.; Lyu, S.; Zhao, Z.H.; Chen, M.F. Effects of MgO nano particles on the mechanical properties and corrosion behavior of Mg-Zn-Ca alloy. Mater. Chem. Phys. 2023, 297, 127380. [Google Scholar] [CrossRef]
- GB/T 24176-2009; Metallic Materials—Fatigue Testing—Statistical Planning and Analysis of Data. China Quality and Standards Publishing & Media Co., Ltd.: Beijing, China, 2010.
- Lindström, R.; Johansson, L.G.; Thompson, G.E.; Skeldon, P.; Svensson, J.E. Corrosion of magnesium in humid air. Corros. Sci. 2004, 46, 1141–1158. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, F.; Wang, Y.; Wang, S.; Patel, J.B. Grain refinement of Mg-alloys by native MgO particles: An overview. J. Magnes. Alloy. 2022, 10, 2919–2945. [Google Scholar] [CrossRef]
- Shen, G.X.; Lyu, S.Y.; Zhao, Y.; You, C.; Wang, X.W.; Chen, M.F. The first principle research of CaO and MgO particulate heterogeneous nucleation in Mg alloys. Appl. Surf. Sci. 2022, 593, 153224. [Google Scholar] [CrossRef]
- Abazari, S.; Shamsipur, A.; Bakhsheshi-Rad, H.R.; Keshavarz, M.; Kehtari, M.; Ramakrishna, S.; Berto, F. MgO-incorporated carbon nanotubes-reinforced Mg-based composites to improve mechanical, corrosion, and biological properties targeting biomedical applications. J. Mater. Res. Technol. 2022, 20, 976–990. [Google Scholar] [CrossRef]
- Tang, C.; Lyu, S.; Zheng, R.; Li, G.; Liu, Z.; Chen, M.; Jiang, B. Realizing ultra-high strength and excellent ductility in a low-alloyed biomedical Mg-Zn-Ca-MgO composite. J. Magnes. Alloy. 2024. [Google Scholar] [CrossRef]
- Pul, M.; Erdem, U.; Turkoz, M.B.; Yildirim, G. The effect of sintering parameters and MgO ratio on structural properties in Al7075/MgO composites: A review. J. Mater. Sci. 2023, 58, 664–684. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, E.L. Biocorrosion behavior of magnesium alloy in different simulated fluids for biomedical application. Mater. Sci. Eng. C-Mater. Biol. Appl. 2009, 29, 1691–1696. [Google Scholar] [CrossRef]
- Habibi, M.K.; Joshi, S.P.; Gupta, M. Hierarchical magnesium nano-composites for enhanced mechanical response. Acta Mater. 2010, 58, 6104–6114. [Google Scholar] [CrossRef]
- Op’T Hoog, C.; Birbilis, N.; Estrin, Y. Corrosion of Pure Mg as a Function of Grain Size and Processing Route. Adv. Eng. Mater. 2008, 10, 579–582. [Google Scholar] [CrossRef]
- Zhang, H.F.; Ding, Y.T.; Li, R.M.; Gao, Y.B. Enhanced strength-ductility synergy and activation of non-basal slip in as-extruded Mg-Zn-Ca alloy via heterostructure. J. Mater. Res. Technol. 2024, 28, 1841–1851. [Google Scholar] [CrossRef]
- Dobkowska, A.; Adamczyk Cieślak, B.; Kuc, D.; Hadasik, E.; Płociński, T.; Ura-Bińczyk, E.; Mizera, J. Influence of bimodal grain size distribution on the corrosion resistance of Mg–4Li–3Al–1Zn (LAZ431). J. Mater. Res. Technol. 2021, 13, 346–358. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Miao, B.; Wang, Z.; Huang, H.; Guan, S.; Yuan, G. Mechanical and corrosion properties of biodegradable magnesium mini-tubes with different grain morphologies: Size and distribution. J. Mater. Sci. Technol. 2024, 183, 165–174. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, C.; Ni, J.; Zhang, S.; Liu, J.; Yan, J.; Chen, Y.; Zhang, X. Research of a novel biodegradable surgical staple made of high purity magnesium. Bioact. Mater. 2016, 1, 122–126. [Google Scholar] [CrossRef]
- Ravi Kumar, N.V.; Blandin, J.J.; Desrayaud, C.; Montheillet, F.; Suéry, M. Grain refinement in AZ91 magnesium alloy during thermomechanical processing. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2003, 359, 150–157. [Google Scholar] [CrossRef]
- Saha, P.; Roy, M.; Datta, M.K.; Lee, B.; Kumta, P.N. Effects of grain refinement on the biocorrosion and in vitro bioactivity of magnesium. Mater. Sci. Eng. C-Mater. Biol. Appl. 2015, 57, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; Hang, R.Q.; Wang, C.; Yu, Z.T.; Li, Z.Y.; Xiao, Y. Biodegradable Metallic Wires in Dental and Orthopedic Applications: A Review. Metals 2018, 8, 212. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, Z.; Yin, Z.; Wang, K.; Liu, Q.D.; Sun, P.F.; Yan, H.; Song, H.G.; Luo, C.; Guan, H.; et al. Corrosion behavior characterization of as extruded Mg-8Li-3Al alloy with minor alloying elements (Gd, Sn and Cu) by scanning Kelvin probe force microscopy. Corros. Sci. 2020, 176, 108923. [Google Scholar] [CrossRef]
- Baek, S.; Kang, J.S.; Shin, H.; Yim, C.D.; You, B.S.; Ha, H.; Park, S.S. Role of alloyed Y in improving the corrosion resistance of extruded Mg–Al–Ca-based alloy. Corros. Sci. 2017, 118, 227–232. [Google Scholar] [CrossRef]
- Holweg, P.; Berger, L.; Cihova, M.; Donohue, N.; Clement, B.; Schwarze, U.; Sommer, N.G.; Hohenberger, G.; van den Beucken, J.J.J.P.; Seibert, F.; et al. A lean magnesium–zinc–calcium alloy ZX00 used for bone fracture stabilization in a large growing-animal model. Acta Biomater. 2020, 113, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, J.F.; Chen, C.; Wang, C.; Sun, Y.F.; Zhu, S.J.; Guan, S.K. Initial micro-galvanic corrosion behavior between Mg2Ca and α-Mg via quasi-in situ SEM approach and first-principles calculation. J. Magnes. Alloy. 2023, 11, 958–965. [Google Scholar] [CrossRef]
- Cha, P.R.; Han, H.S.; Yang, G.F.; Kim, Y.C.; Hong, K.H.; Lee, S.C.; Jung, J.Y.; Ahn, J.P.; Kim, Y.Y.; Cho, S.Y.; et al. Biodegradability engineering of biodegradable Mg alloys: Tailoring the electrochemical properties and microstructure of constituent phases. Sci. Rep. 2013, 3, srep02367. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xia, M.; Arumuganathar, S. Enhanced heterogeneous nucleation in AZ91D alloy by intensive melt shearing. Acta Mater. 2009, 57, 4891–4901. [Google Scholar] [CrossRef]
- Ma, Z.Q.; Li, C.B.; Du, J.; Zhan, M.Y. Grain Refinement of Mg-Al Alloys Inoculated by MgO Powder. Int. J. Met. 2019, 13, 674–685. [Google Scholar] [CrossRef]
- Lin, G.; Liu, D.; Chen, M.; You, C.; Li, Z.; Wang, Y.; Li, W. Preparation and characterization of biodegradable Mg-Zn-Ca/MgO nanocomposites for biomedical applications. Mater. Charact. 2018, 144, 120–130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).