Molecular Dynamics Research on Fe Precipitation Behavior of Cu95Fe5 Alloys during Rapid Cooling
Abstract
1. Introduction
2. Simulation Method
3. Results and Discussion
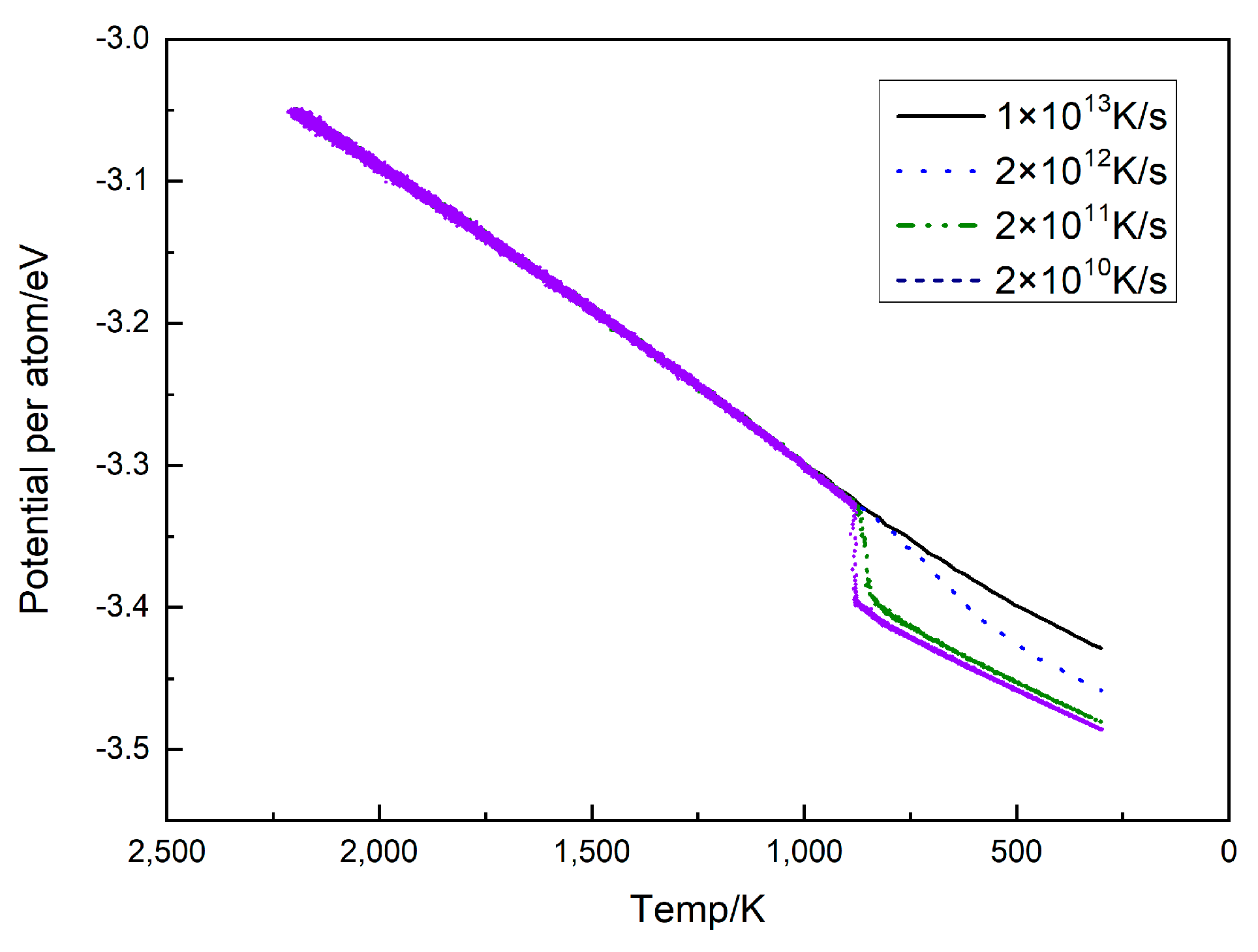
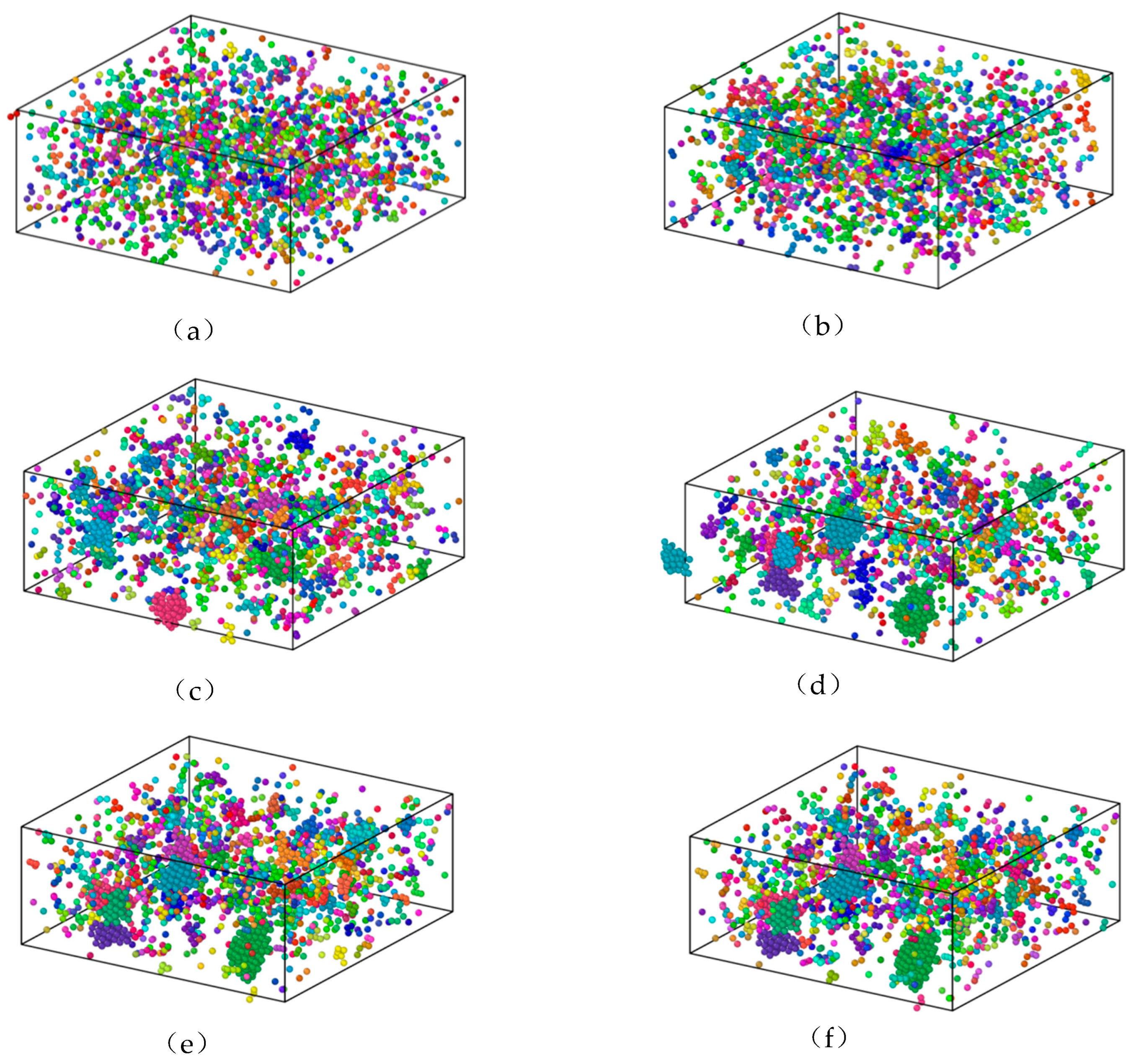
3.1. The Influence of the Cooling Rate on the Cu95Fe5 Alloy Structure
3.2. Structural Changes in the Cu95Fe5 Alloy System during Cooling
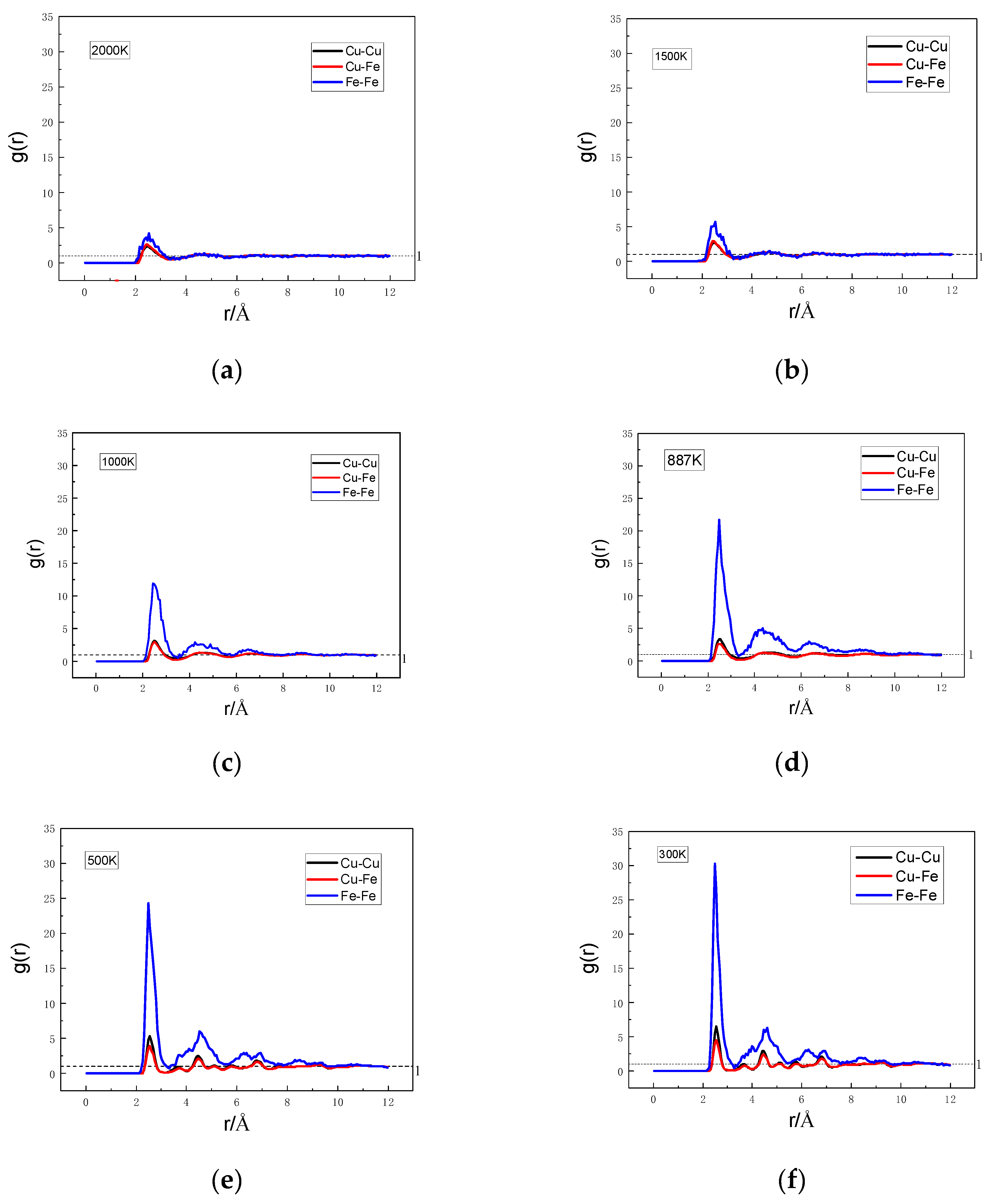
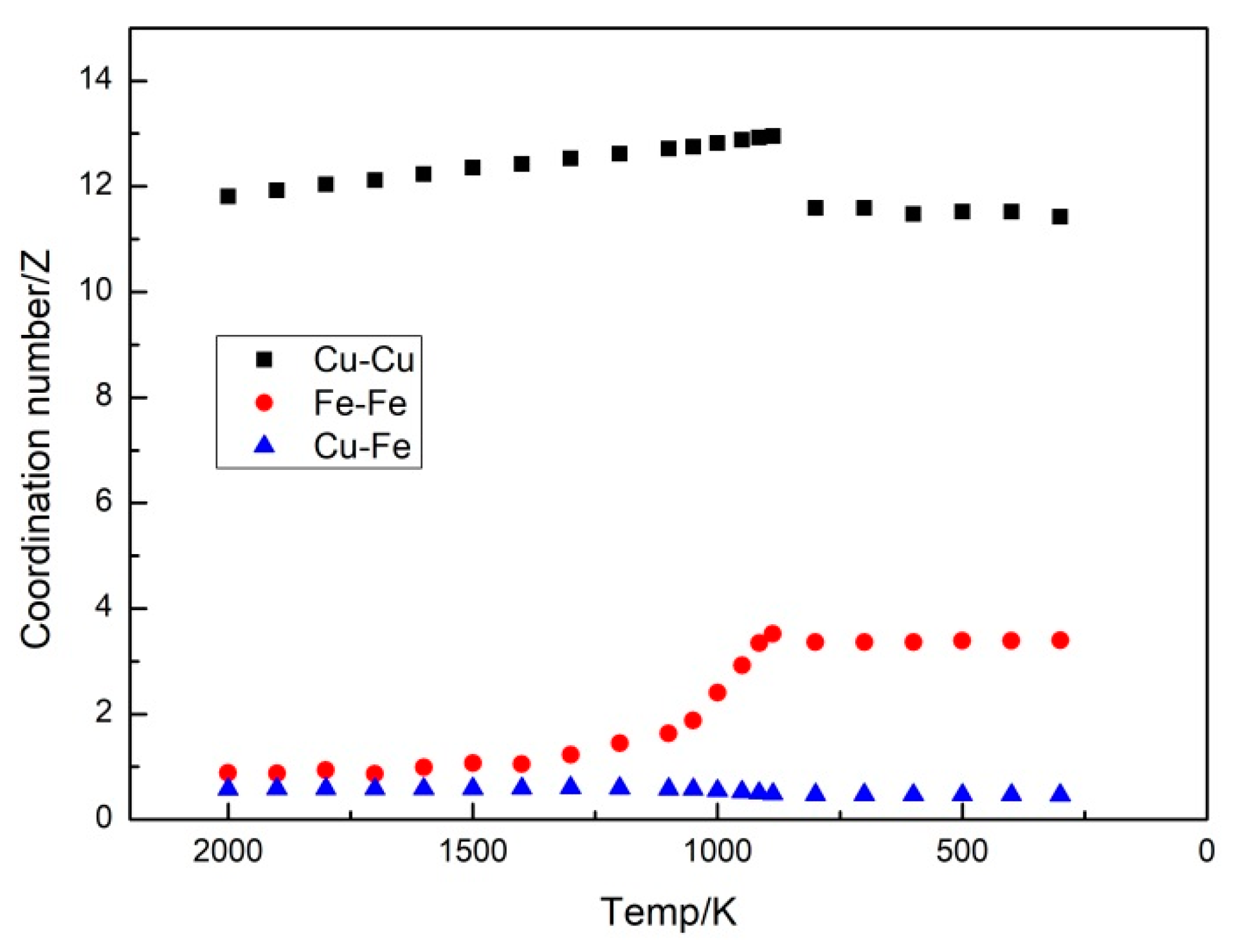
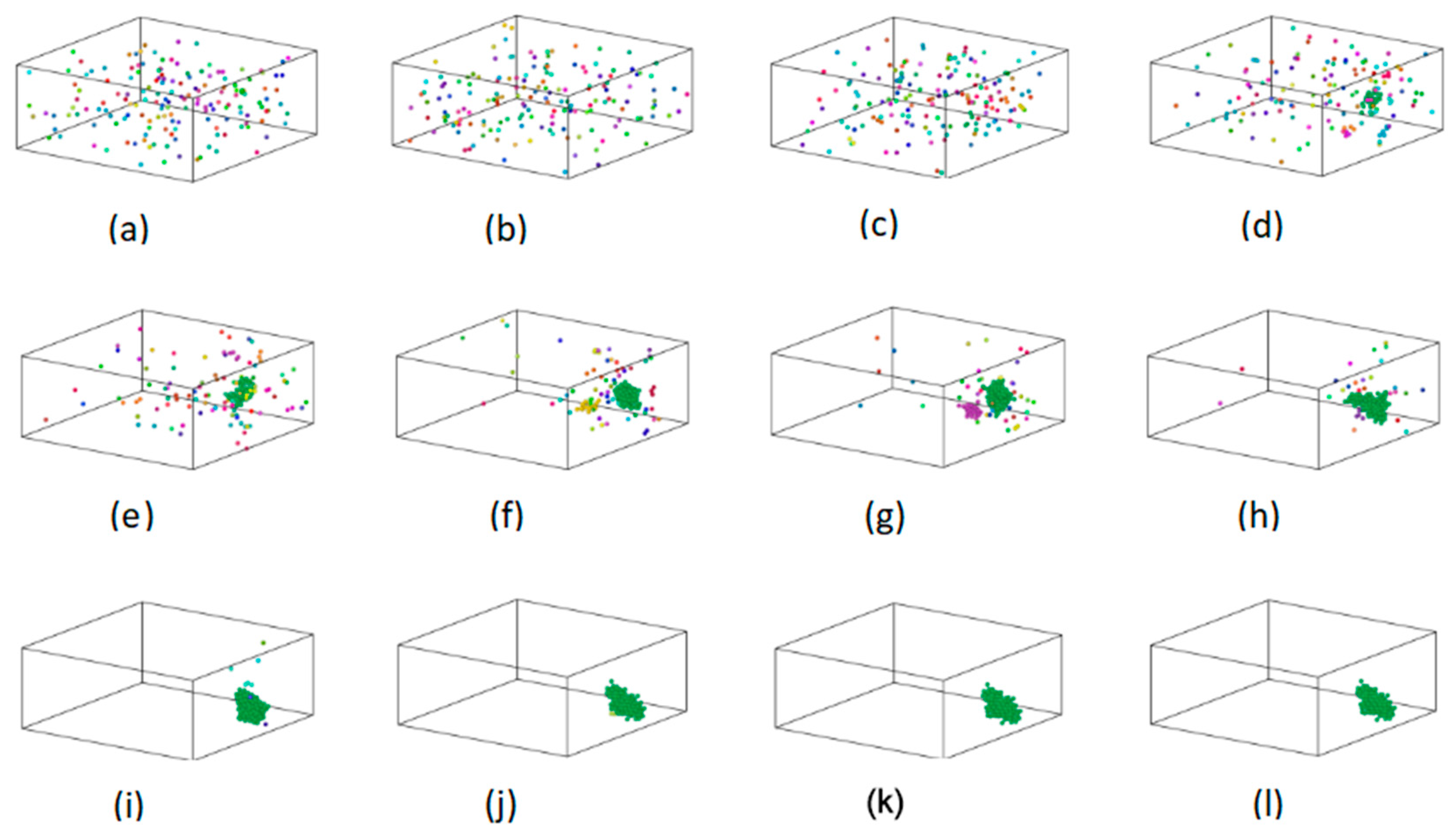
3.3. Formation Mechanism of Fe Clusters in Cu95Fe5 Alloys during Rapid Cooling
4. Conclusions
- (1)
- The phase transition temperature of the alloys varied with the changing cooling rate. As the cooling rate decreased, the phase transition temperature increased. At a lower cooling rate, the average atomic potential energy after phase transition was lower, the system structure was more stable, and the proportion of atoms in the crystal structure was larger. At the cooling rate of 2 × 1010 K/s, the alloy was liquid above 887 K. When the temperature reached about 887 K, the alloy began to crystallize. In the temperature range of 887–850 K, the proportion of FCC and HCP crystals rapidly increased at first, and then the growth rate decreased. The content of BCC crystals first increased, before decomposition into FCC and HCP crystals. Finally, a Cu-Fe alloy was generated, primarily comprising FCC and HCP crystals, accompanied by a limited quantity of BCC crystals.
- (2)
- The interaction between the Fe atoms served as the driving force for the formation of the Fe clusters. The radial distribution function and coordination number of the simulation system structure parameters were analyzed. The results reveal that with the lowering temperature, the difference between the first peak value of and that of and enlarged, the coordination number of the Fe-Fe pairs increased steadily, and the coordination number of the Cu-Fe pairs decreased gradually. Such results indicate that the interaction between the Fe atoms was stronger than that between the Cu atoms and between the Cu and Fe atoms, and there was a repulsion force between the Cu and Fe atoms. With the decrease in the temperature, the second peak value of increased, but that of and decreased. At 300 K, all parts of the curve were located above 1, while the second peak values of and reduced below 1. Such findings suggest that the nearest and second-nearest neighbors of the Fe atoms were mostly occupied by Fe atoms, and the Fe atoms were more prone to form nearest-neighbor structures with Fe atoms.
- (3)
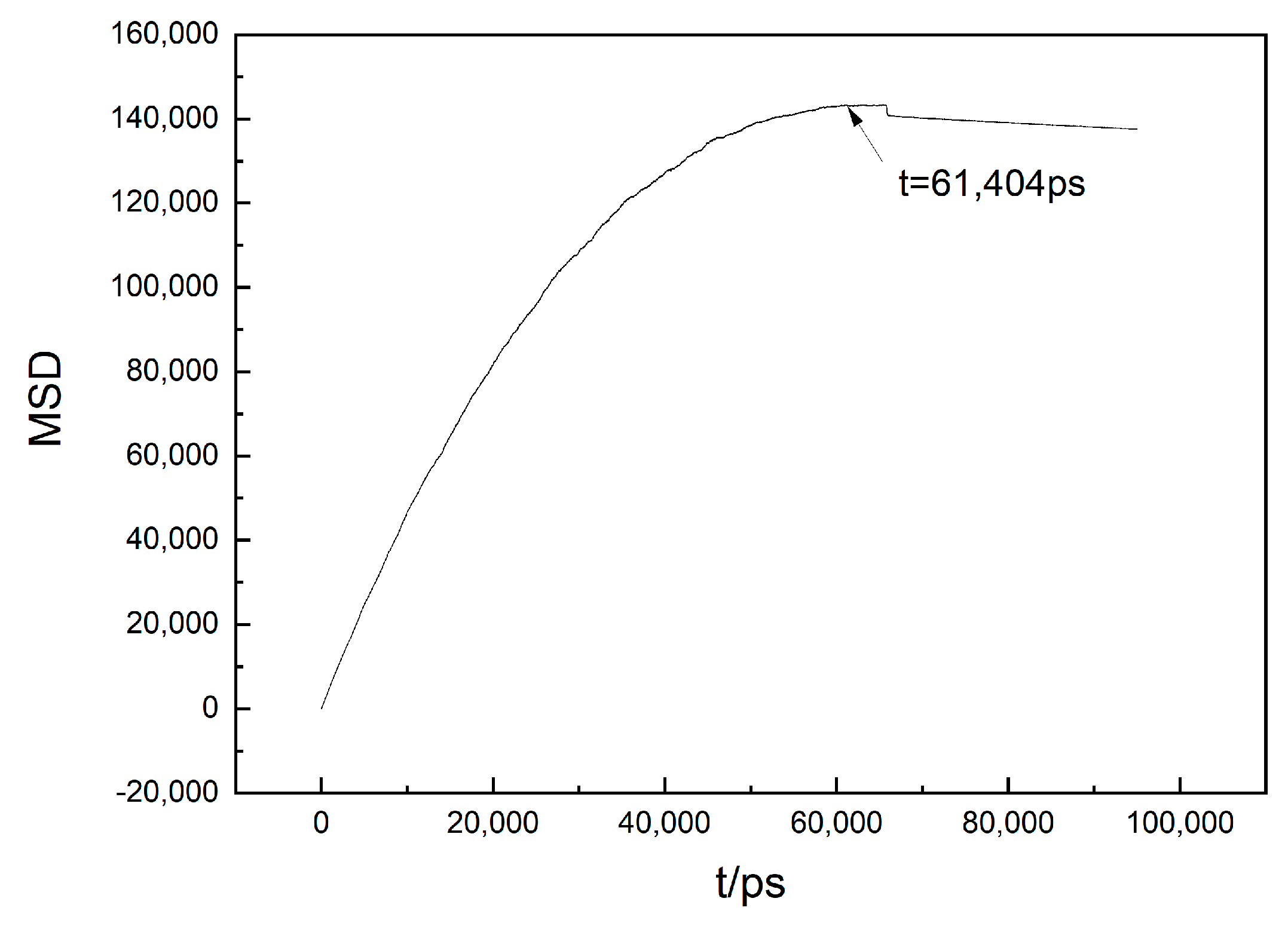
- Temperate changes affected the thermal activities of the atoms. The diffusion coefficient of the atoms influenced the growth of the Fe clusters. A smaller diffusion coefficient indicated a stronger bond between the atoms and a more stable cluster. When the temperature dropped but still remained above 967 K, the diffusion coefficient of the Fe atoms decreased. The diffusion coefficient of the Fe atoms approached 0 within the temperature range of 967–887 K. During this period, the principal structural modification within the system was attributable to the localized rearrangements of the atoms, rather than diffusion-driven processes. This stage represents a crucial period for cluster expansion.
- (4)
- The formation and growth of the Fe clusters were governed by the nucleation and growth mechanism. When the alloy temperature was above 1400 K, more Fe clusters were formed due to the strong interaction between the Fe atoms, and the large diffusion coefficient of the Fe atoms inhibited the growth of Fe clusters. In the temperature range of 1400–1050 K, both the number of atoms in the largest cluster and the number of clusters increased due to the interaction between the Fe atoms. In the temperature range of 1050–887 K, there were two reasons for the growth of Fe clusters. One reason for such a phenomenon is that the interaction among the Fe atoms facilitated the aggregation of individual Fe atoms into Fe clusters. Another contributing factor is the coalescence and fusion of pre-existing clusters. The minimization of the surface area energy promoted the formation and expansion of Fe clusters. When the temperature fell below 887 K, the alloy underwent crystallization, and subsequent alterations in the magnitude and quantity of Fe clusters were minimal.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, H.Y.; Ma, Z.C.; Lei, C.H.; Meng, L.; Fang, Y.T.; Liu, J.B.; Wang, H.T. High strength and high conductivity Cu alloys: A review. Sci. China (Technol. Sci.) 2020, 63, 2505–2517. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Sun, Q.; Li, M.; Yan, M.; Cheng, X.; Li, M.; Zhang, M.-X. Laser powder bed fusion of copper matrix iron particle reinforced nanocomposite with high strength and high conductivity. J. Mater. Sci. Technol. 2023, 134, 50–59. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.L.; Wang, Z.M. Progress in Research on the Alloying of Binary Immiscible Metals. Acta Metall. Sin. 2020, 56, 801–820. [Google Scholar]
- Wang, M.; Yang, Q.-R.; Jiang, Y.-B.; Li, Z.; Xiao, Z.; Gong, S.; Wang, Y.-R.; Guo, C.-L.; Wei, H.-G. Effects of Fe content on microstructure and properties of Cu-Fe alloy. Trans. Nonferrous Met. Soc. China 2021, 31, 3039–3049. [Google Scholar] [CrossRef]
- Tian, Y.Z.; Yang, Y.; Peng, S.Y.; Pang, X.Y.; Li, S.; Jiang, M.; Li, H.X.; Wang, J.W.; Qin, G.W. Managing mechanical and electrical properties of nanostructured Cu-Fe composite by aging treatment. Mater. Charact. 2023, 196, 112600. [Google Scholar] [CrossRef]
- Bao, H.; Xu, H.; Li, Y.; Bai, H.; Ma, F. The interaction mechanisms between dislocations and nano-precipitates in CuFe alloys: A molecular dynamic simulation. Int. J. Plast. 2022, 155, 103317. [Google Scholar] [CrossRef]
- Sunbul, S.E.; Akyol, S.; Onal, S.; Ozturk, S.; Sozeri, H.; Icin, K. Effect of Co, Cu, and Mo alloying metals on electrochemical and magnetic properties of Fe-B alloy. J. Alloys Compd. 2023, 947, 169652. [Google Scholar] [CrossRef]
- Pennell, S.M.; Mack, J.B.; Dunand, D.C. Evolution of lamellar architecture and microstructure during redox cycling of Fe-Co and Fe-Cu foams. J. Alloys Compd. 2022, 918, 165606. [Google Scholar] [CrossRef]
- Ma, H.; Liu, Z.; Li, J.; Liu, Q.; Zhang, J.; Wei, T. Optimization design of environmental-friendly Cu-Fe laser cladding coating for self-grown microchannel in a marine corrosive environment. J. Alloys Compd. 2023, 940, 168820. [Google Scholar] [CrossRef]
- Sahoo, D.; Paliwal, M.; Srivastava, A.; Mishra, S. Study on the composition effect upon the microstructure development in Cu-Fe alloys prepared using Aerodynamic Levitation. Mater. Today Commun. 2023, 37, 107288. [Google Scholar] [CrossRef]
- Chen, K.X.; Li, Z.X.; Wang, Z.D. Morphological Evolution of Fe-Rich Precipitates in a Cu-2.0Fe Alloy during Isothermal Treat ment. Acta Metall. Sin. 2023, 59, 1665–1674. [Google Scholar]
- Syarif, J.; Gillette, V.; Hussien, H.A.; Badawy, K.; Jisrawi, N. Molecular dynamics simulation of the amorphization and alloying of a mechanically milled Fe-Cu system. J. Non-Cryst. Solids 2022, 580, 121410. [Google Scholar] [CrossRef]
- Chatterjee, A.; Popov, D.; Velisavljevic, N.; Misra, A. Phase Transitions of Cu and Fe at Multiscales in an Additively Manufactured Cu-Fe Alloy under High-Pressure. Nanomaterials 2022, 12, 1514. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Structural Evolution of Iron-Copper (Fe-Cu) Bimetallic Janus Nanoparticles during Solidification: An Atomistic Investigation. J. Phys. Chem. C 2020, 124, 1053–1063. [Google Scholar] [CrossRef]
- Wu, C.-D.; Liao, C.-F. Molecular dynamics simulation of the direct bonding of (111)-oriented nanotwinned Cu and its related mechanical behavior. J. Phys. Chem. Solids 2024, 187, 111872. [Google Scholar] [CrossRef]
- Su, L.; Yao, M.; Tao, H.; Yang, J.; Zhang, E.; Han, X. Tribological behavior of copper-iron particles as third body additives on copper matrix composites: Experiment link third body morphology to accommodation mechanism. Tribol. Int. 2024, 191, 109118. [Google Scholar] [CrossRef]
- Adam, O.; Jan, V.; Spotz, Z.; Cupera, J.; Pouchly, V. Ultrafine-grained Cu50(FeCo)50 immiscible alloy with excellent thermal stability. Mater. Charact. 2021, 182, 111532. [Google Scholar] [CrossRef]
- Zhang, H.C.; Wang, X.F.; Li, H.R.; Li, C.Q.; Li, Y.G. Molecular Dynamics Study on the Impact of Cu Clusters at the BCC-Fe Grain Boundary on the Tensile Properties of Crystal. Metals 2020, 10, 1533. [Google Scholar] [CrossRef]
- Hirel, P. Atomsk: A tool for manipulating and converting atomic data files. Comput. Phys. Commun. 2015, 197, 212–219. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; In’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Bonny, G.; Pasianot, R.C.; Castin, N.; Malerba, L. Ternary Fe-Cu-Ni many-body potential to model reactor pressure vessel steels: First validation by simulated thermal annealing. Philos. Mag. 2009, 89, 3531–3546. [Google Scholar] [CrossRef]
- Bonny, G.; Bakaev, A.; Terentyev, D. The combined effect of carbon and chromium enrichment on (100) loop absorption in iron. Comput. Mater. Sci. 2022, 211, 111533. [Google Scholar] [CrossRef]
- Mitsui, Y.; Onoue, M.; Kobayashi, R.; Sato, K.; Kuzuhara, S.; Ito, W.; Takahashi, K.; Koyama, K. High Magnetic Field Effects on Cu-precipitation Behavior of Fe-1mass%Cu at 773 K. ISIJ Int. 2022, 62, 413–417. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Zhang, X.X.; Ma, F.; Dong, S.S.; Yang, W.; Wu, M.H. Molecular dynamics simulation of the crystal structure evolution of titanium under different Tdamp values and heating/cooling rates. Chem. Phys. Lett. 2021, 763, 138187. [Google Scholar] [CrossRef]
- Wang, M.J.; Huang, X.X.; Wu, S.P.; Dai, G.X. Molecular dynamics simulations of tensile mechanical properties and microstructures of Al-4.5Cu alloy: The role of temperature and strain rate. Model. Simul. Mater. Sci. Eng. 2022, 30, 045004. [Google Scholar] [CrossRef]
- Jiao, Y.; Dan, W.J.; Zhang, W.G. Effects of hydrogen on the deformation mechanism of face-centred cubic Fe-C single crystal with nanovoid: A molecular dynamics simulation. J. Alloys Compd. 2021, 870, 159330. [Google Scholar] [CrossRef]
- Yu, B.Y.; Liang, Y.C.; Tian, Z.A.; Liu, R.S.; Gao, T.H.; Xie, Q.; Mo, Y.F. MD simulation on crystallization mechanisms of rapidly supercooled Fe-Ni alloys. J. Cryst. Growth 2020, 535, 125533. [Google Scholar] [CrossRef]
- Tang, J.B.; Ahmadi, A.; Alizadeh, A.; Abedinzadeh, R.; Abed, A.M.; Smaisim, G.F.; Hadrawi, S.K.; Nasajpour-Esfahani, N.; Toghraie, D. Investigation of the mechanical properties of different amorphous composites using the molecular dynamics simulation. J. Mater. Res. Technol.-JMRT 2023, 24, 1390–1400. [Google Scholar] [CrossRef]
- Xu, H.D.; Bao, H.W.; Li, Y.; Bai, H.Z.; Ma, F. Atomic scale insights into the rapid crystallization and precipitation behaviors in FeCu binary alloys. J. Alloys Compd. 2021, 882, 160725. [Google Scholar] [CrossRef]
- Celtek, M.; Domekeli, U.; Sengul, S.; Canan, C. Effects of Ag or Al addition to CuZr-based metallic alloys on glass formation and structural evolution: A molecular dynamics simulation study. Intermetallics 2021, 128, 107023. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Y.C.; Zhou, L.L.; Mo, Y.F.; Wu, R.L.; Tian, Z.A. Effect of topologically close-packed clusters on the pair distribution function of amorphous Zr under different pressures. J. Non-Cryst. Solids 2023, 612, 122354. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhao, T.X.; Yu, X.Q.; Huang, J.K. The Al-Fe Intermetallic Compounds and the Atomic Diffusion Behavior at the Interface of Aluminum-Steel Welded Joint. Metals 2023, 13, 334. [Google Scholar] [CrossRef]
- Polak, W.Z. Efficiency in identification of internal structure in simulated monoatomic clusters: Comparison between common neighbor analysis and coordination polyhedron method. Comput. Mater. Sci. 2022, 201, 110882. [Google Scholar] [CrossRef]
- Duan, L.J.; Liu, Y.C.; Duan, J.S. Calculation of radii and atom numbers of different coordination shells in cubic crystals. Mater. Today Commun. 2020, 22, 100768. [Google Scholar] [CrossRef]
- Ranaweera, S.A.; Donnadieu, B.; Henry, W.P.; White, M.G. Effects of electron-donating ability of binding sites on coordination number: The interactions of a cyclic Schiff base with copper ions. Acta Crystallogr. Sect. C Struct. Chem. 2023, 79, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Trong, D.; Nguyen-Tri, P. Factors affecting the structure, phase transition and crystallization process of AlNi nanoparticles. J. Alloys Compd. 2020, 812, 152133. [Google Scholar] [CrossRef]
- Yang, Z.M.; Qiu, N.; Yang, H.M.; Wang, Y. Vacancies-mediated atomic diffusion controlled by the interfacial structure of FCC/BCC HEA multilayers. J. Alloys Compd. 2023, 947, 169512. [Google Scholar] [CrossRef]
- Simonnin, P.; Schreiber, D.K.; Uberuaga, B.P.; Rosso, K.M. Atomic diffusion, segregation, and grain boundary migration in nickel-based alloys from molecular dynamics simulations. Mater. Today Commun. 2023, 35, 105768. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Cheng, W.; Meng, A.; Zhang, S.; Wang, P. Tissue evolution of Al0.67Cu0.33 alloy during melting and solidification by molecular dynamics simulation. Chem. Phys. 2023, 575, 112049. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Pu, Z.; Li, Y.; Xu, B.Q.; Yang, B. Ab Initio Molecular Dynamic Simulation of Zn-Al-Fe ALLOYS. J. Min. Metall. Sect. B—Metall. 2019, 55, 79–84. [Google Scholar] [CrossRef]
- Wang, L.P.; Yang, W.; Ma, Z.B.; Zhu, J.H.; Li, Y.T. First-principles study of chromium diffusion in the ferritic Fe-Cr alloy. Comput. Mater. Sci. 2020, 181, 109733. [Google Scholar] [CrossRef]
- Syarif, J.; Badawy, K.; Hussien, H.A. Atomistic simulation of the diffusion behavior in Al-Fe. Nucl. Mater. Energy 2021, 29, 101073. [Google Scholar] [CrossRef]
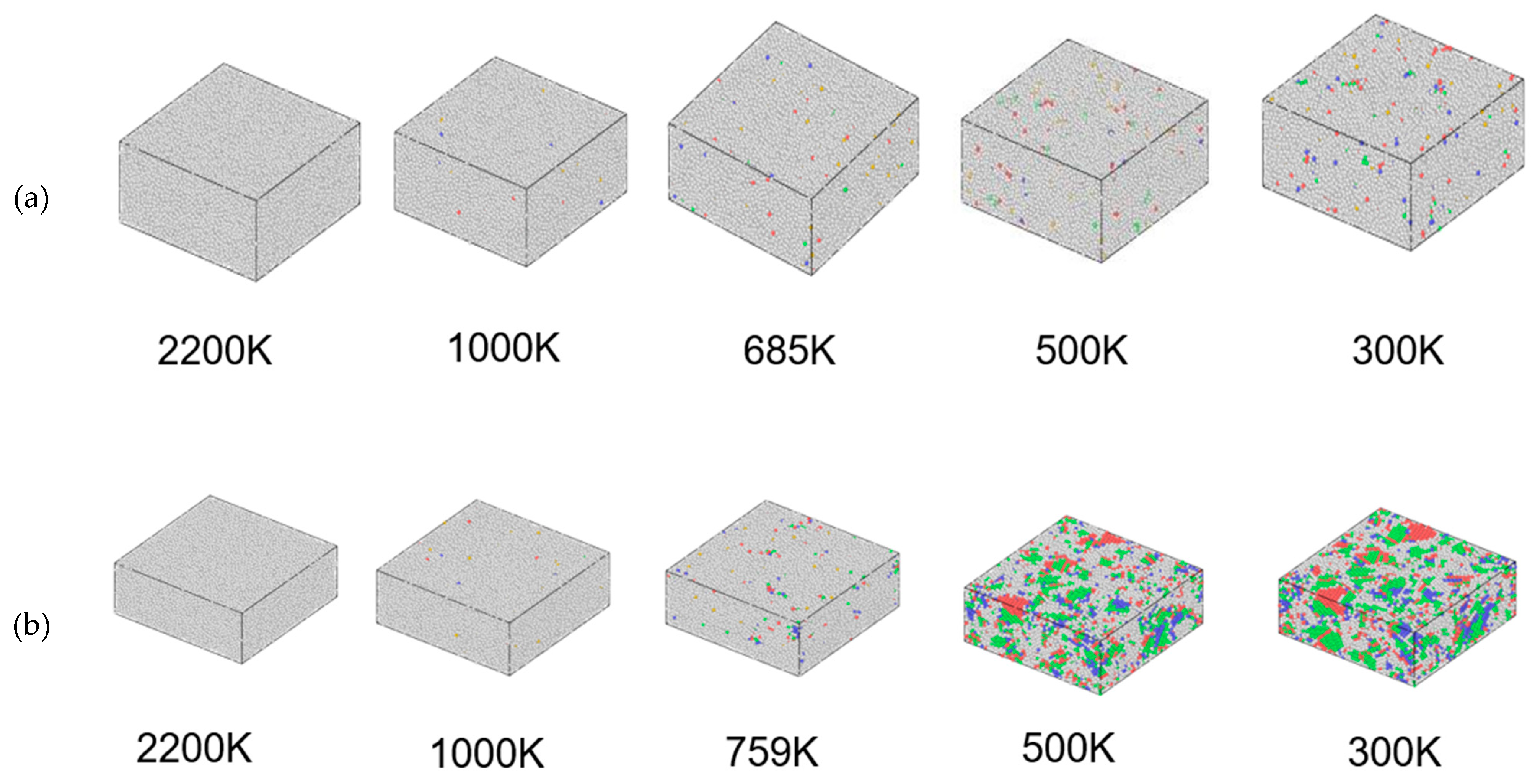
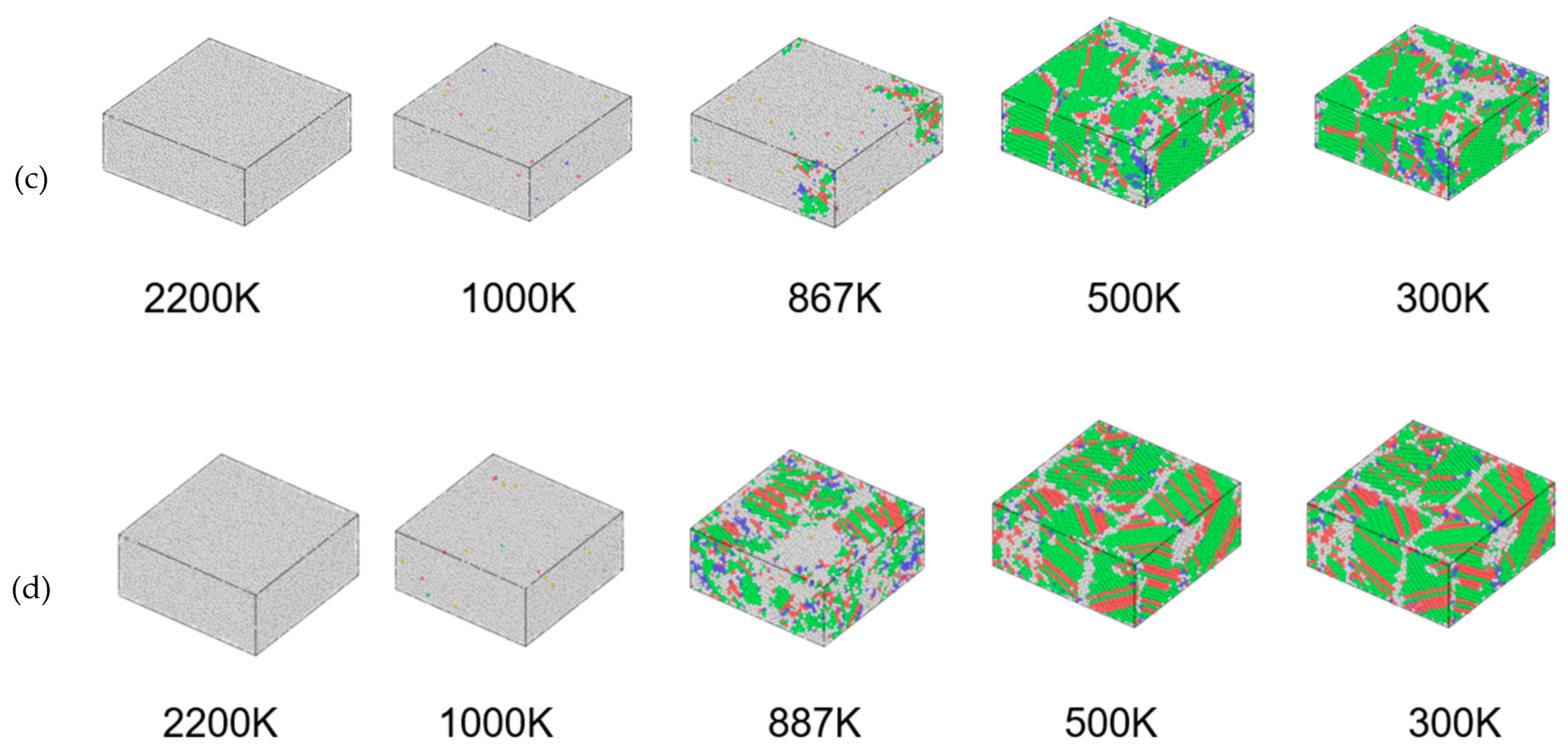


| Temperature | 2200 K | 1000 K | Phase Transition Temperature | 500 K | 300 K | |
|---|---|---|---|---|---|---|
| Cooling Rates | ||||||
| 1 × 1013 K/S | 0 | 0.5% | 1.5% | 3.3% | 4.8% | |
| 2 × 1012 K/S | 0 | 0.5% | 3.4% | 43.1% | 54.1% | |
| 2 × 1011 K/S | 0 | 0.5% | 6.4% | 75.5% | 78.9% | |
| 2 × 1010 K/S | 0 | 0.5% | 20.6% | 79.7% | 82% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Gao, X.; Lai, Z.; Han, Z.; Li, Y. Molecular Dynamics Research on Fe Precipitation Behavior of Cu95Fe5 Alloys during Rapid Cooling. Metals 2024, 14, 228. https://doi.org/10.3390/met14020228
Wang X, Gao X, Lai Z, Han Z, Li Y. Molecular Dynamics Research on Fe Precipitation Behavior of Cu95Fe5 Alloys during Rapid Cooling. Metals. 2024; 14(2):228. https://doi.org/10.3390/met14020228
Chicago/Turabian StyleWang, Xufeng, Xufeng Gao, Zhibo Lai, Zongen Han, and Yungang Li. 2024. "Molecular Dynamics Research on Fe Precipitation Behavior of Cu95Fe5 Alloys during Rapid Cooling" Metals 14, no. 2: 228. https://doi.org/10.3390/met14020228
APA StyleWang, X., Gao, X., Lai, Z., Han, Z., & Li, Y. (2024). Molecular Dynamics Research on Fe Precipitation Behavior of Cu95Fe5 Alloys during Rapid Cooling. Metals, 14(2), 228. https://doi.org/10.3390/met14020228





