Effect of Al2O3 on Microstructure and Corrosion Characteristics of Al/Al2O3 Composite Coatings Prepared by Cold Spraying
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Test Methods
3. Results
3.1. XRD Analysis
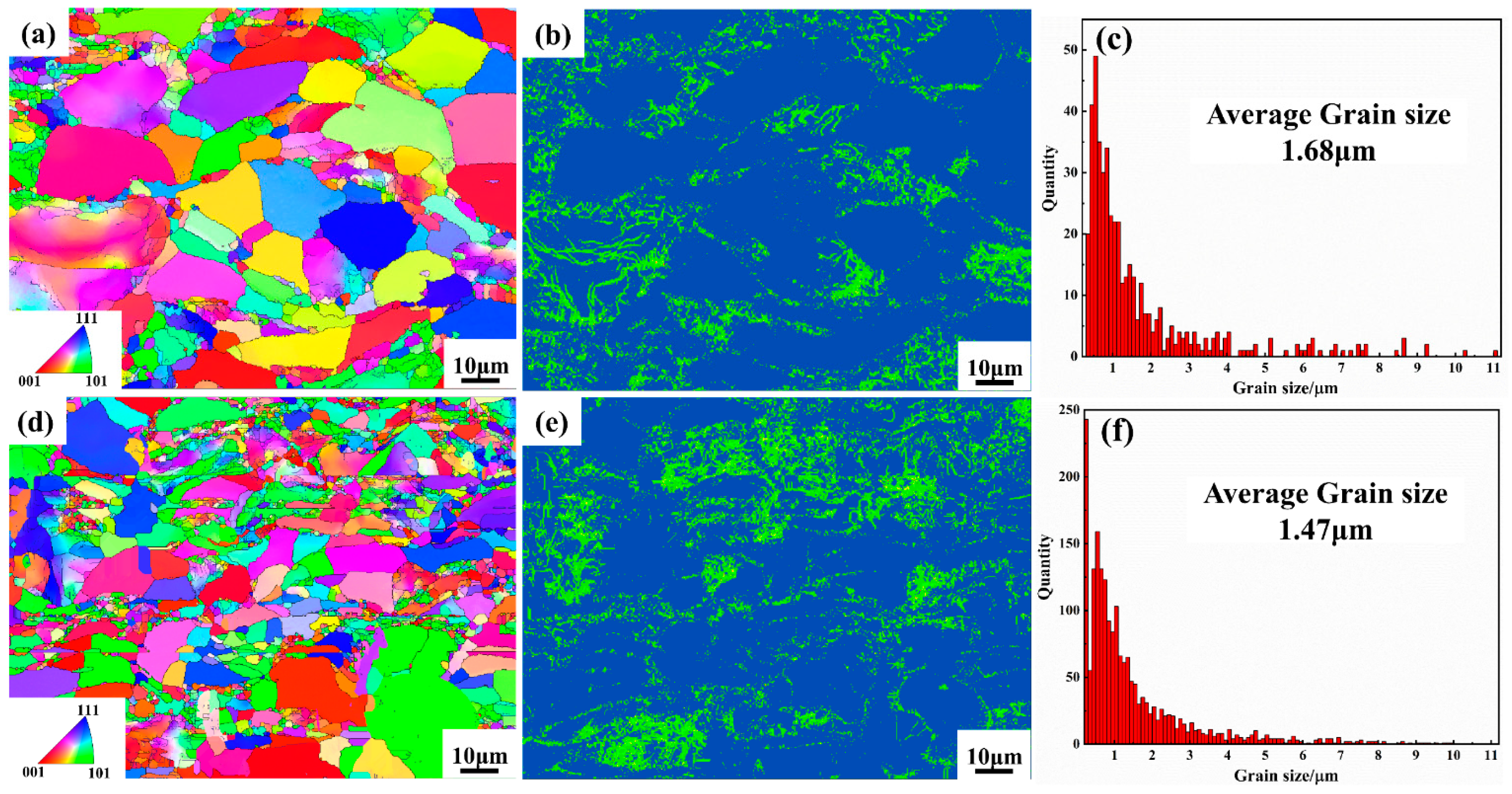
3.2. Surface Morphology of the Coating
3.3. Coating Hardness and Roughness
3.4. Corrosion Experiment
3.4.1. Coating Electrochemical Corrosion Behavior
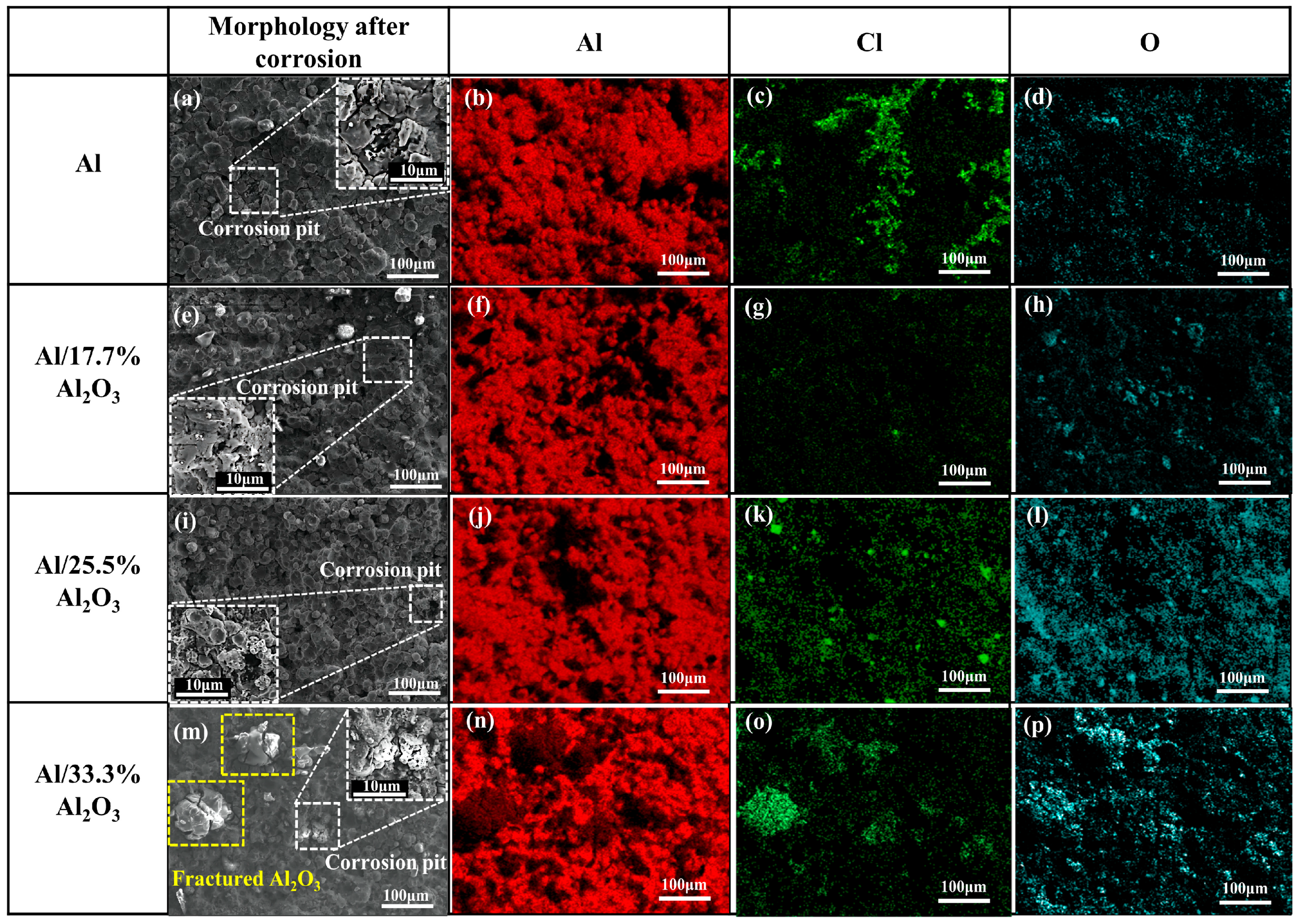
3.4.2. Surface Morphology after Electrochemical Corrosion
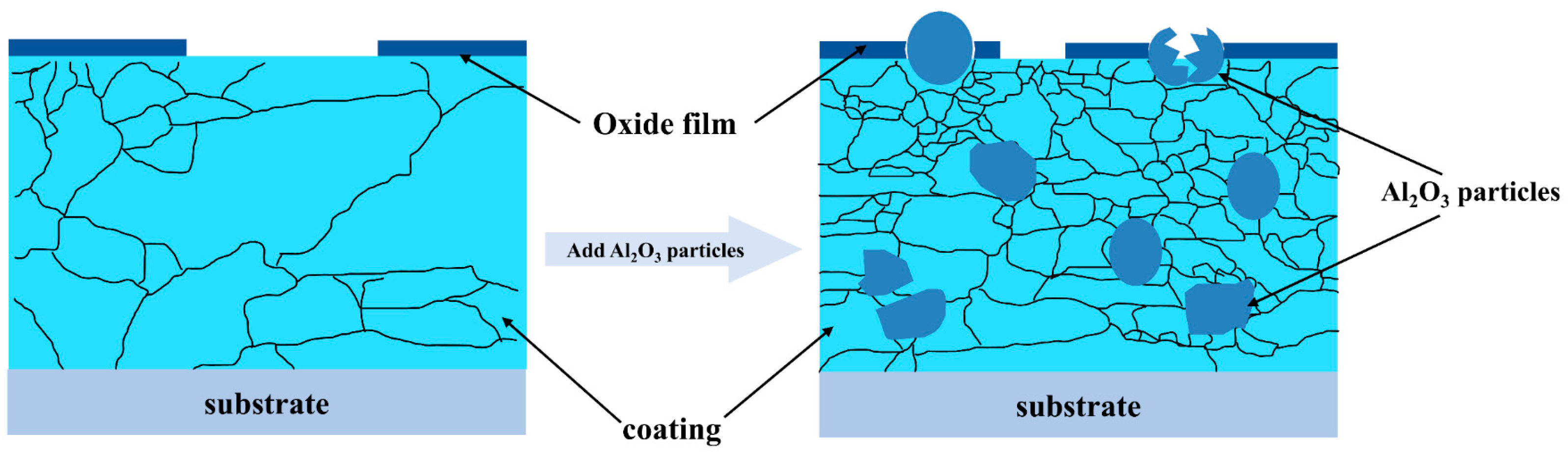
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trueba, M.; Trasatti, S.P. Study of Al alloy corrosion in neutral NaCl by the pitting scan technique. Mater. Chem. Phys. 2010, 121, 523–533. [Google Scholar] [CrossRef]
- Diab, M.; Pang, X.; Jahed, H. The effect of pure aluminum cold spray coating on corrosion and corrosion fatigue of magnesium (3% Al-1% Zn) extrusion. Surf. Coat. Technol. 2017, 309, 423–435. [Google Scholar] [CrossRef]
- Fernandez, R.; Jodoin, B. Cold Spray Aluminum-Alumina Cermet Coatings: Effect of Alumina Content. J. Therm. Spray Technol. 2018, 27, 603–623. [Google Scholar] [CrossRef]
- Bu, H.Y.; Yandouzi, M.; Lu, C.; MacDonald, D.; Jodoin, B. Cold spray blended Al+Mg17Al12 coating for corrosion protection of AZ91D magnesium alloy. Surf. Coat. Technol. 2012, 207, 155–162. [Google Scholar] [CrossRef]
- Liu, H.H.; Tariq, N.U.; Ren, Y.P.; Zhao, L.J.; Yang, Y.; Cui, X.Y.; Wang, J.Q.; Xiong, T.Y. Influence of Al2O3 content on microstructure, electrical conductivity and adhesion strength of cold sprayed Al-Al2O3 coatings on PEEK substrate. Surf. Coat. Technol. 2022, 446, 128752. [Google Scholar] [CrossRef]
- Li, Q.L.; Yuan, S.M.; Li, Z.; Gao, X.X.; Chen, B.C. Mechanical response and microstructure evolution of SiC particle-reinforced Al-MMCs under ultrasonic loading. Compos. Part A Appl. Sci. Manuf. 2023, 173, 107657. [Google Scholar] [CrossRef]
- Zhao, L.J.; Tariq, N.U.; Ren, Y.P.; Liu, H.H.; Han, R.F.; Cui, X.Y.; Wang, J.Q.; Xiong, T.Y. Effect of Particle Size on Ceramic Particle Content in Cold Sprayed Al-Based Metal Matrix Composite Coating. J. Therm. Spray Technol. 2022, 31, 2505–2516. [Google Scholar] [CrossRef]
- Liu, H.J.; Fu, M.K.; Pang, S.Z.; Zhu, H.Q.; Zhang, C.; Ming, L.J.; Liu, X.Y.; Ding, M.H.; Fu, Y.D. Effect of Ball-Milled Feedstock Powder on Microstructure and Mechanical Properties of Cu-Ni-Al-Al2O3 Composite Coatings by Cold Spraying. Coatings 2023, 13, 948. [Google Scholar] [CrossRef]
- Witharamage, C.S.; Alrizqi, M.A.; Chirstudasjustus, J.; Darwish, A.A.; Ansell, T.; Nieto, A.; Gupta, R.K. Corrosion-resistant metallic coatings for aluminum alloys by cold spray. Corros. Sci. 2022, 209, 110720. [Google Scholar] [CrossRef]
- Sayahlatifi, S.; Zaiemyekeh, Z.; Shao, C.W.; McDonald, A.; Hogan, J.D. Micromechanical damage analysis of Al-Al2O3 composites via cold-spray additive manufacturing. Int. J. Mech. Sci. 2023, 259, 108573. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Chu, X.; Zhang, T.; Zheng, J. Corrosion Behavior and Microstructure of Cu-Based Composite Coatings Deposited by Cold Spraying. Metals 2022, 12, 955. [Google Scholar] [CrossRef]
- Aubanel, L.; Delloro, F. Tribological behavior of steel-based composite coatings produced by cold spray. Surf. Coat. Technol. 2023, 470, 129815. [Google Scholar] [CrossRef]
- Bakar, I.A.A.; Omar, N.I.; Yusuf, Y.; Rahim, T.A. Reflection and Future Perspectives in Cold Spray Technology: A Bibliometric Analysis. J. Therm. Spray Technol. 2023, 32, 1576–1595. [Google Scholar] [CrossRef]
- Huang, C.J.; List, A.; Wiehler, L.; Schulze, M.; Gärtner, F.; Klassen, T. Cold spray deposition of graded Al-SiC composites. Addit. Manuf. 2022, 59, 17. [Google Scholar] [CrossRef]
- Ilyushchanka, A.P.; Baray, S.G.; Letsko, A.I.; Talako, T.L.; Manoila, Y.D.; Janu, Y.; Chauhan, V.S.; Patra, M.K.; Saini, L. High-Temperature-Resistant, Mechanically Stable FeCrNiAl/Al2O3 Thermally Sprayed Thick Ceramic Coatings for Stealth Applications over X-Band. Adv. Eng. Mater. 2022, 24, 103116. [Google Scholar] [CrossRef]
- Gül, H.; Kiliç, F.; Aslan, S.; Alp, A.; Akbulut, H. Characteristics of electro-co-deposited Ni-Al2O3 nano-particle reinforced metal matrix composite (MMC) coatings. Wear 2009, 267, 976–990. [Google Scholar] [CrossRef]
- Jue, J.B.; Gu, D.D.; Chang, K.; Dai, D.H. Microstructure evolution and mechanical properties of Al-Al2O3 composites fabricated by selective laser melting. Powder Technol. 2017, 310, 80–91. [Google Scholar] [CrossRef]
- Konstantiniuk, F.; Tkadletz, M.; Czettl, C.; Schalk, N. Fracture Properties of α– and ĸ–Al2O3 Hard Coatings Deposited by Chemical Vapor Deposition. Coatings 2021, 11, 1359. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Normand, B.; Mary, N.; Yu, M.; Liao, H.L. Effects of ceramic particle size on microstructure and the corrosion behavior of cold sprayed SiC P/Al5056 composite coatings. Surf. Coat. Technol. 2017, 315, 314–325. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Yu, S.; Xu, Y.; Hu, K.; Zhao, Y. Electrochemical characterization and microstructure of cold sprayed AA5083/Al2O3 composite coatings. J. Mater. Sci. Technol. 2020, 59, 117–128. [Google Scholar] [CrossRef]
- Xie, X.L.; Hosni, B.; Chen, C.Y.; Wu, H.J.; Li, Y.L.; Chen, Z.; Verdy, C.; Kedim, O.E.I.; Zhong, Q.D.; Addad, A.; et al. Corrosion behavior of cold sprayed 7075Al composite coating reinforced with TiB2 nanoparticles. Surf. Coat. Technol. 2020, 404, 126460. [Google Scholar] [CrossRef]
- Qiu, X.; Tariq, N.U.; Qi, L.; Tang, J.R.; Cui, X.Y.; Du, H.; Wang, J.Q.; Xiong, T.Y. Effects of Dissimilar Alumina Particulates on Microstructure and Properties of Cold-Sprayed Alumina/A380 Composite Coatings. Acta Metall. Sin. Engl. Lett. 2019, 32, 1449–1458. [Google Scholar] [CrossRef]
- Wang, Q.; Birbilis, N.; Huang, H.; Zhang, M.X. Microstructure characterization and nanomechanics of cold-sprayed pure Al and Al-Al2O3 composite coatings. Surf. Coat. Technol. 2013, 232, 216–223. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Liu, F.C.; Han, E.H.; Xu, L. Mechanical and corrosion properties in 3.5% NaCl solution of cold sprayed Al-based coatings. Surf. Coat. Technol. 2020, 385, 125372. [Google Scholar] [CrossRef]
- Qiu, X.; Tariq, N.U.; Wang, J.Q.; Tang, J.R.; Gyansah, L.; Zhao, Z.P.; Xiong, T.Y. Microstructure, microhardness and tribological behavior of Al2O3 reinforced A380 aluminum alloy composite coatings prepared by cold spray technique. Surf. Coat. Technol. 2018, 350, 391–400. [Google Scholar] [CrossRef]
- Lu, F.F.; Ma, K.; Li, C.X.; Yasir, M.; Luo, X.T.; Li, C.J. Enhanced corrosion resistance of cold-sprayed and shot-peened aluminum coatings on LA43M magnesium alloy. Surf. Coat. Technol. 2020, 394, 125865. [Google Scholar] [CrossRef]
- Liu, F.; Han, E.H.; Xu, L.; Uzoma, P.C. Effects of Al2O3 on the microstructures and corrosion behavior of low-pressure cold gas sprayed Al 2024-Al2O3 composite coatings on AA 2024-T3 substrate. Surf. Coat. Technol. 2019, 9, 735–742. [Google Scholar]
- Mansfeld, F.; Jeanjaquet, S.L.; Kendig, M.W. An electrochemical impedance spectroscopy study of reactions at the metal/coating interface. Corros. Sci. 1986, 26, 735–742. [Google Scholar] [CrossRef]
- da Silva, F.S.; Bedoya, J.; Dosta, S.; Cinca, N.; Cano, I.G.; Guilemany, J.M.; Benedetti, A.V. Corrosion characteristics of cold gas spray coatings of reinforced aluminum deposited onto carbon steel. Corros. Sci. 2017, 114, 57–71. [Google Scholar] [CrossRef]
- Conde, A.; de Damborenea, J.J. Electrochemical impedance spectroscopy for studying the degradation of enamel coatings. Corros. Sci. 2002, 44, 1555–1567. [Google Scholar] [CrossRef]
- Chen, H.X.; Kong, D.J. Comparison on electrochemical corrosion performances of arc and laser thermal sprayed Al–Ti–Ni coatings in marine environment. Mater. Chem. Phys. 2020, 251, 123200. [Google Scholar]
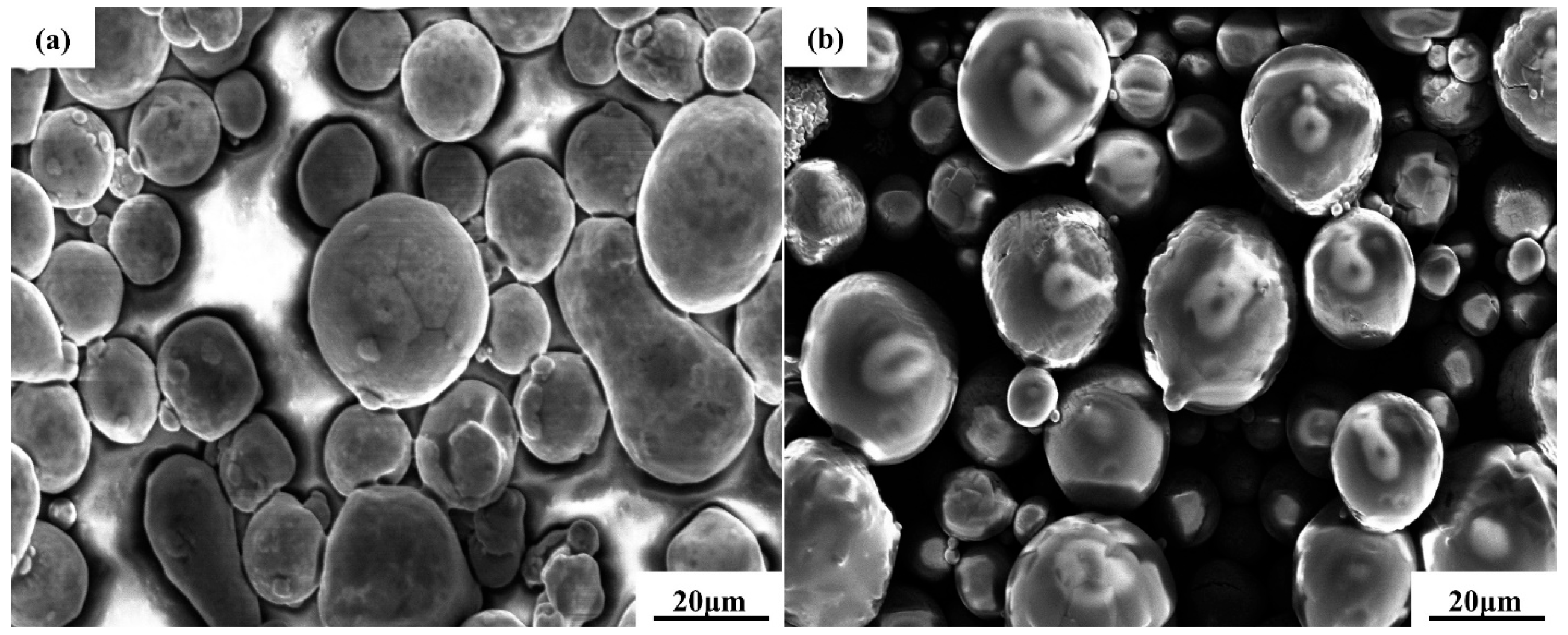

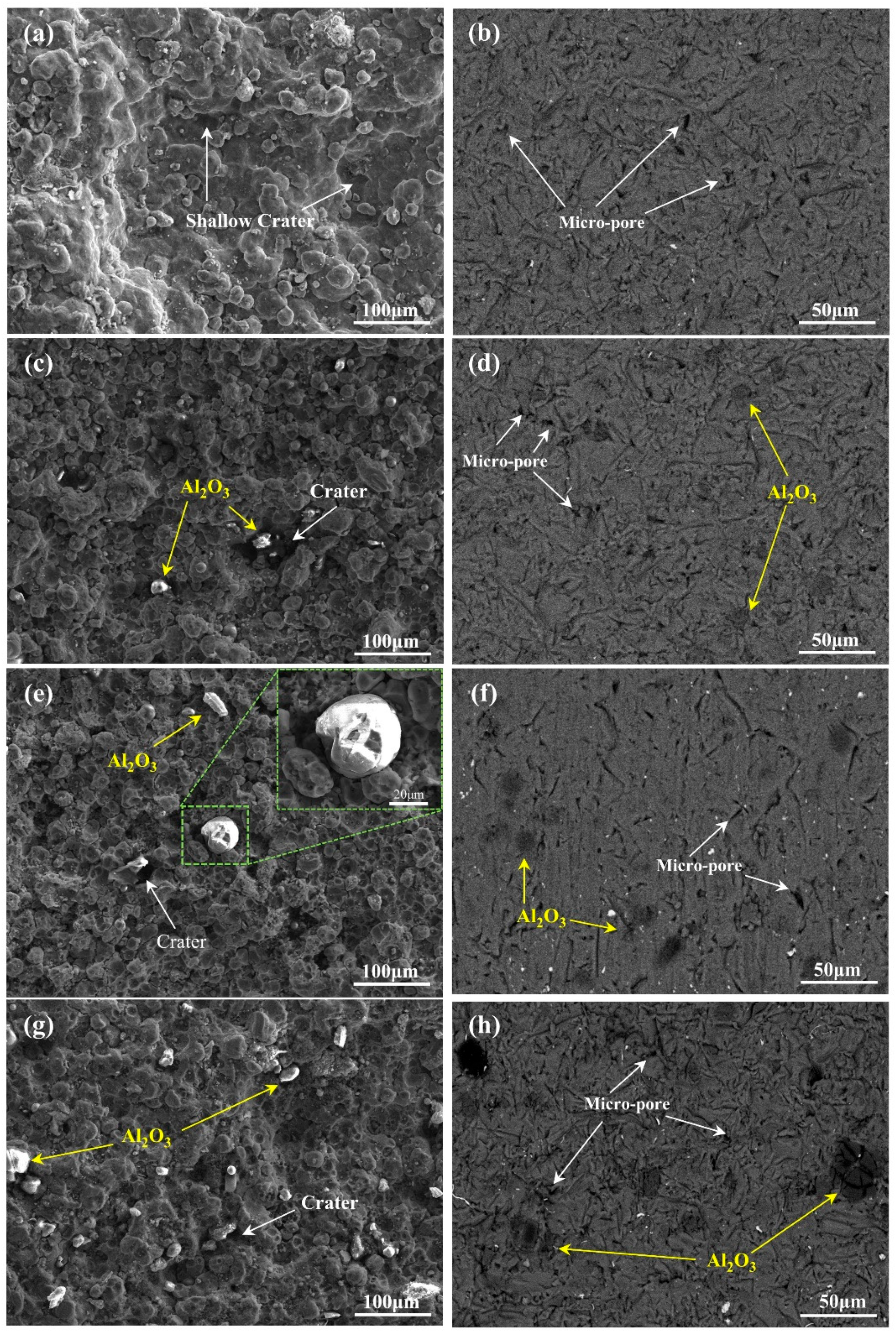
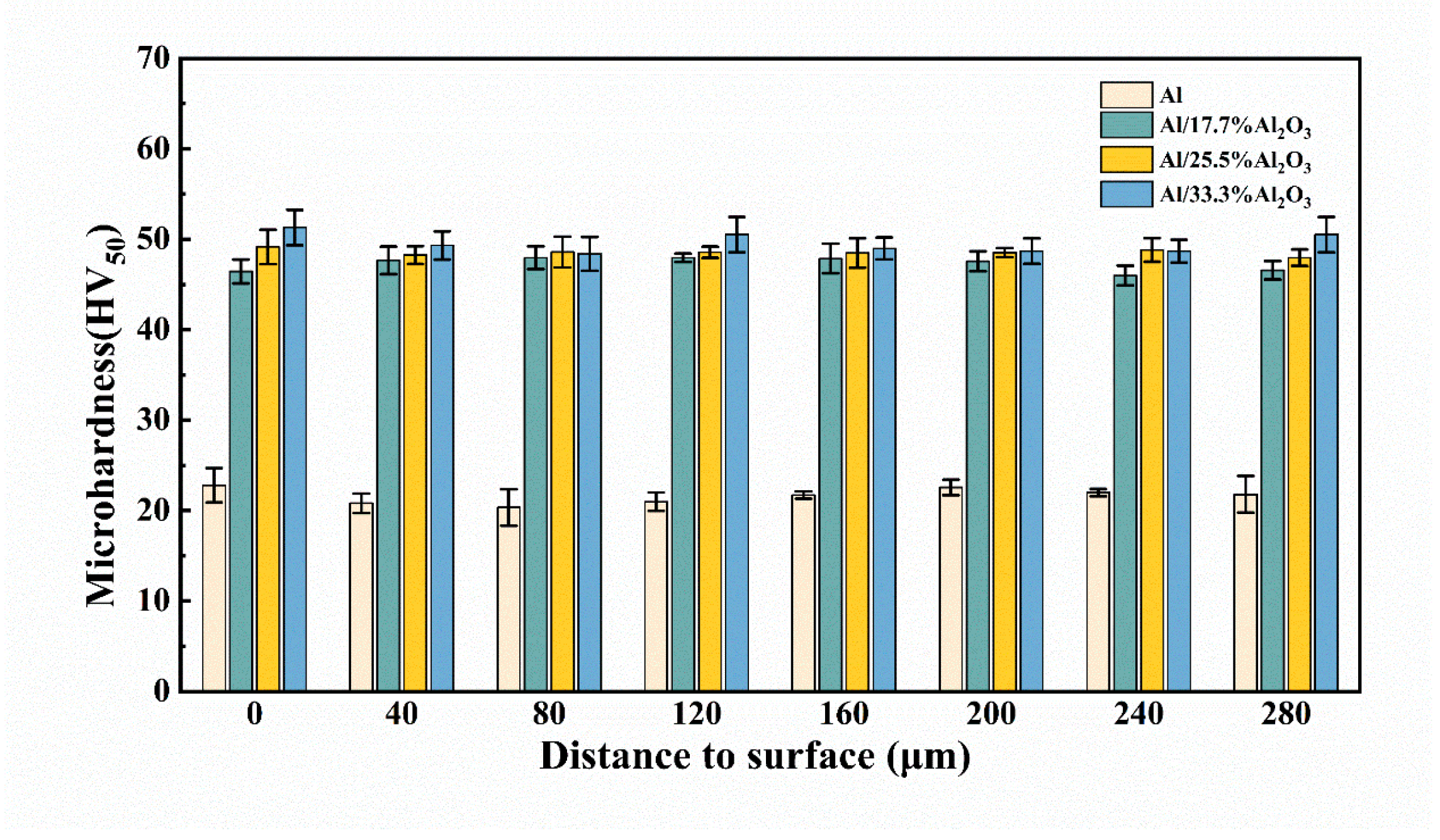
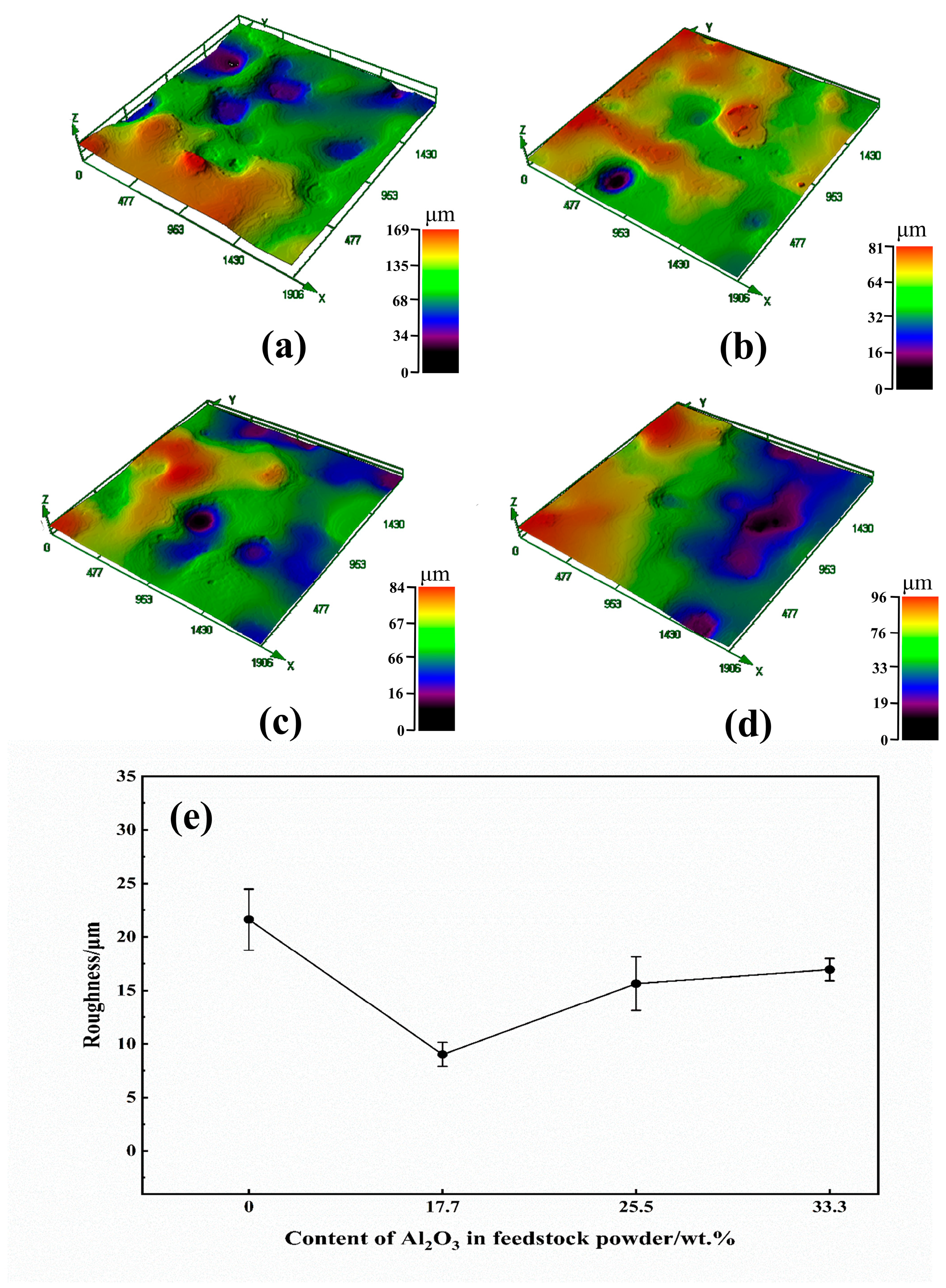

| C | Mn | Si | Cr | Ni | Cu |
|---|---|---|---|---|---|
| 0.42–0.50% | 0.50–0.80% | 0.17–0.37% | ≤0.25% | ≤0.30% | ≤0.25% |
| Specimen | Al | Al/17.7% Al2O3 | Al/25.5% Al2O3 | Al/33.3% Al2O3 |
|---|---|---|---|---|
| Porosity (%) | 6.34 ± 0.08 | 2.07 ± 0.12 | 3.39 ± 0.07 | 3.59 ± 0.10 |
| Specimen | Ecorr (V) | Icorr (A/cm2) | Rp (Ω·cm2) | Rs (kΩ·cm2) | RI (kΩ·cm2) | Qc (μF·cm−2) | nc | Rct (kΩ·cm2) | Qdl (μF·cm−2) | ndl |
|---|---|---|---|---|---|---|---|---|---|---|
| Al | −1.57 | 1.09 × 10−3 | 139.4 | 1.72 × 10−2 | 0.071 | 229.7 | 0.71 | 2.20 | 596.9 | 0.62 |
| Al/17.7%Al2O3 | −1.14 | 2.67 × 10−6 | 21,447.7 | 1.58 × 10−2 | 7.82 | 35.1 | 0.87 | 3.41 | 487.4 | 0.92 |
| Al/25.5%Al2O3 | −1.26 | 2.12 × 10−5 | 2579.6 | 1.82 × 10−2 | 2.04 | 50.4 | 0.70 | 2.91 | 25.9 | 0.98 |
| Al/33.3%Al2O3 | −1.22 | 1.54 × 10−5 | 6967.8 | 1.84 × 10−2 | 3.49 | 45.6 | 0.83 | 3.01 | 164.2 | 0.61 |
| Specimen | Al (wt. %) | Cl (wt. %) | O (wt. %) |
|---|---|---|---|
| Al | 79.22 | 2.66 | 18.12 |
| Al/17.7%Al2O3 | 80.26 | 0.40 | 19.33 |
| Al/25.5%Al2O3 | 73.01 | 1.18 | 25.82 |
| Al/33.3%Al2O3 | 69.08 | 1.86 | 37.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Shen, X.; Wang, Z.; Liu, Y.; Zhang, X.; Wang, E.; Zhang, J. Effect of Al2O3 on Microstructure and Corrosion Characteristics of Al/Al2O3 Composite Coatings Prepared by Cold Spraying. Metals 2024, 14, 179. https://doi.org/10.3390/met14020179
Jiang W, Shen X, Wang Z, Liu Y, Zhang X, Wang E, Zhang J. Effect of Al2O3 on Microstructure and Corrosion Characteristics of Al/Al2O3 Composite Coatings Prepared by Cold Spraying. Metals. 2024; 14(2):179. https://doi.org/10.3390/met14020179
Chicago/Turabian StyleJiang, Wei, Xin Shen, Zhiyuan Wang, Yang Liu, Xiaohua Zhang, Enhao Wang, and Junxin Zhang. 2024. "Effect of Al2O3 on Microstructure and Corrosion Characteristics of Al/Al2O3 Composite Coatings Prepared by Cold Spraying" Metals 14, no. 2: 179. https://doi.org/10.3390/met14020179
APA StyleJiang, W., Shen, X., Wang, Z., Liu, Y., Zhang, X., Wang, E., & Zhang, J. (2024). Effect of Al2O3 on Microstructure and Corrosion Characteristics of Al/Al2O3 Composite Coatings Prepared by Cold Spraying. Metals, 14(2), 179. https://doi.org/10.3390/met14020179





