Abstract
In high-speed steel, carbides are essential phase constituents, which have a direct impact on engineering performance and qualities of high-speed steel. The formation, morphology, and distribution of carbides are dictated by alloying elements. In this paper, various types of carbides in high-speed steel are presented. The effects of different alloying elements such as C, W, Mo, Cr, and V on the formation of carbides in high-speed steel are discussed. Research progresses on carbide improvement by microalloying elements such as N, B, Mg, and rare earth (RE) elements are reviewed. It is reported that Cr promotes the precipitation of M2C, N enhances the formation of fibrous M2C, Mg effectively shatters the large-size carbide grid, Nb refines granular carbide MC, and rare earth elements encourage the formation of M6C, resulting in irregular M2C lamellae. The incorporation of microalloying elements improves the distribution and size of carbides and also refines the solidification structure of high-speed steel.
1. Introduction
F. W. Taylor and M. White invented high-speed steel in 1898 [1,2,3]. High-speed steels are classified into three series based on their alloy compositions: W-series high-speed steel, Mo-series high-speed steel, and W-Mo-series high-speed steel. Vanadium (V) high-speed steel and cobalt (Co) high-speed steel are created by combining vanadium and cobalt. In recent years, with the continuous development of high-speed steel, boron series high-speed steel has also been developed. In general, the engineering performance of W-Mo-series high-speed steel is better than those of W-series and Mo-series high-speed steels. The most extensively used W-Mo-series high-speed steel is M2 (W6Mo5Cr4V2).
The mechanical properties of high-speed steel mainly depend on the chemical composition and microstructure. Due to the harsh service environment of high-speed steel, the wear resistance, hardness, and heat resistance of high-speed steel are highly required. Therefore, it is essential to improve the quality and engineering performance of high-speed steel and establish the matching relationship between carbides as an important phase component and alloying elements. The type, morphology, distribution, and microstructural characteristics of carbides in high-speed steel are influenced by alloying elements. As a result, carbides have been the focus of high-speed steel research for more than a century, dating back to the first introduction of the material due to their impact on the engineering performance of high-speed steel [4,5,6,7,8,9,10,11].
This article gives an overview in terms of classification and microstructural characteristics of high-speed steel. The different types of carbides in high-speed steel are presented. The effects of alloying (C, W, Mo, Cr, and V) and microalloying (N, B, Mg, and RE) elements on the carbide formation and mechanical properties of high-speed steel are discussed, and topics for future studies on high-speed steel are proposed.
2. Types of Carbides in High-Speed Steel
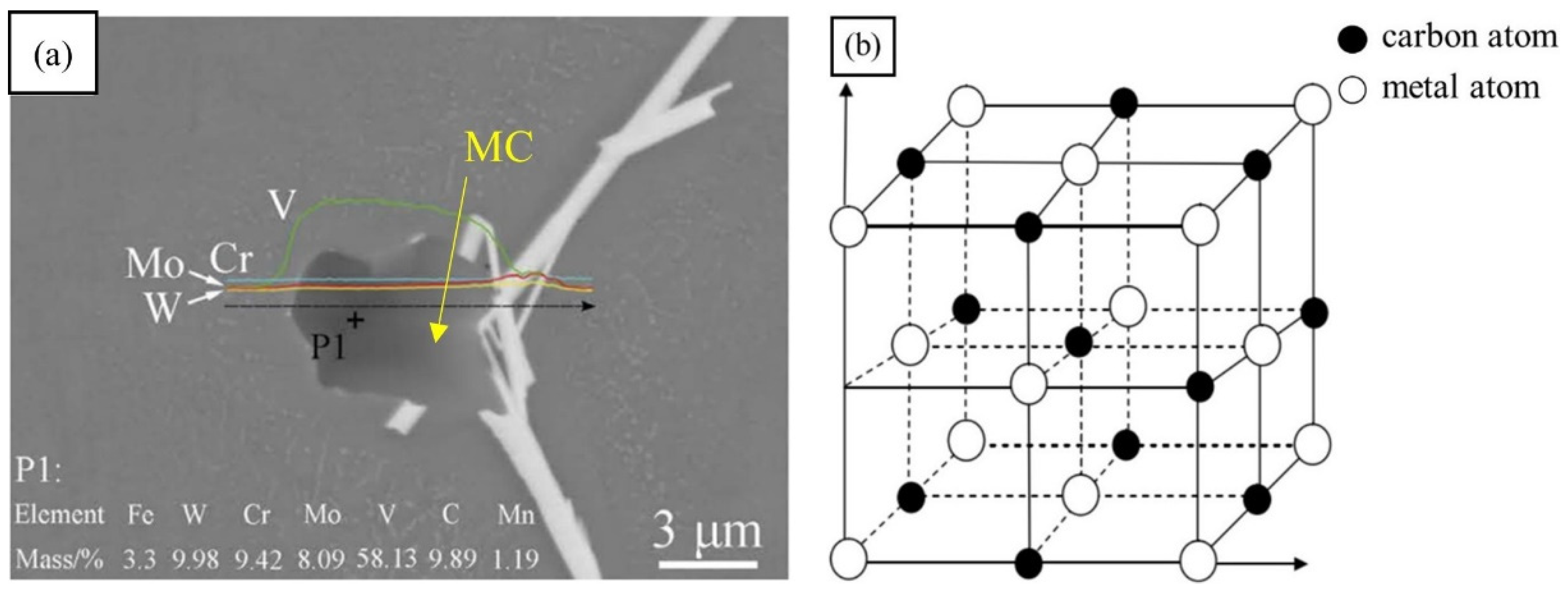
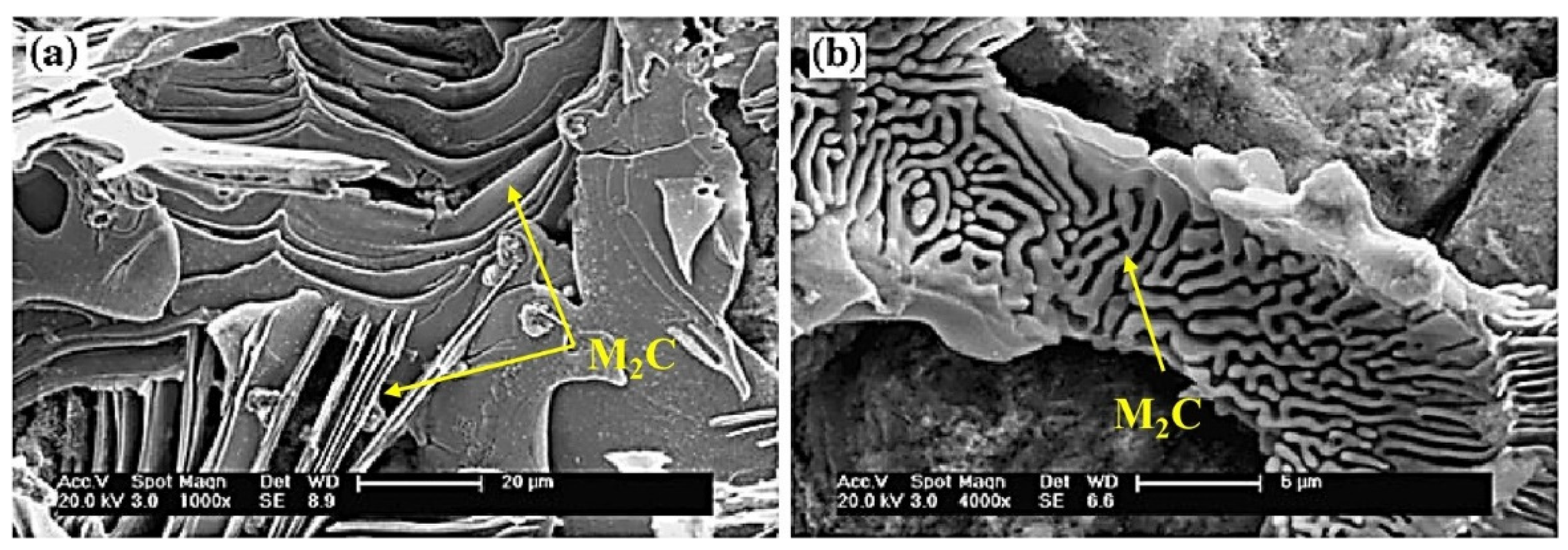
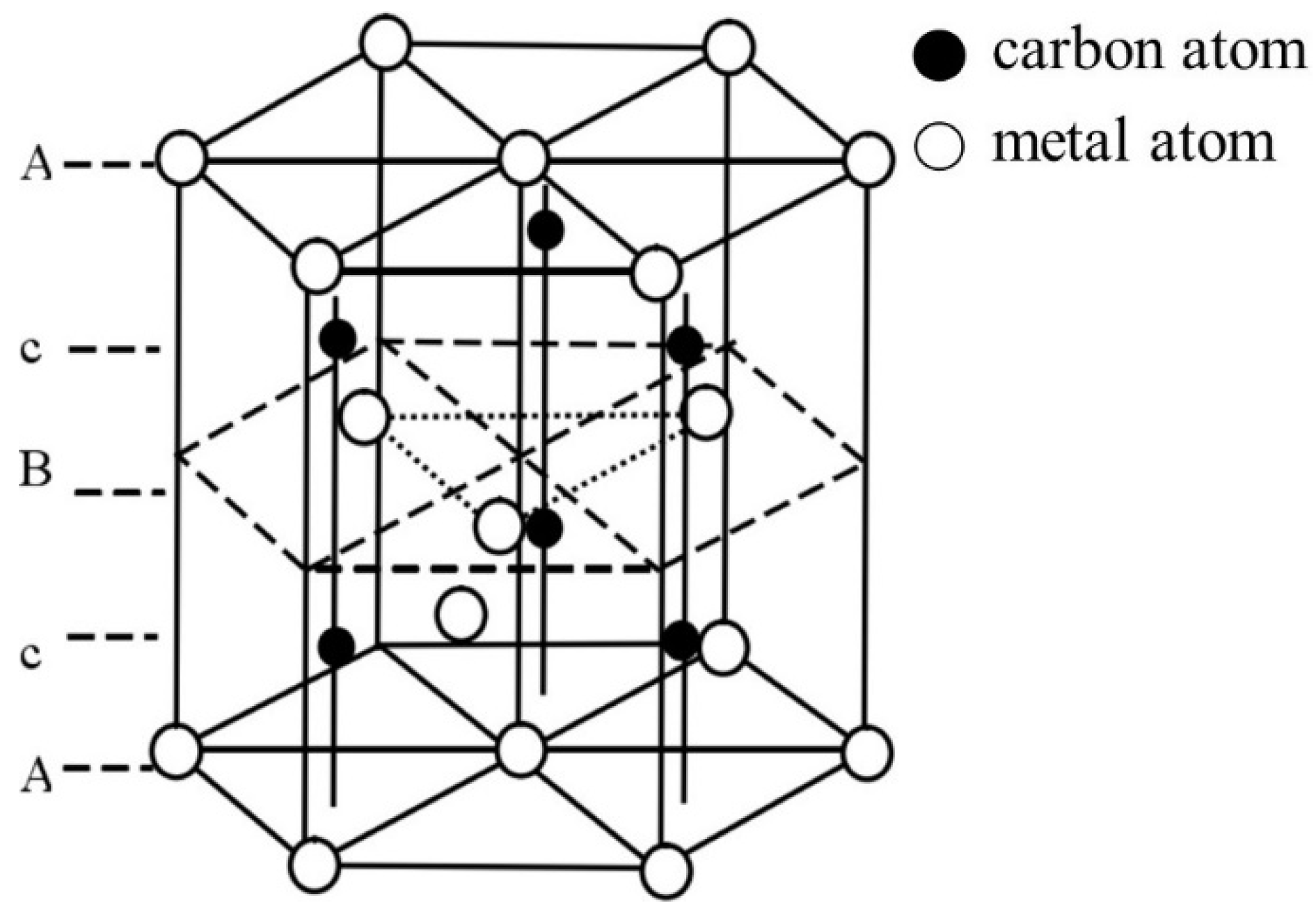
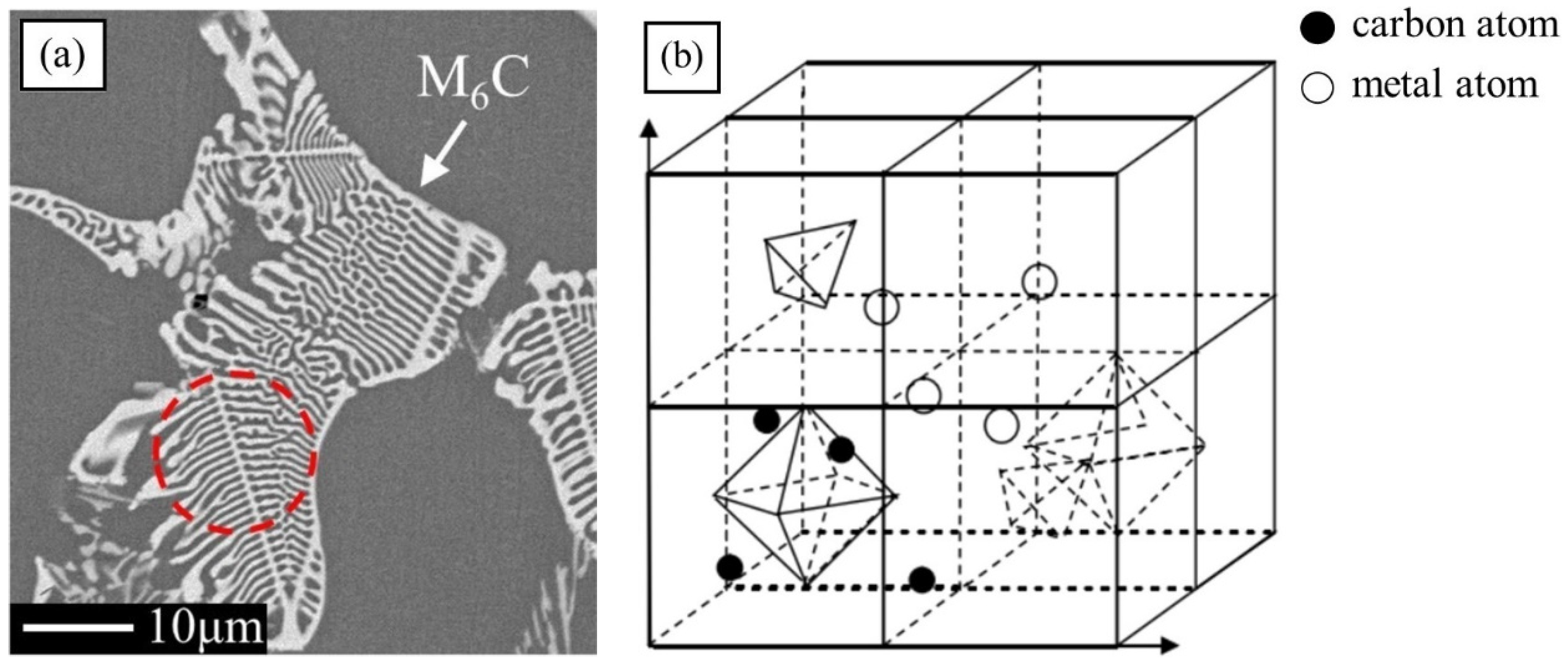
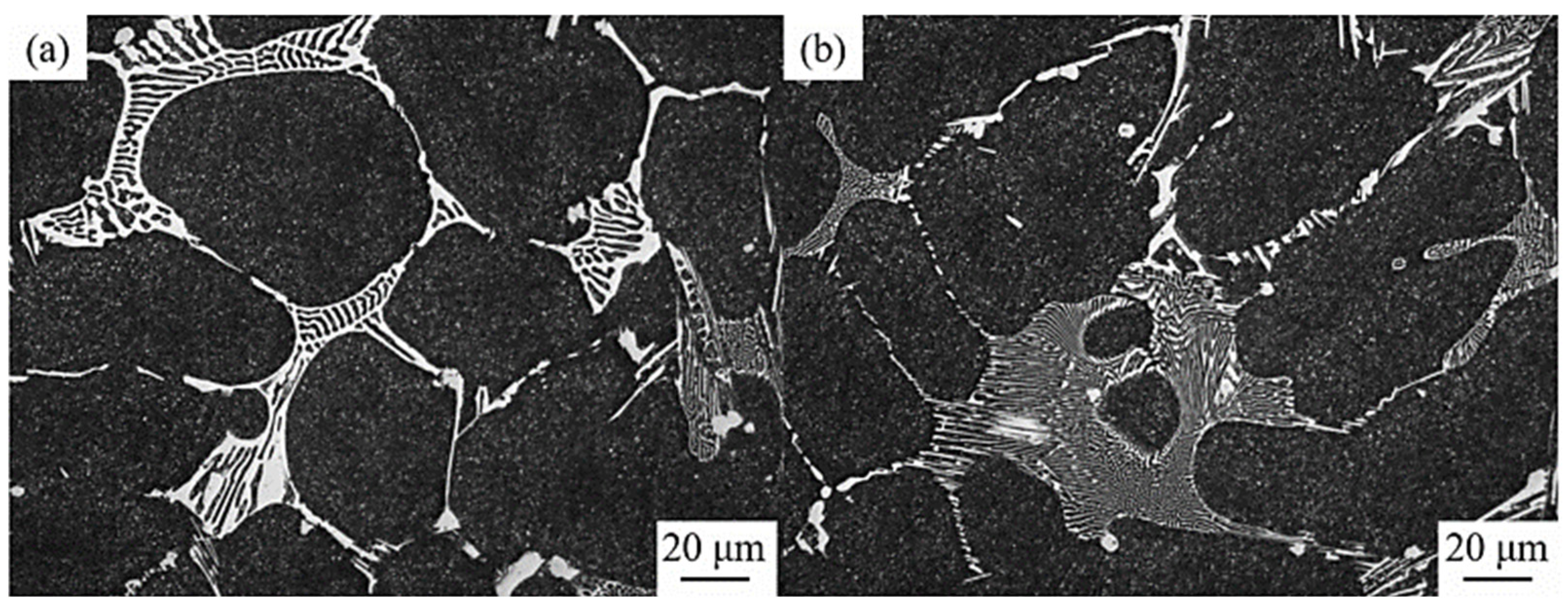
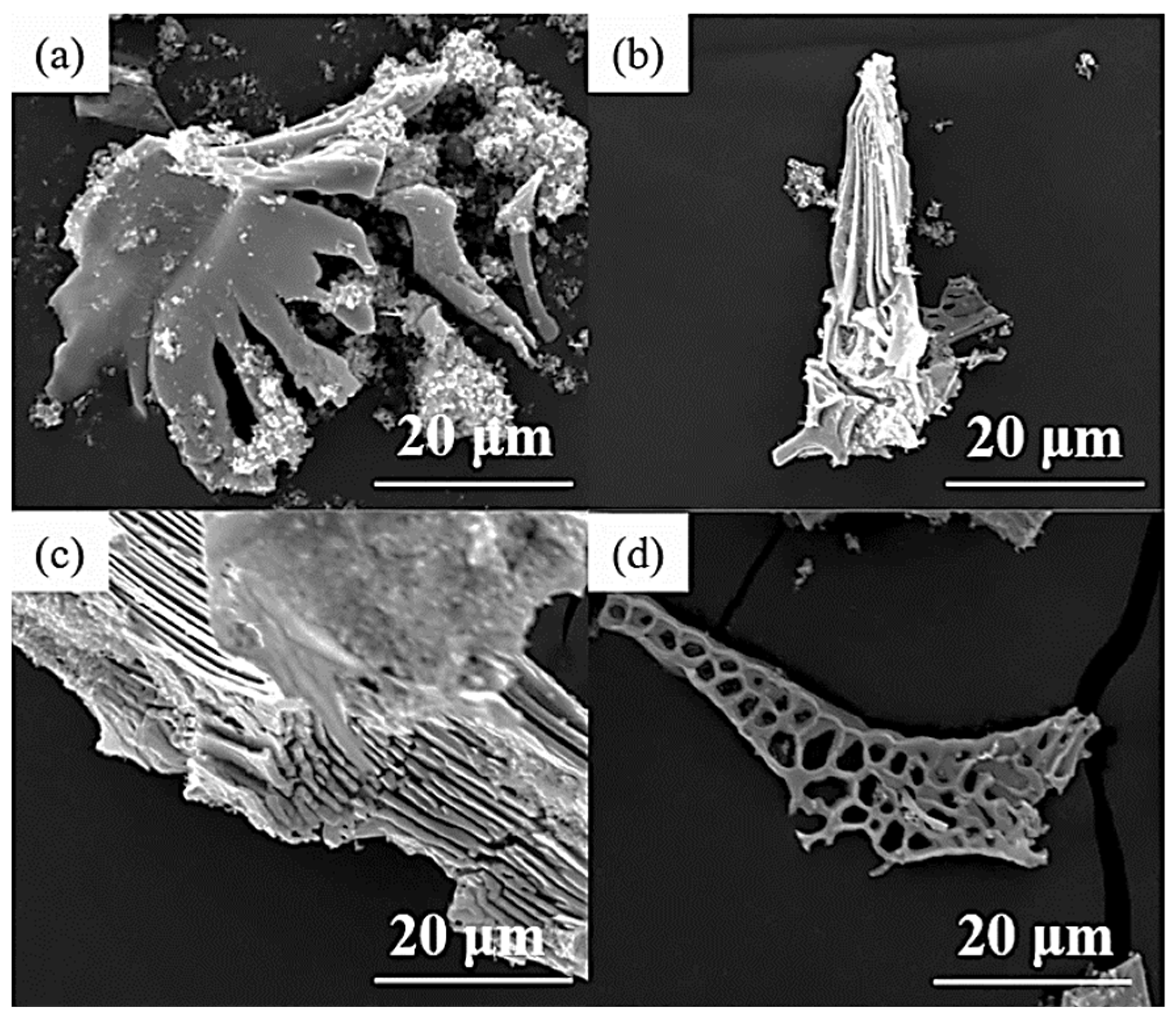
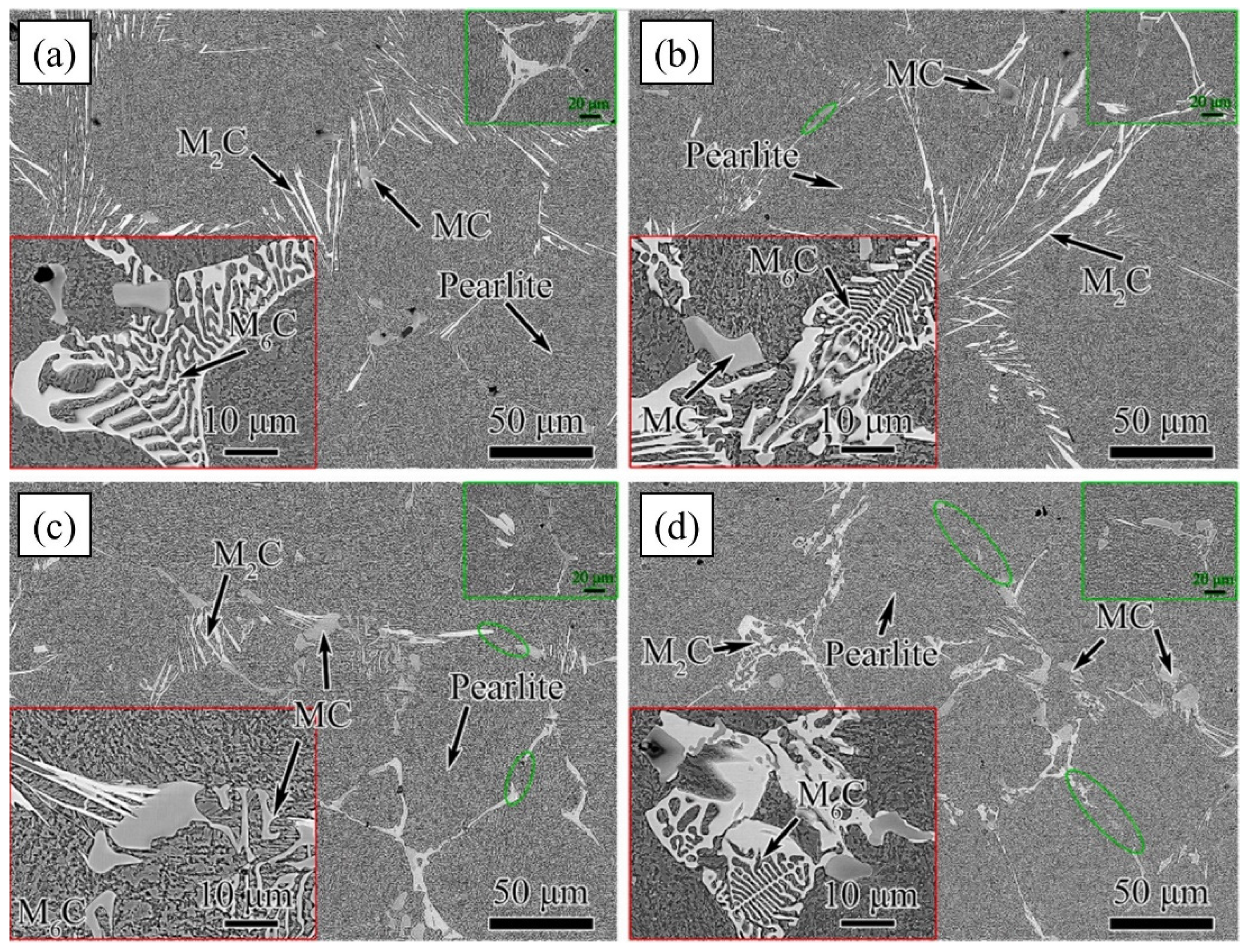
The types of carbides in high-speed steel include MC, M2C, M3C, M4C, M6C, M7C3, M23C6, etc. [1,2,3,4,5,6,7,8]. As shown in Figure 1, MC has a face-centered crystal (FCC) lattice structure, of which the composition varies from MC to M8C7. The metal element M is predominantly V among them, and it can dissolve a tiny amount of W, Mo, and Cr. Ti, Nb, and Zr are the elements that can also make MC carbides [9,12]. As depicted in Figure 2, M2C is made up of thin lamellae or fibers [10,13]. Figure 3 illustrates a hexagonal close-packed (HCP) crystal structure of M2C carbide. Mo, W, V, Cr, Fe, and other metal atoms generate M2C [14]. The metastable condition of M2C eutectic carbides causes them to break down at high temperatures. M6C shaped like a fishbone (Figure 4) is a carbide found mostly in W-series and W-Mo-series high-speed steels and has a complicated cubic lattice structure [14,15]. M7C3 is a complicated orthogonal structure that is mainly found in steels with high carbon and chromium content, appearing as a fishbone or strip. Cr and Fe are the most common metal components in M23C6, which has a complicated FCC structure. Fe concentrations can be extremely high, even exceeding Cr concentrations, and some W, Mo, and trace quantities of V can also be dissolved in M23C6. The primary carbides in W-Mo M2 high-speed steel (W6Mo5Cr4V2) are MC, M2C, and M6C [16,17], whereas M6C, MC, M7C3, and M23C6 are the main carbides in M42 high-speed steel (W2Mo9Cr4Co8). M7C3 is the most common carbide in high-tungsten high-speed steel, while MC, M6C, and M7C3 are the most common carbides in Nb-containing high-speed steel [18,19,20,21,22,23,24,25,26].
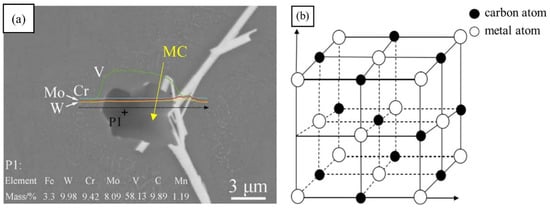
Figure 1.
SEM micrograph of MC morphology and the crystal structure of MC. (a) Morphology; (b) crystal structure (Reprinted with permission from Ref. [12]. 2024, Springer Nature).

Figure 2.
SEM micrograph of M2C morphology and the crystal structure of M2C. (a) Lamellar; (b) fibrous (Reprinted with permission from Ref. [13]. 2024, Springer Nature).
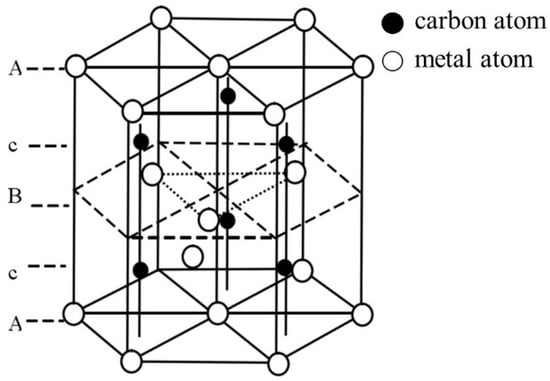
Figure 3.
Crystal structure of M2C (Reprinted from Ref. [14]). A, B and c represent the hexagonal closepacked structure of metal and carbon atom.
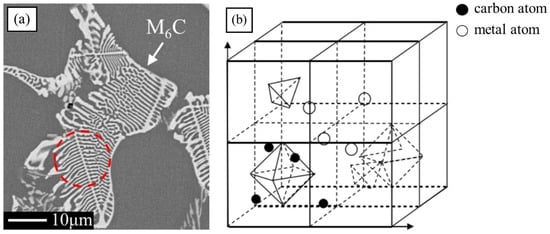
Figure 4.
SEM micrograph of M6C morphology and the crystal structure of M6C. (a) Morphology; (b) crystal structure (Reprinted from Ref. [14]).
It should be pointed out that carbides in high-speed steel have a wide range of compositions. Various high-speed steels can have different compositions of the same type of carbides. Even with the same steel, the application of different manufacturing conditions and techniques can vary the morphology of carbide formation. Ikawa et al. [27] studied the difference in microstructure and properties between the spray deposition method and traditional casting high-speed steel rolls (2.5% C, 6.0% V). Compared with the large bulk carbides processed by the traditional casting process, the carbides processed using the spray deposition method showed a dispersed distribution. The size of the carbide was less than 10 μm, which not only had better mechanical properties but also had a 2~3 times longer service life than the ordinary casting roll.
3. Effect of Alloying on Carbides in High-Speed Steel
The most effective method to regulate carbides in high-speed steel is to rationally adjust the content of alloying elements. Due to the consideration of the performance and cost of high-speed steel, the focus of researchers has been to replace particularly rare and expensive alloying elements with low-price alloying elements that have abundant supplies. The goal of this type of research is to improve the performance of high-speed steel and tools by adding inexpensive alloying elements. The refined morphology and homogenized distribution of carbides ensure the high engineering performance of high-speed steel for a wider range of tooling applications with an extended life.
3.1. Carbon
One of the most important components in high-speed steel is C. C content in high-speed steel ranges from 0.70 to 1.65%, while the C level in high-speed steel for casting rolls ranges from 1.5 to 3.5%. The development of carbides is aided by an increase in carbon content. Carbides are generally spread in the form of a network in high-speed steels with low carbon content. Carbides are predominantly granular and uniformly distributed in high-carbon high-speed steels, with a few feathery, lamellar, and/or fishbone morphologies [28]. An increase in C content changes lamellar Mo-rich M2C to massive V-rich MC. The capacity of C to produce carbides with strong carbide-forming elements like W, Mo, and V improves dramatically as C content rises. C creates carbides with a somewhat lower microhardness than Cr and Fe, and at the same time, increases the volume fraction of carbides. Too much C promotes carbide aggregation and development, disrupts carbide uniform distribution, reduces high-speed tool steel plasticity, and reduces high-speed steel performance. The influence of C on the solidification process is primarily evident in the sequence of primary phase precipitation, according to Wei et al. [29,30,31]. When C is less than 1.64%, ferrite precipitates first. When C is between 1.64 and 2.09%, ferrite and VC precipitate first. When C becomes more than 2.5%, the precipitation of VC takes place. The temperature at which carbides, particularly V-rich MC, precipitate is affected by their C concentration. The quantity of carbides in high-vanadium high-speed steel grows dramatically as the carbon content rises, and the morphology of carbides shifts from rod-like and strip-like to block-like and spherical. Wu et al. [32] prepared M2 high-speed steels using powder metallurgy with various carbon contents and discovered that as the carbon content increased, the formation of regular-shaped granular carbides in M2 high-speed steels increased as well, but that too much carbon resulted in carbides precipitating at the grain boundary. As the main constituent of carbides, the change in C content will mainly affect the precipitation order of different types of carbides. For different types of high-speed steel, the content of certain specific carbides can be controlled by adjusting the C content [28,29,30,31]. Beyond that, because carbide is a faceted phase, its growth mode is also significantly affected by its constituent elements, and a change in C content will also affect the morphology of different types of carbides. Therefore, for the control of different types of carbides, it is a feasible approach to reasonably adjust the C content in high-speed steel.
3.2. Tungsten and Molybdenum
Both W and Mo have a strong carbonizing ability in high-speed steel, allowing them to not only create carbides but also modify the carbide structure. The fundamental characteristics of Mo and W are identical. In most cases, 1%Mo is capable of substituting 2%W, and the carbides generated are identical. The tungsten equivalent [W], where [W] = W + 2Mo, can describe the role of W and Mo in the alloy. Fe4W2C and a tiny amount of W2C constitute the major product of W. During quenching, a portion of Fe4W2C dissolves into the matrix, and during tempering, it disperses and precipitates in the form of W2C [33,34]. When the tungsten concentration is 0.6% or 2%, the carbides at the grain borders are mostly feathery M2C and MC-M2C type complex carbides. When the tungsten content is 4% or 6%, the carbides at the grain boundaries are more fishbone-like M6C. The creation of M6C is aided by an increase in W content in high-speed steel, whereas the precipitation of MC is hindered. When the amount of W added to high-speed steel is too high, however, MC with a high hardness, coarse texture, and skeletal shape forms during solidification. The influence of Mo content on carbides in high-speed steel for rolls was investigated by Chen et al. [25]. In addition, researchers have added Mo2S powder to the vacuum sintering process of powder metallurgy M3/2 and M35 high-speed steel powder mixtures [35]. Mo2S enhanced densification by reacting with the steel matrix and improved machinability and abrasion performance by forming a fine dispersion of complex sulfides (Cr, V, and Fe). Granular MC in high-speed steel reduced as the W concentration rose, but networked carbides that precipitated along grain boundaries increased. In Mo-alloyed and W-Mo-alloyed high-speed steels, Mo primarily forms metastable M2C eutectic carbides, which can be dissolved into smaller-sized M6C and MC during subsequent hot working [36]. The eutectic carbide net in Mo-containing high-speed steel is thin, easy to treat, and breaks easily because the Mo ability to compete for carbon is less than that of W. When Mo increases and W decreases in the steel, the overall quantity of carbides and skeleton M6C drop, and the number of MC carbides grows linearly. Steel with superior comprehensive qualities can be obtained when the added contents of W and Mo are correctly matched.
3.3. Chromium
Cr is found in high-speed steel at a concentration of approximately 4%, and its primary function is to improve the hardenability of the material. Cr has good synergy with other alloying elements, which can double the strengthening effect in high-speed steel by increasing the recrystallization temperature and significantly refining the recrystallized grains. M6C, M7C3, and M23C6 are the carbides generated by Cr in high-speed steel. At a lower quenching temperature, M23C6 can be entirely dissolved, allowing the solid solution to approach C-Cr saturation without impacting grain size. These carbides are basically soluble in the matrix during quenching and heating, which increases the matrix stability. The secondary carbides MC and M2C precipitated by tempering both contain more Cr. Cr can reduce the lattice parameters of each carbide, reduce the mismatch between the carbide and the matrix, lower the nucleation activation energy, and promote the precipitation of secondary carbides, resulting in denser tempered precipitated carbides. However, as the Cr content becomes too high, unstable carbides form in the high-speed steel during tempering, thereby reducing the thermal stability and red hardness of the high-speed steel. In W-Mo-alloyed M2 high-speed steel, increasing Cr promotes the precipitation of M2C, and at the same time, the precipitation of MC is suppressed. In the annealed structure, Cr is mainly dissolved into M23C6. Since the content of Cr in M23C6 is much higher than other types of carbides, it is generally believed that M23C6 is dominated by Cr of carbides [37]. A certain content of Cr is usually added to high-speed steel to promote the precipitation of secondary carbides, thereby enhancing and improving the secondary hardening ability of high-speed steel.
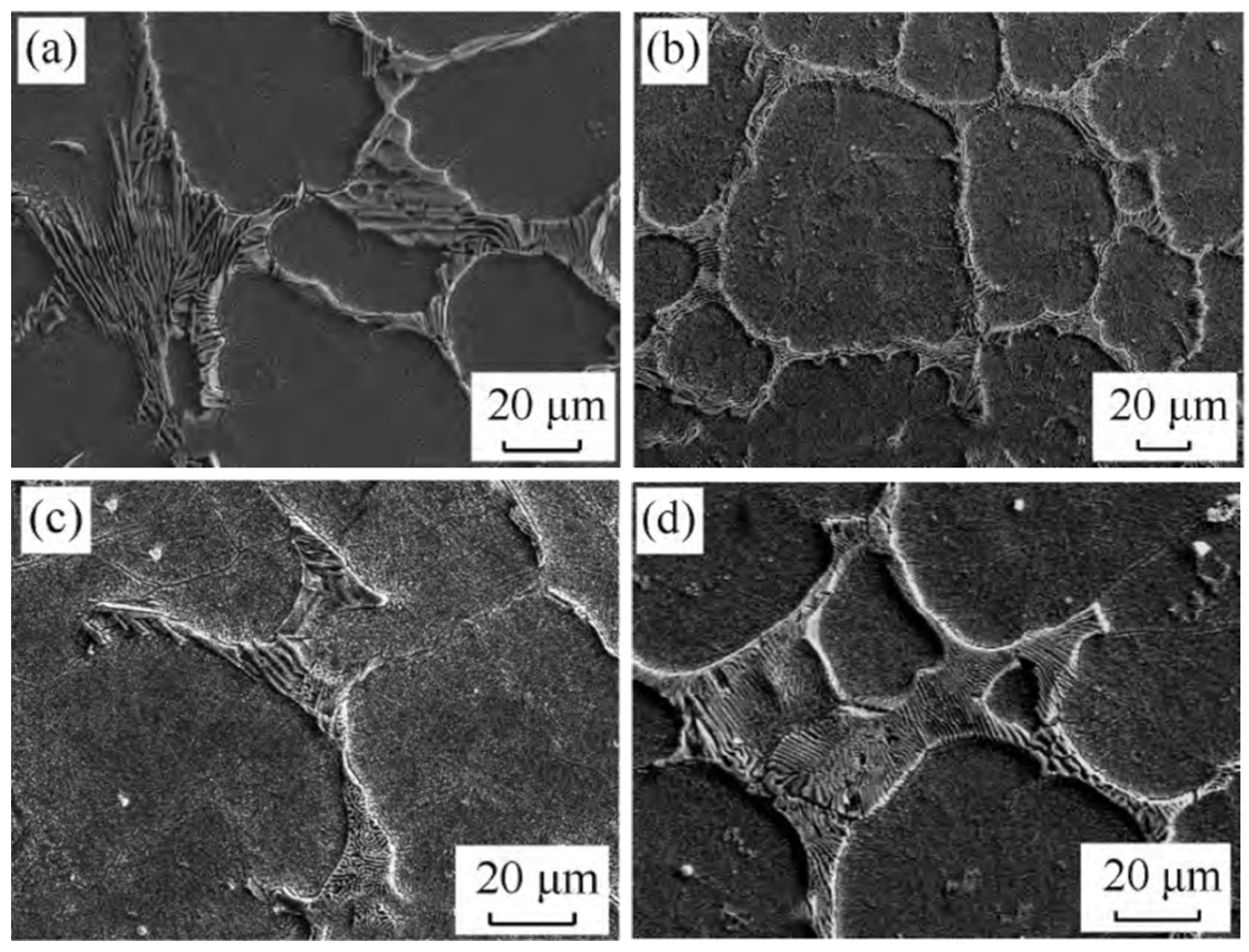
3.4. Vanadium
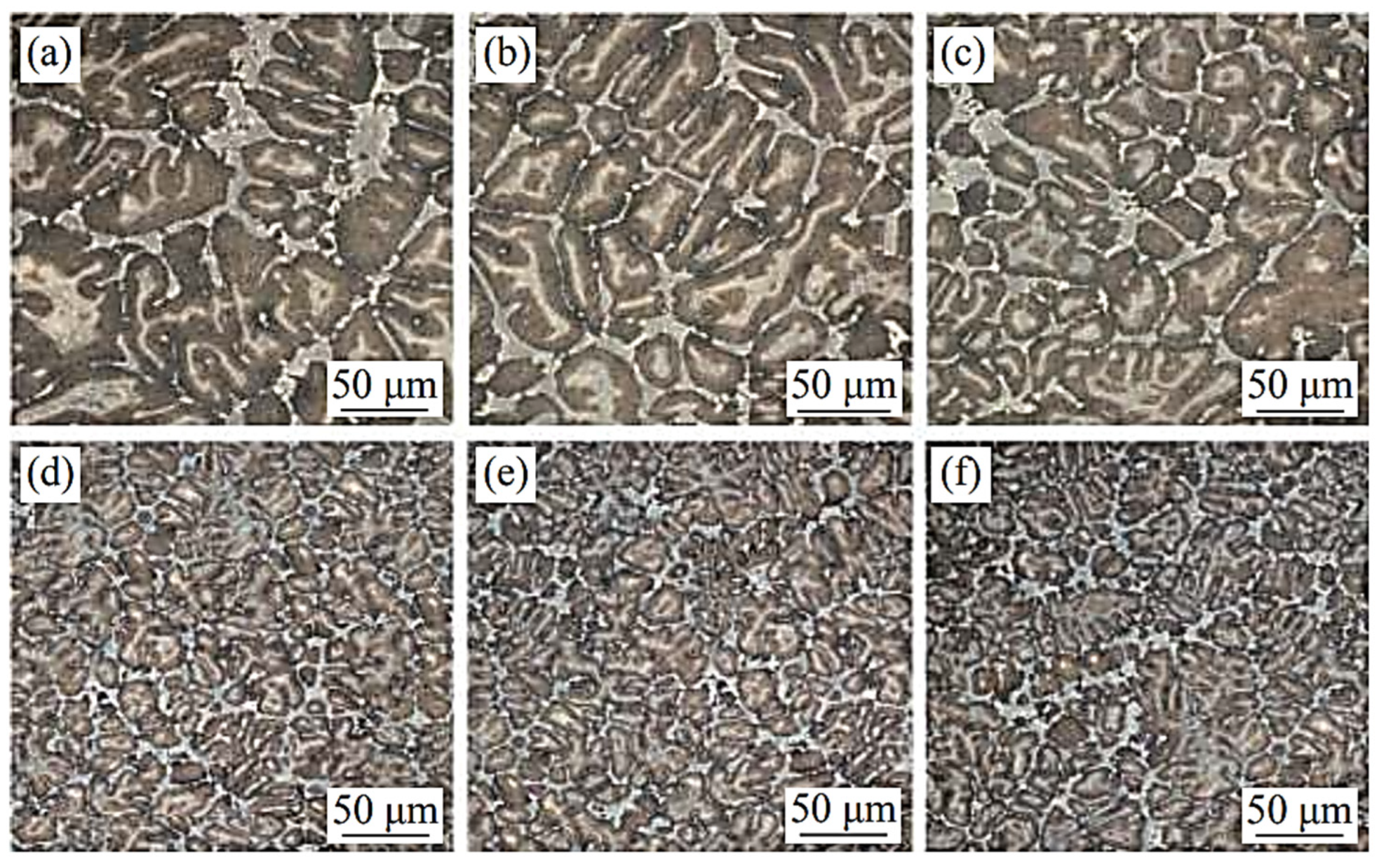
V and C have the strongest bonding capacity of all the high-speed steel alloying elements, and the carbides that they generate are also the most stable. The hardness of VC is high, the grains are microscopic, and it is difficult to break. It is first precipitated during solidification and is mostly disseminated in the matrix as particles or nearly spherical particles [38]. The impact toughness, hardness, and wear resistance of the high-speed steel can all be improved by using VC. Hao et al. [39] tried to use vanadium carbide particles instead of V powder to improve the properties of high-speed steel in powder metallurgy. Increasing the proportion of VC particles was helpful in improving the mechanical properties. The maximum strength was 2597 MPa when the proportion was 150% and the sintering time was 120 min. The hardness of high-speed steel prepared by this method was maintained at 51~52 HRC. Wang et al. [40] experimented with various V contents in W6Mo5Cr4Vx high-speed steel (Figure 5). The inclusion of V refined the high-speed steel grains as well as the network carbides scattered in the intergranular space. The microstructure difference in the high-speed steel was not sensitive to the change in V content when the V content was less than 2%. However, when the V content in the steel increased to more than 3%, the grain refining phenomena became apparent. More carbides were spread in a net or near net shape as the V content increased. The carbides in the steel were increasingly coarse when the V content in the steel increased from 3% to 5%. Notably, as the V content reached 5%, the carbides in the steel were extremely coarse. With high vanadium contents, Xu [41] observed a unique phenomenon called a “black microstructure” in high-speed steel. When the V content of high-speed steel was high and the C content was low, the steel created a “black structure” after quenching, which stayed in the tempered structure, resulting in low hardness. In my opinion, this type of “black structure” could be generated by the high proportion of V in the high-speed steel, which makes MC expand rapidly. In high-speed steel, V not only supports the production of MC but also promotes the creation of lamellar M2C and inhibits the formation of skeletal M6C. When the V content in the high-speed steel is less than 2%, MC is mostly in the form of eutectic carbides and secondary carbides, with secondary carbides accounting for the majority of the MC. As the V content in the secondary carbides exceeds 2%, only a small increase in MC occurs, and the majority of the V is used to form eutectic MC, which are dispersed around the primary M2C and M6C in granular and massive shapes, and some of it forms massive complex carbides with larger sizes when combined with M6C. While the V concentration in the high-speed steel exceeds 3%, the primary MC becomes the largest carbide. The wear resistance of high-speed steel is substantially increased by these carbides with huge particles and exceptionally high hardness, while grindability is greatly improved. With the continuous improvement of the scientific research level, it has been possible to calculate the influence of the content of various elements on precipitated phases such as carbides by different methods. The thermodynamic and kinetic factors of V content on MC and other kinds of carbides can be revealed by first-principle calculations and verified by experiments. Various approaches have provided us with more and more ways to control carbides. As one of the important constituent elements of MC, the content of V has a significant impact on the high-hardness carbides of MC, from the macro aspects of carbide precipitation, distribution, and morphology or from the micro aspects of carbide nucleation and growth.
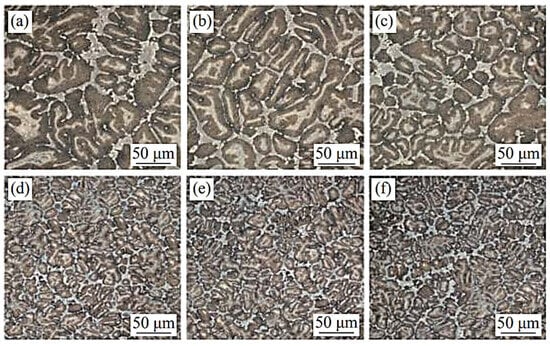
Figure 5.
SEM micrograph of the microstructure of high-speed steel with different vanadium contents. (a) 0% V; (b) 1% V; (c) 2% V; (d) 3% V; (e) 4% V; and (f) 5% V (Reprinted from Ref. [40]).
3.5. Cobalt
Co is incapable of directly producing carbides, and its primary role is to raise the nucleation rate of MC and M2C precipitated during tempering while also slowing the aggregation rate. The form and size of carbides in the as-cast high-speed steel are primarily affected by the Co concentration. Although the addition of Co can refine MC, the refinement effect is limited. The size of MC carbides decreases if the threshold is exceeded. The curved lamellar M2C carbides rapidly disappear as the Co concentration in high-speed steel increases, and the size of large angular MC becomes smaller and disc-shaped. When the Co content is approximately 0.82%, the size of M2C becomes fine. But the shape of the MC carbide is ring-shaped in high-speed steel with a Co concentration of 5.08% and the size of the carbide is coarsened. The change in carbide shape and size is also reflected in the mechanical properties of high-speed steel. With the increase in cobalt content from 0% to 0.82%, the bending strength of high-speed steel increases from 4424 MPa to 5251 MPa. When the cobalt content continued to increase to 1.63% and 5.08%, the bending strength only increased 7 MPa and 43 MPa, respectively [42]. Therefore, based on the consideration of the actual strengthening effect and the cost of high-speed steel, cobalt should be added appropriately. Co can lower the size of secondary MC and M2C carbides in the subsequent hot processing and heat treatment processes of high-speed steel, which is advantageous to improving the hardness and red hardness of the high-speed steel. The influence of Co on the composition of secondary carbides is mostly responsible for the hardness improvement. The presence of Co reduces the content of W and Mo in secondary carbides while increasing the content of Fe and Cr, resulting in a reduction in carbide crystallinity. Carbide tempering precipitation is promoted by the lattice constant and the nucleation barrier, which leads to an increase in the number of carbide precipitations [43]. Co can prevent the formation of Cr-rich M3C and M6C and the solubilization of Cr, resulting in the presence of MC and M2C. The larger the number of fine carbides in the microstructure of the tested steel after quenching, the higher the Co content in M2 high-speed steel. Fine carbides have a pinning effect on grain boundaries from motion. Moon et al. [44] showed that adding Co to P/M high-speed steel accelerated the precipitation of M2C carbides while having no influence on M2C coarsening. Co addition in high-speed steel accelerated the aging kinetic, promoting the precipitation of M2C carbides, as well as increasing the overall hardness. Moreover, the addition of Co as an alloying element led to better hot work properties of P/M high-speed steel [45].
3.6. Silicon and Aluminum
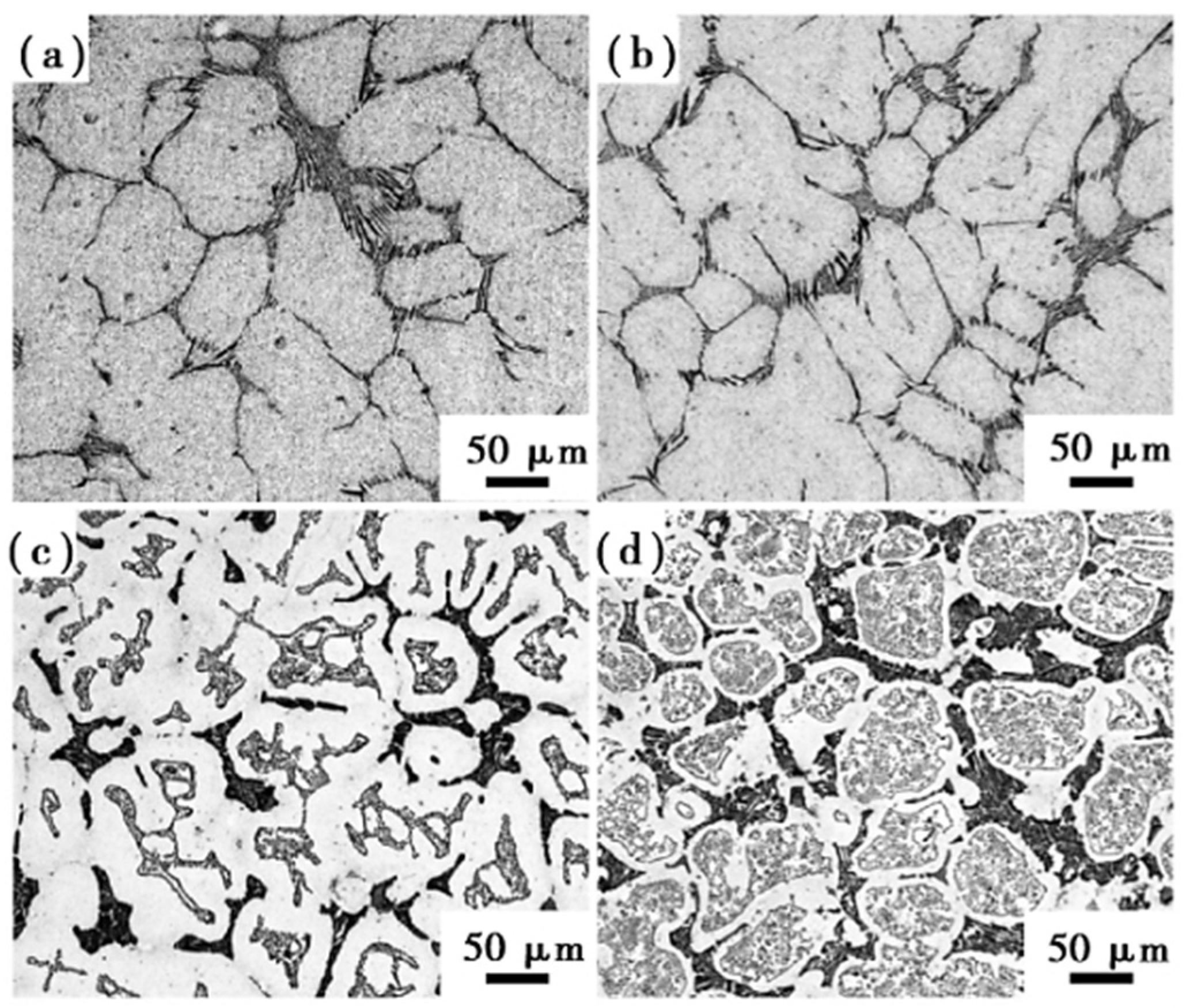
Although Si is not a carbide-forming element, it does increase the number of tempered secondary carbides and refine them when added to high-speed steel. The study by Frisk et al. [37] showed that Si increased carbon activity in high-speed steel, which encouraged carbide nucleation. The inclusion of Si effectively boosted the disintegration of M2C during the heating process in W-Mo-alloyed high-speed steel and Mo-alloyed high-speed steel, resulting in fine secondary carbide particles. Wang et al. [46,47] added silicon to M2 high-speed steel and studied the effect of silicon content on the microstructure and mechanical properties of high-speed steel. They found that adding silicon to high-speed steel promoted the breakdown of M2C eutectic carbide at low temperatures. The carbides in the M2-0.8Si high-speed steel forging billet were finer and more uniform after forging than those in the M2 high-speed steel without silicon. After quenching at 1180 °C and tempering at 540 °C, the bending strength of M2-0.8Si high-speed steel could reach 4183 MPa and the hardness could reach 65.5 HRC. However, there were still large carbides in M2-1.6Si and M2-2.4Si high-speed steels, which was bad for mechanical properties (Figure 6).
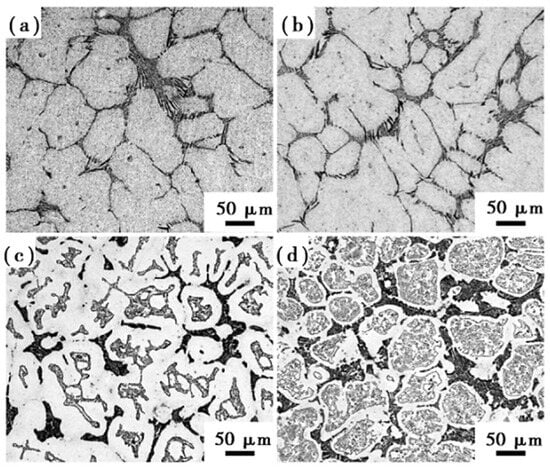
Figure 6.
SEM micrograph of the microstructure of annealed high-speed steel with different silicon content. (a) M2-0.3Si; (b) M2-0.8Si; (c) M2-1.6Si; (d) M2-2.4Si (Reprinted from Ref. [47]).
Many researchers have looked into the use of aluminum in high-speed steel. Pure Al was added to M2 high-speed steel by Li et al. [48]. It was discovered that Al increased the area fraction of ferrite and accelerated the growth of columnar dendrites. Negative segregation was mostly found in M2 high-speed steel, where Al contributed to the creation of M2C eutectic carbides. Li et al. [21] observed that adding varied amounts of Al to the high-speed steel lowered the eutectic carbide breakdown temperature. Carbides disintegrated into MC and M6C after 3 h of heating at 900 °C. The M2C eutectic carbides were degraded and granulated to a great extent. Yang et al. [49] discovered that increasing the aluminum content promoted the development of ferrite. As a result, the hardness dropped from 48HRC to 38HRC, and the carbide broke away from the network.
3.7. Summary of Alloying
As discussed above, the five most essential alloying elements in high-speed steel are C, W, Mo, Cr, and V. The influence of these five alloying elements on carbides is mostly manifested in terms of their impact on the content of various carbide types. C, Mo, and V promote MC precipitation, with C promoting MC precipitation by inhibiting M2C precipitation, as Mo and V enhance MC precipitation by inhibiting M6C precipitation. Cr and V encourage M2C precipitation, while Cr inhibits MC precipitation. V, which inhibits the precipitation of MC and promotes the precipitation of M6C, evidently affects carbide size. MC is refined and then coarsened when the Co concentration changes. M2C is converted from a curved lamella to a long straight layer. Co lowers the size of secondary MC and M2C carbides after further heat treatment. Si and Al mostly aid in M2C decomposition during heat treatment.
4. Effects of Microalloying on Carbides in High-Speed Steel
In most cases, the number of microalloying elements introduced into steel is less than 0.2%. They have the ability to interact with elements including C, N, O, and S, and are distributed in the matrix as second-phase precipitation. Various process parameters can govern their solid solution, precipitation behavior, and precipitate size, consequently varying the characteristics of steel [50,51,52,53,54,55,56]. Microalloying elements were mostly employed in cast iron before high-speed steel microalloying elements were substantially explored and manufactured. Different numbers of microalloying materials can be added to graphite, which has the same faceted phase as carbide. From flakes to spherical, the size is further refined, and the distribution becomes more uniform [57,58,59,60,61,62]. Since microalloying elements are capable of changing graphite morphology and distribution, the study of adding trace elements like N, Mg, and rare earth to high-speed steel has become a new mainstream means to improve carbide distribution and morphology.
4.1. Nitrogen
N shares many properties with C. When alloyed with other elements, N can produce stable nitrides like VN, as well as an interstitial solid solution in some complicated carbides. A modest amount of N can refine the eutectic network and primary carbides in the as-cast structure of high-speed steel. At the same time, adding N to M2C in high-speed steel can change its morphology from lamellar to fibrous, as shown in Figure 7. While the N content is low, the size of M2C in high-speed steel is large and rough. However, when the N content is increased, the thickness and spacing of the carbide lamellar become less than 1 μm (Figure 8) [63]. Compared to layered M2C, fibrous M2C is easily disintegrated into more stable M6C and MC during forging and annealing. Hara et al. [64] investigated the effect of N on the solidification structure of high-speed steel. They successfully introduced nitrogen at a concentration ranging from 48 ppm to 1542 ppm, as found in high-speed steel, by mixing Cr2N into the molten alloy. With the addition of N, the principal M2(C, N)-type carbonitrides are formed and the number of MC and M2C eutectic carbides decreased. Halfa et al. [65] added nitrogen to AlSI41 high-speed steel, and the carbides found in N-containing AlSI41 high-speed steel were primarily M6C and M7C3. Because the properties of N and C are similar, N in high-speed steel mostly replaces part of the C in carbides, making carbides become carbonitrides and changing the crystal structure of carbides from the level of the microstructure. As mentioned earlier, carbide is a kind of faceted phase, and its size and morphology are more affected by crystal structure. The change in surface energy, formation energy, and other energy makes N significantly regulate and control the carbides in high-speed steel. Through the addition and control of N, the control of carbide is feasible, especially the MC-type carbide.
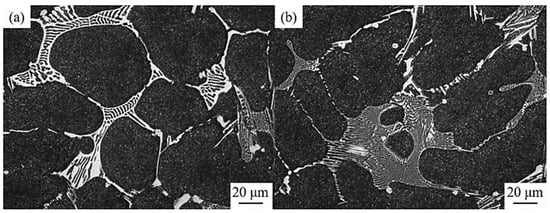
Figure 7.
SEM micrograph of the morphology of carbides in high-speed steel with different N content. (a) 0.006%; (b) 0.011% (Reprinted from Ref. [63]).
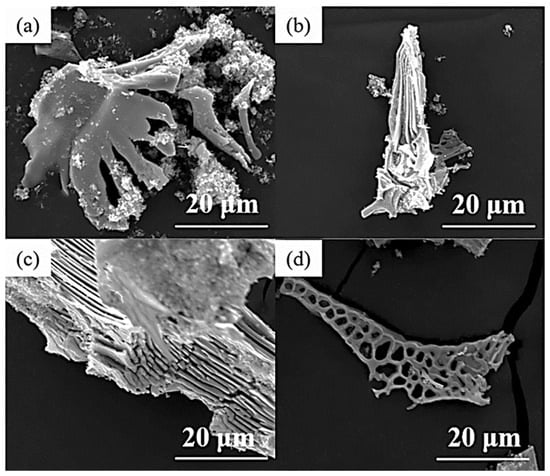
Figure 8.
SEM micrograph of the typical three-dimensional morphology of carbides in high-speed steel with different N content. (a,b) 0.006%; (c,d) 0.011% (Reprinted from Ref. [63]).
4.2. Boron
Just like nitrogen, boron reacts with metal elements to produce a large number of borides with high hardness and thermal durability. In addition, a small amount of B is dissolved in the matrix to partially replace C and other expensive alloying elements to increase the hardenability of the matrix. In high-speed steel, B mostly segregates on eutectic carbides at the grain boundary, promotes eutectic carbide segregation, increases the number of carbides, and reduces the agglomeration of eutectic carbides during heating. Adding trace amounts of B to M2 high-speed steel increased the formation of lamellar M2C carbides, according to the work by Li et al. [66]. The carbides changed from needle-like to coarse flakes as the amount of added B increased. Based on the results of first-principles calculations and the corresponding experimental verification, Yuan [67] confirmed that boron-containing high-speed steel was composed of pearlite, ferrite, retained austenite, a small amount of martensite, and boron carbide, in which the boron-carbon compounds were mainly distributed along the grain boundaries and the hardness of the B carbide phase increased significantly with increasing B content. Astini, V et al. [68] added 0.04% boron to 6.5% V–5% W high-speed steel and found that the grain size of high-speed steel was obviously refined, and the carbide volume fraction increased by approximately 1.2%, which made the microhardness of as-cast high-speed steel increase from 490 HV to 534 HV. The bending strength increased by more than 10%.
4.3. Magnesium
Adding Mg to cast iron causes graphite to grow in different directions, transforming flake graphite into spheroidal graphite [69]. The influence of Mg on carbides with the same faceted phase is mostly reflected in the following aspects. Mg tends to segregate in steel during solidification, changes the carbide phase boundary energy, and promotes carbide nucleation. At the same time, Mg inclusions can serve as heterogeneous carbide nucleation centers, enhancing carbide nucleation while restricting carbide development. When Mg is added to high-speed steel, the size of primary carbides is reduced, their edges become rounded, and massive carbide grids are shattered. Although the kind of carbides in high-speed steel are unchanged, Mg promotes the transformation of carbides from MC to M2C. Duan et al. and Zhang et al. [70,71] added Mg to M2 high-speed steel and discovered the refinement of carbides. The flaky M2C-type eutectic carbides were converted into fish bones when a suitable amount of Mg was introduced. Mg, on the other hand, prevented the spheroidization of fishbone-like eutectic carbides at high temperatures (Figure 9). The effect of Mg on carbide refinement varied depending on the amount of Mg present; 0.008% of Mg mass fraction had the best refinement effect. As the Mg content increased, the influence of Mg on carbide size disappeared. Different from nitrogen and boron, magnesium affects the growth process of carbides in the interface between carbides and other phases during solidification and improves the morphology of carbides by changing the interface energy. Molecular dynamics simulations revealed that the high energy interface of graphite changed from prism to basal with the increase in Mg content. Therefore, for carbides, Mg must have a similar effect, but the mystery is still waiting for us to reveal.
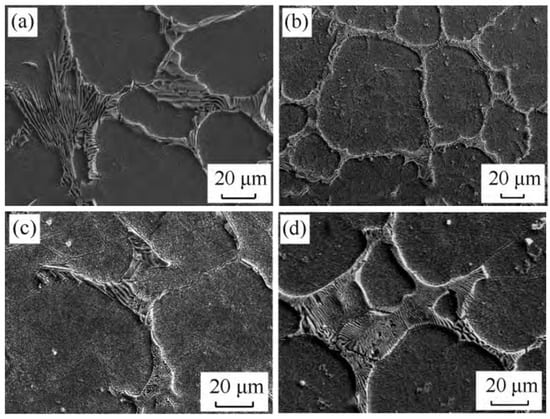
Figure 9.
SEM micrograph of the morphology of carbides in high-speed steel with different Mg contents. (a) 0; (b) 0.008 wt.%; (c) 0.015 wt.%; (d) 0.022 wt.% (Reprinted with permission from Ref. [70] 2024, Elsevier).
4.4. Niobium
Nb has a comparable impact on carbide formation as V. The chemical affinity of Nb to C is higher than V to C. In high-speed steel, the inclusion of Nb causes the formation of the NbC phase, which is often a tiny block carbide. It has been demonstrated [22,65,72,73] that Nb improved the shape, size, and distribution of precipitated carbides in the ingot of alloyed high-speed steel. With the addition of Nb, the degradation of M2C into MC and M6C was accelerated. The form of eutectic carbides was suppressed as Nb was added. NbC particles, on the other hand, served as heterogeneous nucleation sites, reducing grain size and uniformizing the microstructure of Nb-containing high-speed steel. Parallel distributed flake, rod-shaped M2C, and skeleton M6C were found in the steel containing a low content of niobium (0.0847%). However, as the niobium level increased to 0.621%, only one type of rod-shaped carbide was present and was placed in a short and thin layer in the Nb-alloyed steel (Figure 10). The addition of Nb to crystalline carbides caused the formation of rod-like carbides. Because the binding force of Nb to C was greater than that of other alloying elements, NbC precipitated early in the solidification process, inhibiting grain and carbide growth in high-speed steel.
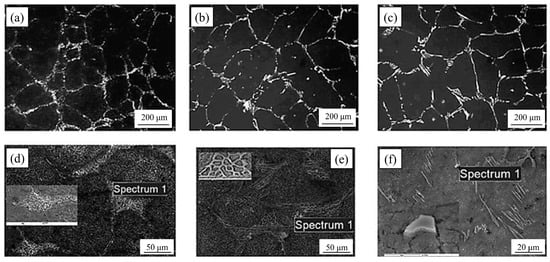
Figure 10.
SEM micrograph of the microstructure of high-speed steel with different Nb contents. (a) and (d) 0.0847%, (b) and (e) 0.213%, and (c) and (f) 0.645% Nb (Reprinted with permission from Ref. [65] 2024, Jon Wiley and Sons).
4.5. Titanium
Ti is chemically active with C and N. When added to steel as a microalloying element, Ti forms very stable TiC, TiN, and Ti(C, N) with C and/or N in molten steel. The small mismatch between Ti-based carbides and Fe enables them to serve as heterogeneous nucleation sites of austenite, substantially improving the microstructure. It was discovered that after Ti modification of high-speed steel with a C concentration of more than 2.0%, a considerable amount of TiC was generated between Ti and the carbon in molten steel at high temperatures. The fine dispersion and precipitation of MC were promoted by the heterogeneous nucleation of MC and austenite. The carbides were distributed in spherical and granular form after heat treatment, which improved the mechanical, wear, and thermal fatigue properties of high-speed steel. According to reports, the impact toughness of high-speed steel can reach 10.3 J/cm2, an increase of more than 39% [72,73,74,75,76,77]. When Ti was added to 11-0-2, 11-2-2, and 9-2-2 type W-Mo-V high-speed steels, Dobrzański et al. [72] found that this behavior led to a chemistry change in MC-type primary carbides. When the concentration in the steel was sufficiently high, VC was turned into a solid solution, resulting in a strong secondary hardness effect. Nitrogen, boron, and niobium elements are mainly used to replace elements in the carbide to achieve regular control of the carbide during the formation of the carbide. Magnesium and rare earth elements are mainly used to improve the structure of the carbide by segregating and aggregating on its surface during the growth of the carbide and affecting its interface energy. In the nucleation process of the carbide, some elements form high-temperature precipitated phases as the core of the heterogeneous nucleation of carbides to refine the size of the carbide. Titanium is one of these. In the solidification process, the formation of carbides is promoted by non-uniform nucleation, which can refine carbides, improve the secondary hardening effect of high-speed steel, further improve the hardness of high-speed steel, and improve the hot-working performance.
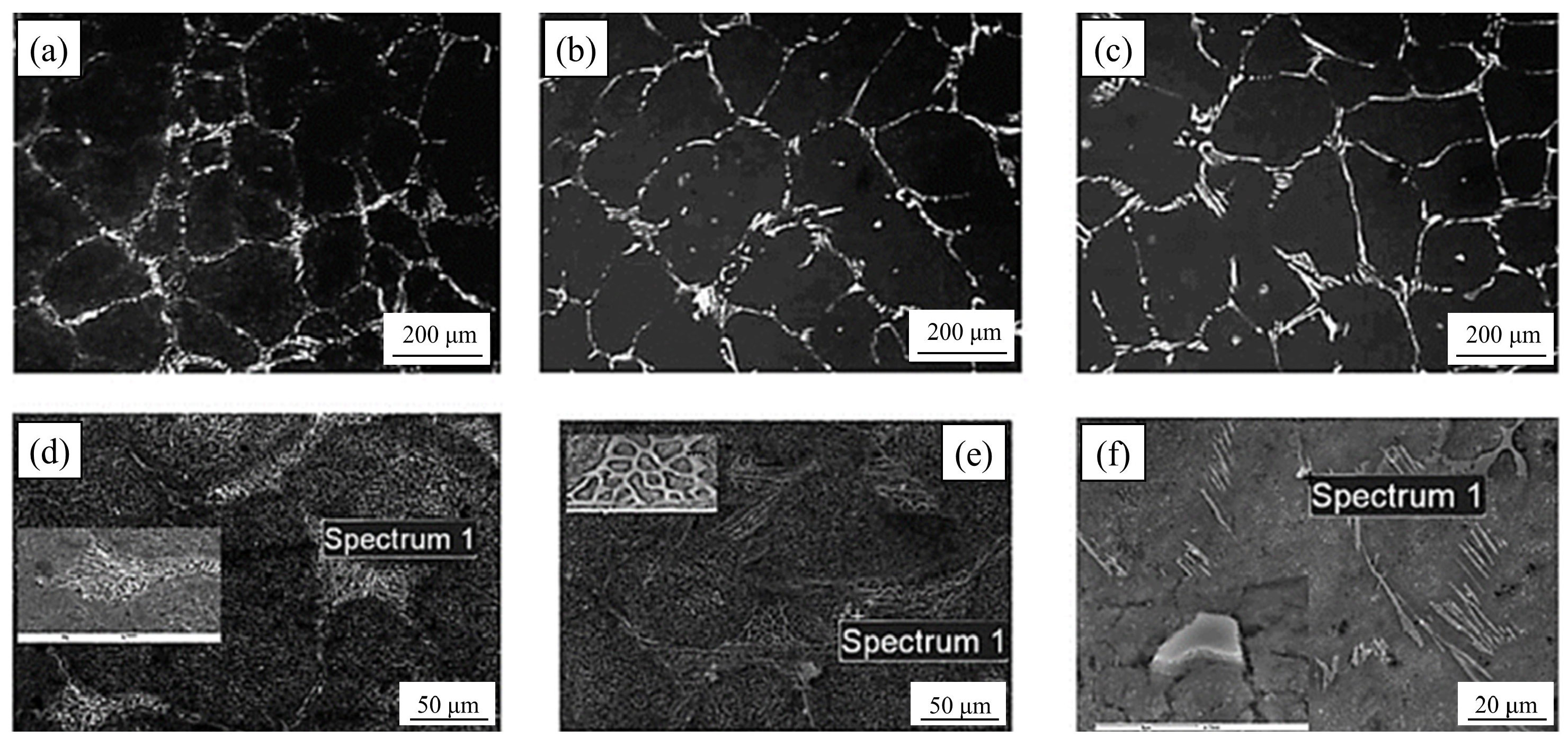
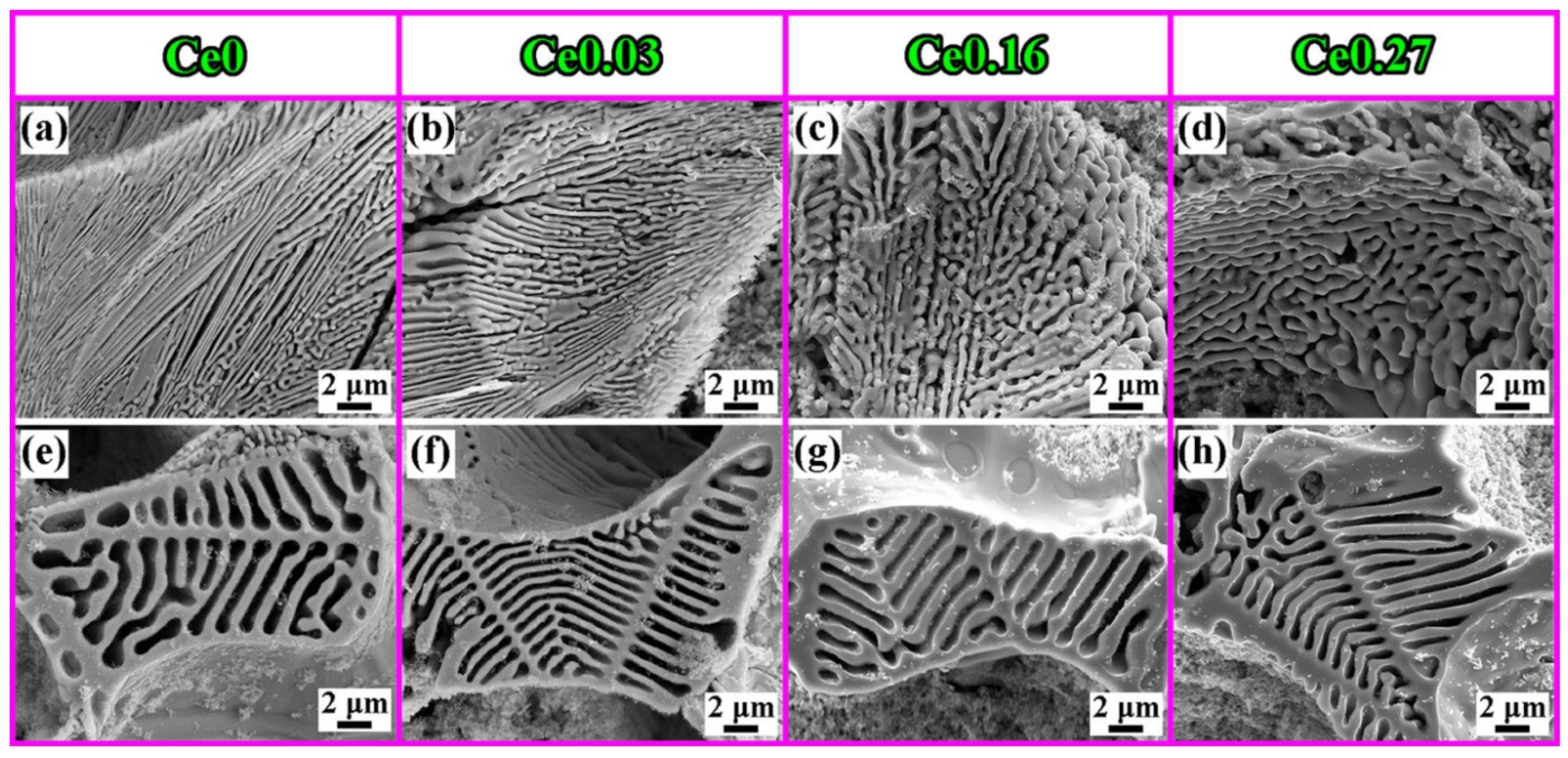
4.6. Rare Earth
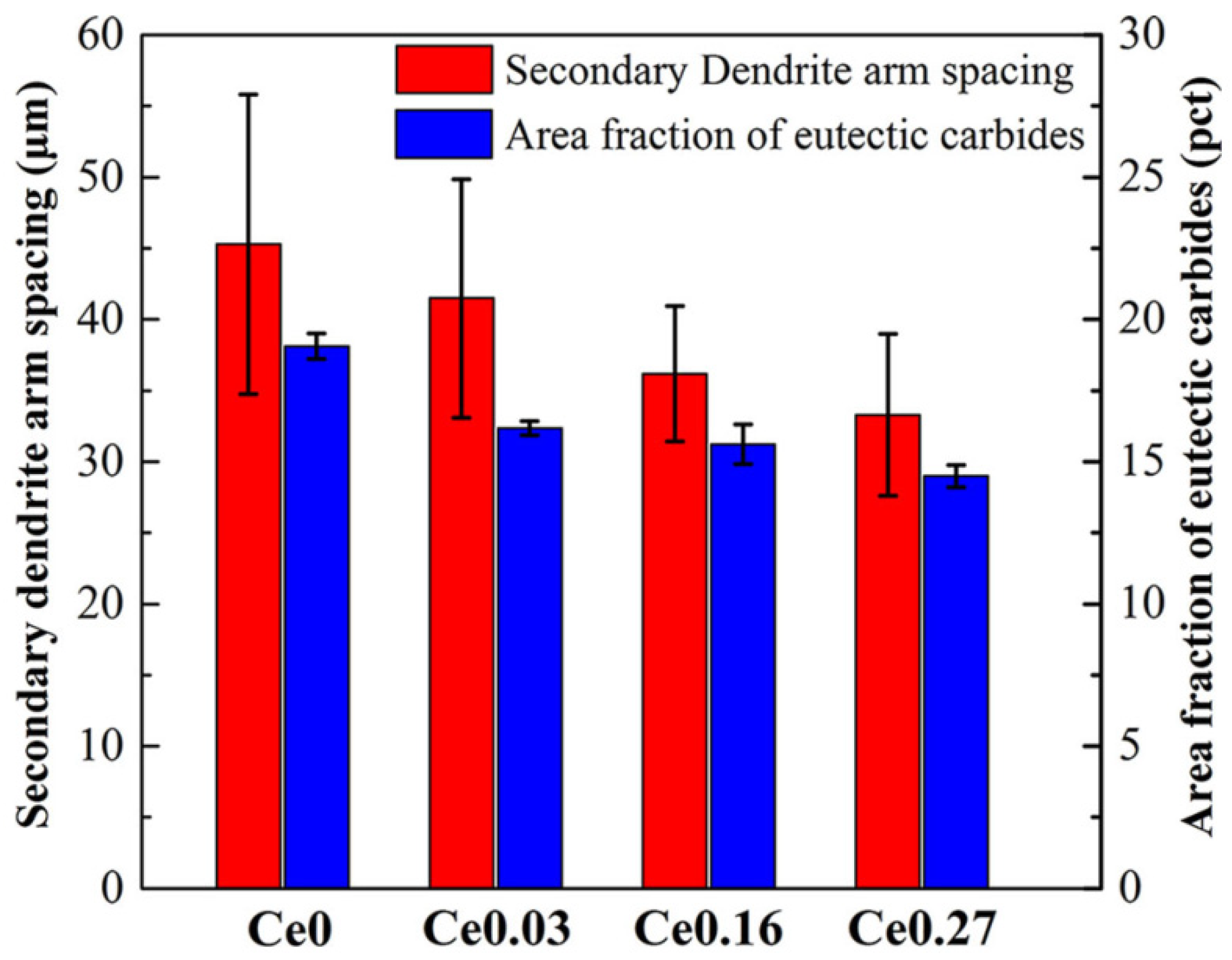
It has been reported [78,79,80,81,82,83,84] that La-Ce rare earth is capable of improving the microstructure and characteristics of a variety of steels. Rare earth elements are concentrated in the front of molten steel dendrites during the solidification process due to their low solubility in austenite, resulting in constitutional supercooling and an increase in the degree of supercooling [85], which increases the nucleation rate of austenite. The dendrite structure refinement inhibits the growth of carbides, achieving the goal of refining the carbides. At the same time, the growth rates of austenite and M2C increase. The growth rate of eutectic austenite as a non-faceted phase is greater than that of M2C carbides (faceted phase). Because M2C is the dictating phase of the eutectic reaction, it bends and branches continually to prevent eutectic austenite from overgrowing [86]. As a result, when the rare earth content in high-speed steel increases, the morphology of M2C changes from long flake or straight rod shapes to short curved rod or honeycomb shapes (Figure 11). Rare earth elements make it easy to form high-melting-point oxides and sulfides with O and S in high-speed steel. Regarding rare earth oxides and rare earth sulfides such as Ce2O3, Ce2O2S, etc., the lattice dislocation between (0001) Ce2O2S and (100) c-Fe is approximately 5.57 pct and the lattice dislocation between (0001) Ce2O3 and (100) c-Fe is approximately 5.57 pct. The lattice dislocation is approximately 6.33 pct [87,88]. When the lattice dislocation is less than 12 pct, it can serve as a site for heterogeneous nucleation, according to Bramfitt’s requirement [89]. As a result, Ce2O2S and Ce2O3 are used as heterogeneous nucleation sites of primary austenite in the solidification process to refine the dendritic structure, which mitigates carbide growth and fulfills the goal of refining carbides. Also, Ce2O2S and Ce2O3 operate as the nucleation core of M6C, increasing its content and reducing its size. Rare earth elements diminish the segregation of alloying elements like W and Mo, and rare earth Ce lowers C activity. The precipitation temperature of carbides decreases, the content of eutectic carbides (especially W-rich M2C) is reduced, and the microstructure is further refined as the segregation of W, Mo, and other alloy elements improves and the activity of C decreases. The presence of rare earth enhances the crystallization of primary austenite and MC carbides [90,91,92,93,94], leading to the refinement of dendrites, the formation of a discontinuous eutectic carbide network, and fine MC, causing dispersion (Figure 12). In general, rare earth elements lower the carbide concentration, promote M6C precipitation, change the M2C morphology from long flaky or straight rod-like morphologies to shorter curved rod-like or honeycomb-like morphologies, and refine austenite branch crystals. Among them, the refining effect of Ce on carbides is more obvious, and the refining effect of La is relatively weak.
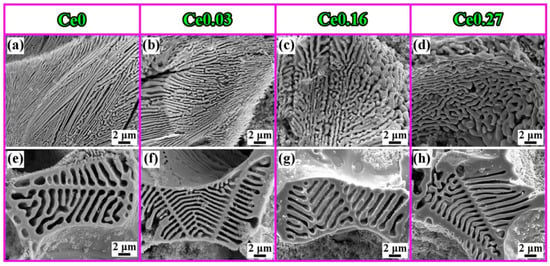
Figure 11.
SEM micrograph of (a–d) M2C and (e–h) M6C in high-speed steels with different Ce contents (Reprinted with permission from Ref. [87] 2024, Springer Nature).
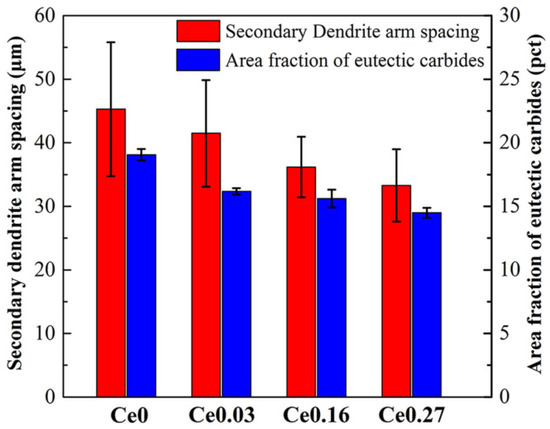
Figure 12.
The SDAS and eutectic carbides area fraction of M42 cast ingots with different Ce contents (Reprinted with permission from Ref. [87] 2024, Springer Nature).
The role of rare earth in high-speed steel has been confirmed by many scholars. Chaus [95] attempted to modify R6M5 high-speed steel by using rare earth Ce. The introduction of Ce considerably disintegrated the dendritic structure of the high-speed steel. After hardening and tempering, the dendrite-refining effect of high-speed steel modified by rare earth Ce was retained, which determined that rare earth modification could affect the final mechanical properties of R6M5 high-speed steel. Chen et al. [59] observed that after Y was added to M2 high-speed steel, the network carbide tended to neck down with the increase in Y. The addition of Y made the net-like carbide tend to disconnect, and the carbide was refined and distributed, as shown in Figure 13, but the addition of Y did not change the carbide type. The carbides in the as-cast M2 high-speed steel were still MC, M2C, and M6C. Liu et al. [96] discovered that rare earth addition to M2 high-speed steel promoted the creation of M6C, and rare earth made the M2C lamella irregular, reduced its thermal stability, and improved the carbide distribution. Wang et al. [97] studied the effect of rare earth elements on the microstructure of M35 high-speed steel. The ledeburite network in the microstructure of the electroslag ingot without rare earth was completely closed. Between the ledeburite networks, rod-shaped eutectic carbides were present. In the microstructure of the 0.1% RE-alloyed M35 high-speed steel, the ledeburite network was partially divorced and became thin. There were some fish bone-shaped eutectic carbides between the partially divorced ledeburite networks. When the rare earth addition was increased to 0.2%, the ledeburite network in the microstructure of the electroslag ingot was completely divorced, the network thickness was reduced, and most of the eutectic carbides were in the shape of fishbones. As the rare earth level rose to 0.4%, the ledeburite network was partially broken, and all of the eutectic carbides became fishbones. The form and distribution of the carbides in the forged samples varied dramatically with the levels of the rare earth addition. The carbide distribution in the wire rod samples without rare earth was uneven, as the majority of carbides were in large angular shapes. The wire rod samples containing 0.2% of rare earth had a homogenous distribution of fine carbides. According to Wang et al. [97], the inclusion of rare earth elements reduced the number of macro eutectic carbides in high-speed steel while proportionally increasing the content of spherical and rod-like MC. To reduce the segregation of W, Mo, and other alloying elements, Li et al. [98] added a tiny amount of pure Ce or RE-Si alloy to M2 high-speed steel. As a result, the number of eutectic carbides was considerably reduced. When heated to a high temperature, the carbides were distributed in a broken network. Ce from rare earth minerals was mostly segregated on the eutectic carbides of the grain boundaries, while M2C carbides with a tiny quantity of Ce also formed.
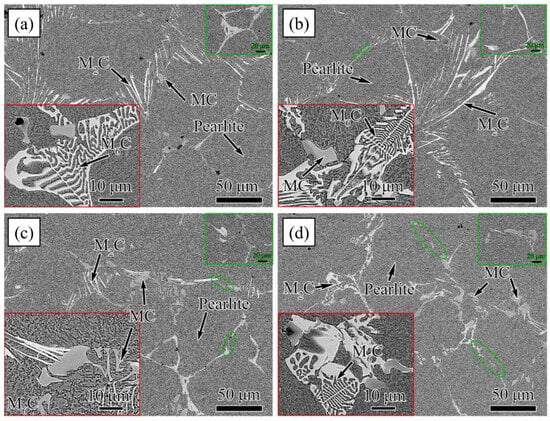
Figure 13.
SEM micrograph of Carbide morphology of high-speed steel with different Y contents. (a) 0%, (b) 0.047% Y, (c) 0.096 Y, and (d) 0.229% Y (Reprinted with permission from Ref. [59] 2024, Elsevier).
4.7. Summary of Microalloying
Overall, the effect of microalloying on the formation of carbides is different from that of alloying elements. N, Mg, and Nb improve carbide dispersion, encourage M2C to convert from long straight lamellae to fibrous or rod-like forms, and refine M2C. Such a change lowers the thermal stability of M2C and speeds up its disintegration after heat treatment. N also impedes carbide precipitation and lowers carbide content. B coarsens M2C but enhances carbide hardness. The heterogeneous nucleation of carbides promoted by Nb, Ti, and rare earth elements increases carbide content (especially MC). Meanwhile, rare earth elements improve the M2C morphology, promote M6C and MC precipitation, decrease the carbide content, and regulate and control the carbide distribution.
5. Outlook
The high demand for novel engineering applications requires advanced high-speed steel. The long-term goal of high-speed steel development must be focused on high quality and low cost. Carbides, as phase constituents of high-speed steel, have a direct impact on its performance. The thermal stability of M2C carbides is unstable, and the decomposition of M2C can start when the quenching temperature reaches 900 °C. All the M2C becomes MC carbides at 1200 °C. MC carbides have high hardness, which helps to improve the hardness and wear resistance of high-speed steel. The size of MC precipitated directly from the liquid phase is usually coarse, and the thermal stability of MC is stable. Because it is difficult for MC to dissolve at a temperature of 1100 °C, it will remain in the steel in large sizes, which will lead to a reduction in mechanical properties. However, MC carbides formed by M2C decomposition are small in size and dispersed, which is an ideal secondary strengthening phase in high-speed steel. M6C carbides precipitated from the liquid phase are often considered to deteriorate the performance of high-speed steel, which is similar to MC. M6C requires a higher quenching temperature to dissolve into austenite and plays a role in improving hardenability and red-hardness. M7C3 can be completely dissolved in austenite at 1100 °C, and the effect of secondary hardening can appear after a high-temperature tempering process, which can precipitate fine nanoscale carbides at the same time. The regulation of the size, shape, distribution, and type of carbides will continue to be the focus of high-speed steel research in the future because the addition of different alloying and microalloying elements in high-speed steel has a different impact on the formation of carbide types, so the design and optimization of future high-quality high-speed steel compositions will also become the focus of research.
The introduction of the powder metallurgy manufacturing technique in the 1960s revolutionized high-speed steel, effectively resolving the problem of coarse carbide segregation during solidification. Meanwhile, carbides were controlled to be spherical and uniformly distributed in the microstructure of high-speed steel [99]. This technology will become more commonly employed in high-end high-speed steel, with a focus on excellent cleanliness and low cost. Electroslag remelting technology [100,101,102] can successfully enhance carbides in high-speed steel while also enhancing cleanliness, and it will continue to be one of the mainstays of high-speed steel billet manufacturing in the future. Optimizing the electroslag casting process to improve the carbide’s shape and size and refine the solidified microstructure will be essential to enhance this technology. The physical field represented by electromagnetic stirring and pulse magneto-oscillation has made remarkable achievements in improving the quality of metallurgical billets [103,104,105,106,107,108,109,110,111]. These technologies, especially pulse magneto-oscillation solidification homogenization technology, show greater advantages in low-cost, large-scale, high-speed steel metallurgical manufacturing. Other techniques of physical external fields, such as pulsed current and magnetic field, will also be of concern in the manipulation of carbides in high-speed steel [112,113].
The application of alloying, particularly microalloying technology in high-speed steel, should be further explored for practical use, although it has produced some positive outcomes. As learned from the production of advanced cast iron, in the 1920s, the inoculation technology of cast iron refined and dispersed the graphite structure with a faceted phase in cast iron, thus doubling the mechanical properties of cast iron. Only 20 years later, the spheroidizing treatment of cast iron has changed the graphite structure in cast iron from flake to spherical, thus doubling the mechanical properties of cast iron. The inoculation and graphite-spheroidizing technologies via microalloying effectively triple the mechanical properties of cast iron. This means that while the morphology of the faceted phase precipitated during solidification is primarily determined by its own crystallographic properties, the morphology and distribution of the faceted phase can be altered through appropriate composition adjustments and microalloying processing technology. It is worth emphasizing that the microalloying treatment technology is not only low-cost but also well-suited to large-scale manufacturing production. As a result, carbide modification via microalloying should be an important future research area in the development of novel high-speed steel with high engineering performance.
Author Contributions
Conceptualization, Q.Z.; project administration, Q.Z.; funding acquisition, Q.Z.; validation, X.C. and H.H.; supervision, X.C.; resources, X.C.; visualization, X.C. and H.H.; writing—original draft preparation, Y.C.; writing—review and editing, C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key project of the National Natural Science Foundation of China (No.52130109), Hebei Key Technology Research and Development Program (20311006D).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chaus, A.S.; Dománková, M. Unknown high-speed steel. Mater. Lett. 2021, 292, 129653. [Google Scholar] [CrossRef]
- Wang, Q.M.; Cheng, G.G.; Huang, Y. Morphological characteristics and precipitation mechanism of large-sized carbides in M2 high-speed steel. Iron Steel 2018, 53, 65–71. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Cao, H.J.; Zheng, X.P.; Ma, B. Research and application of high vanadium high speed steel. Hot Working Technology. 2014, 43, 23–26. [Google Scholar] [CrossRef]
- Liang, W.; Li, J.; Yan, W.; Shi, C.B.; Zhang, J. Evolution behavior and comparison of carbide in ingot casting and ESR M2 high speed steel. China Metall. 2020, 30, 28–34. [Google Scholar] [CrossRef]
- Li, Z.C.; Chen, Y.M.; Chen, X.R.; Zhong, H.G.; Li, L.J.; Zhai, Q.J. Thermal simulation of carbide and macrosegregation in continuous casting M2 high-speed steel. J. Iron Steel Res. 2023, 35, 1228–1240. [Google Scholar] [CrossRef]
- Dai, Y.H.; Man, T.H.; Li, P.; Xu, L.Q.; Liu, Y.; Wei, X.C. Effect of Rare Earth Alloying on High-Carbon and High-Alloy Tool and Die Steel. Mater. Rep. 2023. Available online: http://kns.cnki.net/kcms/detail/50.1078.TB.20231209.1355.002.html (accessed on 11 December 2023).
- Chen, J.Y.; Zhu, L.M.; Yin, Z.H.; Xiao, J.; Liu, H.N.; Zhong, H.G. Production and quality control technologies for high-speed steel. Shanghai Met. 2022, 44, 8–14+23. [Google Scholar] [CrossRef]
- Zhou, X.F.; Liu, D.; Zhu, W.L.; Fang, F.; Tu, Y.Y.; Jiang, J.Q. Morphology, microstructure and decomposition behavior of M2C carbides in high speed steel. J. Iron Steel Res. Int. 2017, 24, 43–49. [Google Scholar] [CrossRef]
- Kazunori, K.; Shinya, I.; Hirofumi, M.; Yuji, K. Effect of MC Type Carbides on Wear Resistance of High Wear Resistant Cast Iron Rolls Developed for Work Rolls of Hot Strip Mills. ISIJ Int. 2021, 61, 2597–2604. [Google Scholar] [CrossRef]
- Ye, H.L.; Wang, Q.; Liu, B.; Li, Z.M.; Chen, X.; Feng, B.D. Research on morphologies and stability of eutectic carbide in M2 high speed steel. Hot Work. Technol. 2022, 51, 83–85. [Google Scholar] [CrossRef]
- Cheng, X.L.; Hou, J.Q.; Fu, H.G. Microstructure evolution and wear resistance of boron-bearing high speed steel roll. Arch. Metall. Mater. 2022, 67, 113–119. [Google Scholar] [CrossRef]
- Yin, F.X.; Su, M.; Ji, F.; Tian, Q.C.; Bai, Y.G.; Feng, J.H.; Xiao, Z.X. Effect of melting rate on microsegregation and primary MC carbides in M2 high-speed steel during electroslag remelting. China Foundry 2021, 18, 163–169. [Google Scholar] [CrossRef]
- Zhou, X.F.; Fang, F.; Li, F.; Jiang, J.Q. Morphology and microstructure of M2C carbide formed at different cooling rates in AISI M2 high speed steel. J. Mater. Sci. 2011, 46, 1196–1202. [Google Scholar] [CrossRef]
- Zheng, L.C.; Yan, B.Q.; Lou, J.; Li, H.B.; Jiang, Z.H. Formation Behavior of M2C and M6C Eutectic Carbides in M42 High-Speed Steel. ISIJ Int. 2023, 63, 294–302. [Google Scholar] [CrossRef]
- Liu, W.F.; Guo, Y.F.; Cao, Y.F.; Wang, J.Q.; Hou, Z.Y.; Sun, M.X.; Xu, B.; Li, D.Z. Transformation behavior of primary MC and M2C carbides in Cr4Mo4V steel. J. Alloys Compd. 2022, 889, 161755. [Google Scholar] [CrossRef]
- Yao, J.; Man, T.H.; Liu, Y.; Zhu, X.D.; Dong, H. Effect of continuous casting on segregation and carbide of M2 high speed steel. China Metall. 2023, 33, 77–84. [Google Scholar] [CrossRef]
- Zheng, T.X.; Li, W.Q.; Qi, W.T.; Xia, Z.B.; Li, Q.; Shen, Z.; Guo, Y.F.; Zhong, Y.B.; Wang, H.; Wang, Q.L. Morphology transition of eutectic carbide assisted by thermoelectric magnetic force during the directional solidification of M2 high-speed steel. Ironmak. Steelmak. 2021, 48, 885–892. [Google Scholar] [CrossRef]
- Luo, Y.W.; Guo, H.J.; Guo, J. Effect of cooling rate on the transformation characteristics and precipitation behaviour of carbides in AISI M42 high-speed steel. Ironmak. Steelmak. 2019, 46, 698–704. [Google Scholar] [CrossRef]
- Ma, J.Y.; Li, H.B.; Wang, H.J.; Jiao, W.C.; Wan, X.D.; Liu, S.Q. Effect of cryogenic treatment on microstructure and properties of high-nitrogen M42 high-speed steel. J. Iron Steel Res. 2023, 35, 1282–1290. [Google Scholar] [CrossRef]
- Qin, Z.; Xin, R.; Zhang, J.; Lan, H.; Xue, Q. Effect of preheat treatment on carbide morphologies of cast HSS. Hot Work. Technol. 2014, 43, 201–203. [Google Scholar] [CrossRef]
- Lin, Y.H.; Li, H.; Ju, J.; Jiang, C.C.; Lei, Y.P.; Fu, H.G. Effect of boron content on high-temperature oxidation resistance of B-bearing high-speed steel. Surf. Rev. Lett. 2020, 27, 12. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, D.S.; Liu, J.H.; Chen, Z.Z.; Yong, Q.L. Microstructure and mechanical properties of W4Mo2Cr4VNb steel. J. Iron Steel Res. 2008, 20, 36–40. [Google Scholar] [CrossRef]
- Fu, H.G.; Liu, X.N.; Yang, Y.W.; Cheng, X.L.; Qu, Y.H. Effect of heat treatment on microstructure and properties of high-boron high-speed steel. Trans. Indian Inst. Met. 2018, 71, 2423–2432. [Google Scholar] [CrossRef]
- Wei, S.Z.; Xu, L.J. The effect of heat treatment on the microstructure of cast high tungsten high-speed steel. Acta Met. Sin. 2020, 56, 523–538. [Google Scholar] [CrossRef]
- Chen, J.B.; Liu, Y.F.; Wang, Y.J.; Xu, R.; Shi, Q.Y.; Chen, J.S.; Wu, Y.P. Design and Optimization of Heat Treatment Process Parameters for High-Molybdenum-Vanadium High-Speed Steel for Rolls. Materials 2023, 16, 7103. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.N.; Cui, C.X.; Zhang, J.J.; Zhao, L.C.; Zheng, G.X.; Cui, S. Enhanced grain refinement of W18Cr4V high-speed steel using in situ TiN–Nb–Cr@Graphene/Fe nanocomposite inoculant. Steel Res. Int. 2021, 92, 2100094. [Google Scholar] [CrossRef]
- Ikawa, Y.; Itami, T.; Kumagai, K.; Kawashima, Y.; Leatham, A.G.; Coombs, J.S.; Brooks, R.G. Spray Deposition Method and Its Application to the Production of Mill Rolls. ISIJ Int. 1990, 30, 756–763. [Google Scholar] [CrossRef][Green Version]
- Wu, Z.J.; Chen, Z.G.; Xiang, Y.; Wei, X.; Zhang, J.S. Effect of small account of Ce addition on microstructure and mechanical properties of high-boron high speed steel. Chin. J. Nonferrous Met. 2013, 23, 1289–1294. [Google Scholar] [CrossRef]
- Wei, S.Z.; Zhu, J.H.; Xu, L.J.; Long, R. Effects of carbon on microstructures and properties of high vanadium high-speed steel. Mater. Des. 2006, 27, 58–63. [Google Scholar] [CrossRef]
- Shao, K.Z.; Wei, S.Z.; Long, R.; Ni, F.; Peng, T. The Effect of Carbon on the Solidification Process of High Vanadium High Speed Steel. Foundry Technol. 2005, 11, 30–34. [Google Scholar] [CrossRef]
- Cao, Y.L.; Zhao, Z.R.; Ma, C.S.; Li, G.Q. Precipitation Behavior and Elemental Distribution of MC Carbides in High Carbon and Vanadium High-Speed Steel. J. Mater. Eng. Perform. 2022, 31, 4444–4458. [Google Scholar] [CrossRef]
- Wu, W.D.; Xiong, X.; Liu, R.T.; Luan, H.Z.; Hao, Y.R. The effect of carbon content on the microstructure and properties of M2 high-speed steel prepared by elemental powder method. Mater. Sci. Eng. Powder Metall. 2019, 24, 273–281. [Google Scholar] [CrossRef]
- Xu, L.J.; Zhou, H.; Wei, S.Z. Crystal structure and interface feature of carbide in high speed steel with high tungsten content. Trans. Mater. Heat Treat. 2016, 37, 123–128. [Google Scholar] [CrossRef]
- Chaus, A.S.; Porubski, Y. Effect of modifying tungsten additions on formation of primary structure of R6M5-type high-speed steel. Phys. Met. Metall. 2012, 113, 1068–1078. [Google Scholar] [CrossRef]
- Šuštaršič, B.; Kosec, L.; Kosec, M.; Podgornik, B.; Dolinsek, S. The influence of MoS2 additions on the densification of water-atomized HSS powders. J. Mater. Process. Technol. 2006, 173, 291–300. [Google Scholar] [CrossRef]
- Yang, W.Y.; Xie, Z.B.; Wang, K.; Wang, Z.Y.; Liang, J.B.; Shao, Q.L. Study on rare earth modification of carbide structure of high speed steel. Hebei Metall. 2021, 11, 32–37+54. [Google Scholar] [CrossRef]
- Frisk, K.; Bratberg, J.; Markström, A. Thermodynamic modelling of the M6C carbide in cemented carbides and high-speed steel. Calphad 2005, 29, 91–96. [Google Scholar] [CrossRef]
- Zhang, J.W.; Zhao, Z.G.; Wang, W.X.; Wang, Y.T. Comparison of the Microstructure of M2 Steel Fabricated by Continuous Casting and with a Sand Mould. Metals 2019, 9, 560. [Google Scholar] [CrossRef]
- Hao, Y.R.; Liu, R.T.; Xiong, X. Effect of vanadium carbide on sintering properties and microstructure evolution of powder high speed steel. J. Northwestern Polytech. Univ. 2020, 38, 838–845. [Google Scholar] [CrossRef]
- Wang, Z.G. Effect of vanadium content on the microstructure and properties of W6Mo5Cr4Vx high-speed steel for bit. Iron Steel Vanadium Titan. 2021, 42, 139–143. [Google Scholar] [CrossRef]
- Xu, Q. Research on the Application of VN Alloy in High-Speed Steel. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2005; pp. 27–31. [Google Scholar]
- Li, L.J. Study on the Effect of Cobalt Content on the Microstructure and Properties of M2 High-Speed Steel. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2021; pp. 20–28. [Google Scholar]
- Chi, H.X.; Ma, D.S.; Zhan, L.C.; Xu, H.X.; Zhu, W.L. Effect of heat treatment on microstructure and mechanical property of a P/M high speed steel W6Mo5Cr4V3Co8. Trans. Mater. Heat Treat. 2012, 33, 105–109. [Google Scholar] [CrossRef]
- Moon, H.K.; Lee, K.B.; Kwon, H. Influences of Co addition and austenitizing temperature on secondary hardening and impact fracture behavior in P/M high speed steels of W–Mo–Cr–V(–Co) system. Mater. Sci. Eng. A 2007, 474, 328–334. [Google Scholar] [CrossRef]
- Toptop, G.; Kisasoz, A.; Karaaslan, A. Effect of alloying elements on the tooling capabilities of high speed steels fabricated by powder metallurgy. Mater. Test. 2013, 55, 43–46. [Google Scholar] [CrossRef]
- Wang, W.Q.; Pan, F.S.; Wu, L.Z.; Tang, A.T.; Liu, T.T. Effect of silicon on eutectic carbides in as-heated M2 high speed steel. J. Chongqing Univ. 2011, 34, 44–49. [Google Scholar] [CrossRef]
- Pan, F.S.; Wang, W.Q.; Tang, A.T.; Wu, L.Z.; Liu, T.T.; Cheng, R.J. Phase transformation refinement of coarse primary carbides in M2 high speed steel. Prog. Nat. Sci. Mater. Int. 2011, 21, 180–186. [Google Scholar] [CrossRef]
- Li, Y.J.; Jiang, Q.C.; He, Z.M.; Zhao, Y.G.; Ge, L.H. The effect of Al on the solidification process of M2 high-speed steel. CJMR 1997, 2, 216–218. [Google Scholar]
- Yang, Y.W.; Fu, H.G.; Wang, K.M.; Lei, Y.P.; Zhu, L.L.; Jiang, L. Effect of aluminum on phase diagram and solidification microstructure of high boron high speed steel. Mater. Heat Treat. 2016, 37, 48–54. [Google Scholar] [CrossRef]
- Pant, G.; Sonia, P.; Kumar, S.H. Investigation of the use of micro-alloy and as-cast micro-alloy steel in automotive application. IOP Mater. Sci. Eng. 2021, 1116, 012018. [Google Scholar] [CrossRef]
- Klemm-Toole, J.; Benz, J.; Thompson, S.W.; Findley, K.O. A quantitative evaluation of microalloy precipitation strengthening in martensite and bainite. Mater. Sci. Eng. 2019, 763, 138145. [Google Scholar] [CrossRef]
- Zhang, L.; Kannengiesser, T. Influence of microalloy design on heat-affected zone toughness of S690QL steels. Weld. World 2018, 62, 339–350. [Google Scholar] [CrossRef]
- Taylor, T.; Fourlaris, G.; Clough, A. Effect of carbon and microalloy additions on hot-stamped boron steel. Mater. Sci. Technol. 2017, 33, 1964–1977. [Google Scholar] [CrossRef]
- Xiong, W.M.; Song, R.B.; Yu, P.; Liu, Z.J.; Qin, S.; Zhang, Y.C.; Quan, S.Y.; Huo, W.F.; Zhao, Z.Y.; Su, S.R.; et al. Hot deformation behavior of V–Ti microalloy Steels. Steel Res. Int. 2020, 92, 2000225. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, M.; Tang, J.P.; Cao, J.; Wei, X.C.; Wang, W.R.; Ren, J.A. Correlation of wear resistance with carbides for Cr12MoV steel. Shanghai Met. 2021, 43, 42–49. [Google Scholar] [CrossRef]
- Wang, J.; Shi, H.S.; Yin, J.L.; Zhang, J.G. Effect of alloy element on microstructure of spray forming ultra high carbon steel. Shanghai Met. 2011, 33, 37–40+44. [Google Scholar] [CrossRef]
- Ueda, Y.; Wade, N.; Kawada, S. Graphite shape and eutectic cell structure of cast iron with incompletely spheroidal graphite. J. Jpn. Foundrymen’s Soc. 1974, 46, 212–217. [Google Scholar] [CrossRef]
- Guo, E.J.; Song, L.; Wang, L.P. Effect of Ce-Mg-Si and Y-Mg-Si nodulizers on the microstructures and mechanical properties of heavy section ductile iron. J. Rare Earths 2014, 32, 738–744. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Y.Z.; Ping, X.Z.; Li, W.; Yi, Y.L. Influence mechanism of Y addition on microstructure, adsorbability, mechanical properties of as-cast high speed steel. Mater. Sci. Eng. A 2023, 863, 144520. [Google Scholar] [CrossRef]
- Labrecque, C.; Gagné, M. Review ductile iron: Fifty years of continuous development. Can. Metall. Q. CMQ 1998, 37, 343–378. [Google Scholar] [CrossRef]
- Franzen, D.; Pustal, B.; Bührig-Polaczek, A. Influence of graphite-phase parameters on the mechanical properties of high-silicon ductile iron. Int. J. Met. 2023, 17, 4–21. [Google Scholar] [CrossRef]
- Soedarsono, J.W.; Soemardi, T.P.; Suharno, B.; Sulamet-Ariobimo, R.D. Effects of carbon equivalent on the microstructures of thin wall ductile iron. J. Mater. Sci. Eng. 2011, 5, 266–270. [Google Scholar] [CrossRef]
- Luo, Y.W.; Guo, H.J.; Sun, X.L.; Guo, J. Influence of the nitrogen content on the carbide transformation of AISI M42 high-speed steels during annealing. Sci. Rep. 2018, 8, 4328–4329. [Google Scholar] [CrossRef] [PubMed]
- Hara, R.; Yamamoto, M.; Ito, G.; Kamimiyada, K.; Narita, I.; Miyahara, H. Effect of nitrogen on the microstructure and hardness of high-carbon high-speed tool steel type alloys. Mater. Trans. 2016, 57, 1945–1951. [Google Scholar] [CrossRef]
- Halfa, H.; Eissa, M.; Fawakhry, K.; Mattar, T. Effect of nitrogen and niobium on the structure and secondary hardening of super hard high speed tool steel. Steel Res. Int. 2012, 83, 32–42. [Google Scholar] [CrossRef]
- Li, Y.J.; Jiang, Q.C.; Zhao, Y.G.; He, Z.M.; Zhong, X.Y. Influence of B on solidification structure of M2 high speed steel. J. Mater. Res. 1999, 13, 183–187. [Google Scholar]
- Yuan, Z.T.; Jiang, Y.H.; Li, L.; Feng, J. First-principles study on the phase stability and mechanical properties of boron carbides in boron-bearing high-speed steel. Sci. Adv. Mater. 2018, 10, 1475–1483. [Google Scholar] [CrossRef]
- Astini, V.; Prasetyo, Y.; Baek, E.R. Effect of boron addition on the microstructure and mechanical properties of 6.5% V-5% W high speed steel. Met. Mater. Int. 2012, 18, 923–931. [Google Scholar] [CrossRef]
- Tewary, U.; Paul, D.; Mehtani, H.K.; Bhagavath, S.; Alankar, A.; Mohapatra, G.; Sahay, S.S.; Panwar, A.S.; Karagadde, S.; Samajdar, I. The origin of graphite morphology in cast iron. Acta Mater. 2022, 226, 117660. [Google Scholar] [CrossRef]
- Duan, J.T.; Jiang, Z.Q.; Fu, H.G. Effect of RE-Mg complex modifier on structure and performance of high speed steel roll. J. Rare Earths 2007, 25, 259–263. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, K.H.; Chang, L.Z.; Chen, J.S.; Zheng, F.Z. The effect of Mg on carbides in M2 high speed tool steel. J. Iron Steel Res. 2019, 31, 1046–1052. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Zarychta, A.; Ligarski, M. High-speed steels with addition of niobium or titanium. J. Mater. Process. Technol. 1997, 63, 531–541. [Google Scholar] [CrossRef]
- Shahram, K. Effect of Ti and Nb on the formation of carbides and the mechanical properties in as-cast AISI-M7 high-speed steel. ISIJ Int. 2001, 41, 1502–1509. [Google Scholar] [CrossRef]
- Chaus, A.S.; Bogachik, M.; Uradnik, P. Structural transformations during heat treatment of W-Mo cast high-speed steel modified using titanium diboride. J. Phys. Met. Metallogr. 2011, 112, 470–479. [Google Scholar] [CrossRef]
- Chaus, A.S.; Dománková, M. Precipitation of secondary carbides in M2 high-speed steel modified with titanium diboride. J. Mater. Eng. Perform. 2013, 22, 1412–1420. [Google Scholar] [CrossRef]
- Fu, H.G.; Xiao, Q.; Kuang, J.C.; Jiang, Z.Q.; Xing, J.D. Effect of rare earth and titanium additions on the microstructures and properties of low carbon Fe-B cast steel. Mater. Sci. Eng. A 2007, 466, 160–165. [Google Scholar] [CrossRef]
- Fu, H.G.; Jiang, Z.Q.; Li, M.W.; Zhang, Y.; Xing, J.D. Investigations on structure and performance of high speed steel roll modified by titanium additions. Foundry 2007, 56, 590–593. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Zhang, D.L.; Shan, Q.; Zhang, F.; Wang, X.Y. Influence of rare-earth element doping on interface and mechanical properties of WC particles reinforced steel matrix composites. Mater. Res. Express 2021, 8, 036512. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, C.J. Agglomeration characteristics of various inclusions in Al-killed molten steel containing rare earth element. Metall. Mater. Trans. B 2020, 51, 2585–2595. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Z.B.; Zheng, K.H.; Long, J.; Wang, J.; Ren, Y.Y.; Li, Y.M. High temperature oxidation behavior of heat resistant steel with rare earth element Ce. Mater. Res. Express 2020, 7, 016571. [Google Scholar] [CrossRef]
- Wang, C.G.; Ma, R.Y.; Zhou, Y.T.; Liu, Y.; Daniel, E.F.; Li, X.F.; Wang, P.; Dong, J.H.; Ke, W. Effects of rare earth modifying inclusions on the pitting corrosion of 13Cr4Ni martensitic stainless steel. J. Mater. Sci. Technol. 2021, 93, 232–243. [Google Scholar] [CrossRef]
- Deng, X.T.; Misra, R.D.K.; Wang, Z.D.; Jiang, Y.Y. Microstructure and mechanical properties of low alloy ultra-high strength steel microalloyed with erium. Key Eng. Mater. 2021, 871, 53–58. [Google Scholar] [CrossRef]
- Wei, W.Z.; Wu, K.M.; Zhang, X.; Liu, J.; Qiu, P.; Cheng, L. In-situ characterization of initial marine corrosion induced by rare-earth elements modified inclusions in Zr-Ti deoxidized low-alloy steels. J. Mater. Res. Technol. 2020, 9, 1412–1424. [Google Scholar] [CrossRef]
- Wang, D. Effects of Rare Earth on the Microstructure and Properties of M35 High-Speed Steel. Ph.D. Thesis, Southeast University, Nanjing, China, 2005; pp. 41–51. [Google Scholar]
- Zhou, X.F.; Fang, F.; Tu, Y.Y.; Jiang, J.Q.; Zhu, W.L.; Yin, S.Y. Carbide refinement in M42 high speed steel by rare earth metals and spheroidizing treatment. J. Southeast Univ. (Engl. Ed.) 2014, 30, 445–448. [Google Scholar]
- Gu, J.B.; Li, J.Y.; Chang, R.J. Enhanced refinement of Cr23C6 by heterogeneous nucleation in annealed nitrogen-alloyed 4Cr5Mo2V die steel. Metall. Mater. Trans. A 2019, 50, 518–522. [Google Scholar] [CrossRef]
- Jiao, W.C.; Li, H.B.; Feng, H.; Jiang, Z.H.; Xia, L.F.; Zhang, S.C.; Zhu, H.C.; Wu, W. Evolutions of micro- and macrostructure by cerium treatment in as-Cast AISI M42 high-speed Steel. Metall. Mater. Trans. B 2020, 51, 2240–2251. [Google Scholar] [CrossRef]
- Ji, Y.P.; Li, Y.M.; Zhang, M.X.; Ren, H.P. Crystallography of the heterogeneous nucleation of δ-Ferrite on Ce2O2S particles during solidification of an Fe-4Si alloy. Metall. Mater. Trans. A 2019, 50, 1787–1794. [Google Scholar] [CrossRef]
- Zhou, X.F.; Yin, X.Y.; Fang, F.; Jiang, J.Q.; Zhu, W.L. Influence of rare earths on eutectic carbides in AISI M2 high speed steel. J. Rare Earths 2012, 30, 1075–1078. [Google Scholar] [CrossRef]
- Hufenbach, J.; Helth, A.; Lee, M.H.; Wendrock, H.; Giebeler, L.; Choe, C.Y.; Kim, K.H.; Kühn, U.; Kim, T.S.; Eckert, J. Effect of cerium addition on microstructure and mechanical properties of high-strength Fe85Cr4Mo8V2C1 cast steel. Mater. Sci. Eng. A 2016, 674, 366–374. [Google Scholar] [CrossRef]
- Wang, M.J.; Mu, S.M.; Sun, F.F.; Wang, Y. Influence of rare earth elements on microstructure and mechanical properties of cast high-speed steel rolls. J. Rare Earths 2007, 25, 490–494. [Google Scholar] [CrossRef]
- Boccalini, M., Jr.; Correa, A.V.O.; Goldenstein, H. Rare earth metal induced modification of γ-M2C, γ-M6C, and γ-MC eutectics in as cast M2 high speed steel. Mater. Sci. Technol. 1999, 15, 621–626. [Google Scholar] [CrossRef]
- Zelič, K.; Burja, J.; McGuiness, P.J.; Godec, M. Effect of rare earth elements on the morphology of eutectic carbides in AISI D2 tool steels: Experimental and modelling approaches. Sci. Rep. 2018, 8, 9233–9238. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.C.; Zhang, W.L.; Hu, B.T.; Zhang, Z.H.; Gao, S.T.; Liu, B.W. Influence of rare earth elements on microstructure and mechanical properties of high speed steel. Powder Metall. Technol. 2017, 35, 416–421. [Google Scholar] [CrossRef]
- Chaus, A.S. Use of REM-Based Modifying Agents for Improving the Structure and Properties of Cast Tungsten-Molybdenum High-Speed Steels. Metal. Sci. Heat Treat. 2004, 46, 415–422. [Google Scholar] [CrossRef]
- Liu, B.L.; Lyu, Z.Q.; Feng, W.W.; Ren, T.Z.; Fu, W.T. Precipitation and decomposition behaviors of carbides in AISI M2 high-speed steel with nitrogen and mischmetal. J. Cent. South. Univ. 2017, 24, 782–788. [Google Scholar] [CrossRef]
- Wang, M.J.; Li, Y.M.; Wang, Z.X.; Bao, E. Effect of rare earth elements on the thermal cracking resistance of high speed steel rolls. J. Rare Earths 2011, 29, 489–493. [Google Scholar] [CrossRef]
- Li, Y.J.; Jiang, Q.C.; Zhao, Y.G.; He, Z.M.; Zhong, X.Y. Influence of cerium on solidification structure of M2 high speed steel. J. Chin. Soc. Rare Earths 1999, 17, 54–57. [Google Scholar] [CrossRef]
- Kunz, J.; Köhler, M.L.; Herzog, S.; Kaletsch, A.; Broeckmann, C. Influence of an increasing alloying content on the microstructure of a high-speed steel in the Laser-Powder Bed Fusion process. Steel Res. Int. 2021, 92, 2100438. [Google Scholar] [CrossRef]
- Guo, Y.F.; Qi, W.T.; Xia, Z.B.; Zhao, X.H.; Li, Q.; Liu, C.M.; Ding, B.; Shen, Z.; Zheng, T.X.; Zhong, Y.B. Morphology tailoring of metal pool and eutectic carbides in magnetic-controlled electroslag remelted M2 high-speed steel. J. Mater. Res. Technol. 2022, 16, 1122–1135. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, W.J.; Zhang, Z.H.; Xie, J.X. Composition, microstructure, and inclusion characteristics of H13 steel ingot by electroslag remelting with rare earth slag. J. Iron Steel Res. 2021, 33, 966–978. [Google Scholar] [CrossRef]
- Cao, Y.L.; Zhao, Z.R.; Wan, X.L.; Li, G.Q.; Jiang, Z.H.; Dong, Y.W. Carbide characteristics of high vanadium high-speed steel manufactured by electroslag remelting. ISIJ Int. 2022, 62, 1430–1438. [Google Scholar] [CrossRef]
- Shi, H.; Li, L.J.; Wang, H.Y.; Zhu, L.M.; Zhai, Q.J. Effect of PMO on rating and distribution of banded carbides in GCr15 steel bars. Shanghai Met. 2020, 42, 57–62+70. [Google Scholar] [CrossRef]
- Li, K.C.; Li, L.J.; Cai, C.Q.; Xu, Y.H.; Xu, H.; Zhai, Q.J. Effect of M-PMO on inclusion of HRB400EG thread steel continuous casting billet. Iron Steel 2023, 58, 58–68. [Google Scholar] [CrossRef]
- Liu, Y.J.; Xu, G.D.; Wang, Y.C.; Zhong, H.G.; Li, L.J.; Wang, B.; Zhai, Q.J. Effects of Pulse magneto-oscillation on GCr15 bearing steel continuous casting billet. J. Iron Steel Res. Int. 2022, 29, 144–150. [Google Scholar] [CrossRef]
- Liu, H.N.; Chen, Y.M.; Chen, X.R.; Li, L.J.; Zhai, Q.J. Effect of PMO on dendritic structure and carbide of high-speed steel. Iron Steel 2023. [Google Scholar] [CrossRef]
- Xu, H.; Li, L.J.; Cai, C.Q.; Zheng, Y.S.; Zhong, H.G.; Zhai, Q.J. Study on improving quality of continuous casting billet by using mould PMO. Shanghai Met. 2019, 41, 75–79. [Google Scholar] [CrossRef]
- Wang, Y.C.; Xu, R.J.; Zhong, H.G.; Xu, G.D.; Xu, Z.S.; Li, R.X.; Zhai, Q.J. Effects of pulsed magneto-oscillation on the homogeneity of low carbon alloy steel continuous casting round billet. Metals 2022, 12, 833. [Google Scholar] [CrossRef]
- Wei, R.X.; Zhang, H.; Li, R.X.; Chen, Y.Q.; Li, C.B.; Liu, H.N. Influence of PMO on solidification structure and central segregation of hard wire steel billet. China Metall. 2023, 33, 115–121. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, L.F.; Wang, Y.; Chen, W.; Wang, X.D.; Zhang, J.Y. Effect of electromagnetic stirring on inclusions in continuous casting blooms of a gear steel. Metall. Mater. Trans. B 2021, 52, 2341–2354. [Google Scholar] [CrossRef]
- Li, P.C.; Zhang, G.F.; Yan, P.; Tian, N.; Feng, Z.H. Numerical and experimental study on carbon segregation in shaped billet of medium carbon steel with combined electromagnetic stirring. Materials 2023, 16, 7464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.H.; Sun, Z.H.; Xia, Z.B.; Liu, C.M.; Shen, Z.; Ding, B.; Zheng, T.X.; Guo, Y.F.; Li, Q.; Zhong, Y.B. Morphology control of metal pool and eutectic carbides in electroslag remelted M2 HSS with an external axial static magnetic field. Metals 2023, 13, 912. [Google Scholar] [CrossRef]
- Chen, D.H.; Xu, X.F.; Zhao, Y.; Fu, X.G.; Wei, L.; Zhou, Y.C.; Wu, Z.C. Superior mechanical properties of M35 high-speed steel obtained by controlling carbide precipitation and distribution via electropulsing treatment. Mater. Sci. Eng. A 2023, 888, 145691. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).