Abstract
Aluminium–magnesium alloys find widespread application in diverse industrial and technological fields owing to their unique characteristics such as lightweight nature, favourable physical and mechanical properties, corrosion resistance and cost-effectiveness. During production, these alloys often undergo various forming processes that significantly affect the morphology and microstructure of their surface layers. Consequently, the surface properties, including corrosion resistance, are notably influenced by these treatments. In this study, the impact of cold rolling on the corrosion behaviour of the 5083 aluminium alloy, which is considered as an important alloy for the aerospace and naval industry, was investigated. The 5083 Al alloy underwent a cold-rolling process, resulting in specimens with reduced average thicknesses of 7% and 15%, respectively. The microstructure of the alloy was examined by using X-ray diffraction, optical and scanning electron microscopy techniques. Furthermore, the corrosion behaviour of both the as-received and cold-rolled aluminium alloy specimens was evaluated through potentiostatic and potentiodynamic corrosion measurements. The experimental results demonstrated that higher cold deformation percentages, within the specified experimental parameters, led to an enhanced corrosion resistance for the alloy. This improvement was primarily attributed to the reduction in grain size induced by recrystallization and to the formation of a passivating aluminium oxide film.
1. Introduction
Aluminium–magnesium alloys have been widely used in various technological and industrial applications, due to their light weight, good physical and mechanical properties, corrosion resistance and low cost [1]. Among these alloys, 5083 aluminium wrought alloy is considered as one of the most important materials of this series [1]. It consists primarily of aluminium, along with small amounts of magnesium, manganese, chromium and other trace elements. Its unique combination of mechanical properties and corrosion resistance makes it well-suited for the aerospace [2,3] naval [4] and construction [5] industries. Some relevant reports concerning the formability and corrosion behaviour of this alloy are presented below:
Ezuber et al. [6] studied the corrosion behaviour of 5083 and 1100 aluminium alloys in seawater at 23 and 60 °C. These researchers found that the breakdown potential of the two alloys decreased with an increase in test temperature, whereas 1100 aluminium alloy had slightly better corrosion resistance than 5083 aluminium alloy. In addition, they observed that both alloys suffered from pitting corrosion. Aballe et al. [7] investigated the influence of surface finishing on the corrosion behaviour of 5083 aluminium alloy in a 3.5% NaCl aerated solution. The aluminium alloy specimens were polished from 80 to 1200 grit, and their corrosion behaviour was studied by using weight loss, linear polarizations and electrochemical noise measurement (ENM) techniques. They observed that the alloy specimens that were polished up to 80 grit showed greater susceptibility to localised alkaline corrosion than the samples that had been polished up to 1200 grit. The decrease of the corrosion resistance with the increase in polishing was attributed to the number of intermetallic particles exposed per materials surface.
Panagopoulos and Georgiou [8] studied the corrosive wear behaviour of 5083 wrought aluminium alloy in a 3.5% NaCl solution (pH = 5.5). The counterface material was a stainless steel pin. They observed that for a constant value of sliding speed, the increase of applied load resulted in a decrease of the friction coefficient of the tribo-system stainless steel–5083 Al alloy. They also observed that the dominant wear mechanisms of this aluminium alloy were plastic deformation and abrasion. These wear mechanisms coexisted with pitting corrosion phenomena on the surface of the alloy. Zazi et al. [9] investigated the corrosion behaviour of an aluminium 5083–H321 alloy that had undergone annealing at 420 °C for 1 h 30 min, followed by various types of thermo-mechanical treatments. The aim was to see the effect of intermetallic phases on the corrosion of their alloy in 3.5% NaCl solution. They observed that deformation resulted in a significant increase in the density of intermetallics and micropores, which degrade the corrosion resistance. However, by adding intermediate annealing steps that bring about recrystallization, the corrosion behaviour can be controlled.
Li et al. [10] reported on the effect of texturing on the stress corrosion cracking of 5083 aluminium alloy sheets. They found that texturing influenced mainly the stress corrosion properties of the alloy, whereas no significant differences were observed in terms of intergranular corrosion. Specifically, samples dominated by the brass texture had higher stress corrosion cracking resistance compared to low-intensity textures. The different performance was linked to the propagation path of the cracks, which, in the case of brass textures cracks, is more tortuous. Faraji et al. [11] investigated the effect of heating time and rate on the stabilization of the 5083 aluminium alloy sheets. They found that by using an appropriate combination of these two factors, both the mechanical properties and corrosion resistance of this alloy can be improved. This improvement was linked to a fine-grained structuring after thermomechanical processing.
Abdulstaar et al. [12] studied the effect of plastic deformation of both the surface layers and bulk material on the fatigue, corrosion, and corrosion fatigue performance of 5083 aluminium alloys. Plastic deformation of the surface layers was performed by applying ball-burnishing and shot peening methods, whereas, to achieve severe deformation of the bulk alloy, rotary swaging was used. They observed that the mechanical properties and fatigue life were significantly improved after surface and/or bulk deformation, but the ductility was reduced due to work-hardening. In addition, the surface deformation techniques showed higher fatigue life enhancement in air than rotary swaging, attributed to the higher ductility of the bulk region. On the contrary, the selected surface deformation techniques exhibited higher corrosion rates, caused by localised pitting corrosion around the Fe-rich phases. Rotary swaging had a higher corrosion resistance, since the secondary phase size became smaller and more well distributed. When a synergism between electrochemical and mechanical phenomena took place, the surface deformation processes resulted in superior corrosion fatigue performance than the bulk rotary swagging.
Beura et al. [13] studied the influence of mechanical deformation on the corrosion behaviour of 5083-H131 aluminium alloys by combining electrochemical and microstructural characterizations. The deformation of the alloy was performed with forward Taylor anvil experiments. The main research outcome of this work was that significant differences were found in the corrosion performance between the impacted region and the undeformed part. This phenomenon was attributed to the local fragmentation of Fe-based intermetallic particles and to an increase in intragranular misorientation near the deformed area. In particular, the fractured intermetallic colonies were identified as catalysts for corrosion reactions, serving as sites for extensive trenching corrosion.
Recently, some publications [14,15] focused on the effect of additive manufacturing processes on the corrosion behaviour of 5083 aluminium alloys. Zhou et al. [14] reported on the effect of adding 0.7 wt% and 1.0 wt% Zr as an alloying element on the printability and buildability of AA5083 alloys produced by laser powder bed fusing. They found that the addition of Zr reduces cracking and improves grain refinement. These microstructural changes improved the corrosion resistance of alloy in a 3.5 wt% NaCl aqueous solution. Li et al. [15] investigated the corrosion behaviour of 5083 Al-Mg alloys manufactured by additive friction stir deposition. They observed that this additive manufacturing method reduced pitting corrosion sensitivity due to refinement and re-dissolution of Mn-rich secondary phase particles. However, the intergranular corrosion susceptibility of the alloy increased due to exfoliation of the fine equiaxed grains.
To conclude, complex corrosion phenomena can be significantly affected by various factors, such as heat treatment [16], surface treatments [17], forming processes [18], synergism between electrochemical phenomena and mechanical stresses (e.g., stress corrosion cracking) [19,20], etc. It is evident that any change in the microstructure of the surface or near surface layers [21,22,23] (e.g., grain structure, secondary phase composition and distribution, texturing etc.), dislocation density, surface topography, generation of localized stresses and/or micro-defects can have a detrimental effect on the corrosion performance of a material that undergoes any forming process. This has been reported not only for aluminium alloys, but also for other extensively applied materials such as steels [24], magnesium [25] and cobalt-based [26] alloys. Thus, this research investigation focuses on the effect of cold rolling on the corrosion behaviour of 5083 aluminium alloy in a 3.5% NaCl solution. Up to date, there is limited work published on the effect of purely cold rolling on the electrochemical performance of this alloy. In addition, it should be taken into consideration that such aluminium alloys are submitted to various forming treatments before being used in industrial applications. Linking forming processes to technological properties is essential for understanding and optimizing the performance of these alloys.
2. Materials and Methods
The material investigated in this research work was a commercially supplied 5083 aluminium alloy, with the following weight percentage (wt.%) chemical composition: 95.2% Al, 3.5% Mg, 0.5% Mn, 0.3% Si, 0.26% Cr and 0.24% Fe. Sheets of the aluminium alloy, measuring 50 cm × 30 cm × 0.3 cm, underwent a cold rolling process in a Mario Di Maio LS630x350 cold rolling mill (Mario Di Maio, Milan, Italy). The average thickness of the sheers was reduced by 7% and 15%, respectively, in a single pass, at a rolling mill speed of 10 m/min. Specimens of dimensions 5 cm × 2 cm × 0.3–0.25 cm were then prepared for both structural and corrosion studies.
To ensure uniform surface topography, all aluminium alloy specimens underwent polishing using SiC papers with incrementally finer finishes and 3 μm diamond paste. The resulting average roughness (Ra), measured with a Mahr-Perthen Stylus Profilometer (Mahr-Perthen, Göttingen, Germany), was approximately 0.25 μm. Following specimen preparation, a stress relief treatment was applied by annealing the specimens at 200 °C for 2 h, with gradual cooling in an automatic furnace under an Ar inert atmosphere. The chosen annealing temperature of 200 °C was selected to avoid exceeding the recrystallization temperature of aluminium. Microhardness tests were conducted on both the surface and cross-section of the samples using a Shimadju Vickers indenter (Shimadju, Kyoto, Japan), applying a force of 0.15 N for 15 s. Five independent measurements were taken for each presented average value.
Potentiostatic and potentiodynamic corrosion experiments were conducted in a 3.5% NaCl solution at 20 °C using an EG&G Potentiostat-Galvanostat instrument (Princeton Applied Research, Oak Ridge, TN, USA). The reference electrode employed was a Standard Calomel Electrode (SCE), while the cathode used was a Platinum Electrode. The scan rate was set at 0.2 mV/sec, and triplicate tests were performed for each condition. For the analysis of the as-received, cold-rolled, and corroded alloys, a Siemens D 5000 X-ray Diffractometer (XRD) (Bruker, Billerica, MA, USA) with Cu Ka radiation, Cu filter and a graphite monochromator was utilized. Additionally, the surface and microstructure of 5083 aluminium alloy, before and after the cold-rolling process, was evaluated with an optical microscope and a Jeol 6100 Scanning Electron Microscope (SEM) (Jeol, Tokyo, Japan), connected to a Noran TS 5500 Electron Dispersive X-ray Analyzer (EDS) (Thermo Fisher Scientific, Waltham, MA, USA). Grain structuring was revealed through chemical etching with Graff’s reagent (84 mL H2O, 15.5 mL HNO3, 0.5 mL HF, and 3 g Cr2O3) for 90 sec at room temperature. Intermetallic structuring was studied via chemical etching with Keller’s reagent (2 mL HF, 3 mL HCl, 5 mL HNO3, 190 mL H2O) for 15 sec at room temperature. Finally, to evaluate changes in grain size and secondary phase content, before and after the cold-rolling process, optical and SEM images were analysed with Image Pro Plus software (4.5, Media Cybernetics, Rockville, MD, USA).
3. Results and Discussion
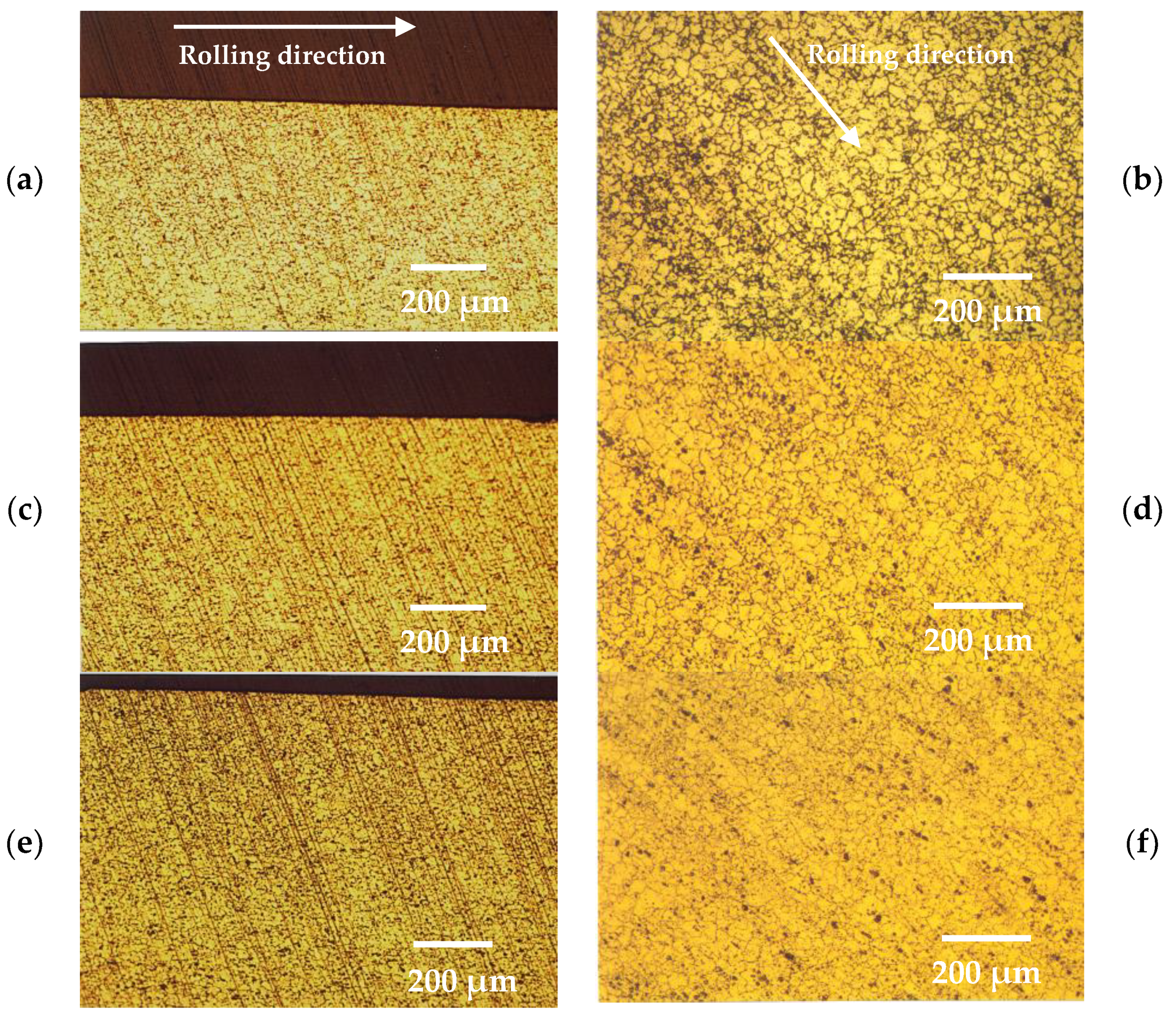
The metallographic microstructure of the surface layers in the 5083 aluminium alloy, both prior to and following the thickness reduction and deformation, is illustrated in Figure 1. It is evident from these micrographs that the increase in the percentage of cold rolling resulted in a decrease of the average grain size in the surface layers of the alloy. This reduction can be attributed to grain recrystallization induced by stress during the cold-forming process [27,28].
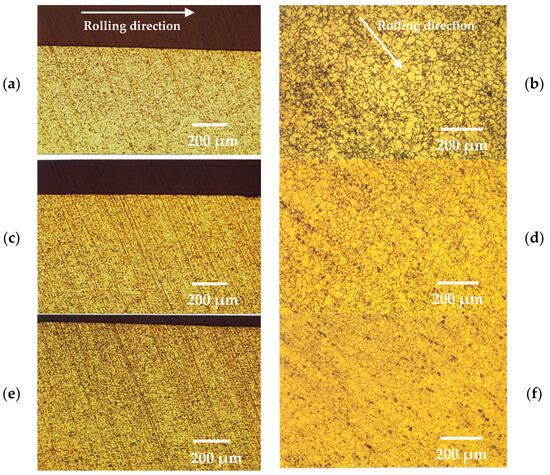
Figure 1.
Optical metallography of surface layers from (a) cross section and (b) surface of as-received 5083 alloy, (c) cross section and (d) surface of 7% cold-rolled 5083 alloy and, (e) cross section and (f) surface of 15% cold-rolled 5083 alloy (Chemical etching Graff’s reagent).
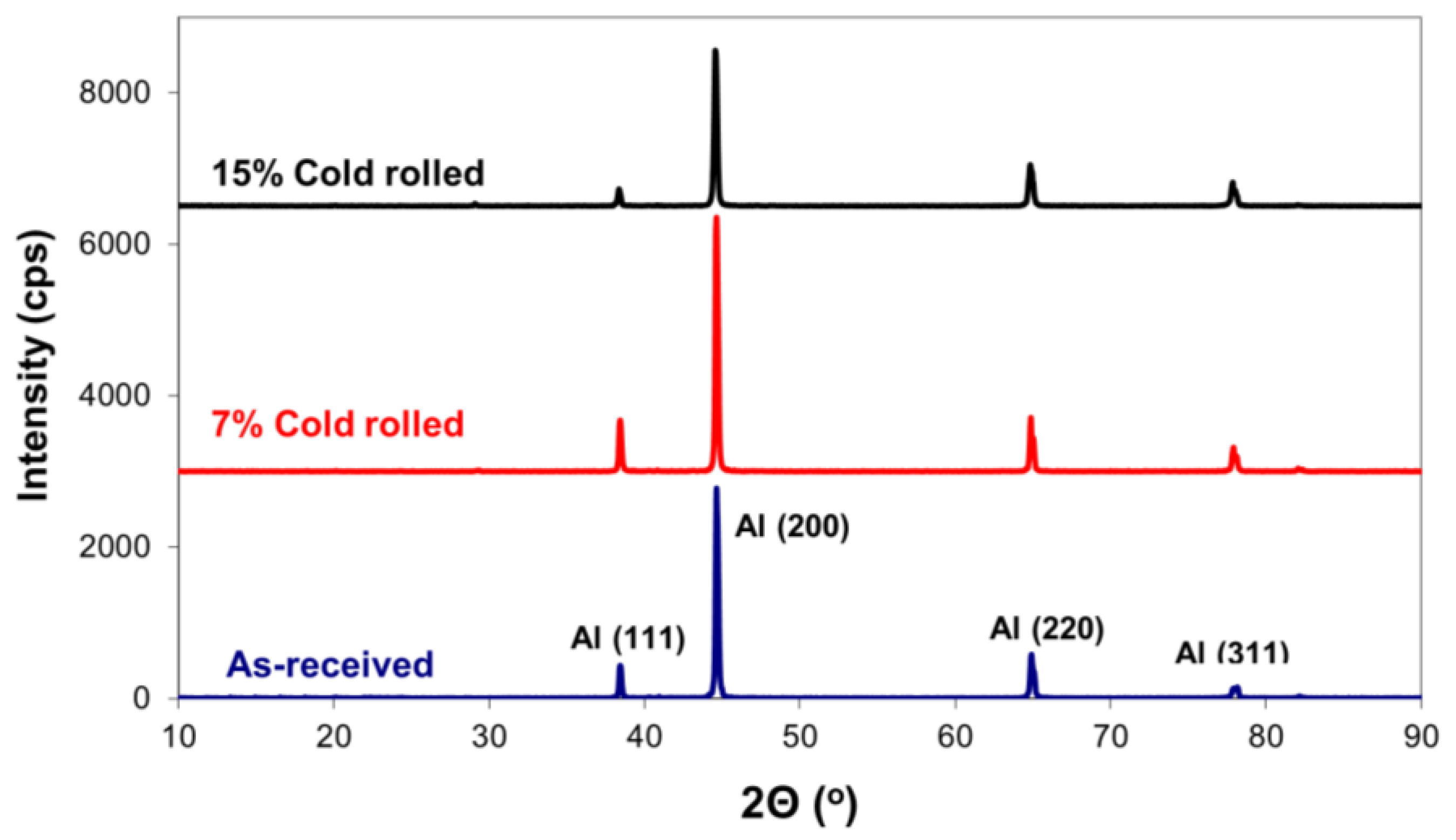
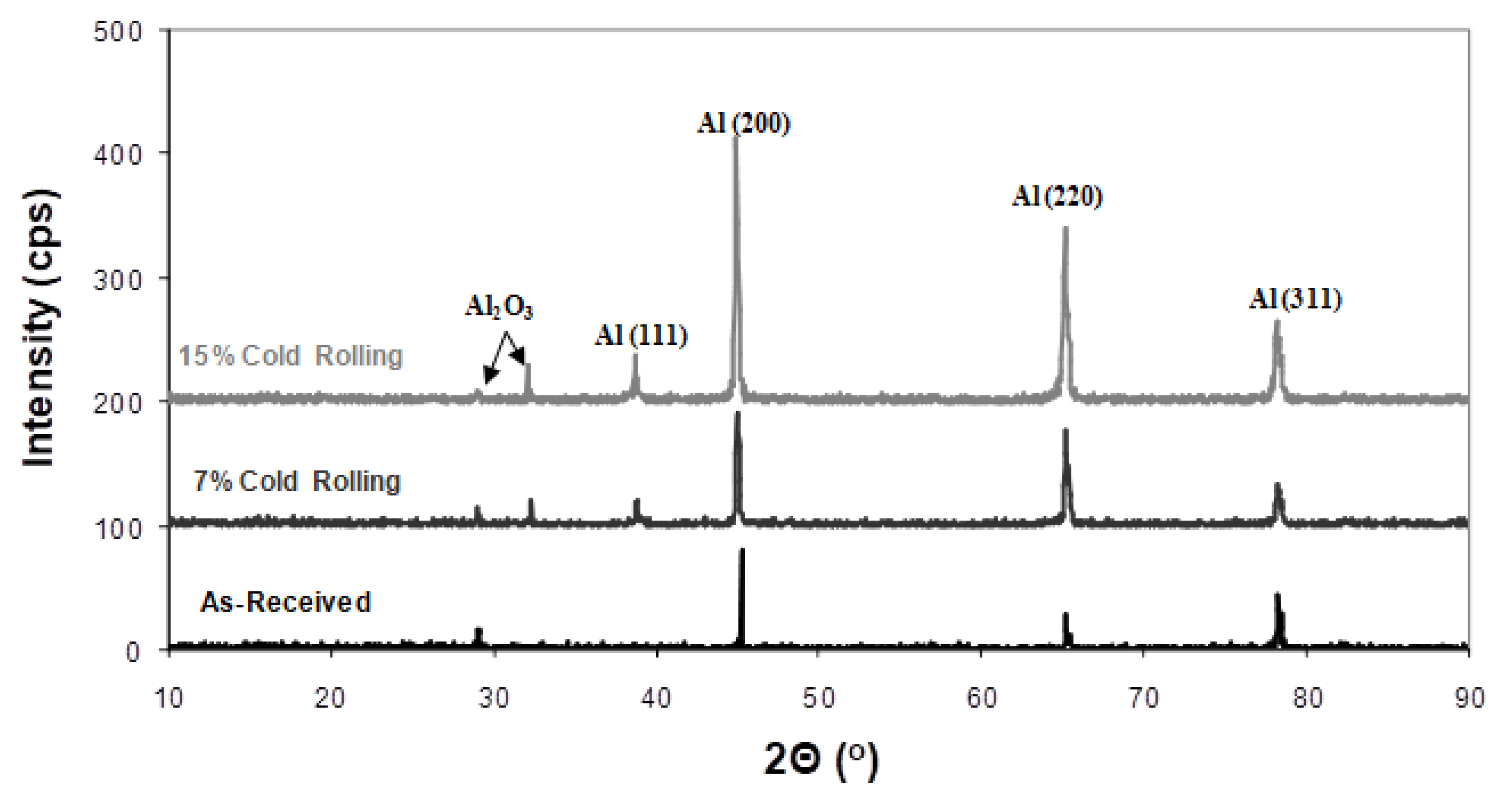
X-ray diffraction analysis of 5083 alloy, both with and without cold rolling, revealed identical aluminium crystallographic planes, Figure 2. No secondary intermetallic phases were detected, suggesting concentrations below the method’s detection limit [29]. However, it is important to highlight that the relative intensity among these aluminium peaks changed, indicating the development of crystallographic texturing. To evaluate this phenomenon, texture coefficients (TC) were calculated for each orientation, as presented in Table 1 [30]. In accordance with these calculations, texture coefficients exceeding one signify a preferred orientation. Therefore, under the specific cold-rolling conditions, preferred orientations for texture development were observed for crystallographic planes (200) and (220).
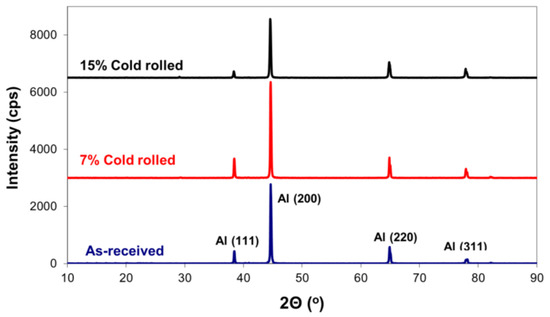
Figure 2.
Effect of cold rolling on the X-ray diffraction patterns of 5083 Al alloy.

Table 1.
Effect of cold-rolling percentage on the crystallographic texture coefficient (TC) of 5083 aluminium alloy.
To obtain a better understanding of the microstructure of this alloy, scanning electron microscopy and EDS chemical spot analysis techniques were used. Across all instances, a similar microstructure was noticed, with the alloy comprising (1) aluminium solid solution, (2) Mg2Si intermetallic phases and (3) insoluble Si, as indicated in Figure 3.

Figure 3.
SEM image showing an indicative microstructure of as-received 5083 aluminium alloy (chemical etching Keller’s reagent).
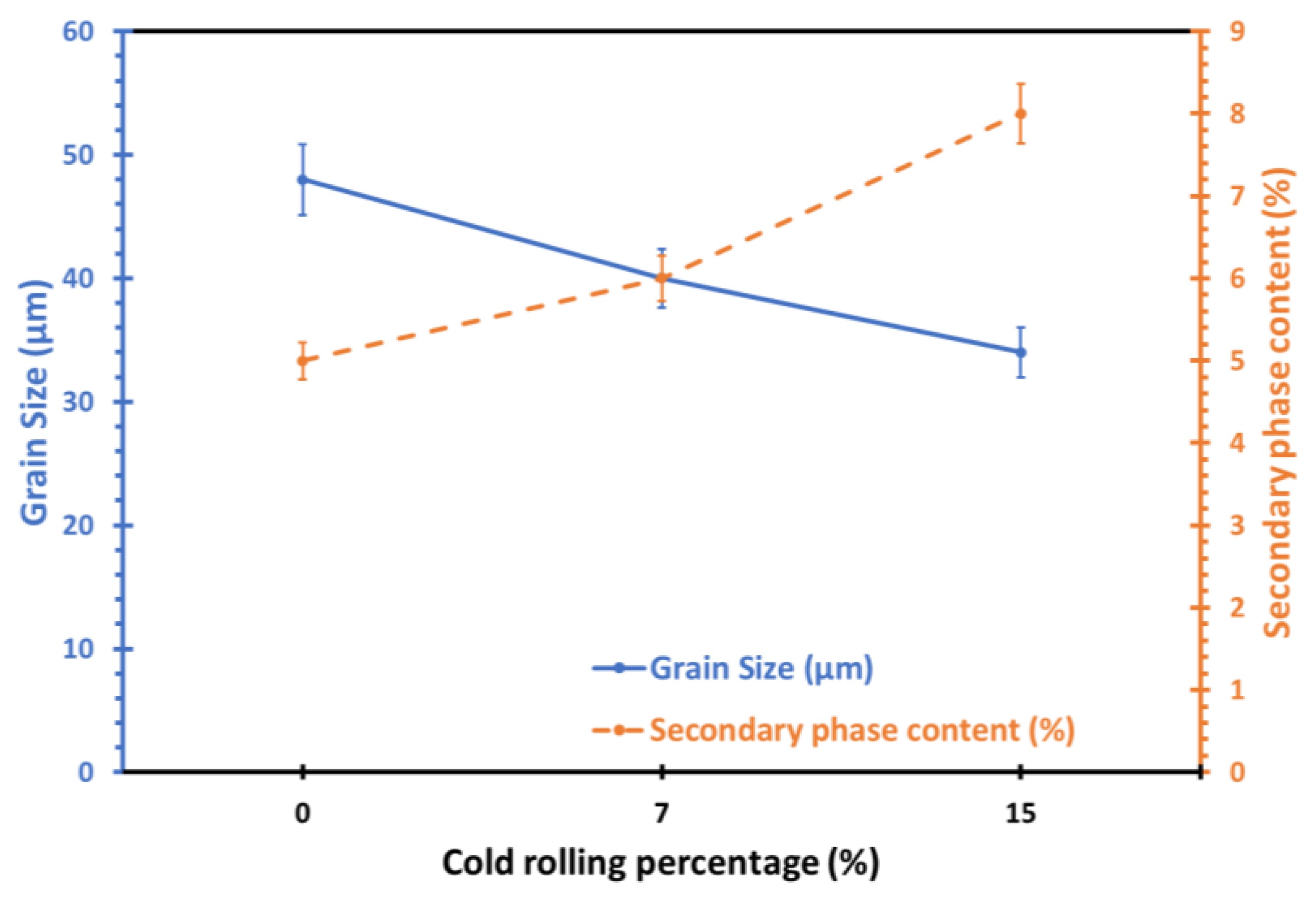
To quantify the changes in grain size and intermetallic content, post analysis of the optical microscopy and SEM images was conducted with the use of Image Pro Analysis software, Figure 4. The results revealed that the average grain size in the surface and deformed layers decreased from 48 µm in the non-rolled alloy to 40 µm with 7% cold rolling and further to 34 µm with 15% cold rolling for the same alloy. Conversely, the percentage of secondary phases increased, rising from approximately 5% in the as-received alloy to around 6% and 8% for the 7% and 15% cold-rolled samples, respectively.
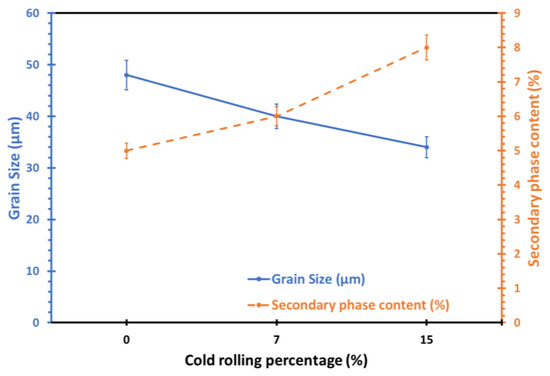
Figure 4.
Effect of cold-rolling percentage on the average grain size and secondary phase content of 5083 Al alloy.
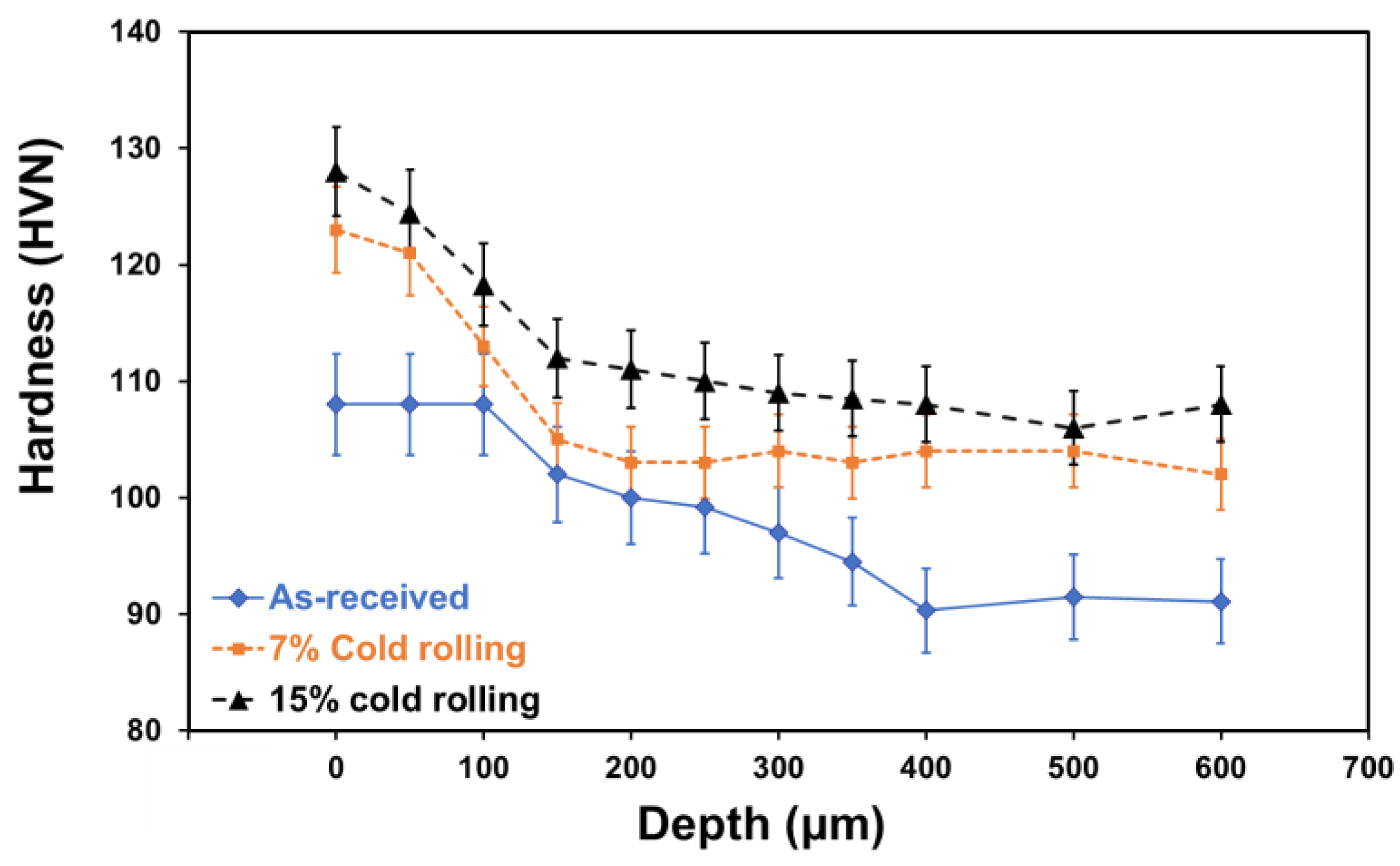
Microhardness measurements were carried out on both the surface and cross section of the 5083 alloy with and without cold rolling, as shown in Figure 5. It is evident from this figure that the increase of cold deformation resulted in an increase of microhardness of the alloy. Moreover, the depth of strain hardening became more pronounced with a higher deformation percentage (thickness reduction). This phenomenon can be explained by the reduction in grain size and the increase in secondary phases, as illustrated in Figure 4. Notably, grain boundaries act as obstacles to dislocation movement and multiplication, and according to the Hall–Petch theory [31], the smaller the grain diameter, the greater the material’s hardness. Additionally, higher concentration and better distribution of intermetallic phases in the alloy [32] contribute to increased hardness, attributed to precipitation hardening mechanisms [33].
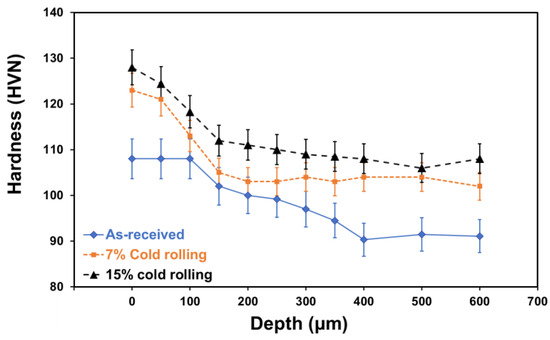
Figure 5.
Effect of cold rolling on the microhardness profile of 5083 aluminium alloy.
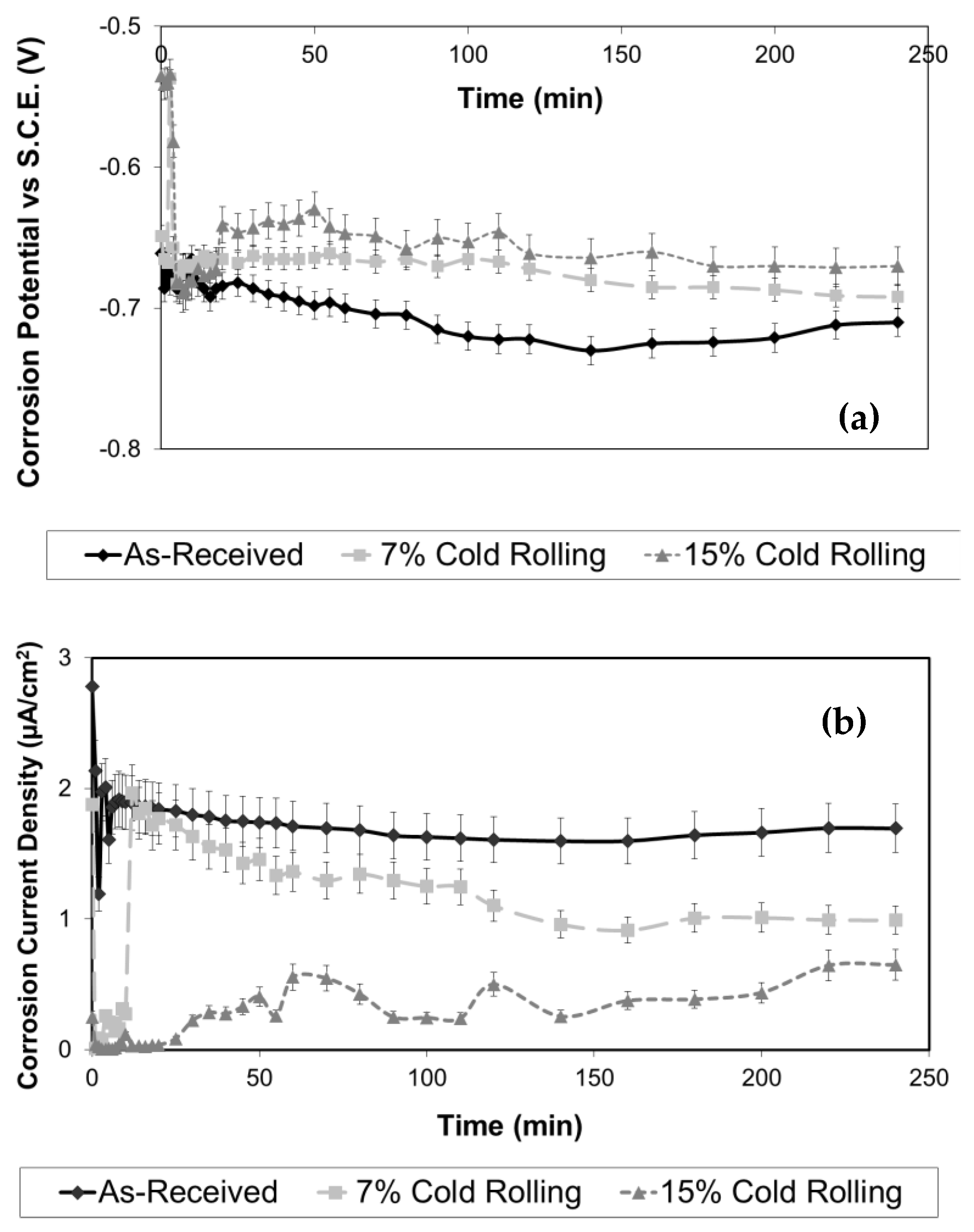
In Figure 6a, typical potentiostatic V(t) corrosion curves are presented for both the as-received and cold-rolled 5083 aluminium alloy specimens. Upon comparison of these curves, it can be observed that the increase in cold deformation (thickness reduction) resulted in a shift of the potentiostatic curves towards more noble corrosion potential values versus the SCE electrode. It is well established that as the corrosion potential of a metallic material becomes more noble, its susceptibility to corrosion decreases. Consequently, the aluminium alloy specimens subjected to 15% cold rolling exhibited the highest corrosion resistance, followed by the specimen with 7% cold rolling and the as-received specimen. Furthermore, all the potentiostatic curves exhibited a similar shape. This observation may be attributed to the fact that the cold-rolling process did not significantly affect the chemical composition of the surface layers of this alloy, indicating minimal impact on micro-segregation phenomena.
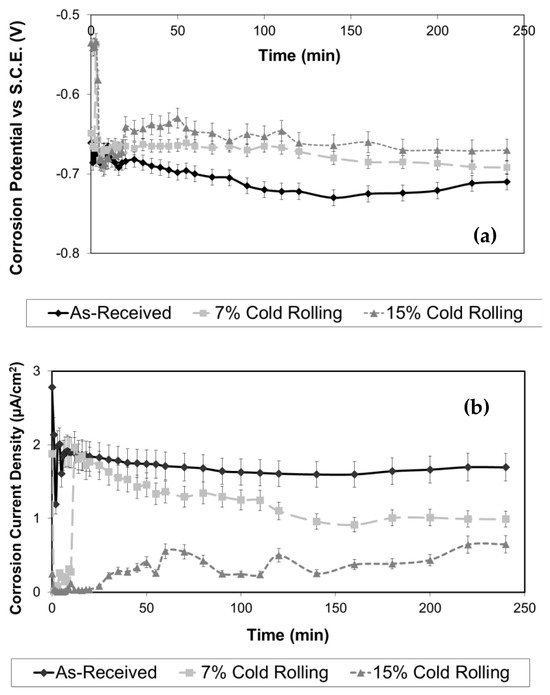
Figure 6.
Effect of cold-rolling percentage on the evolution of (a) corrosion potential and (b) corrosion current density under free corrosion conditions in 3.5% NaCl solution.
To assess the potentiostatic experiments, during the free corrosion experiments the corrosion current density of both the as-received and cold-rolled aluminium alloy specimens was continuously recorded, as shown in Figure 6b. It is apparent from this figure that the increase of the cold-rolling rate has led to lower corrosion current density values, indicating enhanced corrosion resistance of the alloy. These results are in full agreement with the potentiostatic V(t) curves presented previously, in Figure 6a.
This improvement in the corrosion behaviour of the cold-rolled 5083 aluminium alloy, under the specified experimental conditions, can be explained in the following terms:
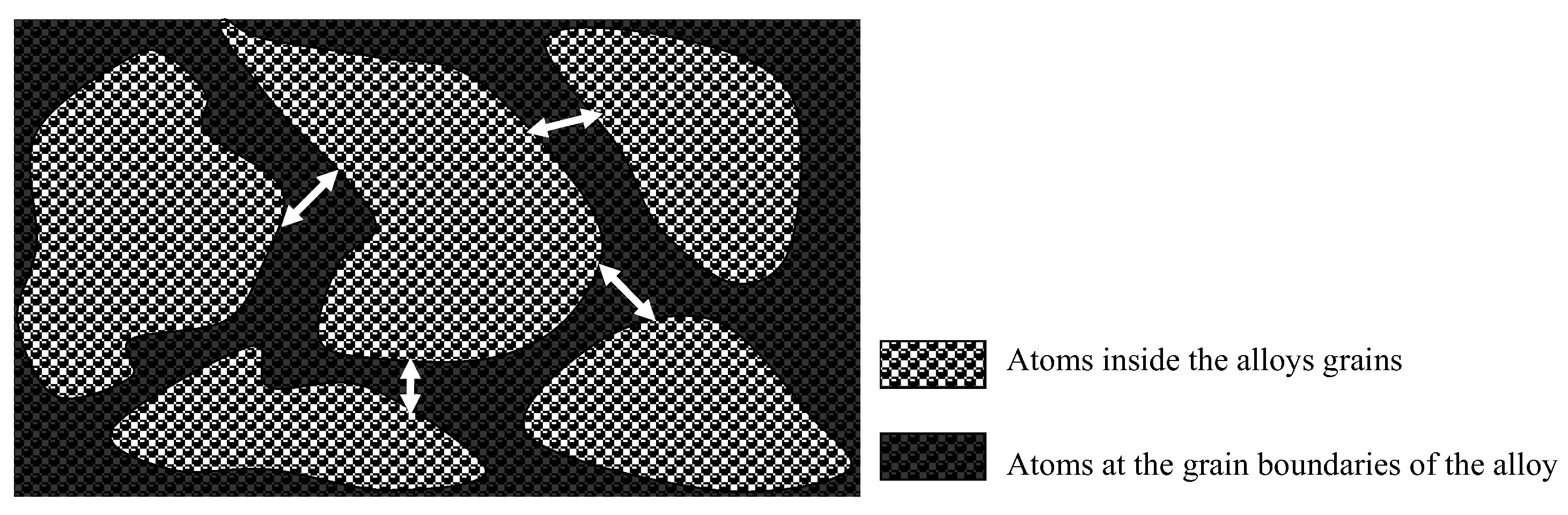
- Grain refinement of the alloy. The grain boundaries in metallic materials are believed to play a crucial role in the corrosion process, acting as ‘easy paths’ for the diffusion of halide ions from the corrosive solution to the surface layers and bulk material. In this case, the cold-rolling process contributed to a decrease in the average grain size of the material (as shown in Figure 4). As the average grain size increases, the grain boundaries become enlarged, as indicated in Figure 7. According to Wagner’s metal oxidation theory [34], the oxidation/corrosion process is determined by diffusion mechanisms that take place in the oxide phase (passivating film) and along the grain boundaries of the bulk material. If the grains are enlarged, more ions can diffuse over time. Moreover, a larger grain size results in the extension of individual grain boundaries deeper into the surface layers of the bulk material. Thus, this enlargement of the grain boundaries is thought to accelerate the corrosion process, as halide ions (Cl-) can diffuse more easily from the corrosive solution deeper into the surface layers of the material.
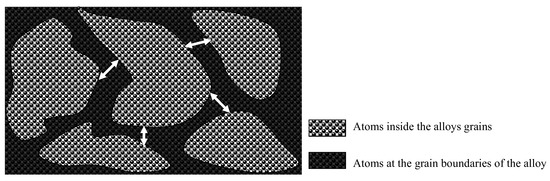 Figure 7. Schematic illustration of the increase of grain boundary width with enlargement of grains.
Figure 7. Schematic illustration of the increase of grain boundary width with enlargement of grains.
- 2.
- Formation of corrosion products. Another aspect to consider is the generation of corrosion products on the alloy’s surface during corrosion. By utilizing X-ray diffraction (XRD), a structural analysis of the corroded surface layers in both the as-received and cold-rolled specimens was performed and is shown in Figure 8. The primary corrosion product identified was aluminium oxide (Al2O3). Peaks from the aluminium solid solution substrate (Mg dissolved in the Al matrix) were also noticeable. However, a comparison of X-ray diffraction patterns in Figure 8 reveals an increase in aluminium oxide peaks with a higher cold-rolling rate. The formation of aluminium oxides on the alloy’s surface is believed to hinder the diffusion of halide ions and, consequently, the corrosion process.
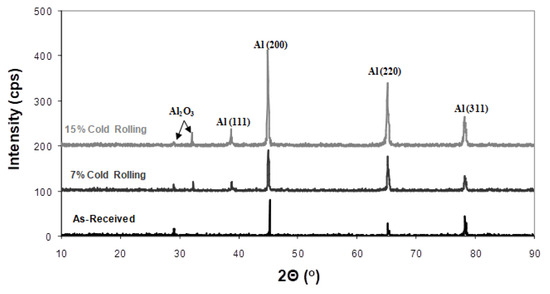 Figure 8. X-ray diffraction of corroded 5083 samples having different rolling percentages.
Figure 8. X-ray diffraction of corroded 5083 samples having different rolling percentages. - 3.
- Crystallographic texturing. The development of crystallographic texturing has been found to impact the corrosion performance of alloys, as observed in magnesium alloys where basal planes exhibit a lower corrosion rate [35]. However, in the case of 5083 aluminium alloy, findings by Li et al. [10] indicate that crystallographic orientation did not considerably influence the corrosion behaviour of this alloy.
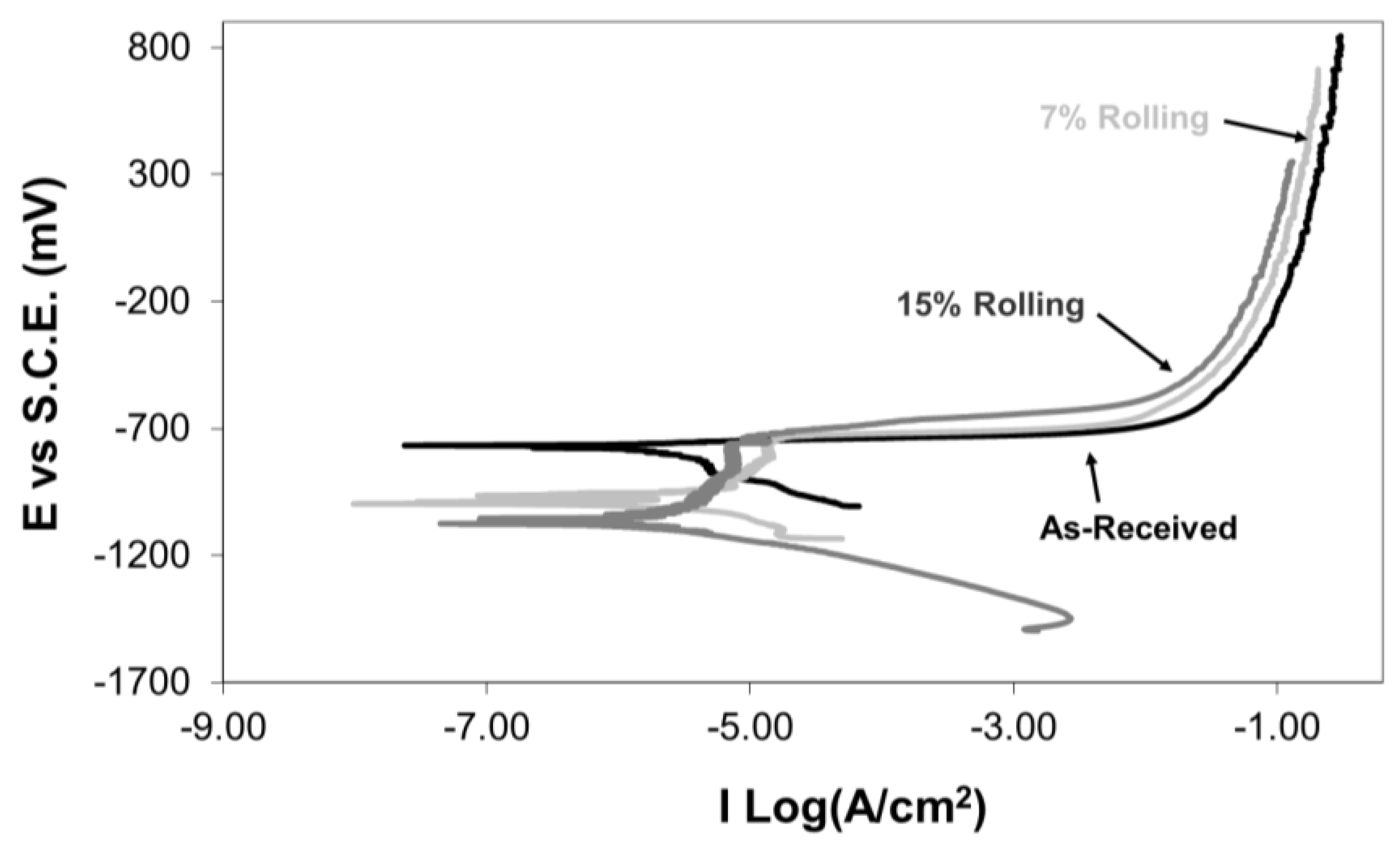
For further investigation of the corrosion behaviour of as-received and cold-rolled aluminium alloys, potentiodynamic corrosion experiments were conducted in a 3.5% NaCl solution. Figure 9 presents typical potentiodynamic corrosion graphs. The as-received specimen showed no passivity regions, indicating continuous corrosion along the anodic branch of the potentiodynamic graph. Conversely, specimens with 7% and 15% cold rolling exhibited small passivity areas at −1000 mV vs SCE and −1070 mV vs SCE, extending up to −720 mV vs SCE and −760 mV vs SCE, respectively. During the passivity region, it is believed that the developed aluminium oxide layer dissolves uniformly. However, after this electrochemical process, pitting occurs with subsequent repassivation up to the breakdown potential. Stable pit growth occurs at potentials higher than the breakdown potential [10]. Furthermore, the rolling process appears to have an influence on the breakdown potential of the passive film. According to the work of Szklarska-Smialowska [36], the breakdown potential depends upon the interaction between the halide ions of the corrosive solution and the structural characteristics of the oxide passive film. In this work, the cold deformation of the surface layers has resulted in an increase of micro-defects (vacancies, voids, etc.) at the surface layers of the alloy [37]. The existence of such micro-defects at and/or near the interface between the oxide film and the base material has been reported to affect the electronic properties and breakdown potential of the passive film [38].
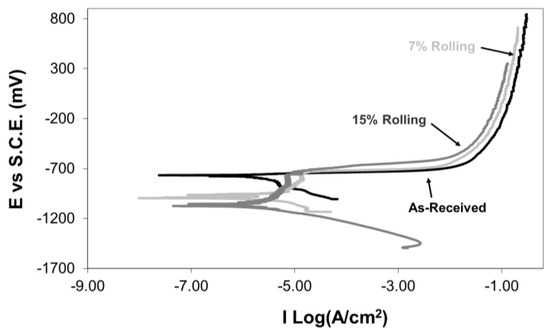
Figure 9.
Effect of cold rolling on the potentiodynamic corrosion curves of 5083 Al alloy in 3.5% NaCl solution.
Comparing the potentiodynamic corrosion curves for the as-received and cold-rolled aluminium specimens, an increase in cold deformation resulted in a shift of the potentiodynamic curves to lower corrosion current values. Thus, the chosen cold-rolling process improved the corrosion resistance of this alloy. This enhancement is attributed to the grain refinement of the alloy and the formation of a protective aluminium oxide film, as explained earlier. However, the open circuit potential of the cold-rolled specimens was observed to be less noble than that of the as-received specimen. The corrosion current (Icorr) and corrosion potential (Ecorr) of the as-received and cold-rolled aluminium alloy specimens obtained from the potentiodynamic curves in Figure 9 are presented in Table 2.

Table 2.
Effect of cold rolling on average corrosion current and potential of 5083 Al alloy in 3.5% NaCl solution, derived from potentiodynamic curves.
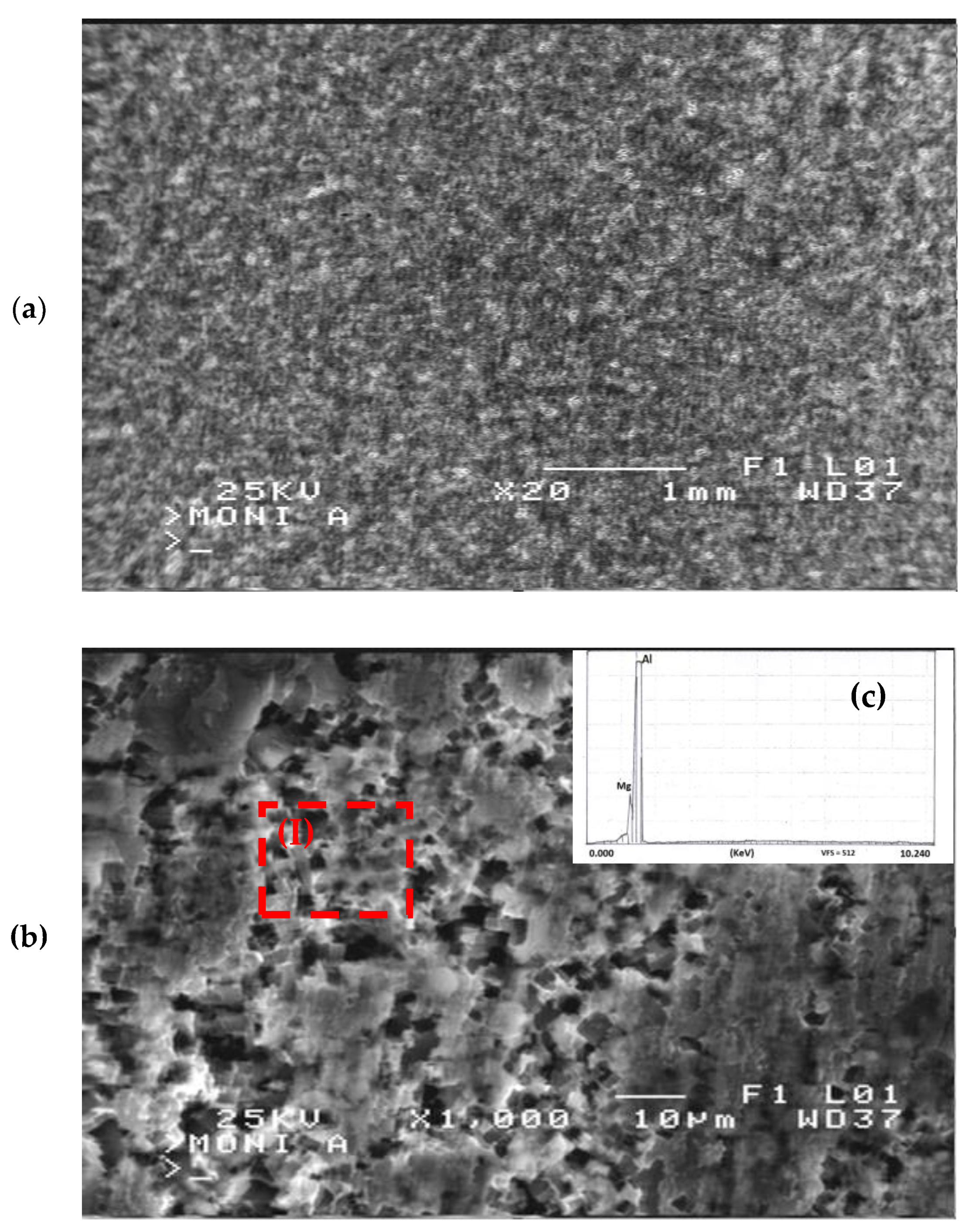
In Figure 10a, a representative metallographic structure of the corroded surface of 15% cold-rolled 5083 aluminium alloy is presented. It is apparent from this figure that the dominant corrosion mechanism was dissolution–precipitation, as corrosion products were observed to form uniformly throughout the surface of the alloy.
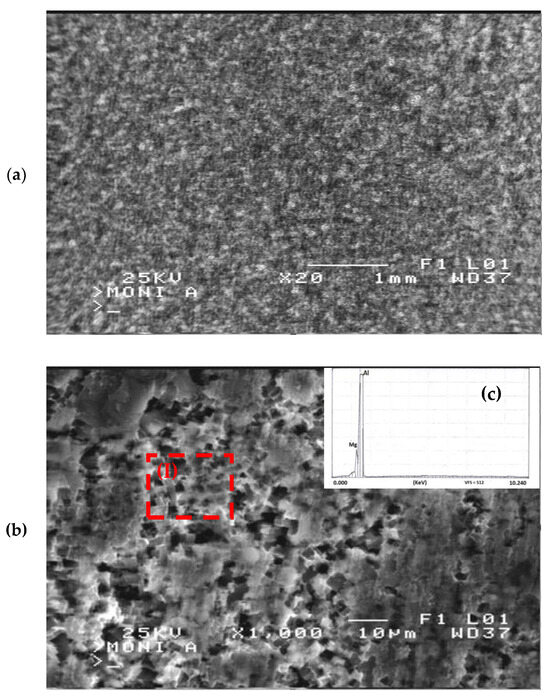
Figure 10.
SEM surface morphology of 15% cold-rolled Al alloy after potentiodynamic corrosion in 3.5% NaCl solution at (a) lower and (b) higher magnification. (c) EDS chemical analysis on area (I) of Figure 9b.
Upon examining the corroded areas, as shown in Figure 10b, extensive pitting corrosion phenomena were identified. This pitting corrosion mechanism can be attributed to the presence of localized galvanic corrosion between the aluminium solid solution and Mg2Si intermetallic phases. According to Yasakau et al. [39], the difference in corrosion potential between the aluminium solid solution and Mg2Si intermetallic phase led to the formation of localized galvanic cells between them, subsequently resulting in the dissolution of the intermetallic phase. The electrochemical reactions that occur at the interface between the alloy and the corrosive solution can be described by the following corrosion mechanism:
Mg2Si + 4H2O → 2Mg(OH)2 +SiH4
SiH4 + mH2O → SiO2nH2O + 4H2↑
Mg2Si + 2H2O → 2Mg+ + SiO2 + 4H+ + 8e−
The dissolution of the Mg2Si intermetallic phase was further validated by conducting localized chemical analyses using the EDS technique. Chemical analyses on area (I) of Figure 10b revealed 97.5 wt. % Al and 2.5 wt. % Mg, shown in Figure 10c. Consequently, the decrease in Mg concentration, coupled with the inability to detect Si, suggests that a portion of the Mg2Si intermetallic phases dissolved during the corrosion process, contributing to the formation of pits.
In addition to the dissolution–precipitation mechanism described earlier, pits can also form due to complex interactions between Cl− halide ions and the surface layers of the aluminium alloy. According to the theory proposed by E. McCafferty [40], chloride ions are adsorbed from the corrosive solution onto the passivating alumina layer and diffuse towards the interface of the alumina and the alloy. The mechanisms of chloride diffusion through the oxide layer have not been fully elucidated [41]. The prevailing theories suggest that chloride ions diffuse through easy paths of the protective layer (e.g., grain boundaries, continuous pores, etc.). However, other researchers [42] propose that corrosive ions move through atomic oxygen vacancies (solid-state diffusion) in the lattice of the protective alumina layer. Earlier theories [42] argued that the diffusion of chloride ions occurs through the local destruction of the protective oxide layer. Another, and perhaps the most prevalent mechanism [43], assumes that chloride ions diffuse through the aluminium protective layer and react with other ions at the interface of the protective layer with the aluminium alloy. Considering that chlorides diffuse through atomic oxygen vacancies in the lattice of the alumina protective layer, complex electrochemical reactions occur [44]. These chemical reactions result in the creation of molecular hydrogen (H2), generating high localized pressures that subsequently rupture the protective layer, forming corrosion pits.
4. Conclusions
This research work focuses on the effect of cold rolling (7% and 15% thickness reduction) on the corrosion behaviour of 5083 aluminium alloys. The selected forming process has led to grain refinement of the alloy due to stress-induced phenomena and to an increase of secondary intermetallic phases. In addition, the development of preferential crystallographic texturing was observed. These structural changes resulted in considerably higher microhardness of the alloy. Furthermore, the selected cold-rolling process also led to an improvement of the corrosion resistance of 5083 aluminium alloy. The increase of corrosion resistance was attributed mainly to the decrease of grain size and structuring, as grain boundaries act as diffusion paths. Finally, in all cases, an alumina layer was formed on the surface of the alloy, whereas the dominant corrosion mechanisms were identified to be dissolution–precipitation and pitting corrosion.
Author Contributions
C.N.P. conceived and designed the experiments; E.P.G. and C.N.P. wrote the paper; C.N.P. and E.P.G. analysed the data; E.P.G. performed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Davis, J.R. Metals Handbook, Properies and Selection of Non-Ferrous Alloys, 9th ed.; ASM: Metals Park, OH, USA, 1985; Volume 2. [Google Scholar]
- Barnes, A.; Raman, H.; Lowerson, A.; Edwards, D. Recent Application of Superformed 5083 Aluminium Alloy in the Aerospace Industry. Mater. Sci. Forum 2012, 735, 361–371. [Google Scholar] [CrossRef]
- Li, S.-S.; Yue, X.; Li, Q.-Y.; Peng, H.-L.; Dong, B.-X.; Liu, T.-S.; Yang, H.-Y.; Fan, J.; Shu, S.-L.; Qiu, F.; et al. Development and applications of aluminum alloys for aerospace industry. J. Mater. Res. Technol. 2023, 27, 944–983. [Google Scholar] [CrossRef]
- Jebaraj, A.V.; Aditya, K.V.V.; Sampath Kumar, T.; Ajaykumar, L.; Deepak, C.R. Mechanical and corrosion behaviour of aluminium alloy 5083 and its weldment for marine applications. Mater. Today Proc. 2020, 22, 1470–1478. [Google Scholar] [CrossRef]
- Georgantzia, E.; Gkantou, M.; Kamaris, G.S. Aluminium alloys as structural material: A review of research. Eng. Struct. 2021, 227, 111372. [Google Scholar] [CrossRef]
- Ezuber, H.; El-Houd, A.; El-Shawesh, F. A study on the corrosion behaviour of aluminium alloys in seawater. Mater. Des. 2008, 29, 801–805. [Google Scholar] [CrossRef]
- Aballe, A.; Bethencourt, M.; Botana, F.J.; Marcos, M.; Sanchez-Amaya, J.M. Influence of the degree of polishing of alloy AA 5083 on its behaviour against localised alkaline corrosion. Corros. Sci. 2004, 46, 1909–1920. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P. Wear behaviour of 5083 wrought aluminium alloy under free corrosion conditions. Tribol.-Mater. Surf. Interfaces 2007, 1, 161–164. [Google Scholar] [CrossRef]
- Zazi, N.; Chopart, J.-P.; Bouabdallah, A. Thermomechanical Treatments Effect on Corrosion Behaviour of Aluminium-magnesium Alloy AA5083–H321. Prot. Met. Phys. Chem. Surf. 2015, 51, 267–274. [Google Scholar] [CrossRef]
- Li, Z.; Yi, D.; Tan, C.; Wang, B. Investigation of the stress corrosion cracking behaviour in annealed 5083 aluminium alloy sheets with different texture types. J. Alloys Compd. 2020, 815, 152690. [Google Scholar] [CrossRef]
- Faraji, M.; Shabestari, S.G.; Razavi, S.H. The Effects of Cold Rolling and Stabilization Treatment on the Improvement of Microstructure, Mechanical Properties, and Corrosion Behaviour of AA5083 Sheet. J. Mater. Eng. Perform. 2023, 32, 2097–2108. [Google Scholar] [CrossRef]
- Abdulstaar, M.; Mhaede, M.; Wollmann, M.; Wagner, L. Investigating the effects of bulk and surface severe plastic deformation on the fatigue, corrosion behaviour and corrosion fatigue of AA5083. Surf. Coat. Technol. 2014, 254, 244–251. [Google Scholar] [CrossRef]
- Beura, V.K.; Kale, C.; Srinivasan, S.; Williams, C.L.; Solanki, K.N. Corrosion behavior of a dynamically deformed Al–Mg alloy. Electrochim. Acta 2020, 354, 136695. [Google Scholar] [CrossRef]
- Zhou, L.; Hyer, H.; Chang, J.; Mehta, A.; Huynh, T.; Yang, Y.; Sohn, Y. Microstructure, mechanical performance, and corrosion behavior of additively manufactured aluminum alloy 5083 with 0.7 and 1.0 wt% Zr addition. Mater. Sci. Eng. A 2021, 823, 141679. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Zhang, M.; Wang, H.; Gong, W.; Lai, R.; Li, Y.; Teng, J. The corrosion behavior and mechanical properties of 5083 Al-Mg alloy manufactured by additive friction stir deposition. Corros. Sci. 2023, 213, 110972. [Google Scholar] [CrossRef]
- Panagopoulos, C.N.; Georgiou, E.P.; Giannakopoulos, K.I. The effect of heat treatment on the corrosion behaviour of 319 cast aluminium alloy. Mater. Corros. 2009, 60, 415–418. [Google Scholar] [CrossRef]
- Dabalá, M.; Ramous, E.; Magrini, M. Corrosion resistance of cerium-based chemical conversion coatings on AA5083 aluminium alloy. Mater. Corros. 2004, 55, 381–386. [Google Scholar] [CrossRef]
- Afseth, A.; Nordlien, J.H.; Scamans, G.M.; Nisancioglu, K. Effect of thermo-mechanical processing on filiform corrosion of aluminium alloy AA3005. Corros. Sci. 2002, 11, 2491–2506. [Google Scholar] [CrossRef]
- Wang, L.; Liang, J.; Li, H.; Cheng, L.; Cui, Z. Quantitative study of the corrosion evolution and stress corrosion cracking of high strength aluminum alloys in solution and thin electrolyte layer containing Cl−. Corros. Sci. 2021, 178, 109076. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Yang, W.; Liu, Y.; Terryn, H.; Cui, Z. Study on the roles of bisulfite in the stress corrosion cracking of 7050-T7451 aluminum alloy in the thin electrolyte layer environment. Corros. Sci. 2023, 215, 111030. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Zhou, X.; Hashimoto, T.; Wang, J. The corrosion behaviour of machined AA7150-T651 aluminium alloy. Corros. Sci. 2017, 126, 265–271. [Google Scholar] [CrossRef]
- Liu, Y.; Hashimoto, T.; Zhou, X.; Thompson, G.E.; Scamans, G.M.; Rainforth, W.M.; Hunter, J.A. Influence of near-surface deformed layers on filiform corrosion of AA3104 aluminium alloy. Surf. Interface Anal. 2013, 45, 1553–1557. [Google Scholar] [CrossRef]
- Liu, E.; Pan, Q.; Liu, B.; Ye, J.; Wang, W. Microstructure Evolution of the Near-Surface Deformed Layer and Corrosion Behavior of Hot Rolled AA7050 Aluminum Alloy. Materials 2023, 16, 4632. [Google Scholar] [CrossRef]
- Ramoul, C.E.; Ghelloudj, O.; Gharbi, A.; Tlili, S.; Beliardouh, N.E.; Chouchane, T. Plastic Deformation Effect on Wear and Corrosion resistance of Super Martensitic Stainless Steel. J. Bio-Tribo-Corros. 2021, 7, 114. [Google Scholar] [CrossRef]
- Bahmani, A.; Lotfpour, M.; Taghizadeh, M.; Kim, W.-J. Corrosion behavior of severely plastically deformed Mg and Mg alloys. J. Magnes. Alloys 2022, 10, 2607–2648. [Google Scholar] [CrossRef]
- Uddin, M.; Santifoller, R.; Hall, C.; Schlaefer, T. Effect of Combined Grinding–Burnishing Process on Surface Integrity, Tribological, and Corrosion Performance of Laser-Clad Stellite 21 Alloys. Adv. Eng. Mater. 2023, 25, 2201332. [Google Scholar] [CrossRef]
- Nah, J.J.; Kang, H.G.; Huha, M.Y.; Engler, O. Effect of strain states during cold rolling on the recrystallized grain size in an aluminium alloy. Scr. Mater. 2008, 58, 500–503. [Google Scholar] [CrossRef]
- Sezek, S.; Aksakal, B. Deformation and temperature behaviour during cold, warm and hot flat rolling of AA5454-O alloy. Mater. Des. 2009, 30, 3450–3459. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Pearson Education Limited: Essex, UK, 2014. [Google Scholar]
- Bérubé, L.P.; Éspérance, G.L. A quantitative method of determining the degree of texture of zinc electrodeposits. J. Electrochem. Soc. 1989, 136, 2314–2315. [Google Scholar] [CrossRef]
- Dieter, G.E. Mechanical Metallurgy; McGraw-Hill: London, UK, 1988. [Google Scholar]
- She, X.; Jiang, X.; Zhang, R.; Wang, P.; Tang, B.; Du, W. Study on microstructure and fracture characteristics of 5083 aluminium alloy thick plate. J. Alloys Compd. 2020, 825, 153960. [Google Scholar] [CrossRef]
- Fine, M.E. Precipitation hardening of aluminium alloys. Metall. Trans. A 1975, 6, 625–630. [Google Scholar] [CrossRef]
- Zhang, X.Y. Understanding the corrosion resistance of nanocrystalline materials: The influence of grain size. In Corrosion Protection and Control Using Nanomaterials; Woodhead Publishing Series in Metals and Surface Engineering; Woodhead Publishing: Sawston, UK, 2012; pp. 34–58. [Google Scholar] [CrossRef]
- Gerashi, E.; Alizadeh, R.; Langdon, T.G. Effect of crystallographic texture and twinning on the corrosion behaviour of Mg alloys: A review. J. Magnes. Alloys 2022, 10, 313–325. [Google Scholar] [CrossRef]
- Szklarska-Smialowska, Z. Pitting corrosion of aluminum. Corros. Sci. 1999, 41, 1743–1767. [Google Scholar] [CrossRef]
- Kenmochi, K.; Yarita, I.; Abe, H.; Fukuhara, A.; Komatu, T.; Kaito, H. Effect of micro-defects on the surface brightness of cold-rolled stainless-steel strip. J. Mater. Proc. Technol. 1998, 69, 106–111. [Google Scholar] [CrossRef]
- Parangusan, H.; Bhadra, J.; Al-Thani, N. A review of passivity breakdown on metal surfaces: Influence of chloride- and sulfide-ion concentrations, temperature, and pH. Emergent Mater. 2021, 4, 1187–1203. [Google Scholar] [CrossRef]
- Yasakau, K.A.; Zheludkevich, M.L.; Lamaka, S.V.; Ferreira, M.G.S. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- McCafferty, E. The electrode kinetics of pit initiation on aluminium. Corros. Sci. 1995, 37, 481–492. [Google Scholar] [CrossRef]
- Marichev, V.A. Kinetics of chloride ion adsorption on stainless alloys by in situ contact electric resistance technique. Electrochim. Acta 2008, 53, 6304–6316. [Google Scholar] [CrossRef]
- Korb, L.J.; Olson, D.L. Metals Handbook, Corrosion, 9th ed.; ASM: Metals Park, OH, USA, 1987; Volume 13. [Google Scholar]
- McCafferty, E. Sequence of steps in the pitting of aluminium by chloride ions. Corros. Sci. 2003, 45, 1421–1438. [Google Scholar] [CrossRef]
- Bargeron, C.B.; Benson, R.C. Analysis of the Gases Evolved during the Pitting Corrosion of Aluminium in Various Electrolytes. J. Electrochem. Soc. 1995, 127, 2528–2530. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).